Abstract
Cytochrome P450s (P450s) comprise a superfamily of proteins that catalyze numerous monooxygenase reactions in animals, plants, and bacteria. In eukaryotic organisms, these proteins not only carry out reactions necessary for the metabolism of endogenous compounds, but they are also important in the oxidation of exogenous drugs and other foreign compounds. Eukaryotic P450 system proteins generally reside in membranes, primarily the endoplasmic reticulum or the mitochondrial membrane. These membranes provide a scaffold for the P450 system proteins that facilitate interactions with their redox partners as well as other P450s. This review focuses on the ability of specific lipid components to influence P450 activities, as well as the role of the membrane in P450 function. These studies have shown that P450s and NADPH–cytochrome P450 reductase appear to selectively associate with specific phospholipids and that these lipid–protein interactions influence P450 activities. Finally, because of the heterogeneous nature of the endoplasmic reticulum as well as other biologic membranes, the phospholipids are not arranged randomly but associate to generate lipid microdomains. Together, these characteristics can affect P450 function by 1) altering the conformation of the proteins, 2) influencing the P450 interactions with their redox partners, and 3) affecting the localization of the proteins into specific membrane microdomains.
Introduction
Cytochrome P450s (P450s) are clinically of great importance because they are responsible for the phase I metabolism of a majority of xenobiotics (Bertz and Granneman, 1997). Variability in P450 activities can be attributable to modulation of P450 protein levels, inhibition of activities by other chemicals (Lin and Lu, 1998), and genetic differences resulting from both structural and expression polymorphisms (Guttendorf and Wedlund, 1992). P450 primarily catalyzes monooxygenation reactions of both xenobiotics and endogenous substrates (Guengerich, 1992). These enzymes function as the terminal component of an electron transport chain that includes interactions with the redox partners, NADPH–cytochrome P450 reductase (CPR) and cytochrome b5, as well as other P450 enzymes (Lu et al., 1969; Hildebrandt and Estabrook, 1971; Backes et al., 1998; Reed et al., 2010). The components of this electron transfer chain are anchored in the endoplasmic reticulum (ER) membrane, which orients the proteins for more efficient electron transfer (Black and Coon, 1982). Phospholipid, which was originally thought to be an obligatory component of this system (Strobel et al., 1970), has been shown to not be required (Müller-Enoch et al., 1984) but, in most cases, supports P450 activities. Phospholipid serves as a matrix for incorporation of P450 and CPR (Causey et al., 1990), promotes the proper orientation of the P450s with their redox partners, and generally facilitates substrate binding (Ingelman-Sundberg et al., 1983; Taniguchi and Pyerin, 1988). Consequently, study of the interactions among P450 system components as well as the interactions between P450 enzymes in their lipid environment have become increasingly relevant for a more complete understanding of P450 function. This review examines the lipid components comprising the ER, as well as the effect of lipid composition on the P450 system.
Structure of the ER
The ER is a complex network of lipids, containing many membrane bound proteins, including those of the P450 enzyme system (Glaumann and Dallner, 1968). Electron microscopy shows the ER membrane to be 50–80 Å thick (Yamamoto, 1963), with a surface area 37 times that of the plasma membrane (Weibel et al., 1969). The lipid composition of rabbit liver microsomes was reported to be approximately 60% phosphatidylcholine (PC), 20% phosphatidylethanolamine (PE), 1% phosphatidylserine (PS), 1% phosphatidic acid (PA), 10% phosphatidylinositol (PI), 4% sphingomyelin (SM), and 5% cholesterol (Brignac-Huber et al., 2011). These values are similar to those found in liver microsomes of humans (Waskell et al., 1982) and rats (Dallner and Ernster, 1968; Glaumann and Dallner, 1968; Davison and Wills, 1974). Additional studies characterized the asymmetry of the phospholipid species and reported that approximately 40% of the PC, 90% of the PE, 14% of the SM, 90% of the PS, and 20% of the PI are located on the outer leaflet of the membrane (Nilsson and Dallner, 1975). Although phospholipids of the rough and smooth ER were reported to be very similar (Glaumann and Dallner, 1968), cholesterol was found to be twice as high in the smooth ER (Pascaud, 1958; Glaumann and Dallner, 1968). Moreover, the ER is a dynamic organelle that can proliferate in response to xenobiotic exposure (Remmer and Merker, 1963).
The lipid bilayer has been shown to play an essential role in protein function at the plasma membrane and similar findings have been reported for the ER. It is estimated that the ER from rat liver is composed of approximately 70% protein and 30% lipid by weight (Glaumann and Dallner, 1968). In vivo studies suggested that phospholipids in the immediate vicinity of P450 molecules are more organized than the bulk membrane and that the P450 is enclosed in a “phospholipid halo,” possibly affecting the diffusion of substrates and products to and from the P450 molecule (Stier and Sackmann, 1973). The exact functional role of the interaction between the phospholipid and the P450 system has been the subject of many studies, which have provided a better understanding of the system in vivo. These studies have employed reconstitution of the proteins into compositionally defined mixtures of phospholipids, as well as natural membranes that are heterogeneous in nature with regard to both lipid and protein content. Numerous lipid species have been used in reconstituted systems that have led to differences in P450 stability and catalytic activities (Ingelman-Sundberg et al., 1981, 1996; Yun et al., 1998; Ahn et al., 2005; Brignac-Huber et al., 2011). This is not surprising, considering that the ER membrane is composed of a variety of lipids. Although PC was found to be a major component that facilitates P450 interactions with their redox partners (Strobel et al., 1970), it is known that the other ER lipids play a critical role in ER membrane structure and monooxygenase function.
Phosphatidylcholine
PC is reported to be the major phospholipid constituent of the ER bilayer (Glaumann and Dallner, 1968) with about one-half of this lipid species located in the outer leaflet (Nilsson and Dallner, 1975). Most PC species contain one unsaturated acyl chain that increases membrane fluidity at room temperature. The cylindrical shape of PC facilitates its spontaneous incorporation into a bilayer (van Meer et al., 2008). PC has been the most studied phospholipid in the context of the P450 system, owing to its prevalence in the ER membrane. Numerous studies both in vivo and in vitro have strengthened our understanding of the role of PC in the monooxygenase system.
In vitro studies by Strobel et al. (1970) established PC as an important lipid constituent in the P450 monooxygenase system, illustrating that optimal electron transfer from CPR to P450 was dependent on the presence of this microsomal lipid. A number of synthetic PC variants produced similar P450 activities when substituted for the microsomal lipid, with dilauroylphosphatidylcholine (DLPC) producing the highest activity (Strobel et al., 1970). This finding led to the use of DLPC as a standard lipid for many P450 studies. Although DLPC is commonly used to prepare preformed lipid vesicles, it was illustrated rather early that certain methods of lipid preparation and protein addition can prevent the enzymes from integrally incorporating into the lipid bilayer (Autor et al., 1973; Ingelman-Sundberg and Glaumann, 1980; Reed et al., 2006). Consequently, many studies have characterized improved methodologies for reconstituting P450 and CPR into PC lipid membranes. The use of the longer chain phospholipids, along with detergent to facilitate CPR and P450 reconstitution, allowed for their more efficient physical incorporation into lipid vesicles. Ultimately, the integration of P450s into phospholipid bilayers affected membrane binding and catalytic characteristics of the P450 enzymes (Taniguchi et al., 1979; Ingelman-Sundberg and Johansson, 1980; Reed et al., 2006, 2008).
