Abstract
Renal transporter–mediated drug-drug interactions (DDIs) are of significant clinical concern, as they can adversely impact drug disposition, efficacy, and toxicity. Emerging evidence suggests that human renal organic cation transporter 2 (hOCT2) and multidrug and toxin extrusion proteins 1 and 2-K (hMATE1/2-K) exhibit substrate-dependent inhibition, but their impact on renal drug secretion and intracellular accumulation is unknown. Using metformin and atenolol as the probe substrates, we found that the classic inhibitors (e.g., cimetidine) of renal organic cation secretion were approximately 10-fold more potent for hOCT2 when atenolol was used, suggesting that atenolol is a more sensitive in vitro substrate for hOCT2 than metformin. In contrast, inhibition of hMATE1/2-K was influenced much less by the choice of substrate. Cimetidine is a much more potent inhibitor for hMATE1/2-K when metformin is the substrate but acts as an equally potent inhibitor of hOCT2 and hMATE1/2-K when atenolol is the substrate. Using hOCT2/hMATE1 double-transfected Madin-Darby canine kidney cells, we evaluated the impact of substrate-dependent inhibition on hOCT2/hMATE1-mediated transepithelial flux and intracellular drug accumulation. At clinically relevant concentrations, cimetidine dose dependently inhibited basal-to-apical flux of atenolol and metformin but impacted their intracellular accumulation differently, indicating that substrate-dependent inhibition may shift the major substrate-inhibitor interaction site between apical and basolateral transporters. Cimetidine is effective only when applied to the basal compartment. Our findings revealed the complex and dynamic nature of substrate-dependent inhibition of renal organic cation drug transporters and highlighted the importance of considering substrate-dependent inhibition in predicting transporter-mediated renal drug interaction, accumulation, and toxicity.
Introduction
Renal excretion is a major elimination pathway for many drugs and drug metabolites. Besides glomerular filtration, circulating drugs are actively secreted by carrier-mediated pathways in the renal proximal tubules. In humans, secretion of organic cation (OC) drugs is primarily accomplished by basolateral uptake via the electrogenic human organic cation transporter 2 (hOCT2) followed by apical efflux via the proton/OC exchangers human multidrug and toxin extrusion proteins 1 and 2-K (hMATE1 and 2-K) (Li et al., 2006; Giacomini et al., 2010; Morrissey et al., 2013; Motohashi and Inui, 2013). Anionic drug molecules, on the other hand, are generally first transported into tubular cells by the basolateral organic anion transporters 1 and 3 (hOAT1 and 3) and then effluxed into the lumen by apical transporters such as the multidrug resistance-associated proteins 2 and 4 (Li et al., 2006; Giacomini et al., 2010; Morrissey et al., 2013). These kidney transporters are important pharmacokinetic and pharmacodynamic determinants for a wide array of clinically used drugs (Giacomini et al., 2010; Morrissey et al., 2013). Furthermore, an imbalance between transporter-mediated uptake and efflux may result in drug accumulation in proximal tubule cells, leading to drug-induced nephrotoxicity and kidney injury (Li et al., 2006; Morrissey et al., 2013).
Numerous clinically significant drug-drug interactions (DDIs) in the kidney are attributed to the inhibition of renal organic cation or anion secretion systems (Masereeuw and Russel, 2001; Li et al., 2006; Morrissey et al., 2013). Historically, cimetidine has been used as the classic inhibitor of the OC system, whereas probenecid is the prototypical inhibitor of the anion system (Masereeuw and Russel, 2001; Li et al., 2006; Morrissey et al., 2013). Renal transporter–mediated DDIs are of significant clinical concern, as they can adversely impact drug disposition, efficacy, and toxicity. Recognizing the importance of transporters in drug disposition and interactions, the US Food and Drug Administration (FDA) and the International Transporter Consortium (ITC) have published a series of recommendations to guide industry in assessing the drug interaction potentials of new molecular entities (NMEs) toward clinically important transporters, including hOCT2, hOAT1/3, and hMATE1/2-K (Giacomini et al., 2010; Zhang et al., 2011; FDA, 2012; Brouwer et al., 2013; Hillgren et al., 2013). In general, if an NME is an in vitro inhibitor for these transporters and its unbound maximal plasma concentration (Cmax) is greater than one-tenth of its half-maximal inhibitory concentration (IC50), further in vivo DDI assessment is recommended (Giacomini et al., 2010; FDA, 2012). A key parameter in the prediction of DDI risk is the IC50 (or the inhibition constant Ki) of the NME, which is typically determined in transporter-expressing cell lines using a recommended probe substrate (Brouwer et al., 2013). Several in vitro substrates, including metformin and 1-methyl-4-phenylpyridinium (MPP+), have been recommended as the probe substrates in preclinical DDI assessment with hOCT2 and hMATEs (FDA, 2012; Hillgren et al., 2013). This approach assumes that the Ki or IC50 value of an NME determined with a probe substrate is a constant and can be extrapolated to predict the in vivo interaction of the NME with clinically used drugs. However, several drug-metabolizing enzymes, including CYP3A4 and 2D6, are known to exhibit substrate-dependent inhibition, where the Ki or IC50 value of an inhibitor can vary considerably depending on the probe substrate used (Kenworthy et al., 1999; VandenBrink et al., 2012). To mitigate the risk of false-negative prediction, the use of two or more probe substrates or a sensitive drug substrate for these enzymes has been suggested (Yuan et al., 2002; FDA, 2012).
Emerging evidence suggests substrate-dependent inhibition can also occur with drug transporters (Belzer et al., 2013; Martinez-Guerrero and Wright, 2013; Izumi et al., 2015). Using metformin and several experimental compounds as probe substrates, Wright and coworkers showed that inhibition of hOCT2 and hMATE1 is substrate-dependent (Belzer et al., 2013; Martinez-Guerrero and Wright, 2013). However, except for metformin, all other substrates used in these studies were experimental compounds not used in humans, making it difficult to infer the clinical impact of substrate-dependent inhibition on DDI prediction. Moreover, the influence of substrate-dependent inhibition on overall tubular secretion has never been studied. Its impact on intracellular drug accumulation, which is an important predictor for drug-induced nephrotoxicity, is also unknown. In this study, we investigated the influence of probe substrate choice on predicting transporter-mediated DDIs using metformin and a newly identified drug substrate of hOCT2 and hMATEs, atenolol (Yin et al., 2015). The overall impact of substrate-dependent inhibition on tubular secretion and intracellular accumulation was also investigated using a double-transfected cell culture model of renal OC secretion.
Materials and Methods
Materials.
[3H]Atenolol (3.3 Ci/mmol) and [14C]metformin (98 mCi/mmol) were purchased from Moravek Biochemicals, Inc. (Brea, CA). [3H]Cimetidine (80 Ci/mmol) and [3H]mannitol (20 Ci/mmol) were purchased from American Radiolabeled Chemicals, Inc. (St. Louis, MO). All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO) and were of analytical grade.
