Abstract
Lifelong weekly infusions of human α1-antitrypsin (hAAT) are currently administered as augmentation therapy for patients with genetic AAT deficiency (AATD). Several recent clinical trials attempt to extend hAAT therapy to conditions outside AATD, including type 1 diabetes. Because the endpoint for AATD is primarily the reduction of risk for pulmonary emphysema, the present study explores hAAT dose protocols and routes of administration in attempt to optimize hAAT therapy for islet-related injury. Islet-grafted mice were treated with hAAT (Glassia; intraperitoneally or subcutaneously) under an array of clinically relevant dosing plans. Serum hAAT and immunocyte cell membrane association were examined, as well as parameters of islet survival. Results indicate that dividing the commonly prescribed 60 mg/kg i.p. dose to three 20 mg/kg injections is superior in affording islet graft survival; in addition, a short dynamic descending dose protocol (240→120→60→60 mg/kg i.p.) is comparable in outcomes to indefinite 60 mg/kg injections. Although pharmacokinetics after intraperitoneal administration in mice resembles exogenous hAAT treatment in humans, subcutaneous administration better imitated the physiologic progressive rise of hAAT during acute phase responses; nonetheless, only the 60 mg/kg dose depicted an advantage using the subcutaneous route. Taken together, this study provides a platform for extrapolating an islet-relevant clinical protocol from animal models that use hAAT to protect islets. In addition, the study places emphasis on outcome-oriented analyses of drug efficacy, particularly important when considering that hAAT is presently at an era of drug-repurposing toward an extended list of clinical indications outside genetic AATD.
Introduction
α1-Antitrypsin (AAT) is an acute phase protein administered as an augmentation therapy for patients with genetic AAT deficiency (AATD). However, preclinical data suggest that AAT treatment may benefit conditions outside AATD, including transplant rejection, as well as ischemia-reperfusion injury, collagen-induced arthritis, graft-versus-host disease, experimental autoimmune encephalomyelitis, lupus, and preeclampsia (Daemen et al., 2000; Lewis et al., 2005; Lewis et al., 2008a; Grimstein et al., 2010; Subramanian et al., 2011; Tawara et al., 2012; Gao et al., 2014; Feng et al., 2015; Elshikha et al., 2016; Feng et al., 2016). AAT displays particular benefit in the case of inflamed pancreatic islets both in vitro and in vivo, as reviewed in Fleixo-Lima et al. (2014).
Several clinical trials presently address the potential benefit of AAT therapy to individuals without AATD, including islet and lung transplantation, type 1 diabetes (T1D), graft-versus-host disease, acute myocardial infarction, and cystic fibrosis (Lior et al., 2016). In the case of T1D clinical trials, the initial dosing plan for assessing islet protection by AAT was directly borrowed from the long-standing protocols of AAT augmentation therapy for AATD patients, e.g., weekly infusions of 60–80 mg/kg plasma-derived affinity-purified human AAT (Balbi et al., 2016). Limited by these constraints, it is a rather unexpected that clinical trials that introduced serial AAT infusions to patients with recent onset T1D presented data suggestive of a possible positive change in disease course. Specifically, in a 12-participant trial (ages 12–39 years old), the duration of treatment consisted of 8 consecutive weekly infusion sessions (Gottlieb et al., 2014), and 80 mg/kg AAT; an 18-month follow up established improved circulating c-peptide levels in 5 of the patients. Consistent with these findings, a 24-participant trial (ages 9–17 years old) tested a total of 18 infusions in a decreasing frequency of infusion sessions: 12 consecutive weekly infusion sessions of AAT, then four infusions 2 weeks apart, followed by two infusions 4 weeks apart (Wewers et al., 1987). The dose of AAT was 40, 60, and 80 mg/kg in three randomized groups; according to the 37-week long follow up, improvement in individual HbA1c levels was established in all participants. Eight patients displayed a 2.94 ± 1.55% decline in HbA1c (compared with a 0.95 ± 1.83% decline in HbA1c in the remainder of the cohort) independent of dose. Both studies displayed remarkable safety and compliance at ≤80 mg/kg. However, not being placebo-controlled, diabetes-related outcomes must be interpreted accordingly.
More recent clinical trials were designed to evaluate the safety and efficacy of several doses of AAT for T1D, including 120 and 180 mg/kg (NCT02005848 and NCT02093221, respectively); in parallel, the 120 mg/kg dose is evaluated as replacement therapy for AATD patients (Sorrells et al., 2015). Although treatment of AATD patients is lifelong, and its success is based solely on decreased prevalence of emphysema (Balbi et al., 2016), the timing and duration of the treatment course required for pancreatic islet preservation has no precedent that may help in extrapolating a relevant treatment protocol. Also, unlike T1D, lung emphysema is not an immune disorder, rendering the course of AAT treatment of both these conditions distinct. Ideally, an islet-protective protocol would be tailored in a mechanism-oriented and function-specific manner, aiming at an endpoint that incorporates islet viability and function.
During a physiologic acute phase response, circulating AAT levels rise 4- to 6-fold for the duration of about 2 weeks, depending on the severity of the underlying inflammatory trigger; the levels gradually decline, coincide with its 3- to 4-day half-life in human plasma. It was reported that hAAT binds to cell surface to execute its activity (Subramaniyam et al., 2010). In contrast, the kinetics of exogenous AAT in humans is comprised of a narrow 24-hour–long spike-like surge that is followed by a sharp decline and subsequent plateau; it has yet to be determined where the molecule is sequestered upon decline under these conditions. Unlike exogenous delivery, continuous release of AAT into the circulation, as achieved by gene delivery of a human AAT-expressing plasmid to mice, despite markedly lower levels of circulating AAT compared with those attained by infusion-based approaches, results in protection of islets (Shahaf et al., 2011). In this regard, it is possible that a distinct distribution of infused AAT might represent an important parameter for diabetes-related trials.
In the present study, we explore several parameters of AAT treatment protocol in an islet allograft transplantation model. In this model, we address the initial days of engraftment (≤14 days) as representing rapid inflammation-related compromised islet survival and function. In contrast, we consider the subsequent advanced weeks to represent the immune response toward the graft. Because this is the first study to directly examine a nonlyophilized preparation of human AAT for islet protection (Glassia, Kamada Ltd., Ness-Ziona, Israel), we also provide an in vitro exploration of its protective effects on primary islets.
Materials and Methods
Animals.
Seven-week-old transgenic hAAT-heterozygote female mice, background strain C57BL/6, a kind gift from Prof. Churg A, University of British Columbia, Vancouver, Canada (Dhami et al., 1999), were used as graft recipients as described (Ashkenazi et al., 2013). Circulating levels of hAAT in these mice are below detection, as determined by specific ELISA for human AAT (sensitivity 10 ng/ml in serum, Immunologic Consultants Laboratory, Inc., Portland, OR). Pancreatic islets and dendritic cells were isolated from 7- to 8-week-old wild-type female CBA/2 mice (Jackson Laboratories, Bar Harbor ME). Experiments were approved by the Ben-Gurion University of the Negev Animal Care and Use Committee.
Pancreatic Islet Culture Experiments.
Pancreatic islets were isolated as described elsewhere (Lewis et al., 2008b). Briefly, donor mice were anesthetized, and pancreata were inflated with collagenase (1 mg/ml, type XI, Sigma-Aldrich, Rehovot, Israel), excised, and incubated for 28 minutes at 37°C. Digested pancreata were vortexed and filtered through a 500-µm sieve and the pellet was washed in Hanks’ balanced salt solution containing 0.5% bovine serum albumin (Sigma-Aldrich). The pellet was resuspended in RPMI 1640 medium supplemented with 10% fetal calf serum, 50 U/ml penicillin, and 50 μg/ml streptomycin, all from Biologic industries, Beit-Haemek, Israel. Islets were collected on a 100-µm cell strainer (BD Biosciences, Bedford, MA) and hand-picked under a stereomicroscope. For in vitro studies, 35 islets per well in triplicates were stimulated with 5 ng/ml recombinant murine interferon (IFN)-γ and 5 ng/ml recombinant murine IL-1β (R&D Systems, Minneapolis, MN) in the absence or presence of 0.5 mg/ml human AAT (Glassia, Kamada Ltd., Ness-Ziona, Israel). Forty-eight hours later, supernatants were collected for analysis by Q-Plex mouse cytokines chemiluminescence-based 8-p ELISA (Quansys Biosciences, Logan, UT). Each cytokine was quantified by densitometry using Quansys Q-View software (Quansys Biosciences). Supernatant nitric oxide levels were evaluated by nitrite measurement using Griess reagent (Promega, Madison, WI). Islet viability was determined by XTT assay, according to manufacturer’s instructions (Sigma-Aldrich).
