Abstract
Protein expression of renal uptake and efflux transporters was quantified by quantitative targeted proteomics using the surrogate peptide approach. Renal uptake transporters assessed in this study included organic anion transporters (OAT1–OAT4), organic cation transporter 2 (OCT2), organic/carnitine cation transporters (OCTN1 and OCTN2), and sodium-glucose transporter 2 (SGLT2); efflux transporters included P-glycoprotein, breast cancer resistance protein, multidrug resistance proteins (MRP2 and MRP4), and multidrug and toxin extrusion proteins (MATE1 and MATE2-K). Total membrane was isolated from the cortex of human kidneys (N = 41). The isolated membranes were digested by trypsin and the digest was subjected to liquid chromatography–tandem mass spectrometry analysis. The mean expression of surrogate peptides was as follows (given with the standard deviation, in picomoles per milligram of total membrane protein): OAT1 (5.3 ± 1.9), OAT2 (0.9 ± 0.3), OAT3 (3.5 ± 1.6), OAT4 (0.5 ± 0.2), OCT2 (7.4 ± 2.8), OCTN1 (1.3 ± 0.6), OCTN2 (0.6 ± 0.2), P-glycoprotein (2.1 ± 0.8), MRP2 (1.4 ± 0.6), MRP4 (0.9 ± 0.6), MATE1 (5.1 ± 2.3), and SGLT2 (3.7 ± 1.8). Breast cancer resistance protein (BCRP) and MATE2-K proteins were detectable but were below the lower limit of quantification. Interestingly, the protein expression of OAT1 and OAT3 was significantly correlated (r > 0.8). A significant correlation was also observed between expression of multiple other drug transporters, such as OATs/OCT2 or OCTN1/OCTN2, and SGLT2/OCTNs, OCT, OATs, and MRP2. These renal transporter data should be useful in deriving in vitro to in vivo scaling factors to accurately predict renal clearance and kidney epithelial cell exposure to drugs or their metabolites.
Introduction
Approximately 30% of approved drugs are predominantly (>50% of total body clearance) cleared by the kidneys (Brater, 2002; Feng et al., 2010). Renal drug clearance is the net effect of glomerular filtration plus net tubular secretion (secretion minus reabsorption). Tubular secretion of hydrophilic drug molecules is primarily mediated by both solute carrier and ATP-binding cassette transporters of the proximal tubular cells, located in the renal cortex. For example, the major influx transporters such as organic anion transporters 1 and 3 (OAT1 and OAT3) and organic cation transporter 2 (OCT2) are located in the basolateral membrane, whereas the major efflux transporters such as multidrug and toxin extrusion 1 (MATE1) and P-glycoprotein (P-gp) are located in the apical membrane of the proximal tubules (Feng et al., 2010; Hillgren et al., 2013). These transporters often work in tandem to drive vectorial transport (and therefore secretion) of drugs from the blood into the urine (Meyer zu Schwabedissen et al., 2010). For example, cimetidine and pyrimethamine reduce the renal clearance of metformin by inhibiting OCT2 and MATE1 or MAT1/2-K, respectively (Wang et al., 2008; Tsuda et al., 2009; Kusuhara et al., 2011; Ito et al., 2012; Feng et al., 2013).
Proximal tubule drug transporters have also been associated with the renal toxicity of drugs (Takeda et al., 1999; Enomoto et al., 2002; Ludwig et al., 2004; Iwata et al., 2012; Moss et al., 2014; Mandíková et al., 2016). For example, cisplatin produces renal toxicity due to its accumulation in the tubular epithelial cells mediated by OCT2. This accumulation occurs because cisplatin is not a good substrate of the kidney epithelial efflux transporters, MATE1 or MATE2-K (Yonezawa and Inui, 2011). However, analogs of cisplatin such as carboplatin and nedaplatin lack significant nephrotoxicity, likely due to their poor affinity for OCT2 (Yokoo et al., 2007).
For the reasons cited above, during the drug development process, it is important to predict whether a drug is likely to be cleared in humans by renal secretion and the extent of the secretion. For instance, if the renal clearance of a drug exceeds its filtration clearance, secretion of the drug by renal transporters should be considered. In addition, it is important to determine whether a drug will accumulate in the kidney epithelial cells. Although in vitro tools such as primary two-dimensional cultures or three-dimensional models (organoid models) of renal proximal tubular cells are becoming available or are being developed (Brown et al., 2008; Verhulst et al., 2008; Kelly et al., 2013), they are not currently routinely available or validated. Instead, we and others have proposed a generic in vitro to in vivo extrapolation method for the prediction of transporter-mediated drug disposition, including renal secretory clearance (Prasad and Unadkat, 2014b). This potential approach is based on measurement of in vitro clearance of a drug in cells expressing individual proximal tubule transporters. The in vitro clearance from expressed cell lines is scaled to in vivo clearance using transporter expression levels measured in the human kidney cortex. Since data from the latter are currently limited (Nakamura et al., 2016), this study aimed to quantify proximal tubule–expressed transporters by quantitative targeted proteomics (based on the surrogate peptide approach) and liquid chromatography (LC) coupled to tandem mass spectrometry (MS/MS). The selectivity and reproducibility of this approach are discussed herein.
