Abstract
The recruitment of neural stem/progenitor cells (NSPCs) for brain restoration after injury is a promising regenerative therapeutic strategy. This strategy involves enhancing proliferation, migration and neuronal differentation of NSPCs. To date, the lack of biomaterials, which facilitate these processes to enhance neural regeneration, is an obstacle for the cell replacement therapies. Our previous study has shown that NSPCs grown on poly-L-ornithine (PO) could proliferate more vigorously and differentiate into more neurons than that on Poly-L-Lysine (PLL) and Fibronectin (FN). Here, we demonstrate that PO could promote migration of NSPCs in vitro, and the underlying mechanism is PO activates α-Actinins 4 (ACTN4), which is firstly certified to be expessed in NSPCs, to promote filopodia formation and therefore enhances NSPCs migration. Taken together, PO might serve as a better candidate for transplanted biomaterials in the regenerative therapeutic strategy, compared with PLL and FN.
Neural stem/progenitor cells (NSPCs), bearing the potential of proliferation and differentiation into all neural lineage cell types1,2,3, especially homing to the lesions restoring damaged neurovascular structure, have aroused great enthusiasm of investigators to keep an eye on their advantages of regenerating various neurological diseases and injuries, meanwhile, provide a feasible cell source for transplantation therapies4. To date, there are two culture systems to collect NSPCs, known as neurosphere culture system and adherent culture system coated with different substrates to mimic the physiological microenvironment. Meanwhile, NSPCs might exhibit different characteristics among various substrates they grown on.
Different substrates hold disparate effects on proliferation, migration and differentiation of NSPCs. Several studies have illustrated that NSPCs, cultured on different substrates, exhibit different proliferation and differentiation potential5,6,7,8. We and our colleagues have previously indicated that NSPCs grown on poly-L-ornithine (PO) could proliferate more vigorously and differentiate into more neurons than that on Poly-L-Lysine (PLL) and Fibronectin (FN)9. Furthermore, study has shown that NSPCs proliferate in the Subventricular Zone (SVZ), but only a few proliferated NSPCs could migrate to the lesions after ischemic stroke10. It implies that the shortage of functional NSPCs in lesions might due to the inhibition of NSPCs migration potential after brain injury. Therefore, how to promote NSPCs migration to the lesions is another significant issue for the use of NSPCs in cell-based therapies. Hence, it is necessary to explore the influence of migration potential of NSPCs on various substrates and its underlying mechanism(s) to look for a better biomaterial for cell replacement therapies.
The isoforms of α-Actinins, approximately 100 kDa and widely expressed in various mammalian cells, interact with actin filaments to regulate cell motility11. To date, there are four human α-actinin genes: actinin 1, 2, 3 and 412. Meanwhile, their encoded proteins involve in the organization of cell cytoskeleton to regulate the motility of muscle and non-muscle cells via interacting with various cytoskeletal and membrane-associated proteins and crosslinking actin filaments13. Especially, α-Actinins 4 (ACTN4), expressed in non-muscle cells, could enhance cancer cell migration and invasion. Previous studies have shown that ACTN4 promotes metastatic potential in numerous malignant cancers cells, including cervical cancer14, human pancreatic cancer15 and non-small cell lung cancer16, by directly linking the actin filaments17 and/or indirectly driving the actin filaments to focal adhesion sites through the cross-link with adhesion plaque proteins18. Whether the NSPCs, one type of non-muscle cells, express ACTN4 is still unclear. Meanwhile, the role of ACTN4 playing in regulating NSPCs migration on different substrates needs to be further elucidated.
In this present study, three substrates (PO, PLL and FN) were tested for their effects on migration of NSPCs. Meanwhile, the expression of ACTN4 in NSPCs was investigated and its role in regulating actin filaments formation was explored. The aim of this study is to investigate the different effects of the three widely used substrates on the migration of NSPCs, therefore, look for a more suitable biomaterial candidate to mimic the physiological microenvironment for NSPCs transplantation strategies. Furthermore, try to elucidate the possible underlying mechanism(s), which might provide more intervention targets for basic and preclinical research to promote functional recovery after various neurological diseases and injuries.
Results
Characteristics of NSPCs isolated from rats
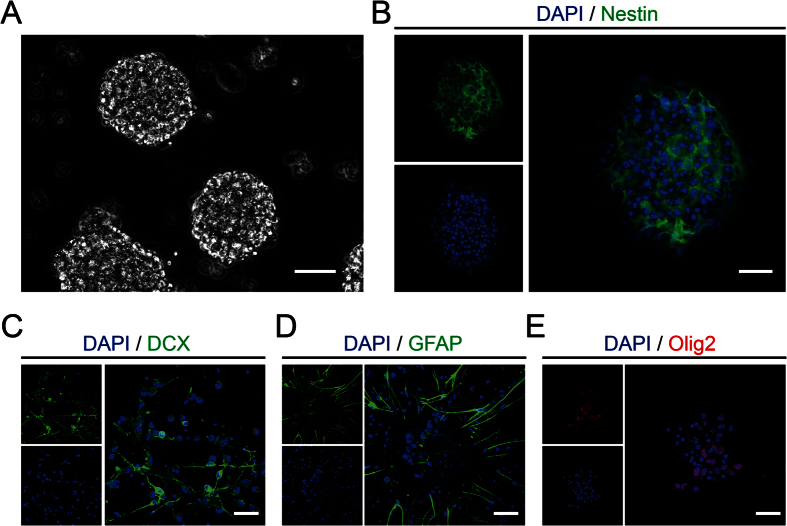
NSPCs, were obtained from neocortical tissues from E14.5 Sprague–Dawley rats as previously described9. They were cultivated in enrichment medium (20 ng/ml FGF-basic and 20 ng/ml EGF) and the suspended growth of neurospheres were notably observed after 3 days (Fig. 1A). Meanwhile, immunofluorescence identified cells expressing Nestin (Fig. 1B). In order to test the differentiation potential, the cells from neurospheres were seeded on PO feeded with conditioned medium (without 20 ng/ml FGF-basic and 20 ng/ml EGF) for 10 days. The immunofluorescence results demonstrated that the cells holded the potential to differentiate into neurons (Fig. 1C), astrocytes (Fig. 1D) and oligodendrocytes (Fig. 1E).
Figure 1. Characteristics and differentiation potential of NSPCs isolated from rats.
