Abstract
Traditional in vitro human liver cell culture models lose key hepatic functions such as metabolic activity during short-term culture. Advanced three-dimensional (3D) liver coculture platforms offer the potential for extended hepatocyte functionality and allow for the study of more complex biologic interactions, which can improve and refine human drug safety evaluations. Here, we use a perfusion flow 3D microreactor platform for the coculture of cryopreserved primary human hepatocytes and Kupffer cells to study the regulation of cytochrome P450 3A4 isoform (CYP3A4) activity by chronic interleukin 6 (IL-6)–mediated inflammation over 2 weeks. Hepatocyte cultures remained stable over 2 weeks, with consistent albumin production and basal IL-6 levels. Direct IL-6 stimulation that mimics an inflammatory state induced a dose-dependent suppression of CYP3A4 activity, an increase in C-reactive protein (CRP) secretion, and a decrease in shed soluble interleukin-6 receptor (IL-6R) levels, indicating expected hepatic IL-6 bioactivity. Tocilizumab, an anti-IL-6R monoclonal antibody used to treat rheumatoid arthritis, has been demonstrated clinically to impact small molecule drug pharmacokinetics by modulating cytochrome P450 enzyme activities, an effect not observed in traditional hepatic cultures. We have now recapitulated the clinical observation in a 3D bioreactor system. Tocilizumab was shown to desuppress CYP3A4 activity while reducing the CRP concentration after 72 hours in the continued presence of IL-6. This change in CYP3A4 activity decreased the half-life and area under the curve up to the last measurable concentration (AUClast) of the small molecule CYP3A4 substrate simvastatin hydroxy acid, measured before and after tocilizumab treatment. We conclude that next-generation in vitro liver culture platforms are well suited for these types of long-term treatment studies and show promise for improved drug safety assessment.
Introduction
Prediction of drug safety in humans remains one of the biggest challenges facing the drug development industry. In particular, preclinical in vivo models fall short of recapitulating human liver biology sufficiently well to anticipate the complex forms of drug-induced liver injury (DILI) or to forecast clinical pharmacokinetic (PK) properties when liver function is modulated (Peters 2005; Navarro and Senior, 2006). In response, researchers have focused on developing human-based in vitro liver platforms in an effort to improve accurate prediction of drug safety.
Primary human hepatocytes cultured on flat, collagen-coated plates are widely used for PK and toxicity studies, but in this culture format the cells lose key hepatic functions such as metabolic activity after only a few days in culture. Recently, there have been several platforms introduced that offer the potential for long-term, multiweek primary human hepatocyte culture, which would provide researchers the ability to model more complex interactions involving chronic dosing effects, low-clearance compound and drug accumulation, human-specific metabolite identification, DILI, and the effects of chronic inflammation (Chao et al., 2009; Kostadinova et al., 2013; Messner et al., 2013; Ballard et al., 2016). There are several approaches that can increase in vitro hepatic performance and concomitant physiologic relevance, including three-dimensional (3D) cell culture, application of media flow and shear conditions to mimic natural tissue, and cocultures with liver nonparenchymal cell types such as Kupffer cells (KCs), liver sinusoidal endothelial cells, and hepatic stellate cells (LeCluyse et al., 2012; Ebrahimkhani et al., 2014).
Hepatocytes express cytochrome P450 (CYP) enzymes, which are critical to the phase I metabolism of many xenobiotics. In particular, CYP3A4 is primarily responsible for the metabolism of over 50% of marketed small-molecule drugs (Ingelman-Sundberg 2004). Inflammation is known to down-regulate CYP activity, a normal adaptation to a stressed state mediated by transcription factors such as pregnane X receptor (PXR), constitutive androstane receptor (CAR), and nuclear factor κB (NF-κB) as well as reactive oxygen species (Morgan et al., 2008). Inflammatory cytokines have been linked to the suppression of specific CYP enzymes (Huang et al., 2010; Zhou and Li, 2014), which has potential implications for modulating drug exposure and thus safety.
Interleukin 6 (IL-6) is an inflammatory cytokine elevated in a number of diseases such as rheumatoid arthritis, inflammatory bowel disease, diabetes, and several forms of cancer (Ishihara and Hirano, 2002; Heikkila et al., 2008). The regulation of IL-6 is an important aspect of normal physiology. In the liver, KCs secrete IL-6 locally in response to inflammatory stimuli such as lipopolysaccharide, which can travel to the liver from the intestinal microbiota (Xu et al., 2004), but hepatocytes are also capable of secreting IL-6 (Norris et al., 2014). Hepatocytes respond to systemically high levels of IL-6 caused by inflammatory diseases through the IL-6 receptor (IL-6R) (Morgan et al., 2008). This receptor exists in both a cell membrane–bound form and a shed soluble form (sIL-6R), both of which bind IL-6 with equivalent affinity and mediate the same downstream signaling cascade (Rose-John, 2012; Scheller et al., 2014). C-reactive protein (CRP), an acute phase protein secreted by hepatocytes in response to IL-6, is commonly used as an in vitro marker of IL-6 signaling in hepatocytes (Bode et al., 2012; Nguyen et al., 2015).
In traditional culture models, IL-6 has been shown to decrease activity or expression for a number of CYP enzymes in hepatocytes, including CYP3A4 (Jover et al., 2002; Huang et al., 2010; Dickmann et al., 2011). However, conventional in vitro liver platforms do not allow for the study of complex inflammation-related drug-drug interactions (DDIs) because of their limited capacity for long-term culture. Dickmann et al. (2011) were able to demonstrate IL-6–based modulation of CYP activities using hepatocytes on collagen-coated plates, but these effects were limited to 96 hours of treatment or less. This short time scale prevents the analysis of changes in small-molecule PK before and after CYP modulation with inflammatory/anti-inflammatory stimuli in the same cell populations over time to determine whether there is a functional consequence of those changes in apparent CYP activity. Those studies also used an anti-IL-6 neutralizing antibody codosed with IL-6 over 72 hours to prevent CYP suppression. Long-term culture would allow for both phases of the process to be observed, first the suppression by cytokine application, then the desuppression of CYPs in hepatocytes.
Cytokines such as IL-6 continue to be targets of large-molecule–based therapies for chronic inflammatory conditions. For example, tocilizumab is an anti-IL-6R monoclonal antibody approved to treat rheumatoid arthritis and juvenile idiopathic arthritis. Because it interferes with cytokine signaling and its downstream effects on CYP expression levels, tocilizumab has the potential to desuppress CYP activity in the liver (Morgan, 2009; Lee et al., 2010). Tocilizumab has been shown clinically to increase CYP3A4 activity in rheumatoid arthritis patients and alter corresponding small-molecule PK, particularly of simvastatin (SVS), a common medication for that patient population.
The prodrug SVS and its biologically active form simvastatin hydroxy acid (SVA) are part of the statin family of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors used to treat high cholesterol. Statins are metabolized primarily by CYP3A4 and both SVS and SVA showed reduced exposure in rheumatoid arthritis patients after tocilizumab administration (Schmitt et al., 2011). U.S. Food and Drug Administration guidance has encouraged the exploration of these types of large-molecule/small-molecule DDIs to support regulatory filings and improve human drug safety evaluation (CDER, 2012).
Here, we used the LiverChip, a next-generation in vitro hepatic culture system, to study the effect of chronic IL-6 inflammation on primary human hepatocyte/KC cocultures. Cells in the LiverChip are cultured within 3D microchannels in a scaffold exposed to continuous medium perfusion flow used to simulate a liver sinusoid (Powers et al., 2002; Sivaraman et al., 2005; Hwa et al., 2007; Domansky et al., 2010). The LiverChip has been previously reported to preserve human hepatocyte metabolic activity (Vivares et al., 2015) and to model hepatic inflammation (Sarkar et al., 2015; Yu et al., 2015), but to date human hepatocyte data have been limited to 1 week of culture. In these studies, we use the LiverChip to directly demonstrate both an IL-6–based reduction in CYP3A4 activity and a tocilizumab-mediated effect on SVA exposure through manipulation of CYP3A4 activity over a period of 2 weeks.