Materials and Methods
Chemicals and Reagents.
The ProteoExtract native membrane protein extraction kit was procured from Calbiochem (Temecula, CA). The protein quantification BCA kit and the in-solution trypsin digestion kit were purchased from Pierce Biotechnology (Rockford, IL). Synthetic light and heavy peptides (Supplemental Table 1) for renal transporter quantification were obtained from New England Peptides (Boston, MA) and Thermo Fisher Scientific (Rockford, IL), respectively. High-performance LC–grade acetonitrile was purchased from Fisher Scientific (Fair Lawn, NJ), and formic acid was purchased from Sigma-Aldrich (St. Louis, MO). All reagents were of analytical grade.
Procurement of Kidney Cortices.
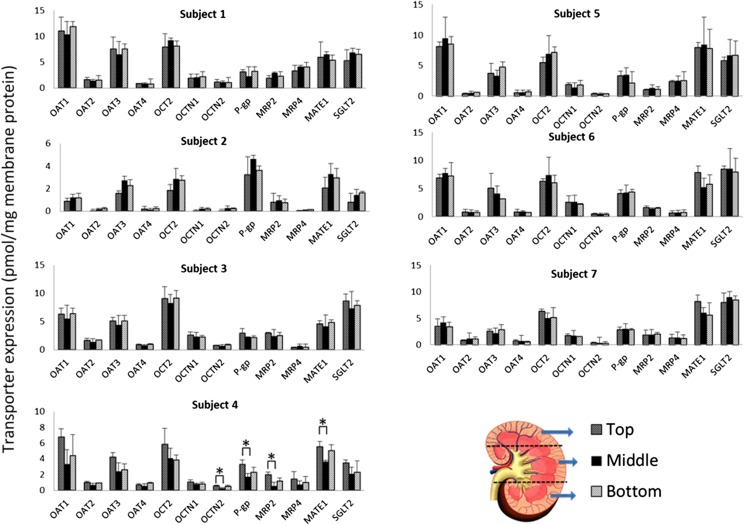
Noncancerous portions (by pathologic examination) of the human kidney cortex (n = 20) from nephrectomies (due to kidney cancer) were collected at the University of Washington Medical Center (UW; Seattle, WA) (Supplemental Table 2). In addition, cortices of kidneys initially targeted for transplant purposes, but eventually not transplanted, were obtained by Ardea Biosciences (AB; n = 7 samples; San Diego, CA) and Newcastle University (NU; n = 14; Newcastle, UK). To assess whether the transporter expression was sample-site dependent, the kidney cortices of seven subjects, collected by AB, were sampled (approximately 100 mg) from three different locations (top, middle, and bottom of the kidney; Fig. 1). The NU samples were available to us as homogenates in a buffer consisting of 25 mM Tris-HCl, 0.5 mM EDTA, 5 mM histidine, and 0.25 M sucrose, pH 7.4. All of the above samples were immediately flash frozen or stored in ice-cold saline buffer for a maximum of 24 hours prior to storage at −80°C. Tissue collection was approved by the respective human subjects division. Although demographic information was available for the UW and AB samples, such information was not available for the NU samples due to human subject restrictions.
Fig. 1.
Transporter protein expression in human cortices obtained from three different parts of the human kidney from seven subjects. Except for the few samples labeled with an asterisk (*P < 0.05, analysis of variance followed by Bonferroni’s multiple comparison test), transporter protein expression was independent of the sampling site. Data are presented as means ± S.D. of triplicate determinations.
Membrane Protein Extraction and Trypsin Digestion.
Total membrane was isolated from the kidney cortex (approximately 100 mg) using a previously described protocol (Prasad et al., 2014). The NU samples were also processed similarly except, instead of the tissue homogenization, a 100-μl aliquot of the cortex homogenate was diluted with 1.9 ml extraction buffer I containing 10 μl protease inhibitor cocktail (ProteoExtract Kit; Calbiochem). The final membrane fraction was diluted to a working concentration of 2 µg membrane protein/µl as quantified by the BCA assay. Total membrane proteins were reduced, denatured, alkylated, and digested as per our previously reported protocol (Wang et al., 2015). All samples were digested and processed in triplicate. The surrogate peptides generated by trypsin digestion were quantified by LC-MS/MS as described below.
Surrogate Peptide Selection and Quantification by LC-MS/MS.
Peptides unique for each transporter and markers of the proximal tubule (proteins predominately located in the proximal tubule) (Supplemental Table 1) were selected based on in silico selection criteria (Kamiie et al., 2008; Prasad and Unadkat, 2014b) and used as calibrators. The corresponding peptides, heavy at [13C615N2]-lysine and [13C615N4]-arginine residues, were used as the internal standards. Eight calibrators ranging from approximately 0.1 to 50.0 fmol (on-column) were prepared by spiking the extraction buffer II of the membrane protein extraction kit with the peptide standards and the internal standards (10 μl).
