Abstract
Background: Cardiovascular diseases (CVDs) are the greatest cause of death globally, and their reduction is a key public-health target. High blood pressure (BP) affects 1 in 3 people in the United Kingdom, and previous studies have shown that milk consumption is associated with lower BP.
Objective: We investigated whether intact milk proteins lower 24-h ambulatory blood pressure (AMBP) and other risk markers of CVD.
Design: The trial was a double-blinded, randomized, 3-way–crossover, controlled intervention study. Forty-two participants were randomly assigned to consume 2 × 28 g whey protein/d, 2 × 28 g Ca caseinate/d, or 2 × 27 g maltodextrin (control)/d for 8 wk separated by a 4-wk washout. The effects of these interventions were examined with the use of a linear mixed-model ANOVA.
Results: Thirty-eight participants completed the study. Significant reductions in 24-h BP [for systolic blood pressure (SBP): −3.9 mm Hg; for diastolic blood pressure (DBP): −2.5 mm Hg; P = 0.050 for both)] were observed after whey-protein consumption compared with control intake. After whey-protein supplementation compared with control intake, peripheral and central systolic pressures [−5.7 mm Hg (P = 0.007) and −5.4 mm Hg (P = 0.012), respectively] and mean pressures [−3.7 mm Hg (P = 0.025) and −4.0 mm Hg (P = 0.019), respectively] were also lowered. Flow-mediated dilation (FMD) increased significantly after both whey-protein and calcium-caseinate intakes compared with control intake [1.31% (P < 0.001) and 0.83% (P = 0.003), respectively]. Although both whey protein and calcium caseinate significantly lowered total cholesterol [−0.26 mmol/L (P = 0.013) and −0.20 mmol/L (P = 0.042), respectively], only whey protein decreased triacylglycerol (−0.23 mmol/L; P = 0.025) compared with the effect of the control. Soluble intercellular adhesion molecule 1 and soluble vascular cell adhesion molecule 1 were reduced after whey protein consumption (P = 0.011) and after calcium-caseinate consumption (P = 0.039), respectively, compared with after control intake.
Conclusions: The consumption of unhydrolyzed milk proteins (56 g/d) for 8 wk improved vascular reactivity, biomarkers of endothelial function, and lipid risk factors. Whey-protein supplementation also lowered 24-h ambulatory SBP and DBP. These results may have important implications for public health. This trial was registered at clinicaltrials.gov as NCT02090842.
Keywords: blood pressure, cardiometabolic health, milk protein, vascular function, dairy, whey, caseinate, angiotensin-converting enzyme inhibition, flow-mediated dilatation, augmentation index
INTRODUCTION
Cardiovascular disease (CVD)6 remains the main cause of death in Western countries, although there has been a substantial decrease in its incidence in the past 2 decades. Despite the reduction in incidence, the prevalence of CVD is growing because of the increasing aging population in these countries (1). Of the modifiable risk factors of CVD, elevated blood pressure (BP) is a key risk factor. In the United Kingdom, nearly 30% of adults have hypertension, and a high percentage remain untreated (1). This lack of treatment can have serious consequences such as stroke, organ damage, or organ failure and death, which places an immense burden on the healthcare system. Note that even a small decrease in SBP of 2–5-mm Hg can translate into considerable reductions in CVD and total mortality (2). An improved quality of diet has been linked to a decrease in BP (3), and epidemiologic studies have shown an inverse association between milk consumption and BP (4). Milk is a complex food that contains several potential bioactive compounds; therefore, it remains to be confirmed which constituents may be responsible for the beneficial effect. Previous studies have linked milk-protein consumption with reduced BP (5); however, there has been an imbalance in the literature, because most attention has focused on the casein-derived lactotripeptides or other milk-protein hydrolysates (6) with a clear evidence gap on the effects of intact milk-protein isolates on BP (7). Milk proteins in liquid milk exist in intact forms, and evidence from robust randomized controlled trials (RCTs) would provide valuable information on the potential mechanism by which milk may affect BP. Furthermore, there is a clear need for a better assessment of BP, because the use of clinic BP may be confounded by the so-called white-coat effect. The 24-h ambulatory blood pressure (AMBP) is considered to be the gold standard, and AMBP predicts cardiovascular events, mortality, and morbidity that are associated with hypertension better than does clinic BP (8–10).
Vascular dysfunction is defined as the suppression of endothelium-dependent vasodilation or arterial stiffness (11, 12), and several factors such as hypertension, atherosclerosis, or diabetes can influence its rate of progression (13). Little attention has focused on the potential impact of dairy proteins on vascular function. Pal and Ellis (7) reported a decrease in BP after both whey-protein and sodium-caseinate supplementation for 12 wk; however, they showed improvements in arterial stiffness only after whey-protein consumption. This result may indicate that whey protein has an additional beneficial effect on the cardiovascular system compared with that of caseinate; however, this possibility remains to be confirmed.
Therefore, our hypothesis was that whey-protein and calcium-caseinate supplementation, compared with control intake, would reduce 24-h AMBP, improve vascular function, and beneficially affect other cardiometabolic risk markers. This hypothesis was tested in a randomized, controlled, double-blinded, 3-way–crossover dietary intervention in prehypertensive and mildly hypertensive men and women.
METHODS
Subjects
Healthy, nonsmoking men and women with mildly elevated BP of 120/80–159/99 mm Hg who were aged 30–77 y and were not taking antihypertensive or cholesterol-lowering medications were recruited for this study through database, local newspaper, and electronic advertisements. This study was conducted at the Hugh Sinclair Unit of Human Nutrition, University of Reading, between February 2014 and February 2015. Inclusion criteria were as follows: a signed consent form; BP from 120/80 to 159/99 mm Hg; aged 30–77 y; BMI (in kg/m2) from 20 to 40; glucose concentration <7 mmol/L (not diagnosed with diabetes); cholesterol concentration <8 mmol/L; triacylglycerol concentration <4 mmol/L; normal liver and kidney function; and hemoglobin concentration >110 and >140 g/dL for women and men, respectively. Participants were excluded on the basis of the following criteria: a milk allergy or lactose intolerance; celiac disease or renal, gastrointestinal, respiratory, endocrine, or liver disease or cancer; surgery in the previous 6 mo; secondary hypertension; excess alcohol consumption (drinking >280 mL and >210 mL ethanol/wk for men and women, respectively); smoking; being a vegan; taking a nutritional supplement (e.g., fish oil, protein shakes, or vitamins); anemia; and pregnancy, lactation, or planning a pregnancy. Blood donation was not allowed for 3 mo before or during the study. All volunteers provided a signed consent form at the screening visit before their inclusion in the study and agreed to adhere to the trial guidelines and give notification of any noncompliance. To detect the arithmetic mean ± SEM SBP intergroup difference (primary outcome) of 4.9 ± 6.5 mm Hg (7, 14) with 90% power and at a 5% significance level, 37 participants were required, which increased to 42 participant to account for a 12% dropout rate (n = 5).
Study design
The study was approved by the Research Ethics Committee at the University of Reading (Research Ethics Committee project 12/40), and the protocol was conducted in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. This trial was registered at clinicaltrials.gov as NCT02090842. The study was a randomized, double-blinded, controlled, 3-way–crossover, dietary intervention study with a 2-wk run-in period before the beginning of the trial. There were two 4-wk washout periods that separated the 3 intervention arms of 8 wk. The total duration of the study was 32 wk. The study design is shown in Supplemental Figure 1. The volunteers were randomly assigned by an independent researcher with the use of an Excel-based random-assignment program (ExtendOffice 12.5) (15) who was also responsible for the treatment allocation. Equal numbers of volunteers were allocated within 6 treatment sequences (ABC, ACB, BAC, BCA, CAB, and CBA). A double-blinded protocol was maintained throughout the study, and during the statistical analysis. The codes for the study drinks were kept under lock at Volac International Ltd. (Cambridge) and the University of Reading.
The primary outcome was 24-h AMBP, and secondary outcomes were vascular reactivity measured with the use of FMD and changes in plasma lipids, markers of insulin resistance, inflammatory markers, and arterial stiffness measured with the use of a pulse wave analysis and digital volume pulse (DVP).
