Abstract
Opioid receptors expressed by peripheral pain-sensing neurons are functionally inactive for antinociceptive signaling under most basal conditions; however, tissue damage or exposure to inflammatory mediators (e.g., bradykinin) converts these receptors from a nonresponsive state to a functionally competent state. Here we tested the hypothesis that the basal, nonresponsive state of the mu- and delta-opioid receptors (MOR and DOR, respectively) is the result of constitutive receptor activity that activates desensitization mechanisms, resulting in MOR and DOR receptor systems that are constitutively desensitized. Consistent with our previous findings, under basal conditions, neither the MOR agonist [d-Ala2,N-MePhe4,Gly-ol5]-enkephalin nor the DOR agonist [d-Pen2,5]-enkephalin, inhibited prostaglandin E2 (PGE2)-stimulated cAMP accumulation in peripheral sensory neurons in culture (ex vivo) or inhibited PGE2-stimulated thermal allodynia in the rat hind paw in vivo. Prolonged treatment with naloxone induced MOR and DOR responsiveness both in vivo and ex vivo to a similar magnitude as that produced by bradykinin. Also similar to bradykinin, the effect of naloxone persisted for 60 minutes after washout of the ligand. By contrast, prolonged treatment with 6β-naltrexol, did not induce functional competence of MOR or DOR but blocked the effect of naloxone. Treatment with siRNA for β-arrestin-2, but not β-arrestin-1, also induced MOR and DOR functional competence in cultured peripheral sensory neurons. These data suggest that the lack of responsiveness of MOR and DOR to agonist for antinociceptive signaling in peripheral sensory neurons is due to constitutive desensitization that is likely mediated by β-arrestin-2.
Introduction
Opioids continue to be frontline drugs for the treatment of moderate to severe pain. However, substantial central nervous system (CNS)-mediated adverse effects constrain effective therapy with opioid medications. Consequently, there is considerable interest in targeting opioid receptors expressed by peripheral pain-sensing neurons (nociceptors) with peripherally restricted opioids to treat pain and avoid adverse effects mediated by the CNS. Although µ (MOR)-, δ (DOR)-, and κ-opioid receptors are expressed by nociceptors (Chen et al., 1997; Li et al., 1998), these peripheral receptors are regulated differently from those in the CNS. Whereas opioid agonists applied directly in the CNS readily produce antinociceptive effects (see Pasternak and Pan, 2013), opioid receptors expressed by peripheral sensory neurons are typically unresponsive to inhibitory effects of opioid agonists (Stein and Zollner, 2009). However, responsiveness of peripheral opioid receptors can be enhanced in vivo by tissue damage or by inflammatory mediators (Fields et al., 1980; Chen et al., 1997; Obara et al., 2009; Rowan et al., 2009; Stein and Lang, 2009; Stein and Zollner, 2009; Sullivan et al., 2015a). Similarly, opioid agonists do not inhibit adenylyl cyclase activity or decrease release of calcitonin gene-related peptide in peripheral sensory neurons in culture unless neurons are treated briefly with inflammatory mediators (Patwardhan et al., 2005; Berg et al., 2007, 2011, 2012; Sullivan et al., 2015a).
The pioneering work of Cerione et al. (1984) with purified β-adrenergic receptors and Gαs proteins and that of Costa and Herz (1989) using membranes from cells expressing DOR established the concept that G protein-coupled receptors can regulate cellular signaling pathways in the absence of an activating ligand (i.e., constitutive receptor activity). Although initially viewed with skepticism (Milligan et al., 1995), it is now generally accepted that most, if not all, receptors possess some level of ligand-independent (constitutive) receptor activity that can be decreased by drugs with inverse agonist properties (Kenakin, 2004; Bond and Ijzerman, 2006). Accordingly, several studies have demonstrated constitutive activity of both MOR and DOR in vivo and in vitro (Devlin et al., 2004; Sadee et al., 2005; Wang et al., 2007; Bilsky et al., 2010; Connor and Traynor, 2010).
In addition to regulating classic signaling mechanisms (e.g., adenylyl cyclases, phospholipases, ion channels, etc.), constitutive receptor activity, like agonist-dependent activity, has also been shown to activate desensitization mechanisms. This receptor activity results in receptor systems that are “constitutively desensitized,” as evidenced by constitutive receptor phosphorylation, internalization, and decreased responsiveness to agonist stimulation (Pei et al., 1994; Berg et al., 1999; Barak et al., 2003; Sullivan et al., 2015b). Prolonged reduction of constitutive receptor activity that activates desensitization mechanisms by treatment with inverse agonists allows receptor systems to resensitize, leading to increased responsiveness to agonists (Milligan and Bond, 1997; Berg et al., 1999; Miserey-Lenkei et al., 2002; Chanrion et al., 2008; Nijmeijer et al., 2010; Sullivan et al., 2015b).
In this study, we tested the hypothesis that the reduced responsiveness of MOR and DOR expressed by peripheral sensory neurons is due to constitutive desensitization. Reduction in constitutive receptor activity by prolonged treatment with an inverse agonist increased responsiveness of both MOR and DOR in vivo and in vitro. The inverse agonist effect was blocked with a neutral antagonist. Moreover, treatment with siRNA against β-arrestin 2, but not β-arrestin 1, also increased responsiveness of MOR and DOR. These data support the hypothesis that MOR and DOR exist in a constitutively desensitized, nonresponsive state in peripheral nociceptors.
Methods
Drugs and Chemicals.
[d-Ala2, N-MePhe4, Gly-ol5]-enkephalin (DAMGO), [d-Pen2,5]-enkephalin (DPDPE), 6β-naltrexol, and naloxone were purchased from Sigma-Aldrich (St. Louis, MO) or Bachem (Torrance, CA). Prostaglandin E2 (PGE2) was purchased from Cayman Chemicals (Ann Arbor, MI). [125I]-cAMP was purchased from PerkinElmer Life and Analytical Sciences (Boston, MA). Fetal bovine serum was from Invitrogen-Life Technologies Corp. (Grand Island, NY). Collagenase was from Worthington (Lakewood, NJ). All other tissue culture reagents were purchased from Invitrogen Corp. All other drugs and chemicals (reagent grade) were purchased from Sigma-Aldrich.
In certain experiments described below, naloxone was used for its inverse agonist properties to reduce constitutive activity of MOR and DOR toward desensitization mechanisms, whereas 6β-naltrexol was used for its antagonist properties. Several studies have shown that naloxone has inverse agonist properties (Liu and Prather, 2001, 2002; Raehal et al., 2005; Sadee et al., 2005; Walker and Sterious, 2005; Wang et al., 2007; Sirohi et al., 2009; Connor and Traynor, 2010) and 6β-naltrexol has properties of an antagonist (Raehal et al., 2005; Sadee et al., 2005; Wang et al., 2007; Sirohi et al., 2009; Connor and Traynor, 2010). It is important to note that display of the pharmacological properties of a ligand are dependent upon several system factors, including receptor density, receptor-effector coupling efficiency, cell phenotype, cell physiologic state, the particular signaling response measured, and the magnitude of constitutive receptor activity toward the response (for reviews, see Kenakin, 2013, 2015; Kenakin and Williams, 2014). Thus naloxone can behave as an inverse agonist, an antagonist, or even a partial agonist, depending upon the system under study. In this work, we found that naloxone acted as an inverse agonist to enhance agonist responsiveness but not to enhance basal or PGE2-stimulated cAMP accumulation (signal transduction pathway dependence). 6β-Naltrexol behaved as an antagonist, blocking the effects of naloxone and of agonist responses, without changing agonist responsiveness or cAMP accumulation.
Animals.
Adult male Sprague-Dawley rats (Charles River, Wilmington, MA), weighing 250–300 g, were used in this study. The animal study protocol was approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio and conformed to International Association for the Study of Pain and federal guidelines. Animals were housed for 1 week, with food and water available ad libitum, before experiments.
Primary Culture of Rat Peripheral Sensory Neurons.
Primary cultures of adult rat trigeminal ganglion cells were prepared as described previously (Berg et al., 2007, 2011, 2012; Jamshidi et al., 2015; Sullivan et al., 2015a). Ganglia were washed with Hanks' balanced salt solution (HBSS; Ca2+, Mg2+ free), digested with 3 mg/ml collagenase for 30 minutes at 37°C, and centrifuged at 200 g for 1 minute. The pellet was further digested with 0.1% trypsin (15 minutes) and 167 µg/ml DNase (10 minutes) at 37°C in the same solution. Cells were pelleted by centrifugation for 2 minutes at 500 g and resuspended in Dulbecco’s modified Eagle’s medium (high glucose) containing 100 ng/ml nerve growth factor, 10% fetal bovine serum, 1× Pen/Strep, 1× l-glutamine, and the mitotic inhibitors 7.5 µg/ml uridine and 17.5 mg/ml 5-fluoro-2′-deoxyuridine. The cell suspension was seeded on polylysine-coated 48-well plates. Media were changed 24 and 48 hours after plating. On the fifth day of culture, cells were refed with serum-free Dulbecco’s modified Eagle’s medium without nerve growth factor. Cells were used for experiments on the sixth day of culture.
Measurement of Cellular cAMP Accumulation.