Surrogate peptides were quantified using the Waters Xevo TQ-S tandem mass spectrometer coupled to an Acquity ultra-performance liquid chromatography (UPLC) system (Waters, Hertfordshire, UK). Briefly, a UPLC column (Acquity UPLC HSS T3 1.8 µm, 2.1 × 100 mm; Waters), with a Security Guard column (C18, 4 mm × 2.0 mm; Phenomenex, Torrance, CA), was eluted (0.3 ml/min) with a gradient mobile phase consisting of water and acetonitrile (with 0.1% formic acid; Supplemental Table 1). The injection volume was 5 µl (approximately 10 μg total protein). Optimized LC-MS/MS parameters (Supplemental Table 1) in positive electrospray ionization mode were used to monitor the parent to product ion transitions for the analyte peptides and their respective heavy peptides.
Data Analyses.
The data were processed by integrating the peak areas generated from the reconstructed ion chromatograms for the analyte peptides and the respective heavy internal standards using MassLynx software (Waters). For quantification of samples or standards, the peak response from two transitions of each peptide was averaged. As justified in our previous publication (Prasad et al., 2014), the peptide yielding the higher value of transporter expression was reported. Protein expression from different sites (top, middle, and bottom parts) of seven subjects was compared by one-way analysis of variance followed by Bonferroni’s multiple comparisons test. A protein–protein expression correlation (r2) > 0.4, estimated using the Spearman correlation, was considered significant. Proteins predominantly expressed in the proximal renal tubules (aquaporin 1, dipeptidase 1, arginosuccinate synthase 1, and dicarbonyl/l-xylulose reductase) were also quantified. Since the expression of all of these markers showed high correlation with each other (r2 > 0.75, data not shown), aquaporin 1, which is expressed in high abundance and is a membrane protein, was used as a proximal tubule membrane marker. The Grubbs’ test was used to identify outliers (Verma et al., 2014). The transporter protein abundance data are presented as means ± S.D.
Results
Comparison of Transporter Expression in Cortex Samples Isolated from Different Parts of the Kidney.
The expression of all of the transporters was not significantly different between the sampling sites except for the few cortices sampled from the top of subject 4’s kidney (Fig. 1). This site-dependent difference (where present) disappeared when transporter protein expression was expressed relative to that of aquaporin 1 (a marker of proximal tubules). This suggests that some of the observed differences (especially for subject 4) were likely due to contamination of the tissue from other parts of the kidney such as the medulla (containing a part of the loop of Henle and the collecting ducts) where the transporters of interest are poorly or not expressed.
Renal Transporter Quantification.
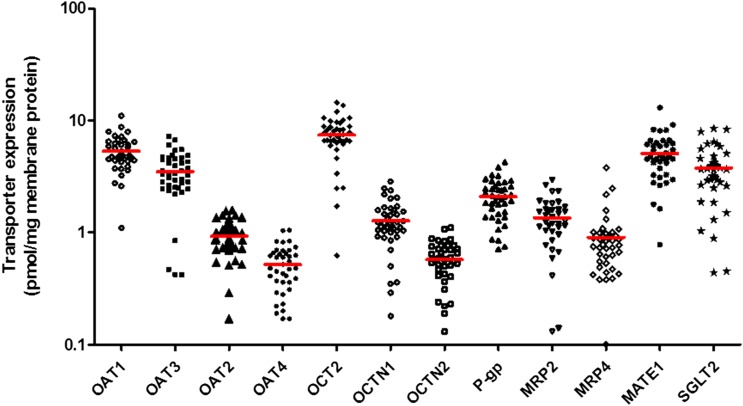
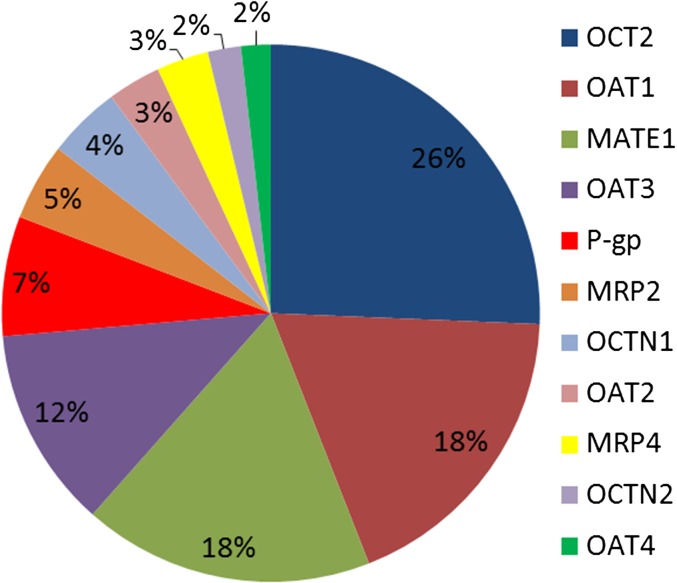
Based on surrogate peptides, the expression of OCT2 in the kidney cortex was found to be the highest, followed by that of OAT1, OAT3, and MATE1 (Figs. 2 and 3; Table 1). Although MATE2-K and breast cancer resistance protein (BCRP) were detected in the cortex, they were below the lower limit of quantification (LLOQ) (signal to noise ratio < 5). Compared with the human liver (Deo et al., 2012; Prasad et al., 2014, 2016; Kumar et al., 2015), P-gp and MATE1 expression in the kidney cortex was 2-fold and 10-fold higher than the liver and multidrug resistance protein 2 (MRP2) expression was 2-fold lower than the liver, respectively. Of the transporters quantified, the protein expression showed moderate variability with a percent coefficient of variation of 34%–51% except for MRP4, which was the most variable transporter with a percent coefficient of variation of 70.7%. Using the limited demographic information available for 27 subjects, no correlation was observed between transporter protein expression and age (donors were aged >40 years), sex (n = 22 men and n = 5 women), or disease condition such as hypertension (n = 12 versus n = 15 controls) or diabetes (n = 7 versus n = 20 controls).