Materials and Methods
Optima liquid chromatography/mass spectrometry–grade water, acetonitrile, methanol, and acetic acid were obtained from Thermo Fisher Scientific (Waltham, MA). Liquid chromatography/mass spectrometry–grade ammonium acetate, SVS (CAS: 79902-63-9), and rifampicin (CAS: 13292-46-1) were obtained from Sigma-Aldrich (St. Louis, MO). SVA (CAS: 139893-43-9) and lovastatin (LV) (CAS: 75330-75-5) were purchased from Toronto Research Chemicals (Toronto, ON, Canada). Recombinant human IL-6 (cat. no. 206-IL) was obtained from R&D Systems (Minneapolis, MN).
LiverChip Platform.
The LiverChip was obtained from CN Bio Innovations (Hertfordshire, United Kingdom). Each platform contains 12 bioreactors with a polysulfone cell culture plate secured to a pneumatic plate (see Fig. 1A). Bioreactors hold a collagen-coated polystyrene scaffold with 300 cylindrical micro-channels of diameter 300 µm and height 250 µm for cell culture (see Fig. 1B). Fluid flow is controlled by pneumatic pumping through the scaffold using a thin polyurethane membrane between the cell culture and pneumatic plates (see Fig. 1C). All LiverChip experiments were performed in a humidified incubator at 37°C with a 5% CO2 atmosphere under a 1 µl/s flow rate.
Fig. 1.
The LiverChip platform. (A) A cell culture plate (yellow) is attached to a pneumatic plate (gray) forming 12 fluidically isolated bioreactors per platform. (B) A collagen-coated polystyrene scaffold containing microchannels is placed into each bioreactor for cell culture. Scaffold diameter is 1 cm. (C) Bioreactor cross-section schematic showing a thin membrane separating the lower pneumatic plate and the upper cell culture plate that houses the scaffold. The medium is pumped through the scaffold and recirculated along a surface channel.
Human Hepatocyte-Kupffer Cell Cocultures.
Cryopreserved human hepatocytes and KCs were purchased from Thermo Fisher Scientific. Donor information is shown in Supplemental Table 1. After thawing, hepatocytes were gently transferred to 50 ml of warm cryopreserved hepatocyte recovery medium (Thermo Fisher Scientific), centrifuged at 100g for 10 minutes, and resuspended in Advanced Dulbecco’s modified Eagle’s medium containing 5% fetal bovine serum, 4 µg/ml insulin, 2 mM GlutaMax, 15 mM HEPES, and 1% penicillin–streptomycin (seeding medium). After thawing, KCs were transferred to ice-cold seeding medium, centrifuged at 400g for 4 minutes at 4°C, and resuspended in cold seeding medium. Viable cells were quantified using a trypan blue exclusion assay. Viability was greater than 90% for all cell lots.
Each reactor well of the LiverChip was seeded with 6.0 × 105 viable hepatocytes and 6.0 × 104 viable KCs in 1.6 ml of seeding medium. After seeding, flow was set to 1.0 µl/s in the down direction for 8 hours, allowing cells to attach to the microchannel scaffolds. After 8 hours, flow was reversed to 1.0 µl/s in the up direction for the duration of the experiment to allow for medium reoxygenation along the bioreactor surface channel in each well. On culture day 1 (24 hours after seeding), the medium in each well was replaced with Advanced Dulbecco’s modified Eagle’s medium containing 6.25 µg/ml insulin, 6.25 µg/ml transferrin, 6.25 µg/ml selenious acid, 1.25 mg/ml bovine serum albumin, 5.25 µg/ml linoleic acid, 2 mM GlutaMax, 15 mM HEPES, and 0.5% penicillin–streptomycin (hepatocyte maintenance supplement; Thermo Fisher Scientific). On culture day 3, the medium was replaced by Williams’ E medium containing maintenance supplement and 100 nM hydrocortisone (maintenance medium), which was subsequently changed every 48 hours. The medium was sampled, aliquoted, and stored at −80°C before analysis.
CYP3A Activity.
CYP3A4 enzyme activity was measured using a P450-Glo kit with a Luciferin-IPA substrate (Promega, Madison, WI). The substrate was diluted 1:1000 to 3 µM in 1.6-ml maintenance medium and was added to each bioreactor after a medium change. After 1 hour of incubation at 37°C, the media were collected from each reactor, and 50 µl was added to a white-walled 96-well plate. We also added 50 µl of standards, prepared using a serial dilution of click beetle luciferin potassium salt (Promega E1601) to the 96-well plate. We added 50 µl of luciferin detection reagent to all samples and standards, after which the plate was covered and incubated for 20 minutes at room temperature. Luminescence was measured using a SpectraMax L plate reader (Molecular Devices, Sunnyvale, CA).
Imaging.
Scaffolds were carefully removed from the LiverChip cell culture plate and washed with phosphate-buffered saline (PBS). Scaffolds were fixed with 2% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA) in PBS for 1 hour at room temperature. After fixation, cells were permeabilized with 0.1% Triton X-100 (Sigma Aldrich, St. Louis, MO) in PBS then stained with a 1:200 dilution of Alexa488 Phalloidin (Thermo Fisher Scientific) for 30 minutes. Scaffolds were counterstained with Hoechst’s dye (Sigma-Aldrich) for 5 minutes. The scaffolds’ confocal reconstructions were acquired on an Olympus Fluoview 1000 scanning confocal microscope (Olympus America, Center Valley, PA), using the 10× and 20× objective magnifications. Images were processed using MetaMorph software (Molecular Devices).
Protein Analysis.
Human albumin secretion was measured using an enzyme-linked immunosorbent assay (ELISA) kit purchased from Bethyl Laboratories (Montgomery, TX). IL-6, CRP, and sIL-6R alpha were measured using human Quantikine ELISA kits (R&D Systems). The assays were developed according to manufacturer’s instructions. Absorbance was measured on a SpectraMax M3 plate reader (Molecular Devices).
IL-6 Stimulation Studies.
Triplicate wells of hepatocytes and KCs cultured in the LiverChip were induced with 10 µM rifampicin on culture day 5 along with 1, 10, 50, 100, 500, 1000, 5000, or 10,000 pg/ml recombinant human IL-6. The media were collected and refreshed with the same components on culture day 7. CYP3A4 was measured on culture day 9 after 96 hours of stimulation. CRP and sIL-6R alpha were measured in exchanged media by ELISA.
Tocilizumab-Simvastatin Hydroxy Acid Interaction Studies.
Hepatocytes and KCs were cultured in the LiverChip for 14 days. Six sample groups were run in triplicate for each of three hepatocyte lots. All wells were induced with 10 µM rifampicin starting on culture day 5. During the first treatment period of days 5–9, sample groups 2, 4, and 6 were stimulated with 1 ng/ml of recombinant human IL-6. On culture day 7, all wells were treated with 909 ng/ml of SVA in 2.0 ml of hepatocyte maintenance medium. This SVA concentration was chosen such that the unbound fraction of drug was set to 10 times human serum Cmax. The final dimethylsulfoxide medium concentration for wells dosed with rifampicin with or without SVA was set to 0.1%. We removed 50 µl of medium at 0.5, 1, 4, 6, 8, 10, 24, and 48 hours, which was stored at −80°C for liquid chromatography with tandem mass spectrometry (LC-MS/MS) analysis of SVA. On culture day 9, CYP3A4 enzyme activity was measured.
