Abstract
Background: Green tea has been suggested to improve cardiovascular disease risk factors, including circulating lipid variables. However, current evidence is predominantly based on small, short-term randomized controlled trials conducted in diverse populations.
Objective: The aim of this study was to examine the efficacy and impact of green tea extract (GTE) supplementation high in epigallocatechin gallate (EGCG) on blood lipids in healthy postmenopausal women.
Design: This was an ancillary study of a double-blind, randomized, placebo-controlled, parallel-arm trial investigating the effects of a GTE supplement containing 1315 mg catechins (843 mg EGCG) on biomarkers of breast cancer risk. Participants were randomly assigned to receive GTE (n = 538) or placebo (n = 537) and were stratified by catechol-O-methyltransferase (COMT) genotype activity (high COMT compared with low or intermediate COMT genotype activity). They consumed either 4 GTE or identical placebo capsules daily for 12 mo. A total of 936 women completed this substudy. Circulating lipid panels including total cholesterol (TC), HDL cholesterol, and triglycerides were measured at baseline and at months 6 and 12.
Results: Compared with placebo, 1-y supplementation with GTE capsules resulted in a significant reduction in circulating TC (−2.1% compared with 0.7%; P = 0.0004), LDL cholesterol (−4.1% compared with 0.9%; P < 0.0001) and non-HDL cholesterol (−3.1% compared with 0.4%; P = 0.0032). There was no change in HDL-cholesterol concentration, but triglyceride concentrations increased by 3.6% in the GTE group, whereas they decreased by 2.5% in the placebo group (P = 0.046). A significant reduction in TC was observed only among women with high (i.e., ≥200 mg/dL) baseline TC concentrations (P-interaction = 0.01) who consumed GTE capsules. The effect of GTE on the increase in triglycerides was mainly observed among obese women and statin users (P-interaction = 0.06).
Conclusion: Supplementation with GTE significantly reduced circulating TC and LDL-cholesterol concentrations, especially in those with elevated baseline TC concentrations. This trial was registered at clinicaltrials.gov as NCT00917735.
Keywords: green tea, EGCG, blood lipids, postmenopausal, cardiovascular, randomized controlled trial
INTRODUCTION
Cardiovascular disease (CVD)7 remains the primary cause of death in the United States and worldwide (1, 2). Hyperlipidemia is considered to be a major risk factor for CVD, and currently a high percentage of US women have elevated concentrations of total cholesterol (TC) and LDL cholesterol (∼45.0% and 32.0%, respectively) (3). Existing guidelines on cholesterol management mainly include LDL-lowering drugs, such as β-hydroxy-β-methylglutaryl coenzyme A reductase inhibitors (statins), and lifestyle changes such as weight loss, physical activity, or diet therapy (4). Due to side effects associated with statin use, there is great interest in dietary modifications to lower cholesterol.
Green tea as a dietary factor has been shown to lower the risk of a number of chronic diseases, including heart disease (5–8). Although not consistent, a number of small short-term human trials showed that green tea and its major bioactive constituent, epigallocatechin gallate (EGCG), exert hypocholesterolemic effects, particularly on lowering the concentrations of TC and LDL cholesterol. Current meta-analyses of green tea and lipid clinical trials, all with short durations (3–24 wk), showed that green tea extract (GTE) or green tea beverage significantly decreased both TC (by 5–7 mg/dL) and LDL cholesterol (by 2–7 mg/dL) in various populations (9–12).
Catechol-O-methyltransferase (COMT) is one of the enzymes involved in the methylation metabolism of tea catechins. Polymorphisms in COMT, in particular a G-to-A transition at codon 158 of COMT (single nucleotide polymorphism rs4680), have been shown to influence the metabolism of tea polyphenols (13), such that individuals with low COMT genotype activity metabolize and excrete catechins more slowly. As a result, they may retain more tea catechins in their bodies and derive greater lipid-lowering benefit from tea consumption.
The present randomized controlled trial (RCT) was an ancillary study of a large RCT conducted to evaluate the effects of a GTE on breast cancer risk biomarkers in postmenopausal women who were at high risk of breast cancer due to high breast density. Blood lipid concentrations in postmenopausal women are of interest due to their increased risk and incidence of developing heart disease (14). We hypothesized that supplementation with a concentrated GTE that included ∼800 mg EGCG for 1 y would significantly reduce circulating concentrations of TC and LDL cholesterol and increase serum HDL-cholesterol concentrations. We also explored the potential modifying effects of the COMT genotype activity on GTE supplementation, speculating that those with the low-activity COMT genotype would show the greatest response to GTE supplementation. To our knowledge, this is the largest and longest RCT to evaluate the effects of green tea catechins on lipids in postmenopausal women. Significant findings may provide additional avenues of treatment or adjunct care for risk management in this and similar populations.
METHODS
Study population and recruitment
The current trial is a substudy of the Minnesota Green Tea Trial (MGTT), which was previously described in detail elsewhere (15). Briefly, the MGTT was a double-blind, placebo-controlled, randomized clinical trial (clinicaltrials.gov; NCT00917735). The purpose of the MGTT was to evaluate the effects of consumption of GTE on a number of breast cancer risk biomarkers, including mammographic density, circulating reproductive hormones, insulin-like growth factor axis proteins, urinary estrogens and estrogen metabolites, and markers of oxidative stress. Participants for this study were women with differing COMT genotypes at high risk of breast cancer due to having dense breast tissue.
Participants were recruited between 2009 and 2013 on the basis of age (50–70 y old) and breast density as recorded in routine mammogram reports (heterogeneously or extremely dense breasts). Exclusionary criteria included the following: current smoker; use of menopausal hormone therapy (within the past 6 mo); weight fluctuation of >10 pounds (4.5 kg) within the past year; had a menstrual period within the past 12 mo; diabetic; positive for hepatitis B or C; current user of >10 prescription medications; current user of methotrexate or etanercept; elevated liver enzymes >1.5 upper limit of normal; intake of >7 alcoholic drinks/wk; any history of breast cancer, ovarian cancer, or proliferative breast disease; history of any other cancer within the past 5 y; BMI (in kg/m2) <18.5 or >40; and consumer of ≥1 cup green tea/wk.
After an initial phone screening, eligible participants attended an orientation session. All of the participants signed a consent form before proceeding with the study. Consented women attended a clinical screening to assess further eligibility. The clinical screening included measurement of anthropometric variables and a small nonfasting blood draw for COMT genotyping, hepatic function, and serologic markers of hepatitis B or C virus. If eligibility was confirmed at their clinical screening, the women were enrolled in the study. Randomization was performed by Investigational Drug Services at the University of Minnesota Medical Center, Fairview, by using a permuted block method in blocks of 8, stratified by COMT genotype. The randomization code was kept confidential from all participants and investigators throughout the study and during analysis, with the exception of the study biostatistician. The MGTT was conducted according to the guidelines described in the Declaration of Helsinki and was approved by the institutional review boards of the University of Minnesota, the Park Nicollet Institute, the University of Pittsburgh, and the University of Southern California.
Study protocol
Nutrient and calorie intakes were determined and analyzed for each participant by completion of a Diet History Questionnaire (developed by the National Cancer Institute) at baseline and month 12 visits. Height was measured at screening, baseline (month 0), and month 12 visits. Weight and vital signs, including systolic and diastolic blood pressure, heart rate, respiration, and temperature, were collected at screening, baseline, and then every 3 mo throughout the study. After randomization, participants attended their baseline visit for fasting blood draws, measurement of vital signs, return of their 24-h urine collections, and to receive their first 3-mo supply of the study supplements. Baseline, month 6, and month 12 were the most comprehensive visits and included a longer overnight fasting blood draw (between 0630 and 1000) for biochemical assays including a lipid panel, a 24-h urine collection, and completion of the Diet History Questionnaire. A comprehensive health history questionnaire was completed at the baseline visit, and a Menopause-Specific Quality of Life questionnaire was completed at baseline and months 6 and 12. A small volume of blood was drawn monthly through month 6 for monitoring of liver function enzymes. After month 6, participants returned to the clinic once every 3 mo for the remainder of the study (totaling 2 visits past the month 6 time point: months 9 and 12). To evaluate treatment compliance, participants were asked to return all unused capsules at each clinic visit. Accordingly, compliance was determined by dividing the number of consumed capsules by the number the participants were supposed to consume. As a secondary measure of compliance, spot urine samples were also collected at months 3 and 9 for all participants and urinary catechins, including epigallocatechin (EGC) and epicatechin (EC), were measured in a randomly selected 10% of participants.