Fig. 2.
Interindividual variability in transporter protein expression in the human kidney cortex (N = 41). Data points indicate observed data and lines indicates mean expression. Of the transporters quantified, the protein expression of MRP4 was the most variable with a percent coefficient of variation of 70.7%.
Fig. 3.
Relative transporter expression shows that OCT2, OAT1, OAT3, and MATE1 are the predominant transporters expressed in the human kidney cortex.
TABLE 1.
Transporter protein expression in the human kidney cortex
Data are presented as means ± S.D. given in picomoles per milligram of membrane protein (N = 41).
| Transporter | Protein Expression | Percent Coefficient of Variation |
|---|---|---|
| Uptake | ||
| OAT1 | 5.33 ± 1.88 | 35.3 |
| OAT2 | 0.93 ± 0.32 | 34.6 |
| OAT3 | 3.50 ± 1.55 | 44.3 |
| OAT4 | 0.52 ± 0.23 | 44.5 |
| OCT2 | 7.42 ± 2.84 | 38.3 |
| OCTN1 | 1.27 ± 0.59 | 46.1 |
| OCTN2 | 0.57 ± 0.24 | 41.2 |
| SGLT2 | 3.67 ± 1.86 | 50.6 |
| Efflux | ||
| P-gp | 2.09 ± 0.82 | 39.3 |
| BCRP | < LLOQ | |
| MRP2 | 1.35 ± 0.63 | 46.4 |
| MRP4 | 0.91 ± 0.64 | 70.7 |
| MATE1 | 5.06 ± 2.27 | 45.0 |
| MATE2-K | < LLOQ |
Protein–Protein Correlation of Kidney Transporter Expression.
Multiple transporters showed correlation (Supplemental Table 3). Notably, protein expression of OAT1 versus OAT3 showed the highest correlation (r > 0.8) (Supplemental Fig. 1; Supplemental Table 3). Other transporters, such as OAT1 versus OAT2, OCT2, or organic/carnitine cation transporter 2 (OCTN2); OAT3 versus OAT2, OCT2, or P-gp; and OCTN1 versus OCTN2 also showed significant correlation (Supplemental Table 3). Interestingly, sodium-glucose transporter 2 (SGLT2) expression was correlated with the expression of multiple transporters (Supplemental Table 3).
Discussion
A sensitive and reproducible LC-MS/MS method was developed for the quantification of the polyspecific drug transporters in the human renal cortex (Fig. 1). Because a blank biologic sample matrix was not available to generate a standard curve, the heavy labeled peptides were used as internal standards to overcome any ion suppression effect produced by the matrix. We have used this approach numerous times to quantify transporter expression in the human liver (Deo et al., 2012; Prasad et al., 2013, 2014, 2016; Prasad and Unadkat, 2014a,b; Kumar et al., 2015). In those studies, we used human liver tissue preparation, spiked with known concentrations of peptides, as quality control samples. These quality control samples (after correcting for the presence of endogenous signal) passed our criteria for accuracy (within 30% of nominal value), indicating that the tissue matrix did not adversely affect our ability to quantify transporters expressed in these tissues.