Cell Lines and Cell Culture.
Control (pcDNA5-transfected) and Flp-In human embryonic kidney (HEK)-293 cell lines stably expressing hOCT2, hMATE1, and hMATE2-K were previously established and maintained in Dulbecco’s modified Eagle’s medium (Duan et al., 2015; Yin et al., 2015). Transporter-expressing cells generally showed transport activities much higher (∼10- to 30-fold) than control HEK-293 cells for a probe substrate (e.g., metformin or MPP+) (Yin et al., 2015). Both transporter-expressing and control HEK-293 cells were maintained in Dulbecco’s modified Eagle’s medium (high glucose) supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 100 IU/ml penicillin, 100 μg/ml streptomycin, and 150 μg/ml hygromycin B. Vectors (pcDNA3.1+ and pcDNA3.1/Hygro(+)) and hOCT2/hMATE1 double-transfected Madin-Darby canine kidney (MDCK) cell lines were previously established in our laboratory (Yin et al., 2015). MDCK cells were maintained in minimum essential medium supplemented with 10% fetal bovine serum, 500 μg/ml G418, and 200 μg/ml hygromycin B. All cell lines were cultured in a humidified incubator at 37°C with 5% CO2.
Uptake and Inhibition Assays in HEK-293 Cells.
In vitro transport and inhibition studies were performed as previously described (Duan and Wang, 2010; Yin et al., 2015). Briefly, transporter-expressing and control HEK-293 cells were seeded in poly-d-lysine–coated plates and allowed to grow for 2–3 days to reach 80–90% confluence. Growth medium was then aspirated, and each well was rinsed two times with prewarmed Krebs-Ringer-Henseleit (KRH) buffer (5.6 mM glucose, 125 mM NaCl, 4.8 mM KCl, 1.2 mM KH2PO4, 1.2 mM CaCl2, 1.2 mM MgSO4, and 25 mM HEPES, pH 7.4). Uptake of [14C]metformin or [3H]atenolol in control and transporter-expressing HEK-293 cells were performed at 37°C in KRH for 2 minutes in the presence or absence of an inhibitor. Metformin and atenolol are highly hydrophilic compounds (LogD7.4 of –5.5 and –2.0, respectively) with low nonspecific binding to cells and cell culture plates. The 2-minute incubation time was chosen because our previous studies showed that it is within the initial rate period of atenolol and metformin uptake for all three transporters (Yin et al., 2015). hMATE uptake studies were performed in KRH buffer with pH adjusted to 8.0 to maintain an outwardly directed proton gradient to drive cellular uptake of the OC substrate, because hMATEs function as proton/organic cation exchangers (Otsuka et al., 2005). For hOCT2, uptake was performed at pH 7.4. Uptake was terminated by washing cells three times with ice-cold KRH buffer. Cells were then solubilized with 0.5 ml of 1 M NaOH at 37°C and neutralized with an equal volume of 1 M HCl. The radioactivity of cell lysates was quantified by a liquid scintillation counter (Tri-Carb B3110TR; PerkinElmer, Waltham, MA) and the protein content was determined by a BCA Protein Assay Kit (Pierce Chemical, Rockford, IL). Cellular uptake was normalized by the total protein content in each well. In all studies, uptake in vector-transfected cells was performed at each data point and used to control for nonspecific substrate uptake and binding. Transporter-specific uptake was calculated by subtracting background uptake in vector-transfected cells from the uptake measured in transporter-expressing cells. Each data measurement was performed in triplicate in three different wells. Each experiment was repeated at least two times.
Transwell Studies in MDCK-hOCT2/hMATE1 Cells.
Transepithelial flux of atenolol or metformin across MDCK cell monolayers was determined as previously described (Yin et al., 2015). Briefly, control and hOCT2/hMATE1-expressing MDCK cells were seeded on Falcon cell culture inserts at a density of 2 × 105 cells/cm2. Transport experiments were performed 5 days after seeding in Transwell apparatuses. The integrity of the MDCK monolayer was verified by measuring transepithelial electrical resistance (TEER) using a Millicell-ERS system (EMD Millipore, Bedford, MA) before and after each experiment. Only data from monolayers with TEER values greater than 150 Ω • cm2 were accepted. In addition, proper formation of tight junctions was also verified by measurement of transepithelial flux of mannitol as described previously (Yin et al., 2015). After removing cell culture medium from both sides of the inserts, cells were preincubated at 37°C for 10 minutes in KRH buffer with the pH in the apical and basal chambers maintained at 6.0 and 7.4, respectively. Transwell transport studies were then initiated after aspirating the preincubation buffer from both apical and basal chambers. For apical-to-basal (A-to-B) transport, 2 ml of KRH buffer (pH 7.4) was first added to the basal chamber, and the transport was initiated by adding 0.8 ml of KRH buffer (pH 6.0) containing a radiolabeled substrate to the apical chamber. Likewise, for basal-to-apical (B-to-A) transport, 0.8 ml of KRH buffer (pH 6.0) was first added to the apical chamber and 2 ml of KRH buffer (pH 7.4) containing a radiolabeled substrate was added to the basal chamber. To measure time-dependent transcellular transport, an aliquot of the incubation medium (100 μl from the basal chamber, 50 μl from the apical chamber) in the receiving chamber was periodically collected and replaced with an equal volume of fresh buffer. For inhibition studies, cimetidine was applied to the basal, apical, or both chambers at the start of the Transwell study. At the end of the Transwell experiment, inserts were rinsed three times with ice-cold KRH buffer and cells were lysed with 1 M NaOH and then neutralized with 1 M HCl. Intracellular accumulation of the radiolabeled substrate was quantified by liquid scintillation counting and normalized to total protein content in cell lysate. Metformin and atenolol are not significantly metabolized in MDCK cells (Zhou et al., 2007; Yin et al., 2015). Experiments were performed in two to three individual Transwell apparatuses and repeated twice. Data were presented as mean ±S.D. of all apparatuses with acceptable TEER values.
Data Analysis.
Uptake studies in HEK-293 cells were performed in triplicate in different wells, whereas Transwell studies in MDCK cells were performed in two to three individual apparatuses. All experiments were repeated at least two times. Data were presented as mean ±S.D. of all pooled measurements (n ≥ 6). The IC50 values were obtained by nonlinear regression fitting of the uptake data to Log (inhibitor concentration) using a four-parameter function model in GraphPad Prism 6.0 using the equation: V = Bottom + (Top – Bottom) / [1 + (I/IC50)nH], where V is the rate of uptake in the presence of inhibitor, Bottom is the residual noninhibitable baseline value, Top is the rate of uptake in the absence of inhibitor, I is the inhibitor concentration, and nH is the Hill coefficient. For transport experiments, the apparent permeability (Papp) of compounds across cell monolayers was calculated as Papp = (dQ/dt) / (A*C0), where Q is the amount of compound transported over time t, A is the insert membrane surface area, and C0 is the initial compound concentration in the donor chamber. Statistical significance was determined using unpaired Student’s t test. A P value less than 0.05 was considered statistically significant.