During the second treatment period of days 9–14, groups 1 and 6 received no treatment, group 2 was treated only with 1 ng/ml IL-6, group 5 was treated only with 1.6 µM pharmaceutic grade tocilizumab (Actemra; Genentech, South San Francisco, CA), and groups 3 and 4 were cotreated with both 1 ng/ml IL-6 and 1.6 µM tocilizumab. This tocilizumab concentration was chosen to represent human serum Cmax. The medium was replaced on culture day 12 to allow for 72 hours of tocilizumab treatment before SVA addition. SVA was dosed to all groups along with corresponding IL-6/tocilizumab treatments on day 12 and sampled similarly to the day-7 dose. The CYP3A4 enzyme activity was measured on culture day 14.
Simvastatin Hydroxy Acid Sample Preparation.
We thawed 50-µl media samples containing SVA and spiked them with 5 µl of 100 ng/ml LV as an internal standard. We added 200 µl of ice cold 50:50 methanol/acetonitrile to each sample, which was then vortexed and placed on ice for 15 minutes. Samples were then spun down at 2000g at 4°C for 15 minutes. The supernatants were removed and used for LC-MS/MS analysis. The standards were prepared in a similar manner, by first spiking SVA and LV into hepatocyte maintenance medium with final concentrations ranging from 50 to 1200 ng/ml SVA before protein extraction.
Simvastatin Hydroxy Acid LC-MS/MS Analysis.
An UltiMate 3000 LC system (Thermo Fisher Scientific) was connected to a Hypersil Gold ultra-high-pressure liquid chromatography column (50 mm × 2.1 mm; 1.9 μm particle size, Thermo Fisher Scientific) and maintained at ambient temperature (∼23°C). The sample injection volume was 20 μl, and the samples were processed using isocratic elution for 10 minutes with a loading pump flow rate of 150 μl/min using 75:25 (% v/v) acetonitrile:100 mM ammonium acetate (adjusted pH to 5.0 with glacial acetic acid), adapted from the method described by Ahmed et al. (2012). The autosampler was maintained at 4°C.
Analytes were detected using a Q Exactive Hybrid Quadrupole-Orbitrap Mass Spectrometer (Thermo Fisher Scientific) equipped with a HESI-II Probe. The HESI-II probe settings were determined using the manufacturer suggested settings at a flow rate of 150 μl/min with sheath gas flow rate 40, auxiliary gas flow rate 10, sweep gas flow rate 2, spray voltage3.5 kV, capillary temperature 250°C, S-lens RF level 65, and heater temperature 300°C. The scan parameters were optimized with full-scan mass spectrometer data collection from time 0–10 minutes and MS/MS data also collected from time 1–3 minutes and operating in positive ion mode.
Full MS-SIM scans were operated in positive ion mode with 70 K resolution, 1.0e6 AGC target, and a scan range of 100–500 m/z; while targeted MS/MS scans operated in positive ion mode with 17.5 K resolution, 1.0e5 AGC target, 4.0 m/z isolation window, and 10.0 NCE. The analytes were quantitated using selected reaction monitoring by detecting the [M+H]+ precursor to product ion transitions. The precursor/product ion transitions were observed at m/z 419.28/199.15 for SVS, 437.29/303.20 for SVA, and 405.26/199.15 for LV. Quantitation was performed using Thermo Xcalibur Quan Browser v2.2 using LV as an internal standard with ICIS peak detection, minimum peak height (S/N) = 3.0, quadratic curve fitting, and 1/X2 weighting.
Statistical Analyses.
Statistical analyses of CYP3A4 activity, SVA half-life, and SVA area under the curve up to the last measurable concentration (AUClast) were performed using a one-way analysis of variance (ANOVA) with Dunnett’s post hoc test in GraphPad Prism software version 7.0 (GraphPad Software, San Diego, CA).
Results
Hepatocyte/Kupffer Cell Dynamic Coculture Characterization.
Hepatocytes and KCs seeded into LiverChip bioreactors attached to the collagen-coated polystyrene scaffold inserts, and downward flow after seeding helped direct them into the scaffold microchannels. Coculture microtissue morphology is shown in Fig. 2 by confocal reconstruction. By culture day 7, microtissues several cell layers thick in the radial direction formed inside the channels, and these tissues extended the 250 µm channel depth. Gaps in the tissue allowed for fluid flow through the scaffold. Cells were also present on the top surface of the scaffolds. Kupffer cells cocultured in this format have previously been demonstrated to be interspersed among the hepatocytes (Wheeler et al., 2014).
Fig. 2.
Representative (A) low-magnification and (B) high-magnification confocal reconstructions of hepatocyte microtissue morphology in a LiverChip scaffold after 7 days of culture stained for f-actin (green) and Hoechst (blue). Scale bars: 100 µm.
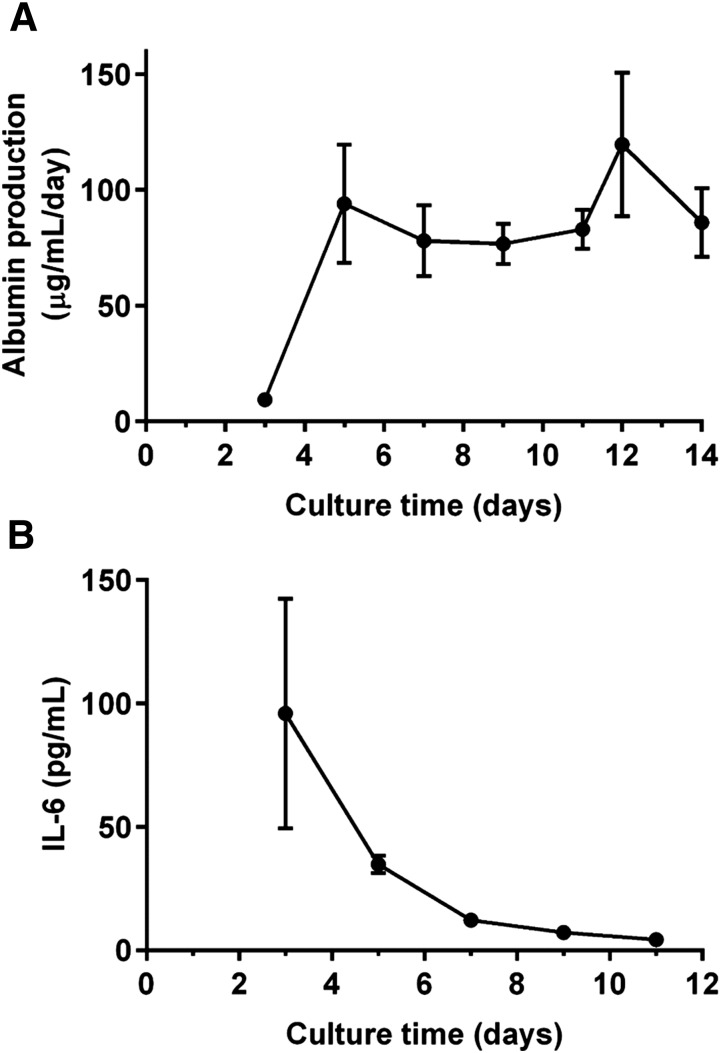
Figure 3A shows the hepatocyte albumin production profile as a function of culture time for hepatocyte donor HU8160. Albumin synthesis is a widely accepted marker of hepatocyte activity for in vitro cultures. Albumin accumulates between media changes, so the measured albumin concentrations shown are from the end of those 24- to 48-hour periods of accumulation and are normalized per day. Albumin production began at low levels before day 3 before subsequently increasing and stabilizing by 5 days. Figure 3B shows that for hepatocyte donor HU8160, basal levels of inflammation, as measured by IL-6 concentration, began high with a mean concentration of 96.0 ± 46.5 pg/ml before decreasing and stabilizing to healthy human physiologic serum levels (<10 pg/ml) by 7 days.