Opioid receptor-mediated inhibition of PGE2-stimulated adenylyl cyclase activity was determined by measuring the amount of cAMP accumulated in the presence of the phosphodiesterase inhibitor, rolipram, and the Gs-coupled prostanoid EP receptor agonist, PGE2, as described previously (Berg et al., 2007, 2011, 2012; Jamshidi et al., 2015; Sullivan et al., 2015a). Briefly, cultures in 48-well plates were washed twice with HBSS containing 20 mM HEPES, pH 7.4 (wash buffer). Cells were preincubated in 250 µl wash buffer per well for 15 minutes at 37°C with or without bradykinin (BK, 10 µM) followed by addition of PGE2 (1 µM) without or with opioid agonists and rolipram (100 µM) and further incubation for 15 minutes. For experiments testing prolonged effects of an opioid inverse agonist/antagonist, naloxone (NAL, 1 µM, ∼100 x Ki), or 6β-naltrexol (6β-NTX, 10 µM, 1000 x Ki) alone or NAL and 6β-NTX in combination were added directly to cultures (after a 24-hour serum-free period) and incubated in a CO2 chamber for the times indicated. Cells were then washed three times with HBSS wash buffer with 3 × 5 minute wash intervals at 37°C to remove the opioid antagonist/inverse agonist and then incubated 15 minutes in 250 µl wash buffer containing rolipram with or without PGE2 or PGE2 plus opioid agonist and rolipram (100 µM). Reactions were terminated by aspiration of the buffer and addition of 500 µl ice-cold absolute ethanol. The ethanol extracts from individual wells were dried under a gentle air stream and reconstituted in 100 µl 50 mM sodium acetate, pH 6.2. The cAMP content of each well was determined by radioimmunoassay.
siRNA-Mediated Reduction of β-Arrestin-1 and β-Arrestin-2 Expression.
Double-stranded “On Targetplus Smart Pool” siRNA targeting β-arrestin-2 (cat #L-080157: rat ARRB2), β-arrestin-1 (cat # L-080156; rat ARRB1), or nontargeting siRNA (cat #001810-10) were purchased from GE Healthcare Dharmacon (Lafayette, CO). Primary cultures (5 days in culture) were transfected with 50 nM siRNA using DharmaFECT reagent 3 (GE Healthcare Dharmacon). The transfection medium was replaced with serum-free medium 24 hours later, and the cells were incubated for an additional 3 hours before experimentation. The effectiveness of siRNA treatment was assessed by measuring the magnitude of reduction of β-arrestin expression with Western analysis.
Western Blot.
For Western analysis of β-arrestin expression, cells were solubilized by boiling in 2× NuPAGE LDS sample buffer (Invitrogen) and resolved on NuPAGE 4 to 12% SDS-polyacrylamide gradient gels (Invitrogen) followed by transfer to polyvinylidene difluoride membranes using the iBlot transfer system (Invitrogen). Membranes were incubated in blocking buffer (1 hour, 23°C; Odyssey; LI-COR Biosciences, Lincoln, NE) and incubated overnight with 0.2 µg/ml of anti-β-arrestin 1/2 antibody (Santa Cruz, cat #sc-28869 lot J241) (Malik and Marchese, 2010), followed by the goat anti-rabbit IR 800 secondary antibody (0.05 µg/ml; IRDye 800CW; LI-COR Biosciences). Images were obtained and analyzed with an Odyssey Infrared Western blot imager (LI-COR Biosciences). The levels of immunoreactivity corresponding to a 55-kDa band representing β-arrestin-1 and -2 were quantified and normalized to actin as a loading control (Santa Cruz, sc-1616). The results are shown in Supplemental Fig. 5.
Behavioral Studies.
Inhibition of PGE2-stimulated thermal allodynia was used to assess the responsiveness of peripheral MOR and DOR to agonist stimulation in vivo as previously described (Rowan et al., 2009; Berg et al., 2011, 2012; Jamshidi et al., 2015). The time (in seconds) for a rat to withdraw its hind paw [paw withdrawal latency (PWL)] in response to a radiant heat stimulus (approximately 5 mm diameter) applied to the ventral surface of the rat hind paw was measured before and after agonist administration. The radiant heat intensity was set such that baseline PWL was 10 ± 2 seconds (25-second cutoff). PWL measurements were obtained in duplicate, at least 30 seconds apart, and the average was used for statistical analysis. Drugs were injected into the plantar surface) of the ipsilateral hind paw in volumes of 50 µl. To induce thermal allodynia, PGE2 (0.3 µg, intraplantar) was injected. This dose of PGE2 produces a mild (≈5 seconds reduction in PWL) and prolonged (>20 minutes) thermal allodynia (Rowan et al., 2009). After baseline measurements of PWL, rats received intraplantar injections with bradykinin (BK, 25 µg), naloxone (0.4 µg), or 6β-naltrexol (4 µg) alone or in combination at various time points (see Results) before coinjection (intraplantar) of PGE2 with vehicle (phosphate-buffered saline) or maximal doses of DAMGO (8 µg) or DPDPE (20 µg). PWL was measured at 5-minute intervals for 20 minutes after the last injection. All measurements were made by observers blinded to the experimental treatments. None of the drugs used altered PWL in the contralateral hind paw, indicating that drugs administered to the hind paw did not reach the systemic circulation in doses high enough to activate opioid receptors in the CNS.
Data Analysis.
Time course data from behavioral and cellular experiments were analyzed by two-way analysis of variance followed by Bonferroni’s post hoc to compare treatment effects over time. Area under the time course curve was calculated using the trapezoidal method with Prism software (GraphPad Software, Inc., San Diego, CA) and is expressed as the mean ± S.E.M. of each group. Statistical analyses of the area under the time course curve data were done with one-way analysis of variance followed by Dunnet’s post hoc test to determine significance from vehicle controls. When only a single time point/concentration was used, statistical significance was assessed using one-way analysis of variance followed by Dunnet’s post hoc or Student t test (paired) using Prism software (GraphPad Software, Inc.). Experiments were repeated at least three times, and data are presented as mean ± S.E.M. P < 0.05 was considered statistically significant.
Results
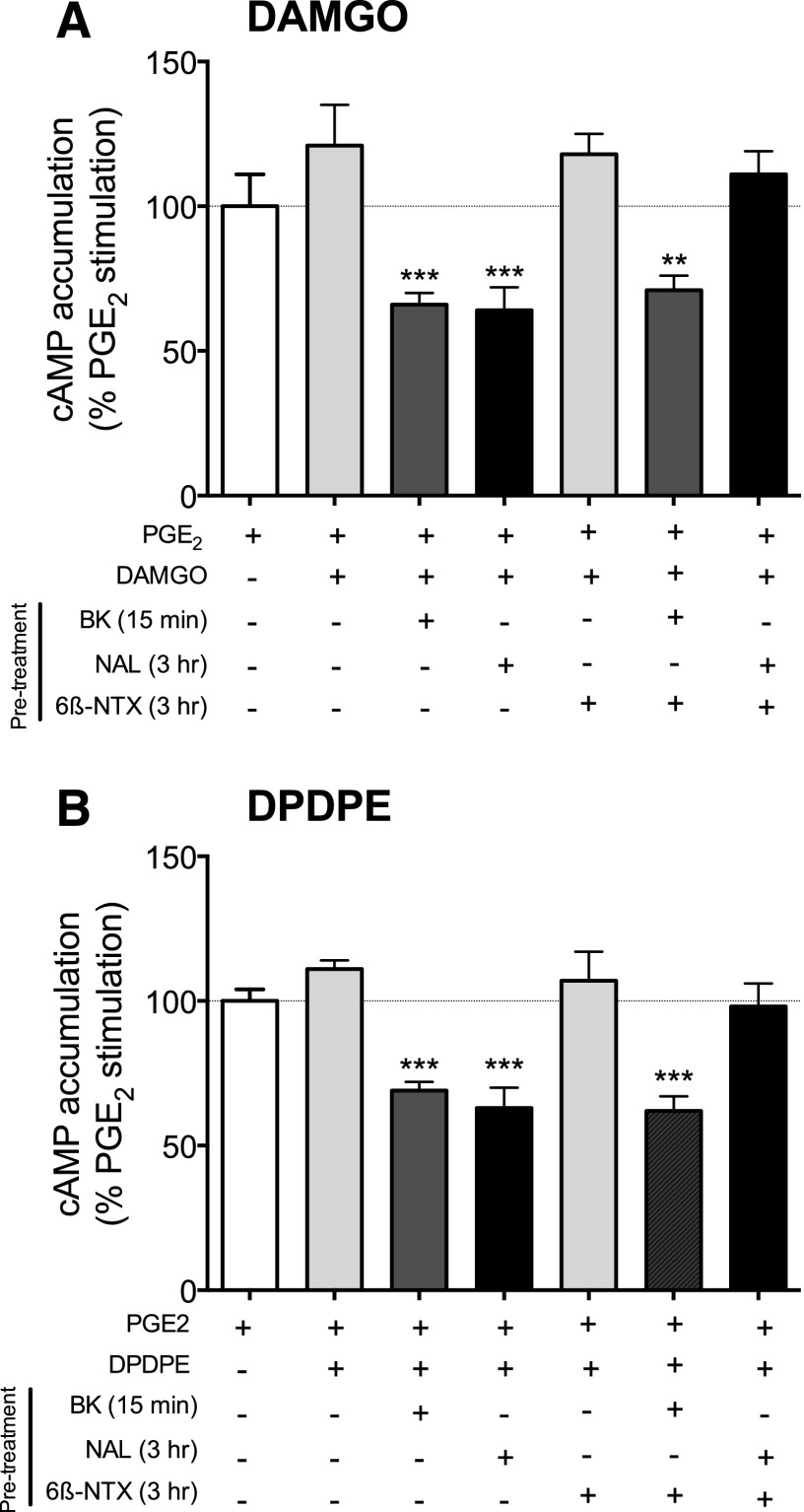
As we reported previously (Patwardhan et al., 2005; Berg et al., 2007, 2011; Sullivan et al., 2015a), in naive rat primary cultures of peripheral sensory neurons (ex vivo model) neither the MOR agonist, DAMGO, nor the DOR agonist, DPDPE, inhibited PGE2-stimulated cAMP accumulation (Fig. 1). However, brief (15 minutes) pretreatment with the inflammatory mediator, bradykinin (BK), increased the responsiveness of both MOR and DOR to stimulation by agonists (induced functional competence of MOR and DOR). After BK pretreatment, both agonists inhibited PGE2-stimulated adenylyl cyclase activity by 34 ± 4 and 31 ± 2% for DAMGO and DPDPE, respectively. Similarly, MOR and DOR functional competence also resulted after prolonged (3 hours) pretreatment with naloxone. After treatment with naloxone (1 µM; 100 × Ki) for 3 hours, followed by a brief (5 minutes) wash to remove it from the receptors, both DAMGO and DPDPE inhibited PGE2-mediated stimulation of cAMP accumulation by 36 ± 8 and 37 ± 8% (DAMGO and DPDPE, respectively). By contrast, prolonged (3 hours) pretreatment with 6β-naltrexol did not enhance the responsiveness of either MOR or DOR to agonist stimulation. Cotreatment of neurons with 6β-naltrexol blocked the naloxone-mediated, but not BK-mediated, induction of functional competence of MOR and DOR. Neither naloxone, BK, nor 6β-naltrexol altered basal or PGE2-stimulated cAMP levels (Supplemental Fig. 1).