(A) The suspended growth of neurospheres was notably observed after 3 days. (B) The immunostaining showed the Nestin expression (green) on NSPCs in neurospheres before seeded on substrates. (C–E) The immunostaining demonstrated the differentiation potential of NSPCs into neurons (DCX, green), astrocytes (GFAP, green), or oligodendrocytes (Olig2, red). Cell nuclei were stained with DAPI in blue. Scale bar: 20 μm.
PO evidently enhanced migration of NSPCs by promoting filopodia formation
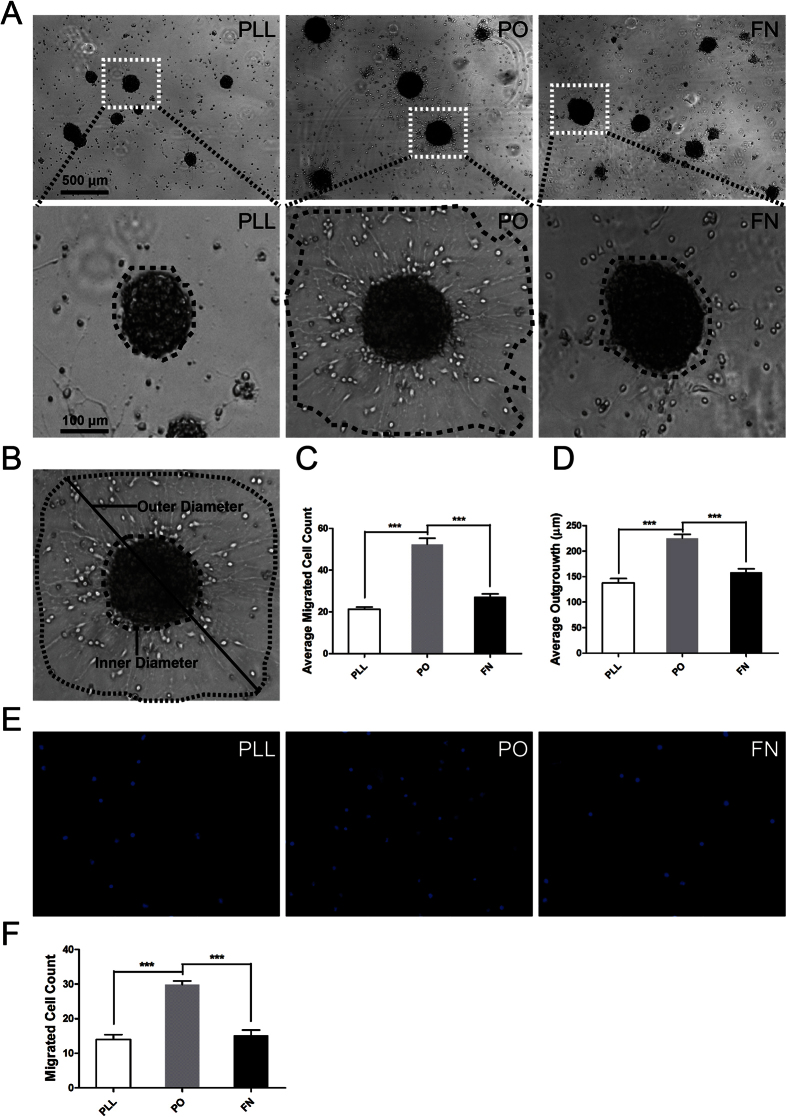
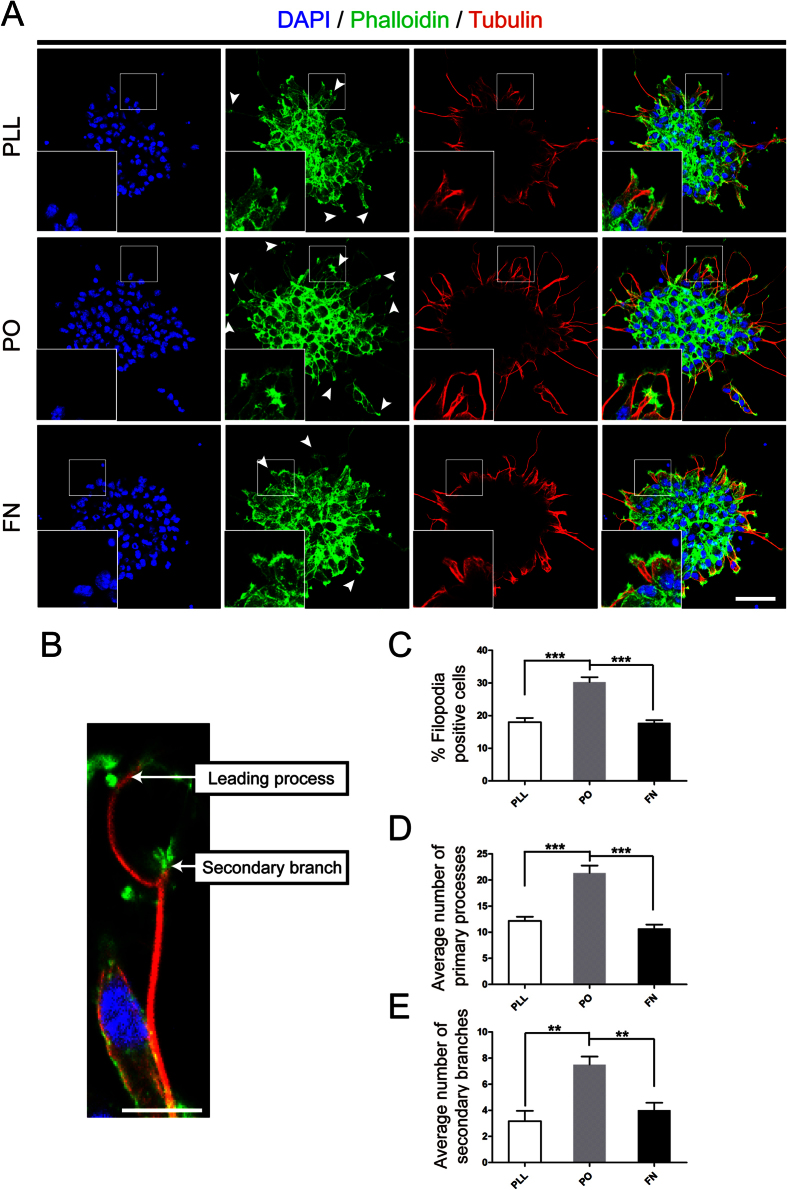
We have previously shown there are no obviously different effect on death or survival of NSPCs among PLL, PO and FN9. In the present study, we used the same adherent culture system to investigate their effects on migration of NSPCs. NSPCs were plated on different substrates in the enrichment medium with 20 ng/ml bFGF and 20 ng/ml EGF according to the well-known standard method19. First, the number and distance of migrated cells were evaluated under phase contrast microscopy. Results indicated that NPSCs on PO migrated from neurospheres were significant increase than that on PLL and FN, both in average migrated cell number and distance (Fig. 2A–D). Next, transwell assays were used to confirm the results collected from previous test. The data demonstrated that Millipore Transwell inserts pre-treated with PO could greatly increase cell mobility, compared with PLL and FN (Fig. 2E,F). Together, these data suggested that PO bear- the capacity to enhance the migration of NSPCs. To understand why PO enhanced the migration of NSPCs, we performed phalloidin staining to assess the filopodia formation, an indicator of cell polarization at the beginning of migration20. Meanwhile, we stained the expression of tubulin, a symbol of cage-like microtubule structure21, to evaluate the morphological structure changes in NSPCs. The results indicated that NSPCs on PO had larger percent of filopodia formation (Fig. 3A,C). Moreover, The average number of processes of NSPCs on PO was sharper increase than that on PLL and FN, including leading processes (Fig. 3A,D) and secondary branches (Fig. 3A,E). While, there were no obvious differentce in cage-like microtubule structure (Fig. 3A). These data demonstrated that PO promoted migration of NSPCs through facilitating filopodia formation.