We focused our study on quantifying protein expression of renal cortex transporters because these transporters are thought to be predominately located in the proximal tubule of the kidney (Giacomini et al., 2010). Transporter expression was found to be consistent across cortex samples obtained from different parts of the kidney (Fig. 1). These results assure that sampling any part of the kidney cortex will result in similar transporter protein expression and demonstrate the reproducibility of our protein quantification method. Based on peptide quantification, the expression of OCT2 in the kidney cortex was found to be the highest, followed by that of OAT1, OAT3, and MATE1 (Fig. 3). Although we quantified the major drug transporters, our study did not include all transporters such as organic anion-transporting polypeptide 4C1, urate transporter 1, and the thiamine transporters. MATE2-K and BCRP were detected in the cortex, but they were below the LLOQ. Our data can be compared with one other study in which only four transporters (P-gp, MRP2, BCRP, and MRP3) in kidneys from four donors were quantified (Fallon et al., 2016). Our P-gp and MRP2 expression data are similar to those reported in that study (Fallon et al., 2016). Consistent with our data, BCRP abundance was found to be close to our LLOQ (Fallon et al., 2016). MRP3 was not detectable in our study perhaps due to poor sensitivity of the surrogate peptide used (Wang et al., 2015). The low expression of MATE2-K, MRP3, and BCRP in the kidneys does not mean that these transporters are not important in the renal clearance of drugs. If the fraction of a drug transported (ft) via these transporters versus passive diffusion or transport by other transporters is large, then these transporters will be important in the renal clearance of the drug. As advances in LC-MS/MS instrumentation and protein sample enrichment methods emerge (e.g., capture by antipeptide antibodies; Razavi et al., 2012), MATE2-K, MRP3, and BCRP may be detected using the surrogate peptides identified in this study.
The mRNA levels of drug transporters in the human kidney have been quantified (Sun et al., 2001; Hilgendorf et al., 2007; Kikuchi et al., 2007; Nozaki et al., 2007). The rank order of mRNA expression is generally consistent with our data on protein expression. The mRNA expression of OAT1 and OAT3 is 5- and 3-fold higher than that of the next highest expressed transporter, OAT4, respectively. The mRNA expression of other transporters in the kidney has the following rank order: MDR1 > MRP2 > OCT2 > OCTN2 > MRP4 (Hilgendorf et al., 2007). As we previously reported, the mRNA expression of transporters does not necessarily correlate with that of the protein (Deo et al., 2012). This lack of correlation could be due to differences in stability, trafficking to the membrane, and/or regulation (Koussounadis et al., 2015). For this reason, and because protein expression is likely to be more representative of transporter activity, our study focused on measurement of transporter protein expression rather than mRNA expression. However, it is important to note that our methodology does not distinguish between transporter protein expression in the plasma membrane versus that in the intracellular compartments.
Consistent with the reported data on mRNA expression (Nozaki et al., 2007), protein expression of OAT1 and OAT3 was significantly correlated (r2 = approximately 0.8) (Supplemental Table 3). Taken together, these findings indicate transcriptional coregulation of these transporters. In addition, the expression of other uptake transporters was also correlated, indicating a common mechanism(s) of regulation of these proteins (Supplemental Table 3). To our surprise, SGLT2 protein expression was found to be correlated with the protein expression of most (but not all) transporters (namely, OCTN2, OCTN1, OAT1, OAT2, OCT2, and MRP2). This selective correlation with only some transporters suggests that these correlations were not due to the quality (e.g., contamination from medulla) of the samples. If the latter was the case, SGLT2 protein expression would be correlated with all of the quantified transporters. Transporter protein expression was not correlated with age, sex, or disease state (hypertension or diabetes) in our limited samples. Although sample AB2 with stage 3 kidney disease showed significantly lower expression of the renal transporters, the limited sample demographic information did not allow us to definitively (due to low power) determine the influence of disease conditions on transporter expression. A larger number of kidney samples will need to be analyzed to determine the influence of these covariates on transporter expression. It would be interesting to compare the data presented here with transporter expression in diseased kidneys to predict the effect of kidney failure on the disposition of renally cleared drugs.
The data presented here, together with the affinity of the transporter for a drug, can help quantify the contribution of a transporter in the in vivo renal clearance of a drug. For example, despite the 7.9-fold lower kidney protein expression of OAT2 versus OCT2, based on the higher affinity of OAT2 for creatinine (Km, = 0.8 mM for OAT2 versus 18.8 mM for OCT2) (Lepist et al., 2014; Shen et al., 2015), OAT2 is likely to be the major contributor to the in vivo renal secretion of creatinine. This assumes that the Vmax of the transporter in vivo is proportional to the expression of the transporter in the kidney cortex and the affinity of the drug for the transporter in vitro is equal to that in vivo.
As illustrated by the extended clearance model, inhibition of efflux transporters in the kidney epithelial cells (e.g., MATE1) could result in a significant increase in drug concentration in these cells (and therefore potential toxicity) without a concurrent change in the concentration of the drug in the systemic circulation (Patilea-Vrana and Unadkat, 2016). That is, these drug–drug interactions (DDIs) cannot be detected by the classic DDI studies in which the plasma concentration of a drug is measured in the presence and absence of an inhibitor. These “silent” drug interactions are a significant challenge in drug development. Although imaging techniques such as positron emission tomography can visualize such DDIs, these studies are not routinely possible. Therefore, alternative methods such as physiologically based pharmacokinetic models, based on transporter expression data, are needed to predict such DDIs. Here, for the first time, we provide such data for the renal cortex transporters in a relatively large sample size. These transporter protein expression and correlation data will be useful in deriving in vitro to in vivo scaling factors to predict the renal clearance of drugs or their metabolites and their renal tissue concentrations via physiologically based pharmacokinetic modeling and simulations. In addition, these data can be used to predict which transporter may be the rate-determining step in the in vivo clearance of the drug and hence predict where a DDI may manifest, such as in the systemic circulation, tissue, or both (Patilea-Vrana and Unadkat, 2016).