Fig. 3.
(A) Albumin production by hepatocytes in the LiverChip over 2 weeks of culture. (B) Basal IL-6 profile for hepatocyte-KC cocultures in the LiverChip. Data points represent the mean ± S.D. for n = 3 technical replicates for hepatocyte donor HU8160.
IL-6 Stimulation Studies.
CYP3A4 activity, CRP secretion, and levels of sIL-6R all showed a strong dose response to IL-6 for hepatocyte donor HU8160 (see Fig. 4). CYP3A4 activity decreased with increasing IL-6 exposure over 96 hours, showing a sigmoidal relationship with an EC50 of 463 pg/ml IL-6 (Fig. 4A). As a result, for subsequent studies modulating CYP3A4 with IL-6, an IL-6 concentration of 1 ng/ml was chosen to saturate the response. CRP, being an acute phase protein, showed an increase in secretion over 48 hours in response to higher levels of IL-6, with a sigmoidal relationship and an EC50 of 354 pg/ml IL-6 (Fig. 4B). The sIL-6R receptor levels showed a first-order decrease in response to increasing IL-6 concentration over 48 hours (Fig. 4C), further demonstrating the bioactivity of IL-6 on the cellular system (Rose-John, 2012).
Fig. 4.
Dose responses of IL-6 on (A) CYP3A4 activity, (B) CRP secretion, and (C) sIL-6R shedding. Data points represent the mean ± S.D. for n = 3 technical replicates for hepatocyte donor HU8160.
Tocilizumab Effects on CYP3A4 Activity.
To parse the ability of the in vitro liver microreactor system to capture the crucial features of known human responses to tocilizumab in patients who also take SVS, we designed an experiment involving six samples groups with two sequential periods of treatment, mimicking the inflammation and resolution of inflammation by tocilizumab treatment, as shown schematically in Fig. 5. For each sample group, the relative CYP3A4 activities for hepatocyte lot HU8160 are represented as a ratio between the activities measured at the ends of the second treatment period (days 12 to 14) to the first treatment period (days 7 to 9) and are plotted in Fig. 6.
Fig. 5.
Tocilizumab–simvastatin interaction study design. A 2-week experiment with six samples groups run in triplicate. This experiment was repeated with three hepatocyte lots. Two dosing periods were used in this study to measure the effects of different treatments in succession on SVA PK and CYP3A4 activity. Treatments in the first dosing period from days 5 to 9 were either with or without 1 ng/ml IL-6. Treatments in the second dosing period from days 9 to 14 included IL-6 and 1.6 µM tocilizumab either separately or dosed together. Ten times Cmax simvastatin hydroxy acid (SVA) was codosed with other treatments on days 7 and 12, with repeated medium sampling taking place over the subsequent 48 hours for PK measurements. CYP3A4 activity assays were run after sampling on days 9 and 14.
Fig. 6.
Ratio of CYP3A4 activities between days 14 and 9 per sample group of tocilizumab–simvastatin interaction study. Columns represent the mean ± S.D. for n = 3 technical replicates per group for hepatocyte donor HU8160. (*P < 0.05 compared with IL-6/IL-6 treatment.)
For hepatocytes not treated throughout the experiment, there was no change in CYP3A4 activity between these two culture periods. Under inflammatory conditions of 1 ng/ml IL-6 throughout the experiment, CYP3A4 activity was depressed (Fig. 4A) but there was no change in the activity measured between treatment periods, so the activity ratio was close to 1. Similarly, CYP3A4 activity was unchanged in cultures that received no treatment in the early period and were subsequently administered 1 ng/ml IL-6 along with 1.6 µM tocilizumab (anti-IL-6R). These results indicate that tocilizumab can block IL-6 signaling in this culture system because the addition of IL-6 as an inflammatory cue would have otherwise decreased the ratio of CYP3A4 activity for day 14 (inflammation cue + inflammation blocker) relative to day 9 (no treatment).
A no-treatment phase followed by tocilizumab alone did not impact CYP3A4, showing that the effect in the presence of IL-6 was specific to inflammatory cytokine inhibition. Initial treatment with IL-6 followed by coadministration of tocilizumab with IL-6 resulted in desuppression of CYP3A4 and a corresponding 2.0 ± 0.5-fold (P < 0.05) increase in the activity ratio on day 14 relative to day 9. Initial treatment with IL-6 followed by a washout period also desuppressed CYP3A4 activity with a mean effect ratio of 1.9 ± 0.5 (P < 0.05). In total, these results demonstrate that the culture system is capable of multiple forms of physiologic adaptation to inflammation.
Testing in a second hepatocyte donor, HU8163 (Supplemental Fig. 1), confirmed the previous results. Similar patterns of CYP3A4 desuppression occurred with IL-6 after tocilizumab treatment, with a mean effect ratio of 1.7 ± 0.1 (P < 0.05), and for the removal of IL-6 with a mean effect ratio of 1.9 ± 0.1 (P < 0.05). In a third hepatocyte donor, HU8196, the IL-6–only controls surprisingly resulted in CYP3A4 desuppression, with higher activity levels measured at the end of the experiment, resulting in an effect ratio approaching that of tocilizumab addition (Supplemental Fig. 2). However, the sIL-6R alpha levels for that donor approached 0 after 7 days of IL-6 treatment (Supplemental Fig. 3), indicating that continued stimulation may have resulted in receptor loss and lack of consistent IL-6 signaling through the proinflammatory sIL-6R over the course of the experiment. Tocilizumab treatment of this donor increased sIL-6R alpha levels, suggesting that treatment reduces receptor internalization and possibly enhances receptor shedding.
Tocilizumab Effects on CRP.
CRP, as a marker of the acute phase response to inflammation, should increase with IL-6 application. Indeed, after 48 hours the sample groups stratified into increasing or decreasing CRP levels based on the respective presence or absence of a 1 ng/ml IL-6 stimulus, as illustrated in Fig. 7 for each of the six sample groups in the tocilizumab experiment for hepatocyte donor HU8160. High levels of IL-6 produced an initial 4-fold increase in CRP, whereas hepatocytes with no inflammatory stimulus throughout the experiment produced CRP levels that decreased and approached 0 over time, which is consistent with the basal IL-6 decrease seen in Fig. 3B. High CRP was maintained for hepatocytes dosed only with IL-6 throughout the experiment while treatment with tocilizumab caused CRP levels to decrease within 2 days, with near-basal levels of CRP measured 3 days after application of antibody. This pattern was mirrored in samples where IL-6 was removed from the system, providing further evidence that tocilizumab successfully inhibited proinflammatory IL-6 signaling. These results were corroborated in hepatocyte donor HU8163 (Supplemental Fig. 4). The fold changes for that donor were even higher due to lower basal levels of CRP.
Fig. 7.
Fold changes in CRP secretion over time. Data points represent the mean ± S.D. for n = 3 technical replicates per group for hepatocyte donor HU8160.
Simvastatin Hydroxy Acid Metabolism in the LiverChip.
With IL-6–mediated CYP3A4 suppression and subsequent tocilizumab-induced CYP3A4 desuppression established in the LiverChip, we turned our attention to measuring SVA metabolism in this model with the aim of directly demonstrating the impact of tocilizumab on SVA PK, as observed in human patients. The SVA concentration was measured from bioreactor samples using an LC-MS/MS method with LV as an internal standard. Supplemental Fig. 5 shows a representative liquid chromatography elution profile for product ions of SVS, SVA, and LV, which all demonstrated distinct elution times.