The PC lipid component of the ER can be altered by xenobiotic exposure. Young et al. (1971) examined changes in microsomal phospholipid and protein components in rats after phenobarbital (PB) treatment. The investigators found an increase in PC levels as a result of the methylation of PE. Although this study did not focus on P450 levels, the authors did report a significant increase in the total microsomal protein content after induction (Young et al., 1971). A more detailed study by Davison and Wills (1974) reported that both PB and 20-methylcholanthrene caused an increase in PC levels days after injections, with the proportion of linoleic acid of both PC and PE being significantly increased by PB treatment. This increase in linoleic acid corresponded to increased concentrations and activities of P450 and CPR (Davison and Wills, 1974). This group suggested that PC may be more significant than PE for drug metabolism. An additional but more recent finding suggests that CYP1A2 possesses phospholipase D (PLD) activity, converting PC to PA. PA serves as a precursor for the formation of diacylglycerol, an important molecule in signal transduction events. CYP1A2-mediated PLD activity was not dependent on the presence of CPR and was not inhibited by the use of specific P450 inhibitors, suggesting that there may be an alternate active site that catalyzes this reaction. This group suggests that the PLD activity of CYP1A2 could affect membrane organization of the ER by metabolizing PC to other lipid products (Yun et al., 1999).
Phosphatidylethanolamine
PE comprises about 25% of the ER bilayer in vivo and is almost exclusively located in the outer leaflet (Nilsson and Dallner, 1975). The presence of this phospholipid has a profound effect on protein organization and function.
Phospholipid packing into bilayers is a function of the general shape of the molecules. This shape is dependent on the size of the polar head group compared with the size of the phospholipid side chains. Side chains that are unsaturated will displace a larger area in the nonpolar region of the membrane, whereas saturated fatty acids would occupy a smaller space. For example, PC, which has polar and nonpolar regions of similar size, would better pack into a bilayer. In contrast, PE, which has a smaller polar head group, would be shaped more like a cone. This would lead to a more negative curvature of the membrane and would generate nonlamellar phases in the purified form (Luzzati and Husson, 1962). These nonlamellar-forming lipids affect the fluidity of the membrane and have been reported to modulate the activities of numerous proteins, including rhodopsin (Epand, 1998), protein kinase C (Epand, 1987), and CYP11A1 (Schwarz et al., 1997), as a few examples.
Generally speaking, the addition of PE tends to increase the fluidity of the membrane, which can be deduced by changes in the temperature of the transition from the lamellar to nonlamellar phases. The temperature of this transition, as measured using 31P-nuclear magnetic resonance (NMR), is generally lower for PE than PC and is further decreased by an increase in unsaturation of the fatty acid chains and the addition of cholesterol (Dekker et al., 1983). In another 31P-NMR study, Bayerl et al. (1985) used hexane phosphonic acid diethyl ester as a 31P-NMR probe. Using an upfield shift in the 31P-NMR signal as an indicator of protein binding to the membrane, systems in which CPR and P450 were reconstituted into PC vesicles did not produce this upfield shift; however, the upfield shift was observed when PE was included in the reconstituted system. On the basis of this result, the authors concluded that P450 and CPR interact specifically with PE. Interestingly, this shift was not observed when only one of the proteins was present, suggesting that PE may play an integral role in the interaction between these proteins.
The importance of PE in reconstitution of P450s was first described by Bösterling et al. (1979), in which the reconstitution of CYP2B4 and CPR was made with a lipid mixture of PC, PE, and PA. As opposed to the PC membranes frequently used (Taniguchi et al., 1979), this group found that the mixed system prevented vesicle aggregation, possibly owing to the structural stability presented by the PC/PE mixture and the negative surface charge due to the presence of PA. This system also produced hydrogen peroxide at levels comparable to those in microsomes, which led this group to conclude that their mixed system more closely mimicked the in vivo membrane (Bösterling et al., 1979). Ingelman-Sundberg et al. (1981) showed that CYP2B4 was more active when reconstituted into mixtures of dioleoylphosphatidylcholine and dioleoylphosphatidylethanolamine (DOPE), with the reaction rates increasing as the fraction of DOPE was elevated. Furthermore, there was a direct relationship between the presence of DOPE in the reconstituted system and the rate of first electron transfer (Ingelman-Sundberg et al., 1981). This led the investigators to suggest that the presence of PE led to more efficient electron transfer, possibly through a more efficient interaction between CPR and CYP2B4.
CYP1A2 and CYP3A4 have been studied in PE-containing membranes. Ahn et al. (2005) reported that the addition of PE to PC vesicles caused an increase in CYP1A2 stability and insertion into the membrane, with a concomitant decrease in CYP1A2 sensitivity to trypsin digestion. Likewise, the time-dependent decrease in catalytic activity seen in PC membranes was stabilized by the inclusion of PE in the reconstituted system. The addition of diacylglycerol, another nonlamellar-prone lipid, to the PC/PE reconstituted system furthered augmented these results (Ahn et al., 2005). CYP3A4 studies suggested a role for domain formation by PE. In these studies, CYP3A4 activity in PC-containing reconstituted systems was unaffected by the addition of PE; however, when the anionic phospholipids PA or PS were included, the addition of PE to the PC/PA or PC/PS reconstituted systems increased CYP3A4 activities. This led the investigators to suggest that the presence of PE in a ternary system of PC/PE/PA or PC/PE/PS led to the formation of anionic lipid-enriched domains, which led to enhanced membrane binding and catalytic activity of CYP3A4 (Kim et al., 2003).
Anionic Phospholipids
In the above studies, PE elicited many of its effects in conjunction with the anionic phospholipids. Although PI, PS, and PA make up only a small percentage of the ER bilayer lipids (Dallner and Ernster, 1968; Waskell et al., 1982; Brignac-Huber et al., 2011), they provide a negatively charged environment that has been suggested to be an effector of P450-catalyzed reactions (Ingelman-Sundberg et al., 1981). In general, anionic phospholipids produce a negatively charged interface that has been shown to facilitate protein insertion into membranes (de Kruijff, 1997). Moreover, these phospholipids play a key role in regulation of cellular functions of the plasma membrane as well as intracellular organelles (Buckland and Wilton, 2000).
Ingelman-Sundberg and Johansson (1980) first established that the minor ER phospholipids play an important role in the P450 system when replacement of PC with microsomal lipids caused an increase in CYP2B4 activity. Further experiments by this group incorporated different lipids into reconstituted systems, including PC, PE, and a mixture of PE with negatively charged PS. Not only did PE stimulate 7-ethoxycoumarin and p-nitroanisole metabolism (as mentioned above), but these activities were further stimulated by the presence of negatively charged PS (Ingelman-Sundberg et al., 1981).
The importance of PE in facilitating CPR/P450 complex formation was further examined by Blanck et al. (1984), who reported that a 3:1 lipid mixture of PE/PS produced a dissociation constant for the CPR/P450 complex that was comparable to systems made with microsomal lipid extracts, which was 10 times lower than in neutral dioleoylphosphatidylcholine membranes. These results could be attributable to the negative charge imposed by the PS or may be a function of PE in the mixed system.
The involvement of negatively charged phospholipids in the incorporation of P450 system proteins was supported by 31P-NMR studies showing that PS and PI were associated with CPR upon purification (Balvers et al., 1993). Subsequent in vitro experiments illustrated that the addition of PS to these reconstituted systems led to a significantly increased rate of CYP2B1-mediated 7-pentoxyresorufin dealkylation compared with PC and PC/PE systems, results that are consistent with those of Ingelman-Sundberg et al. (1981) using CYP2B4. However, these results could not be exclusively attributed to the negative charge of the phospholipid, since the presence of PI did not illicit the same results (Balvers et al., 1993). Das and Sligar (2009) more recently came to similar conclusions when they found that the redox potential of CPR was more negative when 50 mol% PS was added to PC-containing nanodiscs, illustrating that the anionic environment favored electron transfer from CPR.
