Abstract
Background: Previous work demonstrated that a soy-dairy protein blend (PB) prolongs hyperaminoacidemia and muscle protein synthesis in young adults after resistance exercise.
Objective: We investigated the effect of PB in older adults. We hypothesized that PB would prolong hyperaminoacidemia, enhancing mechanistic target of rapamycin complex 1 (mTORC1) signaling and muscle protein anabolism compared with a whey protein isolate (WPI).
Methods: This double-blind, randomized controlled trial studied men 55–75 y of age. Subjects consumed 30 g protein from WPI or PB (25% soy, 25% whey, and 50% casein) 1 h after leg extension exercise (8 sets of 10 repetitions at 70% one-repetition maximum). Blood and muscle amino acid concentrations and basal and postexercise muscle protein turnover were measured by using stable isotopic methods. Muscle mTORC1 signaling was assessed by immunoblotting.
Results: Both groups increased amino acid concentrations (P < 0.05) and mTORC1 signaling after protein ingestion (P < 0.05). Postexercise fractional synthesis rate (FSR; P ≥ 0.05), fractional breakdown rate (FBR; P ≥ 0.05), and net balance (P = 0.08) did not differ between groups. WPI increased FSR by 67% (mean ± SEM: rest: 0.05% ± 0.01%; postexercise: 0.09% ± 0.01%; P < 0.05), decreased FBR by 46% (rest: 0.17% ± 0.01%; postexercise: 0.09% ± 0.03%; P < 0.05), and made net balance less negative (P < 0.05). PB ingestion did not increase FSR (rest: 0.07% ± 0.03%; postexercise: 0.09% ± 0.01%; P ≥ 0.05), tended to decrease FBR by 42% (rest: 0.25% ± 0.08%; postexercise: 0.15% ± 0.08%; P = 0.08), and made net balance less negative (P < 0.05). Within-group percentage of change differences were not different between groups for FSR, FBR, or net balance (P ≥ 0.05).
Conclusions: WPI and PB ingestion after exercise in older men induced similar responses in hyperaminoacidemia, mTORC1 signaling, muscle protein synthesis, and breakdown. These data add new evidence for the use of whey or soy-dairy PBs as targeted nutritional interventions to counteract sarcopenia. This trial was registered at clinicaltrials.gov as NCT01847261.
Keywords: sarcopenia, aging, muscle protein turnover, protein supplementation, leucine
Introduction
As we age, reduced strength and muscle mass, also known as sarcopenia, are predictors of early mortality (1). With the aging of the baby boomer generation, the prevalence of sarcopenia will increase. A sarcopenic population will add to the ever-increasing health care costs as these older individuals will require enhanced care from weakness, bedrest, and loss of independence. Additionally, reduced muscle mass and weakness increase the risk of falls in older adults (1). A fall can lead to hospitalization and placement in a care facility (2, 3).
Amino acid (AA)11/protein supplementation and resistance exercise (RE) are well-studied interventions for maximizing muscle protein synthesis in adults of all ages (4–10). Unfortunately, these stimuli do not enhance muscle protein synthesis in the elderly as robustly as in younger individuals. This phenomenon has been classified as anabolic resistance (11–15). Although the mechanism for anabolic resistance is unknown, evidence suggests that a dose threshold must be reached for protein supplementation to maximally stimulate muscle protein synthesis in older individuals (16, 17). Current research suggests that an intake <20 g protein is insufficient for maximal stimulation of protein synthesis (18, 19). Therefore, any supplement provided to older individuals should contain 20 g protein at a minimum (20). Muscle protein breakdown is less well studied, especially in older individuals. The fractional breakdown rate increases after RE alone or exercise combined with feeding (21–23). Therefore, protein synthesis must be maximized to counteract these increases in protein breakdown to shift protein metabolism into an anabolic state and reach a positive net balance.
Adequate protein intake is only one aspect of using protein supplementation as a tool to overcome anabolic resistance and improve muscle protein turnover. The protein source is also important because different protein sources contain different compositions of amino acids (24, 25). In addition, certain proteins vary in their digestion rates (26). Because activation of skeletal muscle protein synthesis is contingent on the AAs being taken up by the muscle from the blood, the overall length of prolonged blood hyperaminoacidemia is crucial (27).
The most well-studied protein supplement, whey protein, contains high levels of branched-chain AAs compared with other protein sources. It is especially high in leucine, an AA demonstrated to be responsible for activating protein synthesis (28). Whey protein is a rapidly digested protein and thus results in a rapid spike in blood AA concentrations (29). The other milk protein, casein, has a slower digestion profile than whey. As a result, casein prolongs hyperaminoacidemia longer than whey protein. Although the increase in blood AAs after casein ingestion does not reach the magnitude seen with whey ingestion, protein synthesis is still activated postexercise (30, 31). Milk is not the only protein source for postexercise supplementation. The plant protein soy is also capable of stimulating muscle protein synthesis (32). In addition, soy contains many antioxidants and is a good alternative source of protein for those on a vegetarian diet (33). Because whey, casein, and soy all have different AA profiles and digestion rates, a blended protein supplement may potentially provide the benefit of all 3 proteins (34).
A study using this soy-dairy protein blend (PB) supplement in young adults demonstrated that the PB prolonged AA net balance across the leg for ≤2 h postingestion compared with only 20 min for the whey-only group (35). In addition, the PB increased muscle protein synthesis for 4 h post ingestion compared with 2 h for the whey-only group (34). This prolonged stimulus may hold the key for improving skeletal muscle protein anabolism and attenuating sarcopenia in older adults.
The purpose of this study was to determine whether soy-dairy PB ingestion after RE in older adults promotes muscle protein anabolism. We hypothesized that soy-dairy PB ingestion in older adults after a bout of RE would prolong hyperaminoacidemia and enhance muscle mechanistic target of rapamycin complex 1 (mTORC1) signaling and protein synthesis compared with a single protein isolate. To test our hypothesis we conducted a double-blind, randomized controlled clinical trial in men aged 55–75 y and compared the effects of whey protein isolate (WPI) to a soy-dairy PB ingested 1 h after a bout of high-intensity RE.
Methods
Screening of participants.
