Abstract
In vivo, guanine moieties in DNA, RNA, guanine nucleotides, or guanosine or guanine per se can undergo nitration (for example, by peroxynitrite) or hydroxylation (for example, by superoxide anion) on position 8 of the purine ring. Subsequent catabolism of these modified biomolecules leads to the production of a diverse group of 8-nitro, 8-amino, and 8-hydroxy guanosine and guanine compounds. Indeed, studies suggest the in vivo existence of 8-nitroguanosine, 8-nitroguanine, 8-aminoguanosine, 8-aminoguanine, 8-hydroxyguanosine, 8-hydroxy-2′-deoxyguanosine, and 8-hydroxyguanine. Since a multitude of these compounds exist in vivo, and since the renal effects of 8-substituted guanosine and guanine compounds are entirely unknown, we examined the effects of guanosine, guanine, 8-nitroguanosine, 8-nitroguanine, 8-hydroxyguanosine, 8-hydroxyguanine, 8-hydroxy-2′-deoxyguanosine, 8-aminoguanosine, and 8-aminoguanine (33.5 µmol/kg/min; intravenous infusion for 115 minutes) on excretion of sodium, potassium, and glucose in rats. Guanosine, 8-nitroguanosine, and 8-hydroxy-2′-deoxyguanosine had minimal natriuretic activity. Guanine, 8-nitroguanine, 8-hydroxyguanosine, and 8-hydroxyguanine had moderate natriuretic activity (increased sodium excretion by 9.4-, 7.8-, 7.1-, and 8.6-fold, respectively). In comparison with all other compounds, 8-aminoguanosine and 8-aminoguanine were highly efficacious and increased sodium excretion by 26.6- and 17.2-fold, respectively, exceeding that of a matched dose of amiloride (13.6-fold increase). 8-Aminoguanosine and 8-aminoguanine also increased glucose excretion by 12.1- and 12.2-fold, respectively, and decreased potassium excretion by 69.1 and 71.0%, respectively. Long-term radiotelemetry studies demonstrated that oral 8-aminoguanosine and 8-aminoguanine (5 mg/kg/day) suppressed deoxycorticosterone/salt-induced hypertension. These experiments demonstrate that some naturally occurring 8-substitued guanosine and guanine compounds, particularly 8-aminoguanosine and 8-aminoguanine, are potent and efficacious potassium-sparing diuretics/natriuretics that may represent a novel class of antihypertensive diuretics.
Introduction
Peroxynitrite (ONOO−) is formed in vivo from the diffusion-controlled reaction between superoxide anion (O2·−) and nitric oxide (NO) (Carballal et al., 2014). ONOO− is a highly reactive nitrogen species (RNS) that can mediate nitration (i.e., insertion of NO2) of a number of endogenous compounds, including those containing a guanine moiety (Yermilov et al., 1995; Szabo and Ohshima, 1997; Ohshima et al., 2006). In this regard, ONOO− nitrates guanine moieties in position 8 of the purine ring to produce 8-nitroguanine units in DNA, RNA, and the guanine nucleotide pool (Yermilov et al., 1995; Szabo and Ohshima, 1997; Ohshima et al., 2006). It is also conceivable that free guanine per se could be subjected to nitration in the 8 position. In addition to RNS-mediated modification of guanine-containing compounds, reactive oxygen species such as O2·− can also modify position 8 of guanine moieties by inserting a hydroxyl functional group (Szabo and Ohshima, 1997; Misiaszek et al., 2004).
After modification of guanine moieties by RNS or reactive oxygen species, subsequent catabolism of RNA, DNA, and the guanine nucleotide pool would release 8-nitroguanosine, 8-nitro-2′-deoxyguanosine, 8-hydroxyguanosine, and 8-hydroxy-2′-deoxyguanosine. Theoretically, reduction of 8-nitro groups could yield 8-aminoguanosine and 8-amino-2′-deoxyguanosine, and purine nucleoside phosphorylase (PNPase) can convert such compounds to 8-aminoguanine (Osborne and Barton, 1986). PNPase might also convert 8-nitroguanosine and 8-nitro-2′-deoxyguanosine to 8-nitroguanine, and reduction of 8-nitroguanine would yield 8-aminoguanine. Similarly, PNPase might produce 8-hydroxyguanine from 8-hydroxyguanosine or 8-hydroxy-2′-deoxyguanosine. Taken together, these considerations suggest the metabolic framework summarized in Fig. 1. Consistent with this framework are studies confirming the presence of 8-nitroguanosine (Akaike et al., 2003), 8-aminoguanosine (Sodum et al., 1993), 8-hydroxyguanosine (Park et al., 1992), 8-nitroguanine (Ohshima et al., 2006), 8-hydroxyguanine (Fraga et al., 1990), and 8-hydroxy-2′-deoxyguanosine (Lam et al., 2012) in tissues or urine. Although 8-aminoguanine per se has not been reported in tissues, in vivo exogenous 8-aminoguanosine is rapidly converted to 8-aminoguanine (Osborne and Barton, 1986). Therefore, since 8-aminoguanosine exists in tissues (Sodum et al., 1993), 8-aminoguanine likely does also.
Fig. 1.
Potential biochemical pathways for the formation of endogenous 8-substituted guanine and guanosine compounds. ROS, reactive oxygen species.