Other investigators have found that the P450s, in the absence of CPR, have a greater affinity for negatively charged phospholipids. Enhanced membrane insertion mediated by anionic phospholipids was reported for CYP1A2 (Ahn et al., 1998), CYP3A4 (Kim et al., 2003), and CYP2B1 (Kim et al., 2007). The CYP1A2 study also demonstrated that the anionic lipids increased cumene hydroperoxide–mediated CYP1A2 activity, with PA having the greatest effect. These results were accompanied by an observed structural change in CYP1A2 (Ahn et al., 1998). Altogether, this particular study illustrated that anionic lipids could effectively change the catalytic potential of CYP1A2 and was not only a function of altered affinity for CPR.
More recently, Sevrioukova and Poulos (2015) characterized a citrate binding site that was located near the F-G loop of CYP3A4. This is an area where anionic membrane phospholipids may interact with the P450. The presence of citrate affects the ability of substrates to bind and produce a low-to-high spin conversion. This effect is substrate dependent with the testosterone-induced low-to-high spin conversion being significantly enhanced; however, the effect of citrate on the 7-benzyloxy-4-(trifluoromethyl) coumarin–induced conversion was much less prominent. Citrate did not appear to influence the oligomeric state of CYP3A4. CYP3A4 activities and first electron transfer to CYP3A4 were also stimulated by citrate (when CPR was present). However, CPR reduction by NADPH was also stimulated by citrate, making it difficult to parse out the specific effects of citrate on the P450 itself. Taken together, it is plausible that citrate and anionic phospholipids are interacting at a similar region of the P450 molecule.
The role of the F-G loop on association with the membrane was underscored for CYP2J2. Using molecular dynamics simulations, McDougle et al. (2015) showed the proposed binding orientation of CYP2J2 to the membrane, suggesting an interaction among several hydrophobic residues in the F-G loop and the membrane. On the basis of these results, specific F-G loop mutations were generated, replacing hydrophobic Ile-236 and Phe-239 with Asp and His, respectively. When expressed in Escherichia coli, the mutants localized to the cytosolic fraction to a greater extent than Δ34-CYP2J2, consistent with the molecular dynamics simulations. These results support the role of the F-G loop of CYP2J2 with its interaction with the membrane.
The studies discussed above have led to the consideration that membrane lipid heterogeneity can affect not only the interactions between protein and membrane but also protein localization through the formation of distinct lipid domains that can modulate the protein interactions. For instance, it was found that CYP3A4 activity was increased as a function of the concentrations of the anionic phospholipids, PS and PA. These results correlated with an increase in membrane insertion of CYP3A4 and it was concluded that these results were due to the ability of anionic phospholipids to form lipid domains that allowed for proper positioning of the proteins within the lipid bilayer (Ahn and Yun, 1998; Kim et al., 2003). Similarly, CYP2B1 was shown to modulate membrane properties in reconstituted systems by inducing segregation of anionic phospholipids leading to distinct lipid domains. These domains led to alterations in the activity and conformation of CYP2B1 (Kim et al., 2007).
Until our recent publications (Brignac-Huber et al., 2011, 2013; Park et al., 2014, 2015), there were no studies illustrating the effects of such lipid domains in microsomal tissue and their potential to affect P450 function. However, the existence of ER lipid domains has become more widely recognized and can significantly influence the localization of P450 system proteins.
Lipid Microdomains
Early concepts of lipid bilayer structure were established by the fluid mosaic model (Singer and Nicolson, 1972), which described the bulk of the phospholipids as being organized discontinuously with a small fraction of the lipid specifically interacting with integrally incorporated proteins. It also suggested the possibility of cooperative effects with the membrane and specific ligands. Recent studies have greatly increased our understanding of the organization of the lipid membrane, which has been shown to play fundamental roles in protein–protein and protein–lipid interactions. The long saturated fatty acid chains of sphingolipids facilitate their association with cholesterol to form heterogeneities in the membrane that are referred to as liquid-ordered (lo) domains or lipid rafts. The lipids that surround these ordered “raft” regions tend to be more fluid, being enriched in phospholipids containing unsaturated fatty acids. The lo domains have been characterized by their resistance to solubilization by nonionic detergents and have been designated as detergent-resistant membranes (DRMs) (Simons and van Meer, 1988; Simons and Ikonen, 1997; Diaz-Rohrer et al., 2014). Such ordered membrane regions have been implicated in numerous cellular processes, such as lipid and protein sorting and transmembrane signaling through glycosylphosphatidylinositol (GPI)–anchored proteins and secretory and endocytic pathways (Brown and London, 1998). These organized domains have been described primarily in the plasma membrane (Simons and Ikonen, 1997; Brown and London, 1998; Pike, 2003, 2004), Golgi apparatus (Rixon et al., 2004; Vetrivel et al., 2004), and mitochondria (Bini et al., 2003; Foster et al., 2003). The potential for SM- and cholesterol-enriched lo domains to be found in the ER was thought to be less likely, owing to the low concentrations of these constituents in this organelle (Holthuis et al., 2001; Prinz, 2002). Consequently, lipid microdomain formation has only been identified in the ER more recently.
Sevlever et al. (1999) first described organized microdomains of the ER, referred to as Triton-insoluble membranes (TIMs), upon studying the GPI-anchor biosynthetic pathway that occurs in the ER (Sevlever et al., 1999). These domains were isolated by solubilization with 1% Triton X-100 followed by centrifugation using a discontinuous sucrose gradient (Brown and Rose, 1992). Under these conditions, the TIMs would remain intact and float to the 5%/38% interface of the sucrose gradient, whereas the more disordered membrane regions are solubilized. In a study examining the localization of GPI-anchored proteins, ER samples were isolated and shown to be enriched in several of the GPI-anchored intermediates as well as the ER marker calnexin, indicating that the GPI intermediates localized in the ER. When the ER fraction was then subjected to Triton X-100 solubilization/centrifugation, the TIM fractions were shown to be enriched in SM (85%), cholesterol (73%), and some of the GPI intermediates (Sevlever et al., 1999). These results show that the ER contains SM- and cholesterol-enriched ordered domains. Subsequently, these investigators demonstrated that ER-TIMs could be oriented to either the cytoplasmic or luminal face, suggesting that this type of domain is not exclusive to one side of the ER bilayer leaflet (Pielsticker et al., 2005).
Sarnataro et al. (2004) found that specialized ER domains may play a protective role in minimizing protein misfolding. They studied the conversion of cellular prion protein into the infectious misfolded protein, scrapie prion protein, which plays a role in the pathology of transmissible spongiform encephalopathies. Their results showed that disruption of these domains by cholesterol depletion slowed protein maturation and increased protein misfolding to the infectious scrapie prion. These results led the authors to conclude that association of cellular prion protein with cholesterol-enriched ER domains allows for correct protein folding (Sarnataro et al., 2004).
Finally, Browman et al. (2006) characterized two novel proteins, erlin-1 and erlin-2, which were found to reside in lipid-raft-like domains in the ER. These two proteins were found to be new members of the prohibitin family of proteins by the presence of a conserved prohibitin-homology domain of approximately 160 amino acids. Shared properties of these prohibitin family proteins include detergent insolubility, flotation on sucrose gradients, association with membranes, and the ability to form oligomers (Browman et al., 2006).
