Abstract
Here we provide, at least to our knowledge, the first evidence that aripiprazole (APPZ) in the water blunts the stress response of exposed fish in a concentration ten times lower than the concentration detected in the environment. Although the mechanism of APPZ in the neuroendocrine axis is not yet determined, our results highlight that the presence of APPZ residues in the environment may interfere with the stress responses in fish. Since an adequate stress response is crucial to restore fish homeostasis after stressors, fish with impaired stress response may have trouble to cope with natural and/or imposed stressors with consequences to their welfare and survival.
The consumption of antipsychotic drugs has been growing gradually, especially due to the increasing the number of diagnostics of psychotic disorders in recent decades1. These drugs induce serious extrapyramidal effects in patients who are treated in a continuous and prolonged manner2. In 2002, the US FDA approved the use of aripiprazole (APPZ), an atypical antipsychotic with considerably lower occurrence and intensity of adverse effects3,4. APPZ has been approved for the treatment of schizophrenia, bipolar mania and major depressive disorder5,6,7,8,9, and is useful for controling positive and negative symptoms in schizophrenia10, and irritability, hyperactivity and stereotypies in autism11.
Due to its safety and lower incidence of unwanted side effects, the prescription of APPZ is increasing to replace classical antipsychotics. This increased use justify the concern about the accumulation of APPZ residues in wastewater and, consequently, potential negative effects in non-target organisms such as fish. There are a few studies reporting APPZ concentrations detected in effluents12,13, and only one study of cardiovascular risk using zebrafish larvae14. In addition, there are no reports on the impact of this drug in adult zebrafish (Danio rerio).
Endocrine disruptors are compounds that alter the normal functioning of the endocrine system of both humans and wildlife. We have previously shown that other psychotropic drugs such as risperidone15, fluoxetine, diazepam16,17 as well as alcohol18 blunted the response to stress follow acute stress”.
Despite the knowledge on the effects of APPZ in vitro18,19,20, in rodents21,22 and humans11,23,24, little is known about its impact as a possible environmental contaminant. Coupled with the increasing use, interest in the study of psychiatry drugs as environmental contaminants13,25,26 and the impact they have on the aquatic fauna has risk27,28,29,30,31. Thus, here we describe, for the first time, the effects of different concentrations of APPZ on the hypothalamic-pituitary-interrenal (HPI) axis in adult zebrafish.
Results
Two-way ANOVA revealed significant main effects for stress (F1,123 = 10.33, p < 0.0001), as well as a significant interaction between the factors (F5,123 = 101.9, p < 0.0001), but not for drug (F5,123 = 1.54; p = 0.1822) (Fig. 1). At concentrations of 0.556 and 556 ng/L, APPZ prevented the increase in cortisol levels in stressed zebrafish. However, this effect was not observed at other concentrations. On the other hand, APPZ per se did not interfere with whole-body cortisol levels. We can also observe that the stressor stimulus was effective.
Figure 1. Whole-body cortisol levels in zebrafish exposed to different concentrations of aripiprazole (APPZ, in ng/L) without stressor stimulus (S−) and with stressor stimulus (S+).
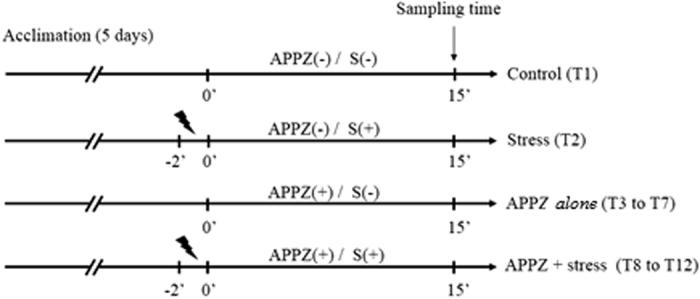
Each dot represents one independent measurement and the black lines represent the mean. Two-way ANOVA followed by Tukey post hoc test. n = 9–15 **p < 0.01; ***p < 0.001; ****p < 0.0001 vs. control group (S−).