Currently, nothing is known regarding the cardiovascular and renal effects of naturally occurring 8-substituted guanosine and guanine compounds. Since a multitude of these compounds exist in vivo, and since the cardiovascular and renal effects of these compounds are unknown, we examined the in vivo effects of guanosine, guanine, 8-nitroguanosine, 8-nitroguanine, 8-hydroxyguanosine, 8-hydroxyguanine, 8-hydroxy-2′-deoxyguanosine, 8-aminoguanosine, and 8-aminoguanine on the cardiovascular system and kidneys. Because some of these compounds produced “amiloride-like” effects, we also included an amiloride-treated group for comparison.
Materials and Methods
Materials.
Amiloride, guanosine, guanine, thiobutabarbital (Inactin), and deoxycorticosterone acetate were purchased from Sigma-Aldrich (St. Louis, MO). 8-Nitroguanosine, 8-nitroguanine, 8-aminoguanosine, and 8-aminoguanine were purchased from Toronto Research Chemicals (Toronto, Ontario, Canada). 8-Hydroxyguanosine, 8-hydroxyguanine, and 8-hydroxy-2′-deoxyguanosine were purchased from Cayman Chemical (Ann Arbor, MI).
Animals.
This study used male Sprague-Dawley rats (Charles River, Wilmington, MA) that were approximately 16 weeks of age. The Institutional Animal Care and Use Committee approved all procedures. The investigation conforms to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Pub. No. 85-23, revised 1996).
Protocol 1.
Rats were anesthetized with Inactin (90 mg/kg, i.p.), placed on an isothermal pad, and body temperature was monitored with a rectal probe thermometer and kept at 37°C with a heat lamp. The trachea was cannulated with polyethylene 240 (PE-240) to facilitate respiration, and a PE-50 cannula was inserted into the carotid artery and connected to a digital blood pressure analyzer (Micro-Med, Inc., Louisville, KY) for continuous measurement of mean arterial blood pressure (MABP) and heart rate (HR). A PE-50 cannula was inserted into the jugular vein, and an infusion of 0.9% saline at 25 μl/min was initiated. PE-10 tubing was placed in the left ureter for urine collection. Noncannulating, transit-time flow probes (Transonic Systems, Inc., Ithaca, NY) were placed on the left renal (1 mm) and mesenteric (2 mm) arteries and were connected to a two-channel small animal transit-time flowmeter (model T-206; Transonic Systems, Inc.) for measurement of renal blood flow (RBF) and mesenteric blood flow (MBF). After a 1-hour stabilization period, urine was collected for 30 minutes while MABP and HR were time-averaged (period 1: 0–30 minutes into protocol). Next, the test compounds dissolved in vehicle (0.9% saline containing 0.03 N HCl) were administered as an intravenous bolus at 33.5 µmol/kg. The dose was selected based on preliminary experiments with 8-aminoguanosine. One group received only vehicle to confirm that the vehicle did not affect any of the measured variables, and to confirm that our model was stable for the 115-minute observation period. Each group of rats (n of at least 6) received only one treatment. Ten minutes after the test agents were administered, urine was collected for another 30 minutes (period 2: 40–70 minutes into protocol). After a 15-minute wait period, urine was again collected for 30 minutes (period 3: 85–115 minutes into protocol). During each urine-collection period, MABP and HR were continuously recorded, and RBF and MBF were recorded at 10, 20, and 30 minutes into the urine-collection period. Sodium and potassium in urine were measured by flame photometry (Model IL-943; Instrumentations Laboratory Inc., Lexington, MA), and glucose in urine was measured by a glucose colorimetric assay kit (Cayman Chemical).
Protocol 2.
Rats were instrumented for long-term and continuous measurement of arterial blood pressure by radiotelemetry as recently described by us (Jackson et al., 2015). Animals were randomized to three groups. In the control group, baseline blood pressures and heart rates were recorded for 27 days from the time of implantation of the radiotransmitters; then, hypertension was induced in the animals by removing one kidney, subcutaneously administering deoxycorticosterone acetate (30 mg/kg twice weekly), and providing 1% NaCl as drinking water [i.e., deoxycorticosterone acetate (DOCA)–salt hypertension]. In the two other groups, baseline blood pressures and heart rates were recorded for 14 days, and animals were then treated with either 8-aminoguanosine or 8-aminoguanine (both at 5 mg/kg/day in drinking water) beginning 13 days before inducing DOCA-salt hypertension and continuing for 49 days after induction of DOCA-salt hypertension.
Statistics.
In the acute studies, statistical analysis was performed using one-factor repeated-measures analysis of variance (ANOVA) followed by Fisher’s least significant difference test if the overall effects in the ANOVA were significant. For the radiotelemetry studies, statistical analysis was conducted by comparing the control group to a treatment group beginning at the time of induction of hypertension using a two-factor (time and treatment) repeated-measures ANOVA. P < 0.05 was considered statistically significant. Absolute values are presented as mean ± S.E.M.
Results
Protocol 1.
To ensure the reliability of our results, we conducted two experimental series to confirm that our animal preparation was both stable and responsive. In the first quality-control series, baseline urine was collected in six rats during period 1, the vehicle for test compounds was then injected intravenously, and urine was collected postinjection during periods 2 and 3. As shown in Fig. 2, urine volume, sodium excretion, potassium excretion, glucose excretion, MABP, HR, MBF, and RBF were not affected by time or vehicle. These experiments indicated that our preparation was stable. In the second quality-control series, baseline urine was collected in another six rats during period 1, the diuretic amiloride (33.5 µmol/kg; same dose used for the guanine and guanosine analog experiments) was then injected intravenously, and urine was collected postinjection during periods 2 and 3. As illustrated in Fig. 3, amiloride caused a robust diuresis and natriuresis while suppressing potassium excretion. Compared with period 1 (baseline period), urine volume and sodium excretion increased by 4.5- and 13.6-fold, respectively, during period 3 (85–115-minute urine collection), whereas potassium excretion decreased by 96.5%. Amiloride did not change glucose excretion (Fig. 3) but slightly decreased MABP and HR (Fig. 3), as well as MBF and RBF (Fig. 3). These experiments indicated that our preparation was responsive to a known diuretic.