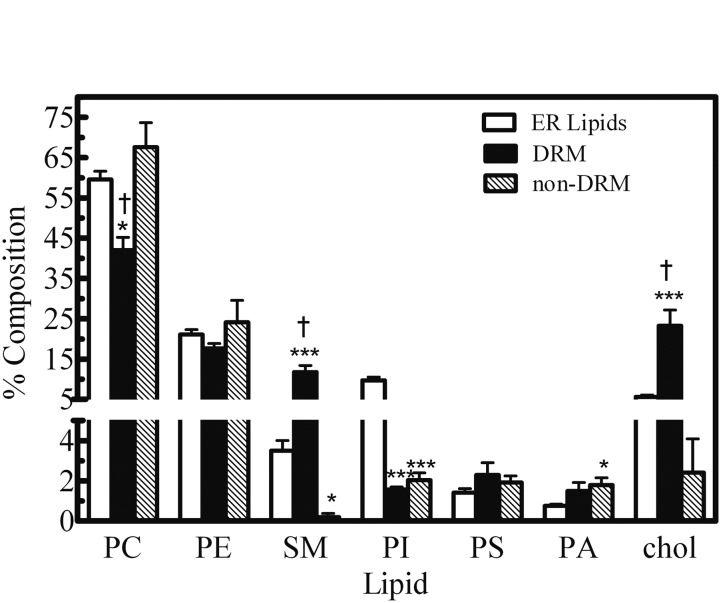
The selective association of proteins into specific domains of the ER has become more widely accepted; however, microdomain localization of P450 system proteins has not been extensively examined. Bae et al. (2004) performed a large proteomics study of liver tissue and found 37 microsomal proteins including CYP1A2 and CPR to reside in ER-DRMs (Bae et al., 2004). More recently, our laboratory has found similar results, in which approximately 70% of CYP1A2 and CPR were found in DRM fractions (i.e., ordered regions) of microsomes from rabbits after detergent treatment with 1% Brij 98 and discontinuous sucrose gradient centrifugation (Brignac-Huber et al., 2011). Lipid analysis of these membrane fractions showed that the composition of the DRM fractions differed from those of the ER lipids, having higher levels of SM and cholesterol and lower levels of PC and PI (Fig. 1). The stability of the DRMs was dependent on cholesterol, as seen by disruption of the domains after cholesterol depletion with methyl-β-cyclodextrin. After cholesterol depletion, both CYP1A2 and CPR were found in the non-DRM fractions of the sucrose gradient, suggesting that cholesterol was an integral component of the CYP1A2-containing ordered microdomains. In addition, microsomal substrate metabolism was significantly lower after cholesterol depletion. Interestingly, reincorporating cholesterol back into the microsomal tissue, after depletion, allowed for reassociation of CYP1A2 and CPR into the DRM fractions and recovery of microsomal substrate metabolism. Moreover, incorporation of purified CYP1A2 and CPR into lipid vesicles having a lipid composition that mimicked that of the DRM exhibited a lower apparent Km for the CPR-CYP1A2 complex compared with when the proteins were incorporated into PC vesicles (Brignac-Huber et al., 2011). Our studies suggest that the specific lipid composition of ER-DRMs allows for more efficient substrate metabolism by increased binding efficiency between CYP1A2 and CPR.
Fig. 1.
Comparison of the phospholipid composition of liver microsomes with that of the ordered microdomains. Rabbit liver microsomes were subjected to solubilization using 1% Brij 98 followed by discontinuous sucrose density gradient centrifugation to isolate the ordered (DRM) regions of the membrane. The phospholipid composition of the ordered region was determined and compared with that of untreated microsomes. This research was originally reported by Brignac-Huber et al. (2011).
Other groups have also suggested that organization of P450s into lipid microdomains could influence P450 function. Cardiolipin (CL), which has been shown to be a constituent of lipid raft-like domains in mitochondria (Sorice et al., 2009), has been implicated as a functional modulator of CYP2E1 and CYP1B1. Cho et al. (2008) reported that the presence of 30 mol% CL in reconstituted systems allowed for approximately a 90% decrease in CYP2E1-mediated reactive oxygen species production. The presence of CL did not affect CYP2E1 activity or NADPH oxidation, which led the group to propose that the specific interaction of CYP2E1 with CL decreased reactive oxygen species production, although the molecular mechanism was undetermined. Similarly, CYP1B1’s catalytic activity, thermal stability, and membrane binding were affected by the presence of CL (Jang et al., 2010). In both studies, the membrane-spanning region of the P450s was found to be important in these CL-induced effects, as illustrated by experiments with the truncated form of the two proteins. Although the CL concentration used in the above studies was much higher than what is found physiologically in the ER, there is increasing evidence that the ER contains enzymes that play a major role in CL metabolism (Cao et al., 2004; Zhao et al., 2009) and may be physiologically relevant in P450 regulation (Jang et al., 2010). CL is known to exist primarily in the inner mitochondrial membrane, although low levels have been reported in the ER (Baraud and Maurice, 1980). The low levels of CL in the ER likely diminish its potential role with regard to microsomal P450 function; however, the significant concentrations of CL (approximately 14%) in liver mitochondria (Paradies et al., 1991) may influence the localization and function of P450s that normally reside in the mitochondria, or even microsomal forms that are alternatively targeted to the mitochondria.
Our laboratory has conducted studies using a variety of fluorescent-labeled probes to determine: 1) whether the lipid microdomains observed in the ER could be detected in reconstituted systems at lipid compositions similar to those found in microsomes and 2) whether microdomains from reconstituted systems similarly affected localization of P450 system proteins. Utilizing lipid probes, which have been characterized in model membranes as localizing to lo or liquid-disordered regions of a membrane, we showed that ordered lipid domains do form in reconstituted systems and that lipid phase separation was seen at lipid compositions similar to those found in liver ER. Furthermore, CYP1A2 was found to reside in the lo regions of these reconstituted systems in a manner analogous to that observed with the ER (Brignac-Huber et al., 2011, 2013; Park et al., 2014, 2015).
More recently, the localization of other P450 enzymes was examined (Park et al., 2014). In this study, rabbits were treated with either PB to induce CYP2B4 or pyrazole to induce CYP2E1. CYP2B4 was shown to distribute roughly equally between both the ordered and disordered membrane regions after PB treatment. In contrast, CYP2E1 was shown to reside predominantly in the disordered regions of microsomes from pyrazole-treated rabbits. CYP1A2 and CPR localization was not affected by either inducer, remaining in the ordered microdomains. Interestingly, CYP1A1 was found to reside in the disordered regions of the ER. Taken together, these results clearly show that P450 enzymes reside in specific regions of the membrane and that their localization can influence P450 function.