Islet Allograft Transplantation.
Recipient mice were rendered hyperglycemic by single dose streptozotocin (STZ, 225 mg/kg i.p., Sigma-Aldrich), and 450 islets were grafted under the renal capsule, as described (Lewis et al., 2008b). Briefly, recipient mice were anesthetized and an abdominal-wall incision was made over the left kidney. Isolated islets were then released into the renal subcapsular space through a puncture in the capsule, which was rapidly sealed with 1-mm3 sterile absorbable gelatin sponge (Surgifoam, Ethicon, Somerville, NJ). Blood glucose levels were determined three times a week from tail blood by a standard glucometer (Roche Pharmaceuticals, Hod Hasharon, Israel).
Generation of Bone Marrow-Derived Dendritic Cells.
Dendritic cells were generated from bone marrow progenitors, as described elsewhere (Lewis et al., 2008b). Briefly, bone marrow was prepared from femurs and tibias of donor mice. Cells were seeded at 3 × 103 cells per culture plate, in 10 ml RPMI 1640 medium supplemented with 10% fetal calf serum, 2 mM l-glutamine, 50 U/ml penicillin and 50 μg/ml streptomycin. Medium was added 10 ng/ml recombinant granulocyte macrophage colony stimulating factor (GM-CSF, Prospec, Rehovot, Israel). After 8 days, BMDC entered in vitro assays.
DC Maturation Assays.
Bone marrow-derived dendritic cells (BMDC) were stimulated with IFNγ and IL-1β (5 ng/ml each, Prospec), in the absence or presence of human AAT (0.5 mg/ml). Forty-eight hours later, supernatants were collected for cytokine and nitrite analysis. In the same manner, 24 hours after stimulation, cells were examined by flow cytometry, as described (Lewis et al., 2008b). The following antibodies were used for staining: anti-CD86-FITC, anti-MHC class II-PE and anti-CD11c-APC (all from eBioscience, San Diego, CA).
hAAT Treatment Protocol.
All in vivo hAAT treatments begun 1 day before islet transplantation and were repeated every 3 days, based on previously reported islet transplantation experiments (Lewis et al., 2005; Lewis et al., 2008a; Ashkenazi et al., 2013), unless otherwise specified. The route of administration included either intraperitoneally or subcutaneously, as indicated, and the doses included 15, 20, 30, 60, 120, and 240 mg/kg, as specified in each experimental group.
hAAT Distribution Study.
Serum from nongrafted hAAT-treated mice was collected using a designated microvette (Fisher Scientific, Waltham, MA). Circulating hAAT levels were detected using species-specific ELISA for human AAT (Immunologic Consultants Laboratory, Inc.). Membrane-associated hAAT was determined by flow cytometry of thioglycolate-elicited peritoneal cell lavages using anti-hAAT-FITC (Bethyl Laboratories, Inc., Montgomery, TX) and anti-CD45-PE (eBioscience) antibodies. Peritoneal macrophages were pulsed with Glassia for indicated time points and lyzed, and hAAT content was depicted by Western blot analysis using goat anti-human AAT (Bethyl Laboratories, Inc.) and mouse anti-β-actin (MP Biomedicals, Santa Ana, CA) antibodies.
Histology.
Islet graft-baring kidneys were removed 7 days after graft failure or >90 days after normoglycemia and preserved in 10% formalin (Sigma-Aldrich) for 24 hours and then transferred into 70% ethanol for another 3 days. Samples were embedded in paraffin and then cut into 5-µm sections. Histologic sections were stained by hematoxylin and eosin (H&E, Dako, Carpinteria, CA).
Statistics.
Two-way ANOVA or Student’s t test was used to assess differences between groups. P < 0.05 was considered statistically significant. Results are presented as mean ± S.E.M.
Results
Glassia Improves Primary Islet Function, Decreases the Degree of Inflammation, and Reduces Dendritic Cell Maturation.
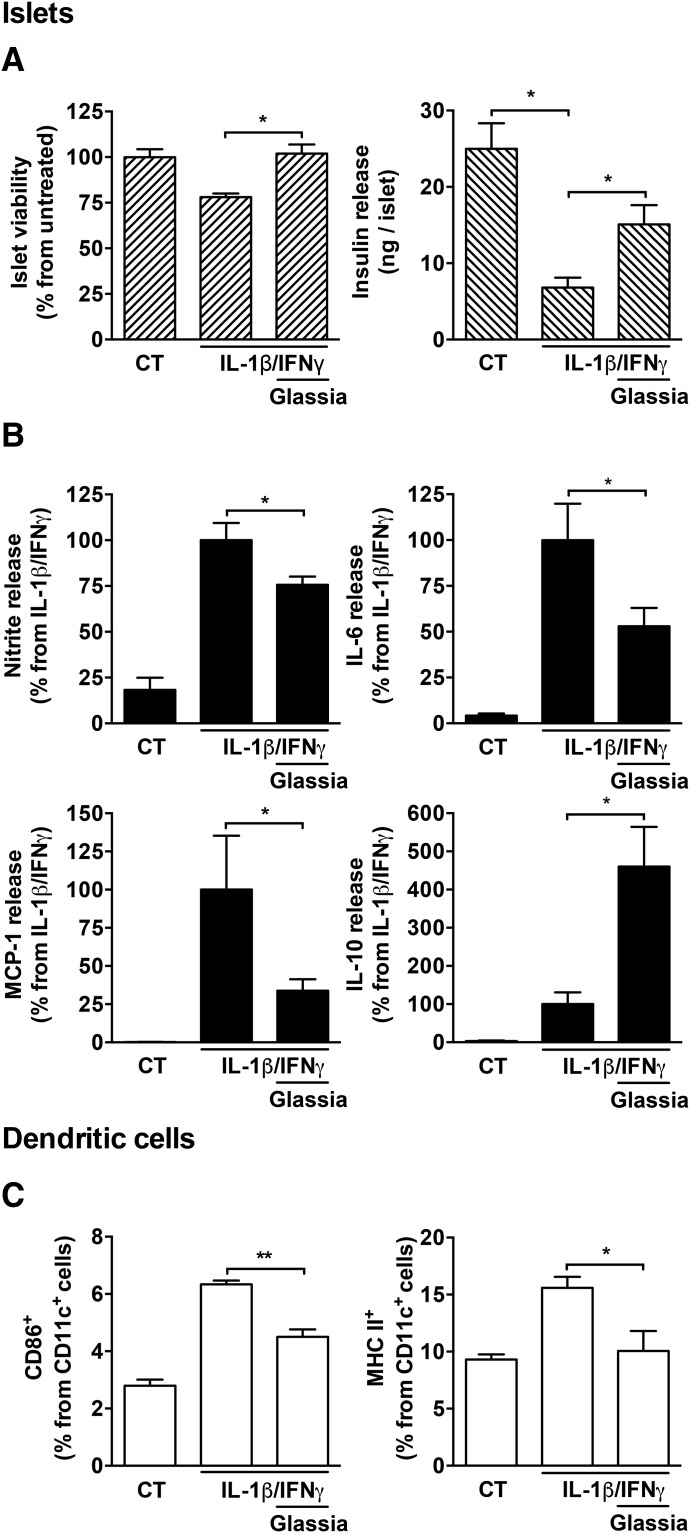
To assess the function of inflamed islets in the presence of Glassia, primary mouse islets were stimulated with interleukin (IL)-1β and interferon γ (IFNγ; 5 ng/ml each) in the absence or presence of Glassia (0.5 mg/ml). As shown in Fig. 1A, 48 hours after stimulation islet viability was reduced, as expected, in the presence of IL-1β/IFNγ (78 ± 0.01% viability compared with non-stimulated islets, albeit without reaching statistical significance). However, in the presence of Glassia, islet viability was significantly improved and restored to near control levels. Accordingly, levels of insulin per islet released into the supernatants were significantly diminished by IL-1β/IFNγ-stimulation (a reduction of 3.67 ± 0.05-fold from nonstimulated islets) and significantly increased in stimulated islets 2.21 ± 0.36-fold in the presence of Glassia.