Results
Inhibition of hOCT2 by Classic Inhibitors Is Highly Dependent on Substrate.
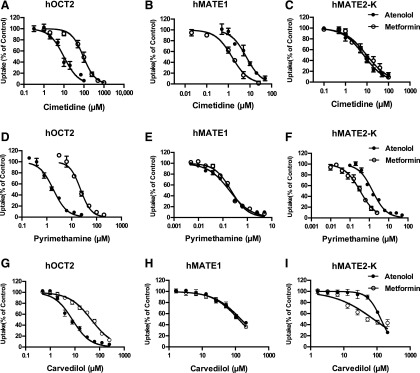
To determine if cimetidine, the classic inhibitor of renal OC secretion, exhibits substrate-dependent inhibition of hOCT2 and hMATE1/2-K, inhibition studies were conducted using two drug substrates, metformin and atenolol. Metformin is a widely used antidiabetic drug and a well-established probe substrate for hOCT2 and hMATE1/2-K, and atenolol is a major antihypertensive that was recently identified as an excellent substrate for these transporters (Yin et al., 2015). Two additional known drug inhibitors of these transporters, pyrimethamine (Kusuhara et al., 2011) and carvedilol (Zolk et al., 2009; Wittwer et al., 2013), were also tested. Inhibition assays of the two substrates were conducted side-by-side in control and transporter-expressing HEK-293 cells and the IC50 values were determined at a substrate concentration much lower than their Km values (see Supplemental Table 1). For hOCT2, substantial substrate-dependent inhibition was observed for all three tested inhibitors (Fig. 1, Table 1). Cimetidine and pyrimethamine were approximately 10-fold more potent toward hOCT2 when atenolol is used as the substrate. Carvedilol also showed 6-fold higher potency for hOCT2 when atenolol is used. In contrast, substrate-dependent inhibition was much less pronounced for hMATE1 and 2-K (Fig. 1, Table 1). Not all inhibitors showed substrate-dependent inhibition of hMATE transporters. Those that showed dependency had much smaller differences in IC50 (<5-fold) compared with hOCT2. Interestingly, atenolol is a more sensitive substrate to detect hOCT2 inhibition, whereas metformin appears to be a more sensitive substrate for hMATE1 and 2-K inhibition.
Fig. 1.
Inhibition of hOCT2, hMATE1, and hMATE2-K by cimetidine, pyrimethamine, and carvedilol using atenolol or metformin as the probe substrate. Cells were incubated in KRH buffer containing either atenolol (1 μM) or metformin (5.5 μM) for 2 minutes in the presence of an inhibitor at graded concentrations. Transporter-specific uptake was calculated by subtracting the uptake in vector-transfected HEK-293 cells and presented as percentage of the uptake determined in the absence of an inhibitor. All experiments were performed in triplicate and repeated two times. Data were presented as mean ±S.D. of all pooled measurements (n = 9).
TABLE 1 .
Cimetidine IC50 values for atenolol and metformin
Experiments were performed in triplicate and repeated two times. Data are presented as mean ± S.D. of all pooled measurements (n = 9).
| Inhibitor | Transporter | IC50 (μM) |
P Value | Fold Difference | |
|---|---|---|---|---|---|
| Atenolol | Metformin | ||||
| Cimetidine | hOCT2 | 9.9 ± 1.1 | 93.5 ± 1.1 | P < 0.001 | 9.4a |
| hMATE1 | 6.7 ± 1.2 | 1.5 ± 1.0 | P < 0.01 | 4.5b | |
| hMATE2-K | 7.1 ± 1.1 | 9.7 ± 1.3 | P = 0.057 | 1.4a | |
| Pyrimethamine | hOCT2 | 1.8 ± 0.2 | 22.9 ± 1.9 | P < 0.001 | 12.7a |
| hMATE1 | 0.18 ± 0.006 | 0.22 ± 0.01 | P < 0.01 | 1.2a | |
| hMATE2-K | 1.5 ± 0.07 | 0.3 ± 0.06 | P < 0.001 | 5.0b | |
| Carvedilol | hOCT2 | 7.9 ± 0.6 | 46.4 ± 0.4 | P < 0.001 | 5.9a |
| hMATE1 | 146.4 ± 0.9 | 124.2 ± 4.3 | P < 0.001 | 1.2b | |
| hMATE2-K | 131.2 ± 4.2 | 78.6 ± 12.5 | P < 0.001 | 1.7b | |
IC50 for metformin/IC50 for atenolol.
IC50 for atenolol/IC50 for metformin.
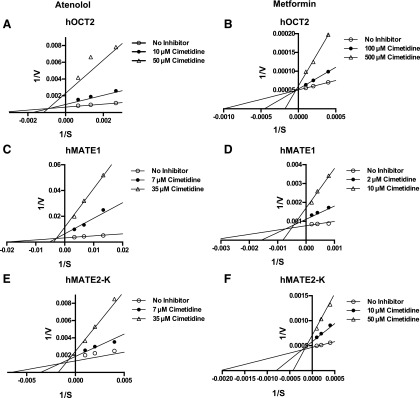
To examine the inhibition mechanisms of cimetidine toward hOCT2 and hMATE1/2-K, concentration-dependent uptake of atenolol or metformin was performed in the presence of different concentrations of cimetidine (0, IC50, and 5 × IC50). The complete kinetic data along with Lineweaver-Burk plots are also shown (Supplemental Figs. 1 and 2). A close-up view of the double-reciprocal plots at the lower x and y range (Fig. 2) indicates that cimetidine acted as a mixed-type inhibitor of hOCT2 for atenolol but became a competitive inhibitor when metformin was used. Cimetidine acted as a mixed-type inhibitor for both hMATE1 and 2-K regardless of the substrate used.
Fig. 2.
Effect of cimetidine inhibition on atenolol or metformin transport kinetics of hOCT2, hMATE1, and hMATE2-K. Concentration-dependent uptake of atenolol and metformin was measured in both transporter-expressing and vector-transfected (control) HEK-293 cells after a 2-minute incubation in the presence of cimetidine at specified concentration (0, IC50, and 5 × IC50). Transporter-specific uptake was obtained by subtracting the uptake in control cells from the uptake in transporter-expressing cells. All experiments were performed in triplicate and repeated twice. The mean values of the kinetic data were used to transform data into Lineweaver-Burk plots. Although all kinetic data points were used for the generation of Lineweaver-Burk plots, only the close-up view at lower x and y ranges was presented to clearly show the intersection point. The kinetic data and corresponding Lineweaver-Burk plots with all data points are provided in Supplemental Figs. 1 and 2.