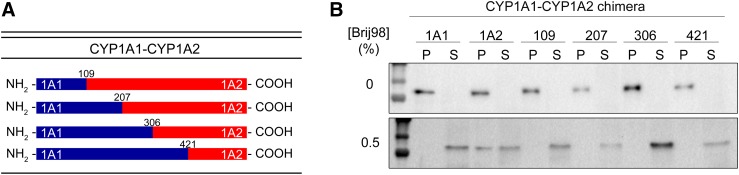
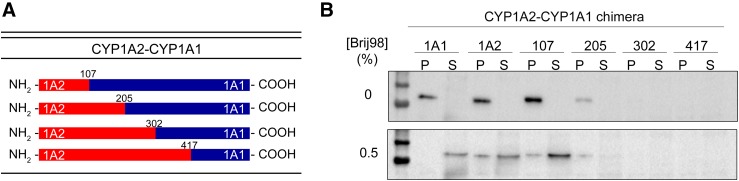
The partitioning of P450 system proteins into different ER microdomains led to questions regarding the presence of amino acid sequences that directed proteins into either ordered or disordered regions. To address this question, we focused on two closely related P450s, CYP1A1 and CYP1A2, that exhibit a high degree of sequence similarity but localize into different microdomains. When examining the sequences of these proteins, two regions of variability were noted: 1) the early portion of the NH2 terminus and 2) an internal region from amino acids 207–306. To test whether these regions were responsible for their differential localization, NH2-CYP1A2-CYP1A1 and NH2-CYP1A1-CYP1A2 chimeric proteins were generated (Fig. 2A). CYP1A2, which resides predominantly in the ordered regions of the ER, could be relocalized to the disordered regions by replacement of 100 amino acids of the NH2 terminus with that of CYP1A1 (Fig. 2B). Conversely, replacement of the NH2 terminus of CYP1A1 with the first 107 amino acids of CYP1A2 caused its partial relocalization to the ordered regions (Fig. 3).
Fig. 2.
Replacement of the NH2-terminal region of CYP1A2 with that of CYP1A1 changes its membrane localization. The microdomain localization CYP1A2 and chimeric proteins where the NH2 terminus was substituted with that of CYP1A1 was measured by expression of the proteins in human embryonic kidney 293T cells and partial membrane solubilization using Brij 98. After Brij treatment, the samples were subjected to centrifugation and the proteins remaining in the membranes were detected in the pellet using PAGE. (A) Schematic of the chimeric proteins generated. (B) Immunoblot of the native CYP1A proteins and the chimeras. Proteins in the pellet remain associated with the membrane, whereas those in the supernatant were solubilized. P, pellet; S, supernatant. This research was originally published in the Journal of Biological Chemistry [Park JW, Reed JR, and Backes WL. The localization of cytochrome P450s CYP1A1 and CYP1A2 into different lipid microdomains is governed by their NH2-terminal and internal protein regions. Journal of Biological Chemistry. (2015) 290, 29449–29460. © American Society for Biochemistry and Molecular Biology].
Fig. 3.
Replacement of the NH2-terminal region of CYP1A1 with that of CYP1A2 changes its membrane localization. The microdomain localization CYP1A1 and chimeric proteins where the NH2 terminus was substituted with that of CYP1A2 was measured as described in the legend for Fig. 1. (A) Schematic of the chimeric proteins generated. (B) Immunoblot of the native CYP1A proteins and the chimeras. P, pellet; S, supernatant. This research was originally published in the Journal of Biological Chemistry (Park et al., 2015).
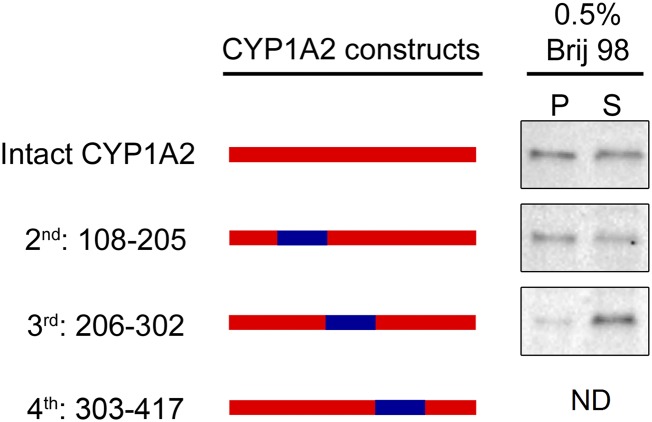
The ability of the CYP1A2 NH2 terminus to only partially cause relocalization of CYP1A1 to the ordered domains suggested that other regions of the protein may also participate in governing their microdomain localization. To address this issue, internal segments of CYP1A2 were replaced by the complementary segment of CYP1A1. Although substitution of amino acids 108–205 did not influence the relative amount of CYP1A2 in the ordered microdomains, substitution of amino acids 206–302 caused a dramatic alteration in chimera localization (Fig. 4). Taken together, these results showed that there are at least two regions that participate in the microdomain localization of CYP1A proteins: the NH2 terminus and an internal segment spanning amino acids 206–302.
Fig. 4.
Importance of the internal regions in directing the microdomain localization of CYP1A proteins. The microdomain localization of chimeric proteins where the internal segment of CYP1A2 was substituted with the corresponding region of CYP1A1 was measured as described in Fig. 1. P, pellet; S, supernatant. This research was originally published in the Journal of Biological Chemistry (Park et al., 2015).
In conclusion, P450 activities are affected by the membrane environment in which they reside. Some lipid-mediated effects result from direct interactions of P450 system proteins with specific phospholipids. These interactions can affect P450 function by promoting protein incorporation into the membrane, affecting the interactions among P450s and their redox partners and altering the redox potential of the electron donor CPR. However, other effects on P450 function appear to result from the heterogeneity of the ER membrane, which leads to the formation of lipid microdomains. The illustration in Fig. 5 provides a summary of our current knowledge on how the lipid membrane influences the localization of P450 system proteins. The presence of lipid microdomains can lead to the selective clustering of proteins into the same regions, which could augment the interactions among colocalizing proteins. In contrast, segregation of proteins into different domains could lessen these interactions. In considering the interactions between P450s and their redox partners, microdomain colocalization would be expected to decrease the apparent KDCPR, which could increase substrate metabolism. In contrast, P450 activities would likely be diminished by segregation of P450 and its electron donors. This raises numerous questions regarding the organization of the P450 system proteins. One question is, “If CPR localizes predominantly in the ordered membrane regions, how does it reduce P450s that exist in the disordered membranes?” There are several possible explanations. First, P450s in the disordered regions are reduced by the smaller fraction of CPR found in the liquid-disordered membranes. This situation would limit the CPR available to the P450 enzymes in this region and would diminish their ability to metabolize substrates. Second, CPR may reside at the interface between the ordered and disordered microdomains, being accessible to P450s in both regions. Third, because lipid microdomains are dynamic in nature, they could constantly be forming and dispersing, leading to the continual constant creation and dissociation of CPR⋅P450 complexes. Finally, there could be factors that govern the migration of proteins in and out of the lo phases. The most likely agents that may lead to altered protein migration are the presence of substrate and complexes with other proteins. First, substrate may cause a conformational change that alters the KD of the complex between CPR and a particular P450 enzyme. Therefore, the substrate would lead to a mass-action alteration in protein partitioning between the microdomains and would change protein localization. A second possible mechanism is by alteration of the supramolecular structure of the P450 system protein complex. The ability of P450 enzymes to form both homomeric and heteromeric complexes has been well established (Davydov, 2011; Reed and Backes, 2012). Any factor that affects the size of these complexes can influence their mobility and possibly their microdomain localization. A better understanding of the relationship between the lipid microenvironment of the ER and the P450 system can only improve our ability to better appreciate how the membrane affects interactions among P450 system proteins and, consequently, drug and xenobiotic metabolism.
Fig. 5.