Fig. 2.
Bar graphs depict the urine volume (A), urinary sodium excretion (B), urinary potassium excretion (C), urinary glucose excretion (D), MABP (E), HR (F), MBF (G), and RBF (H) in anesthetized rats before (0–30 minutes) and after (40–70 and 85–115 minutes) administration of the vehicle used in subsequent experiments. These results confirmed that the vehicle had no effect, and that the preparation was stable for the duration of the experiment. Values are the means and S.E.M.s (n = 6).
Fig. 3.
Bar graphs depict the urine volume (A), urinary sodium excretion (B), urinary potassium excretion (C), urinary glucose excretion (D), MABP (E), HR (F), MBF (G), and RBF (H) in anesthetized rats before (0–30 minutes) and after (40–70 and 85–115 minutes) administration of amiloride (33.5 µmol/kg, intravenous bolus). These results confirmed that the preparation was responsive to a known potassium-sparing diuretic. P values are from one-factor analysis of variance. aSignificant difference (P < 0.05; Fisher’s least significant difference test) between basal period (0–30 minutes) and designated treatment period. Values are the means and S.E.M.s (n = 6).
The most efficacious 8-substituted guanosine compound was 8-aminoguanosine (Fig. 4), which, similar to amiloride, markedly increased urine volume and sodium excretion while suppressing potassium excretion. In this regard, compared with period 1, 8-aminoguanosine increased urine volume and sodium excretion by 4.2- and 26.6-fold, respectively, during period 3, whereas 8-aminoguanosine decreased potassium excretion by 69.1%. Unlike amiloride, 8-aminoguanosine caused a striking increase in glucose excretion during period 3 (12.1-fold). 8-Aminoguanosine did not affect MABP or HR (Fig. 4), but slightly increased MBF and slightly decreased RBF (Fig. 4).
Fig. 4.
Bar graphs depict the urine volume (A), urinary sodium excretion (B), urinary potassium excretion (C), urinary glucose excretion (D), MABP (E), HR (F), MBF (G), and RBF (H) in anesthetized rats before (0–30 minutes) and after (40–70 and 85–115 minutes) administration of 8-aminoguanosine (33.5 µmol/kg, intravenous bolus). P values are from one-factor analysis of variance. aSignificant difference (P < 0.05; Fisher’s least significant difference test) between basal period (0–30 minutes) and designated treatment period. bSignificant difference (P < 0.05; Fisher's least significant difference test) compared with 2nd (40–70 minutes) period. Values are the means and S.E.M.s (n = 6).
The most efficacious 8-substituted guanine compound was 8-aminoguanine (Fig. 5), which, similar to amiloride and 8-aminoguanosine, robustly increased urine volume and sodium excretion while attenuating potassium excretion. Compared with period 1, 8-aminoguanine increased urine volume and sodium excretion by 3.6- and 17.2-fold, respectively, during period 3, whereas 8-aminoguanine decreased potassium excretion by 71.0%. Unlike amiloride, but similar to 8-aminoguanosine, 8-aminoguanine increased glucose excretion 12.2-fold during period 3. 8-Aminoguanine did not affect MABP or HR (Fig. 5), and had little effect on MBF or RBF (Fig. 5).
Fig. 5.
Bar graphs depict the urine volume (A), urinary sodium excretion (B), urinary potassium excretion (C), urinary glucose excretion (D), MABP (E), HR (F), MBF (G), and RBF (H) in anesthetized rats before (0–30 minutes) and after (40–70 and 85–115 minutes) administration of 8-aminoguanine (33.5 µmol/kg, intravenous bolus). P values are from one-factor analysis of variance. aIndicates significant difference (P < 0.05; Fisher’s least significant difference test) between basal period (0–30 minutes) and designated treatment period. Values are the means and S.E.M.s (n = 6).