Study drinks
The 3 treatment products were 90% whey-protein isolate (Volac International Ltd.), 90% calcium caseinate (Garret Ingredients), and maltodextrin (control) (MyProtein) and were packed into identical foil sachets that were labeled with study codes. All products were commercially available. Each protein-containing sachet contained 28 g powder and 27 g maltodextrin in the control sachets, which provided closely matched amounts of energy (1596 kJ/100 g for whey protein, 1519 kJ/100 g for calcium caseinate, and 1628 kJ/100 g for maltodextrin) (for nutrient compositions, see Supplemental Table 1). Calcium caseinate was used in this study instead of sodium caseinate to reduce the potential bias toward the effect of whey protein because sodium may unfavorably affect BP. Volunteers were asked to consume 2 sachets/d mixed with 250 mL H2O. Participants were also given a choice of sugar-free flavor concentrates [vanilla, banana, tropical fruit, or chocolate (MyProtein)] that were to be added before consumption to mask any possible taste of the supplements and to increase compliance. Furthermore, volunteers were informed at the start of the study that all 3 treatments were substantially different from one another in terms of taste and appearance to reduce the possibility of them identifying the protein interventions. Subjects were required to refrain from mixing the sachet content with milk to avoid increasing their milk intake. Participants were also asked to maintain isoenergetic diets to account for the extra energy intake from the consumption of the study drinks. Volunteers were provided with individual nutritional advice by a dietitian and a nutritionist on specific strategies for the incorporation of study drinks into their diets without a substantial change to their habitual food intakes. Participants recorded the exchanged foods (types and weights) on tailored diary sheets, which were evaluated at each study visit, and feedback was provided to the volunteers as to their compliance to the interventions. Subjects were also required to record their consumption of the sachets by marking tick boxes as well as by keeping empty sachets to monitor compliance.
Study visits
At the screening visit, data on height and weight were collected. Height was measured to the nearest 0.5 cm with the use of a wall-mounted stadiometer. Furthermore, weight and BMI were measured with the use of a regularly calibrated bioelectroimpedence scale (Tanita BC-418 digital scale; Tanita Europe) with the use of standard settings (normal body type and +1 kg for clothing) while wearing light clothing. A fasted serum blood sample was collected to assess serum glucose, total cholesterol, triacylglycerol, creatinine, bilirubin, uric acid, alanine aminotransferase, γ-glutamyl transpeptidase, and alkaline phosphatase with the use of an autoanalyzer [ILAB600; Werfen (UK) Ltd.]. Participants were also checked for anemia by a full blood count. After 20 min rest, BP measurement was taken on the upper left arm with the use of an automatic BP monitor (A/A-grade automated oscillometric BP monitors; A&D Instruments Ltd.), which recorded BP readings every 5 min up to 30 mins in a temperature-controlled, quiet environment. The mean of 6 readings was used to assess resting BP to the nearest 0.5 mm Hg. Participants were required to complete a food-frequency questionnaire (FFQ) (European Prospective Investigation into Cancer and Nutrition-Norfolk FFQ v.6) and were asked to remain seated and to refrain from speaking. FFQs were subsequently analyzed with the use of FETA (EPIC, Norfolk) for Windows GLP v2.53 software (16).
The run-in period started with a familiarization visit (visit 0) in the clinical room where the study visits were conducted at the Hugh Sinclair Unit of Human Nutrition to familiarize participants with the environment and, thus, decrease the so-called white-coat effect on the clinic BP and vascular measurements. All volunteers had a one-by-one consultation with the study researcher. During this session, volunteers were provided with verbal and written instructions on the consumption and recording of the consumption of the study drinks, the maintenance of an isocaloric diet, the use of the 24-h AMBP, and the recording of 4-d estimated dietary intake (including 3 weekdays and 1 weekend day). Participants were required to complete the diaries on 6 occasions before each study visit. Portion sizes were estimated with the use of household measures or portion-size images (17), which were later quantified by a trained dietitian with the use of food-portion tables (18). Dietplan 6.6 software (Forestfield Software) was used to assess nutrient intakes of participants for which the National Diet and Nutrition Survey nutrient databank was implemented (19). Volunteers were specifically asked their body weight and reminded at each study visit to maintain their body weights and physical activities and to continue any permitted medication use without any change in the dose during the interventions of which they had to keep record. All vascular measurement techniques and their implementation were clearly explained and shown to the participants.
At baselines (visits 1, 3, and 5) and at the end of all 3 intervention periods (visit 2, 4, and 6), overnight (12-h) fasting venous blood samples were collected for biochemical analysis. Participants were required to refrain from alcohol and aerobic exercises for 24-h before visits, and they had to orally confirm that they adhered to this protocol. Volunteers consumed a low-fat and low-protein meal the evening before each visit and drank only low-nitrate water (Buxton Mineral Water; Nestlé Waters UK). Body weight and body composition were measured as specified previously upon arrival to the nutrition unit. Hand-grip strength was assessed with the use of a hand-grip dynamometer (SU424; JP Lennard Ltd.). Volunteers were asked to perform 3 maximum hand-grip tests with each hand in a standing position with their arms along their bodies. The mean of the 3 measurements calculated the hand-grip strength. Participants rested for 30 min in a supine position in a quiet, temperature-controlled room (20°C ± 1°C) before vascular testing. Before the commencement of vascular measurements, clinic BP was taken on the left upper arm with the use of a validated BP monitor (Omron MX2 Digital Automatic Upper Arm Blood Pressure Monitor; OMRON), and the mean of 3 consecutive measurements was recorded. All measurements were conducted by the same researcher (except for the DVP, which was operated by multiple trained researchers) and at the same time of day for all visits. Premenopausal women attended all visits at the same phase of their menstrual cycles.
Twenty-four-hour AMBP and assessments of vascular function
AMBP was measured with the use of A/A-grade automated oscillometric AMBP monitors (A&D Instruments Ltd.). Twenty-four-hour AMBP and heart rate were recorded every 30 min from 0700 to 2159 and every 60 min from 2200 to 0659 no later than 48 h before clinical visits. Mean 24-h day and night measurements were assessed with the use of sleep times that were recorded on the volunteer activity forms. Pulse pressure was expressed as the difference between SBP and DBP.
FMD was performed by a single, trained researcher as previously described (20). The technique assessed the endothelial-dependent vasodilation of the vasculature with the use of a CX50 Ultrasound System (Philips) according to standard guidelines (21). Electrocardiogram-gated image acquisition was performed at 0.25 frames/s with the use of image-grabbing software (Medical Imaging Applications LLC). The analysis of the obtained images was conducted by a single researcher with the use of wall-tracking software (Vascular Research Tools 5; Medical Imaging Applications LLC). FMD was calculated as the maximum change in the postocclusion brachial artery diameter, which was expressed as the percentage of the baseline diameter [flow-medated dilatation percentage (%FMD)]. The %FMD was assessed 3 times on each image, and the mean was calculated. The arterial stiffness of peripheral vessels was assessed in triplicate as detailed elsewhere (22) with the use of a radial pulse-wave analysis (SphygmoCor; AtCor Medical). The pulse-wave analysis determined the augmentation index, which was corrected for a heart rate of 75 beats/min (percentage). The digital volume pulse (Pulse Trace PCA2; Micro Medical Ltd.) provided an indication of the stiffness index (meters per second) and reflection index (percentage) as measures of arterial stiffness and vascular tone, respectively (20).
Biochemical analyses
Fasted blood samples were centrifuged at 1800 × g for 15 min at 20°C (serum separator blood collection tube; VACUETTE, Greiner Bio-One) to obtain serum and at 1800 × g for 10 min at 4°C (blood collection tubes containing lithium heparin and EDTA; VACUETTE; Greiner Bio-One) to obtain plasma. Samples were kept at −20°C. Lipids (total cholesterol, HDL cholesterol, and triacylglycerol), glucose, nonesterified fatty acid, and C-reactive protein were quantified from serum with the use of an autoanalyzer [ILAB600; Werfen (UK) Ltd.; reagents and analyzer: Instrumentation Laboratory Ltd.; nonesterified fatty acid reagent: Alpha Laboratories). LDL cholesterol was estimated with the use of Friedewald’s formula (23). Insulin resistance was determined with the use of HOMA-IR, and insulin sensitivity was assessed with the original and revised quantitative insulin sensitivity check indexes with the use of standard equations (24). ELISA kits were used to determine serum insulin (Dako Ltd.), intercellular and vascular adhesion molecules, E-selectin, and P-selectin; high-sensitivity kits were used to determine IL-6 and TNF-α (R&D Systems Europe Ltd.). Plasma nitrite and nitrate were measured with the use of HPLC with online reduction of nitrate to nitrite and postcolumn derivatization with the Griess reagent (ENO-30 Analyzer; Eicom) as described elsewhere (25). Serum angiotensin-converting enzyme (ACE) activity was determined by a fluorometric assay as described elsewhere (26). Mean intra-assay and interassay CVs were <5% for the automated assays and <10% for other assays.
In vitro ACE inhibitory effects of intervention products
To assess the ACE inhibitory (ACEi) effect of intervention proteins and the control, the study products were digested in vitro on the basis of the method of Mills et al. (27) with minor modifications. The total digesta were filtered with the use of-3kDa VIVASPIN 20 centrifuge tubes (Sartorius AG) to mimic in vivo intestinal absorption. The ACEi effect of intervention proteins and the control were measured with the use of a spectrophotometric assay with the substrate furanacryloyl-Phe-Gly-Gly and ACE, which were extracted from rabbit lung (both from Sigma-Aldrich) (28, 29). The ACEi effect was expressed as
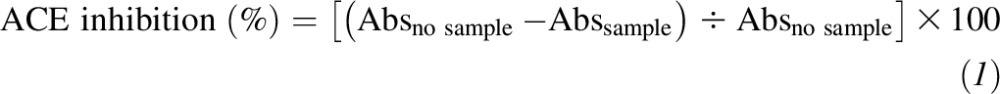 |
Absno sample is the absorbance of the enzyme-substrate mixture in the absence of digested protein, and Abssample is the absorbance of the enzyme-substrate mixture in the presence of digested protein.