Illustration of P450 system protein localization in the ER. Lipid membranes are thought to be segregated into liquid-ordered (lo) and liquid-disordered domains. The ordered domains (which also are referred to as lipid rafts) tend to be more enriched in cholesterol and SM, tend to pack more tightly, and are more resistant to solubilization by detergents (Diaz-Rohrer et al., 2014). P450 system proteins do not distribute randomly throughout the membrane, but they localize in regions of differing phospholipid composition. P450s and CPR have also been reported to be more closely associated with PE and the anionic phospholipids PS and PI, the phospholipids that surround P450 enzymes. The phospholipid regions with the predominant yellow head groups represent the lo microdomains with the disordered regions being largely in gray. Cholesterol (aqua) and SM (black) are intercalated into the lo regions. The anionic phospholipids are indicated by the red polar head groups. CYP1A2 and CPR localize predominantly in the lo regions, whereas CYP1A1 and CYP2E1 are found mainly in the disordered membranes. CYP2B4 distributes between both regions. The figure also illustrates the tendency of different P450 enzymes to form P450⋅P450 complexes.
Abbreviations
- CL
cardiolipin
- CPR
NADPH–cytochrome P450 reductase
- DLPC
dilauroylphosphatidylcholine
- DOPE
dioleoylphosphatidylethanolamine
- DRM
detergent-resistant membrane
- ER
endoplasmic reticulum
- GPI
glycosylphosphatidylinositol
- lo
liquid-ordered domain
- NMR
nuclear magnetic resonance
- PA
phosphatidic acid
- PB
phenobarbital
- PC
phosphatidylcholine
- PE
phosphatidylethanolamine
- P450
cytochrome P450
- PI
phosphatidylinositol
- PLD
phospholipase D
- PS
phosphatidylserine
- SM
sphingomyelin
- TIM
Triton-insoluble membrane
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Brignac-Huber, Park, Reed, Backes.
Footnotes
This research was supported in part by the National Institutes of Health National Institute of Environmental Health Sciences [Grants R01ES004344 and P42ES013648 (to W.L.B.)].
References
- Ahn T, Guengerich FP, Yun CH. (1998) Membrane insertion of cytochrome P450 1A2 promoted by anionic phospholipids. Biochemistry 37:12860–12866. [DOI] [PubMed] [Google Scholar]
- Ahn T, Yun CH. (1998) Phase separation in phosphatidylcholine/anionic phospholipid membranes in the liquid-crystalline state revealed with fluorescent probes. J Biochem 124:622–627. [DOI] [PubMed] [Google Scholar]
- Ahn T, Yun CH, Oh DB. (2005) Involvement of nonlamellar-prone lipids in the stability increase of human cytochrome P450 1A2 in reconstituted membranes. Biochemistry 44:9188–9196. [DOI] [PubMed] [Google Scholar]
- Autor AP, Kaschnitz RM, Heidema JK, Coon MJ. (1973) Sedimentation and other properties of the reconstituted liver microsomal mixed-function oxidase system containing cytochrome P-450, reduced triphosphopyridine nucleotide-cytochrome P-450 reductase, and phosphatidylcholine. Mol Pharmacol 9:93–104. [PubMed] [Google Scholar]
- Backes WL, Batie CJ, Cawley GF. (1998) Interactions among P450 enzymes when combined in reconstituted systems: formation of a 2B4-1A2 complex with a high affinity for NADPH-cytochrome P450 reductase. Biochemistry 37:12852–12859. [DOI] [PubMed] [Google Scholar]
- Bae TJ, Kim MS, Kim JW, Kim BW, Choo HJ, Lee JW, Kim KB, Lee CS, Kim JH, Chang SY, et al. (2004) Lipid raft proteome reveals ATP synthase complex in the cell surface. Proteomics 4:3536–3548. [DOI] [PubMed] [Google Scholar]
- Balvers WG, Boersma MG, Vervoort J, Ouwehand A, Rietjens IM. (1993) A specific interaction between NADPH-cytochrome reductase and phosphatidylserine and phosphatidylinositol. Eur J Biochem 218:1021–1029. [DOI] [PubMed] [Google Scholar]
- Baraud J, Maurice A. (1980) Phospholipid synthesis and exchange between rat liver microsomes and mitochondria in the presence of benzo(a)pyrene. J Lipid Res 21:347–353. [PubMed] [Google Scholar]
- Bayerl T, Klose G, Ruckpaul K, Schwarze W. (1985) Interaction of hexane phosphonic acid diethyl ester with phospholipids in hepatic microsomes and reconstituted liposomes as studied by 31P-NMR. Biochim Biophys Acta 812:437–446. [DOI] [PubMed] [Google Scholar]
- Bertz RJ, Granneman GR. (1997) Use of in vitro and in vivo data to estimate the likelihood of metabolic pharmacokinetic interactions. Clin Pharmacokinet 32:210–258. [DOI] [PubMed] [Google Scholar]
- Bini L, Pacini S, Liberatori S, Valensin S, Pellegrini M, Raggiaschi R, Pallini V, Baldari CT. (2003) Extensive temporally regulated reorganization of the lipid raft proteome following T-cell antigen receptor triggering. Biochem J 369:301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black SD, Coon MJ. (1982) Structural features of liver microsomal NADPH-cytochrome P-450 reductase. Hydrophobic domain, hydrophilic domain, and connecting region. J Biol Chem 257:5929–5938. [PubMed] [Google Scholar]
- Blanck J, Smettan G, Ristau O, Ingelman-Sundberg M, Ruckpaul K. (1984) Mechanism of rate control of the NADPH-dependent reduction of cytochrome P-450 by lipids in reconstituted phospholipid vesicles. Eur J Biochem 144:509–513. [DOI] [PubMed] [Google Scholar]
- Bösterling B, Stier A, Hildebrandt AG, Dawson JH, Trudell JR. (1979) Reconstitution of cytochrome P-450 and cytochrome P-450 reductase into phosphatidylcholine-phosphatidylethanolamine bilayers: characterization of structure and metabolic activity. Mol Pharmacol 16:332–342. [PubMed] [Google Scholar]
- Brignac-Huber L, Reed JR, Backes WL. (2011) Organization of NADPH-cytochrome P450 reductase and CYP1A2 in the endoplasmic reticulum--microdomain localization affects monooxygenase function. Mol Pharmacol 79:549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brignac-Huber LM, Reed JR, Eyer MK, Backes WL. (2013) Relationship between CYP1A2 localization and lipid microdomain formation as a function of lipid composition. Drug Metab Dispos 41:1896–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browman DT, Resek ME, Zajchowski LD, Robbins SM. (2006) Erlin-1 and erlin-2 are novel members of the prohibitin family of proteins that define lipid-raft-like domains of the ER. J Cell Sci 119:3149–3160. [DOI] [PubMed] [Google Scholar]
- Brown DA, London E. (1998) Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol 14:111–136. [DOI] [PubMed] [Google Scholar]
- Brown DA, Rose JK. (1992) Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell 68:533–544. [DOI] [PubMed] [Google Scholar]
- Buckland AG, Wilton DC. (2000) Anionic phospholipids, interfacial binding and the regulation of cell functions. Biochim Biophys Acta 1483:199–216. [DOI] [PubMed] [Google Scholar]
- Cao J, Liu Y, Lockwood J, Burn P, Shi Y. (2004) A novel cardiolipin-remodeling pathway revealed by a gene encoding an endoplasmic reticulum-associated acyl-CoA:lysocardiolipin acyltransferase (ALCAT1) in mouse. J Biol Chem 279:31727–31734. [DOI] [PubMed] [Google Scholar]
