Abstract
Background: Prenatal alcohol exposure results in a broad range of cognitive and behavioral impairments. Because of the long-lasting problems that are associated with fetal alcohol spectrum disorders (FASDs), the development of effective treatment programs is critical. Preclinical animal studies have shown that choline, which is an essential nutrient, can attenuate the severity of alcohol-related cognitive impairments.
Objective: We aimed to translate preclinical findings to a clinical population to investigate whether choline supplementation can ameliorate the severity of memory, executive function, and attention deficits in children with FASDs.
Design: In the current study, which was a randomized, double-blind, placebo-controlled clinical trial, we explored the effectiveness of a choline intervention for children with FASDs who were aged 5–10 y. Fifty-five children with confirmed histories of heavy prenatal alcohol exposure were randomly assigned to either the choline (n = 29) or placebo (n = 26) treatment arms. Participants in the choline group received 625 mg choline/d for 6 wk, whereas subjects in the placebo group received an equivalent dose of an inactive placebo treatment. Primary outcomes, including the performance on neuropsychological measures of memory, executive function, and attention and hyperactivity, were assessed at baseline and postintervention.
Results: Compared with the placebo group, participants in the choline group did not differentially improve in cognitive performance in any domain. Treatment compliance and mean dietary choline intake were not predictive of treatment outcomes.
Conclusions: Findings of the current study do not support that choline, administered at a dose of 625 mg/d for 6 wk, is an effective intervention for school-aged (5–10 y old) children with FASDs. This research provides important information about choline’s therapeutic window. Combined with other studies of choline and nutritional interventions in this population, this study emphasizes a further need for the continued study of the role of nutritional status and supplementation in children with FASDs and the contributions of nutrition to neurocognition. This trial was registered at clinicaltrials.gov as NCT01911299.
Keywords: choline, clinical trial, fetal alcohol spectrum disorders, fetal alcohol syndrome, nutrition, prenatal alcohol exposure, supplementation
INTRODUCTION
Alcohol exposure during pregnancy disrupts fetal development and results in a range of outcomes that are known as fetal alcohol spectrum disorders (FASDs)7. Despite known adverse consequences and public health warnings, women continue to consume alcohol during pregnancy (1). In the United States, 7.6% of women have reported drinking during pregnancy, and 1.4% of women have reported binge patterns of drinking, which is particularly of high risk to the developing fetus (2). Effective, evidence-based interventions are critically needed to ameliorate adverse consequences in children who are affected by prenatal alcohol exposure.
Much attention has been given to the role of nutrition as a protective factor against alcohol teratogenesis. Notably, our group was the first, to our knowledge, to examine the effects of a nutritional intervention of choline with the use of a rodent model of FASDs (3). Through a series of studies, we showed that choline reduces the severity of alcohol’s adverse effects on behavioral development whether administered perinatally (4, 5) or postnatally after alcohol exposure has occurred (3, 6, 7). Choline mitigates behavioral deficits on tasks that depend on the functional integrity of the hippocampus and prefrontal cortex including spatial learning and memory (8), hyperactivity (8), trace classical conditioning (9, 10), and working memory (3). Although research has shown the clinical potential of choline and other nutritional interventions in alcohol-exposed subjects, the majority of work has been in the preclinical phase. To our knowledge, only 2 published studies have documented the effects of choline supplementation in clinical populations with or at risk of FASDs. The first study was published as part of a larger clinical trial that explored micronutrient supplementation in alcohol-consuming pregnant women in the Ukraine (11, 12) and showed that choline supplementation improved neurophysiologic encoding and memory in both alcohol-exposed and -unexposed infants (13). The second study was a randomized clinical trial of choline supplementation in preschool-aged children with FASDs and revealed that choline improved memory function in young children between the ages of 2.5 and 5 y (14). Therefore, given that previous clinical data suggest some cognitive benefits of choline supplementation in early developmental periods, we aimed to investigate the effectiveness of this intervention in older, school-aged children with FASDs.
The current study was a randomized, double-blinded, placebo-controlled clinical trial that examined the effectiveness of a 6-wk choline supplementation in children with FASDs who were aged 5–10 y. The goals were to 1) examine whether choline improves cognition in children with FASDs and 2) explore moderators of treatment outcomes. We hypothesized that choline treatment would improve memory, executive function, and attention. In addition, a measure of motor performance was included as a negative control, considering that preclinical studies have demonstrated specific effects of choline on hippocampal and frontal cholinergic systems but not on cerebellar function (8, 10). Second, we hypothesized that higher treatment compliance and lower daily dietary choline consumption would be associated with greater benefits. It was expected that children who, at baseline, consumed lower or deficient amounts of dietary choline would have more to gain from supplementation and, therefore, show more benefit. This research extends previous studies and contributes to our understanding of choline’s effects across development from infancy through late childhood. Because FASDs are not often identified until children enter school (15), it is critical to examine treatment options for children at older ages.
METHODS
This study was a multisite, randomized, double-blinded, placebo-controlled, parallel-group study that was conducted through the Center for Behavioral Teratology at San Diego State University. All procedures were approved by the institutional review boards at San Diego State University and the University of California, San Diego. All caregivers and participants provided informed consent and assent, respectively, to participate in the study. Additional oversight was provided by an independent Data Safety Monitoring Board. This trial was registered at clinicaltrials.gov as NCT01911299 on 26 July 2013.
Participants
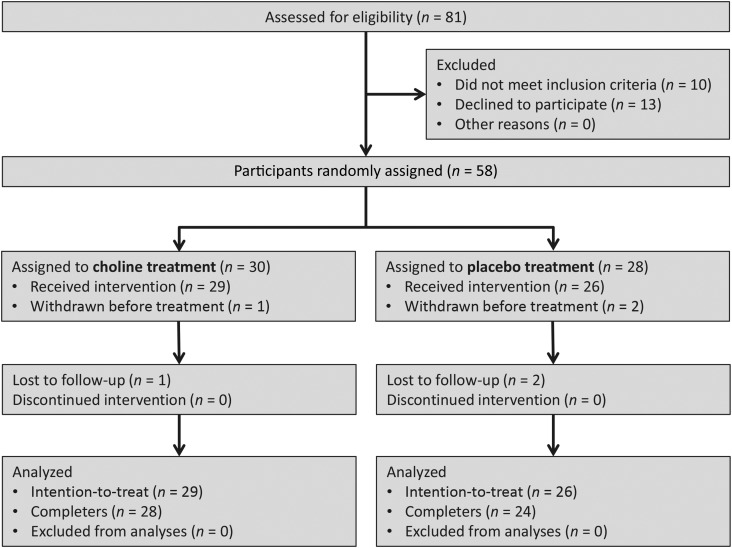
Subjects included 55 children with confirmed histories of heavy prenatal alcohol exposure between the ages of 5 and 10 y (mean ± SD: 8.3 ± 1.75 y). On the basis of expected medium effect sizes (Cohen’s d = 0.29–0.42) that were observed in early phase I analyses by Wozniak et al. (16), we calculated that a total sample size of ∼50 subjects would be needed for 80% power to detect significant differences between treatment groups with an α level of P < 0.05. Individuals were recruited through the following 2 primary sites: the Center for Behavioral Teratology at San Diego State University and Double ARC, which is a nonprofit organization providing services to families of children with FASDs in Toledo, Ohio. Children were also recruited through the Genetics and Dysmorphology Clinic at Rady Children’s Hospital–San Diego and postings on websites and listservs for families of children with FASDs. Age-eligible subjects were recruited from May 2013 to March 2014. Of 55 participants who initiated treatment and received the allocated intervention, 3 subjects were lost to follow-up and did not complete the 6-wk intervention. Figure 1 presents the flow diagram of participants through the phases of the study according to the guidelines for reporting clinical trials of the Consolidated Standards of Reporting Trials (17).