Fig. 1.
The effect of Glassia on pancreatic islet responses and dendritic cell maturation during inflammatory conditions. (A and B) Primary mouse islets (35 per well in triplicate) were cultured for 48 hours in the absence (CT) or presence of IL-1β and IFNγ (5 ng/ml each), with or without overnight pretreatment with Glassia (0.5 mg/ml). (A) Islet viability and insulin release. (B) Supernatant levels of nitric oxide, IL-6, MCP-1, and IL-10. (C) BMDCs (3 × 105 cells per well in triplicates) were stimulated with IL-1β and IFNγ (5 ng/ml each) overnight in the presence or absence of Glassia (0.5 mg/ml). Cells were analyzed by flow cytometry. Representative results of three independent experiments. Mean ± S.E.M., *P < 0.05, **P < 0.01.
We next examined whether the changes in the levels of inducible inflammatory mediators that are released by islet cells, namely, nitrite oxide, IL-6, and MCP-1, and of the anti-inflammatory mediator IL-10, are consistent with changes observed in previous reports. Indeed, as shown in Fig. 1B, nitric oxide production levels were increased by IL-1β/IFNγ 5.48 ± 0.51-fold, unless Glassia was added, which resulted in a significant 32.3% decline in nitric oxide levels on average. Treatment with Glassia also reduced MCP-1 levels (33.8 ± 0.07% from stimulated levels) and IL-6 levels (52.9 ± 0.10% from stimulated levels). Although IL-10 levels increased in the presence of IL-1β/IFNγ, its levels further increased 4.6-fold in the presence of added Glassia.
To assess whether Glassia alters dendritic cell maturation as reported using other preparations of hAAT, cultured bone marrow-derived dendritic cells were treated with IL-1β (5 ng/ml) and IFNγ (5 ng/ml) in the absence or presence of Glassia (0.5 mg/ml) and were then examined for surface activation markers by flow cytometry. As shown in Fig. 1C, stimulated dendritic cells exhibited a marked rise in maturation markers CD86 and MHC class II; however, Glassia treatment resulted in diminished surface CD86 expression (51.4% from stimulated levels, mean), and surface MHC class II reached 13.1±0.11%, nearing control nonstimulated levels.
Glassia Treatment and Mouse Islet Allograft Survival.
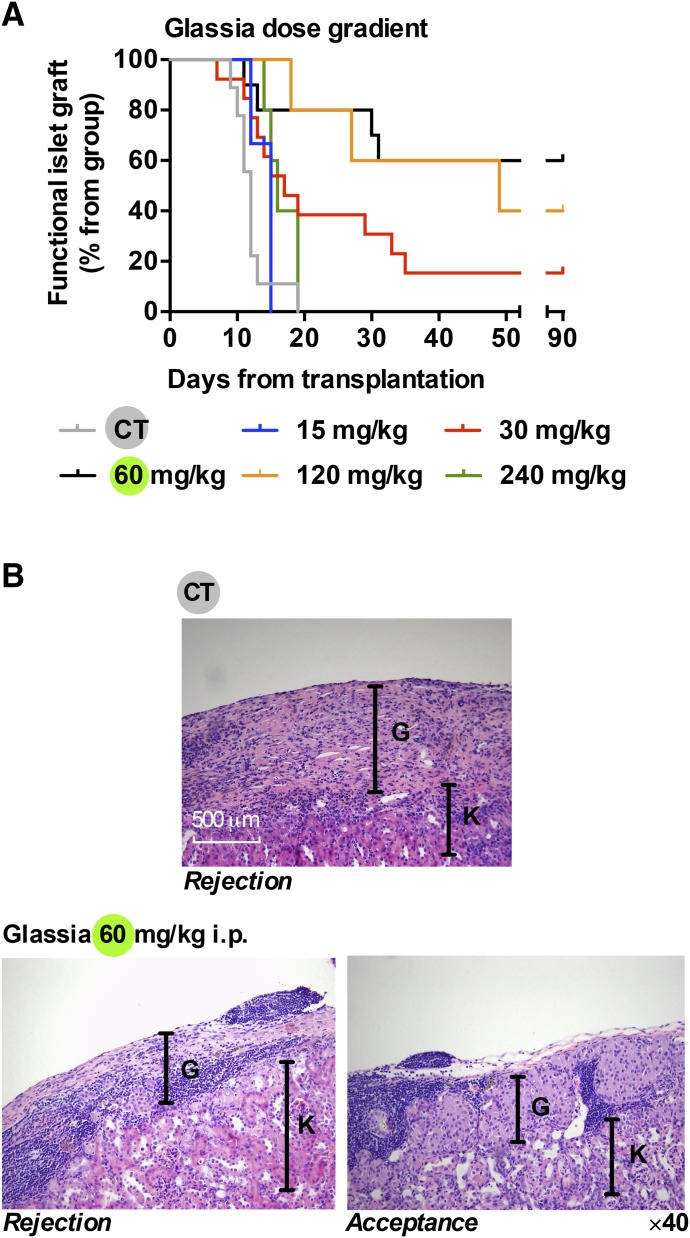
Islet graft survival was examined under the treatment of 60 mg/kg Glassia based on previous protocols (Fig. 2A). To avoid mouse anti-human antibody response, mice that are heterozygous for lung-specific human AAT and that display undetectable circulating levels of human AAT were used as graft recipients. Islet graft survival was defined as the duration of normoglycemia after transplantation, and rejection day was defined as the day circulating glucose levels exceeded 300 mg/dl. Indefinite islet graft survival was defined as normoglycemia in grafted animals that lasted >90 days.
Fig. 2.
Protection of pancreatic islet allografts by Glassia. Mice were rendered hyperglycemic by single-dose STZ (225 mg/kg) and then subjected to islet allograft transplantation. Experimental groups included: 15 mg/kg (n = 3), 30 mg/kg (n = 13), 60 mg/kg (n = 10), 120 mg/kg (n = 5), and 240 mg/kg (n = 5). Control group animals received PBS vehicle (n = 9). (A) Graft survival curve. (B) Graft site histology, K signifies kidney tissue and G signifies graft site. Representative images of rejected untreated mouse graft (top), 60 mg/kg-treated rejected graft (bottom left), and accepted graft (bottom right).
Here, doses were extended above and below 60 mg/kg with adherence to a uniform time course (15, 30, 60, 120, and 240 mg/kg, every 3 days beginning 1 day before transplantation). As shown, islet grafts in all untreated recipients (CT; Fig. 2A) failed to normalize blood glucose levels before day 20 (n = 9). Coinciding with previous reports, administration of 60 mg/kg hAAT resulted in prolonged graft survival (indefinite graft acceptance in 6 of 10 mice). As expected, the extremely low dose of 15 mg/kg exhibited no protective effect on islet allografts, all transplants failed by day 15 (n = 3). The dose of 30 mg/kg resulted in a somewhat limited protection, achieving 2 of 13 mice with indefinite graft acceptance. As expected, twice the standard dose (120 mg/kg) achieved islet graft survival rates that were comparable to the 60 mg/kg group (2 of 5 grafts displayed indefinite graft function). Unexpectedly, the 240 mg/kg dose had no beneficial effect in the long term, because all grafts failed by day 20 (n = 5); nonetheless, the median time point of graft rejection was delayed to 16 days compared with the CT group median time to graft failure (12 days), and the earliest failing graft was on day 14 compared with day 9 in CT, indicating superior early graft function (Table 1).
TABLE 1.