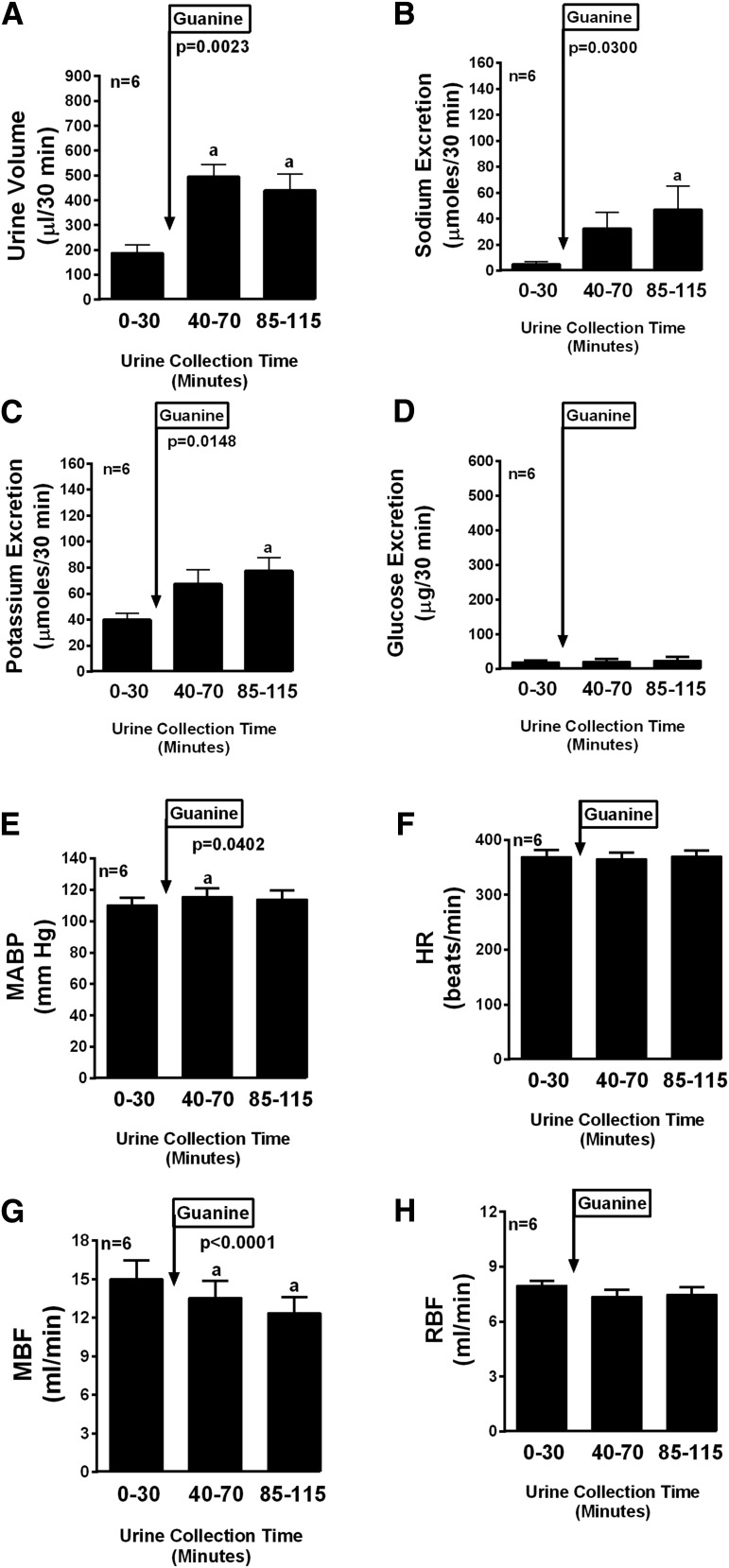
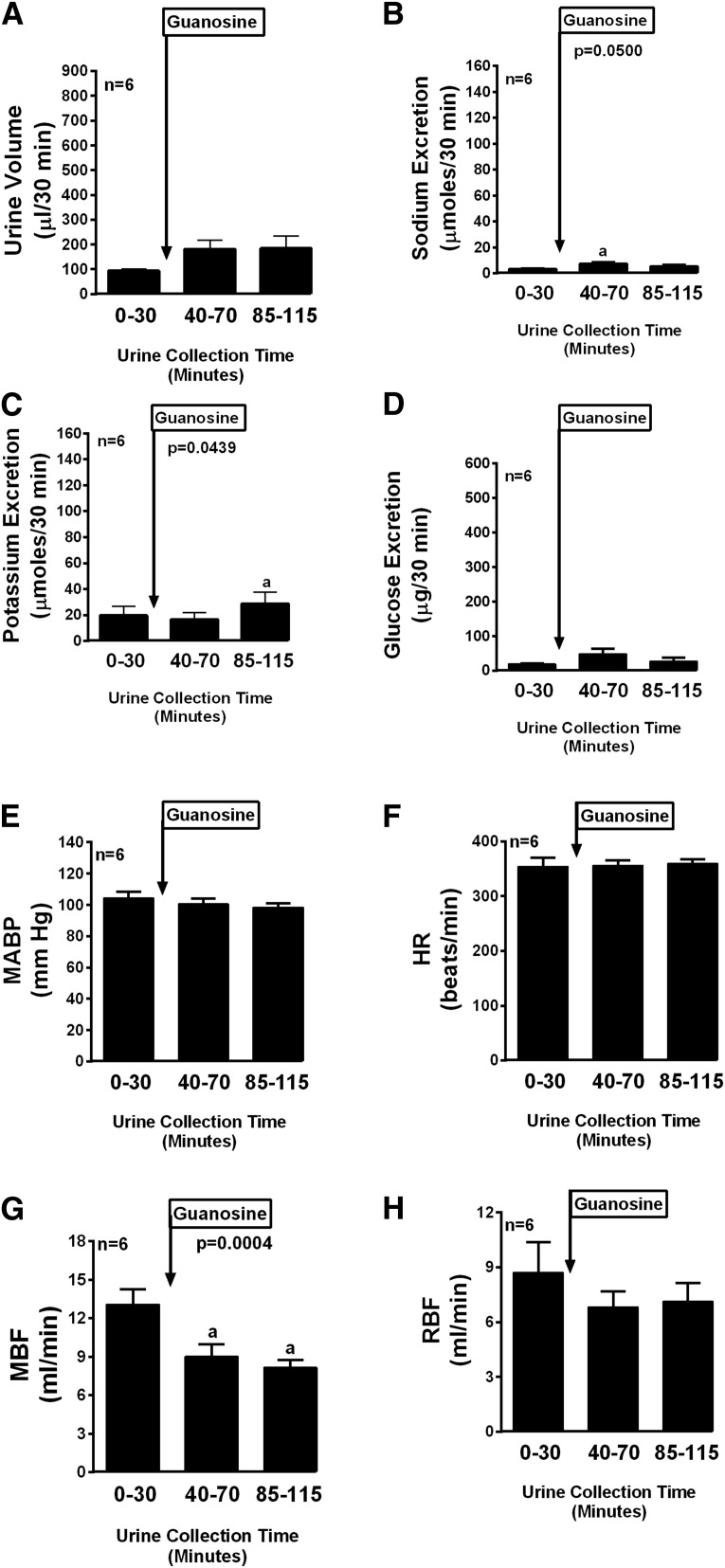
The other compounds tested in this series can be grouped into those with moderate efficacy and those with little or no efficacy. Compounds with moderate efficacy included guanine (Fig. 6), 8-nitroguanine (Fig. 7), 8-hydroxyguanosine (Fig. 8), and 8-hydroxyguanine (Fig. 9). With regard to sodium excretion these compounds caused 9.4-, 7.8-, 7.1-, and 8.6-fold increases, respectively (period 3 compared with period 1). These compounds had no or small and variable effects on potassium and glucose excretion. Compounds with little or no efficacy included guanosine (Fig. 10), 8-nitroguanosine (Fig. 11), and 8-hydroxy-2′-deoxyguanosine (Fig. 12).