FIGURE 1.
Flow diagram of the progression of participants through the study.
All eligible subjects were required to meet the criteria for heavy prenatal alcohol exposure and be primary English speakers for the comprehension of neuropsychological tests. Heavy exposure was defined as ≥4 drinks/occasion ≥1 time/wk or ≥14 drinks/wk during pregnancy. A history of prenatal alcohol exposure was determined retrospectively through a review of available medical, social service, or adoption records as well as from maternal, family, and friend reports when available. In many cases, when detailed information about timing, duration, or quantity of alcohol consumption was unavailable, mothers were reported to be alcoholic, alcohol abusing, or alcohol dependent during pregnancy. Thirty-one participants received a dysmorphology examination conducted by Kenneth Lyons Jones to determine a fetal alcohol syndrome (FAS) diagnosis on the basis of physical, craniofacial, and growth anomalies; FAS was defined as the presence of ≥2 key facial features (short palpebral fissures ≤10th percentile, smooth philtrum, and thin vermillion border of the upper lip), growth deficiency (≤10th percentile for height or weight), and head circumference ≤10th percentile (for further details, see reference 18). In addition, 7 participants had received a formal diagnosis of an FASD (i.e., including a dysmorphologic and physical examination) from other sources (e.g., by dysmorphologists other than Kenneth Lyons Jones or the use of different diagnostic systems such as the 4-Digit-Code (19). The remaining 18 children all met the eligibility criteria of having confirmed histories of heavy prenatal alcohol exposure, as previously defined, but were not evaluated for a diagnosis.
Exclusion criteria were as follows: a history of head injury with a loss of consciousness >30 min; a substantial physical (e.g., uncorrected visual impairment or hemiparesis), neurologic (e.g., seizure disorder), or psychiatric (e.g., active psychosis) disability that precluded involvement in the study; evidence of any other known causes of mental deficiency (e.g., congenital hypothyroidism, neurofibromatosis, or chromosomal abnormalities); or the prescription of medications that were suggestive of or might increase risk of atherosclerosis (e.g., β blockers, hormone supplements, protease inhibitors, and long-term systemic prednisone and cyclosporine) because of a remote associative risk of choline metabolite trimethylamine-N-oxide with atherosclerotic heart disease in cardiac patients (20–24). Medication changes during the treatment period did not preclude study participation but were monitored.
Procedures
Randomized intervention
Participants were randomly assigned to the choline or placebo intervention in a 1:1 allocation ratio that was based on a computer-generated list of random numbers. Random assignment was stratified by sex and age (5–6, 7–8, and 9–10 y) with the use of random block sizes of 2, 4, and 6 to reduce selection bias. Random assignment was handled by an investigator with no clinical involvement in the trial so that clinical research personnel (raters and outcome assessors) and participants were kept blinded to the allocation.
Children in the choline-treatment group received 625 mg choline/d for 6 wk, which was orally administered in the form of a glycerophosphocholine liquid concentrate (5.25 mL/d) (Nutrasal). Glycerophosphocholine is a natural choline compound that is prepared from the hydrolysis of phosphatidylcholine and serves as a precursor to free choline. As the physiologic form of choline in the body, glycerophosphocholine can rapidly cross the blood-brain barrier because of its water solubility. Glycerophosphocholine is an important component in human breast milk (25) and is present in many common food items such as dairy products, olive oil, oat bran, and liver (26). The prescribed dose of choline was 1.7–2.5 times the Adequate Intake (AI) recommended by the Food and Nutrition Board of the Institute of Medicine (27) depending on the child’s age (Table 1). The combined dietary intake (28, 29) and supplementation at 625 mg was unlikely to exceed that Upper Intake Level, which, for school-aged children, is 1000–2000 mg depending on the child’s age. Children in the placebo group received an equivalent dose (5.25 mL/d) of an oral placebo treatment (Nutrasal) that consisted of a liquid mixture of glycerin, water, and xylitol sweetener that was taken daily for 6 wk. The placebo treatment matched the glycerophosphocholine product in taste and consistency. Caregivers were given a 6-wk supply of the allocated treatment at the beginning of the study after the baseline assessment. The glycerophosphocholine and placebo were packaged in identical bottles and labeled with participants’ identification numbers to monitor consumption. Bottles contained no information about the contents other than a code that allowed clinical research personnel to dispense bottles blindly; the code was assigned by Nutrasal, and only the investigator who was involved in the random assignment was provided with the key. Research staff provided caregivers with both verbal and written instructions as well as a demonstration of how to administer the treatment.
TABLE 1.
Summary of the Institute of Medicine’s Dietary Reference Intakes for choline and prescribed treatment doses1
| Age range | AI,2 mg/d | Prescribed dose, mg/d | Upper Intake Level, mg/d |
| 4–8 y | 250 | 625 (2.5 times the AI) | 1000 |
| 9–13 y | 375 | 625 (1.7 times the AI) | 2000 |
Data are from the Institute of Medicine (27).
AI, Adequate Intake.
Cognitive and dietary assessment
Before and immediately after the intervention, participants were administered a standardized neuropsychological test battery that lasted 90 min and consisted of tasks that measured cognitive abilities in the domains of learning and memory, executive function, attention, and fine motor functioning. In addition, caregivers completed the Automated Self-Administered 24-h Dietary Recall (ASA24) (30) at baseline and postintervention to obtain estimates of children’s daily dietary choline intakes. Research personnel interviewed caregivers and entered dietary data into the ASA24 during both study visits. The ASA24 is a web-based 24-h dietary recall interview, which asks caregivers to report all foods and drinks that were consumed by their children on the previous day. The measure provides detailed information and analysis about individual-level nutrients, including choline. Concurrent use of multivitamin and mineral supplements was not an exclusionary criterion and was monitored at both assessment points. No participant was reported to have taken any choline-specific supplements. Caregivers were instructed not to make any changes to their children’s dietary supplement regimens during the course of the study, and all caregivers denied any changes in supplements at follow-up. Information about nutritional supplement use and data on the nutritional status of participants have been previously reported (28). Preliminary analyses were conducted to determine whether dietary choline intake across preassessments and postassessments could be combined. No significant differences were observed over time for either treatment groups (i.e., P-group × time interaction > 0.05). Therefore, dietary choline intake was averaged across the 2 assessment points to obtain an estimate of children’s typical daily intakes.