Acknowledgments
The authors thank Dr. Kimberly Ann Muczynski, Elijah Weber, and Alenka Jaklic for sample procurement and storage. They also acknowledge Marc Vrana for technical help in LC-MS/MS protein quantification.
Abbreviations
- AB
Ardea Biosciences
- BCRP
breast cancer resistance protein
- DDI
drug–drug interaction
- LC
liquid chromatography
- LLOQ
lower limit of quantification
- MATE
multidrug and toxin extrusion
- MRP
multidrug resistance protein
- MS/MS
tandem mass spectrometry
- NU
Newcastle University
- OAT
organic anion transporter
- OCT
organic cation transporter
- OCTN
organic/carnitine cation transporter
- SGLT
sodium-glucose transporter
- UPLC
ultra-performance liquid chromatography
- UW
University of Washington Medical Center
Authorship Contributions
Participated in research design: Prasad, Lee, Kelly, Himmelfarb, Unadkat.
Conducted experiments: Prasad, Johnson, Billington.
Contributed new reagents or analytic tools: Lee, Chung, Brown.
Performed data analysis: Prasad, Johnson, Unadkat.
Wrote or contributed to the writing of the manuscript: Prasad, Johnson, Billington, Lee, Brown, Kelly, Himmelfarb, Unadkat.
Footnotes
This research was supported by the National Institutes of Health National Center for Advancing Translational Sciences [Grant UH2TR000504] and the National Institutes of Health National Institute on Drug Abuse [Grant DA032507].
 This article has supplemental material available at dmd.aspetjournals.org.
This article has supplemental material available at dmd.aspetjournals.org.
References
- Brater DC. (2002) Measurement of renal function during drug development. Br J Clin Pharmacol 54:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CD, Sayer R, Windass AS, Haslam IS, De Broe ME, D’Haese PC, Verhulst A. (2008) Characterisation of human tubular cell monolayers as a model of proximal tubular xenobiotic handling. Toxicol Appl Pharmacol 233:428–438. [DOI] [PubMed] [Google Scholar]
- Deo AK, Prasad B, Balogh L, Lai Y, Unadkat JD. (2012) Interindividual variability in hepatic expression of the multidrug resistance-associated protein 2 (MRP2/ABCC2): quantification by liquid chromatography/tandem mass spectrometry. Drug Metab Dispos 40:852–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto A, Takeda M, Tojo A, Sekine T, Cha SH, Khamdang S, Takayama F, Aoyama I, Nakamura S, Endou H, et al. (2002) Role of organic anion transporters in the tubular transport of indoxyl sulfate and the induction of its nephrotoxicity. J Am Soc Nephrol 13:1711–1720. [DOI] [PubMed] [Google Scholar]
- Fallon JK, Smith PC, Xia CQ, Kim MS. (2016) Quantification of four efflux drug transporters in liver and kidney across species using targeted quantitative proteomics by isotope dilution nanoLC-MS/MS. Pharm Res 33:2280–2288. [DOI] [PubMed] [Google Scholar]
- Feng B, Hurst S, Lu Y, Varma MV, Rotter CJ, El-Kattan A, Lockwood P, Corrigan B. (2013) Quantitative prediction of renal transporter-mediated clinical drug-drug interactions. Mol Pharm 10:4207–4215. [DOI] [PubMed] [Google Scholar]
- Feng B, LaPerle JL, Chang G, Varma MV. (2010) Renal clearance in drug discovery and development: molecular descriptors, drug transporters and disease state. Expert Opin Drug Metab Toxicol 6:939–952. [DOI] [PubMed] [Google Scholar]
- Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, Dahlin A, Evers R, Fischer V, Hillgren KM, et al. International Transporter Consortium (2010) Membrane transporters in drug development. Nat Rev Drug Discov 9:215–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgendorf C, Ahlin G, Seithel A, Artursson P, Ungell AL, Karlsson J. (2007) Expression of thirty-six drug transporter genes in human intestine, liver, kidney, and organotypic cell lines. Drug Metab Dispos 35:1333–1340. [DOI] [PubMed] [Google Scholar]
- Hillgren KM, Keppler D, Zur AA, Giacomini KM, Stieger B, Cass CE, Zhang L, International Transporter Consortium (2013) Emerging transporters of clinical importance: an update from the International Transporter Consortium. Clin Pharmacol Ther 94:52–63. [DOI] [PubMed] [Google Scholar]
- Ito S, Kusuhara H, Yokochi M, Toyoshima J, Inoue K, Yuasa H, Sugiyama Y. (2012) Competitive inhibition of the luminal efflux by multidrug and toxin extrusions, but not basolateral uptake by organic cation transporter 2, is the likely mechanism underlying the pharmacokinetic drug-drug interactions caused by cimetidine in the kidney. J Pharmacol Exp Ther 340:393–403. [DOI] [PubMed] [Google Scholar]