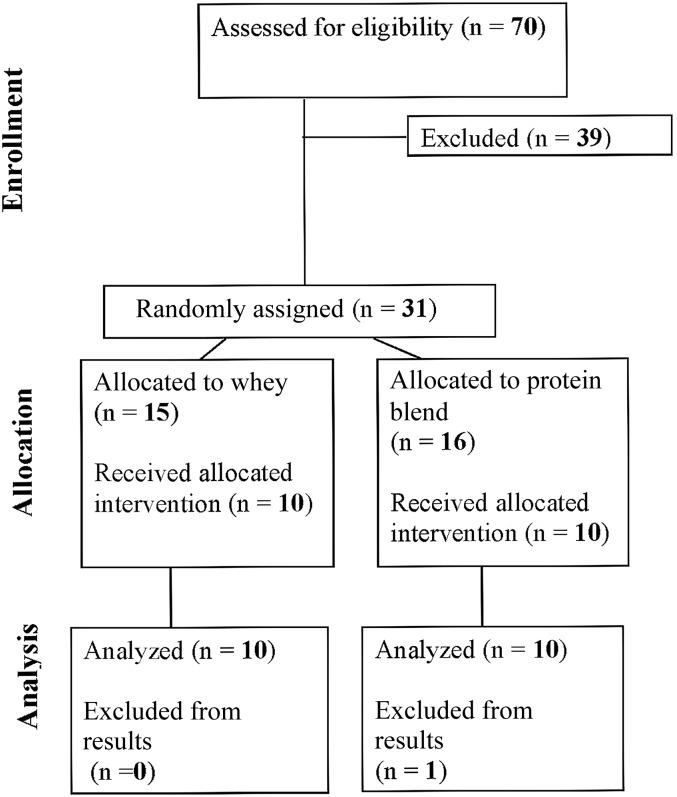
We recruited 20 healthy men aged 55–75 y for this double-blind, randomized clinical trial (Figure 1). Participant characteristics are shown in Table 1. The participants were recruited through flyers, newspaper advertisements, and word of mouth. Participants were required to be healthy, only recreationally active (no high-intensity RE regimen), nonsmoking, and not currently using any protein supplements. Participants were screened on 2 separate days at the Institute for Translational Sciences-Clinical Research Center at the University of Texas Medical Branch. Screening one included laboratory tests (complete blood count with differential, liver and kidney function tests, coagulation profile, fasting blood glucose, hepatitis B and C screening, HIV test, thyroid stimulating hormone, lipid profile, urinalysis, and drug screening), clinical history with physical examination and one-repetition maximum (1RM) testing. The second screening included a DXA scan (Hologic QDR 4500W) to measure lean and fat mass, an additional 1RM test, and a cardiac stress test. The 1RM testing was performed on a leg extension machine (Cybex-VR2). The repetition maximum was considered the average of the heaviest weight lifted from each of the 2 sessions. All participants provided written, informed consent before enrollment in the study. The study was approved by the Institutional Review Board of the University of Texas Medical Branch and is in compliance with the Declaration of Helsinki as revised in 1983.
FIGURE 1.
Consort flow diagram for study recruitment.
TABLE 1.
Participant characteristics1
| n | Age, y | BMI, kg/m2 | Fat, % | Lean mass, kg | 1RM | |
| WPI | 10 | 69.3 ± 2.1 | 26.5 ± 0.5 | 31.5 ± 2.2 | 53.9 ± 1.2 | 175 ± 8.5 |
| PB | 9 | 62.2 ± 1.5* | 25.1 ± 1.1 | 27.3 ± 1.5 | 53.1 ± 3.1 | 179 ± 15.1 |
Values are means ± SEMs. *Different from WPI, P = 0.04. 1RM, one-repetition maximum; PB, soy-dairy protein blend; WPI, whey protein isolate.
Study design.
The infusion protocol for this study was identical for both groups (Supplemental Figure 1). Enrolled participants checked into the Institute for Translational Sciences-Clinical Research Center at ∼0600 on the day of the study. Participants were instructed to refrain from exercise for ≥48 h before admission. Participants fasted for ∼10 h before beginning the infusion but were provided water ad libitum. The participants were randomly assigned to ingest 30 g protein from a soy-dairy PB (n = 9) or WPI beverage (n = 10) at 1 h post-RE. Random assignment was achieved by using the nQuery software (version 3.0); a random block size scheme was used to ensure nonpredictability and interim balance. The rest period between biopsies 1 and 2 before RE was designated as Rest. The 0- to 2-h time period after ingestion of the supplement was designated as Early. The 2- to 4-h time period after supplement ingestion was designated as Late, and the entire 4-h period postingestion was classified as Entire.
Experimental protocol.
The experimental protocol matched the protocol as previously described (34). Briefly, on the morning of the experiment we began the primed, constant infusion (∼10 h) of l-[ring-13C6] phenylalanine and l-[15N] phenylalanine (Sigma-Aldrich). The priming dose for the labeled phenylalanine was 2 μmol/kg, and the infusion rate was 0.05 μmol⋅kg−1 ⋅ min−1. Muscle biopsies were performed on the lateral aspect of the vastus lateralis to determine the resting mixed muscle fractional synthesis rate (FSR) at 2 h and 4 h after infusion initiation. After the second biopsy, the participants were moved to a leg extension machine (Cybex-VR2) for high-intensity RE consisting of 8 sets of 10 repetitions. Sets 4 through 8 were performed at ∼70% 1RM. Three additional muscle biopsies were taken 1, 3, and 5 h after the completion of exercise (0, 2, and 4 h after supplement ingestion). The nutritional supplements were ingested immediately after the 1-h postexercise biopsy. Muscle tissue was immediately blotted, frozen in liquid nitrogen, and stored at −80°C until analysis. Blood samples were collected during the resting (0, 120, 180, 185, 195, 205, 215, 225, 240 min) and postingestion (−60, 0, 20, 40, 60, 80, 100, 120, 140, 160, 180, 185, 195, 205, 215, 225, 240 min) periods (Figure 2) to determine the blood l-[ring-13C6] phenylalanine enrichment (see below) and select AA concentrations. The infusion study concluded with the fifth muscle biopsy, at which time the participants were fed a standard meal.