Islet graft function parameters after hAAT treatment
| Treatment Type | Treatment Group (mg/kg) | n | Median Graft Survival (days) | Average Graft Survival (days) | Indefinite Graft Survival (% from group) | First Occurrence of Graft Failure (day from transplantation) |
|---|---|---|---|---|---|---|
| i.p. dose gradient | Untreated | 9 | 12 | 12.1 | 0 | 9 |
| 15 | 3 | 14 | 13.6 | 0 | 12 | |
| 30 | 13 | 17 | 29.6 | 15.3 | 7 | |
| 60 | 10 | 90 | 62.5 | 60 | 11 | |
| 120 | 5 | 48 | 54.6 | 40 | 18 | |
| 240 | 5 | 16 | 16.6 | 0 | 14 | |
| i.p. dose distribution | 30/30 | 6 | 15 | 18.3 | 0 | 7 |
| 20/20/20 | 3 | 90 | 65.3 | 66.6 | 16 | |
| s.c. | 60 | 6 | 90 | 77.1 | 83.3 | 13 |
| 30/30 | 5 | 20 | 31.4 | 20 | 11 | |
| 30 | 2 | 15 | 15 | 0 | 14 | |
| 15 | 1 | 17 | 17 | 0 | 17 | |
| dynamic dose | 240→0 i.p. | 6 | 40 | 48.3 | 33.3 | 14 |
| 240→0 s.c. | 3 | 17 | 40.3 | 33.3 | 14 |
Graft histology is depicted in Fig. 2B. As shown, rejected grafts in the CT group displayed subcapsular islet graft regions (G) and kidney parenchyma (K) with the prominent presence of a mononuclear infiltrate (representative image, 2 days after graft failure). Upon examining hAAT-treated mice, two patterns emerged: those that were explanted upon graft rejection and those that were accepted. As expected, hAAT-treated graft-rejected mice displayed robust cellular infiltrate throughout the graft site (representative image from the 60 mg/kg group 2 days after graft failure). However, indefinitely accepted grafts exhibited intact islet morphology with no evidence for cellular infiltrate within islet borders but rather a prominent noninvasive concentration of mononuclear cells at junctions of capsule-graft-kidney parenchyma (shown in representative day 110 explant), agreeing with the distinctive profile of active long-term immune tolerance.
Exploration of Transplantation Outcomes using a Fixed Dose of Glassia with Altered Frequency of Rationed Injections.
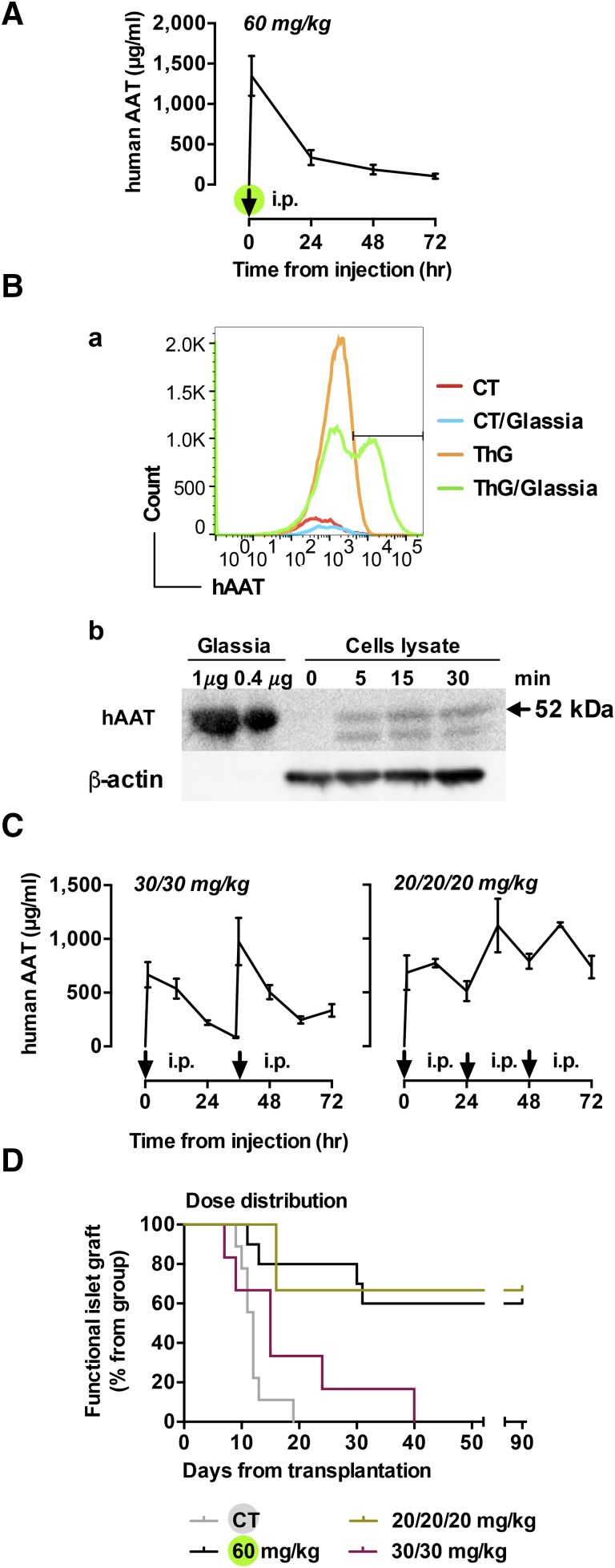
In light of the outcomes in the dose-screening experiment, we sought to examine the impact of hAAT treatment using distinct layouts of dose rations. Namely, we sought to better emulate the behavior of circulating hAAT during an acute phase response, i.e., stably elevated levels and not fluctuating levels obtained during exogenous hAAT treatment. In addition, the pharmacokinetics of oddly distributed injections of hAAT in mice has yet to be determined. Here, mice received either single dose of 60 mg/kg i.p or the same total amount of hAAT distributed as two or three separate rations. Tail blood was collected and serum hAAT levels were determined. As shown in Fig. 3A, the obtained mouse hAAT serum pharmacokinetics were similar to that reported in humans in that soon after administration circulating hAAT levels peaked (1,348.38 ± 247.11 μg/ml), followed by dramatic reduction over the following 72 hours, reaching 103.98 ± 31.67 μg/ml.
Fig. 3.
The effect of intraperitoneal Glassia dose distribution treatment on hAAT serum concentration and islet graft survival. (A) Mice (n = 3–5) were treated with 60 mg/kg i.p Glassia as indicated (arrow). Serum levels of human AAT were determined with specific ELISA. Results of 3 independent experiments, mean ± S.E.M. (B) Mice were stimulated with ThG for 72 hours after treatment with Glassia (240 mg/kg i.p.). Peritoneal lavage was performed 16 hours later, and membrane-associated hAAT levels were measured by flow cytometry (top panel). Groups included nonstimulated, untreated mice (CT, red); ThG-stimulated untreated mice (ThG, yellow); nonstimulated Glassia-treated mice (CT/Glassia, blue), and ThG-stimulated Glassia-treated mice (ThG/Glassia, green), n = 3. Results are representative of 3 independent repeats. For cell-associated analysis (bottom panel), peritoneal macrophages (1×106, in triplicate) were pulsed with Glassia (0.5 mg/ml). hAAT binding was evaluated by Western blotting. (C) Mice were treated with 30 mg/kg (left) or 20 mg/kg (right) Glassia intraperitoneally at indicated time points (arrow; n = 3 or 4 in each group). Serum levels of human AAT were determined. Results of 3 independent experiments, mean ± S.E.M. (D) Islet graft survival curve. Groups include: ×3 of 20 mg/kg i.p (n = 3, yellow) and ×2 of 30 mg/kg i.p (n = 6, red). CT and 60 mg/kg treatment groups are duplicates of Fig. 2A.