Figure 2. PO evidently enhanced migration of NSPCs.
(A) Neurospheres were plated in different substrates pre-coated 24-well plates and images were captured by phase contrast microscopy after 12 hours. Insets were magnified images from each photograph at low magnification. (B) NPSCs migration was determined by the longest outer diameter of neurosphere migration normalized to inner sphere diameter and reported in micron. (C) Summarized graph showed the number of migration cells from neurospheres on PLL, PO and FN. ***P < 0.01, one-way ANOVA followed by Tukey’s post hoc test (n = 6 for each group). (D) Quantitative analysis of migration distance from neurospheres on PLL, PO and FN respectively. ***P < 0.01, one-way ANOVA followed by Tukey’s post hoc test (n = 6 for each group). (E) Transwell assays to evaluated the migration potential of NSPCs on PLL, PO and FN. (F) Summarized graph indicated the number of migration cells from upper chambers to lower ones pre-coated with PLL, PO and FN. ***P < 0.01, one-way ANOVA followed by Tukey’s post hoc test (pooled data from five independent experiments). The data are presented as the mean ± SEM.
Figure 3. PO promoted filopodia formation.
(A) Phalloidin staining (green) assessed the nubmer of filopodia formation and tubulin immunostaining (red) images showed the cage-like microtubule structure in neurospheres growing on PLL, PO and FN. Cell nuclei were stained with DAPI in blue. Arrows indicated the standard filopodia formation in each group. Scale bar: 20 μm. (B) An example of phalloidin and tubulin-labeled NSPCs with a secondary branch and a leading process. Scale bar: 20 μm. (C) Bar graph demonstrated the percent of filopodia formation on PLL, PO and FN. ***P < 0.01, one-way ANOVA followed by Tukey’s post hoc test (n = 6 for each group). (D) Quantitative analysis of average number of primary leading processes on PLL, PO and FN respectively. ***P < 0.01, one-way ANOVA followed by Tukey’s post hoc test (n = 6 for each group). (E) Quantitative analysis of average number of secondary branches on PLL, PO and FN respectively. **P < 0.05, one-way ANOVA followed by Tukey’s post hoc test (n = 6 for each group).
ACTN4 expressed in NSPCs and involved in promoting migration of NSPCs on PO
ACTN4 directly mediates cell migration and filopodia formation in non-muscle cells17. The above results had indicated that PO could enhance the formation of actin filaments. Meanwhile, NSPCs are non-muscle cells. Therefore, we hypothesize that ACTN4 involve in promoting migration of NSPCs.
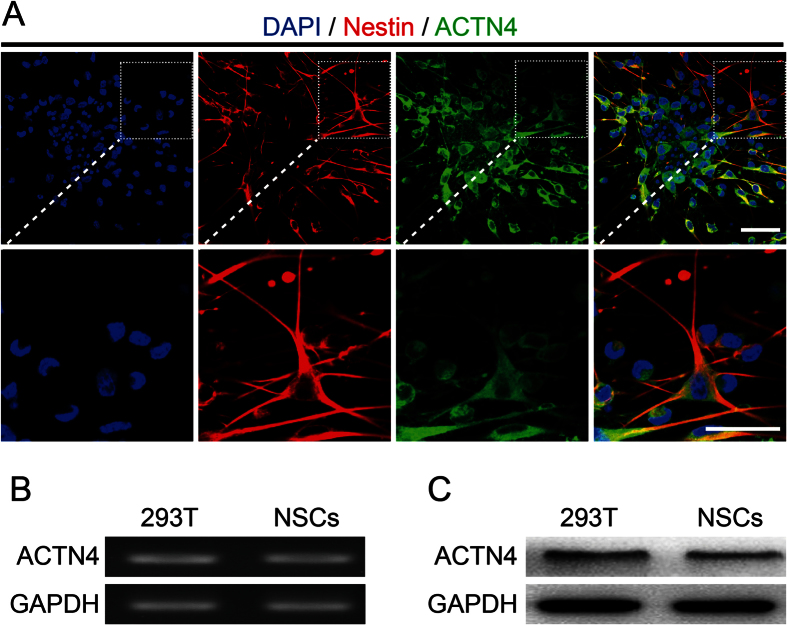
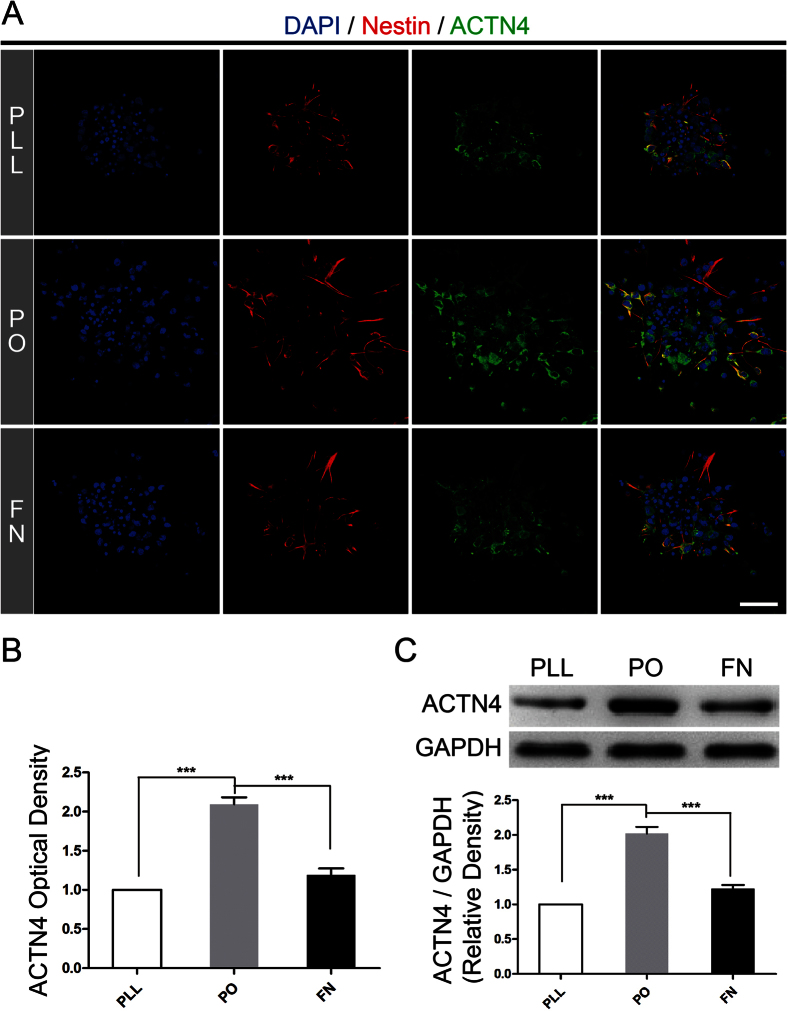
To certify our hypothesis, we firstly identified ACTN4 expressed in NSPCs by immunofluorescence (Fig. 4A). Next, RT-PCR and western blotting assays were used to determine the mRNA and protein expression of ACTN4 in NSPCs, with 293T cell line as positive control to assure the results obtained from immunofluorescence respectively. The data from RT-PCR assays showed that ACTN4 mRNA expressed in NSPCs was almost the same level, compared to the positive control (Fig. 4B). Meanwhile, the western blotting assays indicated the same tendency as mRNA expression (Fig. 4C). Furthermore, immunostaining images showed that NSPCs expressed the highest level of ACTN4, compared with PLL and FN (Fig. 5A,B). In addition, western blotting assays were used to determine the expression level of ACTN4 among PLL, PO and FN. The results illustrated that NSPCs grown on PO expressed the highest level of ACTN4 (Fig. 5C), which confirmed the data obtained from immunofluorescence. In general, these data indicated that ACTN4 expressed in NSPCs and involved in PO induced migration of NSPCs through promoting filopodia formation. As far as we knew, it was the first time to identify the expression of ACTN4 and its function in NSPCs.