- Causey KM, Eyer CS, Backes WL. (1990) Dual role of phospholipid in the reconstitution of cytochrome P-450 LM2-dependent activities. Mol Pharmacol 38:134–142. [PubMed] [Google Scholar]
- Cho EY, Yun CH, Chae HZ, Chae HJ, Ahn T. (2008) Anionic phospholipid-induced regulation of reactive oxygen species production by human cytochrome P450 2E1. FEBS Lett 582:1771–1776. [DOI] [PubMed] [Google Scholar]
- Dallner G, Ernster L. (1968) Subfractionation and composition of microsomal membranes: a review. J Histochem Cytochem 16:611–632. [DOI] [PubMed] [Google Scholar]
- Das A, Sligar SG. (2009) Modulation of the cytochrome P450 reductase redox potential by the phospholipid bilayer. Biochemistry 48:12104–12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison SC, Wills ED. (1974) Studies on the lipid composition of the rat liver endoplasmic reticulum after induction with phenobarbitone and 20-methylcholanthrene. Biochem J 140:461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davydov DR. (2011) Microsomal monooxygenase as a multienzyme system: the role of P450-P450 interactions. Expert Opin Drug Metab Toxicol 7:543–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kruijff B. (1997) Lipid polymorphism and biomembrane function. Curr Opin Chem Biol 1:564–569. [DOI] [PubMed] [Google Scholar]
- Dekker CJ, Geurts van Kessel WS, Klomp JP, Pieters J, De Kruijff B. (1983) Synthesis and polymorphic phase behaviour of polyunsaturated phosphatidylcholines and phosphatidylethanolamines. Chem Phys Lipids 33:93–106. [DOI] [PubMed] [Google Scholar]
- Diaz-Rohrer B, Levental KR, Levental I. (2014) Rafting through traffic: membrane domains in cellular logistics. Biochim Biophys Acta 1838:3003–3013. [DOI] [PubMed] [Google Scholar]
- Epand RM. (1987) The relationship between the effects of drugs on bilayer stability and on protein kinase C activity. Chem Biol Interact 63:239–247. [DOI] [PubMed] [Google Scholar]
- Epand RM. (1998) Lipid polymorphism and protein-lipid interactions. Biochim Biophys Acta 1376:353–368. [DOI] [PubMed] [Google Scholar]
- Foster LJ, De Hoog CL, Mann M. (2003) Unbiased quantitative proteomics of lipid rafts reveals high specificity for signaling factors. Proc Natl Acad Sci USA 100:5813–5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaumann H, Dallner G. (1968) Lipid composition and turnover of rough and smooth microsomal membranes in rat liver. J Lipid Res 9:720–729. [PubMed] [Google Scholar]
- Guengerich FP. (1992) Human cytochrome P-450 enzymes. Life Sci 50:1471–1478. [DOI] [PubMed] [Google Scholar]
- Guttendorf RJ, Wedlund PJ. (1992) Genetic aspects of drug disposition and therapeutics. J Clin Pharmacol 32:107–117. [DOI] [PubMed] [Google Scholar]
- Hildebrandt A, Estabrook RW. (1971) Evidence for the participation of cytochrome b 5 in hepatic microsomal mixed-function oxidation reactions. Arch Biochem Biophys 143:66–79. [DOI] [PubMed] [Google Scholar]
- Holthuis JC, Pomorski T, Raggers RJ, Sprong H, Van Meer G. (2001) The organizing potential of sphingolipids in intracellular membrane transport. Physiol Rev 81:1689–1723. [DOI] [PubMed] [Google Scholar]
- Ingelman-Sundberg M, Blanck J, Smettan G, Ruckpaul K. (1983) Reduction of cytochrome P-450 LM2 by NADPH in reconstituted phospholipid vesicles is dependent on membrane charge. Eur J Biochem 134:157–162. [DOI] [PubMed] [Google Scholar]
- Ingelman-Sundberg M, Glaumann H. (1980) Incorporation of purified components of the rabbit liver microsomal hydroxylase system into phospholipid vesicles. Biochim Biophys Acta 599:417–435. [DOI] [PubMed] [Google Scholar]
- Ingelman-Sundberg M, Haaparanta T, Rydström J. (1981) Membrane charge as effector of cytochrome P-450LM2 catalyzed reactions in reconstituted liposomes. Biochemistry 20:4100–4106. [DOI] [PubMed] [Google Scholar]
- Ingelman-Sundberg M, Hagbjörk AL, Ueng YF, Yamazaki H, Guengerich FP. (1996) High rates of substrate hydroxylation by human cytochrome P450 3A4 in reconstituted membranous vesicles: influence of membrane charge. Biochem Biophys Res Commun 221:318–322. [DOI] [PubMed] [Google Scholar]
- Ingelman-Sundberg M, Johansson I. (1980) Catalytic properties of purified forms of rabbit liver microsomal cytochrome P-450 in reconstituted phospholipid vesicles. Biochemistry 19:4004–4011. [DOI] [PubMed] [Google Scholar]
- Jang HH, Kim DH, Ahn T, Yun CH. (2010) Functional and conformational modulation of human cytochrome P450 1B1 by anionic phospholipids. Arch Biochem Biophys 493:143–150. [DOI] [PubMed] [Google Scholar]
- Kim KH, Ahn T, Yun CH. (2003) Membrane properties induced by anionic phospholipids and phosphatidylethanolamine are critical for the membrane binding and catalytic activity of human cytochrome P450 3A4. Biochemistry 42:15377–15387. [DOI] [PubMed] [Google Scholar]
- Kim KH, Kim DH, Jang HH, Kim M, Kim DH, Kim JS, Kim JI, Chae HZ, Ahn T, Yun CH. (2007) Lateral segregation of anionic phospholipids in model membranes induced by cytochrome P450 2B1: bi-directional coupling between CYP2B1 and anionic phospholipid. Arch Biochem Biophys 468:226–233. [DOI] [PubMed] [Google Scholar]
- Lin JH, Lu AY. (1998) Inhibition and induction of cytochrome P450 and the clinical implications. Clin Pharmacokinet 35:361–390. [DOI] [PubMed] [Google Scholar]
- Lu AYH, Junk KW, Coon MJ. (1969) Resolution of the cytochrome P-450-containing omega-hydroxylation system of liver microsomes into three components. J Biol Chem 244:3714–3721. [PubMed] [Google Scholar]
- Luzzati V, Husson F. (1962) The structure of the liquid-crystalline phases of lipid-water systems. J Cell Biol 12:207–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougle DR, Baylon JL, Meling DD, Kambalyal A, Grinkova YV, Hammernik J, Tajkhorshid E, Das A. (2015) Incorporation of charged residues in the CYP2J2 F-G loop disrupts CYP2J2-lipid bilayer interactions. Biochim Biophys Acta 1848:2460–2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Enoch D, Churchill P, Fleischer S, Guengerich FP. (1984) Interaction of liver microsomal cytochrome P-450 and NADPH-cytochrome P-450 reductase in the presence and absence of lipid. J Biol Chem 259:8174–8182. [PubMed] [Google Scholar]
- Nilsson O, Dallner G. (1975) Distribution of constitutive enzymes and phospholipids in microsomal membranes of rat liver. FEBS Lett 58:190–193. [DOI] [PubMed] [Google Scholar]
- Paradies G, Ruggiero FM, Dinoi P. (1991) The influence of hypothyroidism on the transport of phosphate and on the lipid composition in rat-liver mitochondria. Biochim Biophys Acta 1070:180–186. [DOI] [PubMed] [Google Scholar]