Blood collection and processing
Serum collected at the clinic for analysis was separated from whole blood by centrifugation at 1500 × g for 25 min at 4°C, then separated into 1.5-mL aliquots and stored at −80°C until analysis. Frozen samples were sent in batches to Quest Diagnostic laboratories for measurement of a standard lipid panel. Batches were prepared by arranging participants into GTE-placebo pairs, and all samples from each participant and pair in a batch were analyzed together in the same analysis batch, with all staff blinded to the assigned treatment code. Standard lipid panels including TC, HDL cholesterol, and triglycerides were measured in fasting serum samples. All biospecimen analyses were performed at Quest Diagnostics by using the spectrophotometry methodology with the Beckman Olympus AU5400 chemistry analyzer. Non-HDL cholesterol was calculated by subtracting HDL cholesterol from TC concentrations, and LDL cholesterol was calculated by using the Friedewald method for participants with triglycerides <400 mg/dL (16). The interassay CVs obtained from quality-control samples were 1.4% for TC, 2.6% for HDL cholesterol, and 2.1% for triglycerides. DNA was extracted from buffy coat fractions by using the Qiagen DNeasy Blood and Tissue Kit method (Qiagen, Inc.), and COMT genotyping (rs4680) was conducted by a TaqMan PCR Core Reagent kit (Applied Biosystems) according to the manufacturer’s instructions.
Study supplement
The study supplement, Green Tea Extract Catechin Complex (Corban Complex GTB; abbreviated as “GTE” in this article), was manufactured and provided by Corban Laboratories/Eniva Nutraceutics. The GTE was made from green tea leaves grown by Youshan Tea Farm in China and supplied by Taiyo Green Power Co. Ltd. in China. Corban provided 8 batches of green tea supplement over the course of the trial. Samples were randomly drawn from each new batch for catechin composition analysis by 2 independent laboratories: Covance Laboratories and the laboratory of CS Yang (Rutgers University). Results for catechin compositions of capsules were similar between the 2 laboratories. A detailed description of catechin and caffeine composition of the treatment and placebo capsules was presented previously (15). GTE capsules were decaffeinated with <4 mg caffeine/capsule and, on average, provided 1315 mg catechins/d, including 843 mg EGCG, 202 mg epicatechin gallate, 107 mg EGC, and 107 mg EC. Placebo capsules were identical in appearance to the GTE and mainly composed of maltodextrin and cellulose (50.0% and 49.5% of each capsule’s weight, respectively) with no caffeine included. Supplements were dispensed by the Investigational Drug Services’ pharmacy at the University of Minnesota, and both participants and investigators were blinded to individual participant assignment throughout the entirety of the study. All of the capsules were stored and dispensed on the basis of individual patient schedules and were stored in a temperature-controlled, locked cabinet until given to participants by professional clinic staff at regular clinic visits. Participants were provided a 3-mo supply of supplement at the baseline, month 3, month 6, and month 9 visits (135 capsules of GTE or placebo were provided for each month). Each participant was instructed to take 2 capsules (either GTE or placebo) in the morning after breakfast and 2 capsules in the evening after dinner.
Statistical analyses
Statistical analyses were performed after both intention-to-treat (ITT) and per-protocol paradigms, but the results presented in this article are based on the ITT principle (Figure 1). The differences in the distributions of baseline demographic characteristics and dietary intakes between GTE and placebo participants were examined by Pearson’s chi-square test (categorical or nominal variables) or by Student’s t test (continuous variables).
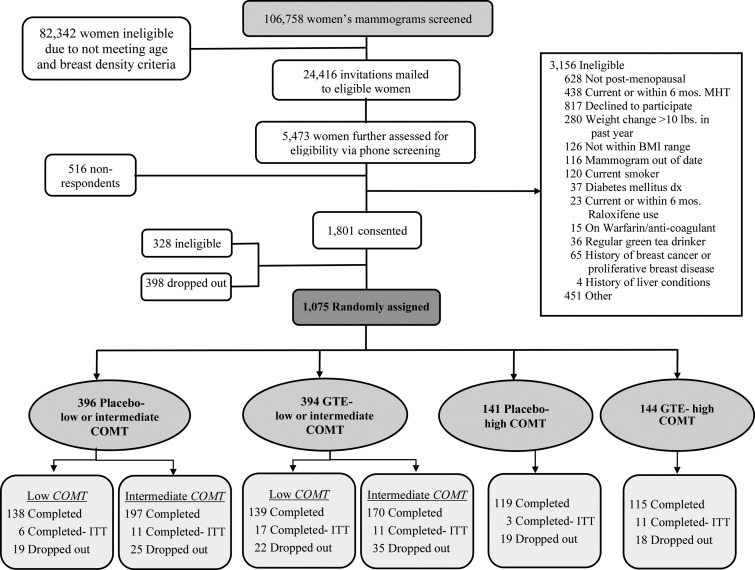
FIGURE 1.
Study participant diagram of recruitment, screening, randomization, and follow-up of the Minnesota Green Tea Trial. COMT, catechol-O-methyltransferase; dx, diagnosis; GTE, green tea extract; ITT, intention-to-treat; MHT, menopausal hormone therapy. Adapted from reference 17 with permission.
All serum lipids are expressed in milligrams per deciliter. The differences in the distributions of serum lipid concentrations at baseline between GTE and placebo participants were examined by using ANOVA, with adjustment for age and BMI. To assess the effect of GTE on lipid concentration, we first calculated the absolute change in concentration of a given serum lipid from baseline to months 6 and 12, respectively. We then used mixed-effects repeated-measures ANOVA to assess the magnitude of these changes in circulating lipid concentrations among participants in the GTE group compared with those in the placebo group. This model simultaneously examined the effect of treatment (i.e., GTE compared with placebo) on lipid concentration change overall as well as at months 6 and 12. We used the same ANOVA for stratified analysis to investigate the potential impact of certain participants’ characteristics at baseline, such as TC, BMI, regular statin use, triglycerides, COMT genotype, and time since menopause, on the effect of GTE on serum lipid concentrations. In all subgroup analyses, age and BMI at baseline were also adjusted for. The potential interaction effect of baseline characteristics and GTE on the change in lipid concentrations was examined by including a product term in the ANOVA. If the interaction was significant, we included the product term in the final model; otherwise, we dropped it from the final model that produced the results presented.
The MGTT sample size was based on a power calculation conducted for the primary endpoint of the MGTT (i.e., change in mammographic density). We further performed post hoc power calculations for the changes in blood lipids as primary outcome measures of this substudy. Assuming a 2-sided significance level of 0.05 and a total sample size of 936 women (468 in the GTE and 468 in the placebo group), we would have an 80% statistical power to detect a 6.1-mg/dL reduction in TC and a 5.1-mg/dL decrease in LDL-cholesterol concentrations in the GTE group compared with the placebo group. However, we did not have sufficient statistical power to examine the modifying effects of COMT genotype activity on GTE supplementation, which, in fact, was part of the exploratory nature of this substudy.
Statistical analyses were carried out by using SAS software version 9.3 (SAS Institute). Reported P values were 2-sided, and P < 0.05 was considered significant for lifestyle and dietary intake (Tables 1 and 2). Given the exploratory nature of the present analysis on the effect of GTE on blood lipid concentrations, Bonferroni correction was used to adjust for multiple comparisons, and P < 0.05 was considered significant (Tables 3–6, Supplemental Tables 1 and 2).
TABLE 1.