Being that the sharp decline in circulating levels of exogenous hAAT upon each dose administration does not represent the physiologic pattern of circulating hAAT during acute phase responses, we sought to examine the possibility that injected hAAT is readily sequestered onto cell membranes. Here, C57BL/6 wild-type mice were treated with single-dose Glassia (240 mg/kg i.p). In half the groups, the peritoneal compartment was preconditioned using thioglycolate for 3 days so as to elicit activated immunocytes with more expressive membrane profiles. Sixteen hours later, lavaged cells were examined for membrane-associated hAAT levels by flow cytometry. As shown in Fig. 1B, top, nonstimulated untreated mice (CT), as well as thioglycolate-preconditioned mice (ThG) displayed no detectible surface levels of hAAT. In contrast, thioglycolate-stimulated Glassia-treated mice (ThG/AAT) displayed detectible membrane-associated hAAT levels. Surprisingly, cells derived from nonstimulated Glassia-treated mice (AAT) did not show any detectible levels of hAAT.
The presence of cell-associated hAAT was also examined in cell lysates. For this, 1×106 peritoneal mouse macrophages were pulsed with 0.5 mg/ml Glassia in vitro and then washed with PBS after various time points. Upon lysis, levels of hAAT were evaluated by Western blot analysis. As shown in Fig. 1B, bottom, hAAT can be recovered from cell lysates as early as 5 minutes after treatment.
Different dose distribution protocols were compared: ×1 of 60 mg/kg, ×2 of 30 mg/kg, and ×3 of 20 mg/kg within 72 hours. As shown in Fig. 3C, the 30 mg/kg dose displayed the predicted circulating levels of 667.05 ± 117.95 μg/ml and a kinetic that overlaps the full dose, and the additional dose of 30 mg/kg increased circulating hAAT levels to 976.1 ± 221.03 μg/ml and a value of 69.32 μg/ml hAAT at the lowest measurement. However, administration of Glassia at 20 mg/kg every 24 hours over a period of 3 days resulted in a relatively stable range of concentrations, all above 226.65 μg/ml hAAT.
The ability of these dose distribution protocols to prolong islet graft survival was next examined. As shown in Fig. 3D, administration of Glassia at 30 mg/kg every 36 hours had a minor effect on graft survival, and all mice rejected the grafts by day 40 (n = 6). Surprisingly, when mice were treated with daily 20 mg/kg doses, 66% displayed indefinite graft survival, comparable to the 60 mg/kg group (n = 3). Table 1 depicts the influence of dose distribution on islet allograft survival.
Subcutaneous Administration of Glassia.
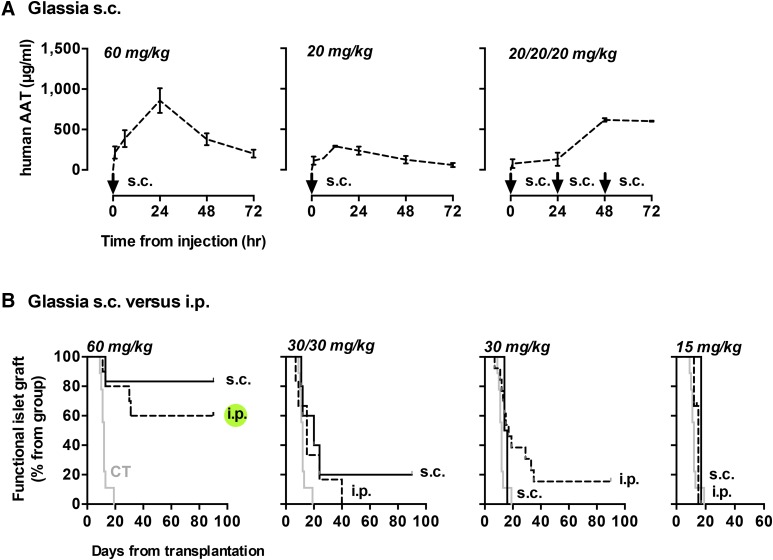
Because the somewhat uniform distribution of hAAT appears to have provided a positive trend in as far as islet protection, using a lower dose per injection, we explored subcutaneous administration of hAAT as a clinically-favorable route that results in a relatively slow release of injected material. As shown in Fig. 4A, circulating hAAT was distributed along the 72-hour experiment in a distinct manner based on the frequency of injected material. The standard dose of 60 mg/kg displayed a delayed peak followed by a gradual decrease. The 20 mg/kg dose was tested alone, and in a serial manner that reaches the same total of 60 mg/kg per 72 hours; as shown, single dose 20 mg/kg conforms well to the pattern obtained with 60 mg/kg s.c.. However, daily administration of 20 mg/kg over 3 days resulted in a well-anticipated build-up of circulating hAAT followed by stabilization at 601.27 ± 6.232 μg/ml.
Fig. 4.
The effect of subcutaneous Glassia treatment on hAAT serum concentration and islet graft survival. (A) Mice were treated with 60 and 20 mg/kg Glassia subcutaneously at indicated time points (arrow; n = 3 or 4 in each group). Serum hAAT levels were measured. Results are representative of three independent experiments, mean ± S.E.M. (B) Islet grafted mice were treated with Glassia either intraperitoneally (dashed) or subcutaneously (solid). Groups include untreated mice (CT; gray. n = 9); ×1 of 60 mg/kg i.p (n = 10); ×1 of 60 mg/kg s.c (n = 6); ×2 of 30 mg/kg i.p. (n = 6); ×2 of 30 mg/kg s.c. (n = 5); ×1 of 30 mg/kg i.p (n = 13); ×1 of 30 mg/kg s.c. (n = 2); ×1 of 15 mg/kg i.p (n = 3); ×1 of 15 mg/kg s.c. (n = 1). Islet graft survival curve.
The effect of switching to the subcutaneous route of administration on islet graft survival was next examined (Fig. 4B). Compared with the established outcomes of intraperitoneal administration of Glassia, graft survival profile was superior in the 60 mg/kg s.c. group alone and to a lesser extent in the ×2 of 30 mg/kg group. The rest of the subcutaneous dosing protocols resulted in grafts failing by day 40 with no apparent advantage over intraperitoneal injections of equivalent doses, as summarized in Table 1.
Dynamic versus Static Dose Protocol.
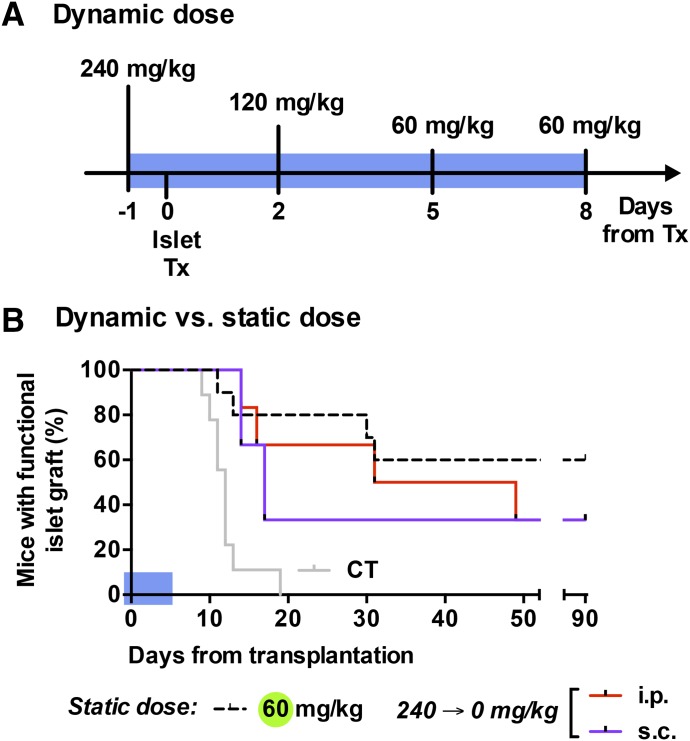
Based on an apparent benefit to high dose Glassia (240 mg/kg) on early graft function and the benefit of lower doses thereafter (×3 of 20 mg/kg), we were interested in examining whether their incorporation into a short dynamic-dose protocol may prolong graft survival in a manner superior to the flat 60 mg/kg dose plan. Figure 5A displays the treatment plan, which runs through days −1 to 7 from islet grafting, in descending doses. The rationale for cutting the treatment short was to facilitate the isolation of any potential advantage of the examined course of treatment over the standard prolonged 60 mg/kg plan. In addition, the subcutaneous route was compared with the intraperitoneal route.