Fig. 6.
Bar graphs depict the urine volume (A), urinary sodium excretion (B), urinary potassium excretion (C), urinary glucose excretion (D), MABP (E), HR (F), MBF (G), and RBF (H) in anesthetized rats before (0–30 minutes) and after (40–70 and 85–115 minutes) administration of guanine (33.5 µmol/kg, intravenous bolus). P values are from one-factor analysis of variance. aSignificant difference (P < 0.05; Fisher’s least significant difference test) between basal period (0–30 minutes) and designated treatment period. Values are the means and S.E.M.s (n = 6).
Fig. 7.
Bar graphs depict the urine volume (A), urinary sodium excretion (B), urinary potassium excretion (C), urinary glucose excretion (D), MABP (E), HR (F), MBF (G), and RBF (H) in anesthetized rats before (0–30 minutes) and after (40–70 and 85–115 minutes) administration of 8-nitroguanine (33.5 µmol/kg, intravenous bolus). P values are from one-factor analysis of variance. aSignificant difference (P < 0.05; Fisher’s least significant difference test) between basal period (0–30 minutes) and designated treatment period. Values are the means and S.E.M.s (n = 6).
Fig. 8.
Bar graphs depict the urine volume (A), urinary sodium excretion (B), urinary potassium excretion (C), urinary glucose excretion (D), MABP (E), HR (F), MBF (G), and RBF (H) in anesthetized rats before (0–30 minutes) and after (40–70 and 85–115 minutes) administration of 8-hydroxyguanosine (33.5 µmol/kg, intravenous bolus). P values are from one-factor analysis of variance. aSignificant difference (P < 0.05; Fisher’s least significant difference test) between basal period (0–30 minutes) and designated treatment period. bSignificant difference (P < 0.05; Fisher's least significant difference test) compared with 2nd (40–70 minutes) period. Values are the means and S.E.M.s (n = 10).
Fig. 9.
Bar graphs depict the urine volume (A), urinary sodium excretion (B), urinary potassium excretion (C), urinary glucose excretion (D), MABP (E), HR (F), MBF (G), and RBF (H) in anesthetized rats before (0–30 minutes) and after (40–70 and 85–115 minutes) administration of 8-hydroxyguanine (33.5 µmol/kg, intravenous bolus). P values are from one-factor analysis of variance. aSignificant difference (P < 0.05; Fisher’s least significant difference test) between basal period (0–30 minutes) and designated treatment period. Values are the means and S.E.M.s (n = 6).
Fig. 10.
Bar graphs depict the urine volume (A), urinary sodium excretion (B), urinary potassium excretion (C), urinary glucose excretion (D), MABP (E), HR (F), MBF (G), and RBF (H) in anesthetized rats before (0–30 minutes) and after (40–70 and 85–115 minutes) administration of guanosine (33.5 µmol/kg, intravenous bolus). P values are from one-factor analysis of variance. aSignificant difference (P < 0.05; Fisher’s least significant difference test) between basal period (0–30 minutes) and designated treatment period. Values are the means and S.E.M.s (n = 6).
Fig. 11.
Bar graphs depict the urine volume (A), urinary sodium excretion (B), urinary potassium excretion (C), urinary glucose excretion (D), MABP (E), HR (F), MBF (G), and RBF (H) in anesthetized rats before (0–30 minutes) and after (40–70 and 85–115 minutes) administration of 8-nitroguanosine (33.5 µmol/kg, intravenous bolus). P values are from one-factor analysis of variance. aSignificant difference (P < 0.05; Fisher’s least significant difference test) between basal period (0–30 minutes) and designated treatment period. Values are the means and S.E.M.s (n = 6).
Fig. 12.
Bar graphs depict the urine volume (A), urinary sodium excretion (B), urinary potassium excretion (C), urinary glucose excretion (D), MABP (E), HR (F), MBF (G), and RBF (H) in anesthetized rats before (0–30 minutes) and after (40–70 and 85–115 minutes) administration of 8-hydroxy-2′-deoxyguanosine (33.5 µmol/kg, intravenous bolus). Values are the means and S.E.M.s (n = 6).
Protocol 2.