Statistical analyses
Statistical analyses were performed with the use of SAS version 9.4 software (SAS Institute Inc.). Suitable checks for normality were performed (evaluated with the use of histograms and quantile-quantile plots) with IBM SPSS Statistics version 21 software (SPSS Inc.), and data were logarithmically transformed as appropriate. The data from participants who withdrew from the study were excluded in the analysis because a per-protocol analysis was used. The effects of treatments were evaluated by implementing a linear mixed-model ANOVA with the use of the difference from baseline (Δ visit 2 − visit 1) as a dependent variable and baseline values of the variable of interest, BMI, age, sex, and intervention treatment as prognostic variables. P ≤ 0.05 was considered significant. Data presented in the text, tables, and figures represent arithmetic means ± SEMs.
RESULTS
Study participation and compliance
A total of 42 volunteers were randomly assigned to interventions, of whom 38 completed the study. Four participants withdrew during the study [2 subjects dropped out because of a dislike of calcium caseinate, 1 subject dropped out because of a dislike of whey protein, and 1 subject dropped out because of a loss of interest (see Figure 1 for flowchart)]. Table 1 summarizes the baseline characteristics of completing participants. On the basis of sachet counting, compliance was 91.0% ± 1.7%, 88.6% ± 2.1%, and 91.8% ± 1.4% for whey protein, calcium-caseinate, and control consumption, respectively (n = 38); and on the basis of tick-sheet recordings, compliance was 91.7% ± 1.9%, 89.9% ± 2.2%, and 93.6% ± 1.8% for whey protein, calcium-caseinate, and control consumption, respectively (n = 37; 1 volunteer failed to complete the form). There were no significant differences between intervention proteins and the control in terms of compliance assessed both with the use of a sachet count (overall treatment effect: P = 0.400) and tick sheet (overall treatment effect: P = 0.545).
FIGURE 1.
Participant inclusion flowchart of the Whey2Go study.
TABLE 1.
Baseline characteristics of participants1
| Value | |
| Total (men/women), n | 38 (20/18) |
| Age, y | 52.9 ± 2.12 |
| BMI, kg/m2 | 27.1 ± 0.8 |
| SBP, mm Hg | 135.5 ± 2.2 |
| DBP, mm Hg | 84.0 ± 1.9 |
| Total cholesterol, mmol/L | 5.11 ± 0.2 |
| Triacylglycerol, mmol/L | 1.12 ± 0.1 |
| Glucose, mmol/L | 5.51 ± 0.1 |
DBP, diastolic blood pressure; SBP, systolic blood pressure.
Mean ± SEM (all such values).
Anthropometric measurements
The body composition of participants remained stable during interventions, and there were no significant treatment effects on those variables (for details, see Supplemental Table 2).
Twenty-four-hour AMBP
For the primary outcome 24-h AMBP, there were significant overall treatment effects (P = 0.027) (Table 2). Whey-protein consumption reduced 24-h SBP to a greater extent than did the control and also reduced daytime SBP compared with the effect of calcium caseinate (P = 0.048), and there was a tendency for lower daytime SBP after whey-protein supplementation than after control intake (P = 0.053). Similarly, whey protein lowered 24-h DBP compared with the effect of the control (P = 0.050) and lowered daytime DBP compared with the effect of calcium caseinate (P = 0.035), and there was a tendency for lower daytime DBP compared with the effect of the control (P = 0.077). No treatment effect was seen for nighttime SBP, DBP, pulse pressure, or heart rate. However, there was a significant treatment effect for clinic SBP, whereby whey protein decreased SBP compared with the effect of maltodextrin (Table 2; for heart-rate and pulse-pressure results, see Supplemental Table 3).
TABLE 2.
Twenty-four-hour ambulatory and clinic blood pressure at baseline (week 0) and postintervention (week 8) for whey protein, calcium-caseinate, and control interventions1
| Whey protein |
Calcium caseinate |
Control |
||||||||
| Baseline | Post | Δ | Baseline | Post | Δ | Baseline | Post | Δ | P2 | |
| 24-h SBP, mm Hg | 130.0 ± 2.2 | 127.1 ± 2.3 | −2.9 ± 1.1a | 129.7 ± 2.0 | 130.3 ± 2.6 | 0.6 ± 1.7a,b | 129.5 ± 2.3 | 130.5 ± 2.3 | 1.0 ± 1.1b | 0.041 |
| Day SBP, mm Hg | 134.2 ± 2.2 | 131.3 ± 2.4 | −2.8 ± 1.2a | 133.7 ± 2.2 | 135.4 ± 2.7 | 1.7 ± 1.7b | 133.4 ± 2.2 | 134.8 ± 2.4 | 1.3 ± 1.2a,b | 0.027 |
| Night SBP, mm Hg | 115.2 ± 2.4 | 112.2 ± 2.5 | −3.3 ± 1.5 | 115.5 ± 2.4 | 113.6 ± 3.0 | −2.0 ± 2.5 | 114.7 ± 2.3 | 115.7 ± 2.4 | 0.9 ± 1.4 | 0.110 |
| 24-h DBP, mm Hg | 78.9 ± 1.5 | 76.9 ± 1.5 | −2.0 ± 0.7a | 78.6 ± 1.3 | 79.0 ± 1.6 | 0.3 ± 1.0a,b | 78.0 ± 1.5 | 78.4 ± 1.6 | 0.5 ± 0.6b | 0.039 |
| Day DBP, mm Hg | 81.9 ± 1.6 | 79.8 ± 1.6 | −2.1 ± 0.8a | 81.6 ± 1.5 | 82.8 ± 1.7 | 1.2 ± 1.1b | 80.8 ± 1.5 | 81.5 ± 1.6 | 0.7 ± 0.7a,b | 0.027 |
| Night DBP, mm Hg | 68.0 ± 1.5 | 66.2 ± 1.5 | −1.8 ± 0.8 | 67.4 ± 1.6 | 66.1 ± 1.8 | −1.3 ± 1.5 | 67.2 ± 1.5 | 67.7 ± 1.5 | 0.5 ± 1.0 | 0.086 |
| Clinic SBP, mm Hg | 132.1 ± 2.1 | 127.9 ± 2.0 | −4.2 ± 1.3a | 129.3 ± 2.0 | 128.4 ± 2.0 | −0.9 ± 1.5a,b | 130.2 ± 2.2 | 130.9 ± 2.2 | 0.7 ± 1.4b | 0.035 |
| Clinic DBP, mm Hg | 76.7 ± 1.6 | 74.6 ± 1.5 | −2.1 ± 0.8 | 74.9 ± 1.6 | 73.6 ± 1.7 | −1.3 ± 0.8 | 74.8 ± 1.5 | 75.2 ± 1.8 | 0.5 ± 0.9 | 0.100 |
| Clinic PP, mm Hg | 55.5 ± 1.6 | 53.3 ± 1.3 | −2.2 ± 1.1 | 54.4 ± 1.9 | 54.8 ± 1.3 | 0.4 ± 1.1 | 55.4 ± 1.6 | 55.7 ± 1.5 | 0.2 ± 1.0 | 0.061 |
All values are means ± SEMs. n = 38. Baselines were significantly different (P ≤ 0.05) from one another except for 24-h SBP and DBP and day DBP. Different superscript letters within a row refer to treatment groups different from one another (P ≤ 0.05). DBP, diastolic blood pressure; PP, pulse pressure; SBP, systolic blood pressure; Δ change from baseline.
Overall between-group treatment effects for each Δ were obtained with the use of a linear mixed-model ANOVA with baseline values for the variable of interest and prognostic values such as age, sex, and BMI. Tukey-Kramer correction was used for the post hoc analysis to adjust for multiple testing.
Vascular function
There was a significant treatment effect on the %FMD whereby both whey protein and calcium caseinate increased the %FMD compared with the effect of the control. The augmentation index, which is a measure of arterial stiffness, was not affected by the study drinks; however, compared with the control, whey protein reduced both peripheral and central systolic and mean pressures. Other measures of arterial stiffness that were assessed with the use of the DVP were not different between treatment groups (Table 3).
TABLE 3.