Compliance
Information about treatment adherence was collected through treatment diaries that were completed by caregivers and by measuring the remaining liquid volume in treatment bottles on study completion. Each day, caregivers reported whether the treatment was taken or missed. Full and empty treatment bottles were collected at study completion. Any remaining liquid was measured in mL, and calculations were performed to determine the amounts of treatment consumed and missed throughout the treatment period.
Tolerability and adverse events
Families were contacted weekly to assess any problems with the treatment administration and monitor any adverse events. Adverse events were assessed with the use of a standardized protocol. Caregivers were asked general, open-ended questions about whether they had noticed any adverse events since beginning treatment. Subsequently, they were prompted about the occurrence of specific events from a predefined list of 21 symptoms across 11 domains (e.g., upset stomach or fishy body odor). In addition, caregivers were asked if they had noticed any positive events since beginning treatment. All adverse and positive events were recorded.
Outcome measures
A description of primary cognitive outcome measures and variables included in the analyses is presented in Table 2. All scores used in the analyses were raw scores with the exception of the Grooved Pegboard, which provides age-corrected z scores.
TABLE 2.
Description of neuropsychological measures and variables included in analyses1
| Domain | Subdomain | Measure | Dependent variable | Description |
| Learning and memory | Visuospatial memory | CANTAB Paired Associates Learning | Total errors | Total number of errors are adjusted for stages not completed; lower scores are indicative of better memory |
| Executive function | Cognitive flexibility | NEPSY-2 Design Fluency | Number correct | Total number of correct designs across both structured and unstructured conditions; higher scores are indicative of better cognitive fluency and flexibility |
| Working memory | CANTAB Spatial Working Memory | Total errors | Total number of errors; lower scores are indicative of better working memory | |
| Planning | CANTAB Spatial Working Memory | Strategy score | Number of times a participant begins a new search with a different box; lower scores represent more efficient planning | |
| Attention | Sustained attention | Quotient ADHD System | Accuracy | Percentage of correct responses; higher scores represent better attention |
| Motor | Fine motor dexterity | Grooved Pegboard | Completion time | Time taken to complete task with dominant hand; lower scores are indicative of better motor ability |
ADHD, Attention-Deficit/Hyperactivity Disorder; CANTAB, Cambridge Neuropsychological Test Automated Battery.
Learning and memory
Visuospatial memory was assessed with the use of the Paired Associates Learning test from the Cambridge Neuropsychological Test Automated Battery (CANTAB) (31). The CANTAB is a computerized, touch-screen system that is frequently used in child psychopharmacologic studies because of its extensive validation, availability in parallel forms for repeat testing, and sensitivity to pharmacologic manipulation (32–34).
Executive function
Several domains of executive function were assessed including cognitive flexibility, initiation and generation, working memory, and planning. The NEPSY-2 Design Fluency test was used to assess cognitive flexibility and the ability to initiate and generate ideas (35). Spatial working memory (SWM) and planning were measured with the use of the SWM test from the CANTAB.
Attention
The Quotient Attention-Deficit/Hyperactivity Disorder (ADHD) System (36) was used to assess sustained attention. The task uses a go/no-go paradigm in which subjects were presented with one of 2 geometric shapes in spatially random positions and asked to respond when the target shape appears and to withhold a response when the non-target shape appears.
Motor
Psychomotor function was assessed with the use of the Grooved Pegboard, which is a task of manual dexterity, hand-eye coordination, and fine motor speed.
Statistical analyses
Statistical analyses were conducted with the use of SPSS Statistics version 20.0 software (37). An α level of P < 0.05 (2 tailed) was used to determine significance.
Demographic data were analyzed with the use of chi-square test for categorical variables (sex, race, ethnicity, handedness, FAS diagnosis, and home placement), and independent-samples t tests were used for continuous variables [age, body weight, and socioeconomic status (SES)]. Demographic variables were included as covariates if they were significantly correlated with the dependent variable and did not interact with either dependent or independent variables. Treatment-adherence data were analyzed with the use of Mann-Whitney U tests because of the nonnormal distribution of compliance variables. Group comparisons for the number of participants who reported adverse and positive events were analyzed with the use of Fisher’s exact tests.
Primary outcome: cognitive performance
To evaluate the efficacy of the choline intervention, cognitive data were analyzed as a 2 × 2 mixed-model design with treatment group (choline and placebo) as a between-subjects factor and time (pretest and posttest) as a within-subjects factor. Primary outcome measures included performance on cognitive tasks of memory, executive function, and attention (outcome variables are shown in Table 2). The primary analysis was an intention-to-treat (ITT) analysis that used linear mixed-effects models and included all participants who received the allocated treatment (n = 55; choline: n = 29; placebo n = 26). ITT is a strategy for the analysis of randomized controlled trials that compares participants in the groups in which they were originally randomly assigned, which minimizes selection bias and preserves the random assignment. All participants were included in analyses according to their treatment group as designated by the randomization procedure regardless of adherence to the entry criteria or deviation from the protocol (38). All possible main and interaction effects were explored.
Predictors of treatment outcomes
Secondary analyses were conducted to examine moderators of treatment outcomes. Moderator variables of interest included intervention adherence (e.g., the percentage of days the treatment was taken as reported in the treatment diaries and the percentage of liquid consumed as measured from treatment bottles), and dietary choline intake (i.e., mean dietary choline intake from the ASA24 across pretest and posttest). Bivariate Pearson correlations were first performed to assess the association between predictor variables and cognitive outcomes at posttest. Correlations that were significant at P < 0.05 were considered for inclusion in subsequent hierarchical multiple linear regression analyses to assess the unique influence of each predictor on cognitive performance at posttest after accounting for age, group, and cognitive performance at pretest. Age (if significantly related to the dependent variable), treatment group, and cognitive performance at pretest were entered as covariates at step 1 (model 1), the predictor variable was entered at step 2 (model 2), and the interaction term between the predictor variable and group was entered at step 3 (model 3).
RESULTS
Demographic data and sample characteristics
Table 3 presents descriptive data as well as statistical analyses for the demographic characteristics of the ITT sample at baseline. As expected, treatment groups did not differ on any demographic characteristic. At baseline, groups did not significantly differ for any outcome variable (P > 0.05).
TABLE 3.