For bioreactor studies, interconversion from SVA to the simvastatin lactone form was negligible (<1%), and freeze-thaw did not significantly impact measurements (data not shown). Supplemental Fig. 6 shows representative parent and product ion spectra for SVA and LV with expected ion peaks identified for SVA and LV (Li et al., 2006). The linear range of detection for this assay was 50–1200 ng/ml SVA (data not shown). The MS peaks associated with known phase I metabolites of SVA such as 3′-hydroxy SVA, 6ʹ-exomethylene SVA, and 3′,5′-dihydrodiol SVA were not detected in this platform, likely due to large bioreactor media volumes diluting their signals below background (data not shown).
In the hepatocyte maintenance medium, SVA protein binding was measured to be 66% using an ultracentrifugation protocol (Nakai et al., 2004). An SVA dose of 909 ng/ml was chosen for bioreactor studies to set the free drug fraction in the medium to 10 times the reported average human serum Cmax (30.9 ng/ml) for an 80-mg SVS dose (Pharmapendium). Figure 8 shows the SVA concentration profiles from hepatocyte lot HU8160 under normal and inflammatory conditions during treatment periods starting on day 7 (Fig. 8A) and day 12 (Fig. 8B).
Fig. 8.
SVA concentration profiles in the LiverChip after treatment on culture day 7 (A) and culture day 12 (B). Data points represent the mean ± S.D. for n = 3 technical replicates per group for hepatocyte donor HU8160.
The actual measured concentration at time 0 and for no-cell controls was slightly less than 909 ng/ml due to an expected small loss in recovery (Ahmed et al., 2012). The SVA half-life was found to be 5.0 ± 0.3 hours on day 7 and 4.9 ± 0.3 hours on day 12. Under inflammatory conditions with 1 ng/ml IL-6, the mean SVA half-life was increased to 6.4 ± 1.2 hours on day 7 and 6.8 ± 1.5 hours on day 9, consistent with decreasing CYP3A4 activity. SVA AUClast was 4841.0 ± 142.8 ng·h/ml on day 7 and 4961.2 ± 127.5 ng·h/ml on day 12. Under inflammatory conditions, SVA AUClast was 5688.7 ± 831.2 ng·h/ml on day 7 and 6034.7 ± 889.7 ng·h/ml on day 12. The increasing half-life and AUClast under inflammatory conditions is consistent with CYP3A4 depression.
Tocilizumab-Simvastatin Hydroxy Acid Interaction.
For the LiverChip experiment detailed in Fig. 5, SVA was codosed during two treatment periods, and the media were repeatedly sampled over the next 48 hours for measurements of how tocilizumab impacts SVA PK in the microreactor. The half-life and AUClast results for hepatocyte donor HU8160 are shown in Fig. 9. Each parameter is represented as a ratio to demonstrate the changes induced by tocilizumab.
Fig. 9.
Tocilizumab effect on SVA half-life (A) and AUClast (B). Columns represent the mean ± S.D. for n = 3 technical replicates per group for hepatocyte donor HU8160. (*P < 0.05 compared with IL-6/IL-6 treatment.)
Coadministration of tocilizumab after an initial IL-6 treatment reduced the mean SVA half-life ratio by 29.8% (P < 0.05) (see Fig. 9A) and reduced the mean AUClast ratio by 21.0% compared with IL-6 treatment alone (P < 0.05) (see Fig. 9B). Similar effect sizes were observed for hepatocyte donor HU8163, where tocilizumab decreased the mean SVA half-life ratio by 20.6% (Supplemental Fig. 7A) and mean AUClast ratio by 19.0% compared with IL-6 treatment alone (P < 0.05) (Supplemental Fig. 7B).
Discussion
Dynamic flow culture and in vitro 3D liver tissue engineering have been proposed as solutions to overcome limitations in the functional capacity of primary human hepatocytes (LeCluyse et al., 2012; Dash et al., 2013; Ebrahimkhani et al., 2014). These methods have shown promise in the evaluation of hepatic drug metabolism, DILI, and PK (Chao et al., 2009; Kostadinova et al., 2013; Messner et al., 2013; Ballard et al., 2016). The LiverChip platform offers continuous perfusion of oxygenated medium through a scaffold containing microchannels for 3D cell culture under flow. Human hepatocytes cultured in the LiverChip have previously been shown to express essential metabolic enzymes over 1 week of culture (Vivares et al., 2015), and cocultures with KCs recapitulated key behaviors under inflammatory conditions such as cytokine secretion in the presence of lipopolysaccharide (Sarkar et al., 2015). Here, we have demonstrated hepatocyte functionality and the ability to successfully modulate cytochrome P450 activities in response to an IL-6 inflammatory stimulus over 2 weeks of culture in the LiverChip. In contrast, previous in vitro studies involving IL-6–mediated CYP3A4 suppression in primary human hepatocytes were limited to 96 hours (Dickmann et al., 2011). As a result, those two-dimensional cultures were unable to show subsequent CYP desuppression induced by interfering with inflammatory cytokine signaling, an important effect that has been demonstrated clinically using tocilizumab (Schmitt et al., 2011). The LiverChip platform and its capacity for long-term culture offer the opportunity to directly recapitulate this clinically relevant tocilizumab-mediated CYP desuppression and its impact on small-molecule PK in vitro.
In LiverChip cultures, both albumin and basal IL-6 stabilized over time, with albumin production increasing after 3 days, and basal inflammation decreasing to healthy physiologic levels (<10 pg/ml) after 1 week. This indicates an initial period of adaptation to the culture after cryopreserved hepatocytes were seeded. Similar patterns for decreases in the clinical liver injury markers aspartate aminotransferase and alanine aminotransferase were observed for freshly isolated human hepatocytes in the LiverChip along similar time scales, although these were not measured in our study (Wheeler et al., 2014).
IL-6 induced a dose-dependent decrease in CYP3A4 activity and an increase in CRP synthesis. These effects were similar to those observed for hepatocytes in two-dimensional culture (Dickmann et al., 2011). Our results have shown that the IL-6 EC50 for CYP3A4 and CRP effects fell in the clinical range for inflammatory diseases (10–1500 pg/ml; Machavaram et al., 2013), as did the 1 ng/ml IL-6 dose subsequently chosen for chronic inflammation studies.
KCs were included to build a more complete hepatic cellular microenvironment, and numerous studies have shown their impact on the behavior of in vitro liver systems, such as enhanced IL-1β–mediated cytokine secretion, acute phase protein production, and cytochrome P450 suppression (Hoebe et al., 2001; Sunman et al., 2004; Nguyen et al., 2015; Sarkar et al., 2015; Rose et al., 2016). There are other mechanisms mediated by IL-6 that could potentially be modeled in the LiverChip system such as drug transporter activity. Exploring additional aspects of cytokine signaling in the LiverChip is a logical extension of the current work.
Notably, in our studies dexamethasone, a potent synthetic anti-inflammatory steroid, was not added to the seeding or maintenance medium, a common practice to boost hepatocyte-specific gene expression and activity. We instead used hydrocortisone (HC), a naturally occurring corticosteroid, in maintenance medium beginning on culture day 3. In fact, 100 nM HC is a physiologically relevant concentration, and HC metabolism in LiverChip cocultures has previously been characterized (Sarkar et al., 2015). The absence of HC reduced basal CYP3A4 activity to low levels after 1 week (data not shown). Our studies also used rifampicin as a CYP3A4 induction agent to increase the dynamic range of CYP3A4 activity. Rifampicin acts on PXR, the nuclear receptor that controls the bulk of CYP3A4 transcription (Li and Chiang, 2006). PXR is the same receptor acted upon by IL-6 signaling (Yang et al., 2010), and rifampicin has been previously used to augment the effects of inflammatory agents on CYP3A4 in hepatocytes (Pascussi et al., 2000; Gu et al., 2006).