Vascular measurements at baseline (week 0) and postintervention (week 8) for whey protein, calcium-caseinate, and control interventions1
| Whey protein |
Calcium caseinate |
Control |
||||||||
| Baseline | Post | Δ | Baseline | Post | Δ | Baseline | Post | Δ | P2 | |
| Endothelial function | ||||||||||
| %FMD | 4.79 ± 0.3 | 5.38 ± 0.4 | 0.59 ± 0.2a | 4.83 ± 0.3 | 4.94 ± 0.3 | 0.11 ± 0.2a | 4.79 ± 0.3 | 4.07 ± 0.3 | −0.72 ± 0.2b | <0.001 |
| Arterial stiffness | ||||||||||
| Assessed by PWA | ||||||||||
| Augmentation index at 75 beats/min, % | 17.6 ± 2.2 | 17.0 ± 2.2 | −0.6 ± 0.8 | 18.2 ± 2.3 | 17.1 ± 2.4 | −1.0 ± 0.8 | 18.2 ± 2.3 | 17.2 ± 2.1 | −1.0 ± 0.8 | 0.946 |
| Peripheral SP, mm Hg | 132.3 ± 2.2 | 127.5 ± 2.0 | −4.8 ± 1.4a | 129.1 ± 2.0 | 129.0 ± 2.0 | −0.1 ± 1.4a,b | 130.1 ± 2.2 | 131.0 ± 2.2 | 0.9 ± 1.4b | 0.007 |
| Peripheral DP, mm Hg | 76.6 ± 1.5 | 74.7 ± 1.5 | −1.9 ± 0.8 | 74.7 ± 1.6 | 73.7 ± 1.6 | −1.0 ± 0.8 | 74.7 ± 1.5 | 75.3 ± 1.8 | 0.6 ± 0.9 | 0.096 |
| Peripheral MP, mm Hg | 95.9 ± 2.0 | 92.5 ± 2.0 | −3.4 ± 0.9a | 94.1 ± 1.8 | 92.9 ± 1.9 | −1.2 ± 1.0a,b | 94.3 ± 1.8 | 94.6 ± 2.0 | 0.3 ± 0.9b | 0.032 |
| Central SP, mm Hg | 121.7 ± 2.5 | 116.7 ± 2.3 | −5.0 ± 1.3a | 118.6 ± 2.3 | 117.9 ± 2.3 | −0.7 ± 1.3a,b | 119.2 ± 2.4 | 119.6 ± 2.6 | 0.4 ± 1.3b | 0.011 |
| Central DP, mm Hg | 77.7 ± 1.6 | 75.6 ± 1.5 | −2.1 ± 0.8 | 75.8 ± 1.7 | 74.8 ± 1.7 | −1.0 ± 0.8 | 75.8 ± 1.5 | 76.4 ± 1.8 | 0.6 ± 0.9 | 0.094 |
| Central MP, mm Hg | 96.6 ± 1.8 | 93.3 ± 1.7 | −3.3 ± 0.8a | 94.1 ± 1.8 | 93.4 ± 1.8 | −0.7 ± 0.9a,b | 94.2 ± 1.8 | 94.9 ± 2.0 | 0.7 ± 1.0b | 0.023 |
| Assessed by DVP | ||||||||||
| Stiffness index, m/s | 7.53 ± 0.4 | 7.42 ± 0.4 | −0.11 ± 0.3 | 8.01 ± 0.4 | 7.92 ± 0.4 | −0.09 ± 0.2 | 7.59 ± 0.4 | 7.55 ± 0.4 | −0.04 ± 0.3 | 0.542 |
| Reflection index, % | 62.1 ± 2.9 | 66.2 ± 2.6 | 4.1 ± 2.3 | 65.0 ± 2.3 | 65.3 ± 2.7 | 0.3 ± 1.9 | 64.7 ± 2.4 | 64.7 ± 2.5 | 0.01 ± 1.6 | 0.515 |
| PPT, m/s | 246.5 ± 10.0 | 249.1 ± 9.9 | 2.6 ± 9.3 | 233.1 ± 10.5 | 235.2 ± 10.0 | 2.1 ± 5.2 | 244.2 ± 10.4 | 242.9 ± 9.5 | −1.3 ± 8.6 | 0.463 |
All values are means ± SEMs. n = 38 (except for FMD and DVP, for which n = 36). Baselines were significantly different (P ≤ 0.05) from one another except for peripheral DP and MP and central DP. Different superscript letters within a row refer to treatment groups differing from one another, P ≤ 0.05. DP, diastolic pressure; DVP, digital volume pulse; FMD; flow-mediated dilation; MP, mean pressure; PPT, peak to peak; PWA, pulse-wave analysis; SP, systolic pressure; Δ change from baseline; %FMD, flow-mediated dilatation percentage.
Overall between-group treatment effects for each Δ were obtained with the use of linear mixed-model ANOVA with baseline values for the variable of interest and prognostic values such as age, sex, BMI. Tukey-Kramer correction was used for the post hoc analysis to adjust for multiple testing.
Blood analyses
There was a significant reduction in total cholesterol after whey protein and calcium-caseinate consumption than after control intake. Triacylglycerol also decreased significantly in the whey-protein group compared with in the control group and almost reached significance (P = 0.055) between calcium caseinate and the control. No difference was seen on any other components of the lipid profile or indexes of insulin resistance and sensitivity. Although whey protein, compared with the control, lowered soluble intercellular adhesion molecule-1 (sICAM-1) significantly, calcium caseinate reduced soluble vascular cell adhesion molecule 1 (sVCAM-1). The protein supplements had no effects on any inflammatory markers or on plasma nitric oxide and serum ACE activity (Table 4).
TABLE 4.
Fasting lipid profile, indexes of insulin resistance, and vascular biomarkers at baseline (week 0) and postintervention (week 8) for whey protein, calcium-caseinate, and control interventions1
| Whey protein |
Calcium caseinate |
Control |
||||||||
| Baseline | Post | Δ | Baseline | Post | Δ | Baseline | Post | Δ | P2 | |
| Fasting lipid profile | ||||||||||
| Total cholesterol, mmol/L | 5.05 ± 0.2 | 4.88 ± 0.1 | −0.18 ± 0.1a | 5.01 ± 0.2 | 4.89 ± 0.2 | −0.12 ± 0.1a | 5.05 ± 0.2 | 5.12 ± 0.2 | 0.08 ± 0.1b | 0.010 |
| LDL cholesterol, mmol/L | 3.09 ± 0.1 | 3.00 ± 0.1 | −0.09 ± 0.1 | 3.08 ± 0.1 | 3.05 ± 0.1 | −0.03 ± 0.1 | 3.13 ± 0.1 | 3.15 ± 0.1 | 0.01 ± 0.1 | 0.337 |
| HDL cholesterol, mmol/L | 1.36 ± 0.1 | 1.34 ± 0.1 | −0.02 ± 0.02 | 1.35 ± 0.1 | 1.31 ± 0.1 | −0.04 ± 0.03 | 1.34 ± 0.1 | 1.37 ± 0.1 | 0.02 ± 0.01 | 0.160 |
| Total cholesterol:HDL cholesterol, mmol/L | 4.00 ± 0.2 | 3.81 ± 0.2 | −0.19 ± 0.1 | 3.97 ± 0.2 | 3.95 ± 0.2 | −0.01 ± 0.1 | 4.02 ± 0.2 | 4.02 ± 0.2 | 0.00 ± 0.1 | 0.094 |
| LDL cholesterol:HDL cholesterol, mmol/L | 2.48 ± 0.2 | 2.38 ± 0.1 | −0.10 ± 0.1 | 2.47 ± 0.2 | 2.50 ± 0.2 | 0.03 ± 0.1 | 2.53 ± 0.2 | 2.49 ± 0.2 | −0.04 ± 0.1 | 0.168 |
| Triacylglycerol, mmol/L | 1.31 ± 0.1 | 1.16 ± 0.1 | −0.15 ± 0.0a | 1.28 ± 0.1 | 1.16 ± 0.1 | −0.12 ± 0.1a,b | 1.25 ± 0.1 | 1.34 ± 0.1 | 0.08 ± 0.1b | 0.018 |
| Indexes of insulin resistance | ||||||||||
| Glucose, mmol/L | 5.04 ± 0.1 | 5.02 ± 0.1 | −0.03 ± 0.01 | 5.03 ± 0.1 | 5.10 ± 0.1 | 0.07 ± 0.1 | 5.00 ± 0.1 | 5.12 ± 0.1 | 0.12 ± 0.1 | 0.111 |
| Insulin, pmol/L | 52.4 ± 5.3 | 51.4 ± 5.0 | −1.17 ± 4.1 | 46.1 ± 4.3 | 51.7 ± 4.2 | 5.53 ± 2.7 | 47.6 ± 4.8 | 52.8 ± 5.0 | 5.25 ± 3.2 | 0.498 |
| NEFAs, μmol/L | 487.7 ± 35.0 | 414.4 ± 29.1 | −73.3 ± 30.7 | 528.7 ± 34.1 | 408.6 ± 28.4 | −120.1 ± 22.0 | 533.1 ± 32.8 | 464.0 ± 29.2 | −69.1 ± 25.8 | 0.091 |
| HOMA-IR | 1.99 ± 0.2 | 1.94 ± 0.2 | −0.05 ± 0.1 | 1.75 ± 0.2 | 1.98 ± 0.2 | 0.23 ± 0.1 | 1.79 ± 0.2 | 2.03 ± 0.2 | 0.24 ± 0.1 | 0.378 |
| QUICKI | 0.501 ± 0.0 | 0.502 ± 0.0 | 0.001 ± 0.0 | 0.503 ± 0.0 | 0.500 ± 0.0 | −0.003 ± 0.0 | 0.503 ± 0.0 | 0.500 ± 0.0 | −0.003 ± 0.0 | 0.135 |
| rQUICKI | 0.510 ± 0.0 | 0.511 ± 0.0 | 0.001 ± 0.0 | 0.511 ± 0.0 | 0.509 ± 0.0 | −0.002 ± 0.0 | 0.512 ± 0.0 | 0.509 ± 0.0 | −0.003 ± 0.0 | 0.102 |
| Vascular biomarkers | ||||||||||