Fig. 1.
Induction of MOR and DOR functional competence by treatment with naloxone in cultured peripheral sensory neurons. Cultures were pretreated with either BK (10 μM, 15 minutes), naloxone (NAL, 1 μM, 3 hours), 6β-naltrexol (6β-NTX, 10 μM, 3 hours) or NAL + 6-β-NTX (3 hours) followed by a wash. Opioid receptor functional competence was assessed by measuring DAMGO (1 μM) (A)- or DPDPE (100 nM) (B)-mediated inhibition of PGE2 (1 μM)-stimulated cAMP accumulation as described in Methods and Materials. Data represent the mean ± S.E.M. of 3–5 experiments and are presented as the percentage of the control PGE2-stimulated cAMP levels. None of the pretreatments altered basal or PGE2-stimulated cAMP levels (see Supplemental Fig. 1). **P < 0.01, ***P < 0.001 compared with PGE2 alone control group.
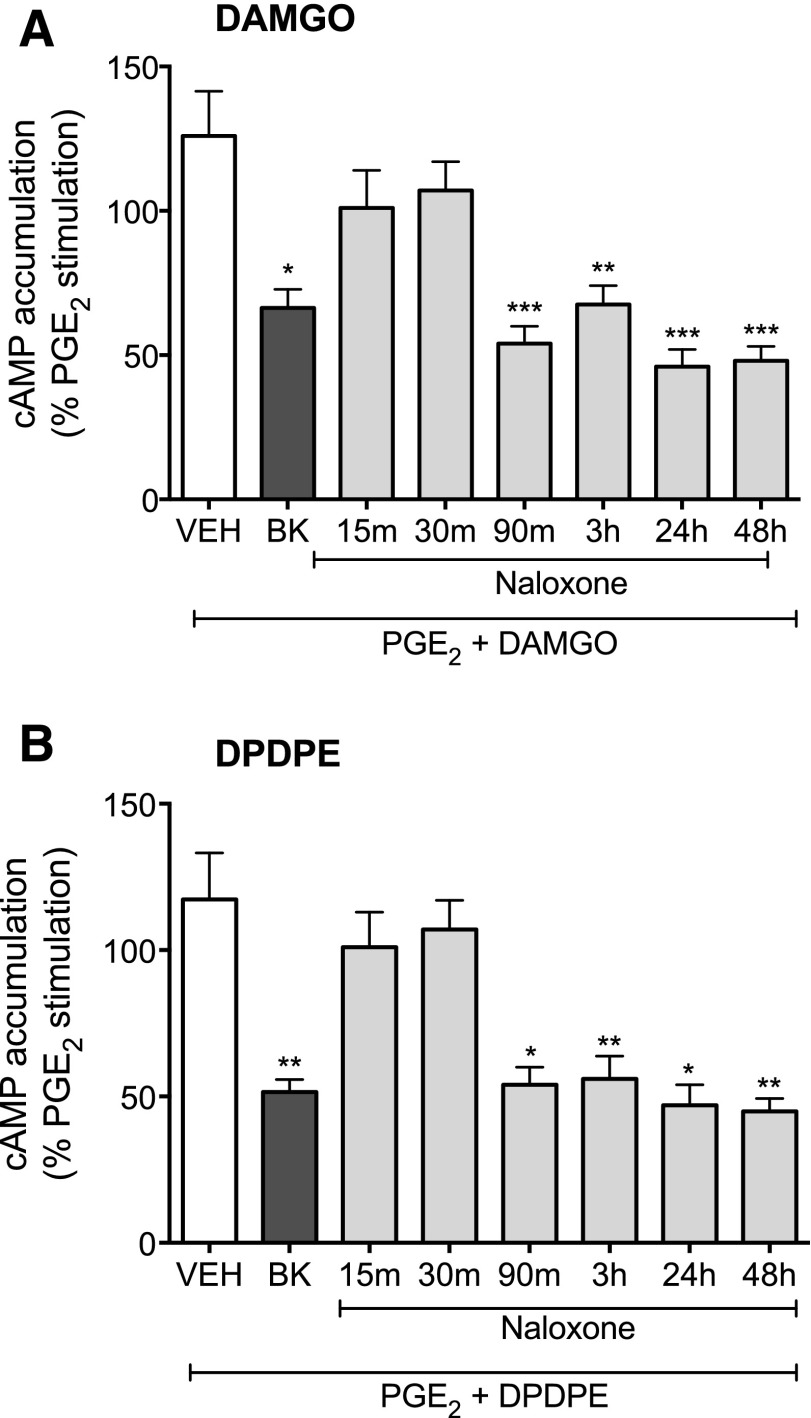
We next examined the time course for naloxone-mediated induction of MOR and DOR system functional competence. Neurons in culture were treated with naloxone for various periods of time (15 minutes–48 hours), washed to remove the ligand, and DAMGO- and DPDPE-mediated inhibition of PGE2-stimulated cAMP accumulation was measured. In contrast to the effect of 15-minute exposure to BK, 15 minutes of naloxone pretreatment did not produce MOR or DOR functional competence. Rather, as shown in Fig. 2, the onset of naloxone-induced functional competence required longer than 30 minutes of pretreatment. Treatment with naloxone had no effect on either basal- or PGE2-stimulated levels of cAMP at any pretreatment duration tested.
Fig. 2.
Time course for onset of naloxone-mediated induction of functional competence for MOR (A) or DOR (B) in primary cultures of peripheral sensory neurons. Cultures were pretreated with vehicle (HBSS), BK (10 μM) for 15 minutes, or naloxone (1 μM) for the times indicated, followed by a wash. Opioid receptor functional competence was assessed by measuring DAMGO (1 µM)- or DPDPE (100 nM)-mediated inhibition of PGE2 (1 µM)-stimulated cAMP accumulation as described in Methods. Data are expressed as the percentage of the control PGE2-stimulated cAMP levels and represent the mean ± S.E.M. of 4–8 experiments. *P < 0.05, **P < 0.01, ***P < 0.001 compared with control PGE2-stimulated cAMP levels.