Figure 4. ACTN4 expressed in NSPCs.
(A) The immunostaining images indicated the co-labeled of the Nestin (red) and ACTN4 (green) of neurospheres after seeded on PO for 12 hours. Insets were magnified images from each photograph at low magnification. Cell nuclei were stained with DAPI in blue. Scale bar: 20 μm. (B) RT-PCR assays demonstrated the mRNA expression of ACTN4 in NSPCs, with 293 T cell line as positive control. (C) Western blotting assays indicated the protein expression of ACTN4 in NSPCs, with 293 T cell line as positive control.
Figure 5. PO upregulated ACTN4 expression, compared to PLL and FN.
(A) The immunostaining images indicated ACTN4 expression (green) on PLL, PO and FN. Cell nuclei were stained with DAPI in blue. Scale bar: 20 μm. (B) Bar graphs summarized semi-quantitative results from (A). ***P < 0.01, one-way ANOVA followed by Tukey’s post hoc test (n = 3 for each group). (C) Western blotting assays demonstrated the different expression level of ACTN4 on PLL, PO and FN after 12 hours for migration. Bands were analyzed using the Image Lab™ software for relative density and normalized to GAPDH control. ***P < 0.01, one-way ANOVA followed by Tukey’s post hoc test (n = 3 for each group).
ACTN4 played a pivotal role in migration induced by PO
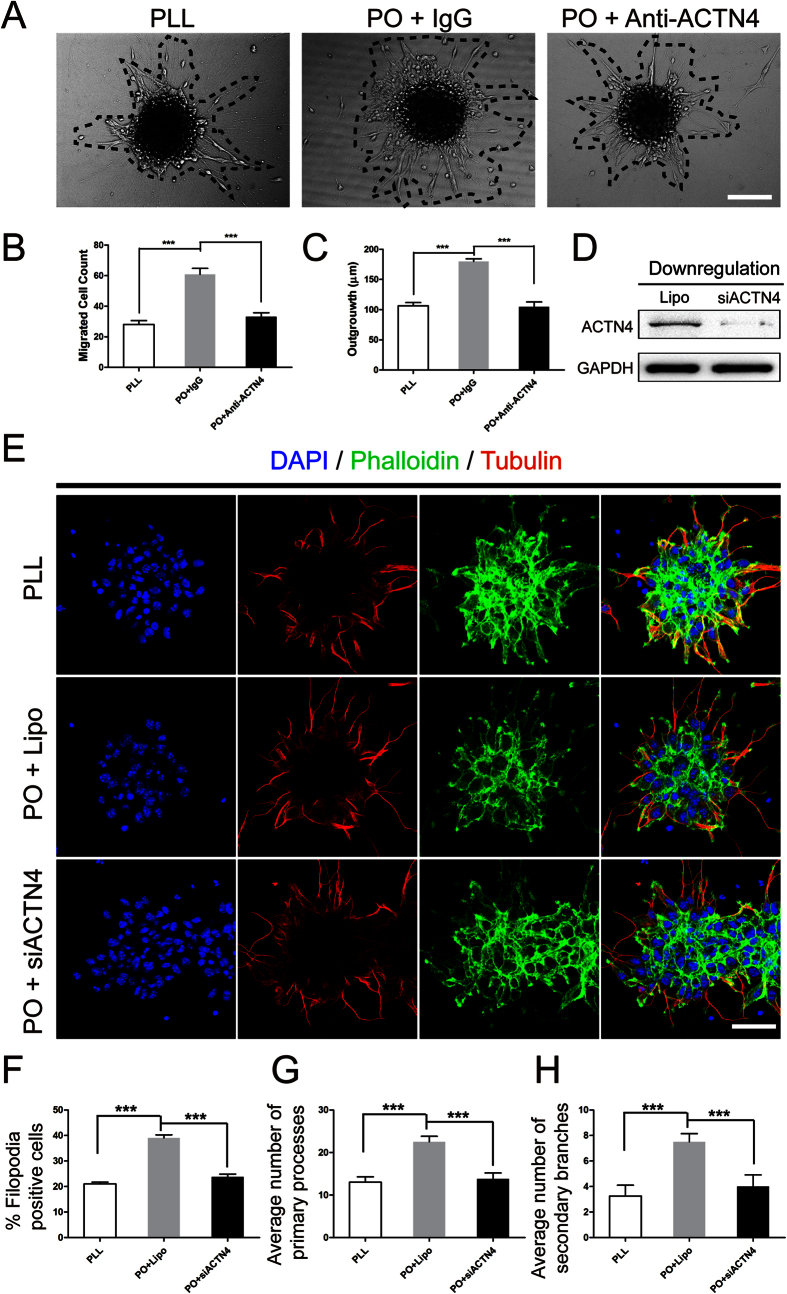
To further unravel the role of ACTN4 playing in PO induced migration of NSPCs, we first used either 1 μg/ml neutralizing antibody against rabbit ACTN4 or control IgG diluted in 1 ml enrichment medium on PO, meanwhile, PLL as control. The data indicated that NSPCs cultured on PO migrated larger number than that on PLL. While, the migrated cell number and distance decreased significantly with the addition of neutralizing antibody after incubation for 12 hours (Fig. 6A–C). Furthmore, to confirm the results above, the downregulation of ACTN4 expression by siRNA were performed. The results indicated the percent of filopodia formation decreased greatly to the PLL level (Fig. 6D,E). At the same time, the migrated cell number and distance showed the same tendency as the treatment of neutralizing antibody (Fig. 6D,F,G). Taken together, these data indicated that ACTN4 played a pivotal role in enhancing migration of NSPCs induced by PO and the underlying mechnism was promoting filopodia formation.
Figure 6. ACTN4 played a pivotal role in migration induced by PO.
(A) Neutralizing Antibody Assays showed the role of ACTN4 playing in migration induced by PO. Neurospheres were plated in PLL or PO pre-coated 24-well plates and images were captured by phase contrast microscopy after 12 hours. Normal rabbit IgG was used as a negative control. (B) Summarized graph indicated the number of migration cells from neurospheres after neutralizing antibody assays, with PLL as control. ***P < 0.01, one-way ANOVA followed by Tukey’s post hoc test (n = 5 for each group). (C) Quantitative analysis of migration distance from neurospheres after neutralizing antibody assays, with PLL as control. ***P < 0.01, one-way ANOVA followed by Tukey’s post hoc test (n = 5 for each group). (D) Blotting bands showed downregulation of ACTN4 using siRNA transfection. Lipofectamine (Lipo) was used as a negative control. (E) The effect of ACTN4 on filopodia formation using phalloidin staining (green) and the cage-like microtubule structure with tubulin immunostaining (red) in neurospheres with/without ACTN4 downregulation using siRNA transfection, compared to PLL control. (F) Bar graph indicated the percent of filopodia formation. ***P < 0.01, one-way ANOVA followed by Tukey’s post hoc test (n = 5 for each group). (G) Quantitative analysis of average number of primary leading processes. ***P < 0.01, one-way ANOVA followed by Tukey’s post hoc test (n = 5 for each group). (H) Quantitative analysis of average number of secondary branches. ***P < 0.01, one-way ANOVA followed by Tukey’s post hoc test (n = 5 for each group).