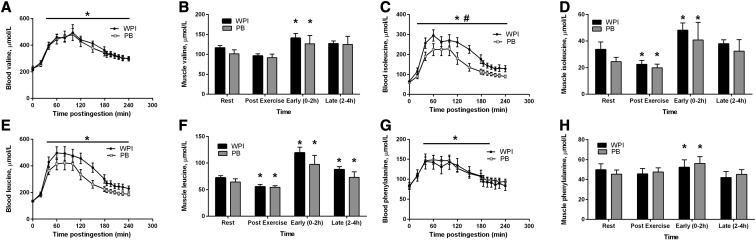
FIGURE 2.
Changes from Rest in blood valine (A), muscle valine (B), blood isoleucine (C), muscle isoleucine (D), blood leucine (E), muscle leucine (F), blood phenylalanine (G), and muscle phenylalanine (H) in older men during the postexercise recovery period after ingestion of whey or protein blend 1 h after a bout of resistance exercise. Data are means ± SEMs, n = 10 (WPI) or 9 (PB). *Different from Rest within treatment group for muscle, P < 0.05. Line with asterisk denotes difference from Rest for all time points with no difference between treatment groups for blood, P < 0.05. Line with hash sign denotes difference between treatment groups for blood, P < 0.05. PB, soy-dairy protein blend; WPI, whey protein isolate.
Protein beverage intervention.
The protein beverages (PB or WPI) were consumed 1 h after exercise. The beverages were dissolved in 300 mL water and enriched (8%) with l-[ring-13C6] and l-[15N] phenylalanine to maintain isotopic steady state in arterialized blood. The PB consisted of 30.5 g total protein (providing 2.7–8 g leucine) composed of 50% protein from sodium caseinate, 25% protein from WPI, and 25% protein from soy protein isolate. The WPI consisted of 30.4 g protein (providing 3.26 g of leucine). Thirty grams of protein was chosen for both supplements because it has been shown to be sufficient to stimulate skeletal muscle protein synthesis in older subjects (17).
Blood and muscle amino acid concentrations.
Concentrations of phenylalanine and the branch-chained AAs (leucine, isoleucine, and valine) were measured in deproteinized whole blood by using gas chromatography–mass spectrometry (GC-MS) as previously described using an internal standard solution (36, 37) for blood AA concentrations. The same free AA concentrations were measured in mixed muscle with the use of GC-MS as previously described by using an internal standard solution (37).
Measurement of lean mass.
Lean mass was estimated using a DXA scan (Hologic QDR 4500W). The CV for repeated measures of lean tissue is <1%.
Measurement of plasma insulin.
Plasma insulin concentrations were measured with Human Insulin ELISA (EMD Millipore) according to the manufacturer instructions. Measured time points were at baseline, immediately after exercise, and at various times after ingestion of protein.
Calculation of muscle protein synthesis.
Muscle proteins and intracellular free AAs were extracted from biopsy samples as previously described (38). Bound muscle and intracellular free concentrations were calculated with the internal standard method by using tracer enrichments for l-[ring-13C6] phenylalanine, l-[15N] phenylalanine and appropriate internal standards (D3 leucine, 13C isoleucine, 13C valine, 15N phenylalanine) via GC-MS (6890 Plus CG, 5973N MSD, 7683 autosampler; Agilent Technologies) (36). Mixed-muscle protein-bound phenylalanine enrichment was analyzed by GC-MS after protein hydrolysis and AA extraction (39) by using the external standard curve approach (40). The FSR of mixed-muscle proteins was calculated from the incorporation rate of l-[ring-13C6] phenylalanine into the mixed-muscle proteins and the free-tissue phenylalanine enrichment by using the formula:
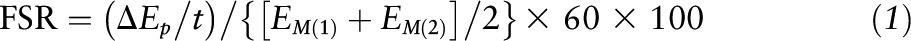 |
where ΔEp/t is the slope of the straight line that fits the protein-bound phenylalanine enrichment across 2 sequential biopsies, t is the time interval encompassing the 2 biopsies, EM(1) and EM(2) are the mean phenylalanine enrichments (tracer/tracee) in the free-muscle pool in 2 biopsies. The results are presented as %/h.
Calculation of muscle protein breakdown.
Muscle protein fractional breakdown rate (FBR) was measured with phenylalanine tracers by using the precursor-product method as previously described (21, 37). To measure FBR at baseline, the l-[ring-13C6] phenylalanine enrichment at 4 h was used as the plateau enrichment and l-[15N] phenylalanine enrichment at 4 h was used for the 1-h decay enrichment. FBR was calculated by using the formula:
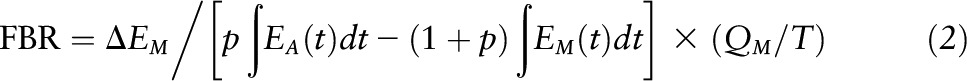 |
where EA(t) and EM(t) are the arterialized and muscle free enrichments at time t. P = EM/(EA − EM) at plateau, and EA and EM are enrichments in the arterial pool and muscle intracellular pool, respectively, and QM/T is the ratio of free to bound phenylalanine in muscle.
Calculation of net protein balance.
Net protein balance was calculated as FSR − FBR for each group during the Rest and Late periods. The Late FBR constituted 3–4 h after supplement ingestion. Late FSR constituted 2–4 h after protein ingestion.
Western blot analysis.
Muscle tissue samples were immediately quick-frozen in liquid nitrogen after the biopsy and kept in liquid nitrogen until analyzed. Phosphorylation of mTOR, 4-E binding protein-1 (4E-BP1), S6K1, and ribosomal protein S6 (rpS6) was measured by using Western blot techniques as previously described (34, 38). The following rabbit polyclonal primary antibodies (Cell Signaling) were used: mTOR (Ser2448), S6K1 (Thr389), 4E-BP1 (Thr37/46), and rpS6 (Ser240/244). Blots were incubated with secondary antibody (Amersham Bioscience) and washed, and then a chemiluminescent solution (ECL plus; Amersham BioSciences) was administered. Optical density measurements were then obtained with a digital imager (Bio-Rad) so that a densitometric analysis (Quantity One software, version 4.5.2; Bio-Rad) could be performed. All data are expressed relative to an internal control sample.
Statistical analysis.