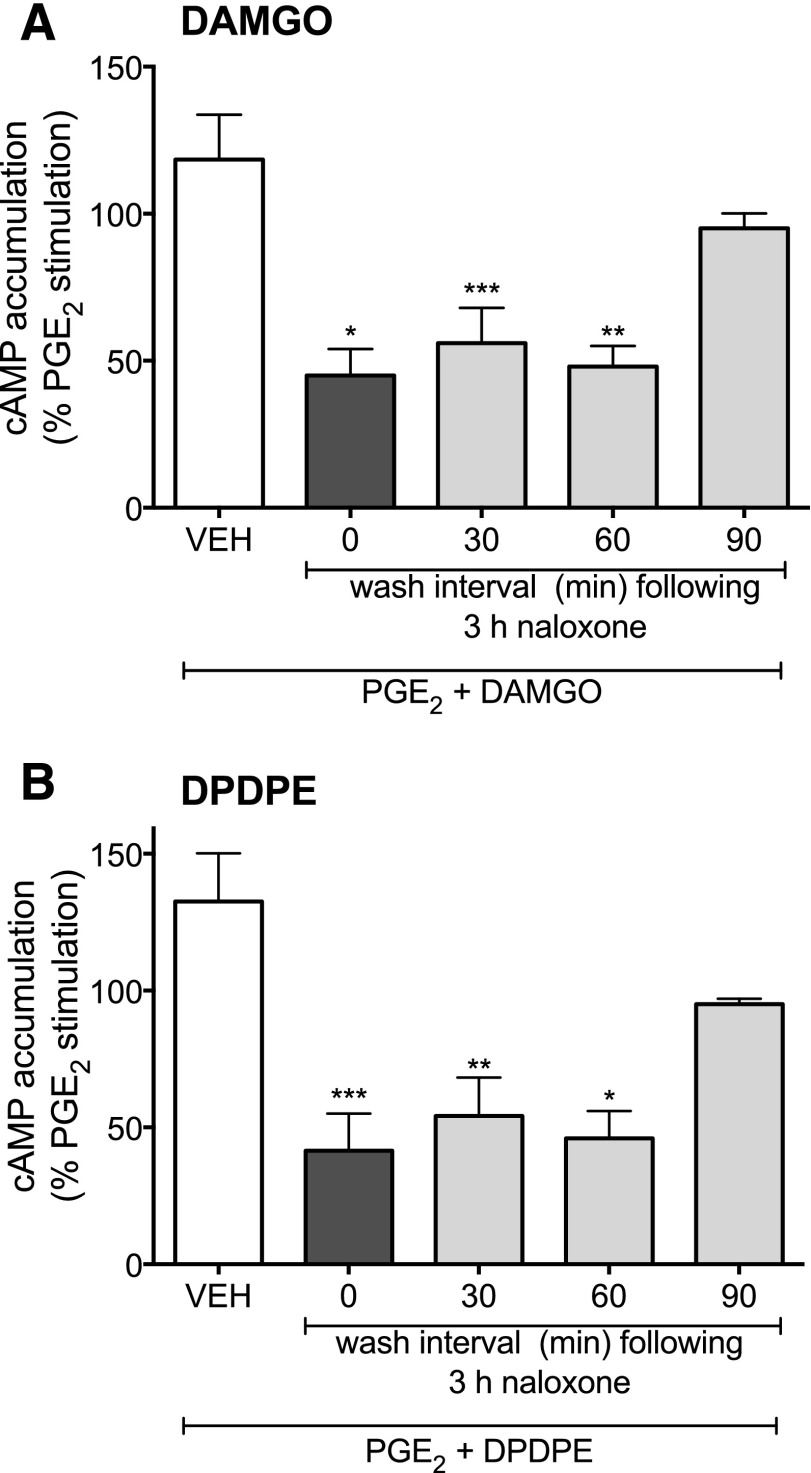
Figure 3 illustrates the persistence of functional competence induced by pretreatment with naloxone. Neurons were pretreated with naloxone for 3 hours, washed to remove the ligand, and then further incubated in the absence of naloxone for 0–90 minutes before measuring the ability of either DAMGO or DPDPE to inhibit PGE2-stimulated cAMP accumulation. Both MOR and DOR functional competence persisted for at least 60 minutes after the 3-hour pretreatment with naloxone. However by 90 minutes, MOR and DOR had reverted to the nonresponsive state.
Fig. 3.
Persistence of MOR (A) and DOR (B) functional competence after induction by naloxone. Cultures were incubated with either vehicle (HBSS) or naloxone (1 μM) for 3 hours. After a 0- to 90-minute washout period after naloxone treatment, opioid receptor functional competence was assessed by measuring DAMGO (1 µM)- or DPDPE (100 nM)-mediated inhibition of PGE2 (1 µM)-stimulated cAMP accumulation. Data are expressed as the percentage of the control PGE2-stimulated cAMP levels and are the mean ± S.E.M. of 3 experiments. *P < 0.05, **P < 0.01, ***P < 0.001 compared with control PGE2-stimulated cAMP levels.
As we have shown before, PGE2 administration to the hind paw of rats by intraplantar injection reduces paw withdrawal latencies in response to a thermal stimulus (thermal allodynia) that persists for at least 20 minutes after the injection (Rowan et al., 2009; Berg et al., 2011; Sullivan et al., 2015a). Similar to the ex vivo model with cultured sensory neurons, in this behavioral model, intraplantar injection of peripherally restricted doses of DAMGO or DPDPE did not alter the PGE2-induced thermal allodynia under basal (vehicle) conditions (Supplemental Fig. 2 and Rowan et al., 2009; Sullivan et al., 2015a). By contrast, 15 minutes after intraplantar injection of BK [which produces a transient (<15 minutes) thermal allodynia], DAMGO and DPDPE were effective at reducing PGE2-mediated thermal allodynia (see Supplemental Fig. 2 and Rowan et al., 2009; Berg et al., 2012; Sullivan et al., 2015a). Coinjection of opioid antagonists along with BK has no effect on PGE2-evoked responses (Rowan et al., 2009; Berg et al., 2011, 2012; Sullivan et al., 2015a), indicating that, under these experimental conditions, BK does not elicit local release of endogenous opioids.
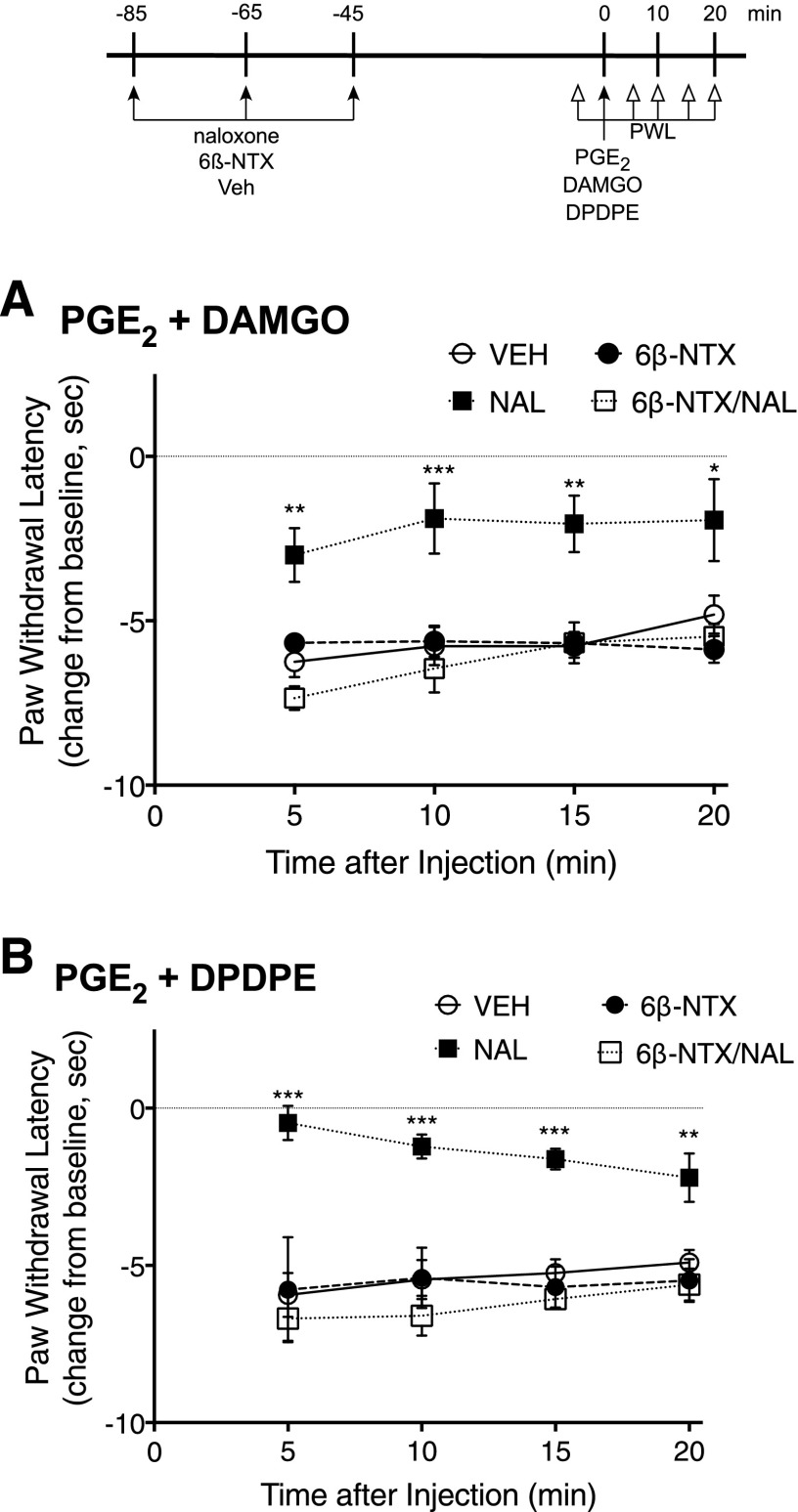
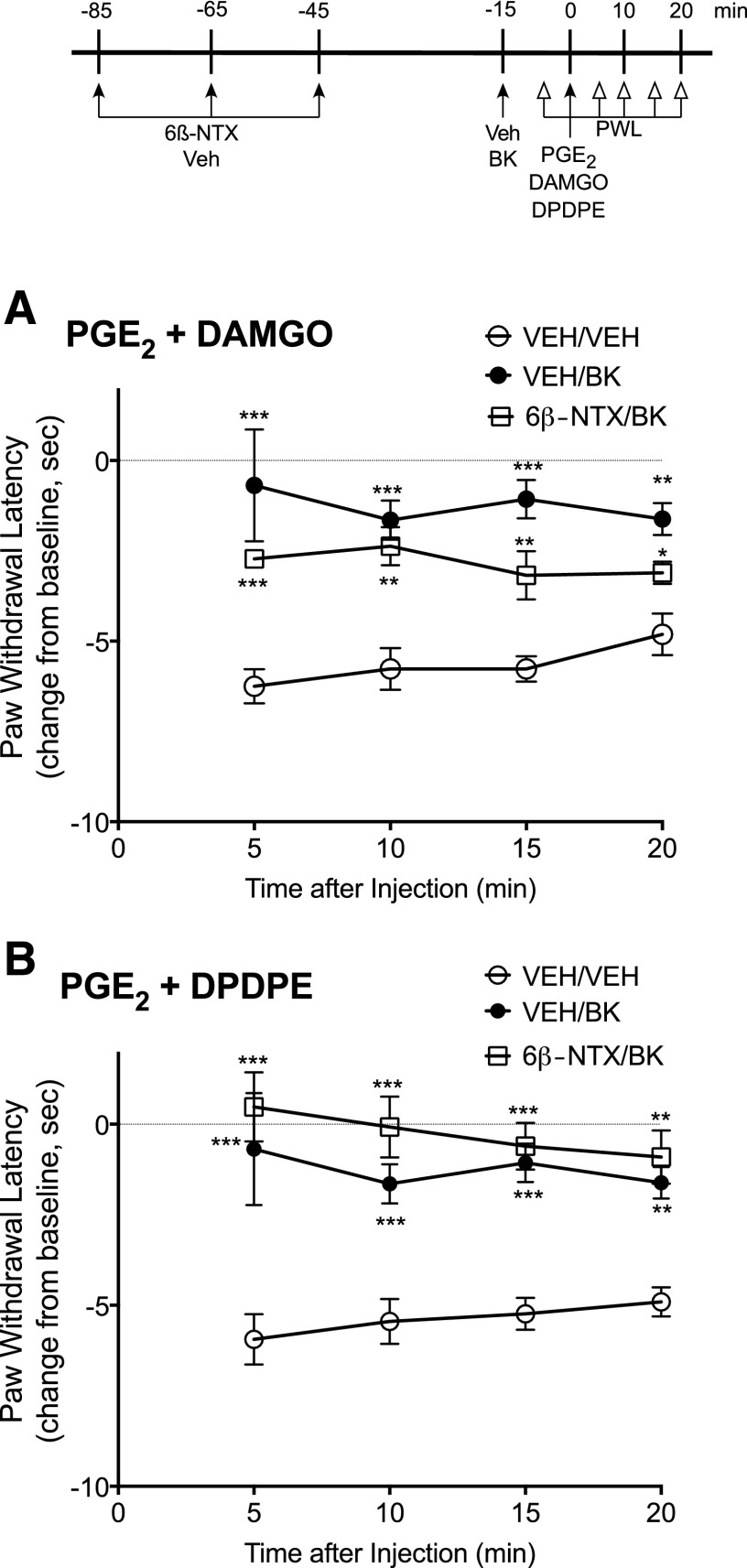
Figure 4 shows the effect of pretreatment with naloxone on MOR and DOR responsiveness in vivo. Rats received pretreatment injections (intraplantar) of naloxone, 6β-naltrexol, naloxone plus 6β-naltrexol, or vehicle, 85, 65, and 45 minutes before testing the ability of DAMGO or DPDPE (intraplantar) to inhibit PGE2-induced thermal allodynia. This repeated injection paradigm was developed to ensure sufficient receptor occupancy with naloxone to reduce constitutive receptor activity toward desensitization mechanisms for a prolonged period of time sufficient to allow resensitization to occur (≈60 minutes) and to allow time (≈45 minutes) for clearance of the ligand before testing responsiveness to agonist. Doses of naloxone and 6β-naltrexol were chosen that acutely blocked the effects of DAMGO and DPDPE after