- Park JW, Reed JR, Backes WL. (2015) The localization of cytochrome P450s CYP1A1 and CYP1A2 into different lipid microdomains is governed by their N-terminal and internal protein regions. J Biol Chem 290:29449–29460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JW, Reed JR, Brignac-Huber LM, Backes WL. (2014) Cytochrome P450 system proteins reside in different regions of the endoplasmic reticulum. Biochem J 464:241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascaud M. (1958) [Renovation of glycerophosphate fatty acids in normal rats]. C R Hebd Seances Acad Sci 246:1312–1315. [PubMed] [Google Scholar]
- Pielsticker LK, Mann KJ, Lin WL, Sevlever D. (2005) Raft-like membrane domains contain enzymatic activities involved in the synthesis of mammalian glycosylphosphatidylinositol anchor intermediates. Biochem Biophys Res Commun 330:163–171. [DOI] [PubMed] [Google Scholar]
- Pike LJ. (2003) Lipid rafts: bringing order to chaos. J Lipid Res 44:655–667. [DOI] [PubMed] [Google Scholar]
- Pike LJ. (2004) Lipid rafts: heterogeneity on the high seas. Biochem J 378:281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz W. (2002) Cholesterol trafficking in the secretory and endocytic systems. Semin Cell Dev Biol 13:197–203. [DOI] [PubMed] [Google Scholar]
- Reed JR, Backes WL. (2012) Formation of P450 · P450 complexes and their effect on P450 function. Pharmacol Ther 133:299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JR, Brignac-Huber LM, Backes WL. (2008) Physical incorporation of NADPH-cytochrome P450 reductase and cytochrome P450 into phospholipid vesicles using glycocholate and Bio-Beads. Drug Metab Dispos 36:582–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JR, Eyer M, Backes WL. (2010) Functional interactions between cytochromes P450 1A2 and 2B4 require both enzymes to reside in the same phospholipid vesicle: evidence for physical complex formation. J Biol Chem 285:8942–8952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JR, Kelley RW, Backes WL. (2006) An evaluation of methods for the reconstitution of cytochromes P450 and NADPH P450 reductase into lipid vesicles. Drug Metab Dispos 34:660–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remmer H, Merker HJ. (1963) Drug-induced changes in the liver endoplasmic reticulum: association with drug-metabolizing enzymes. Science 142:1657–1658. [DOI] [PubMed] [Google Scholar]
- Rixon HW, Brown G, Aitken J, McDonald T, Graham S, Sugrue RJ. (2004) The small hydrophobic (SH) protein accumulates within lipid-raft structures of the Golgi complex during respiratory syncytial virus infection. J Gen Virol 85:1153–1165. [DOI] [PubMed] [Google Scholar]
- Sarnataro D, Campana V, Paladino S, Stornaiuolo M, Nitsch L, Zurzolo C. (2004) PrP(C) association with lipid rafts in the early secretory pathway stabilizes its cellular conformation. Mol Biol Cell 15:4031–4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz D, Kisselev P, Wessel R, Pisch S, Bornscheuer U, Schmid RD. (1997) Possible involvement of nonbilayer lipids in the stimulation of the activity of cytochrome P450SCC (CYP11A1) and its propensity to induce vesicle aggregation. Chem Phys Lipids 85:91–99. [DOI] [PubMed] [Google Scholar]
- Sevlever D, Pickett S, Mann KJ, Sambamurti K, Medof ME, Rosenberry TL. (1999) Glycosylphosphatidylinositol-anchor intermediates associate with triton-insoluble membranes in subcellular compartments that include the endoplasmic reticulum. Biochem J 343:627–635. [PMC free article] [PubMed] [Google Scholar]
- Sevrioukova IF, Poulos TL. (2015) Anion-dependent stimulation of CYP3A4 monooxygenase. Biochemistry 54:4083–4096. [DOI] [PubMed] [Google Scholar]
- Simons K, Ikonen E. (1997) Functional rafts in cell membranes. Nature 387:569–572. [DOI] [PubMed] [Google Scholar]
- Simons K, van Meer G. (1988) Lipid sorting in epithelial cells. Biochemistry 27:6197–6202. [DOI] [PubMed] [Google Scholar]
- Singer SJ, Nicolson GL. (1972) The fluid mosaic model of the structure of cell membranes. Science 175:720–731. [DOI] [PubMed] [Google Scholar]
- Sorice M, Manganelli V, Matarrese P, Tinari A, Misasi R, Malorni W, Garofalo T. (2009) Cardiolipin-enriched raft-like microdomains are essential activating platforms for apoptotic signals on mitochondria. FEBS Lett 583:2447–2450. [DOI] [PubMed] [Google Scholar]
- Stier A, Sackmann E. (1973) Spin labels as enzyme substrates. Heterogeneous lipid distribution in liver microsomal membranes. Biochim Biophys Acta 311:400–408. [DOI] [PubMed] [Google Scholar]
- Strobel HW, Lu AYH, Heidema J, Coon MJ. (1970) Phosphatidylcholine requirement in the enzymatic reduction of hemoprotein P-450 and in fatty acid, hydrocarbon, and drug hydroxylation. J Biol Chem 245:4851–4854. [PubMed] [Google Scholar]
- Taniguchi H, Imai Y, Iyanagi T, Sato R. (1979) Interaction between NADPH-cytochrome P-450 reductase and cytochrome P-450 in the membrane of phosphatidylcholine vesicles. Biochim Biophys Acta 550:341–356. [DOI] [PubMed] [Google Scholar]
- Taniguchi H, Pyerin W. (1988) Phospholipid bilayer membranes play decisive roles in the cytochrome P-450-dependent monooxygenase system. J Cancer Res Clin Oncol 114:335–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meer G, Voelker DR, Feigenson GW. (2008) Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol 9:112–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetrivel KS, Cheng H, Lin W, Sakurai T, Li T, Nukina N, Wong PC, Xu H, Thinakaran G. (2004) Association of gamma-secretase with lipid rafts in post-Golgi and endosome membranes. J Biol Chem 279:44945–44954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waskell L, Koblin D, Canova-Davis E. (1982) The lipid composition of human liver microsomes. Lipids 17:317–320. [DOI] [PubMed] [Google Scholar]
- Weibel ER, Stäubli W, Gnägi HR, Hess FA. (1969) Correlated morphometric and biochemical studies on the liver cell. I. Morphometric model, stereologic methods, and normal morphometric data for rat liver. J Cell Biol 42:68–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T. (1963) On the thickness of the unit membrane. J Cell Biol 17:413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young DL, Powell G, McMillan WO. (1971) Phenobarbital-induced alterations in phosphatidylcholine and triglyceride synthesis in hepatic endoplasmic reticulum. J Lipid Res 12:1–8. [PubMed] [Google Scholar]
- Yun CH, Ahn T, Guengerich FP. (1998) Conformational change and activation of cytochrome P450 2B1 induced by salt and phospholipid. Arch Biochem Biophys 356:229–238. [DOI] [PubMed] [Google Scholar]
- Yun CH, Ahn T, Guengerich FP, Yamazaki H, Shimada T. (1999) Phospholipase D activity of cytochrome P450 in human liver endoplasmic reticulum. Arch Biochem Biophys 367:81–88. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Chen YQ, Li S, Konrad RJ, Cao G. (2009) The microsomal cardiolipin remodeling enzyme acyl-CoA lysocardiolipin acyltransferase is an acyltransferase of multiple anionic lysophospholipids. J Lipid Res 50:945–956. [DOI] [PMC free article] [PubMed] [Google Scholar]