Baseline demographic and other selected characteristics of study participants1
| Variables | GTE (n = 463) | Placebo (n = 473) | P | |
| Age at baseline, y | 60.02 ± 4.89 | 59.65 ± 5.04 | 0.26 | |
| Weight, kg | 67.34 ± 10.66 | 67.40 ± 10.33 | 0.93 | |
| BMI, kg/m2 | 25.16 ± 3.72 | 25.01 ± 3.73 | 0.53 | |
| Waist-to-hip ratio2 | 0.84 ± 0.07 | 0.83 ± 0.07 | 0.22 | |
| Race,3 n (%) | ||||
| White | 453 (98.05) | 457 (97.03) | 0.31 | |
| Nonwhite | 9 (1.95) | 14 (2.97) | ||
| Ethnicity,4 n (%) | ||||
| Hispanic | 5 (1.09) | 4 (0.86) | 0.72 | |
| Non-Hispanic | 453 (98.91) | 463 (99.14) | ||
| Smoking status,5 n (%) | ||||
| Never | 316 (68.55) | 324 (68.64) | 0.97 | |
| Former | 145 (31.45) | 148 (31.36) | ||
| Educational level (degree),6 n (%) | ||||
| High school or less | 27 (5.86) | 31 (6.61) | 0.08 | |
| Some college | 313 (67.90) | 286 (60.98) | ||
| Postgraduate or professional | 121 (26.25) | 152 (32.41) | ||
| Regular use of vitamin and/or mineral supplements,7,8 n (%) | ||||
| No | 42 (9.09) | 66 (13.95) | 0.02 | |
| Yes | 420 (90.91) | 407 (86.05) | ||
| Regular use of aspirin,8,9 n (%) | ||||
| No | 339 (73.22) | 341 (72.25) | 0.74 | |
| Yes | 124 (26.78) | 131 (27.75) | ||
| Regular use of statins,8,10 n (%) | ||||
| No | 360 (78.43) | 378 (80.60) | 0.41 | |
| Yes | 99 (21.57) | 91 (19.40) | ||
| Consumption of soy,8,11 n (%) | ||||
| No | 327 (71.40) | 307 (66.16) | 0.09 | |
| Yes | 131 (28.60) | 157 (33.84) | ||
| Times/wk12 | 2.39 ± 2.68 | 1.98 ± 2.48 | 0.21 | |
| Regular consumption of black and/or green tea,8 n (%) | ||||
| No | 187 (40.39) | 179 (37.84) | 0.21 | |
| Yes | 275 (59.40) | 289 (61.10) | ||
| Unknown | 1 (0.22) | 5 (1.06) | ||
| Years since menopause13 | 11.07 ± 7.63 | 10.32 ± 7.24 | 0.13 | |
| Self-reported physical activity,9 MET-h/wk | 45.71 ± 53.97 | 51.07 ± 106.95 | 0.33 |
Values are means ± SDs for continuous variables or frequencies (percentages) for categorical variables. P values were derived from Student’s t test for continuous variables and Pearson’s chi-square test for categorical variables. GTE, green tea extract; MET-h, metabolic equivalent hours.
2–6Participant numbers for the GTE and placebo groups, respectively, are as follows: 2n = 461 and 473, 3462 and 471, 4458 and 467, 5461 and 472, and 6461 and 469.
n = 462 for the GTE group.
Self-reported on the baseline health history questionnaire.
n = 472 for the placebo group.
10–13Participant numbers for the GTE and placebo groups, respectively, are as follows: 10n = 459 and 469, 11458 and 464, 12119 and 143, and 13440 and 453.
TABLE 2.
Daily nutrient intake at month 0 (baseline) and month 12 (endpoint) by treatment group1
| Nutrient | GTE2 | Placebo3 | P |
| Total energy, kcal | |||
| Baseline | 1438.26 ± 537.50 | 1447.83 ± 525.18 | 0.78 |
| Month 12 | 1370.91 ± 523.00 | 1365.66 ± 468.77 | 0.87 |
| Carbohydrate, % of energy | |||
| Baseline | 50.40 ± 7.49 | 50.36 ± 7.28 | 0.93 |
| Month 12 | 49.72 ± 7.61 | 49.33 ± 7.41 | 0.42 |
| Protein, % of energy | |||
| Baseline | 16.33 ± 2.75 | 15.99 ± 2.67 | 0.06 |
| Month 12 | 16.36 ± 2.79 | 16.08 ± 2.61 | 0.11 |
| Total fat, % of energy | |||
| Baseline | 32.89 ± 6.76 | 33.15 ± 6.48 | 0.54 |
| Month 12 | 33.85 ± 6.75 | 34.02 ± 6.50 | 0.70 |
| Saturated fat, % of energy | |||
| Baseline | 10.13 ± 2.29 | 10.14 ± 2.23 | 0.93 |
| Month 12 | 10.49 ± 2.35 | 10.38 ± 2.33 | 0.47 |
| Monounsaturated fat, % of energy | |||
| Baseline | 12.75 ± 3.22 | 12.87 ± 3.12 | 0.56 |
| Month 12 | 13.13 ± 3.19 | 13.33 ± 3.25 | 0.33 |
| Polyunsaturated fat, % of energy | |||
| Baseline | 7.56 ± 2.28 | 7.70 ± 2.25 | 0.33 |
| Month 12 | 7.74 ± 2.33 | 7.82 ± 2.28 | 0.59 |
| Omega-3 FAs, g | |||
| EPA (20:5) | |||
| Baseline | 0.02 ± 0.03 | 0.02 ± 0.02 | 0.52 |
| Month 12 | 0.02 ± 0.03 | 0.02 ± 0.02 | 0.54 |
| DHA (22:6) | |||
| Baseline | 0.05 ± 0.04 | 0.05 ± 0.04 | 0.44 |
| Month 12 | 0.05 ± 0.05 | 0.05 ± 0.04 | 0.57 |
| ALA (18:3) | |||
| Baseline | 1.03 ± 0.51 | 1.04 ± 0.53 | 0.60 |
| Month 12 | 0.99 ± 0.52 | 0.98 ± 0.45 | 0.82 |
| trans FAs, g | |||
| Baseline | 2.68 ± 1.38 | 2.66 ± 1.33 | 0.79 |
| Month 12 | 2.62 ± 1.39 | 2.53 ± 1.26 | 0.32 |
| Cholesterol, mg | |||
| Baseline | 145.96 ± 72.35 | 142.65 ± 72.36 | 0.47 |
| Month 12 | 141.94 ± 73.35 | 140.30 ± 68.84 | 0.73 |
| Total dietary fiber, g | |||
| Baseline | 17.53 ± 8.21 | 17.72 ± 7.96 | 0.72 |
| Month 12 | 16.83 ± 7.95 | 16.47 ± 6.81 | 0.46 |
| Soluble fiber, g | |||
| Baseline | 5.78 ± 2.78 | 5.86 ± 2.63 | 0.62 |
| Month 12 | 5.56 ± 2.69 | 5.45 ± 2.26 | 0.50 |
| Caffeine,4 mg | |||
| Baseline | 353.59 ± 312.11 | 378.62 ± 322.85 | 0.23 |
| Month 12 | 336.59 ± 296.57 | 361.02 ± 306.99 | 0.22 |
| Alcohol, g | |||
| Baseline | 6.89 ± 15.35 | 6.63 ± 8.57 | 0.75 |
| Month 12 | 5.76 ± 8.00 | 6.36 ± 8.88 | 0.27 |
| Total red meat intake, ounces | |||
| Baseline | 1.18 ± 0.85 | 1.12 ± 0.81 | 0.26 |
| Month 12 | 1.11 ± 0.80 | 1.05 ± 0.71 | 0.22 |
| Total vegetable intake, servings | |||
| Baseline | 3.51 ± 2.28 | 3.38 ± 2.14 | 0.39 |
| Month 12 | 3.41 ± 2.23 | 3.13 ± 1.78 | 0.03 |
Values are means ± SDs. There were no significant interactions between treatment and time for any of the nutrients. P values for differences in mean nutrients between the GTE and placebo groups were derived from Student’s t test. ALA, α-linolenic acid; FA, fatty acid; GTE, green tea extract.
n = 463 and n = 461 for baseline and month 12 data, respectively.
n = 473 and n = 469 for baseline and month 12 data, respectively.
Excluding the small amount of caffeine from the supplement.
TABLE 3.