| sVCAM-1, ng/mL | 637.3 ± 21.0 | 639.6 ± 22.5 | 2.3 ± 11.1a,b | 641.6 ± 22.5 | 618.5 ± 21.4 | −23.1 ± 11.7a | 627.7 ± 20.3 | 649.4 ± 23.2 | 21.7 ± 9.9b | 0.011 |
| sICAM-1, ng/mL | 224.7 ± 10.6 | 213 ± 9.0 | −11.7 ± 5.0a | 221.7 ± 10.4 | 218.4 ± 9.0 | −3.2 ± 5.6a,b | 223.6 ± 8.9 | 228.9 ± 10.7 | 5.3 ± 4.9b | 0.039 |
| E-selectin, ng/mL | 33.7 ± 2.1 | 34.15 ± 2.0 | 0.45 ± 0.8 | 34.46 ± 2.2 | 34.90 ± 2.1 | 0.44 ± 0.7 | 34.43 ± 2.2 | 36.32 ± 2.4 | 1.89 ± 0.8 | 0.303 |
| P-selectin, ng/mL | 33.5 ± 0.4 | 33.6 ± 0.3 | 0.09 ± 0.1 | 33.9 ± 0.3 | 33.5 ± 0.4 | −0.41 ± 0.2 | 33.9 ± 0.3 | 33.6 ± 0.4 | −0.28 ± 0.2 | 0.143 |
| CRP, mg/L | 1.25 ± 0.2 | 1.14 ± 0.2 | −0.11 ± 0.2 | 1.13 ± 0.1 | 1.46 ± 0.3 | 0.33 ± 0.3 | 1.10 ± 0.2 | 1.28 ± 0.2 | 0.18 ± 0.2 | 0.906 |
| IL-6, pg/mL | 1.31 ± 0.1 | 1.20 ± 0.1 | −0.11 ± 0.1 | 1.28 ± 0.1 | 1.27 ± 0.1 | −0.02 ± 0.1 | 1.21 ± 0.1 | 1.25 ± 0.1 | 0.04 ± 0.1 | 0.926 |
| TNF-α, pg/mL | 1.23 ± 0.1 | 1.24 ± 0.1 | 0.01 ± 0.1 | 1.24 ± 0.1 | 1.25 ± 0.1 | 0.01 ± 0.1 | 1.22 ± 0.1 | 1.27 ± 0.1 | 0.05 ± 0.1 | 0.856 |
| NO2, μmol/L | 1.45 ± 0.1 | 1.51 ± 0.1 | 0.06 ± 0.2 | 1.41 ± 0.1 | 1.49 ± 0.1 | 0.08 ± 0.2 | 1.51 ± 0.1 | 1.35 ± 0.1 | −0.17 ± 0.1 | 0.842 |
| NO3, μmol/L | 12.8 ± 1.9 | 12.1 ± 1.6 | −0.75 ± 1.4 | 12.0 ± 1.2 | 10.7 ± 1.1 | −1.30 ± 1.1 | 10.8 ± 1.1 | 11.7 ± 1.7 | −0.98 ± 1.2 | 0.811 |
| ACE activity, U/L | 4.65 ± 0.2 | 4.83 ± 0.2 | 0.18 ± 0.1 | 4.76 ± 0.2 | 5.01 ± 0.2 | 0.25 ± 0.1 | 4.58 ± 0.2 | 5.03 ± 0.2 | 0.45 ± 0.1 | 0.503 |
All values are means ± SEMs. n = 38 (except for sICAM-1 and E-selectin, for which n = 36; for P-selectin and IL-6, for which n = 37; and for CRP, for which n = 35). Baselines were not significantly different (P ≤ 0.05) from one another except for E-selectin and IL-6. Data were log transformed for CRP, IL-6, and TNF-α. Different superscript letters within a row refer to treatment groups different from one another (P ≤ 0.05). ACE, angiotensin-converting enzyme; CRP, C-reactive protein; NEFA, nonesterified fatty acid; QUICKI, quantitative insulin sensitivity index, rQUICKI, revised quantitative insulin sensitivity index; sICAM-1, soluble intercellular adhesion molecule 1; sVCAM-1, soluble vascular cell adhesion molecule 1; Δ, change from baseline.
Overall between-group treatment effects for each Δ were obtained with the use of a linear mixed-model ANOVA with baseline values for the variable of interest and prognostic values such as age, sex, and BMI. Tukey-Kramer correction was used for the post hoc analysis to adjust for multiple testing.
Diet
No overall treatment effect was observed for nutrient intake apart from that for protein (higher for the milk-protein groups than for the control group), carbohydrate (higher for the control than for the whey protein and casein), and calcium intake (higher for the calcium-caseinate group than for the whey-protein and control groups) (Table 5). On the basis of the FFQ, the mean ± SEM milk intake of participants was 312 ± 33 mL/d; consumption of yogurt, fromage frais, and other dairy desserts was 74 ± 13 mL/d; cheese intake was 19.3 ± 3.2 g/d; and butter consumption was 2.8 ± 0.6 g/d.
TABLE 5.
Reported nutrient intake at baseline (week 0) and postintervention (week 8) for whey protein, calcium-caseinate and control interventions1
| Whey protein |
Calcium caseinate |
Control |
||||||||
| Baseline | Post | Δ | Baseline | Post | Δ | Baseline | Post | Δ | P2 | |
| Energy, MJ/d | 9.5 ± 0.5 | 9.4 ± 0.5 | −0.09 ± 0.4 | 9.6 ± 0.5 | 9.2 ± 0.4 | −0.46 ± 0.3 | 9.2 ± 0.5 | 9.7 ± 0.5 | 0.50 ± 0.4 | 0.149 |
| Total fat, % of TE | 34.6 ± 1.0 | 32.2 ± 1.2 | −2.4 ± 0.8 | 34.8 ± 1.2 | 31.8 ± 1.1 | −3.0 ± 0.9 | 35.2 ± 1.2 | 32.3 ± 1.2 | −2.9 ± 1.1 | 0.906 |
| SFA, % of TE | 12.9 ± 0.6 | 12.7 ± 0.8 | −0.2 ± 0.6 | 12.4 ± 0.7 | 11.7 ± 0.7 | −0.7 ± 0.4 | 13.4 ± 0.6 | 12.2 ± 0.7 | −1.2 ± 0.6 | 0.391 |
| MUFA, % of TE | 11.7 ± 0.5 | 10.8 ± 0.4 | −1.0 ± 0.3 | 12.0 ± 0.5 | 10.9 ± 0.4 | −1.1 ± 0.4 | 12.3 ± 0.6 | 11.0 ± 0.5 | −1.3 ± 0.5 | 0.981 |
| n–6 PUFA, % of TE | 4.81 ± 0.2 | 4.11 ± 0.2 | −0.70 ± 0.2 | 5.22 ± 0.4 | 4.23 ± 0.2 | −0.99 ± 0.3 | 4.58 ± 0.3 | 4.37 ± 0.3 | −0.21 ± 0.3 | 0.464 |
| n–3 PUFA, % of TE | 0.78 ± 0.1 | 0.79 ± 0.1 | 0.01 ± 0.6 | 0.84 ± 0.1 | 0.85 ± 0.1 | 0.01 ± 0.6 | 0.74 ± 0.1 | 0.71 ± 0.1 | −0.03 ± 0.6 | 0.495 |
| Total PUFA, % of TE | 5.59 ± 0.3 | 5.04 ± 0.3 | −0.55 ± 0.4 | 6.06 ± 0.4 | 4.81 ± 0.3 | −1.24 ± 0.4 | 5.32 ± 0.3 | 5.08 ± 0.3 | −0.24 ± 0.4 | 0.456 |
| trans Fat, % of TE | 0.88 ± 0.1 | 0.90 ± 0.1 | 0.02 ± 0.1 | 0.97 ± 0.1 | 0.95 ± 0.1 | −0.02 ± 0.1 | 1.00 ± 0.1 | 0.97 ± 0.1 | −0.03 ± 0.1 | 0.972 |
| Protein, % of TE | 16.0 ± 0.5 | 24.4 ± 0.9 | 8.4 ± 0.8a | 15.9 ± 0.5 | 24.4 ± 0.7 | 8.5 ± 0.6a | 16.2 ± 0.6 | 15.2 ± 0.6 | −1.00 ± 0.6b | <0.001 |
| Carbohydrates, % of TE | 49.4 ± 1.3 | 44.0 ± 1.2 | −5.5 ± 1.0a | 49.7 ± 1.4 | 43.7 ± 1.4 | −6.0 ± 1.3a | 48.5 ± 1.7 | 54.8 ± 1.6 | 6.3 ± 1.9b | <0.001 |
| Alcohol, % of TE | 3.02 ± 0.7 | 2.60 ± 0.5 | −0.42 ± 0.5 | 2.59 ± 0.6 | 2.76 ± 0.6 | 0.18 ± 0.4 | 3.20 ± 0.7 | 2.48 ± 0.7 | −0.72 ± 0.6 | 0.066 |
| Dietary fiber (AOAC), g/d | 22.6 ± 1.4 | 20.1 ± 1.2 | −2.49 ± 1.1 | 23.0 ± 1.4 | 20.5 ± 1.2 | −2.56 ± 0.8 | 21.3 ± 1.1 | 20.4 ± 1.3 | −0.92 ± 0.9 | 0.399 |
| Sodium, g/d | 3.18 ± 0.2 | 3.12 ± 0.2 | −0.07 ± 0.2 | 3.37 ± 0.2 | 2.81 ± 0.2 | −0.57 ± 0.2 | 3.57 ± 0.5 | 2.95 ± 0.2 | −0.62 ± 0.5 | 0.215 |
| Potassium, g/d | 5.22 ± 1.2 | 5.21 ± 1.2 | −0.01 ± 0.2 | 4.54 ± 0.7 | 4.71 ± 0.9 | 0.17 ± 0.4 | 4.10 ± 0.6 | 4.80 ± 0.9 | 0.70 ± 0.8 | 0.582 |
| Calcium, g/d | 1.06 ± 0.1 | 1.19 ± 0.1 | 0.13 ± 0.1b | 1.09 ± 0.1 | 1.57 ± 0.1 | 0.48 ± 0.1a | 0.97 ± 0.1 | 1.00 ± 0.1 | 0.03 ± 0.1b | <0.001 |
All values are means ± SEMs. n = 38. Data were log transformed for alcohol intake. Baselines were not significantly different (P ≤ 0.05) from one another. Different superscript letters within a row refer to treatment groups different from one another (P ≤ 0.05). AOAC, Association of Official Analytic Chemists; TE, total energy; Δ change from baseline.