Treatments with 8-aminoguanosine (5 mg/kg/day) or 8-aminoguanine (5 mg/kg/day) were initiated after 14 days of baseline data collection and 13 days before inducing DOCA-salt hypertension. Treatments with 8-aminoguanosine or 8-aminoguanine were maintained for the duration of the protocol, which included 49 days of data collection following induction of DOCA-salt hypertension. The control group was similarly treated, except this group did not receive 8-aminoguanosine or 8-aminoguanine in the drinking water. Pretreatment of rats with 8-aminoguanosine (Fig. 13) did not affect baseline systolic, mean, or diastolic blood pressures or heart rates. Nonetheless, 8-aminoguanosine significantly attenuated DOCA-salt–induced increases in systolic (P < 0.0001) and mean (P = 0.0023) blood pressures and tended (P = 0.0631) to attenuate DOCA-salt–induced increases in diastolic blood pressure. Heart rates drifted downward in both the control group and 8-aminoguanosine group, but there was no significant interaction (P = 0.9993) between time and treatment group in this regard, thus indicating that 8-aminoguanosine did not influence heart rate. Pretreatment of rats with 8-aminoguanine (Fig. 14) also did not affect baseline systolic, mean, or diastolic blood pressures or heart rates. 8-Aminoguanine significantly attenuated DOCA-salt–induced increases in systolic (P < 0.0001), mean (P < 0.0001), and diastolic (P < 0.0001) blood pressures. Although 8-aminoguanine did not affect baseline heart rates and did not initially affect heart rates after induction of DOCA-salt hypertension, beginning approximately 25 days after inducing DOCA-salt hypertension, heart rates were moderately lower in the 8-aminoguanine group (P = 0.0133). Blood samples were taken at the end of the study, and plasma samples were analyzed for sodium, potassium, and glucose. In DOCA-salt rats, chronic treatment with 8-aminoguanosine or 8-aminoguanine did not alter plasma levels of electrolytes or glucose.
Fig. 13.
Line graphs depict the long-term effects of 8-aminoguanosine (5 mg/kg/day in drinking water) on systolic arterial blood pressure (SBP) (A), MABP (B), diastolic arterial blood pressure (DBP) (C), and heart rate (D) in rats with hypertension induced by: 1) removing one kidney, 2) administering twice weekly subcutaneous injections of deoxycorticosterone acetate, and 3) providing 1% NaCl as drinking water. P values are from two-factor analysis of variance with repeated measures (time × treatment interaction).
Fig. 14.
Line graphs depict the long-term effects of 8-aminoguanine (5 mg/kg/day in drinking water) on systolic arterial blood pressure (SBP) (A), MABP (B), diastolic arterial blood pressure (DBP) (C), and heart rate (D) in rats with hypertension induced by 1) removing one kidney, 2) administering twice weekly subcutaneous injections of deoxycorticosterone acetate, and 3) providing 1% NaCl as drinking water. P values are from two-factor analysis of variance with repeated measures (time × treatment interaction).
Discussion
Published reports demonstrate that a number of 8-substituted guanosine and guanine derivatives exist in vivo, including 8-nitroguanosine (Akaike et al., 2003), 8-aminoguanosine (Sodum et al., 1993), 8-hydroxyguanosine (Park et al., 1992), 8-nitroguanine (Ohshima et al., 2006), 8-hydroxyguanine (Fraga et al., 1990), and 8-hydroxy-2′-deoxyguanosine (Lam et al., 2012). Given the well characterized ability of ONOO− and O2·− to modify guanine moieties in the 8-position (Yermilov et al., 1995; Szabo and Ohshima, 1997; Misiaszek et al., 2004; Ohshima et al., 2006) and the known activity of PNPase to release purine bases from purine nucleosides (Osborne and Barton, 1986), the existence of these compounds is not surprising and can be rationalized by the biochemical framework shown in Fig. 1. Despite the fact that 8-substitued guanosine and 8-substitued guanine derivatives exist in vivo, to the best of our knowledge, the cardiovascular and renal effects of these compounds have not been examined. Therefore, we set out to systematically assess the effects of these compounds on cardiovascular and renal parameters in vivo.
Here, we report that five naturally occurring, 8-substituted guanosine and guanine compounds (8-nitroguanine, 8-hydroxyguanosine, 8-hydroxyguanine, 8-aminoguanosine, and 8-aminoguanine) possess diuretic/natriuretic activity. Of these five compounds, three had natriuretic activity somewhat lower than that of amiloride (8-nitroguanine, 8-hydroxyguanosine, and 8-hydroxyguanine), and two (8-aminoguanosine and 8-aminoguanine) had natriuretic activity superior to that of amiloride. Thus the findings represent the discovery of a new class of diuretic/natriuretic compounds that could be of pharmacological significance.
Although speculative, since these compounds exist in vivo, it is conceivable that they function as an endogenous natriuretic system that regulates renal excretion of sodium and thereby modulates arterial blood pressure. However, addressing this hypothesis will require careful development of highly specific and sensitive assays to determine how the endogenous levels of these compounds are regulated and whether pharmacologically active levels of these compounds are achieved in blood, kidneys, or urine under physiological or pathophysiological conditions. In this regard, we are in the process of obtaining internal standards [i.e., heavy-isotope-labeled (13C and 15N) 8-aminoguanosine and 8-aminoguanine] to address this critically important issue using ultraperformance liquid chromatography—tandem mass spectrometry. In addition, we can use this assay to determine the stability and pharmacokinetics of 8-aminoguanosine and 8-aminoguanine, and to determine whether 8-aminoguanosine is a “prodrug” that is converted in vivo to 8-aminoguanine.
Regardless of whether 8-substituted guanosines/guanines serve as an endogenous natriuretic system, the diuretic/natriuretic effects of 8-aminoguanosine and 8-aminoguanine suggest that these agents may be clinically useful drugs. With the notable exceptions of mineralocorticoid antagonists and epithelial sodium channel inhibitors, most clinically useful diuretics/natriuretics (for example, thiazide-type, thiazide-like, and loop diuretics) increase potassium excretion, leading to increased risk of hypokalemia (Reilly and Jackson, 2011). Importantly, 8-aminoguanosine and 8-aminoguanine substantially (approximately 70%) suppress potassium excretion. This is a desirable aspect of the pharmacology of 8-aminoguanosine and 8-aminoguanine and supports the possibility that these compounds may be of clinical utility as diuretics/natriuretics for the treatment of hypertension and edema.