All values are expressed as means ± SEMs. Data were transformed by using the Box-Cox set of transformations to stabilize the variance and make the data approximately normally distributed. To test differences between groups, the data were modeled by using an ANCOVA model with resting or baseline values and age as covariates. The testing of differences was conducted through a t test of the variable indicating the difference between groups. Comparisons with resting values were based on testing contrasts across time by using a mixed model with the subject as a random intercept term and age as a covariate. AUC was calculated by using the AUC function in the flux package in R; the function uses the trapezoidal rule to do the calculation. Significance was set at P < 0.05. All calculations were done in R (41).
Results
Subject characteristics.
Descriptive characteristics for all subjects are shown in Table 1. The participants had similar lean mass, percentage of body fat, and 1RM values. Men in the WPI group were older than those in the PB group (P < 0.05).
Insulin concentrations.
Plasma insulin was elevated for both treatment groups from Rest (P < 0.05) beginning at 20 min postingestion. WPI remained elevated until 150 min postingestion, whereas PB was elevated until 100 min postingestion (Supplemental Figure 2). There was no difference (P ≥ 0.05) between groups for AUC (Supplemental Figure 3).
Blood and muscle AA concentrations.
Blood concentrations for valine (Figure 2A) were elevated from Rest (P < 0.05) for both treatment groups for the entire treatment period postingestion with no difference between groups. Valine intracellular muscle concentrations were elevated in both groups for the Early period compared with Rest, (P < 0.05, Figure 2B). Isoleucine concentrations in the blood were elevated from Rest (P < 0.05) for both treatment groups for the entire treatment period postingestion with the concentration greater at all time points for WPI > PB (P < 0.05, Figure 2C). Both groups had significantly reduced muscle isoleucine concentrations at 1 h postexercise compared with baseline, but these concentrations were significantly increased 2 h postingestion (P < 0.05, Figure 2D). Blood concentrations for leucine were elevated from Rest (P < 0.05) for both treatment groups for the entire treatment period postingestion with no difference between groups (Figure 2E). There was no difference between groups for AUC for blood leucine concentrations (Supplemental Figure 4). Leucine intracellular muscle concentrations were reduced compared with baseline in both groups 1 h post-RE bout, but they were significantly elevated for both the Early and Late periods compared with Rest (P < 0.05, Figure 2F). Blood phenylalanine concentrations were elevated in both groups up to 205 min postingestion with no difference between groups (P < 0.05, Figure 2G). Phenylalanine intracellular muscle concentrations were elevated in both groups for the Early period compared with Rest (P < 0.05, Figure 2H).
Muscle mTORC1 signaling.
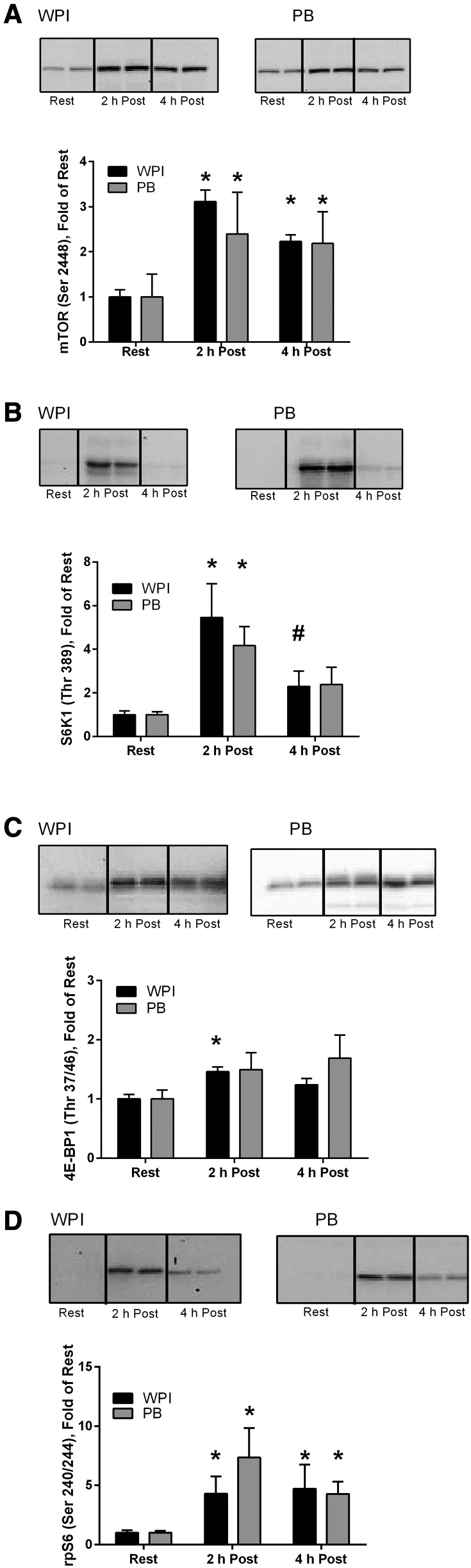
The phosphorylation of mTORC1 (Ser2448) showed no treatment difference between groups at 2 h (P ≥ 0.05) or 4 h postingestion (P ≥ 0.05). Phosphorylation was significantly increased at both 2 h and 4 h postingestion (P < 0.01) for WPI as well as PB at 2 h (P < 0.05) and 4 h (P < 0.05) compared with baseline (Figure 3A). There was no treatment difference between groups at 2 h (P ≥ 0.05) or 4 h (P ≥ 0.05) postingestion for S6K1 (Thr389) phosphorylation. Phosphorylation was elevated at 2 h postingestion in both groups (P < 0.05). At 4 h postingestion, S6K1 phosphorylation in the WPI group was significantly reduced (P < 0.05) from the 2-h time point but did not differ from baseline (Figure 3B). There was no treatment difference between groups at 2 h (P ≥ 0.05) or 4 h (P ≥ 0.05) postingestion for 4E-BP1 (Thr37/42) phosphorylation. 4E-BP1 phosphorylation showed a significant increase only at 2 h (P < 0.05) in the WPI group. (Figure 3C). Lastly, there was no treatment difference between groups at 2 h (P ≥ 0.05) or 4 h (P ≥ 0.05) postingestion for rpS6 (Ser240/244) phosphorylation. Phosphorylation was significantly increased at both 2 h (P < 0.05) and 4 h (P < 0.05) postingestion in WPI and at 2 h (P < 0.05) and 4 h (P < 0.05) for PB compared with baseline. (Figure 3D).
FIGURE 3.