Fig. 5.
The effect of Glassia dynamic dose treatment on graft survival. Islet-grafted mice were treated with Glassia using dynamic dose regimen. (A) Illustration representing the treatment protocol. (B) Islet graft survival curve under Glassia dynamic dose treatment (blue area). Comparison of intraperitoneal (red; n = 6) and subcutaneous (purple; n= 3) dynamic dose treatment.
As shown in Fig. 5B, both routes of administration of the descending dynamic dose resulted in outcomes that were comparable to the 60 mg/kg dose in the first 3 weeks of treatment, at which point, unexpectedly, a week of dynamic 240–60 mg/kg i.p. Glassia remained beneficial to islet function almost as favorably as the 60 mg/kg dose (days 14, 16, 31, 49, >90, and >90). In contrast, subcutaneous administration of descending hAAT doses displayed a trend toward grafts failing before the 3-week time point (days 14, 17, and >90). A similar survival trend was observed in the subcutaneous group, 33% of mice displayed graft survival beyond day 90. Table 1 lists the parameters of comparison between treatment groups.
Discussion
Human α1-antitrypsin has been extensively investigated in preclinical models of autoimmune diabetes and islet allograft transplantation. Administered in the early stages of these pathologies, hAAT appears to shift the profile of the immune system toward tolerance (Lewis et al., 2008a; Koulmanda et al., 2014). These studies provided the rationale for the use of hAAT infusions for patients with T1D (Gottlieb et al., 2014; Rachmiel et al., 2016) and for clinical islet transplantation, as reviewed (Fleixo-Lima et al., 2014).
The dosing for these studies is directly extracted from lifelong hAAT augmentation protocols, designed for patients with genetic AATD. However, these doses are not based on preclinical data, let alone on the specific purpose of protecting islet cells from injury. Specific attention to functional endpoints, e.g., islet cell viability, has yet to be incorporated. Here, we examined the effect of hAAT (Glassia) on mouse islet function and survival both in vitro and in vivo at a wide range of clinically relevant doses and routes of administration. As expected, treatment with Glassia reproduced the previously reported beneficial outcomes using other clinical-grade formulations (e.g., Aralast, Baxter Deerfield, IL; Pileggi et al., 2008; Koulmanda et al., 2012; Koulmanda et al., 2014); islet viability and function had increased, their inflammatory profile decreased, and graft survival was prolonged.
The rationale for exploring not only the dose but also the distribution of doses relates to the dynamic of islet injury. After islet transplantation, grafts endure nonspecific inflammation and subsequently only are destroyed by antigen-specific events; the early phase is as crucial to islet function as the latter. By following the function of grafted islets, we identify both phases of islet injury. As expected, not all doses of hAAT effectively targeted both waves of islet destruction; lower doses were inefficient altogether and the 120 mg/kg dose provided no particular benefit over 60–80 mg /kg dose. The high dose of 240 mg/kg, confirmed to be safe in animals (Ashkenazi et al., 2013; Iskender et al., 2016) and patients (Stolk et al., 2005), displayed an advantage in protecting islets in the first 14 days from grafting; it failed, however, to provide long-lasting benefit, possibly by facilitating concentration-dependent allogeneic mouse anti-human antibodies (Lewis et al., 2005; Lu et al., 2006). Of note, several treatment groups have a low n, including groups treated with extreme subtherapeutic doses of hAAT previously shown to fail in protecting allografts (Shahaf et al., 2011). Thus these particular group outcomes satisfy dose dependency curves rather than novelty.
Based on these observations, we examined the outcomes of a dynamic dose treatment. Exploiting both the favorable effects of an initial high dose of hAAT and the advantage of lower maintenance doses thereafter, a relatively short descending treatment protocol was tested (240→120→60→60). This protocol offered prolonged islet graft survival similar to repeated indefinite injections of 60 mg/kg hAAT. The fact that a long-standing immune tolerance has thus far required at least 21 days of repeated 60 mg/kg hAAT in mice (Lewis et al., 2008a) and is achieved hereby in a short dynamic protocol, sheds light on the importance of peritransplantation protection of islets compared with the finer immunomodulation by hAAT that occurs thereafter and may require low doses.
Unlike during acute phase responses, where hAAT levels rise progressively, patient infusions result in nonphysiologic spikes in circulating hAAT. To better imitate the physiologic pattern, we examined variations in dose distribution. As we demonstrate here, dividing 60 mg/kg dose of hAAT to 30/30 and to 20/20/20 mg/kg injections within the same original timeframe resulted in an interesting phenomenon: when it comes to protection of islet grafts from failure, i.e., the desired outcome/activity of hAAT treatment in the present constellation, the presence of mildly elevated stable circulating hAAT levels (20/20/20 i.p.) was superior to the 30/30 i.p. dose distribution; the latter displayed transient periods of low circulating hAAT levels between injections. Nonetheless, it is established that patient compliance is already inferior in weekly infusion-drip sessions compared with other forms of administration, let alone to more frequent infusions; excluding recent positive hAAT gene therapy attempts (Flotte et al., 2011), there is presently no protocol for systemic administration of hAAT in any other manner outside weekly infusions.
In as far as achieving the desired outcome for augmentation therapy, hAAT infusion appeared thus far to be sufficient, i.e., it reduced the risk for pulmonary emphysema (Balbi et al., 2016). Here, the desired endpoint is distinct and might not be the mere amount of circulating hAAT; rather, we propose that it is its levels between infusions. Thus we explored subcutaneous hAAT; by this we also achieve a slower deposition of hAAT in the serum. Interestingly, subcutaneous is preferable in a list of drugs that used to be administered intravenously/intramuscularly, including heparin and hydrocortisone (Jin et al., 2015). However here, only the 60 mg/kg group displayed prolong graft survival. In the parallel intraperitoneal route of administration, only the 60 mg/kg and the 20/20/20 mg/kg groups displayed prolonged graft survival. These groups have also displayed higher levels of hAAT in the serum (∼1 μg/ml), similar only to the 60 mg/kg s.c group. Thus it is possible that other groups failed to reach therapeutic levels early after transplantation. Considering this dose would translate to an impractical volume of subcutaneous material for human injection (Jin et al., 2015), this outcome suggests that a more concentrated formulation of clinical-grade hAAT, or a slow-release apparatus, may better emulate sustained functional levels of hAAT. The prospect of introducing hAAT subcutaneously has been tested in rabbits (Pamarthi et al., 2008) and mice (Ma et al., 2010) and is evaluated in a phase I/II trial (NCT02503683).
The anti-inflammatory mechanism of hAAT is not entirely clear. Regarding signal transduction, hAAT was reported to elevate cAMP levels in multiple cell types (Janciauskiene et al., 2007; Kalis et al., 2010; Ehlers, 2014) and in this manner is associated with an anti-inflammatory profile (Tilg et al., 1993; Pott et al., 2009). We hypothesize that it is not merely the antiprotease function of hAAT that exerts beneficial effects on islet survival but rather multiple binding activities that were recently described for the molecule, as reviewed in (Guttman et al., 2015). Jonigk et al. (2013) report that hAAT with no antielastase activity reduces responses to lipopolysaccharide. Here we show that exogenous hAAT adheres to immune cells, agreeing with reports of adherence of hAAT to membrane lipid rafts (Subramaniyam et al., 2010; Zhou et al., 2015). Binding to cell surfaces would also provide a mechanism for the rapid sequestration of exogenous hAAT and the superiority of continuously elevated hAAT over interspersed spikes, agreeing with islet preservation using plasmid-derived hAAT in vivo (Lu et al., 2006; Shahaf et al., 2011).
Other binding targets of hAAT include a set of danger-associated molecular pattern molecules (DAMPs), which predominate at sites of cell injury and act as immune adjuvants (Braza et al., 2016), including gp96 and HSP70 (Finotti and Pagetta, 2004; Ochayon et al., 2013). Based on this attribute, it is possible that the narrow surge in hAAT levels would be inferior to its constant supply, in a manner that accommodates local DAMP sequestration. It also supports the concept of a dynamic dose, in that the expected period of peak DAMPs would be the earlier window of massive cell injury, as opposed to the more delicate manipulation of immunocyte functions (Ozeri et al., 2012). Indeed, some overlap between the pathophysiology of emphysema and T1D may exist: lung alveolar wall degradation is observed in AATD and β cell injury in T1D is associated with inactivated glycated-hAAT (Austin et al., 1987).