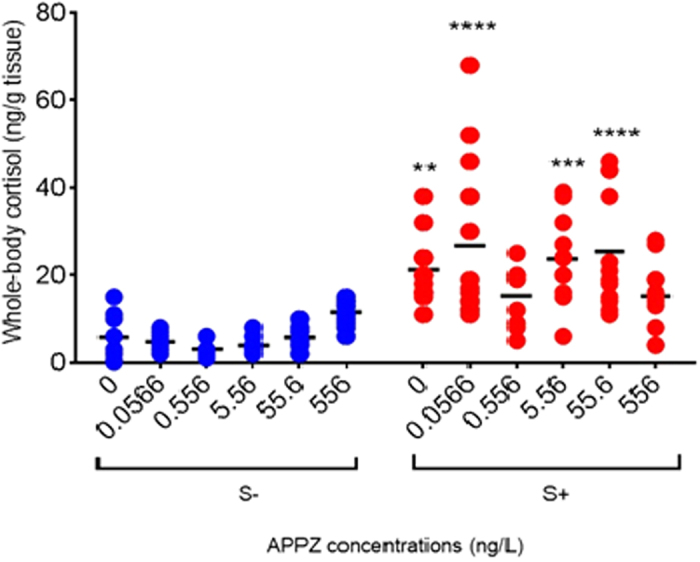
Figure 2. Schematic representation of the experimental design. APPZ (aripiprazole), S(−) without stressor stimulus; S(+) with stressor stimulus.
Discussion
Here we provide, at least to our knowledge, the first evidence that APPZ in the water decreases the stress response of exposed fish. The concentration of 0.556 ng/L, ten times lower than the concentration detected in the environment (5.56 ng/L13), blunted the cortisol increase after an acute stressor. This blunting effect of the lower concentration, in an environmental perspective, points to the necessity of great caution in relation to the delivery of APPZ residues in water bodies.
Interestingly, the HPI-blunting effect was also evident in the higher concentrations of 556 ng/L. This U-shaped concentration-response curve suggests a hormetic effect, similar to other psychotropic drugs such as diazepam16 and risperidone17.
In fact, the mechanism of action of APPZ on the HPI axis of zebrafish is not clear. Our hypothesis is that APPZ has a dopaminergic stabilizer effect20, since it is a partial agonist of dopamine receptors30, and tends to buffer the effects of endogenous dopamine31. Since dopamine receptors regulate hypothalamic-pituitary-adrenal axis activity in rats32, it is possible that APPZ may also act via this mechanism in fish. Our hypothesis based on the dopaminergic regulation of HPI axis is reinforced since in unstressed fish, the APPZ did not cause any change in cortisol levels, as verified in humans25. In addition, zebrafish treated with a dopaminergic antagonist and subjected to stress did not increase their cortisol, reinforcing the dopaminergic involvement in the HPI activation33.
Despite the undetermined mechanism, our results highlight that the presence of APPZ residues in the environment may interfere in the neuroendocrine axis that coordinates the stress responses in fish. In fish, cortisol plays a key role in the intermediary metabolism, osmoregulation and immune function34. Thus, the adequate stress response is crucial to restoring fish homeostasis after stressors; a fish with an impaired response may have trouble to cope with natural and/or imposed stressors with consequences to fish welfare and survival.
A limitation of our study is that we evaluated the effects of acute, but not chronic exposition to AAPZ. Further studies are necessary to better understand the effects of chronic exposition of AAPZ on behavioral and neuroendocrine parameters in zebrafish.
Furthermore, the zebrafish is a model for translational studies of several human diseases, due to the high homology with the human genome35. Although we do not have addressed the mechanism whereby APPZ blocks the stress response, this does not lessen the importance of our initial assessment, since, at least to our knowledge, this is the first report about an in vivo APPZ effect in fish HPI axis.