Discussion
NSPCs, with the ability of multipotency and differentiation into three main subtypes of neural cells, hold promise for cell replacement therapy of many neurological disorders such as Parkinson’s disease22, traumatic brain injury23 and ischemic stroke24,25,26. To date, how to obtain sufficient NSPCs and direct their proper differentiation is a big challenge. Our previous study has indicated that PO could significantly increase NSPCs proliferation and induce preferred differentiation into neurons and oligodendrocytes in vitro9. While, study has shown that NSPCs proliferate in the Subventricular Zone (SVZ), but only a few proliferated NSPCs could migrate to the lesions after ischemic stroke in adult rats10. It implies that the shortage of functional NSPCs in lesions might due to the limitation of NSPCs migration potential after brain injury. Biomaterials for cell replacement therapies should facilitate proliferation, differentiation and migration of NSPCs. Our previous study has uncovered the effect of PO on proliferation and differentiation of NSPCs. Meanwhile, studies have proved that PLL is likely to enhance inflammatory responses27,28,29. And, FN would influence the proliferation potential of NSPCs in vitro30. While, PO usually serves as one component of alginate microcapsule system to deliver medicine and transplanted cells31,32. Hence, it is necessary to find out whether PO could improve the migratory potential of NSPCs and its underlying mechnism might be different from our previous study.
In the present study, we identify ACTN4 expressed in NSPCs and it plays a pivotal role in migration of NSPCs induced by PO. ACTN4, first cloned by Honda et al.33, acts as a significant mediator to regulate cytoskeletal organization of the actin network by cross-linking F-actin17,34. Previous studies have shown that increased ACTN4 expression promotes the motility of various cancer cell migration and metastasis14,15,17,35,36, while its downregulation evidently decreases the migration potential of lung cancer metastasis to the brain36. Here, our results indicate that ACTN4 facilitates NSPCs migration induced by PO, which was consistent with previous studies in malignant phenotypes of cancer cells35,36,37, compared to PLL and FN. It is the first time to provide exact evidence, to our limited knowledge, to illustrate the expression and its function of ACTN4 in NSPCs.
Meanwhile, PO enhances filopodia formation of NSPCs, which exhibits positive correlation with ACTN4 expression. ACTN4, one of actin-bundling proteins, could directly cross-link the filopodia formation20,38. Filopodia-thin, finger-like and highly dynamic actin-rich membrane protrusions, could extend out from cell edge when migration and its extension could be mediated by various molecules such as small GTPases of the Rho family (i.e. Rac1 and Cdc42)33,39. The NSPCs on PLL, PO and FN show different migratory potential due to the different expression levels of ACTN4 to regulate filopodia formation. As we previously showed that NSPCs on PO increased higher level of p-ERK9, which is a regulator to mediate the expression of Rac1 and RhoA40,41. Hence, the underlying mechnism for PO promoting migration of NSPCs might be due to the activition of ERK/small GTPases/ACTN4 signaling cascade. But which member of small GTPases is dominated needs to be addressed in the future investigation.