Baseline demographic characteristics of the ITT sample1
| Variable | Choline (n = 29) | Placebo (n = 26) | P2 |
| Sex, n (%) | 0.50 | ||
| M | 13 (44.8) | 14 (53.8) | |
| F | 16 (55.2) | 12 (46.2) | |
| Age, y | 8.3 ± 1.603 | 8.2 ± 1.94 | 0.87 |
| Age strata, n (%) | 0.69 | ||
| 5–6 y | 6 (20.7) | 8 (30.8) | |
| 7–8 y | 11 (37.9) | 9 (34.6) | |
| 9–10 y | 12 (41.4) | 9 (34.6) | |
| Body weight, kg | 59.8 ± 15.91 | 63.9 ± 25.43 | 0.47 |
| Percentile | 44.7 ± 32.94 | 49.8 ± 35.00 | 0.58 |
| Race, n (%) | 0.78 | ||
| Caucasian | 20 (69.0) | 17 (65.4) | |
| African American | 5 (17.2) | 5 (19.2) | |
| Multiracial | 4 (13.8) | 4 (15.4) | |
| Ethnicity, n (%) | 0.29 | ||
| Hispanic | 2 (6.9) | 3 (11.5) | |
| Not Hispanic | 25 (86.2) | 18 (69.2) | |
| Not reported | 2 (6.9) | 5 (19.2) | |
| SES | 48.5 ± 12.99 | 46.4 ± 13.65 | 0.55 |
| Handedness, n (%) | 0.17 | ||
| Right | 24 (82.8) | 24 (92.3) | |
| Left | 5 (17.2) | 1 (3.8) | |
| Mixed | 0 (0.0) | 1 (3.8) | |
| FAS diagnosis,4 n (%) | 0.96 | ||
| FAS | 3 (10.3) | 3 (11.5) | |
| Prenatally exposed, non-FAS | 16 (55.2) | 15 (57.7) | |
| Not diagnosed | 10 (34.5) | 8 (30.8) | |
| Home placement, n (%) | 0.17 | ||
| Biological | 2 (6.9) | 5 (19.2) | |
| Adopted | 27 (93.1) | 21 (80.8) | |
| Psychiatric comorbidity, n (%) | 0.41 | ||
| None | 12 (41.4) | 8 (30.8) | |
| ≥1 diagnosis | 17 (58.6) | 18 (69.2) | |
| Medications, n (%) | |||
| Noncognitive | 5 (17.2) | 6 (23.1) | 0.63 |
| Cognitive | 19 (65.5) | 17 (65.4) | 0.85 |
| Site, n (%) | 0.58 | ||
| San Diego | 11 (37.9) | 8 (30.8) | |
| Ohio | 18 (62.1) | 18 (69.2) |
FAS, fetal alcohol syndrome; ITT, intention-to-treat; SES, socioeconomic status.
Comparisons between treatment groups were conducted with the use of independent samples t tests for continuous variables and a chi-square test for categorical variables.
Mean ± SD (all such values).
FAS children met 3 diagnostic criteria for FAS on the basis of a dysmorphologic examination. Prenatally exposed non-FAS children did not meet the criteria for full FAS on the basis of a dysmorphologic examination but received a diagnosis on the spectrum. Not diagnosed children were not evaluated for a diagnosis.
In addition, because this was a multisite study, site differences of demographic variables were assessed. Sites differed on age [t(53) = −2.33, P = 0.02], body weight percentile [t(53)= −2.92, P = 0.01], and SES [t(53) = 2.33, P = 0.02]. Compared with the San Diego site, participants at the Ohio site were older (Ohio mean age: 8.7 y; San Diego mean age: 7.6 y), were at a higher body weight percentile (Ohio mean: 56.1th percentile, San Diego mean: 30.0th percentile), and had lower SES (Ohio mean: 44.6, San Diego mean: 53.0). No differences were observed between study locations for sex, race, ethnicity, handedness, FAS diagnosis, home placement, or psychiatric comorbidity (P > 0.07).
Compliance
Treatment diaries were returned by 98% of participants (n = 51) who completed the study. According to returned treatment diaries, the mean compliance rate (i.e., percentage of days the treatment was taken) was 96.4% for the sample. A total of 24 children (47%) missed at least one dose throughout the treatment period. The median number of days on which the treatment was missed was 0.00 d, and the mean ± SD was 1.64 ± 2.59 d. Treatment bottles were returned by 98% of participants (n = 51) who completed the study. According to returned treatment bottles, the mean compliance rate (i.e., percentage of liquid consumed) was 95.7% for the sample. The median amount of liquid missed was 5.38 mL (equivalent to 1.02 d of treatment), and the mean ± SD was 9.31 ± 35.89 mL (equivalent to 1.77 d of treatment). Treatment compliance, on the basis of treatment diaries and liquid measurements, did not differ by group (P > 0.37).
Adverse events
Data on reported adverse and positive events are presented in Table 4. Thirty-six children (65%) reported ≥1 adverse event throughout the treatment period. Groups did not differ for the number of participants with reported adverse events in any category (P > 0.15) although the number of children who reported ≥1 adverse event in any category was significantly higher in the choline group than in the placebo group (P = 0.03). There were no reported serious adverse events in either group.
TABLE 4.
Participants who reported adverse events during the course of the study1
| Adverse event | Choline (n = 29) | Placebo (n = 26) | P2 |
| General health | 4 (13.8) | 2 (7.7) | 0.67 |
| Skin | 11 (37.9) | 5 (19.2) | 0.15 |
| Ear, nose, throat | 1 (3.4) | 0 (0.0) | 1.00 |
| Cardiovascular | 1 (3.4) | 0 (0.0) | 1.00 |
| Respiratory | 0 (0.0) | 1 (3.8) | 0.47 |
| Gastrointestinal | 12 (41.4) | 7 (26.9) | 0.40 |
| Genitourinary | 0 (0.0) | 1 (3.8) | 0.47 |
| Musculoskeletal | 1 (3.4) | 1 (3.8) | 1.00 |
| Neurologic | 3 (10.3) | 0 (0.0) | 0.24 |
| Behavioral | 7 (24.1) | 4 (15.4) | 0.51 |
| Allergy | 1 (3.4) | 1 (3.8) | 1.00 |
| ≥1 adverse event in any category | 23 (79.3) | 13 (50.0) | 0.03 |
| Positive events | 9 (31.0) | 6 (23.1) | 0.56 |
Data are presented as n (%).
Comparisons between treatment groups were conducted with the use of Fisher’s exact test.
Primary outcome: cognitive performance
The effectiveness of the intervention was assessed by comparing the pretest and posttest cognitive performance between groups. Separate 2 (group) × 2 (time) repeated-measures mixed-effects models were performed for each cognitive variable. Mean scores for each group for each cognitive measure are presented in Table 5.
TABLE 5.