Methods
Ethical note
This study was approved at protocol #20/2016, by the Ethics Commission for Animal Use of Universidade de Passo Fundo (Passo Fundo, RS, Brazil) and all methods were carried out in accordance with the guidelines of National Council of Animal Experimentation Control (CONCEA).
Fish
We used a population of 144 mixed-sex adult wild-type zebrafish (Danio rerio), short-fin strain, and 50:50 male and female. Fish were acclimatized for 5 days in 3.9 l glass aquaria (20 × 15 × 14 cm), in groups of three animals, under constant aeration, with a photoperiod of 14 h light: 10 h dark. Water temperature was maintained at 27.4 ± 1.3 °C; pH 6.7 ± 0.3; dissolved oxygen at 5.6 ± 0.5 mg/L; total hardness at 58.9 ± 12.4 mg/L CaCO3, alkalinity at 45 ± 10 mg/L CaCO3 and ionized ammonia was <0.011 mg/L.
Study strategy
Our strategy was to expose zebrafish to five concentrations of APPZ and verify the stress response of these fish to an additional acute stressor. A possible isolated effect of APPZ was accessed by measuring cortisol in exposed unstressed fish.
Experimental procedures
We distributed fish in 12 groups. Each group consist of four glass aquaria with three animals each one (n = 12 per treatment). We then exposed fish to five concentrations of APPZ (Aristab®, Aché, Brazil). We set two lower and two higher concentrations (at 10-fold basis) in relation to the APPZ concentration already detected in the environment (5.56 ng/L13). Thus, the five concentrations were 0.0556; 0.556; 5.556; 55.6 and 556 ng/L.
In the exposed and stressed groups, we administered APPZ directly in the water for a 15 min exposure time and then we applied the acute stress stimulus by chasing fish with a pen net for 2 minutes. After 15 min the animals were euthanized for cortisol measurement (see Fig. 1).
After this period, the fish were gently captured and immediately euthanized with cold water, followed by decapitation and freeze of the whole body at liquid nitrogen for 30 s. Then, we stored samples at −20 °C for cortisol extraction, following the method described in Oliveira et al.18. The tissue extract was suspended in PBS, and whole-body cortisol was measured using a commercially available enzyme-linked immune sorbent (EIAgen CORTISOL test, Bio Chem Immuno Systems). This kit is fully validated for zebrafish extracts using the methodology described by Sink et al.36.
Statistics
The data is expressed as mean ± standard error of mean (S.E.M). The normal distribution of the data was confirmed by Kolmogorov-Smirnov and Levene tests, and results analyzed by two-way ANOVA followed by Tukey’s post hoc test. Two-way ANOVA was used to identify the main effects of stress and treatment, as well as their interactions. Differences were considered significant at p < 0.05”.
Additional Information
How to cite this article: Barcellos, H. H. A. et al. Waterborne aripiprazole blunts the stress response in zebrafish. Sci. Rep. 6, 37612; doi: 10.1038/srep37612 (2016).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
This study was funded by the Universidade de Passo Fundo and CNPq. L.J.G.B. holds CNPq research fellowships (301992/2014-2).
Footnotes
Author Contributions H.H.A.B. and L.J.G.B. conceptualize the experiments, wrote the manuscript and prepare the figures. H.H.A.B., F.K., J.G.S.R., T.A.O., R.I., M.S.A., A.C.V.G., M.F., C.V. and M.R. conducted experimental procedures. A.L.P. analyzed the results. G.K. measured cortisol concentrations. All authors have read and approved the manuscript for publication.
References
- INCB. International Narcotics Control Broad. Availability of Internationally Controlled Drugs: Ensuring Adequate Access for Medical and Scientific Purpose: Analysis of world situation. Cap.3, 49–67 (2015).