Western blot analyses of mTORC1 (A) and mTORC1 downstream effector proteins: S6K1 (B), 4E-BP1 (C), and rpS6 (D), in older men during the treatment period after a bout of resistance exercise. Data are means ± SEMs, n = 10 (WPI) or 9 (PB). *Different from Rest, P < 0.05. #Different from 2 h Post, P < 0.05. There was no difference between groups at any time point. Two representative bands are displayed for each time point because all samples were run in duplicate. mTORC1, mechanistic target of rapamycin complex 1; PB, soy-dairy protein blend; Post, postingestion; rpS6, ribosomal protein S6; WPI, whey protein isolate; 4E-BP1, 4-E binding protein-1.
FSR.
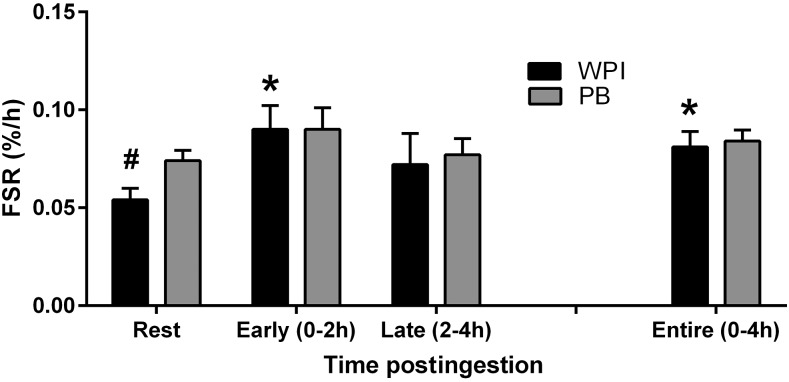
No treatment difference existed postingestion for the Early period (P ≥ 0.05), Late period (P ≥ 0.05), or Entire period (P ≥ 0.05). There was a difference between groups at Rest (P < 0.05) for muscle protein synthesis. The PB group had a higher baseline synthesis rate than the WPI group. Early FSR was elevated from Rest by 67% in the WPI group (P < 0.05) as well as by 50% during the Entire period (P < 0.05) (Figure 4). FSR did not increase in the PB group between Rest and the Early period (P ≥ 0.05). Similarly, FSR did not increase between Rest and the Entire period (P ≥ 0.05). There was no difference (P ≥ 0.05) between groups for percentage of change from Rest to Early, Late, or Entire.
FIGURE 4.
FSR in the vastus lateralis of older men at rest and during the treatment period after an acute bout of resistance exercise and ingestion of WPI or PB 1 h after exercise. Early is 0–2 h postingestion, Late is 2–4 h postingestion, and Entire is 0–4 h postingestion. Data are means ± SEMs, n = 10 (WPI) or 9 (PB). *Different from Rest (P < 0.05). #Different from PB at Rest, P < 0.05. FSR, fractional synthesis rate; PB, soy-dairy protein blend; WPI, whey protein isolate.
FBR.
There was no difference between groups in muscle protein breakdown at preingestion (P ≥ 0.05) or postingestion (P ≥ 0.05). The WPI group showed a significant 46% reduction in FBR during the final hour of the treatment period compared with the baseline breakdown measure (P < 0.05), whereas there was only a tendency for FBR to decrease by 42% in the PB group (P = 0.08). There was no difference between groups for percentage of change from before protein ingestion to after protein ingestion (P ≥ 0.05) (Supplemental Figure 5).
Net protein balance.
The WPI and the PB group both showed a significant change in net protein balance during the final hour of the Treatment period compared with the Rest period (P < 0.05) resulting in a less-negative protein balance after protein ingestion. There was no difference between groups at either time point, Rest (P ≥ 0.05) or Late (P = 0.08). There was no difference between groups for percent change from Rest to Late (P ≥ 0.05) (Supplemental Figure 6).
Blood l-[15N] phenylalanine tracer enrichments.
There was no difference (P ≥ 0.05) between groups at any time point (Supplemental Figure 7).
Protein-bound l-[ring-13C6] phenylalanine tracer enrichments.
There was no difference (P ≥ 0.05) between groups at any time point (Supplemental Figure 8).
Discussion
The stimulation of muscle protein synthesis that RE and/or protein supplementation must provide to overcome anabolic resistance in older populations has become a major focus in the field of sarcopenia. Feeding alone can be effective in increasing muscle protein synthesis in older populations with the proper dosage. Cuthbertson et al. (11) showed a significant increase in FSR ≤3.5 h postingestion using 10–20 g essential AAs (EAAs). Similarly, Katsanos et al. (17) saw a marked increase in FSR in older subjects after ingestion of 6.7 g EAA beverage containing 2.8 g leucine. This was the equivalent leucine concentration of a 25- to 30-g protein supplement. Moore et al. (16) also demonstrated a need for greater protein intake for stimulating myofibrillar protein synthesis in older compared with younger subjects in a retrospective study, with older subjects requiring a protein intake of 0.40 g · kg body mass−1 · meal−1 compared with the 0.24 g/kg body mass the young required.
Unlike feeding, RE alone typically does not promote a robust increase in skeletal muscle protein synthesis in older adults. Using RE, Fry et al. (14) were unable to induce an increase in FSR in older adults that was shown in younger adults after 8 sets of 10 leg extensions at 70% 1RM. Kumar et al. (15) saw a similar disparity between young and old subjects after RE alone and concomitant increases in FSR. Conversely, Kumar et al. (42) did find an increase in myofibrillar protein synthesis for older adults with RE alone when exercise volume was doubled while intensity was held constant.
It would appear that the key factor for promoting skeletal muscle protein synthesis is the leucine concentration in the supplement (9, 17, 28). In older adults, Katsanos et al. (17) showed that 2.8 g leucine was necessary to increase FSR, whereas a 1.7-g leucine dose was insufficient, even when the total EAAs in the beverage remained constant. Bukhari et al. (43) demonstrated significant elevations in muscle protein synthesis in older women using a 3-g EAA beverage containing 1.2 g leucine in combination with RE. The study by Katsanos et al. (17), along with that of Moore et al. (16) cited above, offers further evidence that body mass may play an integral role in protein and leucine dosage in older individuals. The mean lean body mass for subjects in the study by Bukhari et al. (43) was only 40.5 kg compared with ∼53.5 kg for the study by Katsanos et al. (17). This weight discrepancy may explain the increases in muscle protein synthesis after ingestion of 1.2 g leucine in one study not seen with 1.7 g in the other study.