Substrate-Dependent Inhibition Results in Different Prediction of DDI Potential for hOCT2.
According to the latest FDA draft guidance for hOCT2-mediated DDI studies, an in vivo DDI study is recommended for a compound if its unbound Cmax/IC50 is greater than 0.1 (FDA, 2012). Cimetidine is 20% protein bound in the plasma, and the reported unbound Cmax after a typical 400-mg oral dose is around 8 μM (Somogyi et al., 1980, 1989). Our study showed that the IC50 values of cimetidine toward hOCT2 differ by 10-fold when different drug substrates are used. To examine if substrate-dependent inhibition leads to different recommendations for in vivo DDI assessment, the unbound Cmax/IC50 values of cimetidine were calculated for atenolol and metformin. In addition, the reported cimetidine IC50 toward MPP+ (Ito et al., 2012), a recommended in vitro probe for hOCT2, was also used for prediction. Very different recommendations for cimetidine in vivo DDI study were obtained for hOCT2 with a “Yes” for atenolol, a “No” for MPP+, and a borderline value for metformin (Table 2). These data demonstrated that substrate-dependent inhibition can significantly impact the outcome of DDI prediction for hOCT2. Compared with hOCT2, cimetidine is generally a more potent inhibitor for hMATE1/2-K and showed less pronounced substrate-dependent inhibition (Table 1). The unbound Cmax/IC50 values of cimetidine are all greater than 0.1 for hMATE1/2-K, resulting in a consensus in recommendations for all three substrates.
TABLE 2 .
Prediction of hOCT2 DDIs following FDA decision tree
| Atenolol | Metformin | MPP+ | |
|---|---|---|---|
| hOCT2 | |||
| IC50 | 9.9 | 93.5 | 146b |
| Cimetidine unbound Cmax/IC50a | 0.8 | 0.09 | 0.05 |
| Recommendation for in vivo DDI study | Yes | Yes/No | No |
| hMATE1 | |||
| IC50 | 6.7 | 1.5 | 2.7b |
| Cimetidine unbound Cmax/IC50a | 1.2 | 5.3 | 3.0 |
| Recommendation for in vivo DDI study | Yes | Yes | Yes |
| hMATE2-K | |||
| IC50 | 7.1 | 9.7 | 4.0b |
| Cimetidine unbound Cmax/IC50 a | 1.1 | 0.8 | 2.0 |
| Recommendation for in vivo DDI study | Yes | Yes | Yes |
Cimetidine unbound Cmax = 8 μM was used for calculation. Value is from Brunton LL, Chabner BA, Knollman BC eds (2011) Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 12th ed, McGraw-Hill Medical, New York.
Value is from Ito et al. (2012).
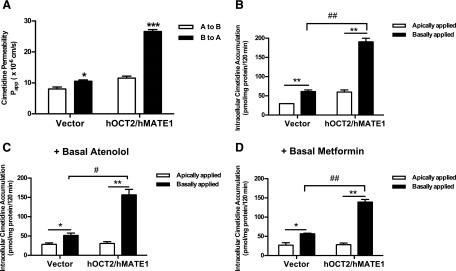
Cimetidine Applied in the Basal Chamber Inhibited B-to-A Flux of Atenolol and Metformin in hOCT2/hMATE1-Expressing MDCK Monolayer.
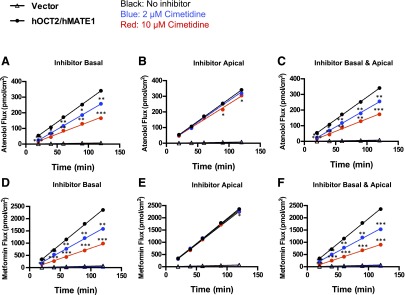
In the human kidney proximal tubule, OC secretion is sequentially mediated by basolateral hOCT2 and apical hMATE1/2-K. To determine the overall effect of cimetidine on hOCT2/hMATE-mediated OC secretion, we examined the inhibitory effect of cimetidine on basal-to-apical (B-to-A) flux of radiolabeled atenolol (1 μM) and metformin (5.5 μM) using an hOCT2/hMATE1 double-transfected MDCK cell line previously established in our laboratory (Yin et al., 2015). Two clinically relevant concentrations of cimetidine (2 and 10 μM) were chosen on the basis of their IC50 values toward hOCT2 and hMATE1 (Table 1). The substrate concentrations used in our study were also within the reported clinical concentration range of atenolol and metformin (Goodman et al., 2011). To evaluate whether different membrane access of the inhibitor plays a role on its inhibitory effect, cimetidine was applied to the basal, apical, or both chambers in the Transwell studies. As expected, B-to-A flux of atenolol and metformin in the absence of cimetidine was much greater in hOCT2/hMATE1-transfected cells than in vector-transfected cells (Fig. 3). When applied to the basal or both chambers, cimetidine dose dependently inhibited B-to-A flux of atenolol and metformin (Fig. 3, A, C, D, F). Surprisingly, when applied to the apical chamber, cimetidine at the concentrations used had little effect on B-to-A flux of either atenolol or metformin (Fig. 3, B and E).
Fig. 3.
Effects of cimetidine on B-to-A flux of atenolol and metformin in hOCT2/hMATE1-expressing MDCK cells. Cells were incubated in KRH buffer containing atenolol (1 μM) or metformin (5.5 μM) in the basal chamber. The pH in the apical and basal chambers was maintained at 6.0 and 7.4, respectively. B-to-A flux of atenolol and metformin was measured in the absence or presence of 2 μM or 10 μM of cimetidine. Cimetidine was added to the basal (A, D), apical (B, E), or both chambers (C, F). At various time points, 50 μl of sample was taken from the apical chamber and replenished with an equal volume of fresh KRH buffer. Experiments were performed in two to three individual Transwell apparatuses and repeated twice. Data were presented as mean ±S.D. (n = 6) of all apparatuses with acceptable TEER values. The B-to-A flux of atenolol and metformin in MDCK-hOCT2/hMATE1 cells in the presence of cimetidine was compared with that in the absence of cimetidine. ***P < 0.001, **P < 0.01, *P < 0.05 indicates significant difference from the no inhibitor control.
Cimetidine Differentially Impacted Intracellular Drug Accumulation in a Substrate-Dependent Manner.