We found that sIL-6R showed an IL-6 dose-dependent decrease. In this platform, membrane-resident IL-6 receptors on hepatocytes are likely outnumbered by high concentrations of soluble receptors (Nesbitt and Fuller, 1992) due to a high medium-volume-to-tissue ratio. Membrane and soluble receptors of IL-6 have equivalent affinity for IL-6 (Scheller et al., 2014) and are both inhibited by tocilizumab (Mihara et al., 2005), making the soluble receptors the key mediators of IL-6 signaling in the LiverChip. This fits with the observed decrease in sIL-6R upon IL-6 treatment. In order for IL-6 to be used as a chronic proinflammatory stimulus in long-term cultures, sIL-6R must not be depleted over the course of the experiment. We observed that when sIL-6R was depleted for hepatocyte donor HU8196 after IL-6 administration, this contributed to a CYP3A4 result in disagreement with other hepatocyte donors.
In our studies, LiverChip culture was used to mimic a large molecule–small molecule DDI, specifically the clinically demonstrated impact of tocilizumab on CYP3A4 and SVA metabolism (Schmitt et al., 2011). High systemic levels of IL-6 in rheumatoid arthritis patients decrease CYP3A4 activity in the liver, but when tocilizumab is administered, IL-6 signaling decreases, leading to a desuppression of CYP3A4 activity. Schmitt et al. (2011) reported PK results for the prodrug SVS and its biologically active HMG CoA reductase inhibitor form SVA before and after 2 weeks of tocilizumab treatment. Clinically, tocilizumab reduced SVA AUClast by 39% (24%–51%, 90% confidence interval [CI]) and SVS AUClast by 57% (45%–66%, 90% CI). No significant changes in half-life of SVS or SVA after tocilizumab were reported, with SVA half-life of 5 ±1 hours before tocilizumab and 4 ±2 hours after tocilizumab. For our studies we focused on SVA because it is the biologically active form and because in cell culture media the SVS parent undergoes a pH-dependent nonenzymatic conversion to SVA (Ahmed et al., 2012), which confounds its analysis. Nonspecific SVA interconversion back to the simvastatin lactone form was negligible in our system (<1%), and metabolic products of SVA were not detected likely due to large bioreactor media volumes diluting low metabolite signals. However, the media volume in this platform was also advantageous because it allowed for repeated PK sampling over 48 hours after SVA treatment.
To mimic the tocilizumab–SVA clinical study in vitro, we treated hepatocytes with 10 times human serum Cmax SVA under inflammatory conditions before and after the addition of tocilizumab at its human serum Cmax. The SVA AUClast was measured and compared for both treatment periods, showing decreases of 20.6% and 21.0% after tocilizumab treatment in two hepatocyte donors. This effect was statistically significant compared with controls but fell just under the 90% CI for the reported clinical tocilizumab effect. CYP3A4 activity showed a 70%–90% increase across donors after tocilizumab addition to IL-6 inflamed hepatocytes. This assay uses a substrate at its CYP3A4 enzyme Km, whereas for SVA a concentration of 2.1 µM was used, well below its Km of 50–80 µM (Prueksaritanont et al., 2003), indicating a relatively low enzyme activity rate. This SVA concentration was chosen based on physiologic relevance balanced with LC-MS/MS assay range of detection, which may help to explain why the effect size for SVA PK after tocilizumab was not larger. The biologic activity of tocilizumab on IL-6 signaling in hepatocytes was confirmed using CRP, which increased under inflammatory conditions and abruptly decreased after tocilizumab addition, mimicking clinical findings (Nishimoto et al., 2008).
In studies with primary cells, donor variability is always a concern. Cryopreserved human hepatocytes are advantageous because they provide interexperimental consistency. Cytochrome P450 metabolic enzyme activity is highly donor dependent, with a 30- to 40-fold observed variation in CYP3A activity (Tracy et al., 2016). Attempting to capture the whole population spectrum of human hepatocytes in vitro would likely prove challenging and costly. Although it is possible to use hepatocyte donor cocktails for short-term cultures in an attempt to represent a population average, these pooled hepatocytes are not necessarily amenable to long-term culture because of the difficulties in controlling cell numbers over time. As an alternative, physiologically based PK models have been used for simulation of IL-6–mediated CYP phenomena (Machavaram et al., 2013; Xu et al., 2015), but notably these models often rely on parameters generated from in vitro datasets.
To our knowledge, the studies presented here have been the first to directly demonstrate the clinically observed tocilizumab–SVA interaction in an in vitro liver culture model, which was made possible due to the extended culture times allowed by a 3D perfusion bioreactor platform. This proof of concept study illustrates the power of next-generation cell culture technologies for the prediction of clinically relevant drug-drug interactions. Advanced in vitro systems in combination with in silico modeling approaches continue to drive improvements in the early drug development process. As these in vitro models increase in complexity and approach greater physiologic relevance, their application should continue to expand from drug PK and toxicity evaluations to pharmacodynamic and human disease models (Ananthanarayanan et al., 2014; Wheeler et al., 2014; Vernetti et al., 2016).
Acknowledgments
The authors thank David Hughes and Emma Large from CN Bio Innovations for assisting with LiverChip installation, hepatocyte lot qualification, and platform optimization. We also thank Josh Dekeyser, John Roberts, and Dean Hickman from Amgen PKDM, Murat Cirit, Jiajie Yu, and Nikos Tsamandouras from MIT and Raman Venkataramanan from the University of Pittsburgh for helpful discussions. We would also like to thank Robert Dawson from the Amgen medical writing team for help with figure preparation.
Abbreviations
- AUClast
area under the curve up to the last measurable concentration
- CI
confidence interval
- CRP
C-reactive protein
- CYP
cytochrome P450 enzymes
- 3D
three dimensional
- DDI
drug-drug interaction
- DILI
drug-induced liver injury
- ELISA
enzyme-linked immunosorbent assay
- HC
hydrocortisone
- HMG-CoA
3-hydroxy-3-methylglutaryl-coenzyme A
- IL-6
interleukin-6
- IL-6R
interleukin-6 receptor
- KC
Kupffer cell
- LC-MS/MS
liquid chromatography with tandem mass spectrometry
- LV
lovastatin
- PBS
phosphate-buffered saline
- PK
pharmacokinetics
- PXR
pregnane X receptor
- sIL-6R
soluble IL-6 receptor
- SVA
simvastatin hydroxy acid
- SVS
simvastatin
Authorship Contributions
Participated in research design: Long, Dunn, Hamadeh, Afshari, McBride, Griffith.
Conducted experiments: Long, Cosgrove.
Contributed new reagents or analytic tools: Cosgrove, Dunn.
Performed data analysis: Long, Cosgrove, Stolz.
Wrote or contributed to the writing of the manuscript: Long, Cosgrove, Dunn, Stolz, Hamadeh, Afshari, McBride, Griffith.
Footnotes
This work was partly supported by the United States Defense Advanced Research Projects Agency (DARPA) [W911NF-12-2-0039] and by the National Institutes of Health [UH3TR000496].
 This article has supplemental material available at dmd.aspetjournals.org.
This article has supplemental material available at dmd.aspetjournals.org.