The 2 groups in this study, WPI and PB, were not matched for leucine content. The WPI group received 3.26 g leucine whereas the PB received 2.78 g. Although the 2 groups did not ingest equal amounts of leucine, according to the work of Katsanos et al. (17), both groups received enough leucine to exceed the minimum threshold to shift protein turnover into an anabolic state. Similarly Mitchell et al. (44) demonstrated an increase in myofibrillar FSR for middle-aged subjects consuming either whey protein alone or a whole-milk (20% whey, 80% casein) supplement with the whole-milk group receiving a smaller leucine dose than the whey protein group.
For the first primary outcome, there was no difference between groups for either blood leucine concentrations or AUC measurements after supplement ingestion. Therefore, leucine availability was comparable for both the WPI group and the PB group. Interestingly, previous work has shown a pronounced and prolonged hyperaminoacidemia in older adults after ingestion of protein or AAs (45), which may have contributed to the similar hyperaminoacidemia between groups found in our study.
The sustained availability of AAs in the blood past 2 h for both groups may explain the increased anabolic signaling through the mTORC1 signaling pathway at 2–4 h postingestion of the supplement. This hyperaminoacidemia is vital for stimulating anabolic signaling in older individuals. Fry et al. (14) showed no increase in mTORC1 signaling or muscle protein synthesis after RE alone in older adults. We found for our second primary outcome that mTORC1 and its downstream targets rpS6 and S6K1 were similarly phosphorylated after supplementation for both groups. Thus both groups activated skeletal muscle mTORC1 signaling in response to exercise and protein ingestion.
Although both hyperaminoacidemia and mTORC1 signaling were similarly elevated between groups, only the WPI group showed a statistically significant increase in FSR, our third primary outcome, during the Early period (P < 0.05). This may have been because of the potentially superior ability of fast-acting whey protein compared with a PB to stimulate muscle protein synthesis in older adults. Yet there was no difference in FSR between groups. The PB group had a higher baseline FSR value and therefore did not show a statistically significant increase during the Early period. A large variance within the baseline measures for PB prevented a significant increase in FSR during the Early period. Still, considering the similarities between groups in AA concentrations, cell signaling, and overall FSR during the Early, Late, and Entire periods, it is possible to speculate that the PB group would have matched the WPI group during the Early period if not for a few unexplained high values during the baseline period.
Protein turnover is not only governed by changes in muscle protein synthesis. Muscle protein breakdown may serve an important role in controlling muscle accretion or atrophy (46). Some studies have shown an increase in FBR after exercise but then an attenuation after feeding in younger adults (5, 6). Research on FBR in older adults is not as comprehensive. One study showed no change in FBR in older adults after RE alone between baseline and 24 h after exercise (21). This study measured protein breakdown both during the rest period and the treatment period. Protein supplementation resulted in an attenuation of the increase in FBR, a secondary outcome measure, which is commonly observed after RE. WPI yielded a significant decrease in FBR with only a trend for a decrease in the PB group (P = 0.08). This attenuation resulted in a shift toward a less negative net protein balance (Supplemental Figure 6).
There are a few limitations to our study. First, we included only men. Although sex differences are not apparent in younger populations for mTORC1 signaling and muscle protein synthesis after RE, we cannot make any inferences on the potential sex differences in older populations when combining RE with a PB (47). Second, our randomization procedure resulted in subjects in the PB group being slightly younger. This may have contributed to the tendency for baseline FSR values to be higher in this group. However, as mentioned previously, the primary factor for not detecting a significant increase in FSR in this group was most likely due to a few subjects with high basal FSR values. In fact, in the PB group 7 of the 9 subjects had an increase in FSR after exercise and protein ingestion.
The precursor-product method used for measuring skeletal muscle protein breakdown in this study has four assumptions that must be met for the FBR equation to be valid: 1) isotopic steady state must be maintained, 2) inward transport and protein breakdown are constant over time, 3) the only source of tracer is from arterialized blood, and 4) the intramuscular free AA pool size is constant. First, as shown in Supplemental Figure 7, the l-[15N] phenylalanine tracer enrichment was in a steady state condition at both time points before termination of the tracer. After tracer cessation, both groups experienced a similar loss of tracer enrichment, and there was no difference between groups at any time point for l-[15N] phenylalanine tracer enrichment. Second, in our study we were unable to measure inward transport because we did not use femoral catheters. However, in our previous study in which we compared these supplements in young men (35), inward transport rates were not significantly different from rest during the 3–4 h after ingestion. Although this may be different in older adults, our earlier data would suggest that inward transport rates returned to resting levels during the time period when we measured FBR for the current study. Third, the contribution of tracer from a source other than arterialized blood is minimal. The protein-bound enrichment was negligible relative to the blood enrichment because it reached a maximum of ∼0.05%, i.e., 3 orders of magnitude smaller than the blood phenylalanine enrichment (typically 8–10%) (Supplemental Figure 8). Fourth, the data in Figure 2 show that intramuscular phenylalanine concentrations had returned to postprandial concentrations by the muscle biopsy at 4 h after ingestion. This suggests that during our postprandial FBR measurement (i.e., 3–4 h after protein ingestion), the intramuscular pool size remained constant. In summary, all assumptions of the FBR model have been met for measuring protein breakdown rates during the postprandial period, and all relevant data for validation of these model assumptions have been included in Figure 2 and Supplemental Figures 5–8.
For our net protein balance results, we used the 2- to 4-h time point (Late period) for FSR in the net balance equation because the FBR measurement occurred during the 3- to 4-h time point after ingestion. Although these time points do not match exactly, they do overlap and thus provide an opportunity to determine net protein balance over the final hour of the study period. We believe that net protein balance remained negative in this study after both RE and protein supplementation because of the timing of the measurement (i.e., FSR peaked during the Early period and returned to baseline values during the Late period). Thus, net protein balance during the first hour after protein ingestion was most likely positive.