Overall between-group treatment effects for each Δ were obtained with the use of a linear mixed-model ANOVA with baseline values for the variable of interest and prognostic values such as age, sex, and BMI. Tukey-Kramer correction was used for the post hoc analysis to adjust for multiple testing.
ACEi effects of study drinks: in vitro study
ACEi effects of the intervention products are presented in Supplemental Figure 2. Whey-protein permeate exhibited significant ACEi activity compared with that of calcium-caseinate permeate, control permeate and of undigested gastric digesta and retentate for whey protein, calcium caseinate, and the control.
DISCUSSION
This novel study revealed that the consumption of whey protein (56 g/d) for 8 wk resulted in clinically relevant reductions in 24-h SBP (−2.9 ± 1.1 mm Hg) and DBP (−2.0 ± 0.7 mm Hg) compared with the effect of the control in adults with prehypertension and mild hypertension. To date, only a very limited number of studies have evaluated the effects of intact dairy proteins on BP. Although Pal and Ellis (7) did not use 24-h AMBP, our findings support their data relating to the efficacy of whey protein to reduce BP. However, contrary to their findings, we did not detect changes in BP after calcium-caseinate supplementation. Although clinic BP may be considered less reliable in relation to future cardiovascular mortality and morbidity (30, 31), we also showed a significant decrease in clinic SBP after whey-protein supplementation than after control intake. The inhibition of ACE has been proposed as a potential mechanism by which dairy proteins reduce BP (32). Data from our in vitro study confirmed the significant ACEi activity of whey protein compared with that of calcium caseinate and the control. However, a reduction in circulating ACE activity was not observed in the intervention study, possibly because of a lack of power, and further studies are require to confirm the importance of ACEi in BP reduction by whey protein.
Significant improvements in the %FMD response after both whey protein and calcium-caseinate supplementation compared with after control intake were also observed, thereby supporting previous studies (33, 34). FMD is the gold standard for endothelial dysfunction assessment and provides additional, independent prognostic data beyond the classic CVD-risk factors (35). It has been estimated that the clinical significance of consuming whey protein and calcium caseinate for 8 wk would be 7.7% and 1.4% reductions in future CVD events, respectively (36). Nitric oxide has been recognized as a mediator of vessel dilation (37). However, we observed no differences in plasma NO2 or NO3 between intervention products, thereby confirming previous data (33). Nevertheless, it has been proposed that vascular dilation is not solely related to nitric oxide (38). Indeed, inflammatory markers and cell adhesion molecules have also been associated with the progression of atherosclerosis and endothelial dysfunction (39). During atherosclerotic plaque formation, inflammatory cells, circulatory soluble adhesion molecules (such as sICAM-1 and sVCAM-1), and cell surface adhesion molecules (E-selectin and P-selectin) are activated (40). We showed a significant decrease in sVCAM-1 and sICAM-1 after calcium-caseinate and whey-protein consumption, respectively, than after control intake. This result suggests that the beneficial effects of dairy proteins on adhesion molecules may be a potential mechanism for improvements in vascular reactivity. This outcome is in agreement with a recent review that summarized the acute and chronic effects of dietary proteins on markers of inflammation and adhesion molecules and reported a reduction in sICAM after a 4-wk high-protein diet compared with the effect of maltodextrin (41).
In contrast with our study, Pal and Ellis (7) reported a decrease in the augmentation index after whey-protein supplementation. Nevertheless, we showed significant decreases in systolic and mean arterial pressures of both peripheral and central pressures that were assessed with the use of a pulse-wave analysis. This result is an important finding because emerging evidence suggests that aortic pressure is more strongly related to future cardiovascular events than is brachial pressure (42–44) because organs such as the heart, brain, and kidneys are exposed to central pressure rather than peripheral pressure (45). Furthermore, the mean arterial pressure has been proposed to be a predictor of stroke (46). Note that, in our study, each intervention period lasted for 8 wk as opposed to in the study of Pal and Ellis (7), which was a 12-wk intervention. At week 6, Pal and Ellis (7) showed no significant treatment effects on the augmentation index, which suggests that a longer intervention period may have been required for significant changes to the augmentation index to be shown. This possibility has been supported by Jauhiainen et al. (47) who reported a lower augmentation index after lactotripeptide consumption in a 12-wk trial. In contrast, improvements in the pulse-wave velocity (which is another measure of arterial stiffness) after 6 and 8 wk of supplementation with lactotripeptides were observed (48, 49).
A significant decrease in total cholesterol after both whey protein and calcium-caseinate supplementation was observed as were reductions in triacylglycerol after whey-protein consumption. In our recent review, we identified 4 RCTs and a pilot study that evaluated the long-term effects of dairy proteins on lipid metabolism (50). Our results are in agreement with the majority of these studies (51–53); however, we failed to detect a decrease in LDL cholesterol or an increase in HDL cholesterol. Longer supplementation periods with milk proteins may have been more informative on the relative effects of dairy proteins on other lipid profiles or on the strength of significance. Calcium intake from dairy has been associated with calcium–fatty acid soap formation in the gastrointestinal tract, which leads to reduced fat absorption (54, 55); thus, calcium intake may well be a mechanism of action for the lipid-lowering effects of calcium caseinate. Although calcium has been linked with a favorable effect on BP (56), calcium-caseinate supplementation had no significant effect on BP despite containing >2.5-fold higher calcium than whey protein. Other potential mechanisms of action have been linked with both whey protein and calcium-caseinate consumption. These mechanisms include the inhibition of genes that are involved in fatty-acid and cholesterol absorption and synthesis in the intestines (57, 58), increased catabolic metabolism via urinary excretion of tricarboxylic acid cycle compounds (59), and the stimulation of gut-bacteria activity resulting in higher short-chain fatty acids (60), which have been proposed as key regulatory metabolites in lipid metabolism (61).
There was no change in body weight or dietary intake apart from the inclusion of study products during the intervention. Isoenergetic intake was successfully achieved through diligent dietetic management, which increased the motivation and compliance of our participants. The habitual milk and dairy consumption of the participants was similar to that of the United Kingdom population (62) and confirmed that the favorable effects of dairy proteins were observed in individuals who habitually consume dairy foods.
To blind the study, maltodextrin was chosen as an appropriate control because it was flavorless and easily dissolved and had an energy content that matched that of the milk proteins. However, the maltodextrin may not have been inert and could have influenced some of the variables measure, which could have been a potential limitation of the study. Nevertheless, the differences observed between the whey protein and calcium-caseinate groups confirmed the effects attributed to the specific properties of the proteins rather than to the differences between macronutrients. Another potential limitation was that the daily dose given in this trial was relatively high, and therefore further studies are required to determine the lowest effective dose of dairy proteins.