pretreatment with BK (Supplemental Fig. 2) and results shown in Supplemental Fig. 3 show that 45 minutes provides enough time for naloxone to be washed from the receptors (i.e., responses to the opioid agonists were no longer antagonized) in the hind paw. As shown in Fig. 4, DAMGO and DPDPE were effective in inhibiting PGE2-induced thermal allodynia after pretreatment with naloxone but not with 6β-naltrexol. The effect of naloxone to increase responsiveness of MOR and DOR to agonist stimulation was blocked by 6β-naltrexol. As shown in Fig. 5, pretreatment injections with 6β-naltrexol did not alter induction of functional competence induced by BK, indicating that 45 minutes provides enough time for washout of the ligand before testing opioid receptor system responsiveness to agonist stimulation.
Fig. 4.
Naloxone-mediated induction of MOR and DOR functional competence in vivo. Rats received injections (intraplantar, 50 µl) of either vehicle (VEH), naloxone (NAL, 0.4 μg), 6β-naltrexol (6βNTX; 4 μg) or NAL + 6βNTX, 85, 65, and 45 minutes before receiving an injection (intraplantar, 50 µl) of PGE2 (0.3 µg) with either DAMGO (8 µg) or DPDPE (20 µg). Paw withdrawal latency (PWL) in response to a thermal stimulus applied to the ventral surface of the hind paw was measured in duplicate before (baseline) and at 5-minute intervals after the PGE2/opioid injection for 20 minutes. Data are expressed as the change in PWL from baseline responses and represent the mean ± S.E.M. of 4–6 rats per group (for some data points, error bars are contained within the size of the symbol). Baseline PWL averaged 9.8 ± 0.4 seconds and was not altered by pretreatment with either naloxone or 6β-naltrexol alone or in combination (Supplemental Fig. 4). *P < 0.05, **P < 0.01, ***P < 0.001 compared with VEH pretreatment group.
Fig. 5.
Treatment with 6β-naltrexol does not alter BK-induced functional competence of MOR or DOR in vivo. Rats received injections (intraplantar, 50 µl) of either vehicle (VEH) or 6β-naltrexol (6βNTX; 4 μg) at 85, 65, and 45 minutes before injection (intraplantar, 50 µl) of vehicle (VEH) or bradykinin (BK, 25 µg) 15 minutes later, rats received intraplantar injection with PGE2 (0.3 µg) and either DAMGO (8 µg) or DPDPE (20 µg). Paw withdrawal latency (PWL) in response to a thermal stimulus applied to the ventral surface of the hind paw was measured in duplicate before (baseline) and at 5-minute intervals after the PGE2/opioid injection for 20 minutes. Data are expressed as the change in PWL from baseline responses and represent the mean ± S.E.M. of 4–8 rats per group. **P < 0.01, ***P < 0.001 compared with VEH/VEH pretreatment group.
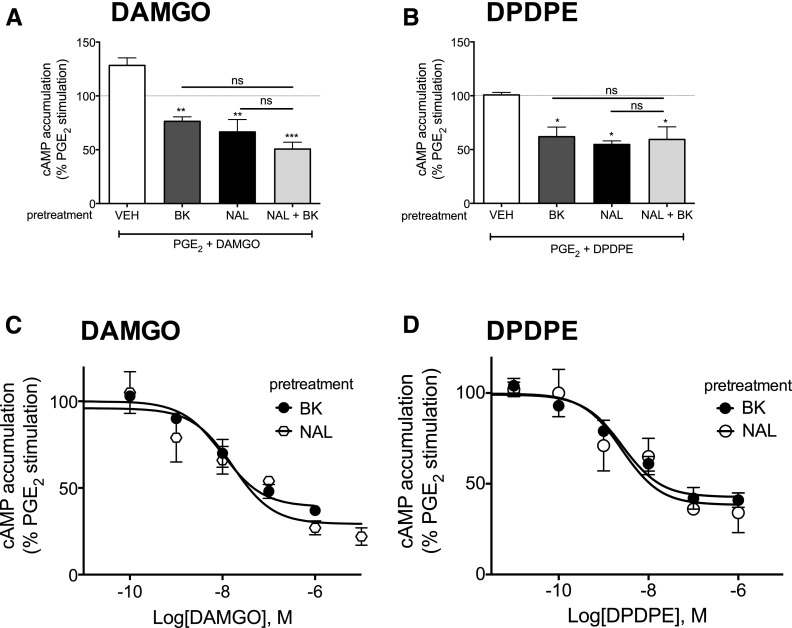
As shown in Fig. 6 and described above, after brief (15 minutes) pretreatment with BK or prolonged (3 hours) pretreatment with naloxone, DAMGO and DPDPE inhibited PGE2-mediated stimulation of cAMP accumulation. The combination of pretreatments (3 hours of naloxone with 15 minutes BK) had no further effect on opioid receptor functional competence. Furthermore, there was no difference in the concentration response curves for DAMGO- or DPDPE-mediated inhibition of cAMP accumulation after pretreatment with BK (15 minutes) compared with naloxone (3 hours).
Fig. 6.