Mean cognitive scores by group at pretest (baseline) and posttest
| Choline |
Placebo |
Analyses, P |
|||||||
| n | Mean ± SD | Cohen’s d | n | Mean ± SD | Cohen’s d | Main effect of group | Main effect of time | Interaction | |
| Paired Associates Learning | −0.17 | −0.23 | 0.78 | 0.02 | 0.73 | ||||
| Pretest | 29 | 37.7 ± 49.95 | 26 | 36.6 ± 53.14 | |||||
| Posttest | 28 | 29.3 ± 48.64 | 23 | 26.0 ± 38.59 | |||||
| Design Fluency | 0.28 | 0.21 | 0.39 | 0.01 | 0.31 | ||||
| Pretest | 29 | 16.1 ± 7.99 | 24 | 15.9 ± 7.71 | |||||
| Posttest | 28 | 18.5 ± 9.36 | 22 | 17.5 ± 7.68 | |||||
| Spatial Working Memory total errors | −0.14 | −0.37 | 0.99 | 0.01 | 0.31 | ||||
| Pretest | 29 | 59.6 ± 12.99 | 26 | 62.0 ± 18.81 | |||||
| Posttest | 28 | 57.5 ± 15.87 | 23 | 54.7 ± 20.84 | |||||
| Spatial Working Memory strategy | 0.36 | −0.08 | 0.20 | 0.59 | 0.32 | ||||
| Pretest | 29 | 37.4 ± 2.62 | 26 | 37.2 ± 3.56 | |||||
| Posttest | 28 | 38.4 ± 2.86 | 23 | 36.9 ± 4.13 | |||||
| Quotient ADHD1 Accuracy | 0.07 | 0.05 | 0.24 | 0.97 | 0.72 | ||||
| Pretest | 27 | 64.8 ± 12.60 | 22 | 70.3 ± 12.69 | |||||
| Posttest | 23 | 65.7 ± 11.71 | 20 | 70.9 ± 12.93 | |||||
| Grooved Pegboard | −0.21 | −0.41 | 0.75 | 0.01 | 0.92 | ||||
| Pretest | 29 | 0.90 ± 1.52 | 25 | 1.1 ± 2.14 | |||||
| Posttest | 27 | 0.57 ± 1.66 | 22 | 0.37 ± 1.30 | |||||
ADHD, Attention-Deficit/Hyperactivity Disorder.
Memory
The demographic variable of age was significantly related to memory performance scores at both pretest and posttest and was included in the analysis as a covariate. The analysis of Paired Associates Learning revealed a main effect of time (F[1,52.2] = 5.85, P = 0.02); both groups significantly improved at posttest compared with at pretest. No main effect of group (F[1,52.6] = 0.076, P = 0.78) or group × time interaction (F[1,52.2] = 0.123, P = 0.73) was observed. Age was a significant covariate (F[1,52.0] = 6.35, P = 0.02).
Executive function
Age was significantly related to executive function performance on both the Design Fluency and SWM tasks and was included in these analyses as a covariate. For all analyses in which age was included, age was a significant covariate (P < 0.001).
For Design Fluency, analyses revealed a significant main effect of time (F[1,48.8] = 6.93, P = 0.01); fluency performance across both groups significantly improved at posttest compared with at pretest. There was no main effect of group (F[1,50.1] = 0.760, P = 0.39) or group × time interaction (F[1,48.8] = 1.05, P = 0.31). The analysis of SWM total errors revealed a significant main effect of time (F[1,48.3] = 4.27, P = 0.04); across both groups, SWM errors significantly declined at posttest compared with at pretest. There was no main effect of group (F[1,50.0] = 0.000, P = 0.99) or group × time interaction (F[1,48.4] = 1.02, P = 0.31). For SWM strategy, analyses did not reveal a significant main effect of group (F[1,51.4] = 1.68, P = 0.20), time (F[1,51.0] = 0.303, P = 0.59), or group × time interaction (F[1,51.0] = 1.03, P = 0.32).
Attention
Age was significantly related to Quotient ADHD Accuracy and was a significant covariate for accuracy (F[1,45.1] = 11.2, P = 0.002). No significant main or interaction effects were observed (group: F[1,45.7] = 1.40, P = 0.24; time: F[1,41.9] = 0.002, P = 0.97; group × time: F[1,41.9] = 0.127, P = 0.72).
Motor
No demographic variables were significantly associated with motor performance; therefore, the analysis was continued without covariates. The analysis of the Grooved Pegboard revealed a significant main effect of time (F[1,45.2] = 7.01, P = 0.01). Motor performance across both groups improved at posttest. No main effect of group (F[1,49.6] = 0.106, P = 0.75) or group × time interaction (F[1,45.2] = 0.010, P = 0.92) was observed.
Post hoc completers analyses
Analyses were repeated with the use of general linear models that included only participants who completed the study (n = 52; choline: n = 28; placebo: n = 24), and results did not change.
Contribution of age
Age was significantly related with most dependent variables and was included as a covariate when appropriate. However, because of the possibility that children in various age groups might have differentially responded to treatment, primary data analyses were repeated with age group (e.g., 5–6, 7–8, and 9–10 y) as a between-subjects factor to investigate if a therapeutic window, during which choline supplementation may have been most effective, was evident; such findings might have been overshadowed by adjusting results for age alone. No significant interaction of group × time × age group was observed for any cognitive outcome variable (P < 0.05), which suggested that the treatment effect did not vary by age.
Predictors of treatment outcome
Treatment compliance, according to returned treatment bottles, showed a significant, positive correlation with SWM strategy at posttest (r = 0.34, P = 0.01). Treatment compliance, according to returned treatment diaries, and mean choline dietary intake, as measured with the use of the ASA24, were not significantly correlated with any cognitive variable.
A hierarchical multiple linear regression analysis was performed with SWM strategy at posttest as the dependent variable. Model 1 was not statistically significant (R2 = 0.05, P = 0.31), but the model fit significantly improved with the addition of the moderator variable (ΔR2 = 0.10, ΔP = 0.02; model 2). Treatment compliance emerged as a significant predictor of SWM strategy (β = 0.32, P = 0.02) after controlling for the performance at pretest and treatment group. However, the direction of this relation was contrary to expectations because increased treatment compliance was associated with less-efficient planning. The model fit did not significantly improve with the addition of the interaction term (ΔR2 = 0.01; ΔP = 0.49; model 3).
DISCUSSION
The current study was a randomized, double-blinded, placebo-controlled trial with which we sought to examine the effectiveness of a 6-wk choline intervention for children with heavy prenatal alcohol exposure. The results did not support the efficacy of choline as a cognitive intervention for 5- to 10-y-old children with FASDs. Both ITT and completer analyses failed to show choline-related improvements in learning and memory, executive function, or sustained attention. Both treatment compliance and dietary choline were not predictive of improved treatment outcomes. An examination of the groups separately revealed that small effect sizes and sample sizes would have to have been inordinately large to confidently draw conclusions from the results; in addition, the possibility of a type II error could not be ruled out (achieved power ranged from 0.13 to 0.84). The null findings of this clinical trial were unanticipated because of the strong evidence that has suggested beneficial effects of choline supplementation in animal models of both typical development (39) and prenatal alcohol exposure (3–8, 10). However, this investigation was only one of a few trials to translate the aforementioned animal research to a human clinical trial of choline supplementation in FASDs, and previous studies, at earlier ages, have suggested some positive beneficial effects (13, 14). Because of the early stage of this field of research, factors may have contributed to the divergent findings that were observed in this study compared with in other clinical studies and with the preclinical data.