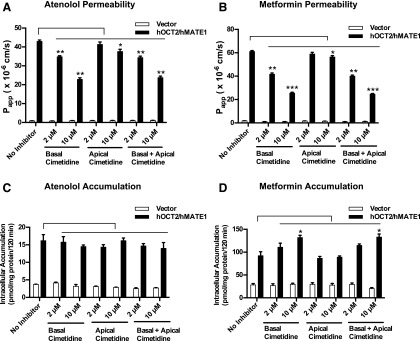
Transporter-mediated drug accumulation within renal tubular cells is a frequent cause underlying drug-induced nephrotoxicity (DeGorter et al., 2012; Morrissey et al., 2013). Depending on whether the interaction occurs at the site of basolateral uptake or apical efflux, an inhibitor can have very different effects on intracellular drug accumulation. Our inhibition data (Table 1 and Fig. 1) showed that cimetidine is an equally potent inhibitor of hOCT2 and hMATE1 when atenolol is the substrate but becomes an hMATE1-selective inhibitor if metformin is used. These data suggest that although cimetidine reduces tubular secretion for hOCT2/hMATE substrates, its effect on intracellular drug accumulation may be substrate-dependent. Indeed, when the apparent permeability was calculated, cimetidine applied to the basal or both chambers showed a clear inhibitory effect on B-to-A permeability of atenolol and metformin (Fig. 4, A and B). When the intracellular drug accumulation was measured in the absence of cimetidine at the end of the Transwell study, intracellular drug accumulation was significantly higher in hOCT2/hMATE1 cells than in vector-transfected cells (Fig. 4, C and D). Interestingly, cimetidine, applied to either basal or both chambers, had no effect on intracellular atenolol accumulation (Fig. 4C), which is consistent with its similar inhibition potencies for hOCT2 and hMATE1 when atenolol is used as the substrate (Table 1). In contrast, cimetidine dose dependently increased the intracellular accumulation of metformin when applied to basal or both chambers (Fig. 4D), suggesting a predominant inhibition of the apical hMATE1 when metformin is used as the substrate (Table 1). These data, obtained from a cell culture model of renal OC secretion, suggest that inhibitors of tubular secretion can impact intracellular drug accumulation in a substrate-dependent manner and the major substrate-inhibitor interacting site for a given inhibitor can shift between the apical and basolateral OC transporters depending on the substrate used.
Fig. 4.
Effect of cimetidine inhibition on permeability and intracellular accumulation of atenolol and metformin in MDCK-hOCT2/hMATE1 cells. The B-to-A permeability of atenolol (A) and metformin (B) was calculated using the equation described in Materials and Methods. Intracellular accumulation of atenolol (C) and metformin (D) was measured at the end of the Transwell study in the absence or presence of cimetidine (2 or 10 μM). Cimetidine was added to basal, apical, or both chambers at the start of the Transwell experiment. ***P < 0.001, **P < 0.01, *P < 0.05 indicates significant difference from no inhibitor control. Experiments were performed in two to three individual Transwell apparatuses and repeated twice. Data were presented as mean ±S.D. (n = 6) of all apparatuses with acceptable TEER values.
Basally Applied Cimetidine Accumulated in MDCK-hOCT2/MATE1 Cells.
An intriguing observation in our studies is that the effect of cimetidine on hOCT2/hMATE1-mediated substrate flux and intracellular accumulation is only manifested when the inhibitor is applied to the basal chamber. To explore what could be the possible cause of the differential effect of cimetidine on substrate transport when placed on a different side of MDCK monolayer, we measured and compared monolayer permeability and intracellular accumulation of 3H-labeled cimetidine under several experimental conditions. Cimetidine showed a minor directional transport in vector-transfected control cells. Consistent with being a reported substrate for hOCT2 and hMATE1 (Tahara et al., 2005; Tanihara et al., 2007), cimetidine exhibited a B-to-A/A-to-B ratio of 2.3 in hOCT2/hMATE1-transfected MDCK cells (Fig. 5A), suggesting the role of hOCT2/hMATE1 in its renal secretion. Importantly, intracellular accumulation of cimetidine (10 μM) is much greater when it was applied to the basal side than the apical side in MDCK-hOCT2/hMATE1 cells (Fig. 5B). Similar results were also observed in the double-transfected cells when cold atenolol (1 μM) or metformin (5.5 μM) was added in the basal chamber (Fig. 5, C and D) to simulate the experimental conditions used in the inhibition studies in Figs. 3 and 4. In vector-transfected control cells, cimetidine intracellular accumulation was also slightly higher when applied basally, which probably results from its uptake by a low level of endogenously expressed canine Oct2 in MDCK cells (Shu et al., 2001). These data suggest that under the experimental condition, when the acidic pH (6.0) in the apical chamber drives hMATE1-mediated substrate efflux from the cells, the configuration of the hMATE1 transporter may allow the substrate/inhibitor binding site(s) to be accessible only from the intracellular side. The lack of an inhibitory effect of apically applied cimetidine on hMATE1 appears to be attributable to a low intracellular accumulation of the inhibitor (Fig. 5, B–D).
Fig. 5.
Cimetidine permeability and intracellular accumulation in MDCK-hOCT2/hMATE1 cells. Vector- and hOCT2/hMATE1-transfected MDCK cells growing on inserts were incubated in KRH buffer with [3H]cimetidine (10 μM) added to either apical (pH 6.0) or basal chamber (pH 7.4). An aliquot of buffer in the receiving chamber was sampled periodically up to 120 minutes. (A) Cimetidine permeability in the B-to-A and A-to-B directions was measured and compared (***P < 0.001, *P < 0.05). (B–D) Intracellular accumulation of [3H]cimetidine (10 μM) after application to basal or apical chamber alone or with cold atenolol (1 μM) or metformin (5.5 μM) added in the basal chamber. **P < 0.01, *P < 0.05 indicates significantly higher accumulation when cimetidine was applied to the basal chamber compared with the apical chamber. ##P < 0.01, #P < 0.05 indicate significantly higher accumulation in hOCT2/hMATE1 cells compared with vector control. Experiments were performed in two to three individual Transwell apparatuses and repeated twice. Data were presented as mean ±S.D. (n = 6) of all apparatuses with acceptable TEER values.
Discussion
In this study, we investigated the impact of substrate-dependent inhibition on transporter-mediated drug interaction and accumulation by focusing on the hOCT2/hMATE-mediated renal secretion pathway. Several novel findings were obtained. First, our data showed that the choice of a probe substrate has a larger impact on hOCT2 than hMATEs in their interactions with inhibitors. Secondly, atenolol is a more sensitive substrate than metformin for evaluating hOCT2-inhibitor interactions in vitro. Further, substrate-dependent inhibition can complicate in vitro-to-in vivo DDI prediction for hOCT2 and lead to different recommendations for in vivo DDI studies. Thirdly, we showed that although cimetidine inevitably reduced hOCT2/hMATE1-mediated transepithelial flux of a drug substrate, its impact on intracellular drug accumulation, thus toxicity, can be unpredictable owing to substrate-dependent shifting of the major interaction site between apical and basolateral transporters. Lastly, our data provided the first evidence that cimetidine concentrations in the basolateral side (i.e., blood) may be more relevant than those in the apical side (i.e., filtrate) for estimating its inhibitory effects on renal hOCT2 and hMATE1/2-K transporters.