Comparison between BK and naloxone for induction of functional competence. (A and B) Cultures of peripheral sensory neurons were pretreated with BK (10 μM, 15 minutes), naloxone (1 μM, 3 hours), or a combination of naloxone (3 hours) + BK (15 minutes). Functional competence of MOR (A) and DOR (B) was by measuring DAMGO (1 μM) (A)- or DPDPE (100 nM) (B)-mediated inhibition of PGE2 (1 μM)-stimulated cAMP accumulation as described in Methods. (C and D) Cultures of peripheral sensory neurons were pretreated with BK (10 μM, 15 minutes) or naloxone (1 μM, 3 hours). After pretreatment, concentration response curves to DAMGO (C) or DPDPE (D) were obtained measuring inhibition of PGE2-stimulated cAMP accumulation. Data represent the mean ± S.E.M. of 3 or 4 experiments and are presented as the percentage of the control PGE2-stimulated cAMP levels (for some data points, error bars are contained within the size of the symbol). *P < 0.05, **P < 0.01, ***P < 0.001 compared with the control PGE2-stimulated cAMP levels.
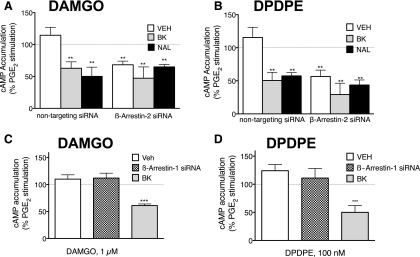
To examine a potential role for β-arrestin in the nonresponsive state of MOR and DOR, we used siRNA designed to reduce expression of β-arrestin-1 or β-arrestin-2 in peripheral sensory neurons in culture using siRNA transfection. Cultures were transfected with siRNA specific for β-arrestin-1 (50 nM) or β-arrestin-2 (50 nM), and cells were harvested 24 hours later. As measured with Western blot analysis, β-arrestin-1 and β-arrestin-2 siRNA transfection resulted in a significant decrease in β-arrestin protein expression (Supplemental Fig. 5). siRNA against β-arrestin-2, but not β-arrestin-1, induced functional competence to DAMGO and DPDPE similar to that induced by BK or naloxone pretreatment (Fig. 7).
Fig. 7.
Treatment with β-arrestin-2, but not β-arrestin-1, siRNA promotes MOR and DOR functional competence in primary cultures of peripheral sensory neurons. (A and B) Sensory neuron cultures were transfected with either β-arrestin-2 siRNA (50 nM) or nontargeting siRNA (50 nM). After 24 hours, cells were washed and pretreated with vehicle, BK (10 μM, 15 minutes) or naloxone (1 μM, 3 hours). After these pretreatments, inhibition of PGE2-stimulated cAMP accumulation by DAMGO (1 μM) or DPDPE (100 nM) was measured. (C and D) Sensory neuron cultures were transfected with β-arrestin-1 siRNA (50 nM). After 24 hours, cells were washed and pretreated with vehicle or BK (10 μM, 15 minutes), and inhibition of PGE2-stimulated cAMP accumulation by DAMGO (1 μM) or DPDPE (100 nM) was measured. Data represent the mean ± S.E.M. of 3 or 4 experiments and are presented as the percentage of control PGE2-stimulated cAMP levels. siRNA treatment reduced expression of β-arrestins by approximately 50% (Supplemental Fig. 5) and had no effect on cAMP levels, which were 0.13 ± 0.03 and 180% ± 40%, basal and PGE2-stimulated, respectively, n = 7. **P < 0.01, ***P < 0.001 compared with VEH control group.
Discussion
The pioneering work of Cerione et al. (1984) and Costa and Herz (1989) provided the first demonstration that G protein-coupled receptors can be active in the absence of an activating ligand (constitutively active) and led the way for the identification of ligands with negative intrinsic efficacy (i.e., inverse agonists) that can decrease constitutive receptor activity. Constitutive receptor activity, like agonist-stimulated activity, can differentially activate multiple cellular signaling mechanisms, including those that lead to desensitization (loss of responsiveness). As a result, receptor systems can exist in a state of constitutive desensitization (Pei et al., 1994; Berg et al., 1999; Barak et al., 2001, 2003; Sullivan et al., 2015b) where responsiveness to agonist stimulation is reduced. Prolonged reduction in constitutive receptor activity produced by treatment with an inverse agonist allows constitutively desensitized receptor systems to resensitize, thereby increasing responsiveness to agonist stimulation once occupancy by the inverse agonist is removed.
In this work, we tested the hypothesis that the lack of responsiveness of MOR and DOR, expressed by peripheral sensory neurons, to stimulation by agonists is due to constitutive desensitization. Under basal conditions, stimulation of MOR, with the agonist DAMGO, or DOR, with the agonist DPDPE, did not inhibit adenylyl cyclase in cultured peripheral sensory neurons or produce antinociception when injected locally into the rat hind paw, as reported previously (Patwardhan et al., 2005; Berg et al., 2007, 2012; Rowan et al., 2009; Sullivan et al., 2015a). Naloxone has been characterized to have inverse agonist properties at MOR and DOR, whereas 6β-naltrexol appears to be an antagonist (i.e., does not change constitutive receptor activity) (Liu and Prather, 2001,2002; Raehal et al., 2005; Sadee et al., 2005; Walker and Sterious, 2005; Wang et al., 2007; Sirohi et al., 2009; Connor and Traynor, 2010). Prolonged exposure of cultured sensory neurons to naloxone (followed by washout) greatly increased MOR and DOR agonist-mediated inhibition of PGE2-stimulated cAMP accumulation to an extent similar to that produced by pretreatment with BK. The effect of naloxone to increase MOR and DOR responsiveness required treatment of cultured neurons for longer than 30 minutes (for resensitization to occur due to reduced constitutive receptor activity), and the effect persisted for at least 60 minutes after naloxone washout. By contrast, treatment with 6β-naltrexol did not augment agonist responses and did not block the effect of bradykinin but did block the increased responsiveness of MOR and DOR produced by naloxone.
Similarly, local injection of DAMGO or DPDPE into the plantar surface of the rat hind paw to activate MOR and DOR, respectively, on peripheral sensory neurons in vivo did not inhibit PGE2-stimulated thermal allodynia. However, after prolonged treatment with intraplantar naloxone, responsiveness of both MOR and DOR increased such that DAMGO and DPDPE produced antinociception. Consistent with the effects seen in cultured peripheral sensory neurons, 6β-naltrexol did not alter opioid receptor responsiveness, but blocked the naloxone-induced augmentation of responsiveness.
These results suggest that the nonresponsive state of the MOR and DOR receptor systems under basal conditions is due to constitutive activity of MOR AND DOR toward activation of desensitization mechanisms in peripheral sensory neurons. Prolonged reduction of constitutive receptor activity with naloxone reduced constitutive activation of desensitization mechanisms and allowed the receptor systems to resensitize, resulting in enhanced responsiveness to agonist stimulation. Importantly, the effect of naloxone to augment opioid receptor responsiveness was blocked by 6β-naltrexol, supporting the conclusion that naloxone’s effect was opioid receptor mediated.
Constitutive activity of MOR and DOR has been reported for receptors in a wide variety of in vitro systems (Sadee et al., 2005; Wang et al., 2007; Connor and Traynor, 2010). Although often criticized for being nonphysiologic, constitutive receptor activity, and consequently, inverse agonist efficacy, are more easily measured when receptors are expressed at high density in heterologous expression systems (Kenakin, 2006). However, MOR and DOR constitutive activity has also been shown in membranes prepared from native tissues using inverse agonist reduction in the binding of GTP[γ35S] as a readout (Wang et al., 2007; Connor and Traynor, 2010), suggesting that opioid receptor constitutive activity occurs when receptors are expressed at physiologic levels. Interestingly, the level of constitutive activity of MOR and DOR is dynamically regulated. Several studies have reported that prolonged exposure to agonists increases MOR and DOR constitutive activity (Liu and Prather, 2001, 2002; Wang et al., 2007; Divin et al., 2009). This effect has been postulated to play a role in the development of tolerance and dependence that occurs after repeated use of opioids in vivo (Liu and Prather, 2001, 2002; Raehal et al., 2005; Walker and Sterious, 2005; Divin et al., 2009; Sirohi et al., 2009; Navani et al., 2011).
Definitive demonstration of constitutive receptor activity in vivo is difficult due to the presence of endogenous agonists. A reduction in a receptor-mediated response by a ligand may be due to simple competitive antagonism of the binding of an endogenous agonist, rather than reduction of constitutive receptor activity (inverse agonism). However, in the absence of constitutive activity, inverse agonists and antagonists have the same efficacy to reduce the receptor-mediated response, because both will block receptor occupancy by endogenous agonists. If, however, there is constitutive activity of a receptor system in vivo, then it is expected that inverse agonists would produce a greater reduction in a receptor-mediated response (blockade of the endogenous agonist and reduction of constitutive receptor activity) than would a simple antagonist. In opioid-dependent rodents, it has been shown that drugs with inverse agonist properties, such as naloxone and naltrexone, produce greater withdrawal symptoms than do antagonists (e.g., 6β-naltrexol), and the effect of inverse agonists to induce withdrawal is reduced by antagonists (Wang et al., 2001; Raehal et al., 2005; Walker and Sterious, 2005; Sirohi et al., 2009; Navani et al., 2011; Corder et al., 2013). Such results provide strong evidence of functional consequences of opioid receptor constitutive activity in vivo.