- Iwata K, Aizawa K, Kamitsu S, Jingami S, Fukunaga E, Yoshida M, Yoshimura M, Hamada A, Saito H. (2012) Effects of genetic variants in SLC22A2 organic cation transporter 2 and SLC47A1 multidrug and toxin extrusion 1 transporter on cisplatin-induced adverse events. Clin Exp Nephrol 16:843–851. [DOI] [PubMed] [Google Scholar]
- Kamiie J, Ohtsuki S, Iwase R, Ohmine K, Katsukura Y, Yanai K, Sekine Y, Uchida Y, Ito S, Terasaki T. (2008) Quantitative atlas of membrane transporter proteins: development and application of a highly sensitive simultaneous LC/MS/MS method combined with novel in-silico peptide selection criteria. Pharm Res 25:1469–1483. [DOI] [PubMed] [Google Scholar]
- Kelly EJ, Wang Z, Voellinger JL, Yeung CK, Shen DD, Thummel KE, Zheng Y, Ligresti G, Eaton DL, Muczynski KA, et al. (2013) Innovations in preclinical biology: ex vivo engineering of a human kidney tissue microperfusion system. Stem Cell Res Ther 4 (Suppl 1):S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi R, Kusuhara H, Hattori N, Kim I, Shiota K, Gonzalez FJ, Sugiyama Y. (2007) Regulation of tissue-specific expression of the human and mouse urate transporter 1 gene by hepatocyte nuclear factor 1 alpha/beta and DNA methylation. Mol Pharmacol 72:1619–1625. [DOI] [PubMed] [Google Scholar]
- Koussounadis A, Langdon SP, Um IH, Harrison DJ, Smith VA. (2015) Relationship between differentially expressed mRNA and mRNA-protein correlations in a xenograft model system. Sci Rep 5:10775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V, Prasad B, Patilea G, Gupta A, Salphati L, Evers R, Hop CE, Unadkat JD. (2015) Quantitative transporter proteomics by liquid chromatography with tandem mass spectrometry: addressing methodologic issues of plasma membrane isolation and expression-activity relationship. Drug Metab Dispos 43:284–288. [DOI] [PubMed] [Google Scholar]
- Kusuhara H, Ito S, Kumagai Y, Jiang M, Shiroshita T, Moriyama Y, Inoue K, Yuasa H, Sugiyama Y. (2011) Effects of a MATE protein inhibitor, pyrimethamine, on the renal elimination of metformin at oral microdose and at therapeutic dose in healthy subjects. Clin Pharmacol Ther 89:837–844. [DOI] [PubMed] [Google Scholar]
- Lepist EI, Zhang X, Hao J, Huang J, Kosaka A, Birkus G, Murray BP, Bannister R, Cihlar T, Huang Y, et al. (2014) Contribution of the organic anion transporter OAT2 to the renal active tubular secretion of creatinine and mechanism for serum creatinine elevations caused by cobicistat. Kidney Int 86:350–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig T, Riethmüller C, Gekle M, Schwerdt G, Oberleithner H. (2004) Nephrotoxicity of platinum complexes is related to basolateral organic cation transport. Kidney Int 66:196–202. [DOI] [PubMed] [Google Scholar]
- Mandíková J, Volková M, Pávek P, Navrátilová L, Hyršová L, Janeba Z, Pavlík J, Bárta P, Trejtnar F. (2016) Entecavir interacts with influx transporters hOAT1, hCNT2, hCNT3, but not with hOCT2: the potential for renal transporter-mediated cytotoxicity and drug-drug interactions. Front Pharmacol 6:304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer zu Schwabedissen HE, Verstuyft C, Kroemer HK, Becquemont L, Kim RB. (2010) Human multidrug and toxin extrusion 1 (MATE1/SLC47A1) transporter: functional characterization, interaction with OCT2 (SLC22A2), and single nucleotide polymorphisms. Am J Physiol Renal Physiol 298:F997–F1005. [DOI] [PubMed] [Google Scholar]
- Moss DM, Neary M, Owen A. (2014) The role of drug transporters in the kidney: lessons from tenofovir. Front Pharmacol 5:248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Hirayama-Kurogi M, Ito S, Kuno T, Yoneyama T, Obuchi W, Terasaki T, Ohtsuki S. (2016) Large-scale multiplex absolute protein quantification of drug-metabolizing enzymes and transporters in human intestine, liver, and kidney microsomes by SWATH-MS: comparison with MRM/SRM and HR-MRM/PRM. Proteomics 16:2106–2117. [DOI] [PubMed] [Google Scholar]
- Nozaki Y, Kusuhara H, Kondo T, Hasegawa M, Shiroyanagi Y, Nakazawa H, Okano T, Sugiyama Y. (2007) Characterization of the uptake of organic anion transporter (OAT) 1 and OAT3 substrates by human kidney slices. J Pharmacol Exp Ther 321:362–369. [DOI] [PubMed] [Google Scholar]