How does the present study translate to humans? Administering hAAT to humans once a week is the equivalent of administering hAAT to mice every 3 days (Lewis et al., 2005). Based on this frequency of infusions, 21 days of treatment (6 infusions in mice) were established as sufficient to achieve immune tolerance (Lewis et al., 2008a), hence the 8–18 infusions in the T1D trials (Gottlieb et al., 2014; Rachmiel et al., 2016). Based on the present results, however, it is possible that future trials may explore a shorter course of hAAT, provided it is distributed so as to better overlap a physiologic acute phase response. For example, patients may be introduced the following course of weekly hAAT infusions: 180, 180, 120, 120, 120, 120, 60, and 60 mg/kg (all representing slow-drip infusion sessions in recently diagnosed patients with high titers of auto-antibodies and detectable c-peptide levels). This shorter protocol may be both more potent in immediate islet protection and superior in as far as patient compliance and procedure cost.
Taken together, we provide a platform for extrapolation of a clinical protocol from animals in which hAAT appears to protect islets from injury. The study places emphasis on function- and outcome-oriented analyses of treatment, a particularly important entity when considering that hAAT is entering an era of drug-repurposing toward an extended list of clinical indications (Lewis, 2012; Lior et al., 2016). More studies are to be undertaken to optimize treatment protocols relevant to diseases other than AATD.
Abbreviations
- AAT
α1-antitrypsin
- AATD
AAT deficiency
- BMDC
bone marrow-derived dendritic cell
- CT
control
- DAMP
danger-associated molecular pattern molecule
- IFN
interferon
- IL
interleukin
- STZ
streptozotocin
- ThG
thioglycolate
- T1D
Type 1 diabetes
Authorship Contributions
Participated in research design: Baranovski, Ozeri, Strauss, Schenker, and Lewis.
Conducted experiments: Baranovski, Ozeri, Shahaf, Ochayon, Schuster, Bahar, Kalay, Cal, and Mizrahi.
Contributed new reagents or analytic tools: Strauss and Schenker.
Performed data analysis: Baranovski, Ozeri, and Lewis.
Wrote or contributed to the writing of the manuscript: Baranovski, Ozeri, Nisim, Strauss, Schenker, and Lewis.
Footnotes
This study was supported by the Juvenile Diabetes Research Foundation (JDRF) and Israel Science Foundation (ISF)–JDRF Joint Program in Type 1 Diabetes Research.
References
- Ashkenazi E, Baranovski BM, Shahaf G, Lewis EC. (2013) Pancreatic islet xenograft survival in mice is extended by a combination of alpha-1-antitrypsin and single-dose anti-CD4/CD8 therapy. PLoS One 8:e63625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin GE, Mullins RH, Morin LG. (1987) Non-enzymic glycation of individual plasma proteins in normoglycemic and hyperglycemic patients. Clin Chem 33:2220–2224. [PubMed] [Google Scholar]
- Balbi B, Ferrarotti I, Miravitlles M. (2016) Efficacy of augmentation therapy for emphysema associated with α1-antitrypsin deficiency: enough is enough. Eur Respir J 47:35–38. [DOI] [PubMed] [Google Scholar]
- Braza F, Brouard S, Chadban S, Goldstein DR. (2016) Role of TLRs and DAMPs in allograft inflammation and transplant outcomes. Nat Rev Nephrol 12:281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daemen MA, Heemskerk VH, van’t Veer C, Denecker G, Wolfs TG, Vandenabeele P, Buurman WA. (2000) Functional protection by acute phase proteins alpha(1)-acid glycoprotein and alpha(1)-antitrypsin against ischemia/reperfusion injury by preventing apoptosis and inflammation. Circulation 102:1420–1426. [DOI] [PubMed] [Google Scholar]
- Dhami R, Zay K, Gilks B, Porter S, Wright JL, Churg A. (1999) Pulmonary epithelial expression of human alpha1-antitrypsin in transgenic mice results in delivery of alpha1-antitrypsin protein to the interstitium. J Mol Med (Berl) 77:377–385. [DOI] [PubMed] [Google Scholar]
- Ehlers MR. (2014) Immune-modulating effects of alpha-1 antitrypsin. Biol Chem 395:1187–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elshikha AS, Lu Y, Chen MJ, Akbar M, Zeumer L, Ritter A, Elghamry H, Mahdi MA, Morel L, Song S. (2016) Alpha 1 antitrypsin inhibits dendritic cell activation and attenuates nephritis in a mouse model of lupus. PLoS One 11:e0156583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Hu L, Xu Q, Yuan H, Ba L, He Y, Che H. (2015) Cytoprotective role of alpha-1 antitrypsin in vascular endothelial cell under hypoxia/reoxygenation condition. J Cardiovasc Pharmacol 66:96–107. [DOI] [PubMed] [Google Scholar]
- Feng Y, Xu J, Zhou Q, Wang R, Liu N, Wu Y, Yuan H, Che H. (2016) Alpha-1 antitrypsin prevents the development of preeclampsia through suppression of oxidative stress. Front Physiol 7:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finotti P, Pagetta A. (2004) A heat shock protein70 fusion protein with alpha1-antitrypsin in plasma of type 1 diabetic subjects. Biochem Biophys Res Commun 315:297–305. [DOI] [PubMed] [Google Scholar]
- Fleixo-Lima G, Ventura H, Medini M, Bar L, Strauss P, Lewis EC. (2014) Mechanistic evidence in support of alpha1-antitrypsin as a therapeutic approach for type 1 diabetes. J Diabetes Sci Technol 8:1193–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flotte TR, Trapnell BC, Humphries M, Carey B, Calcedo R, Rouhani F, Campbell-Thompson M, Yachnis AT, Sandhaus RA, McElvaney NG, et al. (2011) Phase 2 clinical trial of a recombinant adeno-associated viral vector expressing α1-antitrypsin: interim results. Hum Gene Ther 22:1239–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Zhao J, Kim H, Xu S, Chen M, Bai X, Toba H, Cho HR, Zhang H, Keshavjeel Set al. (2014) alpha1-Antitrypsin inhibits ischemia reperfusion-induced lung injury by reducing inflammatory response and cell death. J Heart Lung Transplant 33:309–315. [DOI] [PubMed] [Google Scholar]
- Gottlieb PA, Alkanani AK, Michels AW, Lewis EC, Shapiro L, Dinarello CA, Zipris D. (2014) α1-Antitrypsin therapy downregulates toll-like receptor-induced IL-1β responses in monocytes and myeloid dendritic cells and may improve islet function in recently diagnosed patients with type 1 diabetes. J Clin Endocrinol Metab 99:E1418–E1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimstein C, Choi YK, Satoh M, Lu Y, Wang X, Campbell-Thompson M, Song S. (2010) Combination of alpha-1 antitrypsin and doxycycline suppresses collagen-induced arthritis. J Gene Med 12:35–44. [DOI] [PubMed] [Google Scholar]
- Guttman O, Baranovski BM, Schuster R, Kaner Z, Freixo-Lima GS, Bahar N, Kalay N, Mizrahi MI, Brami I, Ochayon DE, et al. (2015) Acute-phase protein α1-anti-trypsin: diverting injurious innate and adaptive immune responses from non-authentic threats. Clin Exp Immunol 179:161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iskender I, Sakamoto J, Nakajima D, Lin H, Chen M, Kim H, Guan Z, Del Sorbo L, Hwang D, Waddell TK, et al. (2016) Human α1-antitrypsin improves early post-transplant lung function: Pre-clinical studies in a pig lung transplant model. J Heart Lung Transplant 35:913–921. [DOI] [PubMed] [Google Scholar]