Our results suggest that the MOR and DOR systems in peripheral sensory neurons under basal (noninflamed) conditions are constitutively desensitized and unresponsive to agonist stimulation for antinociceptive signaling. In many receptor systems, agonist-induced desensitization involves receptor phosphorylation by G protein receptor kinases that is followed by recruitment of β-arrestin to bind to the phosphorylated receptor; however, receptor phosphorylation is not required for β-arrestin association (DeFea, 2011). With respect to desensitization, β-arrestin binding interferes with G protein binding and activation and can result in receptor internalization and downregulation, both of which result in loss of responsiveness (for reviews, see Gainetdinov et al., 2004; DeFea, 2011). Similarly, constitutive receptor activity also can activate these desensitization mechanisms (Barak et al., 2003). Under basal conditions, opioid receptors have been found to be constitutively phosphorylated (Arden et al., 1995; Hasbi et al., 1998) and associated with β-arrestin (Walwyn et al., 2007; Audet et al., 2012). We found that treatment of cultured peripheral sensory neurons with siRNA for β-arrestin-2, but not β-arrestin-1, increased responsiveness of MOR and DOR, mimicking the effect of naloxone. However caution is needed in relying on manufacturer’s claims of siRNA selectivity. These data provide further support that the lack of responsiveness of MOR and DOR in peripheral sensory neurons is due to constitutive desensitization. These results are also consistent with reports of increased constitutive activity of MOR (Walwyn et al., 2007; Lam et al., 2011) and increased antinociceptive responsiveness of MOR to activation by systemic morphine in β-arrestin-2 knockout mice (Bohn et al., 1999, 2002).
Tissue damage and exposure to some inflammatory mediators will also enhance responsiveness of opioid receptors expressed by peripheral sensory neurons (Fields et al., 1980; Chen et al., 1997; Patwardhan et al., 2005; Berg et al., 2007; Rowan et al., 2009; Stein and Lang, 2009; Stein and Zollner, 2009; Berg et al., 2011, 2012). Brief exposure to bradykinin induces functional competence through a cellular mechanism that involves production of a cyclooxygenase-dependent metabolite of arachidonic acid (Patwardhan et al., 2005; Berg et al., 2007, 2011; Rowan et al., 2009; Sullivan et al., 2015a) and an increase in receptor-G protein coupling efficiency (Berg et al., 2007). It is possible that bradykinin, acting via a cyclooxygenase-dependent arachidonic acid metabolite, may promote dissociation of β-arrestin-2 from MOR and DOR, thereby disinhibiting Gαi-mediated signaling.
In summary, the results of this work suggest that the lack of responsiveness to agonist stimulation of MOR and DOR expressed by peripheral sensory neurons under basal conditions in vivo and ex vivo is the result of constitutive activity of those receptors that leads to constitutive desensitization. Reduction of constitutive activity by treatment with an inverse agonist permits the receptor systems to resensitize, resulting in increased responsiveness to agonist stimulation for antinociceptive signaling. These results add to the growing evidence that constitutive receptor activity has physiologic relevance in vivo. A better understanding of the mechanisms that underlie regulation of the responsiveness of peripheral opioid receptors may lead to new approaches to improve the treatment of pain by targeting opioid receptors expressed by peripheral sensory neurons.
Acknowledgments
We thank Peter LoCoco for helpful comments and Hudson Smith and Wenxin Cai for technical assistance.
Abbreviations
- BK
bradykinin
- CNS
central nervous system
- DAMGO
[d-Ala2,N-MePhe4,Gly-ol5]-enkephalin
- DOR
δ-opioid receptor
- DPDPE
[d-Pen2,5]-enkephalin
- G protein
guanine nucleotide binding protein
- HBSS
Hanks' balanced salt solution
- MOR
µ-opioid receptor
- NAL
naloxone
- 6β-NTX
6β-naltrexol
- PGE2
prostaglandin E2
- PWL
paw withdrawal latency
Authorship Contributions
Participated in research design: Sullivan, Jamshidi, Berg, and Clarke.
Conducted experiments: Sullivan, Chavera, and Jamshidi.
Performed data analysis: Sullivan, Berg, and Clarke.
Wrote or contributed to the writing of the manuscript: Sullivan, Berg, and Clarke.
Footnotes
This work was supported by US Public Health Service grants from the National Institutes of Health National Institute of Drug Abuse [Grant RO1 DA 024865] and National Institute of General Medical Sciences [Grant R01 GM 106035]; the William and Ella Owens Medical Research Foundation; and US Public Health Service training grants from the National Institute of Dental and Craniofacial Research [COSTAR Training Grant T32 DE 14318] and the National Institute on Drug Abuse [Grant T32 DA 031115] to L.C.S.
Portions of this work (naloxone induction of opioid receptor functional competence) were previously presented at the Annual Meeting of the Society for Neuroscience; Washington DC, November 12-16, 2011.
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Arden JR, Segredo V, Wang Z, Lameh J, Sadée W. (1995) Phosphorylation and agonist-specific intracellular trafficking of an epitope-tagged mu-opioid receptor expressed in HEK 293 cells. J Neurochem 65:1636–1645. [DOI] [PubMed] [Google Scholar]
- Audet N, Charfi I, Mnie-Filali O, Amraei M, Chabot-Doré AJ, Millecamps M, Stone LS, Pineyro G. (2012) Differential association of receptor-Gβγ complexes with β-arrestin2 determines recycling bias and potential for tolerance of δ opioid receptor agonists. J Neurosci 32:4827–4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak LS, Oakley RH, Laporte SA, Caron MG. (2001) Constitutive arrestin-mediated desensitization of a human vasopressin receptor mutant associated with nephrogenic diabetes insipidus. Proc Natl Acad Sci USA 98:93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak LS, Wilbanks AM, Caron MG. (2003) Constitutive desensitization: a new paradigm for g protein-coupled receptor regulation. Assay Drug Dev Technol 1:339–346. [DOI] [PubMed] [Google Scholar]
- Berg KA, Patwardhan AM, Sanchez TA, Silva YM, Hargreaves KM, Clarke WP. (2007) Rapid modulation of micro-opioid receptor signaling in primary sensory neurons. J Pharmacol Exp Ther 321:839–847. [DOI] [PubMed] [Google Scholar]
- Berg KA, Rowan MP, Gupta A, Sanchez TA, Silva M, Gomes I, McGuire BA, Portoghese PS, Hargreaves KM, Devi LA, et al. (2012) Allosteric interactions between δ and κ opioid receptors in peripheral sensory neurons. Mol Pharmacol 81:264–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg KA, Rowan MP, Sanchez TA, Silva M, Patwardhan AM, Milam SB, Hargreaves KM, Clarke WP. (2011) Regulation of κ-opioid receptor signaling in peripheral sensory neurons in vitro and in vivo. J Pharmacol Exp Ther 338:92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg KA, Stout BD, Cropper JD, Maayani S, Clarke WP. (1999) Novel actions of inverse agonists on 5-HT2C receptor systems. Mol Pharmacol 55:863–872. [PubMed] [Google Scholar]
- Bilsky EJ, Giuvelis D, Osborn MD, Dersch CM, Xu H, Rothman RB. (2010) In vitro and in vivo assessment of mu opioid receptor constitutive activity. Methods Enzymol 484:413–443. [DOI] [PubMed] [Google Scholar]
- Bohn LM, Lefkowitz RJ, Caron MG. (2002) Differential mechanisms of morphine antinociceptive tolerance revealed in (beta)arrestin-2 knock-out mice. J Neurosci 22:10494–10500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT. (1999) Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science 286:2495–2498. [DOI] [PubMed] [Google Scholar]
- Bond RA, Ijzerman AP. (2006) Recent developments in constitutive receptor activity and inverse agonism, and their potential for GPCR drug discovery. Trends Pharmacol Sci 27:92–96. [DOI] [PubMed] [Google Scholar]
- Cerione RA, Codina J, Benovic JL, Lefkowitz RJ, Birnbaumer L, Caron MG. (1984) The mammalian beta 2-adrenergic receptor: reconstitution of functional interactions between pure receptor and pure stimulatory nucleotide binding protein of the adenylate cyclase system. Biochemistry 23:4519–4525. [DOI] [PubMed] [Google Scholar]
- Chanrion B, Mannoury la Cour C, Gavarini S, Seimandi M, Vincent L, Pujol J-F, Bockaert J, Marin P, Millan MJ. (2008) Inverse agonist and neutral antagonist actions of antidepressants at recombinant and native 5-hydroxytryptamine2C receptors: differential modulation of cell surface expression and signal transduction. Mol Pharmacol 73:748–757. [DOI] [PubMed] [Google Scholar]
- Chen JJ, Dymshitz J, Vasko MR. (1997) Regulation of opioid receptors in rat sensory neurons in culture. Mol Pharmacol 51:666–673. [DOI] [PubMed] [Google Scholar]
- Connor M, Traynor J. (2010) Constitutively active μ-opioid receptors. Methods Enzymol 484:445–469. [DOI] [PubMed] [Google Scholar]