In our study, metformin and atenolol were used as the substrates to probe potential substrate-dependent inhibition of renal OC transporters. Our data showed that cimetidine, as well as pyrimethamine and carvedilol, are much more potent inhibitors of hOCT2 when atenolol is used as the substrate (Fig. 1, Table 1). In contrast, substrate-dependent inhibition was less pronounced for hMATE1 and 2-K (Fig. 1, Table 1). These data suggest that the choice of a probe substrate can greatly influence the inhibition potency of a potential inhibitor (e.g., an NME), especially for hOCT2, leading to very different recommendations for in vivo DDI studies as exemplified in Table 2. Accordingly, it may be necessary to evaluate two or more probe substrates in in vitro studies for drug transporters that display substantial substrate-dependent inhibition. To mitigate the risk of false-negative predictions, a sensitive substrate, which consistently produces lower IC50 or Ki values for a range of known inhibitors, should be included. Lastly, direct in vitro DDI analysis should be performed using a commonly comedicated drug as the substrate if frequent coadministration is anticipated (e.g., two antihypertensive drugs used simultaneously).
In the current International Transporter Consortium and FDA recommendations, MPP+ and metformin are suggested as the probe substrates for assessing the inhibition potential of an NME toward hOCT2 (Giacomini et al., 2010; FDA, 2012; Hillgren et al., 2013). Belzer et al. (2013) previously reported that structurally diverse hOCT2 inhibitors were generally more effective (up to 9- to10-fold) for inhibition of metformin than of MPP+, suggesting that metformin is a more sensitive hOCT2 substrate than MPP+. Our data revealed that atenolol is an even more sensitive (up to 10-fold) hOCT2 substrate than metformin. Like metformin, atenolol is minimally metabolized in vivo and exclusively eliminated unchanged by the kidney. With a LogD7.4 of –2.0, atenolol is highly hydrophilic with minimal passive membrane diffusion and nonspecific binding. We recently showed that atenolol is an excellent hOCT2 substrate and also has a higher apparent affinity for hOCT2 than metformin (Supplemental Table 1) (Yin et al., 2015). These characteristics bestow on atenolol clinical relevance as a probe substrate more sensitive than metformin for assessing hOCT2 inhibitors in vitro. However, available DDI data did not suggest a positive in vivo interaction for atenolol and cimetidine (Houtzagers et al., 1982; Kirch et al., 1982; Mutschler et al., 1984). The total renal clearance of atenolol (∼168 ml/min) is only 1.4-fold of its glomerular filtration clearance (∼120 ml/min), suggesting that active tubular secretion only contributes to a minor (∼30%) part of atenolol total clearance. This makes it difficult to observe clinically significant pharmacokinetic interactions of atenolol in vivo. In contrast, metformin has a total renal clearance of ∼454 ml/min with a much larger (75%) contribution from tubular secretion. Thus, to assess hOCT2-mediated DDIs in vivo, metformin may be more revealing than atenolol for detection of systemic exposure or renal clearance changes caused by inhibition of tubular secretion.
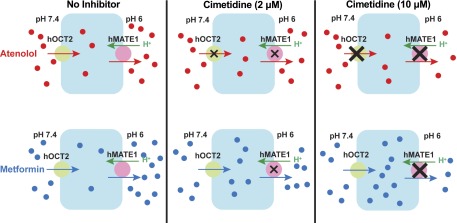
Drug-induced nephrotoxicity is a major concern during drug discovery and development (Morrissey et al., 2013). Renal transporter–mediated DDIs may not only affect the systemic exposure of a victim drug but could also have profound toxicological effect on renal proximal tubule cells. Inhibition of a basolateral uptake transporter is generally nephron-protective as it reduces transporter-mediated drug accumulation within renal tubular cells. Indeed, coadministration of probenecid to inhibit hOAT1-mediated uptake of cidofovir is used clinically to prevent intrarenal accumulation and nephrotoxicity of cidofovir (Cundy et al., 1995; Cihlar et al., 1999). Inhibition of hOCT2-mediated renal cisplatin uptake is also being explored as a strategy to ameliorate cisplatin nephrotoxicity (Filipski et al., 2009; Sprowl et al., 2013; Pabla et al., 2015). On the other hand, inhibition of apical efflux transporters diminishes drug exit from renal cells, which may lead to increased drug accumulation and nephrotoxicity (Yonezawa and Inui, 2011). Therefore, knowing the major site of interaction (i.e., apical versus basolateral) is critical for predicting a nephron-toxic or a nephron-protective effect of a potential inhibitor. In the case of cimetidine, inhibition of hOCT2-mediated uptake was historically thought to underlie its numerous clinical DDIs (Li et al., 2006; Giacomini et al., 2010). However, on the basis of the in vitro Ki or IC50 values of cimetidine determined with metformin and other experimental substrates, Ito et al. (2012) suggested that inhibition of apical hMATEs, but not basolateral hOCT2, is the major mechanism underlying clinically observed renal DDIs with cimetidine. Here, our data revealed that cimetidine is an hMATE-selective inhibitor when metformin is the substrate but acts as an equally potent inhibitor for hOCT2 and hMATE1 when atenolol is the substrate (Table 1). These data suggest that depending on the substrate (i.e., the victim drug), the site of cimetidine interaction is changeable between the apical and basolateral OC transporters. Consequently, the effect of an inhibitor on intracellular accumulation is also substrate-dependent, leading to either a nephron-toxic or a nephron-protective effect. This concept, supported by our Transwell studies on atenolol and metformin (Figs. 3 and 4), is illustrated in Fig. 6. Our studies demonstrated that the effect of a transport inhibitor on intracellular drug accumulation is dynamic and substrate-dependent; and information obtained with one inhibitor-substrate pair may not be extrapolated to other drug combinations containing the same inhibitor.
Fig. 6.
Hypothesized effect and site of interaction for cimetidine on renal tubular secretion and intracellular accumulation of atenolol and metformin. For atenolol, cimetidine inhibits hOCT2 and hMATE1 with equal potencies. At clinically relevant concentrations, cimetidine dose dependently reduces atenolol tubular secretion but has no effect on intracellular accumulation. For metformin, cimetidine mainly inhibits hMATE1. Cimetidine not only reduces metformin tubular secretion but also increases its intracellular accumulation in a dose-dependent manner.