One explanation may be that the age range that was targeted in this study was outside choline’s therapeutic window. Preclinical studies have shown that choline supplementation is effective when administered from postnatal days 11–20 and 21–30 in the developing rat (7); these developmental phases are in parallel with early childhood and late childhood, respectively—the latter of which corresponds to the developmental stage of children in the current study. During this time, choline improved spatial learning and object recognition, which are abilities that are mediated by the hippocampus. Choline continued to ameliorate alcohol’s detrimental effects from postnatal days 40–60 (i.e., equivalent to adolescence) (40); however, effects were restricted to working memory and did not affect problems with overactivity or spatial learning, which suggested that choline targets different brain systems as development progresses and the brain matures (i.e., the prefrontal cortex is one of the last cortical regions to mature, whereas hippocampal development occurs within the first 5 y of life). Thus, although choline’s critical period is quite large, and effects can be seen in later developmental periods, supplementation earlier in development has greater benefits (5, 7). Wozniak et al. (14) observed improved memory in children aged 2.5 to ≤4 y but not in children aged >4 to 5 y, which was consistent with both the timing and pattern that have been shown in preclinical studies. When we examined age strata as a factor, the data did not indicate a differential treatment response within any particular age group of children. Together, the clinical data suggest that choline’s therapeutic potential may not extend beyond 4 y of age, and earlier intervention is likely needed for maximal success. An important caveat is that the sample sizes within each age strata were very limited; thus, it is possible that a therapeutic window exists within the age range examined, but small sample sizes prevented its detection.
Furthermore, the treatment duration examined in this study may have been too short. The treatment duration of previous clinical studies was ∼9 mo (13, 14). In preclinical studies, the period of choline administration ranged from 10 to 20 d, which is equivalent to years in human development. The results from these studies suggest that choline’s effects are due to structural changes in brain development (3) (e.g., increased hippocampal neurogenesis (41–43). However, choline is hypothesized to have multiple mechanisms of action including acute changes in cholinergic activity, changes in cellular membranes and signaling pathways, and epigenetic modification of gene expression (44, 45). The rationale for a short-term intervention was based on the extant literature that has supported the acute effects of choline on cognitive function through the enhancement of brain acetylcholine levels even after treatment periods as short as 90 min (46–49). However, note that these were studies of normal populations. Acute cholinergic supplementation may not be sufficient to overcome developmental brain insult that results from prenatal alcohol exposure. Moreover, the doses administered in the aforementioned studies were quite large [e.g., 10 g, which is ∼18–25 times the AI for adults (49)], which raises another possibility that the dose of choline supplemented in the current study was too low, at least for an acute effect.
Another important consideration in understanding the effect of choline supplementation is the form of choline administered. Preclinical studies have primarily used choline salts (e.g., choline chloride or bitartrate) as did Wozniak et al. (14). We chose glycerophosphocholine for several reasons as follows: 1) glycerophosphocholine is not converted to trimethylamine in the gut, which can impart a fishy body odor and, thereby, may reduce compliance; 2) the relative bioavailability of glycerophosphocholine is higher than that of phosphatidylcholine and choline salts and can more rapidly cross the blood-brain barrier (50, 51); and 3) glycerophosphocholine has been shown to better augment the levels and release of brain acetylcholine, at least in dementia populations (52–54). The administration of choline in different forms has likely contributed to the variability of results across clinical and preclinical studies due to differences in biological effects. Although previous literature has supported the use of glycerophosphocholine, a variety of other factors may interact with the choline form.
A major limitation of the current study is that serum choline concentrations were not measured because of resource limitations. Thus, it was not possible to confirm differences in choline concentration between treatment groups, to compare findings with previous studies, or to determine participant choline intake relative to the AI. However, data were collected on the dietary nutritional status of participants, and children across both groups were observed to have consumed significantly lower amounts of choline and other important nutrients compared with typically developing children in the United States and established AI levels [previously reported (28)]. In addition, to increase the ecologic validity, children with comorbid behavioral and psychiatric disorders (e.g., ADHD) were not excluded. Psychiatric comorbidity is a risk factor that is associated with a poorer response to intervention (55) and can be associated with cognitive impairment independent of prenatal alcohol exposure (56–58). Although concomitant psychopathology in the sample may have diluted the findings, psychiatric comorbidity was equivalent across groups, which minimized its impact. Furthermore, caregivers were asked to refrain from administering any cognitive-enhancing medications on testing days; however, this may not have been sufficient given the cumulative effects of these medications and depending on their half-life or washout time. Finally, single nucleotide polymorphisms can influence metabolic efficiency and, consequently, an individual’s dependency on dietary choline (59–61), which may underlie interindividual variability in responsiveness to supplementation. Despite these limitations, the current study had many notable strengths and adds to the available literature in several respects. A primary strength of this study was its methodologic rigor as a randomized, double-blinded clinical trial. In addition, participants were recruited from 2 centers across the United States and encompassed a wide range of SESs representative of the general FASD population. Other strengths were the low rates of participant dropout and adverse events and the high rate of treatment adherence. Ninety-five percent of participants completed treatment with a compliance rate of 96%. These rates are consistent with those of previous studies (14, 16) and further support that choline supplementation is feasible and tolerable in children with FASDs.
In conclusion, to our knowledge, this investigation is the first study to examine choline supplementation in children of school age around the time that the effects of alcohol are most commonly noticed. Although findings do not support our hypotheses, the study provides important information about the upper limits of choline’s therapeutic window in children with FASDs. Together with a few other investigations of choline and nutritional interventions in this population, this study emphasizes a further need for the continued study of the role of nutritional status and supplementation in children with FASDs and the contributions of nutrition to neurocognition. In addition, future studies should incorporate a genetic analysis of single nucleotide polymorphisms that are related to choline metabolism, which may identify subpopulations of individuals who may be more-readily responsive to treatment or require higher amounts of supplementation. An understanding of these differences may help us to target and customize interventions at the level of the individual rather than solely at the group level.
Acknowledgments
We thank Edward P Riley and the Center for Behavioral Teratology for providing resources and support. Also, we thank Suzette Fisher and Mary Sartor for assisting with recruitment at Double ARC, Kenneth Lyons Jones for providing dysmorphologic evaluations, and Benjamin N Deweese and Jason Dudley for database management. Finally, we thank Loki Natarajan, Mari S Golub, and Judith K Eckerle for their participation on the Data Safety Monitoring Board, as well as Howard Taras for consultation on matters of participant safety.
The authors’ responsibilities were as follows—TTN: analyzed the data and wrote the manuscript; TTN and RDR: conducted the research; TTN, CDC, and JDT: designed the research; TTN and JDT: had primary responsibility for the final content of the manuscript; SNM: provided advice for the test battery development and interpretation of neuropsychological test results; SNM and CDC: provided essential materials, resources, and recruitment support; and all authors: read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: ADHD, attention-deficit/hyperactivity disorder; AI, Adequate Intake; ASA24, Automated Self-Administered 24-h Dietary Recall; CANTAB, Cambridge Neuropsychological Test Automated Battery; FAS, fetal alcohol syndrome; FASD, fetal alcohol spectrum disorders; ITT, intention-to-treat; SES, socioeconomic status; SWM, Spatial Working Memory.