- Patilea-Vrana G, Unadkat JD. (2016) Transport vs. metabolism: what determines the pharmacokinetics (PK) and pharmacodynamics (PD) of drugs? Insights from the extended clearance model. Clin Pharmacol Ther DOI: 10.1002/cpt.437[published ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad B, Evers R, Gupta A, Hop CE, Salphati L, Shukla S, Ambudkar SV, Unadkat JD. (2014) Interindividual variability in hepatic organic anion-transporting polypeptides and P-glycoprotein (ABCB1) protein expression: quantification by liquid chromatography tandem mass spectroscopy and influence of genotype, age, and sex. Drug Metab Dispos 42:78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad B, Gaedigk A, Vrana M, Gaedigk R, Leeder JS, Salphati L, Chu X, Xiao G, Hop CE, Evers R, et al. (2016) Ontogeny of hepatic drug transporters as quantified by LC-MS/MS proteomics. Clin Pharmacol Ther 100:362–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad B, Lai Y, Lin Y, Unadkat JD. (2013) Interindividual variability in the hepatic expression of the human breast cancer resistance protein (BCRP/ABCG2): effect of age, sex, and genotype. J Pharm Sci 102:787–793. [DOI] [PubMed] [Google Scholar]
- Prasad B, Unadkat JD. (2014a) Comparison of heavy labeled (SIL) peptide versus SILAC protein internal standards for LC-MS/MS quantification of hepatic drug transporters. Int J Proteomics 2014:451510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad B, Unadkat JD. (2014b) Optimized approaches for quantification of drug transporters in tissues and cells by MRM proteomics. AAPS J 16:634–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razavi M, Frick LE, LaMarr WA, Pope ME, Miller CA, Anderson NL, Pearson TW. (2012) High-throughput SISCAPA quantitation of peptides from human plasma digests by ultrafast, liquid chromatography-free mass spectrometry. J Proteome Res 11:5642–5649. [DOI] [PubMed] [Google Scholar]
- Shen H, Liu T, Morse BL, Zhao Y, Zhang Y, Qiu X, Chen C, Lewin AC, Wang XT, Liu G, et al. (2015) Characterization of organic anion transporter 2 (SLC22A7): a highly efficient transporter for creatinine and species-dependent renal tubular expression. Drug Metab Dispos 43:984–993. [DOI] [PubMed] [Google Scholar]
- Sun W, Wu RR, van Poelje PD, Erion MD. (2001) Isolation of a family of organic anion transporters from human liver and kidney. Biochem Biophys Res Commun 283:417–422. [DOI] [PubMed] [Google Scholar]
- Takeda M, Tojo A, Sekine T, Hosoyamada M, Kanai Y, Endou H. (1999) Role of organic anion transporter 1 (OAT1) in cephaloridine (CER)-induced nephrotoxicity. Kidney Int 56:2128–2136. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Terada T, Ueba M, Sato T, Masuda S, Katsura T, Inui K. (2009) Involvement of human multidrug and toxin extrusion 1 in the drug interaction between cimetidine and metformin in renal epithelial cells. J Pharmacol Exp Ther 329:185–191. [DOI] [PubMed] [Google Scholar]
- Verhulst A, Sayer R, De Broe ME, D’Haese PC, Brown CD. (2008) Human proximal tubular epithelium actively secretes but does not retain rosuvastatin. Mol Pharmacol 74:1084–1091. [DOI] [PubMed] [Google Scholar]
- Verma SP, Díaz-González L, Rosales-Rivera M, Quiroz-Ruiz A. (2014) Comparative performance of four single extreme outlier discordancy tests from Monte Carlo simulations. Scientific World Journal 2014:746451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Prasad B, Salphati L, Chu X, Gupta A, Hop CE, Evers R, Unadkat JD. (2015) Interspecies variability in expression of hepatobiliary transporters across human, dog, monkey, and rat as determined by quantitative proteomics. Drug Metab Dispos 43:367–374. [DOI] [PubMed] [Google Scholar]
- Wang ZJ, Yin OQ, Tomlinson B, Chow MS. (2008) OCT2 polymorphisms and in-vivo renal functional consequence: studies with metformin and cimetidine. Pharmacogenet Genomics 18:637–645. [DOI] [PubMed] [Google Scholar]
- Yokoo S, Yonezawa A, Masuda S, Fukatsu A, Katsura T, Inui K. (2007) Differential contribution of organic cation transporters, OCT2 and MATE1, in platinum agent-induced nephrotoxicity. Biochem Pharmacol 74:477–487. [DOI] [PubMed] [Google Scholar]
- Yonezawa A, Inui K. (2011) Organic cation transporter OCT/SLC22A and H(+)/organic cation antiporter MATE/SLC47A are key molecules for nephrotoxicity of platinum agents. Biochem Pharmacol 81:563–568. [DOI] [PubMed] [Google Scholar]