- Lieberman J. A. et al. Clinical antipsychotic trials of intervention effectiveness (CATIE) investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. New England Journal of Medicine. 353, 1209–1223 (2005). [DOI] [PubMed] [Google Scholar]
- Grady M. A., Gasperoni T. L. & Kirkpatrick P. Aripiprazole. Nature reviews/drug discovery. 2, 427–428 (2003). [DOI] [PubMed] [Google Scholar]
- Marder S. R. et al. Aripiprazole in the treatment of schizophrenia: safety and tolerability in short-term, placebo-controlled trials. Schizophrenia Research. 61, 123–136 (2003). [DOI] [PubMed] [Google Scholar]
- Keck P. E. et al. Aripiprazole Study Group. A placebo-controlled, double-blind study of the efficacy and safety of aripiprazole in patients with acute bipolar mania. American Journal of Psychiatry. 160, 1651–1658 (2003). [DOI] [PubMed] [Google Scholar]
- Sachs G. et al. Aripiprazole Study Group. Aripiprazole in the treatment of acute manic or mixed episodes in patients with bipolar I disorder: a 3-week placebo-controlled study. Journal of Psychopharmacology. 20, 536–546 (2006). [DOI] [PubMed] [Google Scholar]
- Stroup T. S. et al. CATIE Investigators. Results of phase 3 of the CATIE schizophrenia trial. Schizophrenia Research. 107, 1–12 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X. et al. Comparative efficacy, acceptability, and tolerability of augmentation agents in treatment-resistant depression: systematic review and network meta-analysis. Journal of Clinical Psychiatry. 76, e487–e498 (2015). [DOI] [PubMed] [Google Scholar]
- Stewart T. D. et al. Effect of symptom severity on efficacy and safety of aripiprazole adjunctive to antidepressant monotherapy in major depressive disorder: a pooled analysis. Journal of Affective Disorders. 162, 20–25 (2014). [DOI] [PubMed] [Google Scholar]
- Mailman R. B. & Murthy V. Third generation antipsychotic drugs: partial agonism or receptor functional selectivity? Current Pharmacology Design. 16, 488–501 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Accordino R. E., Kidd C., Henry C. A. & McDougle C. J. Psychopharmacological interventions in autism spectrum disorder. Expert Opinions on Pharmacotherapy. 17, 937–952 (2016). [DOI] [PubMed] [Google Scholar]
- Subedi B., Lee S., Moon H. & Kannan K. Psychoactive pharmaceuticals in sludge and their remission from wastewater treatment facilities in Korea. Environmental Science and Technology. 47, 13321−13329 (2013). [DOI] [PubMed] [Google Scholar]
- Subedi B. & Kannan K. Occurrence and fate of select psychoactive pharmaceuticals and antihypertensives in two wastewater treatment plants in New York State, USA. Science of the Total Environment. 514, 273–280 (2015). [DOI] [PubMed] [Google Scholar]
- Lee S. H., Kim H. R., Han R. X., Oqani R. K. & Jin D. I. Cardiovascular risk assessment of atypical antipsychotic drugs in a zebrafish model. Journal of Applied Toxicology. 22, 466–470 (2013). [DOI] [PubMed] [Google Scholar]
- Idalencio et al. Waterborne risperidone decreases stress response in zebrafish. PLOS One. 16, 1–10 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abreu M. S. et al. Diazepam and fluoxetine decrease the stress response in zebrafish. PLOS One. 9, e103232 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomini A. C. V. V. et al. Fluoxetine and diazepam acutely modulate stress induced-behavior. Behavioural Brain Research. 296, 301–310 (2016). [DOI] [PubMed] [Google Scholar]
- Oliveira T. A. et al. Alcohol impairs predation risk response and communication in zebrafish. PLOS One. 8, e75780 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burris K. D. et al. Aripiprazole, a novel, a high-affinity partial agonist at human dopamine D2 receptors. The Journal of Pharmacology and Experimental Therapeutics. 302, 381–389 (2002). [DOI] [PubMed] [Google Scholar]