REFERENCES
- 1.Tsai J, Floyd RL, Green PP, Denny CH, Coles CD, Sokol RJ. Concurrent alcohol use or heavier use of alcohol and cigarette smoking among women of childbearing age with accessible health care. Prev Sci 2010;11:197–206. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC). Alcohol use and binge drinking among women of childbearing age - United States, 2006-2010. MMWR Morb Mortal Wkly Rep 2012;61:534–8. [PubMed] [Google Scholar]
- 3.Thomas JD, La Fiette MH, Quinn VR, Riley EP. Neonatal choline supplementation ameliorates the effects of prenatal alcohol exposure on a discrimination learning task in rats. Neurotoxicol Teratol 2000;22:703–11. [DOI] [PubMed] [Google Scholar]
- 4.Thomas JD, Abou EJ, Dominguez HD. Prenatal choline supplementation mitigates the adverse effects of prenatal alcohol exposure on development in rats. Neurotoxicol Teratol 2009;31:303–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas JD, Idrus NM, Monk BR, Dominguez HD. Prenatal choline supplementation mitigates behavioral alterations associated with prenatal alcohol exposure in rats. Birth Defects Res A Clin Mol Teratol 2010;88:827–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas JD, Biane JS, O’Bryan KA, O’Neill TM, Dominguez HD. Choline supplementation following third-trimester-equivalent alcohol exposure attenuates behavioral alterations in rats. Behav Neurosci 2007;121:120–30. [DOI] [PubMed] [Google Scholar]
- 7.Ryan SH, Williams JK, Thomas JD. Choline supplementation attenuates learning deficits associated with neonatal alcohol exposure in the rat: effects of varying the timing of choline administration. Brain Res 2008;1237:91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas JD, Garrison M, O’Neill TM. Perinatal choline supplementation attenuates behavioral alterations associated with neonatal alcohol exposure in rats. Neurotoxicol Teratol 2004;26:35–45. [DOI] [PubMed] [Google Scholar]
- 9.Wagner AF, Hunt PS. Impaired trace fear conditioning following neonatal ethanol: reversal by choline. Behav Neurosci 2006;120:482–7. [DOI] [PubMed] [Google Scholar]
- 10.Thomas JD, Tran T. Choline supplementation mitigates trace, but not delay, eyeblink conditioning deficits in rats exposed to alcohol during development. Hippocampus 2012;22:619–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keen CL, Uriu-Adams JY, Skalny A, Grabeklis A, Grabeklis S, Green K, Yevtushok L, Wertelecki WW, Chambers CD. The plausibility of maternal nutritional status being a contributing factor to the risk for fetal alcohol spectrum disorders: the potential influence of zinc status as an example. Biofactors 2010;36:125–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coles CD, Kable JA, Keen CL, Jones KL, Wertelecki W, Granovska IV, Pashtepa AO, Chambers CD, CIFASD. Dose and timing of prenatal alcohol exposure and maternal nutritional supplements: developmental effects on 6-month-old infants. Matern Child Health J 2015;19:2605–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kable JA, Coles CD, Keen CL, Uriu-Adams JY, Jones KL, Yevtushok L, Kulikovsky Y, Wertelecki W, Pedersen TL, Chambers CD, et al. The impact of micronutrient supplementation in alcohol-exposed pregnancies on information processing skills in Ukrainian infants. Alcohol 2015;49:647–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wozniak JR, Fuglestad AJ, Eckerle JK, Fink BA, Hoecker HL, Boys CJ, Radke JP, Kroupina MG, Miller NC, Brearley AM, et al. Choline supplementation in children with fetal alcohol spectrum disorders: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr 2015;102:1113–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olson HC, Jirikowic T, Kartin D, Astley S. Responding to the challenge of early intervention for fetal alcohol spectrum disorders. Infants Young Child 2007;20:172–89. [Google Scholar]
- 16.Wozniak JR, Fuglestad AJ, Eckerle JK, Kroupina MG, Miller NC, Boys CJ, Brearley AM, Fink BA, Hoecker HL, Zeisel SH, et al. Choline supplementation in children with fetal alcohol spectrum disorders has high feasibility and tolerability. Nutr Res 2013;33:897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, Elbourne D, Egger M, Altman DG. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Int J Surg 2012;10:28–55. [DOI] [PubMed] [Google Scholar]
- 18.Jones KL, Robinson LK, Bakhireva LN, Marintcheva G, Storojev V, Strahova A, Sergeevskaya S, Budantseva S, Mattson SN, Riley EP, et al. Accuracy of the diagnosis of physical features of fetal alcohol syndrome by pediatricians after specialized training. Pediatrics 2006;118:e1734–8. [DOI] [PubMed] [Google Scholar]
- 19.Astley SJ, Clarren SK. Diagnosing the full spectrum of fetal alcohol-exposed individuals: introducing the 4-digit diagnostic code. Alcohol Alcohol 2000;35:400–10. [DOI] [PubMed] [Google Scholar]
- 20.Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med 2013;368:1575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011;472:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin JC, Canlet C, Delplanque B, Agnani G, Lairon D, Gottardi G, Bencharif K, Gripois D, Thaminy A, Paris A. 1H NMR metabonomics can differentiate the early atherogenic effect of dairy products in hyperlipidemic hamsters. Atherosclerosis 2009;206:127–33. [DOI] [PubMed] [Google Scholar]
- 23.Rossi M, Zanardi M. An open study on the clinical efficacy of citicoline in patients with chronic cerebral vasculopathy. Clin Ter 1993;142:141–4. [PubMed] [Google Scholar]
- 24.Loscalzo J. Gut microbiota, the genome, and diet in atherogenesis. N Engl J Med 2013;368:1647–9. [DOI] [PubMed] [Google Scholar]
- 25.Holmes-McNary MQ, Cheng WL, Mar MH, Fussell S, Zeisel SH. Choline and choline esters in human and rat milk and in infant formulas. Am J Clin Nutr 1996;64:572–6. [DOI] [PubMed] [Google Scholar]
- 26.Zeisel SH, Mar MH, Howe JC, Holden JM. Concentrations of choline-containing compounds and betaine in common foods. J Nutr 2003;133:1302–7. [DOI] [PubMed] [Google Scholar]
- 27.Institute of Medicine. Dietary reference intakes: the essential guide to nutrient requirements. Washington (DC): The National Academy Press; 2006. [Google Scholar]
- 28.Nguyen TT, Risbud RD, Chambers CD, Thomas JD. Dietary nutrient intake in school-aged children with heavy prenatal alcohol exposure. Alcohol Clin Exp Res 2016;40:1075–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fuglestad AJ, Fink BA, Eckerle JK, Boys CJ, Hoecker HL, Kroupina MG, Zeisel SH, Georgieff MK, Wozniak JR. Inadequate intake of nutrients essential for neurodevelopment in children with fetal alcohol spectrum disorders (FASD). Neurotoxicol Teratol 2013;39:128–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Subar AF, Kirkpatrick SI, Mittl B, Zimmerman TP, Thompson FE, Bingley C, Willis G, Islam NG, Baranowski T, McNutt S, et al. The Automated Self-Administered 24-Hour Dietary Recall (ASA24): a resource for researchers, clinicians, and educators from the National Cancer Institute. J Acad Nutr Diet 2012;112:1134–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cambridge Cognition. CANTABeclipse version 3: test administration guide. Cambridge (United Kingdom): Cambridge Cognition; 2006. [Google Scholar]