To predict transporter-mediated renal DDIs, the unbound plasma concentration of the inhibitor is often used (Giacomini et al., 2010; FDA, 2012). An intriguing observation in our study is that although cimetidine inhibited hMATE1-mediated atenolol or metformin uptake in HEK-293 cells, it had no inhibitory effect on hMATE1-mediated substrate efflux in Transwell studies in MDCK cells when applied apically (Figs. 3 and 4). These seemingly paradoxical observations may be explained by the unique transport mechanism of the MATE transporters, which function as proton/OC antiporters and simultaneously transport proton in the opposite direction of OC substrate (Otsuka et al., 2005). For these transporters, proton binding always occurs on the opposite side of OC substrate binding. In the uptake studies in HEK-293 cells, an extracellular pH of 8.0 was used to create an outwardly directed proton gradient to drive cellular uptake of the OC substrate. Proton binds intracellularly whereas substrate/inhibitor binds from the extracellular side, which allows the measurement of the inhibitory effect of cimetidine in these studies. In contrast, in the Transwell studies in MDCK cells, the pH in the basal and apical chambers was maintained, respectively, at 7.4 and 6.0 to mimic the physiologic environment in the nephron. The acidic pH in the apical compartment creates an inwardly directed proton gradient to drive hMATE1-mediated substrate efflux from MDCK cells. Under this condition, proton binds from the outside whereas substrate/inhibitor binds from the inside of the MDCK cells (see Fig. 6). The configuration of the hMATE1 transporter may allow the substrate/inhibitor binding site(s) to be accessible only from the intracellular side. As apically applied cimetidine cannot diffuse into MDCK cells owing to its low passive diffusion (LogP = 0.4), the inhibitor may not gain access to the intracellularly orientated binding site under this condition. Thus, apically applied cimetidine lacks an effect on hMATE1 whereas basally applied cimetidine, after being transported into cells by hOCT2, can exert an inhibitory effect on hMATE1. This hypothesis is in agreement with data presented in Fig. 5 demonstrating a much higher intracellular accumulation of cimetidine when applied to the basal compartment. Together, our studies suggest that plasma and intracellular concentrations of cimetidine, compared with its filtrate or urine concentrations, may be more relevant for assessing hOCT2/hMATE1-mediated cimetidine-drug interactions in the kidney.
In summary, we demonstrated that the choice of a probe substrate can greatly impact the prediction of hOCT2-mediated renal drug interaction and accumulation. Our findings revealed the complex and dynamic nature of substrate-inhibitor interactions in multispecific drug transporters and highlighted the importance of considering substrate-dependent inhibition in assessing transporter-mediated renal drug interaction, accumulation, and toxicity.
Acknowledgments
The authors thank Dr. Weibin Zha for helping to design Fig. 6.
Abbreviations
- Cmax
maximal plasma concentration
- DDI
drug-drug interaction
- FDA
Food and Drug Administration
- HEK
human embryonic kidney
- hMATE
human multidrug and toxin extrusion protein
- hOAT
human organic anion transporter
- hOCT
human organic cation transporter
- KRH
Krebs-Ringer-Henseleit
- MDCK
Madin-Darby canine kidney
- MPP+
1-methyl-4-phenylpyridinium
- NME
new molecular entity
- OC
organic cation
- TEER
transepithelial electrical resistance
Authorship Contributions
Participated in research design: Yin, Wang.
Conducted experiments: Yin, Duan.
Performed data analysis: Yin, Wang.
Wrote or contributed to the writing of the manuscript: Yin, Wang.
Footnotes
This study was supported by the National Institutes of Health National Institute of General Medical Sciences [Grant R01 GM066233] and the National Center for Advancing Translational Sciences [Grant TL1 TR000422]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Belzer M, Morales M, Jagadish B, Mash EA, Wright SH. (2013) Substrate-dependent ligand inhibition of the human organic cation transporter OCT2. J Pharmacol Exp Ther 346:300–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer KL, Keppler D, Hoffmaster KA, Bow DA, Cheng Y, Lai Y, Palm JE, Stieger B, Evers R, International Transporter Consortium (2013) In vitro methods to support transporter evaluation in drug discovery and development. Clin Pharmacol Ther 94:95–112. [DOI] [PubMed] [Google Scholar]
- Cihlar T, Lin DC, Pritchard JB, Fuller MD, Mendel DB, Sweet DH. (1999) The antiviral nucleotide analogs cidofovir and adefovir are novel substrates for human and rat renal organic anion transporter 1. Mol Pharmacol 56:570–580. [DOI] [PubMed] [Google Scholar]
- Cundy KC, Petty BG, Flaherty J, Fisher PE, Polis MA, Wachsman M, Lietman PS, Lalezari JP, Hitchcock MJ, Jaffe HS. (1995) Clinical pharmacokinetics of cidofovir in human immunodeficiency virus-infected patients. Antimicrob Agents Chemother 39:1247–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGorter MK, Xia CQ, Yang JJ, Kim RB. (2012) Drug transporters in drug efficacy and toxicity. Annu Rev Pharmacol Toxicol 52:249–273. [DOI] [PubMed] [Google Scholar]
- Duan H, Hu T, Foti RS, Pan Y, Swaan PW, Wang J. (2015) Potent and Selective Inhibition of Plasma Membrane Monoamine Transporter by HIV Protease Inhibitors. Drug Metab Dispos 43:1773–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan H, Wang J. (2010) Selective transport of monoamine neurotransmitters by human plasma membrane monoamine transporter and organic cation transporter 3. J Pharmacol Exp Ther 335:743–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA (2012) Guidance for Industry Drug Interaction Studies. http://wwwfdagov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm292362pdf.