In general, our present study has revealed pivotal effect of PO on NSPCs migration and its possible underlying mechanism, which is a significant supplement of our previous study. Together, our results suggest that PO could serve as a better candidate for cultivating NSPCs in vitro, even for transplantation biomaterial in vivo, in comparison with PLL and FN, for basic and preclinical research after various neurological diseases and injuries.
Materials and Methods
Animal
This study was carried out according to the China’s animal welfare legislation for the care and use of animals and approved by the Third Military Medical University Chongqing, China. Every effort was made to minimize the number of animals and relieve their suffering. E14.5 Sprague-Dawley rats were sacrificed after anesthetized with pentobarbital (60 mg/kg intraperitoneally).
Primary Neural Stem/Progenitor Cells Culture
Primary rat NSPCs from the cortices of fetal E14.5 Sprague–Dawley pups were isolated and cultured as previously described9. Neocortical tissues were trypsinized by 0.25% trypsin (GIBCO, Grand Island, NY) for 30 minutes at 37 °C. Soybean trypsin inhibitor (2.8 mg/mL, Roche Diagnosis, Mannheim, Germany) was in addition to inhibit trypsin activity. After washing twice with DMEM/F12 medium (GIBCO, Grand Island, NY), the samples were triturated using a fire-polished Pasteur pipette and passed through a 100 μm Nylon cell strainer (BD Falcon, San Jose, CA) to harvest dissociated cell suspensions. Then, they were seeded at an initial cell density of 1 × 105 cells/mL in DMEM/F12 medium supplemented with 20 ng/ml recombinant murine FGF-basic (Peprotech, Rocky Hill, NJ), 20 ng/ml recombinant murine EGF (Peprotech, Rocky Hill, NJ) and 2% B-27 supplement (GIBCO, Grand Island, NY) for 24 hours at 37 °C under humidified 5% CO2 conditions. Neurospheres were seeded on 35-mm different pre-coated dishes (18 hours) after washing twice with sterile cell culture grade water according to the manufacturer’s instructions respectively. The pre-coated substrates were as follows: 100 μg/ml Poly-L-lysine (Sigma-Aldrich, St. Louis, MO), 10 μg/ml poly-L-ornithine (Sigma-Aldrich, St. Louis, MO) and 20 μg/ml Fibronectin (Sigma-Aldrich, St. Louis, MO). Half volume of culture medium was exchanged every 3 days.
Immunofluorescence
For fluorescence, neurospheres, adhered to different pre-coated coverslips, were fixed with 4% paraformaldehyde in 0.01 M phosphate-buffered saline (pH 7.4) for 2 hours at room temperature and blocked with 5% v/v fetal bovine serum or with 0.5% v/v Triton-X 100 (Sigma-Aldrich, St. Louis, MO) in PBS. Neurospheres were incubated with rabbit polyclonal to Doublecortin (DCX, 1:200, Abcam, Cambridge, UK), rabbit polyclonal to glial fibrillary acidic protein (GFAP, 1:100, Proteintech Group, Inc, Beijing, China), rabbit polyclonal to Olig2 (1:100, Proteintech Group, Inc, Beijing, China), rabbit polyclonal to ACTN4 (1:100, Proteintech Group, Inc, Beijing, China), mouse monoclonal to Tubulin (1:100, Beyotime, Beijing, China) or mouse monoclonal to Nestin (1:200, Abcam, Cambridge, UK) overnight at 4 °C and then relative fluorescence secondary antibodies were incubated for 2 hours at room temperature. Cell nuclei were stained with 4′-6-Diamidino-2-phenylindole (DAPI, Sigma-Aldrich, St. Louis, MO) for 10 minutes at room temperature. Then, coverslips were mounted onto glass slides and the images were captured by confocal microscope (Carl Zeiss, LSM780, Weimar, German) and examined using Zen 2011 software (Carl Zeiss, Weimar, Germany).
NPSCs Migration Assay
For neurospheres migration assay, neurospheres were plated on different substrates pre-coated 24-well plates and the quantification methods were previously described42. Images were captured by phase contrast microscopy at 10 × once every 2 h for 1 day allowing for the tracking of NPSCs migration out of the neurospheres. Phase contrast images (n = 6 per sample well) were analyzed for longest sphere diameter as shown in Fig. 2B using a custom-designed MatLab program (MathWorks, Inc., Natick, MA) and were normalized to baseline measurements taken 2 h after plating. All neurosphere experiments were performed in four wells, and results were representative of at least three independent experiments.
For single cell migration assays, NSPCs were allowed to migrate through 12 μm pore Millicell cell culture inserts (Millipore, Temecula, CA) in accordance with the manufacturer’s instructions as previously43. Insert membranes were coated with substrates as described previously and NPSCs were plated in mitogenic growth-factor free media (n = 5 replicates per group). The upper chambers were seeded with 100 μl (1 × 105) NSPCs. The lower chambers were filled with 600 μl DMEM/F-12 medium supplemented with 10% FBS which served as a chemoattractant. The NSPCs were allowed to migrate from the upper to lower chambers for 12 hours at 37 °C in a humidified incubator with 5% CO2. Non-migratory cells were removed from the top of the membrane with a cotton swab and the cells attached to the lower surface of membrane were fixed in 4% paraformaldehyde at room temperature for 30 min and counterstained with DAPI, and the number was counted under fluorescence microscope. A total of 5 fields were counted for each transwell filter.
Western Blotting
Neurospheres adhered to different precoated dishes were homogenized with RIPA (Sigma-Aldrich, St. Louis, MO) supplemented with protease inhibitor cocktail (Roche, Indianapolia, IN, USA). The protein concentration was measured by enhanced BCA Protein Assay Kit (Beyotime, Beijing, China). Proteins (15 μg/lane) were separated by 10% SDS-PAGE under reducing conditions and electroblotted to polyvinylidene difluoride membranes (Roche, Indianapolia, IN, USA). After membranes were blocked in 5% skimmed milk in TBST at room temperature for 2 hours. Then, they were incubated with rabbit polyclonal to ACTN4 (1:1000, Proteintech Group, Inc, Beijing, China) and mouse monoclonal to GAPDH (1:1000, Santa Cruz Biotechnology, CA, USA) overnight at 4 °C. After incubation with peroxidase-conjugated (HRP)-conjugated secondary IgG (Zsgb-bio, 1:5000) for 2 hours at room temprature. All membranes were detected by ChemiDoc™ XRS+ imaging system (Bio-Rad, California, USA) using the WesternBright ECL Kits (Advansta, Menlo Park, CA, USA). Densitometric measurement of each membrane was performed using Image Lab™ software (Bio-Rad, California, USA). GAPDH, an internal control, was used to normalize the expression level of each protein.