References
- Ahmed TA, Horn J, Hayslip J, Leggas M. (2012) Validated LC-MS/MS method for simultaneous determination of SIM and its acid form in human plasma and cell lysate: pharmacokinetic application. J Pharm Anal 2:403–411 DOI: 10.1016/j.jpha.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ananthanarayanan A, Nugraha B, Triyatni M, Hart S, Sankuratri S, Yu H. (2014) Scalable spheroid model of human hepatocytes for hepatitis C infection and replication. Mol Pharm 11:2106–2114. [DOI] [PubMed] [Google Scholar]
- Ballard TE, Wang S, Cox LM, Moen MA, Krzyzewski S, Ukairo O, Obach RS. (2016) Application of a micropatterned cocultured hepatocyte system to predict preclinical and human-specific drug metabolism. Drug Metab Dispos 44:172–179. [DOI] [PubMed] [Google Scholar]
- Bode JG, Albrecht U, Häussinger D, Heinrich PC, Schaper F. (2012) Hepatic acute phase proteins—regulation by IL-6- and IL-1-type cytokines involving STAT3 and its crosstalk with NF-κB-dependent signaling. Eur J Cell Biol 91:496–505. [DOI] [PubMed] [Google Scholar]
- Chao P, Maguire T, Novik E, Cheng KC, Yarmush ML. (2009) Evaluation of a microfluidic based cell culture platform with primary human hepatocytes for the prediction of hepatic clearance in human. Biochem Pharmacol 78:625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash A, Inman W, Hoffmaster K, Sevidal S, Kelly J, Obach RS, Griffith LG, Tannenbaum SR. (2009) Liver tissue engineering in the evaluation of drug safety. Expert Opin Drug Metab Toxicol 5:1159–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash A, Simmers MB, Deering TG, Berry DJ, Feaver RE, Hastings NE, Pruett TL, LeCluyse EL, Blackman BR, Wamhoff BR. (2013) Hemodynamic flow improves rat hepatocyte morphology, function, and metabolic activity in vitro. Am J Physiol Cell Physiol 304:C1053–C1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickmann LJ, Patel SK, Rock DA, Wienkers LC, Slatter JG. (2011) Effects of interleukin-6 (IL-6) and an anti-IL-6 monoclonal antibody on drug-metabolizing enzymes in human hepatocyte culture. Drug Metab Dispos 39:1415–1422. [DOI] [PubMed] [Google Scholar]
- Domansky K, Inman W, Serdy J, Dash A, Lim MHM, Griffith LG. (2010) Perfused multiwell plate for 3D liver tissue engineering. Lab Chip 10:51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahimkhani MR, Neiman JAS, Raredon MSB, Hughes DJ, Griffith LG. (2014) Bioreactor technologies to support liver function in vitro. Adv Drug Deliv Rev 69-70:132–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Ke S, Liu D, Sheng T, Thomas PE, Rabson AB, Gallo MA, Xie W, Tian Y. (2006) Role of NF-κB in regulation of PXR-mediated gene expression: a mechanism for the suppression of cytochrome P-450 3A4 by proinflammatory agents. J Biol Chem 281:17882–17889. [DOI] [PubMed] [Google Scholar]
- Heikkilä K, Ebrahim S, Lawlor DA. (2008) Systematic review of the association between circulating interleukin-6 (IL-6) and cancer. Eur J Cancer 44:937–945. [DOI] [PubMed] [Google Scholar]
- Hoebe KH, Witkamp RF, Fink-Gremmels J, Van Miert ASJPAM, Monshouwer M. (2001) Direct cell-to-cell contact between Kupffer cells and hepatocytes augments endotoxin-induced hepatic injury. Am J Physiol Gastrointest Liver Physiol 280:G720–G728. [DOI] [PubMed] [Google Scholar]
- Huang SM, Zhao H, Lee JI, Reynolds K, Zhang L, Temple R, Lesko LJ. (2010) Therapeutic protein-drug interactions and implications for drug development. Clin Pharmacol Ther 87:497–503. [DOI] [PubMed] [Google Scholar]
- Hwa AJ, Fry RC, Sivaraman A, So PT, Samson LD, Stolz DB, Griffith LG. (2007) Rat liver sinusoidal endothelial cells survive without exogenous VEGF in 3D perfused co-cultures with hepatocytes. FASEB J 21:2564–2579. [DOI] [PubMed] [Google Scholar]
- Ingelman-Sundberg M. (2004) Human drug metabolising cytochrome P450 enzymes: properties and polymorphisms. Naunyn Schmiedebergs Arch Pharmacol 369:89–104. [DOI] [PubMed] [Google Scholar]
- Ishihara K, Hirano T. (2002) IL-6 in autoimmune disease and chronic inflammatory proliferative disease. Cytokine Growth Factor Rev 13:357–368. [DOI] [PubMed] [Google Scholar]
- Jover R, Bort R, Gómez-Lechón MJ, Castell JV. (2002) Down-regulation of human CYP3A4 by the inflammatory signal interleukin-6: molecular mechanism and transcription factors involved. FASEB J 16:1799–1801. [DOI] [PubMed] [Google Scholar]
- Kostadinova R, Boess F, Applegate D, Suter L, Weiser T, Singer T, Naughton B, Roth A. (2013) A long-term three dimensional liver co-culture system for improved prediction of clinically relevant drug-induced hepatotoxicity. Toxicol Appl Pharmacol 268:1–16. [DOI] [PubMed] [Google Scholar]
- LeCluyse EL, Witek RP, Andersen ME, Powers MJ. (2012) Organotypic liver culture models: meeting current challenges in toxicity testing. Crit Rev Toxicol 42:501–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JI, Zhang L, Men AY, Kenna LA, Huang SM. (2010) CYP-mediated therapeutic protein-drug interactions: clinical findings, proposed mechanisms and regulatory implications. Clin Pharmacokinet 49:295–310. [DOI] [PubMed] [Google Scholar]
- Li C, Subramanian R, Yu S, Prueksaritanont T. (2006) Acyl-coenzyme a formation of simvastatin in mouse liver preparations. Drug Metab Dispos 34:102–110. [DOI] [PubMed] [Google Scholar]
- Li T, Chiang JY. (2006) Rifampicin induction of CYP3A4 requires pregnane X receptor cross talk with hepatocyte nuclear factor 4alpha and coactivators, and suppression of small heterodimer partner gene expression. Drug Metab Dispos 34:756–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machavaram KK, Almond LM, Rostami-Hodjegan A, Gardner I, Jamei M, Tay S, Wong S, Joshi A, Kenny JR. (2013) A physiologically based pharmacokinetic modeling approach to predict disease-drug interactions: suppression of CYP3A by IL-6. Clin Pharmacol Ther 94:260–268. [DOI] [PubMed] [Google Scholar]
- Messner S, Agarkova I, Moritz W, Kelm JM. (2013) Multi-cell type human liver microtissues for hepatotoxicity testing. Arch Toxicol 87:209–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara M, Kasutani K, Okazaki M, Nakamura A, Kawai S, Sugimoto M, Matsumoto Y, Ohsugi Y. (2005) Tocilizumab inhibits signal transduction mediated by both mIL-6R and sIL-6R, but not by the receptors of other members of IL-6 cytokine family. Int Immunopharmacol 5:1731–1740. [DOI] [PubMed] [Google Scholar]
- Morgan ET, Goralski KB, Piquette-Miller M, Renton KW, Robertson GR, Chaluvadi MR, Charles KA, Clarke SJ, Kacevska M, Liddle C, et al. (2008) Regulation of drug-metabolizing enzymes and transporters in infection, inflammation, and cancer. Drug Metab Dispos 36:205–216. [DOI] [PubMed] [Google Scholar]
- Morgan ET. (2009) Impact of infectious and inflammatory disease on cytochrome P450-mediated drug metabolism and pharmacokinetics. Clin Pharmacol Ther 85:434–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai D, Kumamoto K, Sakikawa C, Kosaka T, Tokui T. (2004) Evaluation of the protein binding ratio of drugs by a micro-scale ultracentrifugation method. J Pharm Sci 93:847–854. [DOI] [PubMed] [Google Scholar]