Another important aspect of the pharmacology of 8-aminoguanosine and 8-aminoguanine that relates to their clinical utility is the ability of these compounds to increase glucose excretion. Although this effect is modest (approximately 12-fold increase), the glucosuric effects of 8-aminoguanosine and 8-aminoguanine are in stark contrast to those of thiazide-like and thiazide-type diuretics on glucose homeostasis. The tendency of thiazide-like/-type diuretics to increase plasma glucose has been widely discussed and remains a concern, particularly for patients who are prediabetic (Palmer, 2011; Zhang and Zhao, 2016). The availability of a class of diuretics that decreases potassium excretion while increasing glucose excretion is an attractive prospect.
The present study also demonstrates that 8-aminoguanosine and 8-aminoguanine attenuate the development of DOCA-salt hypertension. Since the DOCA-salt model of hypertension is a salt-sensitive model, and since 8-aminoguanosine and 8-aminoguanine are natriuretics, it is likely that the natriuretic effects of these compounds explain their antihypertensive activity at least in part. However, other mechanisms are certainly possible, and we hypothesize that the antihypertensive effects of these compounds involve multiple mechanisms.
The fact that 8-aminoguanosine and 8-aminoguanine are antihypertensive compounds has important implications. A speculative implication is that, inasmuch as 8-aminoguanosine and 8-aminoguanine exist in vivo, these compounds may participate in the long-term regulation of arterial blood pressure. If so, alterations in the production or metabolism of 8-substituted guanosine and guanine derivatives could play a part in the pathophysiology of hypertension (e.g., salt sensitivity). Another implication is that, because 8-aminoguanosine and 8-aminoguanine are orally active, they could be useful for the long-term management of hypertension. Importantly, neither 8-aminoguanosine nor 8-aminoguanine caused reflex increases in heart rate. Indeed, after chronic treatment, 8-aminoguanine decreases heart rate. Antihypertensive agents that decrease heart rate and do not induce reflex tachycardia may reduce cardiovascular events more than those that increase heart rate (Palatini et al., 2006).
The mechanism by which 8-aminoguanosine and 8-aminoguanine induce diuresis/natriuresis is unknown and is under investigation. Since these compounds do not increase MABP, pressure natriuresis can be excluded as a mechanism of diuresis/natriuresis. Likewise, 8-aminoguanosine and 8-aminoguanine do not increase RBF, which rules out a mechanism involving vasodilation of the preglomerular microvascular circulation. Since 8-aminoguanosine and 8-aminoguanine increase the excretion of glucose (mostly reabsorbed in the proximal tubule) while decreasing the excretion of potassium (secreted in the collecting duct), it is likely that these compounds affect transport at multiple sites in the nephron.
It is worth noting that both 8-aminoguanosine and 8-aminoguanine are inhibitors of PNPase, with 8-aminoguanine being 10-fold more potent than 8-aminoguanosine (Kazmers et al., 1981; Gilbertsen and Dong, 1985). It is conceivable that at least a part of the diuretic/natriuretic mechanism of 8-aminoguanosine and 8-aminoguanine involves inhibition of PNPase. Importantly, inhibition of PNPase suppresses T cell function (Bennett et al., 1993), and inflammation mediated by T cells is now clearly understood to importantly contribute to both the etiology of hypertension and hypertension-induced target organ damage (Guzik and Mikolajczyk, 2014; Ji et al., 2014; Li et al., 2014; Pollow et al., 2014). Therefore, we hypothesize that, because 8-aminoguanosine and 8-aminoguanine inhibit PNPase, these compounds may be effective antihypertensive agents that not only reduce blood pressure but also attenuate target organ damage.
In summary, in vivo reactive nitrogen and oxygen species produce an array of 8-amino and 8-hydroxy guanosine and guanine compounds. Until now, the cardiovascular and renal effects of these compounds were unknown. The present study shows that 8-aminoguanosine and 8-aminoguanine are potent, efficacious, potassium-sparing orally active diuretics/natriuretics that promote urinary glucose excretion and prevent salt-induced hypertension. Thus these findings could result in the development of a safe and effective treatment of cardiovascular diseases and hypertension.
Abbreviations
- ANOVA
analysis of variance
- DOCA
deoxycorticosterone acetate
- HR
heart rate
- MABP
mean arterial blood pressure
- MBF
mesenteric blood flow
- PE
polyethylene
- PNPase
purine nucleoside phosphorylase
- RBF
renal blood flow
- RNS
reactive nitrogen species
Authorship Contributions
Participated in research design: Jackson.
Conducted experiments: Gillespie, Mi.
Performed data analysis: Jackson, Gillespie, Mi.
Wrote or contributed to the writing of the manuscript: Jackson.
Footnotes
The work was supported by the National Institutes of Health [DK091190, HL069846, DK068575, HL109002, and DK079307].