- Filipski KK, Mathijssen RH, Mikkelsen TS, Schinkel AH, Sparreboom A. (2009) Contribution of organic cation transporter 2 (OCT2) to cisplatin-induced nephrotoxicity. Clin Pharmacol Ther 86:396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, Dahlin A, Evers R, Fischer V, Hillgren KM, et al. International Transporter Consortium (2010) Membrane transporters in drug development. Nat Rev Drug Discov 9:215–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman LS, Brunton LL, Blumenthal DK, Murri N, and Hilal-Dandan R (2011) Appendex II. Design and Optimization of Dosage Regimens; Pharmacokinetic Data, in Goodman & Gilman’s The Pharmacological Basis of Therapeutics 12th ed (Brunton LL, Chabner BA, Knollman BC eds) pp 1787–1888, McGraw-Hill Medical, New York. [Google Scholar]
- Hillgren KM, Keppler D, Zur AA, Giacomini KM, Stieger B, Cass CE, Zhang L, International Transporter Consortium (2013) Emerging transporters of clinical importance: an update from the International Transporter Consortium. Clin Pharmacol Ther 94:52–63. [DOI] [PubMed] [Google Scholar]
- Houtzagers JJ, Streurman O, Regårdh CG. (1982) The effect of pretreatment with cimetidine on the bioavailability and disposition of atenolol and metoprolol. Br J Clin Pharmacol 14:67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S, Kusuhara H, Yokochi M, Toyoshima J, Inoue K, Yuasa H, Sugiyama Y. (2012) Competitive inhibition of the luminal efflux by multidrug and toxin extrusions, but not basolateral uptake by organic cation transporter 2, is the likely mechanism underlying the pharmacokinetic drug-drug interactions caused by cimetidine in the kidney. J Pharmacol Exp Ther 340:393–403. [DOI] [PubMed] [Google Scholar]
- Izumi S, Nozaki Y, Maeda K, Komori T, Takenaka O, Kusuhara H, Sugiyama Y. (2015) Investigation of the impact of substrate selection on in vitro organic anion transporting polypeptide 1B1 inhibition profiles for the prediction of drug-drug interactions. Drug Metab Dispos 43:235–247. [DOI] [PubMed] [Google Scholar]
- Kenworthy KE, Bloomer JC, Clarke SE, Houston JB. (1999) CYP3A4 drug interactions: correlation of 10 in vitro probe substrates. Br J Clin Pharmacol 48:716–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirch W, Spahn H, Köhler H, Ohnhaus EE, Mutschler E. (1982) Interaction of metoprolol, propranolol and atenolol with concurrent administration of cimetidine. Klin Wochenschr 60:1401–1407. [DOI] [PubMed] [Google Scholar]
- Kusuhara H, Ito S, Kumagai Y, Jiang M, Shiroshita T, Moriyama Y, Inoue K, Yuasa H, Sugiyama Y. (2011) Effects of a MATE protein inhibitor, pyrimethamine, on the renal elimination of metformin at oral microdose and at therapeutic dose in healthy subjects. Clin Pharmacol Ther 89:837–844. [DOI] [PubMed] [Google Scholar]
- Li M, Anderson GD, Wang J. (2006) Drug-drug interactions involving membrane transporters in the human kidney. Expert Opin Drug Metab Toxicol 2:505–532. [DOI] [PubMed] [Google Scholar]
- Martínez-Guerrero LJ, Wright SH. (2013) Substrate-dependent inhibition of human MATE1 by cationic ionic liquids. J Pharmacol Exp Ther 346:495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masereeuw R, Russel FG. (2001) Mechanisms and clinical implications of renal drug excretion. Drug Metab Rev 33:299–351. [DOI] [PubMed] [Google Scholar]
- Morrissey KM, Stocker SL, Wittwer MB, Xu L, Giacomini KM. (2013) Renal transporters in drug development. Annu Rev Pharmacol Toxicol 53:503–529. [DOI] [PubMed] [Google Scholar]
- Motohashi H, Inui K. (2013) Organic cation transporter OCTs (SLC22) and MATEs (SLC47) in the human kidney. AAPS J 15:581–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutschler E, Spahn H, Kirch W. (1984) The interaction between H2-receptor antagonists and beta-adrenoceptor blockers. Br J Clin Pharmacol 17 (Suppl 1):51S–57S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka M, Matsumoto T, Morimoto R, Arioka S, Omote H, Moriyama Y. (2005) A human transporter protein that mediates the final excretion step for toxic organic cations. Proc Natl Acad Sci USA 102:17923–17928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabla N, Gibson AA, Buege M, Ong SS, Li L, Hu S, Du G, Sprowl JA, Vasilyeva A, Janke LJ, et al. (2015) Mitigation of acute kidney injury by cell-cycle inhibitors that suppress both CDK4/6 and OCT2 functions. Proc Natl Acad Sci USA 112:5231–5236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu Y, Bello CL, Mangravite LM, Feng B, Giacomini KM. (2001) Functional characteristics and steroid hormone-mediated regulation of an organic cation transporter in Madin-Darby canine kidney cells. J Pharmacol Exp Ther 299:392–398. [PubMed] [Google Scholar]
- Somogyi AA, Hovens CM, Muirhead MR, Bochner F. (1989) Renal tubular secretion of amiloride and its inhibition by cimetidine in humans and in an animal model. Drug Metab Dispos 17:190–196. [PubMed] [Google Scholar]
- Somogyi A, Rohner HG, Gugler R. (1980) Pharmacokinetics and bioavailability of cimetidine in gastric and duodenal ulcer patients. Clin Pharmacokinet 5:84–94. [DOI] [PubMed] [Google Scholar]
- Sprowl JA, van Doorn L, Hu S, van Gerven L, de Bruijn P, Li L, Gibson AA, Mathijssen RH, Sparreboom A. (2013) Conjunctive therapy of cisplatin with the OCT2 inhibitor cimetidine: influence on antitumor efficacy and systemic clearance. Clin Pharmacol Ther 94:585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahara H, Kusuhara H, Endou H, Koepsell H, Imaoka T, Fuse E, Sugiyama Y. (2005) A species difference in the transport activities of H2 receptor antagonists by rat and human renal organic anion and cation transporters. J Pharmacol Exp Ther 315:337–345. [DOI] [PubMed] [Google Scholar]
- Tanihara Y, Masuda S, Sato T, Katsura T, Ogawa O, Inui K. (2007) Substrate specificity of MATE1 and MATE2-K, human multidrug and toxin extrusions/H(+)-organic cation antiporters. Biochem Pharmacol 74:359–371. [DOI] [PubMed] [Google Scholar]
- VandenBrink BM, Foti RS, Rock DA, Wienkers LC, Wahlstrom JL. (2012) Prediction of CYP2D6 drug interactions from in vitro data: evidence for substrate-dependent inhibition. Drug Metab Dispos 40:47–53. [DOI] [PubMed] [Google Scholar]
- Wittwer MB, Zur AA, Khuri N, Kido Y, Kosaka A, Zhang X, Morrissey KM, Sali A, Huang Y, Giacomini KM. (2013) Discovery of potent, selective multidrug and toxin extrusion transporter 1 (MATE1, SLC47A1) inhibitors through prescription drug profiling and computational modeling. J Med Chem 56:781–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J, Duan H, Shirasaka Y, Prasad B, Wang J. (2015) Atenolol renal secretion is mediated by human organic cation transporter 2 and multidrug and toxin extrusion proteins. Drug Metab Dispos 43:1872–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonezawa A, Inui K. (2011) Importance of the multidrug and toxin extrusion MATE/SLC47A family to pharmacokinetics, pharmacodynamics/toxicodynamics and pharmacogenomics. Br J Pharmacol 164:1817–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan R, Madani S, Wei XX, Reynolds K, Huang SM. (2002) Evaluation of cytochrome P450 probe substrates commonly used by the pharmaceutical industry to study in vitro drug interactions. Drug Metab Dispos 30:1311–1319. [DOI] [PubMed] [Google Scholar]
- Zhang L, Huang SM, Lesko LJ. (2011) Transporter-mediated drug-drug interactions. Clin Pharmacol Ther 89:481–484. [DOI] [PubMed] [Google Scholar]
- Zhou M, Xia L, Wang J. (2007) Metformin transport by a newly cloned proton-stimulated organic cation transporter (plasma membrane monoamine transporter) expressed in human intestine. Drug Metab Dispos 35:1956–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolk O, Solbach TF, König J, Fromm MF. (2009) Structural determinants of inhibitor interaction with the human organic cation transporter OCT2 (SLC22A2). Naunyn Schmiedebergs Arch Pharmacol 379:337–348. [DOI] [PubMed] [Google Scholar]