Reverse Transcription Polymerase Chain Reaction
Total RNA was extracted from neurospheres with TaKaRa MiniBEST Universal RNA Extraction Kit according to the manufacturer’s instructions (TaKaRa Bio Inc. Tokyo, Japan) and contaminating DNA was depleted with RNase-free DNase (Qiagen, Valencia, CA). Total RNA (2 μg) was reverse transcribed into cDNA with PrimeScript™ II 1st Strand cDNA Synthesis Kit (TaKaRa Bio Inc. Tokyo, Japan) and an aliquot of cDNA mixture (0.2%) was used as polymerase chain reaction (PCR) templates. ACTN4 primers were purchased from Invitrogen (rs138139611, Thermo Fisher Scientific, Waltham, MA, USA), GAPDH (forward, 5′-GGC CCC TCT GGA AAG CTG TG-3′; reverse, 5′-CCA GGC GGC ATG GCA GAT C-3′). The annealing temperature for PCR was 55 °C and carried out for 30 cycles. Gels were imaged by ChemiDoc™ XRS+ System (Bio-Rad, California, USA).
Filopodia Detection
To visualize F-actin for filopodia formation assays, cells were incubated with 488-phalloidin (Life Technologies, Waltham, MA, USA). Samples were mounted in the ProLong Gold Antifade Reagent with DAPI (Sigma-Aldrich, St. Louis, MO), and results were analyzed with a confocal microscope (Carl Zeiss, LSM780, Weimar, German) and examined using Zen 2011 software (Carl Zeiss, Weimar, Germany).
Neutralizing Antibody Assay
For neutralizing antibody group, neurospheres were incubated in 1 μg/ml neutralizing antibody against rabbit ACTN4 antibody and normal rabbit IgG as control before seeding in PO pre-coated 24-well plates. Then neurospheres were plated in 24-well plates and images were captured by phase contrast microscopy at 10 × once every 2 h for 1 day allowing for the tracking of NPSCs migration out of the neurosphere. The quantification methods were as previously described.
ACTN4 siRNA transfection
ACTN4-specific siRNA (sc-43102) was purchased from Santa Cruz Biotechnology (CA, USA). Transfection of siRNA was performed using Lipofectamine RNAiMAX (Lipo, Invitrogen, Waltham, MA, USA) transfection reagent according to the manufacturer’s instructions. And the same amout of Lipofectamine RNAiMAX was used as negative control.
Statistical methods
All data were showed as mean ± SEM and statistical analyses were carried out with SPSS v19.0 (SPSS Inc, Chicago, IL). One-way ANOVA followed by Tukey’s post hoc test was used to define statistical significance. p < 0.05 was considered statistically significant and p < 0.01 was considered as obvious statistical significance.
Additional Information
How to cite this article: Ge, H. et al. Poly-L-ornithine enhances migration of neural stem/progenitor cells via promoting α-Actinin 4 binding to actin filaments. Sci. Rep. 6, 37681; doi: 10.1038/srep37681 (2016).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
This work was supported by grants from National Natural Science Foundation of China (No. 81371340, 81301058 and 81560217) and National Key Basic Research Program of China (2014CB541600). We also say thanks to professor Keith R. Pennypacker (Department of Molecular Pharmacology and Physiology, Morsani College of Medicine, University of South Florida, Tampa, Florida, USA) for reviewing our manuscript and valuable comments.
Footnotes
The authors declare no competing financial interests.
Author Contributions H.F. and R.H. designed the study; H.F.G., A.Y.Y. and J.Y.C. performed the study, data analysis and interpretation; J.C.Y. and Y.Y. performed the data analysis; W.S.D.M. and L.T. performed data analysis and interpretation; Y.Y., W.X.C. and C.L. provided illustrations; H.F., H.F.G., and R.H. contributed in writing the manuscript. All authors reviewed the manuscript.
References
- Keller G. M. In vitro differentiation of embryonic stem cells. Curr Opin Cell Biol 7, 862–9 (1995). [DOI] [PubMed] [Google Scholar]
- Smith A. G. Embryo-derived stem cells: of mice and men. Annu Rev Cell Dev Biol 17, 435–62 (2001). [DOI] [PubMed] [Google Scholar]
- Gadue P., Huber T. L., Nostro M. C., Kattman S. & Keller G. M. Germ layer induction from embryonic stem cells. Exp Hematol 33, 955–64 (2005). [DOI] [PubMed] [Google Scholar]
- Polak J. M. & Bishop A. E. Stem cells and tissue engineering: past, present, and future. Ann N Y Acad Sci 1068, 352–66 (2006). [DOI] [PubMed] [Google Scholar]
- Nakaji-Hirabayashi T., Kato K., Arima Y. & Iwata H. Oriented immobilization of epidermal growth factor onto culture substrates for the selective expansion of neural stem cells. Biomaterials 28, 3517–29 (2007). [DOI] [PubMed] [Google Scholar]
- Nakaji-Hirabayashi T., Kato K. & Iwata H. Surface-anchoring of spontaneously dimerized epidermal growth factor for highly selective expansion of neural stem cells. Bioconjug Chem 20, 102–10 (2009). [DOI] [PubMed] [Google Scholar]
- Chetty S. et al. Stress and glucocorticoids promote oligodendrogenesis in the adult hippocampus. Mol Psychiatry 19, 1275–83 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan L. A., Rebaza L. M., Derzic S., Schwartz P. H. & Monuki E. S. Regulation of human neural precursor cells by laminin and integrins. J Neurosci Res 83, 845–56 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge H. et al. Poly-L-ornithine promotes preferred differentiation of neural stem/progenitor cells via ERK signalling pathway. Sci Rep 5, 15535 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitz M. A., Lo E. H. & Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron 67, 181–98 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylanne J., Scheffzek K., Young P. & Saraste M. Crystal structure of the alpha-actinin rod reveals an extensive torsional twist. Structure 9, 597–604 (2001). [DOI] [PubMed] [Google Scholar]
- Park S. S. et al. C(16)-Ceramide-induced F-actin regulation stimulates mouse embryonic stem cell migration: involvement of N-WASP/Cdc42/Arp2/3 complex and cofilin-1/alpha-actinin. Biochim Biophys Acta 1831, 350–60 (2013). [DOI] [PubMed] [Google Scholar]
- Djinovic-Carugo K., Young P., Gautel M. & Saraste M. Structure of the alpha-actinin rod: molecular basis for cross-linking of actin filaments. Cell 98, 537–46 (1999). [DOI] [PubMed] [Google Scholar]