- Corder G, Doolen S, Donahue RR, Winter MK, Jutras BL, He Y, Hu X, Wieskopf JS, Mogil JS, Storm DR, et al. (2013) Constitutive μ-opioid receptor activity leads to long-term endogenous analgesia and dependence. Science 341:1394–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa T, Herz A. (1989) Antagonists with negative intrinsic activity at delta opioid receptors coupled to GTP-binding proteins. Proc Natl Acad Sci USA 86:7321–7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFea KA. (2011) Beta-arrestins as regulators of signal termination and transduction: how do they determine what to scaffold? Cell Signal 23:621–629. [DOI] [PubMed] [Google Scholar]
- Devlin MG, Smith NJ, Ryan OM, Guida E, Sexton PM, Christopoulos A. (2004) Regulation of serotonin 5-HT2C receptors by chronic ligand exposure. Eur J Pharmacol 498:59–69. [DOI] [PubMed] [Google Scholar]
- Divin MF, Bradbury FA, Carroll FI, Traynor JR. (2009) Neutral antagonist activity of naltrexone and 6beta-naltrexol in naïve and opioid-dependent C6 cells expressing a mu-opioid receptor. Br J Pharmacol 156:1044–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields HL, Emson PC, Leigh BK, Gilbert RF, Iversen LL. (1980) Multiple opiate receptor sites on primary afferent fibres. Nature 284:351–353. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Premont RT, Bohn LM, Lefkowitz RJ, Caron MG. (2004) Desensitization of G protein-coupled receptors and neuronal functions. Annu Rev Neurosci 27:107–144. [DOI] [PubMed] [Google Scholar]
- Hasbi A, Polastron J, Allouche S, Stanasila L, Massotte D, Jauzac P. (1998) Desensitization of the delta-opioid receptor correlates with its phosphorylation in SK-N-BE cells: involvement of a G protein-coupled receptor kinase. J Neurochem 70:2129–2138. [DOI] [PubMed] [Google Scholar]
- Jamshidi RJ, Jacobs BA, Sullivan LC, Chavera TA, Saylor RM, Prisinzano TE, Clarke WP, Berg KA. (2015) Functional selectivity of kappa opioid receptor agonists in peripheral sensory neurons. J Pharmacol Exp Ther 355:174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenakin T. (2004) Efficacy as a vector: the relative prevalence and paucity of inverse agonism. Mol Pharmacol 65:2–11. [DOI] [PubMed] [Google Scholar]
- Kenakin T.(2006) Testing for inverse agonism with constitutive receptor systems. Curr Protoc Pharmacol 32:9.5.1–9.5.13. [DOI] [PubMed] [Google Scholar]
- Kenakin T. (2013) New concepts in pharmacological efficacy at 7TM receptors: IUPHAR review 2. Br J Pharmacol 168:554–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenakin T. (2015) Gaddum Memorial Lecture 2014: receptors as an evolving concept: from switches to biased microprocessors. Br J Pharmacol 172:4238–4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenakin T, Williams M. (2014) Defining and characterizing drug/compound function. Biochem Pharmacol 87:40–63. [DOI] [PubMed] [Google Scholar]
- Lam H, Maga M, Pradhan A, Evans CJ, Maidment NT, Hales TG, Walwyn W. (2011) Analgesic tone conferred by constitutively active mu opioid receptors in mice lacking β-arrestin 2. Mol Pain 7:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JL, Ding YQ, Li YQ, Li JS, Nomura S, Kaneko T, Mizuno N. (1998) Immunocytochemical localization of mu-opioid receptor in primary afferent neurons containing substance P or calcitonin gene-related peptide. A light and electron microscope study in the rat. Brain Res 794:347–352. [DOI] [PubMed] [Google Scholar]
- Liu JG, Prather PL. (2001) Chronic exposure to mu-opioid agonists produces constitutive activation of mu-opioid receptors in direct proportion to the efficacy of the agonist used for pretreatment. Mol Pharmacol 60:53–62. [DOI] [PubMed] [Google Scholar]
- Liu JG, Prather PL. (2002) Chronic agonist treatment converts antagonists into inverse agonists at delta-opioid receptors. J Pharmacol Exp Ther 302:1070–1079. [DOI] [PubMed] [Google Scholar]
- Malik R, Marchese A. (2010) Arrestin-2 interacts with the endosomal sorting complex required for transport machinery to modulate endosomal sorting of CXCR4. Mol Biol Cell 21:2529–2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan G, Bond RA. (1997) Inverse agonism and the regulation of receptor number. Trends Pharmacol Sci 18:468–474. [DOI] [PubMed] [Google Scholar]
- Milligan G, Bond RA, Lee M. (1995) Inverse agonism: pharmacological curiosity or potential therapeutic strategy? Trends Pharmacol Sci 16:10–13. [DOI] [PubMed] [Google Scholar]
- Miserey-Lenkei S, Parnot C, Bardin S, Corvol P, Clauser E. (2002) Constitutive internalization of constitutively active agiotensin II AT(1A) receptor mutants is blocked by inverse agonists. J Biol Chem 277:5891–5901. [DOI] [PubMed] [Google Scholar]
- Navani DM, Sirohi S, Madia PA, Yoburn BC. (2011) The role of opioid antagonist efficacy and constitutive opioid receptor activity in the opioid withdrawal syndrome in mice. Pharmacol Biochem Behav 99:671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijmeijer S, Leurs R, and Vischer HF(2010) Constitutive activity of the histamine H-1 receptor, in Methods in Enzymology: Constitutive Activity in Receptors and Other Proteins, Part A (Conn PM ed) vol. 484, pp 127–147, Elsevier, London. [DOI] [PubMed] [Google Scholar]
- Obara I, Parkitna JR, Korostynski M, Makuch W, Kaminska D, Przewlocka B, Przewlocki R. (2009) Local peripheral opioid effects and expression of opioid genes in the spinal cord and dorsal root ganglia in neuropathic and inflammatory pain. Pain 141:283–291. [DOI] [PubMed] [Google Scholar]
- Pasternak GW, Pan YX. (2013) Mu opioids and their receptors: evolution of a concept. Pharmacol Rev 65:1257–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patwardhan AM, Berg KA, Akopain AN, Jeske NA, Gamper N, Clarke WP, Hargreaves KM. (2005) Bradykinin-induced functional competence and trafficking of the delta-opioid receptor in trigeminal nociceptors. J Neurosci 25:8825–8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei G, Samama P, Lohse M, Wang M, Codina J, Lefkowitz RJ. (1994) A constitutively active mutant beta 2-adrenergic receptor is constitutively desensitized and phosphorylated. Proc Natl Acad Sci USA 91:2699–2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raehal KM, Lowery JJ, Bhamidipati CM, Paolino RM, Blair JR, Wang D, Sadée W, Bilsky EJ. (2005) In vivo characterization of 6beta-naltrexol, an opioid ligand with less inverse agonist activity compared with naltrexone and naloxone in opioid-dependent mice. J Pharmacol Exp Ther 313:1150–1162. [DOI] [PubMed] [Google Scholar]
- Rowan MP, Ruparel NB, Patwardhan AM, Berg KA, Clarke WP, Hargreaves KM. (2009) Peripheral delta opioid receptors require priming for functional competence in vivo. Eur J Pharmacol 602:283–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadée W, Wang D, Bilsky EJ. (2005) Basal opioid receptor activity, neutral antagonists, and therapeutic opportunities. Life Sci 76:1427–1437. [DOI] [PubMed] [Google Scholar]
- Sirohi S, Dighe SV, Madia PA, Yoburn BC. (2009) The relative potency of inverse opioid agonists and a neutral opioid antagonist in precipitated withdrawal and antagonism of analgesia and toxicity. J Pharmacol Exp Ther 330:513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein C, Lang LJ. (2009) Peripheral mechanisms of opioid analgesia. Curr Opin Pharmacol 9:3–8. [DOI] [PubMed] [Google Scholar]
- Stein C, Zöllner C. (2009) Opioids and sensory nerves. Handbook Exp Pharmacol 194:495–518. [DOI] [PubMed] [Google Scholar]
- Sullivan LC, Berg KA, Clarke WP. (2015a) Dual regulation of δ-opioid receptor function by arachidonic acid metabolites in rat peripheral sensory neurons. J Pharmacol Exp Ther 353:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan LC, Clarke WP, Berg KA. (2015b) Atypical antipsychotics and inverse agonism at 5-HT2 receptors. Curr Pharm Des 21:3732–3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker EA, Sterious SN. (2005) Opioid antagonists differ according to negative intrinsic efficacy in a mouse model of acute dependence. Br J Pharmacol 145:975–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walwyn W, Evans CJ, Hales TG. (2007) Beta-arrestin2 and c-Src regulate the constitutive activity and recycling of mu opioid receptors in dorsal root ganglion neurons. J Neurosci 27:5092–5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Raehal KM, Bilsky EJ, Sadée W. (2001) Inverse agonists and neutral antagonists at mu opioid receptor (MOR): possible role of basal receptor signaling in narcotic dependence. J Neurochem 77:1590–1600. [DOI] [PubMed] [Google Scholar]
- Wang D, Sun X, Sadee W. (2007) Different effects of opioid antagonists on mu-, delta-, and kappa-opioid receptors with and without agonist pretreatment. J Pharmacol Exp Ther 321:544–552. [DOI] [PubMed] [Google Scholar]