Means (95% CIs) and changes from baseline concentrations of blood lipids by treatment group1
| Lipid and time point | GTE (n = 463) | Placebo (n = 473) | P2 | P-overall treatment3 |
| TC, mg/dL | ||||
| Baseline | 206 (204, 209) | 209 (206, 212) | 0.17 | |
| Δ Month 6 | −6.64 (−8.52, −4.76) | −0.53 (−2.39, 1.33) | <0.001 | 0.002 |
| Δ Month 12 | −4.35 (−6.35, −2.35) | 1.43 (−0.54, 3.41) | 0.0004 | |
| HDL cholesterol, mg/dL | ||||
| Baseline | 70 (69, 72) | 69 (68, 71) | 0.39 | |
| Δ Month 6 | −1.29 (−2.04, −0.53) | 0.55 (−0.20, 1.30) | 0.0045 | 0.003 |
| Δ Month 12 | −0.12 (−0.88, 0.65) | 0.83 (0.08, 1.59) | 0.50 | |
| LDL cholesterol,4 mg/dL | ||||
| Baseline | 118 (115, 120) | 120 (117, 122) | 0.32 | |
| Δ Month 6 | −5.66 (−7.31, −4.01) | −0.54 (−2.17, 1.10) | 0.0001 | <0.001 |
| Δ Month 12 | −4.81 (−6.58, −3.04) | 1.05 (−0.70, 2.80) | <0.0001 | |
| Triglycerides, mg/dL | ||||
| Baseline | 93 (89, 97) | 102 (98, 106) | 0.001 | |
| Δ Month 6 | 1.57 (−1.17, 4.31) | −2.09 (−4.80, 0.63) | 0.38 | 0.004 |
| Δ Month 12 | 3.31 (0.23, 6.39) | −2.60 (−5.64, 0.45) | 0.046 | |
| TC:HDL-cholesterol ratio | ||||
| Baseline | 3.10 (3.02, 3.18) | 3.22 (3.15, 3.30) | 0.03 | |
| Δ Month 6 | −0.06 (−0.10, −0.02) | −0.03 (−0.07, 0.004) | 1.00 | 0.24 |
| Δ Month 12 | −0.07 (−0.12, −0.02) | −0.03 (−0.08, 0.01) | 1.00 | |
| Non-HDL cholesterol, mg/dL | ||||
| Baseline | 136 (133, 139) | 140 (137, 143) | 0.06 | |
| Δ Month 6 | −5.35 (−7.10, −3.60) | −1.08 (−2.82, 0.65) | 0.0042 | <0.001 |
| Δ Month 12 | −4.23 (−6.16, −2.29) | 0.60 (−1.31, 2.51) | 0.0032 |
Values are least-squares means (95% CIs). Values were Bonferroni-corrected and adjusted for age and BMI at baseline. There were no significant interactions between treatment and time for any blood lipids by using the linear mixed models. GTE, green tea extract; TC, total cholesterol; Δ, change.
P values comparing baseline concentrations of lipids between GTE and placebo groups were derived from ANOVA with adjustment for age and BMI. P values for the assessment of changes in lipids from baseline to months 6 and 12 separately between GTE and placebo groups were derived from a mixed-effects repeated-measures ANOVA with adjustment for age and BMI at baseline.
P values for the overall treatment effect between GTE and placebo groups were derived from a mixed-effects repeated-measures ANOVA with adjustment for age and BMI at baseline.
n = 472 for the placebo group.
TABLE 6.
Treatment effect: mean changes in blood lipids by statin users and nonusers1
| Statin users (n = 190) |
Nonusers of statins (n = 738) |
||||
| Lipid and time point | GTE (n = 99) | Placebo (n = 91) | GTE (n = 360) | Placebo (n = 378) | P-interaction2 |
| TC, mg/dL | 0.35 | ||||
| Baseline | 194 | 190.0 | 210.0 | 214.0 | |
| Δ Month 6 | −1.67 | 3.58 | −7.93 | −1.76 | |
| Δ Month 12 | 1.30 | 3.26 | −5.89 | 0.66 | |
| P3 | 1.00 | <0.0001 | |||
| HDL cholesterol, mg/dL | 0.44 | ||||
| Baseline | 63.0 | 65.0 | 72.0 | 71.0 | |
| Δ Month 6 | −0.001 | 1.24 | −1.60 | 0.38 | |
| Δ Month 12 | 0.15 | 0.26 | −0.21 | 0.96 | |
| P3 | 1.00 | 0.016 | |||
| LDL cholesterol,4 mg/dL | 0.91 | ||||
| Baseline | 108.0 | 103.0 | 120.0 | 124.0 | |
| Δ Month 6 | −2.69 | 3.27 | −6.41 | −1.64 | |
| Δ Month 12 | −0.61 | 3.68 | −5.94 | 0.12 | |
| P3 | 0.17 | <0.0001 | |||
| Triglycerides, mg/dL | 0.06 | ||||
| Baseline | 112.0 | 112.0 | 88.0 | 100.0 | |
| Δ Month 6 | 5.11 | −4.58 | 0.42 | −1.67 | |
| Δ Month 12 | 9.02 | −3.42 | 1.75 | −2.57 | |
| P3 | 0.018 | 0.53 | |||
| TC:HDL-cholesterol ratio | 0.80 | ||||
| Baseline | 3.20 | 3.09 | 3.07 | 3.23 | |
| Δ Month 6 | 0.01 | 0.02 | −0.08 | −0.05 | |
| Δ Month 12 | 0.03 | 0.05 | −0.1 | −0.06 | |
| P3 | 1.00 | 1.00 | |||
| Non-HDL cholesterol, mg/dL | 0.51 | ||||
| Baseline | 130.0 | 125.0 | 138.0 | 144.0 | |
| Δ Month 6 | −1.67 | 2.33 | −6.33 | −2.13 | |
| Δ Month 12 | 1.15 | 3.00 | −5.68 | −0.3 | |
| P3 | 1.00 | 0.0011 | |||
Values are least-squares means, Bonferroni-corrected, and adjusted for age and BMI at baseline. n = 4 in both the GTE and placebo groups had unknown statin use or nonuse. GTE, green tea extract; TC, total cholesterol; Δ, change.
P values for the interaction between treatment and baseline statin use were derived from a mixed-effects repeated-measures ANOVA with adjustment for age and BMI.
P values for the overall changes in lipids from baseline to months 6 and 12 between GTE and placebo groups were derived from a mixed-effects repeated-measures ANOVA with adjustment for age and BMI at baseline within users or nonusers of statin.
One subject was excluded due to missing values at baseline.
RESULTS
Of 1075 participants randomly assigned into either the GTE (n = 538) or placebo (n = 537) groups, 937 women (or 87.2% of the randomly assigned participants) completed the intervention (Figure 1). One participant was excluded from all analyses for this substudy due to missing data on all lipids at baseline. Therefore, the final sample size for this substudy was 936 (GTE: n = 463; placebo: n = 473).
An analysis of baseline characteristics showed no significant differences between treatment groups, with the exception of the GTE group, who reported greater regular intakes of vitamin and/or mineral supplements at baseline than the placebo group (P = 0.02) (Table 1). Intakes of energy, nutrients, and select food groups were also compared both at baseline and after 1 y in the study (Table 2). Only vegetable intake at month 12 showed a significant difference between treatment groups, with a slightly higher intake in the GTE group (P = 0.03). Changes from baseline in energy and nutrient intakes were not different between the 2 groups (data not shown).
Baseline concentrations of TC, HDL cholesterol, LDL cholesterol, and non-HDL cholesterol were not different between the GTE and placebo groups (Table 3). However, triglycerides and the TC-to-HDL-cholesterol ratio were significantly lower in the GTE group than in the placebo group at baseline (P = 0.001 and 0.03, respectively). After 1 y of supplementation, compared with placebo, participants in the GTE group experienced significant reductions in TC (−2.1% compared with 0.7%; P = 0.0004), LDL cholesterol (−4.1% compared with 0.9%; P < 0.0001), and non-HDL cholesterol (−3.1% compared with 0.4%; P = 0.0032). At the same time, participants in the GTE group experienced a significant increase in triglycerides (3.6% compared with −2.5%; P = 0.046), whereas concentrations of HDL cholesterol and the TC-to-HDL-cholesterol ratio did not change significantly between the 2 groups. There were no significant interactions between treatment and time.
Table 4 shows changes in serum lipids when stratifying participants by baseline TC into 1 of 3 clinical subcategories of TC as established by the National Cholesterol Education Program of the NIH (4): <200 mg/dL (“desirable”), 200–239 mg/dL (“borderline high”), and ≥240 mg/dL (“high”). There were significant or borderline significant interactions between treatment and baseline category for TC (P = 0.01), LDL cholesterol (P = 0.06), and non-HDL cholesterol (P = 0.05), suggesting that the lipid-lowering effects of the GTE occurred only in the “borderline high” and “high” cholesterol groups. At 12 mo, for TC, changes in the GTE compared with the placebo groups in the “borderline high” category were −3.6% compared with 0.20%, whereas the changes in the “high” cholesterol group were −8.3% compared with −2.8% (P < 0.0001 and P = 0.0004 for overall treatment effect, respectively). For LDL cholesterol, changes in the GTE compared with placebo groups in the “borderline high” cholesterol group were −5.5% compared with 0.50%, whereas the changes in the “high” cholesterol group were −12.2% compared with −4.4% (P < 0.0001 and P = 0.006 for overall treatment effect, respectively). For non-HDL cholesterol, changes in the GTE compared with placebo groups in the “borderline high” cholesterol group were −4.3% compared with 0.001%, whereas the changes in the “high” cholesterol group were −11.1% compared with −4.4% (P = 0.004 and 0.01 for overall treatment effect, respectively). Results were not markedly affected after excluding statin users among those in the high-baseline-TC category (data not shown).