In conclusion, this novel RCT has several important observations. Compared with the control, whey protein significantly lowered 24-h SBP and DBP, central and peripheral SBP, and mean arterial pressure. Furthermore, compared with the control, both whey protein and calcium caseinate improved endothelial function, reduce adhesion molecules and vascular biomarkers of risk, and improved blood lipids. The magnitude of changes in the CVD risk markers observed is modest but may have important implications for public health.
Acknowledgments
We thank Karen Jenkins, Rada Mihaylova, Kim Jackson, Honglin Dong, Katy Fingleton, Fintan McArdle, Xianying Cui, Emily Gimson, and Davide Gottardo for assisting with the trial. We also thank Attila Tóth and Miklós Fagyas for the serum ACE-activity analysis and Sue Todd for statistical advice.
The authors’ responsibilities were as follows—ÁAF: conducted the research, analyzed the data, and wrote the manuscript; ÁAF, DIG, and JAL: designed the study; CG and YC: substantially contributed to the conduct of the research; YC: analyzed the diet diaries and FFQ; and all authors: modified the writing of the manuscript and read and approved the final manuscript. DIG and JAL previously received funding for research from AHDB Dairy, acted as advisors to the Dairy Council, and received in-kind foods from Arla for a study funded by the Medical Research Council. The remaining authors reported no conflicts of interest related to the study.
Footnotes
Abbreviations used: ACE, angiotensin-converting enzyme; ACEi, angiotensin-converting enzyme inhibitory; AMBP ambulatory blood pressure; BP, blood pressure; CVD, cardiovascular disease; DBP, diastolic blood pressure; DVP, digital volume pulse; FFQ, food-frequency questionnaire; FMD, flow-mediated dilatation; RCT, randomized controlled trial; SBP, systolic blood pressure; sICAM-1, soluble intercellular adhesion molecule 1; sVCAM-1, soluble vascular cell adhesion molecule 1; %FMD, flow-mediated dilatation percentage.
REFERENCES
- 1.Townsend NWJ, Bhatnagar P, Wickramasinghe K, Rayner M. Cardiovascular disease statistics, 2014. London: British Heart Foundation; 2014. [Google Scholar]
- 2.Appel LJ, Brands MW, Daniels SR, Karanja N, Elmer PJ, Sacks FM. Dietary approaches to prevent and treat hypertension: a scientific statement from the American Heart Association. Hypertension 2006;47:296–308. [DOI] [PubMed] [Google Scholar]
- 3.Saneei P, Salehi-Abargouei A, Esmaillzadeh A, Azadbakht L. Influence of Dietary Approaches to Stop Hypertension (DASH) diet on blood pressure: a systematic review and meta-analysis on randomized controlled trials. Nutr Metab Cardiovasc Dis 2014;24:1253–61. [DOI] [PubMed] [Google Scholar]
- 4.Elwood PC, Pickering JE, Givens DI, Gallacher JE. The consumption of milk and dairy foods and the incidence of vascular disease and diabetes: an overview of the evidence. Lipids 2010;45:925–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fekete ÁA, Givens DI, Lovegrove JA. Casein-derived lactotripeptides reduce systolic and diastolic blood pressure in a meta-analysis of randomized clinical trials. Nutrients 2015;7:659–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fekete ÁA, Givens DI, Lovegrove JA. The impact of milk proteins and peptides on blood pressure and vascular function: a review of evidence from human intervention studies. Nutr Res Rev 2013;26:177–90. [DOI] [PubMed] [Google Scholar]
- 7.Pal S, Ellis V. The chronic effects of whey proteins on blood pressure, vascular function, and inflammatory markers in overweight individuals. Obesity (Silver Spring) 2010;18:1354–9. [DOI] [PubMed] [Google Scholar]
- 8.O’Brien E, Asmar R, Beilin L, Imai Y, Mallion JM, Mancia G, Mengden T, Myers M, Padfield P, Palatini P, et al. European Society of Hypertension recommendations for conventional, ambulatory and home blood pressure measurement. J Hypertens 2003;21:821–48. [DOI] [PubMed] [Google Scholar]
- 9.Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, et al. Guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2007;28:1462–536. [DOI] [PubMed] [Google Scholar]
- 10.Clement DL, De Buyzere ML, De Bacquer DA, De Leeuw PW, Duprez DA, Fagard RH, Gheeraert PJ, Missault LH, Braun JJ, Six RO, et al. Prognostic value of ambulatory blood-pressure recordings in patients with treated hypertension. N Engl J Med 2003;348:2407–15. [DOI] [PubMed] [Google Scholar]
- 11.Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation 2004;109(23 Suppl 1):III27–32. [DOI] [PubMed] [Google Scholar]
- 12.Ras RT, Streppel MT, Draijer R, Zock PL. Flow-mediated dilation and cardiovascular risk prediction: a systematic review with meta-analysis. Int J Cardiol 2013;168:344–51. [DOI] [PubMed] [Google Scholar]
- 13.Payne RA, Webb DJ. Arterial blood pressure and stiffness in hypertension: is arterial structure important? Hypertension 2006;48:366–7. [DOI] [PubMed] [Google Scholar]
- 14.Mancia G. Short- and long-term blood pressure variability: present and future. Hypertension 2012;60:512–7. [DOI] [PubMed] [Google Scholar]
- 15.Kang M, Ragan BG, Park JH. Issues in outcomes research: an overview of randomization techniques for clinical trials. J Athl Train 2008;43:215–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mulligan AA, Luben RN, Bhaniani A, Parry-Smith DJ, O’Connor L, Khawaja AP, Forouhi NG, Khaw KT. A new tool for converting food frequency questionnaire data into nutrient and food group values: FETA research methods and availability. BMJ Open 2014;4:e004503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nelson M, Atkinson M, Meyer J. Food portion sizes: a photographic atlas. London: Ministry of Agriculture, Fisheries, and Food; 1997. [Google Scholar]
- 18.Food Standard Agency. Food portion sizes. 2nd revised edLondon: The Stationary Office; 2002. [Google Scholar]
- 19.Henderson LG, Irving J, Swan K. G. The National Diet and Nutrition Survey: adults aged 19 to 64 years. Vol. 2: energy, protein, carbohydrate, fat and alcohol intake. London: The Stationary Office; 2003. [Google Scholar]
- 20.Newens KJ, Thompson AK, Jackson KG, Wright J, Williams CM. DHA-rich fish oil reverses the detrimental effects of saturated fatty acids on postprandial vascular reactivity. Am J Clin Nutr 2011;94:742–8. [DOI] [PubMed] [Google Scholar]
- 21.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 2002;39:257–65. [DOI] [PubMed] [Google Scholar]
- 22.Hobbs DA, Goulding MG, Nguyen A, Malaver T, Walker CF, George TW, Methven L, Lovegrove JA. Acute ingestion of beetroot bread increases endothelium-independent vasodilation and lowers diastolic blood pressure in healthy men: a randomized controlled trial. J Nutr 2013;143:1399–405. [DOI] [PubMed] [Google Scholar]
- 23.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499–502. [PubMed] [Google Scholar]
- 24.Brady LM, Gower BA, Lovegrove SS, Williams CM, Lovegrove JA. Revised QUICKI provides a strong surrogate estimate of insulin sensitivity when compared with the minimal model. Int J Obes Relat Metab Disord 2004;28:222–7. [DOI] [PubMed] [Google Scholar]
- 25.Rassaf T, Bryan NS, Kelm M, Feelisch M. Concomitant presence of N-nitroso and S-nitroso proteins in human plasma. Free Radic Biol Med 2002;33:1590–6. [DOI] [PubMed] [Google Scholar]
- 26.Carmona AK, Schwager SL, Juliano MA, Juliano L, Sturrock ED. A continuous fluorescence resonance energy transfer angiotensin I-converting enzyme assay. Nat Protoc 2006;1:1971–6. [DOI] [PubMed] [Google Scholar]
- 27.Mills DJ, Tuohy KM, Booth J, Buck M, Crabbe MJ, Gibson GR, Ames JM. Dietary glycated protein modulates the colonic microbiota towards a more detrimental composition in ulcerative colitis patients and non-ulcerative colitis subjects. J Appl Microbiol 2008;105:706–14. [DOI] [PubMed] [Google Scholar]
- 28.Murray BA, Walsh DJ, FitzGerald RJ. Modification of the furanacryloyl-L-phenylalanylglycylglycine assay for determination of angiotensin-I-converting enzyme inhibitory activity. J Biochem Biophys Methods 2004;59:127–37. [DOI] [PubMed] [Google Scholar]