- 32.Curtis WJ, Lindeke LL, Georgieff MK, Nelson CA. Neurobehavioural functioning in neonatal intensive care unit graduates in late childhood and early adolescence. Brain 2002;125:1646–59. [DOI] [PubMed] [Google Scholar]
- 33.Luciana M, Nelson CA. The functional emergence of prefrontally-guided working memory systems in four- to eight-year-old children. Neuropsychologia 1998;36:273–93. [DOI] [PubMed] [Google Scholar]
- 34.Rhodes SM, Coghill DR, Matthews K. Acute neuropsychological effects of methylphenidate in stimulant drug-naive boys with ADHD II–broader executive and non-executive domains. J Child Psychol Psychiatry 2006;47:1184–94. [DOI] [PubMed] [Google Scholar]
- 35.Korkman M, Kirk U, Kemp S. NEPSY-II administration manual. San Antonio (TX): PsychCorp; 2007. [Google Scholar]
- 36.Teicher MH, Ito Y, Glod CA, Barber NI. Objective measurement of hyperactivity and attentional problems in ADHD. J Am Acad Child Adolesc Psychiatry 1996;35:334–42. [DOI] [PubMed] [Google Scholar]
- 37.IBM Corporation. IBM SPSS Statistics 20.0; Armonk (NY): IBM Corporation; 2011. [Google Scholar]
- 38.Hollis S, Campbell F. What is meant by intention to treat analysis? Survey of published randomised controlled trials. BMJ 1999;319:670–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCann JC, Hudes M, Ames BN. An overview of evidence for a causal relationship between dietary availability of choline during development and cognitive function in offspring. Neurosci Biobehav Rev 2006;30:696–712. [DOI] [PubMed] [Google Scholar]
- 40.Schneider RD, Thomas JD. Adolescent choline supplementation attenuates working memory deficits in rats exposed to alcohol during the third trimester equivalent. Alcohol Clin Exp Res 2016;40:897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Glenn MJ, Gibson EM, Kirby ED, Mellott TJ, Blusztajn JK, Williams CL. Prenatal choline availability modulates hippocampal neurogenesis and neurogenic responses to enriching experiences in adult female rats. Eur J Neurosci 2007;25:2473–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yen CL, Mar MH, Zeisel SH. Choline deficiency-induced apoptosis in PC12 cells is associated with diminished membrane phosphatidylcholine and sphingomyelin, accumulation of ceramide and diacylglycerol, and activation of a caspase. FASEB J 1999;13:135–42. [PubMed] [Google Scholar]
- 43.Albright CD, Friedrich CB, Brown EC, Mar MH, Zeisel SH. Maternal dietary choline availability alters mitosis, apoptosis and the localization of TOAD-64 protein in the developing fetal rat septum. Brain Res Dev Brain Res 1999;115:123–9. [DOI] [PubMed] [Google Scholar]
- 44.Zeisel SH. Choline: critical role during fetal development and dietary requirements in adults. Annu Rev Nutr 2006;26:229–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zeisel SH, Blusztajn JK. Choline and human nutrition. Annu Rev Nutr 1994;14:269–96. [DOI] [PubMed] [Google Scholar]
- 46.Spiers PA, Myers D, Hochanadel GS, Lieberman HR, Wurtman RJ. Citicoline improves verbal memory in aging. Arch Neurol 1996;53:441–8. [DOI] [PubMed] [Google Scholar]
- 47.Alvarez XA, Laredo M, Corzo D, Fernandez-Novoa L, Mouzo R, Perea JE, Daniele D, Cacabelos R. Citicoline improves memory performance in elderly subjects. Methods Find Exp Clin Pharmacol 1997;19:201–10. [PubMed] [Google Scholar]
- 48.Ladd SL, Sommer SA, LaBerge S, Toscano W. Effect of phosphatidylcholine on explicit memory. Clin Neuropharmacol 1993;16:540–9. [PubMed] [Google Scholar]
- 49.Sitaram N, Weingartner H, Caine ED, Gillin JC. Choline: selective enhancement of serial learning and encoding of low imagery words in man. Life Sci 1978;22:1555–60. [DOI] [PubMed] [Google Scholar]
- 50.Wurtman RJ, Hirsch MJ, Growdon JH. Lecithin consumption raises serum-free-choline levels. Lancet 1977;2:68–9. [DOI] [PubMed] [Google Scholar]
- 51.Cheng WL, Holmes-McNary MQ, Mar MH, Lien EL, Zeisel SH. Bioavailability of choline and choline esters from milk in rat pups. J Nutr Biochem 1996;7:457–64. [Google Scholar]
- 52.Amenta F, Parnetti L, Gallai V, Wallin A. Treatment of cognitive dysfunction associated with Alzheimer’s disease with cholinergic precursors. Ineffective treatments or inappropriate approaches? Mech Ageing Dev 2001;122:2025–40. [DOI] [PubMed] [Google Scholar]
- 53.Sigala S, Imperato A, Rizzonelli P, Casolini P, Missale C, Spano P. L-alpha-glycerylphosphorylcholine antagonizes scopolamine-induced amnesia and enhances hippocampal cholinergic transmission in the rat. Eur J Pharmacol 1992;211:351–8. [DOI] [PubMed] [Google Scholar]
- 54.Parnetti L, Amenta F, Gallai V. Choline alphoscerate in cognitive decline and in acute cerebrovascular disease: an analysis of published clinical data. Mech Ageing Dev 2001;122:2041–55. [DOI] [PubMed] [Google Scholar]
- 55.Shea MT, Widiger TA, Klein MH. Comorbidity of personality disorders and depression: implications for treatment. J Consult Clin Psychol 1992;60:857–68. [DOI] [PubMed] [Google Scholar]
- 56.Castellanos FX, Sonuga-Barke EJ, Milham MP, Tannock R. Characterizing cognition in ADHD: beyond executive dysfunction. Trends Cogn Sci 2006;10:117–23. [DOI] [PubMed] [Google Scholar]
- 57.Clark C, Prior M, Kinsella G. The relationship between executive function abilities, adaptive behaviour, and academic achievement in children with externalising behaviour problems. J Child Psychol Psychiatry 2002;43:785–96. [DOI] [PubMed] [Google Scholar]
- 58.Clark C, Prior M, Kinsella GJ. Do executive function deficits differentiate between adolescents with ADHD and oppositional defiant/conduct disorder? A neuropsychological study using the Six Elements Test and Hayling Sentence Completion Test. J Abnorm Child Psychol 2000;28:403–14. [DOI] [PubMed] [Google Scholar]
- 59.Zeisel SH. Gene response elements, genetic polymorphisms and epigenetics influence the human dietary requirement for choline. IUBMB Life 2007;59:380–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zeisel SH. What choline metabolism can tell us about the underlying mechanisms of fetal alcohol spectrum disorders. Mol Neurobiol 2011;44:185–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zeisel SH. Nutritional genomics: defining the dietary requirement and effects of choline. J Nutr 2011;141:531–4. [DOI] [PMC free article] [PubMed] [Google Scholar]