In summary, WPI ingestion after exercise increased FSR and reduced FBR, resulting in a less-negative net protein balance. PB ingestion after exercise did not significantly increase FSR and tended to decrease FBR; however, this combination caused net balance to become less negative. This resulted in no differences between groups postexercise for FSR, FBR, or net balance. We conclude that protein supplementation, whether a single protein isolate or a PB, enhances mTORC1 signaling and muscle protein anabolism in older men after RE.
Acknowledgments
We thank Junfang Hao, Susan Wilson, Ming Zheng, Chelsea Therrien, and Allyson Schattel for technical assistance and Sarah Toombs Smith for writing assistance. MSB, PTR, MBC, RM, EV, and BBR designed the research; MSB, PTR, SHH, MMM, RRD, ABR, and BSL conducted the research; MSB, PTR, MMM, RRD, BSL, MBC, RM, EV, and BBR reviewed the manuscript; MSB, PTR, ABR, KJ, EV, and BBR analyzed the data; MSB and BBR wrote the manuscript and had primary responsibility for the final content. MBC and RM were not involved with the conducted research or data analysis. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: AA, amino acid; EAA, essential amino acid; FBR, fractional breakdown rate; FSR, fractional synthesis rate; GC-MS, gas chromatography–mass spectrometry; mTORC1, mechanistic target of rapamycin complex 1; PB, soy-dairy protein blend; RE, resistance exercise; rpS6, ribosomal protein S6; WPI, whey protein isolate; 1RM, one-repetition maximum; 4E-BP1, 4-E binding protein-1.
References
- 1.Wandrag L, Brett SJ, Frost G, Hickson M. Impact of supplementation with amino acids or their metabolites on muscle wasting in patients with critical illness or other muscle wasting illness: a systematic review. J Hum Nutr Diet 2015;28:313–30. [DOI] [PubMed] [Google Scholar]
- 2.Hida T, Harada A, Imagama S, Ishiguro N. Managing sarcopenia and its related-fractures to improve quality of life in geriatric populations. Aging Dis 2013;5:226–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morley JE, Anker SD, von Haehling S. Prevalence, incidence, and clinical impact of sarcopenia: facts, numbers, and epidemiology-update 2014. J Cachexia Sarcopenia Muscle 2014;5:253–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drummond MJ, Dreyer HC, Pennings B, Fry CS, Dhanani S, Dillon EL, Sheffield-Moore M, Volpi E, Rasmussen BB. Skeletal muscle protein anabolic response to resistance exercise and essential amino acids is delayed with aging. J Appl Physiol 2008;104:1452–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biolo G, Tipton KD, Klein S, Wolfe RR. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am J Physiol 1997;273:E122–9. [DOI] [PubMed] [Google Scholar]
- 6.Tipton KD, Ferrando AA, Phillips SM, Doyle D, Wolfe RR. Postexercise net protein synthesis in human muscle from orally administered amino acids. Am J Physiol 1999;276:E628–34. [DOI] [PubMed] [Google Scholar]
- 7.Rasmussen BB, Tipton KD, Miller SL, Wolf SE, Wolfe RR. An oral essential amino acid-carbohydrate supplement enhances muscle protein anabolism after resistance exercise. J Appl Physiol 2000;88:386–92. [DOI] [PubMed] [Google Scholar]
- 8.Børsheim E, Tipton KD, Wolfe SE, Wolfe RR. Essential amino acids and muscle protein recovery from resistance exercise. Am J Physiol Endocrinol Metab 2002;283:E648–57. [DOI] [PubMed] [Google Scholar]
- 9.Dreyer HC, Drummond MJ, Pennings B, Fujita S, Glynn EL, Chinkes DL, Dhanani S, Volpi E, Rasmussen BB. Leucine-enriched essential amino acid and carbohydrate ingestion following resistance exercise enhances mTOR signaling and protein synthesis in human muscle. Am J Physiol Endocrinol Metab 2008;294:E392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Symons TB, Sheffield-Moore M, Mamerow MM, Wolfe RR, Paddon-Jones D. The anabolic response to resistance exercise and a protein-rich meal is not diminished by age. J Nutr Health Aging 2011;15:376–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J 2005;19:422–4. [DOI] [PubMed] [Google Scholar]
- 12.Farnfield MM, Breen L, Carey KA, Garnham A, Cameron-Smith D. Activation of mTOR signalling in young and old human skeletal muscle in response to combined resistance exercise and whey protein ingestion. Appl Physiol Nutr Metab 2012;37:21–30. [DOI] [PubMed] [Google Scholar]
- 13.Burd NA, Gorissen SH, van Loon LJ. Anabolic resistance of muscle protein synthesis with aging. Exerc Sport Sci Rev 2013;41:169–73. [DOI] [PubMed] [Google Scholar]
- 14.Fry CS, Drummond MJ, Glynn EL, Dickinson JM, Gundermann DM, Timmerman KL, Walker DK, Dhanani S, Volpi E, Rasmussen BB. Aging impairs contraction-induced human skeletal muscle mTORC1 signaling and protein synthesis. Skelet Muscle 2011;1:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar V, Selby A, Rankin D, Patel R, Atherton P, Hildebrandt W, Williams J, Smith K, Seynnes O, Hiscock N, et al. Age-related differences in the dose-response relationship of muscle protein synthesis to resistance exercise in young and old men. J Physiol 2009;587:211–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moore DR, Churchward-Venne TA, Witard O, Breen L, Burd NA, Tipton KD, Phillips SM. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J Gerontol A Biol Sci Med Sci 2015;70:57–62. [DOI] [PubMed] [Google Scholar]
- 17.Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab 2006;291:E381–7. [DOI] [PubMed] [Google Scholar]
- 18.Yang Y, Breen L, Burd NA, Hector AJ, Churchward-Venne TA, Josse AR, Tarnopolsky MA, Phillips SM. Resistance exercise enhances myofibrillar protein synthesis with graded intakes of whey protein in older men. Br J Nutr 2012;108:1780–8. [DOI] [PubMed] [Google Scholar]
- 19.Pennings B, Groen B, de Lange A, Gijsen AP, Zorenc AH, Senden JM, van Loon LJ. Amino acid absorption and subsequent muscle protein accretion following graded intakes of whey protein in elderly men. Am J Physiol Endocrinol Metab 2012;302:E992–9. [DOI] [PubMed] [Google Scholar]