References
- Akaike T, Okamoto S, Sawa T, Yoshitake J, Tamura F, Ichimori K, Miyazaki K, Sasamoto K, Maeda H. (2003) 8-nitroguanosine formation in viral pneumonia and its implication for pathogenesis. Proc Natl Acad Sci USA 100:685–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett LL, Jr, Allan PW, Noker PE, Rose LM, Niwas S, Montgomery JA, Erion MD. (1993) Purine nucleoside phosphorylase inhibitors: biochemical and pharmacological studies with 9-benzyl-9-deazaguanine and related compounds. J Pharmacol Exp Ther 266:707–714. [PubMed] [Google Scholar]
- Carballal S, Bartesaghi S, Radi R. (2014) Kinetic and mechanistic considerations to assess the biological fate of peroxynitrite. Biochim Biophys Acta 1840:768–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraga CG, Shigenaga MK, Park JW, Degan P, Ames BN. (1990) Oxidative damage to DNA during aging: 8-hydroxy-2′-deoxyguanosine in rat organ DNA and urine. Proc Natl Acad Sci USA 87:4533–4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbertsen RB, Dong MK. (1985) Effects of 8-aminoguanosine, an inhibitor of purine nucleoside phosphorylase, on plasma nucleosides in Wistar rats. Ann N Y Acad Sci 451:313–314. [Google Scholar]
- Guzik TJ, Mikolajczyk T. (2014) In search of the T cell involved in hypertension and target organ damage. Hypertension 64:224–226. [DOI] [PubMed] [Google Scholar]
- Jackson EK, Mi Z, Tofovic SP, Gillespie DG. (2015) Effect of dipeptidyl peptidase 4 inhibition on arterial blood pressure is context dependent. Hypertension 65:238–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H, Zheng W, Li X, Liu J, Wu X, Zhang MA, Umans JG, Hay M, Speth RC, Dunn SE, et al. (2014) Sex-specific T-cell regulation of angiotensin II-dependent hypertension. Hypertension 64:573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazmers IS, Mitchell BS, Dadonna PE, Wotring LL, Townsend LB, Kelley WN. (1981) Inhibition of purine nucleoside phosphorylase by 8-aminoguanosine: selective toxicity for T lymphoblasts. Science 214:1137–1139. [DOI] [PubMed] [Google Scholar]
- Lam PM, Mistry V, Marczylo TH, Konje JC, Evans MD, Cooke MS. (2012) Rapid measurement of 8-oxo-7,8-dihydro-2′-deoxyguanosine in human biological matrices using ultra-high-performance liquid chromatography-tandem mass spectrometry. Free Radic Biol Med 52:2057–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wu Y, Zhang C, Li P, Cui W, Hao J, Ma X, Yin Z, Du J. (2014) γδT Cell-derived interleukin-17A via an interleukin-1β-dependent mechanism mediates cardiac injury and fibrosis in hypertension. Hypertension 64:305–314. [DOI] [PubMed] [Google Scholar]
- Misiaszek R, Crean C, Joffe A, Geacintov NE, Shafirovich V. (2004) Oxidative DNA damage associated with combination of guanine and superoxide radicals and repair mechanisms via radical trapping. J Biol Chem 279:32106–32115. [DOI] [PubMed] [Google Scholar]
- Ohshima H, Sawa T, Akaike T. (2006) 8-nitroguanine, a product of nitrative DNA damage caused by reactive nitrogen species: formation, occurrence, and implications in inflammation and carcinogenesis. Antioxid Redox Signal 8:1033–1045. [DOI] [PubMed] [Google Scholar]
- Osborne WR, Barton RW. (1986) A rat model of purine nucleoside phosphorylase deficiency. Immunology 59:63–67. [PMC free article] [PubMed] [Google Scholar]
- Palatini P, Benetos A, Julius S. (2006) Impact of increased heart rate on clinical outcomes in hypertension: implications for antihypertensive drug therapy. Drugs 66:133–144. [DOI] [PubMed] [Google Scholar]
- Palmer BF. (2011) Metabolic complications associated with use of diuretics. Semin Nephrol 31:542–552. [DOI] [PubMed] [Google Scholar]
- Park EM, Shigenaga MK, Degan P, Korn TS, Kitzler JW, Wehr CM, Kolachana P, Ames BN. (1992) Assay of excised oxidative DNA lesions: isolation of 8-oxoguanine and its nucleoside derivatives from biological fluids with a monoclonal antibody column. Proc Natl Acad Sci USA 89:3375–3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollow DP, Uhrlaub J, Romero-Aleshire MJ, Sandberg K, Nikolich-Zugich J, Brooks HL, Hay M. (2014) Sex differences in T-lymphocyte tissue infiltration and development of angiotensin II hypertension. Hypertension 64:384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly RF, Jackson EK. (2011) Regulation of renal function and vascular volume, in Goodman & Gilman’s The Pharmacological Basis of Therapeutics (Brunton LL, Chabner BA, Knollmann BC. eds) pp 671–719, McGraw Hill, New York. [Google Scholar]
- Sodum RS, Nie G, Fiala ES. (1993) 8-Aminoguanine: a base modification produced in rat liver nucleic acids by the hepatocarcinogen 2-nitropropane. Chem Res Toxicol 6:269–276. [DOI] [PubMed] [Google Scholar]
- Szabó C, Ohshima H. (1997) DNA damage induced by peroxynitrite: subsequent biological effects. Nitric Oxide 1:373–385. [DOI] [PubMed] [Google Scholar]
- Yermilov V, Rubio J, Ohshima H. (1995) Formation of 8-nitroguanine in DNA treated with peroxynitrite in vitro and its rapid removal from DNA by depurination. FEBS Lett 376:207–210. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhao Q. (2016) Association of thiazide-type diuretics with glycemic changes in hypertensive patients: A systematic review and meta-analysis of randomized controlled clinical trials. J Clin Hypertens (Greenwich) 18:342–351. [DOI] [PMC free article] [PubMed] [Google Scholar]