- Janciauskiene SM, Nita IM, Stevens T. (2007) Alpha1-antitrypsin, old dog, new tricks. Alpha1-antitrypsin exerts in vitro anti-inflammatory activity in human monocytes by elevating cAMP. J Biol Chem 282:8573–8582. [DOI] [PubMed] [Google Scholar]
- Jin JF, Zhu LL, Chen M, Xu HM, Wang HF, Feng XQ, Zhu XP, Zhou Q. (2015) The optimal choice of medication administration route regarding intravenous, intramuscular, and subcutaneous injection. Patient Prefer Adherence 9:923–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonigk D, Al-Omari M, Maegel L, Müller M, Izykowski N, Hong J, Hong K, Kim SH, Dorsch M, Mahadeva R, et al. (2013) Anti-inflammatory and immunomodulatory properties of α1-antitrypsin without inhibition of elastase. Proc Natl Acad Sci USA 110:15007–15012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalis M, Kumar R, Janciauskiene S, Salehi A, Cilio CM. (2010) α 1-antitrypsin enhances insulin secretion and prevents cytokine-mediated apoptosis in pancreatic β-cells. Islets 2:185–189. [DOI] [PubMed] [Google Scholar]
- Koulmanda M, Bhasin M, Fan Z, Hanidziar D, Goel N, Putheti P, Movahedi B, Libermann TA, Strom TB. (2012) Alpha 1-antitrypsin reduces inflammation and enhances mouse pancreatic islet transplant survival. Proc Natl Acad Sci USA 109:15443–15448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koulmanda M, Sampathkumar RS, Bhasin M, Qipo A, Fan Z, Singh G, Movahedi B, Duggan M, Chipashvili V, Strom TB. (2014) Prevention of nonimmunologic loss of transplanted islets in monkeys. Am J Transplant 14:1543–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis EC. (2012) Expanding the clinical indications for α(1)-antitrypsin therapy. Mol Med 18:957–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis EC, Mizrahi M, Toledano M, Defelice N, Wright JL, Churg A, Shapiro L, Dinarello CA. (2008a) alpha1-Antitrypsin monotherapy induces immune tolerance during islet allograft transplantation in mice. Proc Natl Acad Sci USA 105:16236–16241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis EC, Mizrahi M, Toledano M, Defelice N, Wright JL, Churg A, Shapiro L, Dinarello CA. (2008b) alpha1-Antitrypsin monotherapy induces immune tolerance during islet allograft transplantation in mice. Proc Natl Acad Sci USA 105:16236–16241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis EC, Shapiro L, Bowers OJ, Dinarello CA. (2005) Alpha1-antitrypsin monotherapy prolongs islet allograft survival in mice. Proc Natl Acad Sci USA 102:12153–12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lior Y, Geyra A, Lewis EC. (2016) Therapeutic compositions and uses of alpha1-antitrypsin: a patent review (2012 - 2015). Expert Opin Ther Pat 26:581–589. [DOI] [PubMed] [Google Scholar]
- Lu Y, Tang M, Wasserfall C, Kou Z, Campbell-Thompson M, Gardemann T, Crawford J, Atkinson M, Song S. (2006) Alpha1-antitrypsin gene therapy modulates cellular immunity and efficiently prevents type 1 diabetes in nonobese diabetic mice. Hum Gene Ther 17:625–634. [DOI] [PubMed] [Google Scholar]
- Ma H, Lu Y, Li H, Campbell-Thompson M, Parker M, Wasserfall C, Haller M, Brantly M, Schatz D, Atkinson M, et al. (2010) Intradermal alpha1-antitrypsin therapy avoids fatal anaphylaxis, prevents type 1 diabetes and reverses hyperglycaemia in the NOD mouse model of the disease. Diabetologia 53:2198–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochayon DE, Mizrahi M, Shahaf G, Baranovski BM, Lewis EC. (2013) Human α1-Antitrypsin Binds to Heat-Shock Protein gp96 and Protects from Endogenous gp96-Mediated Injury In vivo. Front Immunol 4:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozeri E, Mizrahi M, Shahaf G, Lewis EC. (2012) α-1 antitrypsin promotes semimature, IL-10-producing and readily migrating tolerogenic dendritic cells. J Immunol 189:146–153. [DOI] [PubMed] [Google Scholar]
- Pamarthi MF, Taylor GM, Wilson J, Scuderi P, Arora V. (2008) Pharmacokinetics of Subcutaneously Administered Alpha-1 Antitrypsin. J Allergy Clin Immunol 123:S163. [Google Scholar]
- Pileggi A, Molano RD, Song S, Zahr E, SanJose S, Villate S, Wasserfall C, Ricordi C, Atkinson MA, Inverardi L. (2008) Alpha-1 antitrypsin treatment of spontaneously diabetic nonobese diabetic mice receiving islet allografts. Transplant Proc 40:457–458. [DOI] [PubMed] [Google Scholar]
- Pott GB, Chan ED, Dinarello CA, Shapiro L. (2009) Alpha-1-antitrypsin is an endogenous inhibitor of proinflammatory cytokine production in whole blood. J Leukoc Biol 85:886–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachmiel M, Strauss P, Dror N, Benzaquen H, Horesh O, Tov N, Weintrob N, Landau Z, Ben-Ami M, Haim A, et al. (2016) Alpha-1 antitrypsin therapy is safe and well tolerated in children and adolescents with recent onset type 1 diabetes mellitus. Pediatr Diabetes 17:351–359. [DOI] [PubMed] [Google Scholar]
- Shahaf G, Moser H, Ozeri E, Mizrahi M, Abecassis A, Lewis EC. (2011) α-1-antitrypsin gene delivery reduces inflammation, increases T-regulatory cell population size and prevents islet allograft rejection. Mol Med 17:1000–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrells S, Camprubi S, Griffin R, Chen J, Ayguasanosa J. (2015) SPARTA clinical trial design: exploring the efficacy and safety of two dose regimens of alpha1-proteinase inhibitor augmentation therapy in alpha1-antitrypsin deficiency. Respir Med 109:490–499. [DOI] [PubMed] [Google Scholar]
- Stolk J, Nieuwenhuizen W, Stoller JK, Aboussouan L. (2005) High dose intravenous AAT and plasma neutrophil derived fibrinogen fragments. Thorax 60:84. [PMC free article] [PubMed] [Google Scholar]
- Subramanian S, Shahaf G, Ozeri E, Miller LM, Vandenbark AA, Lewis EC, Offner H. (2011) Sustained expression of circulating human alpha-1 antitrypsin reduces inflammation, increases CD4+FoxP3+ Treg cell population and prevents signs of experimental autoimmune encephalomyelitis in mice. Metab Brain Dis 26:107–113. [DOI] [PubMed] [Google Scholar]
- Subramaniyam D, Zhou H, Liang M, Welte T, Mahadeva R, Janciauskiene S. (2010) Cholesterol rich lipid raft microdomains are gateway for acute phase protein, SERPINA1. Int J Biochem Cell Biol 42:1562–1570. [DOI] [PubMed] [Google Scholar]
- Tawara I, Sun Y, Lewis EC, Toubai T, Evers R, Nieves E, Azam T, Dinarello CA, Reddy P. (2012) Alpha-1-antitrypsin monotherapy reduces graft-versus-host disease after experimental allogeneic bone marrow transplantation. Proc Natl Acad Sci USA 109:564–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilg H, Vannier E, Vachino G, Dinarello CA, Mier JW. (1993) Antiinflammatory properties of hepatic acute phase proteins: preferential induction of interleukin 1 (IL-1) receptor antagonist over IL-1 beta synthesis by human peripheral blood mononuclear cells. J Exp Med 178:1629–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wewers MD, Casolaro MA, Sellers SE, Swayze SC, McPhaul KM, Wittes JT, Crystal RG. (1987) Replacement therapy for alpha 1-antitrypsin deficiency associated with emphysema. N Engl J Med 316:1055–1062. [DOI] [PubMed] [Google Scholar]
- Zhou X, Liu Z, Shapiro L, Yang J, Burton GF. (2015) Low-density lipoprotein receptor-related protein 1 mediates α1-antitrypsin internalization in CD4+ T lymphocytes. J Leukoc Biol 98:1027–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]