- Hirose T. & Kikuchi T. Aripiprazole, a novel antipsychotic agent: dopamine D2 receptor partial agonist. Journal of Medical Investigation. 52, 284–290 (2005). [DOI] [PubMed] [Google Scholar]
- Koener B., Focant M. C., Bosier B., Maloteaux J. & Hermans E. Increasing the density of the D2L receptor and manipulating the receptor environment are required to evidence the partial agonist properties of aripiprazole. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 36, 60–70 (2012). [DOI] [PubMed] [Google Scholar]
- Saraei M. et al. In vivo anti‐Toxoplasma activity of aripiprazole. Iranian Journal of Basic Medical Sciences. 18, 938–941 (2015). [PMC free article] [PubMed] [Google Scholar]
- Takahashi et al. Alterations in behavioral responses to dopamine agonists in olfactory bulbectomized mice: relationship to changes in the striatal dopaminergic system. Psychopharmacology. 23, 1311–1322 (2016). [DOI] [PubMed] [Google Scholar]
- Mamo D., Mizrahi R., Shammi C. M., Romever F. & Kapur S. Differential Effects of Aripiprazole on D 2, 5-HT 2, and 5-HT1A Receptor Occupancy in Patients With Schizophrenia: A Triple Tracer PET Study. American Journal of Psychiatry. 164, 1411–1417 (2007). [DOI] [PubMed] [Google Scholar]
- Handley R. et al. Effects of antipsychotics on cortisol, interleukin-6 and hippocampal perfusion in healthy volunteers. Schizophrenia Research. 174, 99–105 (2016). [DOI] [PubMed] [Google Scholar]
- Calisto V. & Esteves V. I. Psychiatric pharmaceuticals in the environment. Chemosphere. 77, 1257–1274 (2009). [DOI] [PubMed] [Google Scholar]
- Yuan S., Jiang X., Xia X., Zhang H. & Zheng S. Detection, occurrence and fate of 22 psychiatric pharmaceuticals in psychiatric hospital and municipal wastewater treatment plants in Beijing, China. Chemosphere. 90, 2520–2525 (2013). [DOI] [PubMed] [Google Scholar]
- Abreu M. S. et al. Effects of waterborne fluoxetine on stress response and osmoregulation in zebrafish. Environmental Toxicology and Pharmacology. 40, 704–707 (2015). [DOI] [PubMed] [Google Scholar]
- Kalichak et al. Waterborne psychoactive drugs impair the initial development of zebrafish. Environmental Toxicology and Pharmacology. 41, 89–94 (2016). [DOI] [PubMed] [Google Scholar]
- Tamminga C. A. & Carlsson A. Partial Dopamine Agonists and Dopaminergic Stabilizers, in the Treatment of Psychosis. Current Drug Targets - CNS & Neurological Disorders. 1, 141–147 (2002). [DOI] [PubMed] [Google Scholar]
- Horacek J. et al. Mechanism of action of atypical antipsychotic drugs and the neurobiology of schizophrenia. CNS Drugs. 20, 389–409 (2006). [DOI] [PubMed] [Google Scholar]
- Borowsky B. & Kuhn C. M. D1 and D2 dopamine receptors stimulate hypothalamo-pituitary-adrenal activity in rats. Neuropharmacology. 31, 671–678 (1992). [DOI] [PubMed] [Google Scholar]
- Idalencio et al. Dopaminergic control of stress response in zebrafish. in preparation.
- Hontela A. Interrenal dysfunction in fish from contaminated sites: in vivo and in-vitro assessment. Environmental Toxicology and Chemistry. 17, 44–48 (1998). [Google Scholar]
- Bradbury J. Small fish, big science. PLOS Biology. 2, 568–572 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sink T. D., Lochmann R. T. & Fecteau K. A. Validation, use, and disadvantages of enzyme-linked immunosorbent assay kits for detection of cortisol in channel catfish, largemouth bass, red pacu, and golden shiners. Fish Physiology and Biochemistry. 34, 95–101 (2007). [DOI] [PubMed] [Google Scholar]