TABLE 4.
Treatment effect: changes in blood lipids in the GTE or placebo groups by TC category at baseline1
| TC, mg/dL |
|||||||
| <200 (n = 388)2 |
200–239 (n = 408) |
≥240 (n = 140) |
|||||
| Lipid and time point | GTE (n = 199) | Placebo (n = 189) | GTE (n = 200) | Placebo (n = 208) | GTE (n = 64) | Placebo (n = 76) | P-interaction3 |
| TC, mg/dL | 0.01 | ||||||
| Baseline | 180 | 180 | 216 | 218 | 259 | 260 | |
| Δ Month 6 | 0.44 | 4.18 | −9.43 | −1.54 | −19.88 | −9.46 | |
| Δ Month 12 | 4.65 | 6.06 | −7.75 | 0.41 | −21.57 | −7.31 | |
| P4 | 1.00 | <0.0001 | 0.0004 | ||||
| HDL cholesterol, mg/dL | 0.19 | ||||||
| Baseline | 65 | 64 | 74 | 72 | 75 | 75 | |
| Δ Month 6 | 0.24 | 1.70 | −2.22 | −0.35 | −3.09 | 0.17 | |
| Δ Month 12 | 1.72 | 1.27 | −1.59 | 0.41 | −1.20 | 0.89 | |
| P4 | 1.00 | 0.083 | 0.37 | ||||
| LDL cholesterol,5 mg/dL | 0.06 | ||||||
| Baseline | 98 | 96 | 124 | 125 | 161 | 163 | |
| Δ Month 6 | −0.40 | 2.99 | −7.82 | −0.71 | −15.23 | −8.93 | |
| Δ Month 12 | 1.92 | 4.81 | −6.80 | 0.58 | −19.58 | −7.20 | |
| P4 | 0.70 | <0.0001 | 0.006 | ||||
| Triglycerides, mg/dL | 0.43 | ||||||
| Baseline | 89 | 99 | 90 | 101 | 116 | 115 | |
| Δ Month 6 | 3.14 | −2.39 | 3.07 | −2.26 | −7.99 | −0.86 | |
| Δ Month 12 | 5.21 | 0.01 | 3.10 | −3.03 | −1.89 | −7.89 | |
| P4 | 0.59 | 0.36 | 1.00 | ||||
| TC:HDL-cholesterol ratio | 0.90 | ||||||
| Baseline | 2.94 | 2.97 | 3.09 | 3.25 | 3.65 | 3.76 | |
| Δ Month 6 | −0.02 | 0.005 | −0.07 | −0.03 | −0.16 | −0.14 | |
| Δ Month 12 | −0.02 | 0.05 | −0.06 | −0.06 | −0.26 | −0.19 | |
| P4 | 1.00 | 1.00 | 1.00 | ||||
| Non-HDL cholesterol, mg/dL | 0.05 | ||||||
| Baseline | 115 | 116 | 142 | 146 | 184 | 185 | |
| Δ Month 6 | 0.20 | 2.48 | −7.21 | −1.18 | −16.79 | −9.63 | |
| Δ Month 12 | 2.94 | 4.79 | −6.16 | −0.001 | −20.37 | −8.20 | |
| P4 | 1.00 | 0.004 | 0.01 | ||||
Values are least-squares means, Bonferroni-corrected, and adjusted for age and BMI at baseline. GTE, green tea extract; TC, total cholesterol; Δ, change.
n = 198 and 189 in the GTE and placebo groups, respectively, for the 12-mo time point.
P values for the interaction between treatment and baseline TC category were derived from a mixed-effects repeated-measures ANOVA with adjustment for age and BMI.
P values for the overall change in lipids from baseline to months 6 and 12 between the GTE and placebo groups were derived from a mixed-effects repeated-measures ANOVA with adjustment for age and BMI at baseline within each category of TC.
One subject was excluded due to missing values at baseline.
Table 5 shows findings stratified into 1 of 3 subcategories of baseline BMI: BMI <25 (normal or healthy), 25–29.9 (overweight), and ≥30.0 (obese). There was a borderline significant interaction between treatment and baseline BMI category only for triglycerides (P = 0.06). GTE supplementation resulted in a significant increase in triglyceride concentrations compared with placebo only in obese participants (P-overall treatment = 0.031). When the analysis was limited to obese statin users, GTE had no effect on triglycerides or any other lipids (data not shown).
TABLE 5.
Treatment effect: changes in blood lipids by baseline BMI category1
| BMI, kg/m2 |
|||||||
| <25 (n = 520)2 |
25–29.9 (n = 313) |
≥30 (n = 103) |
|||||
| Lipid and time point | GTE (n = 256) | Placebo (n = 264) | GTE (n = 147) | Placebo (n = 166) | GTE (n = 60) | Placebo (n = 43) | P-interaction3 |
| TC, mg/dL | 0.28 | ||||||
| Baseline | 207 | 209 | 208 | 210 | 199 | 210 | |
| Δ Month 6 | −5.46 | −0.76 | −7.87 | 0.55 | −8.79 | −3.11 | |
| Δ Month 12 | −2.67 | 1.99 | −7.19 | 1.6 | −4.67 | −2.39 | |
| P4 | 0.058 | 0.0006 | 1.00 | ||||
| HDL cholesterol, mg/dL | 0.96 | ||||||
| Baseline | 75 | 75 | 66 | 64 | 56 | 59 | |
| Δ Month 6 | −1.30 | 0.57 | −1.10 | 0.55 | −1.68 | 0.50 | |
| Δ Month 12 | 0.39 | 1.08 | −0.97 | 0.53 | −0.23 | 0.50 | |
| P4 | 0.61 | 0.76 | 1.00 | ||||
| LDL cholesterol,5 mg/dL | 0.35 | ||||||
| Baseline | 116 | 116 | 120 | 123 | 118 | 126 | |
| Δ Month 6 | −4.41 | −0.60 | −6.50 | 0.26 | −9.07 | −3.05 | |
| Δ Month 12 | −3.49 | 1.05 | −6.62 | 1.74 | −6.16 | −1.40 | |
| P4 | 0.055 | 0.0007 | 1.00 | ||||
| Triglycerides, mg/dL | 0.06 | ||||||
| Baseline | 79 | 90 | 108 | 115 | 119 | 127 | |
| Δ Month 6 | 1.25 | −3.54 | −1.29 | 0.24 | 10.09 | −2.42 | |
| Δ Month 12 | 2.05 | −0.98 | 2.04 | −4.04 | 11.92 | −7.19 | |
| P4 | 1.00 | 1.00 | 0.031 | ||||
| TC:HDL-cholesterol ratio | 0.65 | ||||||
| Baseline | 2.87 | 2.93 | 3.28 | 3.52 | 3.7 | 3.78 | |
| Δ Month 6 | −0.04 | −0.04 | −0.08 | −0.01 | −0.1 | −0.09 | |
| Δ Month 12 | −0.07 | −0.01 | −0.07 | −0.04 | −0.08 | −0.15 | |
| P4 | 1.00 | 1.00 | 1.00 | ||||
| Non-HDL cholesterol, mg/dL | 0.30 | ||||||
| Baseline | 132 | 134 | 142 | 146 | 142 | 151 | |
| Δ Month 6 | −4.17 | −1.33 | −6.76 | 0.01 | −7.08 | −3.58 | |
| Δ Month 12 | −3.06 | 0.89 | −6.22 | 1.07 | −4.41 | −2.86 | |
| P4 | 0.42 | 0.0066 | 1.00 | ||||
Values are least-squares means, Bonferroni-corrected, and adjusted for age and BMI at baseline. GTE, green tea extract; TC, total cholesterol; Δ, change.
n = 255 and 264 in the GTE and placebo groups, respectively, for the 12-mo time point.
P values for the interaction between treatment and baseline BMI category were derived from a mixed-effects repeated-measures ANOVA with adjustment for age and BMI.
P values for the overall changes in lipids from baseline to months 6 and 12 between the GTE and placebo groups were derived from a mixed-effects repeated-measures ANOVA with adjustment for age and BMI at baseline within each BMI category.
One subject was excluded due to missing values at baseline.
Supplementation with GTE did not enhance the lipid-lowering effectiveness of statins in our substudy (Table 6). We observed a borderline significant interaction between treatment and statin-use category for triglycerides (P-interaction = 0.06). Surprisingly, serum triglyceride concentrations increased at both 6 and 12 mo only in the GTE statin users, whereas their placebo counterparts experienced reductions at both time points (P-overall treatment = 0.018). Findings with regard to changes in triglyceride concentrations were in accord with the main results in Table 3, regardless of statin use.