- 29.Vermeirssen V, Van Camp J, Verstraete W. Optimisation and validation of an angiotensin-converting enzyme inhibition assay for the screening of bioactive peptides. J Biochem Biophys Methods 2002;51:75–87. [DOI] [PubMed] [Google Scholar]
- 30.O’Brien E, Asmar R, Beilin L, Imai Y, Mancia G, Mengden T, Myers M, Padfield P, Palatini P, Parati G, et al. Practice guidelines of the European Society of Hypertension for clinic, ambulatory and self blood pressure measurement. J Hypertens 2005;23:697–701. [DOI] [PubMed] [Google Scholar]
- 31.Mancia G, Parati G. Office compared with ambulatory blood pressure in assessing response to antihypertensive treatment: a meta-analysis. J Hypertens 2004;22:435–45. [DOI] [PubMed] [Google Scholar]
- 32.FitzGerald RJ, Meisel H. Milk protein-derived peptide inhibitors of angiotensin-I-converting enzyme. Br J Nutr 2000;84:S33–7. [DOI] [PubMed] [Google Scholar]
- 33.Ballard KD, Bruno RS, Seip RL, Quann EE, Volk BM, Freidenreich DJ, Kawiecki DM, Kupchak BR, Chung MY, Kraemer WJ, et al. Acute ingestion of a novel whey-derived peptide improves vascular endothelial responses in healthy individuals: a randomized, placebo controlled trial. Nutr J 2009;8:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoshizawa M, Maeda S, Miyaki A, Misono M, Choi Y, Shimojo N, Ajisaka R, Tanaka H. Additive beneficial effects of lactotripeptides intake with regular exercise on endothelium-dependent dilatation in postmenopausal women. Am J Hypertens 2010;23:368–72. [DOI] [PubMed] [Google Scholar]
- 35.Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation 2007;115:2390–7. [DOI] [PubMed] [Google Scholar]
- 36.Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging 2010;26:631–40. [DOI] [PubMed] [Google Scholar]
- 37.Lidder S, Webb AJ. Vascular effects of dietary nitrate (as found in green leafy vegetables and beetroot) via the nitrate-nitrite-nitric oxide pathway. Br J Clin Pharmacol 2013;75:677–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Green DJ, Jones H, Thijssen D, Cable NT, Atkinson G. Flow-mediated dilation and cardiovascular event prediction: does nitric oxide matter? Hypertension 2011;57:363–9. [DOI] [PubMed] [Google Scholar]
- 39.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation 2007;115:1285–95. [DOI] [PubMed] [Google Scholar]
- 40.Constans J, Conri C. Circulating markers of endothelial function in cardiovascular disease. Clin Chim Acta 2006;368:33–47. [DOI] [PubMed] [Google Scholar]
- 41.Teunissen-Beekman KFM, Dopheide J, Geleijnse JM, Bakker SJL, Brink EJ, de Leeuw PW, Schalkwijk CG, van Baak MA. Dietary proteins improve endothelial function under fasting conditions but not in the postprandial state, with no effects on markers of low-grade inflammation. Br J Nutr 2015;114:1819–28. [DOI] [PubMed] [Google Scholar]
- 42.Pini R, Cavallini MC, Palmieri V, Marchionni N, Di Bari M, Devereux RB, Masotti G, Roman MJ. Central but not brachial blood pressure predicts cardiovascular events in an unselected geriatric population: the ICARe Dicomano Study. J Am Coll Cardiol 2008;51:2432–9. [DOI] [PubMed] [Google Scholar]
- 43.Safar ME, Blacher J, Pannier B, Guerin AP, Marchais SJ, Guyonvarc’h PM, London GM. Central pulse pressure and mortality in end-stage renal disease. Hypertension 2002;39:735–8. [DOI] [PubMed] [Google Scholar]
- 44.Roman MJ, Devereux RB, Kizer JR, Lee ET, Galloway JM, Ali T, Umans JG, Howard BV. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the strong heart study. Hypertension 2007;50:197–203. [DOI] [PubMed] [Google Scholar]
- 45.McEniery CM, Cockcroft JR, Roman MJ, Franklin SS, Wilkinson IB. Central blood pressure: current evidence and clinical importance. Eur Heart J 2014;35:1719–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Verdecchia P, Schillaci G, Reboldi G, Franklin SS, Porcellati C. Different prognostic impact of 24-hour mean blood pressure and pulse pressure on stroke and coronary artery disease in essential hypertension. Circulation 2001;103:2579–84. [DOI] [PubMed] [Google Scholar]
- 47.Jauhiainen T, Ronnback M, Vapaatalo H, Wuolle K, Kautiainen H, Groop PH, Korpela R. Long-term intervention with Lactobacillus helveticus fermented milk reduces augmentation index in hypertensive subjects. Eur J Clin Nutr 2010;64:424–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cicero AF, Rosticci M, Gerocarni B, Bacchelli S, Veronesi M, Strocchi E, Borghi C. Lactotripeptides effect on office and 24-h ambulatory blood pressure, blood pressure stress response, pulse wave velocity and cardiac output in patients with high-normal blood pressure or first-degree hypertension: a randomized double-blind clinical trial. Hypertens Res 2011;34:1035–40. [DOI] [PubMed] [Google Scholar]
- 49.Nakamura T, Mizutani J, Ohki K, Yamada K, Yamamoto N, Takeshi M, Takazawa K. Casein hydrolysate containing Val-Pro-Pro and Ile-Pro-Pro improves central blood pressure and arterial stiffness in hypertensive subjects: a randomized, double-blind, placebo-controlled trial. Atherosclerosis 2011;219:298–303. [DOI] [PubMed] [Google Scholar]
- 50.Fekete ÁA, Givens DI, Lovegrove JA. Can milk proteins be a useful tool in the management of cardiometabolic health? An updated review of human intervention trials. Proc Nutr Soc. 2016;75:328–41. [DOI] [PubMed] [Google Scholar]
- 51.Pal S, Ellis V, Dhaliwal S. Effects of whey protein isolate on body composition, lipids, insulin and glucose in overweight and obese individuals. Br J Nutr 2010;104:716–23. [DOI] [PubMed] [Google Scholar]
- 52.Weisse K, Brandsch C, Zernsdorf B, Nkengfack Nembongwe GS, Hofmann K, Eder K, Stangl GI. Lupin protein compared to casein lowers the LDL cholesterol:HDL cholesterol-ratio of hypercholesterolemic adults. Eur J Nutr 2010;49:65–71. [DOI] [PubMed] [Google Scholar]
- 53.Gouni-Berthold I, Schulte DM, Krone W, Lapointe JF, Lemieux P, Predel HG, Berthold HK. The whey fermentation product malleable protein matrix decreases TAG concentrations in patients with the metabolic syndrome: a randomized placebo-controlled trial. Br J Nutr 2012;107:1694–706. [DOI] [PubMed] [Google Scholar]
- 54.Bendsen NT, Hother AL, Jensen SK, Lorenzen JK, Astrup A. Effect of dairy calcium on fecal fat excretion: a randomized crossover trial. Int J Obes (Lond) 2008;32:1816–24. [DOI] [PubMed] [Google Scholar]
- 55.Christensen R, Lorenzen JK, Svith CR, Bartels EM, Melanson EL, Saris WH, Tremblay A, Astrup A. Effect of calcium from dairy and dietary supplements on faecal fat excretion: a meta-analysis of randomized controlled trials. Obes Rev 2009;10:475–86. [DOI] [PubMed] [Google Scholar]
- 56.Belizan JM, Villar J, Pineda O, Gonzalez AE, Sainz E, Garrera G, Sibrian R. Reduction of blood pressure with calcium supplementation in young adults. JAMA 1983;249:1161–5. [PubMed] [Google Scholar]
- 57.Chen Q, Reimer RA. Dairy protein and leucine alter GLP-1 release and mRNA of genes involved in intestinal lipid metabolism in vitro. Nutrition 2009;25:340–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang X, Beynen AC. Lowering effect of dietary milk-whey protein v. casein on plasma and liver cholesterol concentrations in rats. Br J Nutr 1993;70:139–46. [DOI] [PubMed] [Google Scholar]
- 59.Lillefosse HH, Clausen MR, Yde CC, Ditlev DB, Zhang X, Du ZY, Bertram HC, Madsen L, Kristiansen K, Liaset B. Urinary loss of tricarboxylic acid cycle intermediates as revealed by metabolomics studies: an underlying mechanism to reduce lipid accretion by whey protein ingestion? J Proteome Res 2014;13:2560–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zheng H, Yde CC, Clausen MR, Kristensen M, Lorenzen J, Astrup A, Bertram HC. Metabolomics investigation to shed light on cheese as a possible piece in the French paradox puzzle. J Agric Food Chem 2015;63:2830–9. [DOI] [PubMed] [Google Scholar]
- 61.Tremaroli V, Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature 2012;489:242–9. [DOI] [PubMed] [Google Scholar]
- 62.Public Health England. National Diet and Nutrition Survey: results from years 1-4 (combined) of the rolling programme for 2008 and 2009 to 2011 and 2012. London: Public Health England; 2014. [Google Scholar]