- Navarro VJ, Senior JR. (2006) Drug-related hepatotoxicity. N Engl J Med 354:731–739. [DOI] [PubMed] [Google Scholar]
- Nesbitt JE, Fuller GM. (1992) Differential regulation of interleukin-6 receptor and gp130 gene expression in rat hepatocytes. Mol Biol Cell 3:103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TV, Ukairo O, Khetani SR, McVay M, Kanchagar C, Seghezzi W, Ayanoglu G, Irrechukwu O, Evers R. (2015) Establishment of a hepatocyte-Kupffer cell coculture model for assessment of proinflammatory cytokine effects on metabolizing enzymes and drug transporters. Drug Metab Dispos 43:774–785. [DOI] [PubMed] [Google Scholar]
- Nishimoto N, Terao K, Mima T, Nakahara H, Takagi N, Kakehi T. (2008) Mechanisms and pathologic significances in increase in serum interleukin-6 (IL-6) and soluble IL-6 receptor after administration of an anti-IL-6 receptor antibody, tocilizumab, in patients with rheumatoid arthritis and Castleman disease. Blood 112:3959–3964. [DOI] [PubMed] [Google Scholar]
- Norris CA, He M, Kang LI, Ding MQ, Radder JE, Haynes MM, Yang Y, Paranjpe S, Bowen WC, Orr A, et al. (2014) Synthesis of IL-6 by hepatocytes is a normal response to common hepatic stimuli. PLoS One 9:e96053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascussi JM, Gerbal-Chaloin S, Pichard-Garcia L, Daujat M, Fabre JM, Maurel P, Vilarem MJ. (2000) Interleukin-6 negatively regulates the expression of pregnane X receptor and constitutively activated receptor in primary human hepatocytes. Biochem Biophys Res Commun 274:707–713. [DOI] [PubMed] [Google Scholar]
- Peters TS. (2005) Do preclinical testing strategies help predict human hepatotoxic potentials? Toxicol Pathol 33:146–154. [DOI] [PubMed] [Google Scholar]
- Powers MJ, Domansky K, Kaazempur-Mofrad MR, Kalezi A, Capitano A, Upadhyaya A, Kurzawski P, Wack KE, Stolz DB, Kamm R, et al. (2002) A microfabricated array bioreactor for perfused 3D liver culture. Biotechnol Bioeng 78:257–269. [DOI] [PubMed] [Google Scholar]
- Prueksaritanont T, Ma B, Yu N. (2003) The human hepatic metabolism of simvastatin hydroxy acid is mediated primarily by CYP3A, and not CYP2D6. Br J Clin Pharmacol 56:120–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose KA, Holman NS, Green AM, Andersen ME, LeCluyse EL. (2016) Co-culture of hepatocytes and Kupffer cells as an in vitro model of inflammation and drug-induced hepatotoxicity. J Pharm Sci 105:950–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose-John S. (2012) IL-6 trans-signaling via the soluble IL-6 receptor: importance for the pro-inflammatory activities of IL-6. Int J Biol Sci 8:1237–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar U, Rivera-Burgos D, Large EM, Highes DJ, Ravindra KC, Dyer RL, Ebrahimkhani MR, Wishnok JS, Griffith LG, Tannenbaum SR. (2015) Metabolite profiling and pharmacokinetic evaluation of hydrocortisone in a perfused three-dimensional human liver bioreactor. Drug Metab Dispos 43:1091–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller J, Garbers C, Rose-John S. (2014) Interleukin-6: from basic biology to selective blockade of pro-inflammatory activities. Semin Immunol 26:2–12. [DOI] [PubMed] [Google Scholar]
- Schmitt C, Kuhn B, Zhang X, Kivitz AJ, Grange S. (2011) Disease-drug-drug interaction involving tocilizumab and simvastatin in patients with rheumatoid arthritis [published correction appears in Clin Pharmacol Ther 2011;90(3):479] Clin Pharmacol Ther 89:735–740. [DOI] [PubMed] [Google Scholar]
- Sivaraman A, Leach JK, Townsend S, Iida T, Hogan BJ, Stolz DB, Fry R, Samson LD, Tannenbaum SR, Griffith LG. (2005) A microscale in vitro physiological model of the liver: predictive screens for drug metabolism and enzyme induction. Curr Drug Metab 6:569–591. [DOI] [PubMed] [Google Scholar]
- Sunman JA, Hawke RL, LeCluyse EL, Kashuba AD. (2004) Kupffer cell-mediated IL-2 suppression of CYP3A activity in human hepatocytes. Drug Metab Dispos 32:359–363. [DOI] [PubMed] [Google Scholar]
- Tracy TS, Chaudhry AS, Prasad B, Thummel KE, Schuetz EG, Zhong XB, Tien YC, Jeong H, Pan X, Shireman LM, et al. (2016) Interindividual variability in cytochrome P450-mediated drug metabolism. Drug Metab Dispos 44:343–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Drug Evaluation and Research (CDER) (2012) Guidance for Industry: Drug Interaction Studies—Study Design, Data Analysis, Implications for Dosing, and Labeling Recommendations [Draft Guidance], U.S. Department of Health and Human Services, Food and Drug Administration, Rockville, MD: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm292362.pdf [Google Scholar]
- Vernetti LA, Senutovitch N, Boltz R, DeBiasio R, Shun TY, Gough A, Taylor DL. (2016) A human liver microphysiology platform for investigating physiology, drug safety, and disease models. Exp Biol Med (Maywood) 241:101–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivares A, Salle-Lefort S, Arabeyre-Fabre C, Ngo R, Penarier G, Bremond M, Moliner P, Gallas JF, Fabre G, Klieber S. (2015) Morphological behaviour and metabolic capacity of cryopreserved human primary hepatocytes cultivated in a perfused multiwell device. Xenobiotica 45:29–44. [DOI] [PubMed] [Google Scholar]
- Wheeler SE, Clark AM, Taylor DP, Young CL, Pillai VC, Stolz DB, Venkataramanan R, Lauffenburger D, Griffith L, Wells A. (2014) Spontaneous dormancy of metastatic breast cancer cells in an all human liver microphysiologic system. Br J Cancer 111:2342–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu DX, Wei W, Sun MF, Wu CY, Wang JP, Wei LZ, Zhou CF. (2004) Kupffer cells and reactive oxygen species partially mediate lipopolysaccharide-induced downregulation of nuclear receptor pregnane x receptor and its target gene CYP3a in mouse liver. Free Radic Biol Med 37:10–22. [DOI] [PubMed] [Google Scholar]
- Xu Y, Hijazi Y, Wolf A, Wu B, Sun YN, Zhu M. (2015) Physiologically based pharmacokinetic model to assess the influence of Blinatumomab-mediated cytokine elevations on cytochrome P450 enzyme activity. CPT Pharmacometrics Syst Pharmacol 4:507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Hao C, Yang D, Shi D, Song X, Luan X, Hu G, Yan B. (2010) Pregnane X receptor is required for interleukin-6-mediated down-regulation of cytochrome P450 3A4 in human hepatocytes. Toxicol Lett 197:219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Cilfone NA, Large EM, Sarkar U, Wishnok JS, Tannenbaum SR, Hughes DJ, Lauffenburger DA, Griffith LG, Stokes CL, et al. (2015) Quantitative systems pharmacology approaches applied to microphysiological systems (MPS): data interpretation and multi-MPS integration. CPT Pharmacometrics Syst Pharmacol 4:585–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Li F. (2014) Potential pharmacokinetic interactions of therapeutic cytokines or cytokine modulators on small-molecule drugs: mechanistic understanding via studies using in vitro systems. Drug Metabol Drug Interact 29:17–28. [DOI] [PubMed] [Google Scholar]