There was no interaction between treatment and baseline triglyceride category for any of the lipids, suggesting that women with different concentrations of baseline triglycerides responded similarly to GTE supplementation (Supplemental Table 1). Likewise, stratifying data by COMT genotype activity yielded inconsistent patterns of changes in lipid concentrations, with no evidence of interaction between GTE intake and COMT genotype activity (Supplemental Table 2).
The main findings from the per-protocol analysis were very similar to those in the ITT analysis in which GTE supplementation for 1 y resulted in a 2.2% reduction in TC and a 3.2% decrease in non–HDL cholesterol concentrations (compared with a 0.8% and 0.6% increase in the placebo group; P = 0.0002 and 0.002, respectively).
DISCUSSION
This double-blind, randomized clinical trial assessed the longer-term effects of supplementation with a high dose of GTE on lipid profiles in healthy postmenopausal women. Daily supplementation of ∼1315 mg green tea catechins (843 mg EGCG) significantly reduced concentrations of TC, LDL cholesterol, and non-HDL cholesterol over the course of 12 mo. These findings are in agreement with the results of most epidemiologic studies (18–21) and currently published meta-analyses (9–12) that indicate that the consumption of green tea significantly lowers serum concentrations of TC and LDL cholesterol.
We observed no change in the concentrations of HDL cholesterol after 1 y of GTE supplementation, which is consistent with the data from meta-analysis studies (9–12) and most of the RCTs (22–43) conducted to date. A few intervention studies (44–46) found increased concentrations of HDL cholesterol after the ingestion of a green tea beverage or extract. However, participants in the latter studies were overweight or obese, with chronic complications such as hypertension or metabolic syndrome. In addition, these studies had very small sample sizes (n < 60) with a shorter duration than our trial and used catechin doses less than half of the dosage that we administered in the MGTT.
Surprisingly, the GTE group had significantly lower triglyceride concentrations than the placebo group at baseline; this difference was unanticipated, given the randomized design and large sample size of the MGTT. Although the GTE group experienced an increase in triglycerides over the course of 12-mo trial, the placebo group experienced a slight decline, providing no evidence of improvement in triglycerides associated with GTE in this study. This is consistent with data from meta-analyses (10–12), but 3 randomized, double-blind, controlled trials reported lowered concentrations of triglycerides after the consumption of GTE or beverage (30, 35, 44). These studies, however, were shorter in length (3 mo each) than our trial, study participants were obese, the treatment dose was much lower (EGCG doses varying from 208 to 214 mg/d), comparisons were mostly made within treatment groups, and/or the green tea supplementation was added to an exercise-induced weight-loss intervention.
Subgroup analyses showed that significant reductions in TC and borderline significant reductions in LDL and non-HDL cholesterol occurred only among those with the “borderline high” and “high” baseline TC categories. The greatest effects were seen in the baseline “high” cholesterol group (−8.3% in TC, −12.2% in LDL cholesterol, and −11.1% in non-HDL cholesterol for those in the GTE group). These results are consistent with an RCT that provided 250 mg GTE/d for 8 wk (22), but are inconsistent with 2 RCTs (37, 40) in which hypercholesterolemic participants drank green tea beverages (3 g tea leaves in 500–600 mL water) daily for 2–3 mo.
Higher triglyceride and cholesterol concentrations have been associated with increasing age and BMI and postmenopausal status (47). To control for this, the subgroup analyses in our study adjusted for baseline age and BMI, and all of the women in our study were postmenopausal. In addition, average years since menopause was taken into consideration in the statistical analysis, but results were not different between the 2 groups (data not shown). To the best of our knowledge, only 2 RCTs (27, 36) have so far examined the effects of green tea catechins on lipids in postmenopausal women. Hill et al. (27) did not observe any changes in lipids after the ingestion of 150 mg EGCG along with moderate-intensity exercise for 12 wk in overweight or obese postmenopausal women. In a second RCT by Wu et al. (36), 103 postmenopausal women were supplemented with either 400 or 800 mg EGCG as Polyphenon E daily for 2 mo. Only the reduction in LDL cholesterol was significant compared with the placebo group (percentage changes were 0.5% with placebo, −7.9% with 400 mg EGCG, and −6.6% with 800 mg EGCG; P = 0.02).
Among the bioactive green tea catechins, EGCG is of great interest because it is the most abundant and purported to be the main bioactive catechin responsible for the hypolipidemic effects of green tea. The exact mechanisms by which EGCG and green tea exert their lipid-lowering effects remain largely unknown. The hypothesized mechanisms are through the suppression of cholesterol biosynthesis (48–50), the interference of lipid absorption (51, 52), and the increase in fecal excretion of cholesterol (53, 54).
Most of the studies on the effects of green tea on cardiometabolic risk factors were conducted in overweight and obese populations, with conflicting findings. We did not observe significant interactions between green tea supplementation and baseline BMI for either TC or LDL cholestserol in our study, which is consistent with most studies conducted in overweight participants (25, 26, 37, 46). At the same time, 2 studies (30, 44) reported lower concentrations of TC and/or LDL cholesterol after the ingestion of GTE in overweight men and/or women. This discrepancy may be due to different dosages or composition of GTE or green tea beverages. GTE supplementation in our study resulted in an increase in triglycerides exclusively in obese participants. We are not aware of any study that reported higher concentrations of triglycerides in obese subjects by taking GTE, so we cannot rule out the possibility of this result by chance.
The use of statins to reduce serum cholesterol and triglycerides is a common clinical practice in the United States. The effects of GTEs on lipids were not modified by statin use, except for a greater increase in triglycerides in the GTE–statin user group than in the GTE–non-statin user group. This modifying effect was observed after a borderline significant interaction between GTE intake and statin use category. Possible contributing factors to these null results may include the following: 1) GTE does not enhance the effects of statin therapy; 2) different statins work through different mechanisms, thus GTE may enhance the function of some statins and not others; and 3) other factors, not monitored in our study, may be important, such as consistency and duration of use. This subgroup analysis was exploratory in nature and took advantage of the unique opportunity to test the potential of a green tea–medication interaction in a large study population. To our knowledge, no previous intervention or observational study has examined the effects of green tea intake on lipid concentrations stratified by statin use; these findings should be further investigated in future studies.
It was expected that women with low COMT genotype activity would show the greatest response to GTE intake because they may retain more tea polyphenols in their bodies. However, in our study, we did not observe an interaction between treatment and COMT genotype, which may be due to the fact that we were not statistically powered to detect this effect. To the best of our knowledge, no other green tea intervention study has evaluated the role of COMT genotype on lipids.
There are some limitations to our study that should be considered. Our study population was predominantly non-Hispanic white and educated. Blood lipid analysis was based on a single measure and samples had been stored for 1–3 y; thus, the effects of daily variations in lipid concentrations (55) and long-term storage of serum (56) on our results cannot be entirely ruled out, although randomization should have helped to minimize this effect.
This study also has some notable strengths. It was a well-designed, comprehensive, double-blind RCT of the effects of daily supplementation of ∼1315 mg green tea catechins (843 mg as EGCG) on components of the lipid profile in postmenopausal women. Although assessing the effectiveness of green tea catechin supplementation on serum lipid concentrations was not the primary outcome of the MGTT, this RCT had statistically adequate power and is the largest study with the longest treatment duration to date. Participants showed excellent compliance, taking, on average, 96.5% of their prescribed supplements. This was confirmed by analysis of urinary catechin content in a random subsample of participants (15), by which participants in the GTE group showed a >10-fold increase in the urinary concentrations of EGC and EC compared with the placebo group.
Taken together, supplementation with GTE for 12 mo was shown to significantly reduce TC, LDL cholesterol, and non-HDL cholesterol in postmenopausal women. GTE appears to generally exert stronger cholesterol-lowering effects in participants with baseline TC >200 mg/dL; in particular, GTE resulted in an 8.5% reduction in TC and a 12.4% decrease in LDL-cholesterol concentrations in hypercholesterolemic participants. GTE did not enhance the lipid-lowering effect of statin therapy in our study, possibly due to the small number of participants that used statins (n = 190). This should be pursued further in other large intervention studies. Current evidence from this trial suggests that GTE may be recommended for cholesterol lowering, in particular in those with borderline or high cholesterol concentrations. Green tea is an inexpensive, easily accessible, and popular drink and may indirectly lead to lower morbidity and mortality rates due to CVD through improving hyperlipidemia outcomes.