- An H. T., Yoo S. & Ko J. alpha-Actinin-4 induces the epithelial-to-mesenchymal transition and tumorigenesis via regulation of Snail expression and beta-catenin stabilization in cervical cancer. Oncogene (2016). [DOI] [PubMed] [Google Scholar]
- Brandi J. et al. Secretome protein signature of human pancreatic cancer stem-like cells. J Proteomics 136, 1–12 (2016). [DOI] [PubMed] [Google Scholar]
- Miura N. et al. Efficacy of adjuvant chemotherapy for non-small cell lung cancer assessed by metastatic potential associated with ACTN4. Oncotarget (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal N. et al. MTBP suppresses cell migration and filopodia formation by inhibiting ACTN4. Oncogene 32, 462–70 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otey C. A. & Carpen O. Alpha-actinin revisited: a fresh look at an old player. Cell Motil Cytoskeleton 58, 104–11 (2004). [DOI] [PubMed] [Google Scholar]
- Reynolds B. A. & Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 255, 1707–10 (1992). [DOI] [PubMed] [Google Scholar]
- Jacquemet G., Hamidi H. & Ivaska J. Filopodia in cell adhesion, 3D migration and cancer cell invasion. Curr Opin Cell Biol 36, 23–31 (2015). [DOI] [PubMed] [Google Scholar]
- Shinohara R. et al. A role for mDia, a Rho-regulated actin nucleator, in tangential migration of interneuron precursors. Nat Neurosci 15, 373-80, s1–2 (2012). [DOI] [PubMed] [Google Scholar]
- Kim J. H. et al. Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson’s disease. Nature 418, 50–6 (2002). [DOI] [PubMed] [Google Scholar]
- Alvarez Z. et al. Neurogenesis and vascularization of the damaged brain using a lactate-releasing biomimetic scaffold. Biomaterials 35, 4769–81 (2014). [DOI] [PubMed] [Google Scholar]
- Park K. J., Park E., Liu E. & Baker A. J. Bone marrow-derived endothelial progenitor cells protect postischemic axons after traumatic brain injury. J Cereb Blood Flow Metab 34, 357–66 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karelina K., Alzate-Correa D. & Obrietan K. Ribosomal S6 kinase regulates ischemia-induced progenitor cell proliferation in the adult mouse hippocampus. Exp Neurol 253, 72–81 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y. et al. Neural stem cell protects aged rat brain from ischemia-reperfusion injury through neurogenesis and angiogenesis. J Cereb Blood Flow Metab 34, 1138–47 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand B. L., Gaserod O., Kulseng B., Espevik T. & Skjak-Baek G. Alginate-polylysine-alginate microcapsules: effect of size reduction on capsule properties. J Microencapsul 19, 615–30 (2002). [DOI] [PubMed] [Google Scholar]
- Tam S. K. et al. Biocompatibility and physicochemical characteristics of alginate-polycation microcapsules. Acta Biomater 7, 1683–92 (2011). [DOI] [PubMed] [Google Scholar]
- Hillberg A. L. et al. Improving alginate-poly-L-ornithine-alginate capsule biocompatibility through genipin crosslinking. J Biomed Mater Res B Appl Biomater 101, 258–68 (2013). [DOI] [PubMed] [Google Scholar]
- Sun T. et al. A comparison of proliferative capacity and passaging potential between neural stem and progenitor cells in adherent and neurosphere cultures. Int J Dev Neurosci 29, 723–31 (2011). [DOI] [PubMed] [Google Scholar]
- McQuilling J. P. et al. New alginate microcapsule system for angiogenic protein delivery and immunoisolation of islets for transplantation in the rat omentum pouch. Transplant Proc 43, 3262–4 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna O., Moya M. L., Opara E. C. & Brey E. M. Synthesis of multilayered alginate microcapsules for the sustained release of fibroblast growth factor-1. J Biomed Mater Res A 95, 632–40 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda K. et al. Actinin-4, a novel actin-bundling protein associated with cell motility and cancer invasion. J Cell Biol 140, 1383–93 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao H., Wu C. & Wells A. Phosphorylation of alpha-actinin 4 upon epidermal growth factor exposure regulates its interaction with actin. J Biol Chem 285, 2591–600 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlicher A. J. et al. Alpha-actinin binding kinetics modulate cellular dynamics and force generation. Proc Natl Acad Sci USA 112, 6619–24 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y. et al. ACTN4 and the pathways associated with cell motility and adhesion contribute to the process of lung cancer metastasis to the brain. BMC Cancer 15, 277 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda K. The biological role of actinin-4 (ACTN4) in malignant phenotypes of cancer. Cell Biosci 5, 41 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arjonen A., Kaukonen R. & Ivaska J. Filopodia and adhesion in cancer cell motility. Cell Adh Migr 5, 421–30 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierro-Gonzalez J. C., White M. D., Silva J. C. & Plachta N. Cadherin-dependent filopodia control preimplantation embryo compaction. Nat Cell Biol 15, 1424–33 (2013). [DOI] [PubMed] [Google Scholar]
- Hetmanski J. H., Zindy E., Schwartz J. M. & Caswell P. T. A MAPK-Driven Feedback Loop Suppresses Rac Activity to Promote RhoA-Driven Cancer Cell Invasion. PLoS Comput Biol 12, e1004909 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slomiany B. L. & Slomiany A. Helicobacter pylori-elicited induction in gastric mucosal matrix metalloproteinase-9 (MMP-9) release involves ERK-dependent cPLA activation and its recruitment to the membrane-localized Rac1/p38 complex. Inflammopharmacology (2016). [DOI] [PubMed] [Google Scholar]
- Addington C. P., Pauken C. M., Caplan M. R. & Stabenfeldt S. E. The role of SDF-1alpha-ECM crosstalk in determining neural stem cell fate. Biomaterials 35, 3263–72 (2014). [DOI] [PubMed] [Google Scholar]
- Wang G. et al. Formylpeptide Receptors Promote the Migration and Differentiation of Rat Neural Stem Cells. Sci Rep 6, 25946 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]