Acknowledgments
The authors’ responsibilities were as follows—HS, ARN, J-MY, AHW, and MSK: designed the research; HS, ARN, and MSK: conducted the study; HS and ARN: wrote the manuscript and analyzed the data; J-MY, AHW, and MSK: contributed to revisions of the manuscript; HS, RW, and J-MY: performed the statistical analyses; MSK: had primary responsibility for final content; and all authors: read, commented on, revised, and approved the final manuscript. The sponsor and the supplement manufacturer had no influence on the execution of the study, analysis and interpretation of data, or the final manuscript. No potential conflicts of interest were disclosed.
Footnotes
Abbreviations used: COMT, catechol-O-methyltransferase; CVD, cardiovascular disease; EC, epicatechin; EGC, epigallocatechin; EGCG, epigallocatechin gallate; GTE, green tea extract; ITT, intention-to-treat; MGTT, Minnesota Green Tea Trial; RCT, randomized controlled trial; TC, total cholesterol.
REFERENCES
- 1.World Health Organization. Global status report on noncommunicable diseases 2010 [cited 2015 Sep 2]. Available from: http://www.who.int/nmh/publications/ncd_report_full_en.pdf. [DOI] [PubMed]
- 2.Heron M. Deaths: leading causes for 2014. National Vital Statistics Reports; vol. 65, no. 5. Hyattsville (MD): National Center for Health Statistics; 2016. [PubMed] [Google Scholar]
- 3.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation 2015;131:e29–322. [DOI] [PubMed] [Google Scholar]
- 4.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III): final report. Circulation 2002;106:3143–421. [PubMed] [Google Scholar]
- 5.Pang J, Zhang Z, Zheng T, Yang YJ, Li N, Bai M, Peng Y, Zhang J, Li Q, Zhang B. Association of green tea consumption with risk of coronary heart disease in Chinese population. Int J Cardiol 2015;179:275–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liang W, Lee AH, Binns CW, Huang R, Hu D, Zhou Q. Tea consumption and ischemic stroke risk: a case-control study in southern China. Stroke 2009;40:2480–5. [DOI] [PubMed] [Google Scholar]
- 7.Wang QM, Gong QY, Yan JJ, Zhu J, Tang JJ, Wang MW, Yang ZJ, Wang LS. Association between green tea intake and coronary artery disease in a Chinese population. Circ J 2010;74:294–300. [DOI] [PubMed] [Google Scholar]
- 8.Nakachi K, Matsuyama S, Miyake S, Suganuma M, Imai K. Preventive effects of drinking green tea on cancer and cardiovascular disease: epidemiological evidence for multiple targeting prevention. Biofactors 2000;13:49–54. [DOI] [PubMed] [Google Scholar]
- 9.Zheng XX, Xu YL, Li SH, Liu XX, Hui R, Huang XH. Green tea intake lowers fasting serum total and LDL cholesterol in adults: a meta-analysis of 14 randomized controlled trials. Am J Clin Nutr 2011;94:601–10. [DOI] [PubMed] [Google Scholar]
- 10.Kim A, Chiu A, Barone MK, Avino D, Wang F, Coleman CI, Phung OJ. Green tea catechins decrease total and low-density lipoprotein cholesterol: a systematic review and meta-analysis. J Am Diet Assoc 2011;111:1720–9. [DOI] [PubMed] [Google Scholar]
- 11.Onakpoya I, Spencer E, Heneghan C, Thompson M. The effect of green tea on blood pressure and lipid profile: a systematic review and meta-analysis of randomized clinical trials. Nutr Metab Cardiovasc Dis 2014;24:823–36. [DOI] [PubMed] [Google Scholar]
- 12.Khalesi S, Sun J, Buys N, Jamshidi A, Nikbakht-Nasrabadi E, Khosravi-Boroujeni H. Green tea catechins and blood pressure: a systematic review and meta-analysis of randomised controlled trials. Eur J Nutr 2014;53:1299–311. [DOI] [PubMed] [Google Scholar]
- 13.Inoue-Choi M, Yuan JM, Yang CS, Van Den Berg DJ, Lee MJ, Gao YT, Yu MC. Genetic association between the COMT genotype and urinary levels of tea polyphenols and their metabolites among daily green tea drinkers. Int J Mol Epidemiol Genet 2010;1:114–23. [PMC free article] [PubMed] [Google Scholar]
- 14.van den Brandt PA, Spiegelman D, Yaun SS, Adami HO, Beeson L, Folsom AR, Fraser G, Goldbohm RA, Graham S, Kushi L, et al. Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am J Epidemiol 2000;152:514–27. [DOI] [PubMed] [Google Scholar]
- 15.Samavat H, Dostal AM, Wang R, Bedell S, Emory TH, Ursin G, Torkelson CJ, Gross MD, Le CT, Yu MC, et al. The Minnesota Green Tea Trial (MGTT), a randomized controlled trial of the efficacy of green tea extract on biomarkers of breast cancer risk: study rationale, design, methods, and participant characteristics. Cancer Causes Control 2015;26:1405–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499–502. [PubMed] [Google Scholar]
- 17.Dostal AM, Samavat H, Bedell S, Torkelson C, Wang R, Swenson K, Le C, Wu AH, Ursin G, Yuan JM, et al. The safety of green tea extract supplementation in postmenopausal women at risk for breast cancer: results of the Minnesota Green Tea Trial. Food Chem Toxicol 2015;83:26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imai K, Nakachi K. Cross sectional study of effects of drinking green tea on cardiovascular and liver diseases. BMJ 1995;310:693–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kono S, Shinchi K, Ikeda N, Yanai F, Imanishi K. Green tea consumption and serum lipid profiles: a cross-sectional study in northern Kyushu, Japan. Prev Med 1992;21:526–31. [DOI] [PubMed] [Google Scholar]
- 20.Kono S, Shinchi K, Wakabayashi K, Honjo S, Todoroki I, Sakurai Y, Imanishi K, Nishikawa H, Ogawa S, Katsurada M. Relation of green tea consumption to serum lipids and lipoproteins in Japanese men. J Epidemiol 1996;6:128–33. [DOI] [PubMed] [Google Scholar]
- 21.Tokunaga S, White IR, Frost C, Tanaka K, Kono S, Tokudome S, Akamatsu T, Moriyama T, Zakouji H. Green tea consumption and serum lipids and lipoproteins in a population of healthy workers in Japan. Ann Epidemiol 2002;12:157–65. [DOI] [PubMed] [Google Scholar]
- 22.Batista G A, Cunha CL, Scartezini M, von der Heyde R, Bitencourt MG, Melo SF. Prospective double-blind crossover study of Camellia sinensis (green tea) in dyslipidemias. Arq Bras Cardiol 2009;93:128–34. [DOI] [PubMed] [Google Scholar]
- 23.Brown AL, Lane J, Coverly J, Stocks J, Jackson S, Stephen A, Bluck L, Coward A, Hendrickx H. Effects of dietary supplementation with the green tea polyphenol epigallocatechin-3-gallate on insulin resistance and associated metabolic risk factors: randomized controlled trial. Br J Nutr 2009;101:886–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown AL, Lane J, Holyoak C, Nicol B, Mayes AE, Dadd T. Health effects of green tea catechins in overweight and obese men: a randomised controlled cross-over trial. Br J Nutr 2011;106:1880–9. [DOI] [PubMed] [Google Scholar]
- 25.Diepvens K, Kovacs EM, Vogels N, Westerterp-Plantenga MS. Metabolic effects of green tea and of phases of weight loss. Physiol Behav 2006;87:185–91. [DOI] [PubMed] [Google Scholar]
- 26.Frank J, George TW, Lodge JK, Rodriguez-Mateos AM, Spencer JP, Minihane AM, Rimbach G. Daily consumption of an aqueous green tea extract supplement does not impair liver function or alter cardiovascular disease risk biomarkers in healthy men. J Nutr 2009;139:58–62. [DOI] [PubMed] [Google Scholar]
- 27.Hill AM, Coates AM, Buckley JD, Ross R, Thielecke F, Howe PR. Can EGCG reduce abdominal fat in obese subjects? J Am Coll Nutr 2007;26:396S–402S. [DOI] [PubMed] [Google Scholar]
- 28.Hsu CH, Liao YL, Lin SC, Tsai TH, Huang CJ, Chou P. Does supplementation with green tea extract improve insulin resistance in obese type 2 diabetics? A randomized, double-blind, and placebo-controlled clinical trial. Altern Med Rev 2011;16:157–63. [PubMed] [Google Scholar]
- 29.Hsu CH, Tsai TH, Kao YH, Hwang KC, Tseng TY, Chou P. Effect of green tea extract on obese women: a randomized, double-blind, placebo-controlled clinical trial. Clin Nutr 2008;27:363–70. [DOI] [PubMed] [Google Scholar]
- 30.Maki KC, Reeves MS, Farmer M, Yasunaga K, Matsuo N, Katsuragi Y, Komikado M, Tokimitsu I, Wilder D, Jones F, et al. Green tea catechin consumption enhances exercise-induced abdominal fat loss in overweight and obese adults. J Nutr 2009;139:264–70. [DOI] [PubMed] [Google Scholar]
- 31.Matsuyama T, Tanaka Y, Kamimaki I, Nagao T, Tokimitsu I. Catechin safely improved higher levels of fatness, blood pressure, and cholesterol in children. Obesity (Silver Spring) 2008;16:1338–48. [DOI] [PubMed] [Google Scholar]
- 32.Nagao T, Hase T, Tokimitsu I. A green tea extract high in catechins reduces body fat and cardiovascular risks in humans. Obesity (Silver Spring) 2007;15:1473–83. [DOI] [PubMed] [Google Scholar]
- 33.Nantz MP, Rowe CA, Bukowski JF, Percival SS. Standardized capsule of Camellia sinensis lowers cardiovascular risk factors in a randomized, double-blind, placebo-controlled study. Nutrition 2009;25:147–54. [DOI] [PubMed] [Google Scholar]
- 34.Sone T, Kuriyama S, Nakaya N, Hozawa A, Shimazu T, Nomura K, Rikimaru S, Tsuji I. Randomized controlled trial for an effect of catechin-enriched green tea consumption on adiponectin and cardiovascular disease risk factors. Food Nutr Res 2011;55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suliburska J, Bogdanski P, Szulinska M, Stepien M, Pupek-Musialik D, Jablecka A. Effects of green tea supplementation on elements, total antioxidants, lipids, and glucose values in the serum of obese patients. Biol Trace Elem Res 2012;149:315–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu AH, Spicer D, Stanczyk FZ, Tseng CC, Yang CS, Pike MC. Effect of 2-month controlled green tea intervention on lipoprotein cholesterol, glucose, and hormone levels in healthy postmenopausal women. Cancer Prev Res (Phila) 2012;5:393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bertipaglia de Santana M, Mandarino MG, Cardoso JR, Dichi I, Dichi JB, Camargo AE, Fabris BA, Rodrigues RJ, Fatel EC, Nixdorf SL, et al. Association between soy and green tea (Camellia sinensis) diminishes hypercholesterolemia and increases total plasma antioxidant potential in dyslipidemic subjects. Nutrition 2008;24:562–8. [DOI] [PubMed] [Google Scholar]
- 38.Erba D, Riso P, Bordoni A, Foti P, Biagi PL, Testolin G. Effectiveness of moderate green tea consumption on antioxidative status and plasma lipid profile in humans. J Nutr Biochem 2005;16:144–9. [DOI] [PubMed] [Google Scholar]
- 39.Miyazaki R, Kotani K, Ayabe M, Tsuzaki K, Shimada J, Sakane N, Takase H, Ichikawa H, Yonei Y, Ishii K. Minor effects of green tea catechin supplementation on cardiovascular risk markers in active older people: a randomized controlled trial. Geriatr Gerontol Int 2013;13:622–9. [DOI] [PubMed] [Google Scholar]
- 40.Vieira Senger AE, Schwanke CH, Gomes I, Valle Gottlieb MG. Effect of green tea (Camellia sinensis) consumption on the components of metabolic syndrome in elderly. J Nutr Health Aging 2012;16:738–42. [DOI] [PubMed] [Google Scholar]
- 41.Fukino Y, Ikeda A, Maruyama K, Aoki N, Okubo T, Iso H. Randomized controlled trial for an effect of green tea-extract powder supplementation on glucose abnormalities. Eur J Clin Nutr 2008;62:953–60. [DOI] [PubMed] [Google Scholar]
- 42.Princen HM, van Duyvenvoorde W, Buytenhek R, Blonk C, Tijburg LB, Langius JA, Meinders AE, Pijl H. No effect of consumption of green and black tea on plasma lipid and antioxidant levels and on LDL oxidation in smokers. Arterioscler Thromb Vasc Biol 1998;18:833–41. [DOI] [PubMed] [Google Scholar]
- 43.Mielgo-Ayuso J, Barrenechea L, Alcorta P, Larrarte E, Margareto J, Labayen I. Effects of dietary supplementation with epigallocatechin-3-gallate on weight loss, energy homeostasis, cardiometabolic risk factors and liver function in obese women: randomised, double-blind, placebo-controlled clinical trial. Br J Nutr 2014;111:1263–71. [DOI] [PubMed] [Google Scholar]
- 44.Bogdanski P, Suliburska J, Szulinska M, Stepien M, Pupek-Musialik D, Jablecka A. Green tea extract reduces blood pressure, inflammatory biomarkers, and oxidative stress and improves parameters associated with insulin resistance in obese, hypertensive patients. Nutr Res 2012;32:421–7. [DOI] [PubMed] [Google Scholar]
- 45.Basu A, Sanchez K, Leyva MJ, Wu M, Betts NM, Aston CE, Lyons TJ. Green tea supplementation affects body weight, lipids, and lipid peroxidation in obese subjects with metabolic syndrome. J Am Coll Nutr 2010;29:31–40. [DOI] [PubMed] [Google Scholar]
- 46.Stendell-Hollis NR, Thomson CA, Thompson PA, Bea JW, Cussler EC, Hakim IA. Green tea improves metabolic biomarkers, not weight or body composition: a pilot study in overweight breast cancer survivors. J Hum Nutr Diet 2010;23:590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hall G, Collins A, Csemiczky G, Landgren BM. Lipoproteins and BMI: a comparison between women during transition to menopause and regularly menstruating healthy women. Maturitas 2002;41:177–85. [DOI] [PubMed] [Google Scholar]
- 48.Cuccioloni M, Mozzicafreddo M, Spina M, Tran CN, Falconi M, Eleuteri AM, Angeletti M. Epigallocatechin-3-gallate potently inhibits the in vitro activity of hydroxy-3-methyl-glutaryl-CoA reductase. J Lipid Res 2011;52:897–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bursill CA, Roach PD. A green tea catechin extract upregulates the hepatic low-density lipoprotein receptor in rats. Lipids 2007;42:621–7. [DOI] [PubMed] [Google Scholar]
- 50.Ge H, Liu J, Zhao W, Wang Y, He Q, Wu R, Li D, Xu J. Mechanistic studies for tri-targeted inhibition of enzymes involved in cholesterol biosynthesis by green tea polyphenols. Org Biomol Chem 2014;12:4941–51. [DOI] [PubMed] [Google Scholar]
- 51.Shishikura Y, Khokhar S, Murray BS. Effects of tea polyphenols on emulsification of olive oil in a small intestine model system. J Agric Food Chem 2006;54:1906–13. [DOI] [PubMed] [Google Scholar]
- 52.Raederstorff DG, Schlachter MF, Elste V, Weber P. Effect of EGCG on lipid absorption and plasma lipid levels in rats. J Nutr Biochem 2003;14:326–32. [DOI] [PubMed] [Google Scholar]
- 53.Bose M, Lambert JD, Ju J, Reuhl KR, Shapses SA, Yang CS. The major green tea polyphenol, (–)-epigallocatechin-3-gallate, inhibits obesity, metabolic syndrome, and fatty liver disease in high-fat-fed mice. J Nutr 2008;138:1677–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang TT, Koo MW. Chinese green tea lowers cholesterol level through an increase in fecal lipid excretion. Life Sci 2000;66:411–23. [DOI] [PubMed] [Google Scholar]
- 55.Demacker PN, Schade RW, Jansen RT, Van ’t Laar A. Intra-individual variation of serum cholesterol, triglycerides and high density lipoprotein cholesterol in normal humans. Atherosclerosis 1982;45:259–66. [DOI] [PubMed] [Google Scholar]
- 56.Shih WJ, Bachorik PS, Haga JA, Myers GL, Stein EA. Estimating the long-term effects of storage at -70 degrees C on cholesterol, triglyceride, and HDL-cholesterol measurements in stored sera. Clin Chem 2000;46:351–64. [PubMed] [Google Scholar]