- 20.Churchward-Venne TA, Burd NA, Phillips SM. Nutritional regulation of muscle protein synthesis with resistance exercise: strategies to enhance anabolism. Nutr Metab (Lond) 2012;9:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fry CS, Drummond MJ, Glynn EL, Dickinson JM, Gundermann DM, Timmerman KL, Walker DK, Volpi E, Rasmussen BB. Skeletal muscle autophagy and protein breakdown following resistance exercise are similar in younger and older adults. J Gerontol A Biol Sci Med Sci 2013;68:599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gundermann DM, Walker DK, Reidy PT, Borack MS, Dickinson JM, Volpi E, Rasmussen BB. Activation of mTORC1 signaling and protein synthesis in human muscle following blood flow restriction exercise is inhibited by rapamycin. Am J Physiol Endocrinol Metab 2014;306:E1198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phillips SM, Parise G, Roy BD, Tipton KD, Wolfe RR, Tamopolsky MA. Resistance-training-induced adaptations in skeletal muscle protein turnover in the fed state. Can J Physiol Pharmacol 2002;80:1045–53. [DOI] [PubMed] [Google Scholar]
- 24.Tang JE, Phillips SM. Maximizing muscle protein anabolism: the role of protein quality. Curr Opin Clin Nutr Metab Care 2009;12:66–71. [DOI] [PubMed] [Google Scholar]
- 25.Paul GL. The rationale for consuming protein blends in sports nutrition. J Am Coll Nutr 2009;28 (Suppl):464S–72S. [DOI] [PubMed] [Google Scholar]
- 26.Dangin M, Boirie Y, Garcia-Rodenas C, Gachon P, Fauquant J, Callier P, Ballevre O, Beaufrere B. The digestion rate of protein is an independent regulating factor of postprandial protein retention. Am J Physiol Endocrinol Metab 2001;280:E340–8. [DOI] [PubMed] [Google Scholar]
- 27.Bos C, Metges CC, Gaudichon C, Petzke KJ, Pueyo ME, Morens C, Everwand J, Benamouzig R, Tome D. Postprandial kinetics of dietary amino acids are the main determinant of their metabolism after soy or milk protein ingestion in humans. J Nutr 2003;133:1308–15. [DOI] [PubMed] [Google Scholar]
- 28.Norton LE, Layman DK. Leucine regulates translation initiation of protein synthesis in skeletal muscle after exercise. J Nutr 2006;136:533S–7S. [DOI] [PubMed] [Google Scholar]
- 29.Burke LM, Hawley JA, Ross ML, Moore DR, Phillips SM, Slater GR, Stellingwerff T, Tipton KD, Garnham AP, Coffey VG. Preexercise aminoacidemia and muscle protein synthesis after resistance exercise. Med Sci Sports Exerc 2012;44:1968–77. [DOI] [PubMed] [Google Scholar]
- 30.Tipton KD, Elliot TA, Cree MG, Wolf SE, Sanford AP, Wolfe RR. Ingestion of casein and whey proteins result in muscle anabolism after resistance exercise. Med Sci Sports Exerc 2004;36:2073–81. [DOI] [PubMed] [Google Scholar]
- 31.Reitelseder S, Agergaard J, Doessing S, Helmark IC, Lund P, Kristensen NB, Frystyk J, Flyvbjerg A, Schjerling P, van Hall G, et al. Whey and casein labeled with L-[1–13C]leucine and muscle protein synthesis: effect of resistance exercise and protein ingestion. Am J Physiol Endocrinol Metab 2011;300:E231–42. [DOI] [PubMed] [Google Scholar]
- 32.Tang JE, Moore DR, Kujbida GW, Tarnopolsky MA, Phillips SM. Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J Appl Physiol 2009;107:987–92. [DOI] [PubMed] [Google Scholar]
- 33.Wang Q, Ge X, Tian X, Zhang Y, Zhang J, Zhang P. Soy isoflavone: the multipurpose phytochemical (Review). Biomed Rep 2013;1:697–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reidy PT, Walker DK, Dickinson JM, Gundermann DM, Drummond MJ, Timmerman KL, Fry CS, Borack MS, Cope MB, Mukherjea R, et al. Protein blend ingestion following resistance exercise promotes human muscle protein synthesis. J Nutr 2013;143:410–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reidy PT, Walker DK, Dickinson JM, Gundermann DM, Drummon MJ, Timmerman KL, Cope MB, Mukherjea R, Jennings K, Volpi E, et al. Soy-dairy protein blend and whey protein ingestion after resistance exercise increases amino acid transport and transporter expression in human skeletal muscle. J Appl Physiol 2014;116:1353–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drummond MJ, Bell JA, Fujita S, Dreyer HC, Glynn El, Volpi E, Rasmussen BB. Amino acids are necessary for the insulin-induced activation of mTOR/S6K1 signaling and protein synthesis in healthy and insulin resistant human skeletal muscle. Clin Nutr 2008;27:447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wolfe RR, Chinkes DL. Isotope tracers in metabolic research: principles and practice of kinetic analysis. 2nd ed Hoboken (NJ): Wiley-Liss; 2005. [Google Scholar]
- 38.Dreyer HC, Fujita S, Cadenas JG, Chinkes DL, Volpi E, Rasmussen BB. Resistance exercise increases AMPK activity and reduces 4E–BP1 phosphorylation and protein synthesis in human skeletal muscle. J Physiol 2006;576:613–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Volpi E, Kobayashi H, Sheffield-Moore M, Mittendorfer B, Wolfe RR. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr 2003;78:250–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Calder AG, Anderson SE, Grant I, McNurlan MA, Garlick PJ. The determination of low d5-phenylalanine enrichment (0.002–0.09 atom percent excess), after conversion to phenylethylamine, in relation to protein turnover studies by gas chromatography/electron ionization mass spectrometry. Rapid Commun Mass Spectrom 1992;6:421–4. [DOI] [PubMed] [Google Scholar]
- 41.Team RC. R version 13.2. Vienna (Austria): R Foundation for Statistical Computing; 2012.
- 42.Kumar V, Atherton PJ, Selby A, Rankin D, Williams J, Smith K, Hiscock N, Rennie MJ. Muscle protein synthetic responses to exercise: effects of age, volume, and intensity. J Gerontol A Biol Sci Med Sci 2012;67:1170–7. [DOI] [PubMed] [Google Scholar]
- 43.Bukhari SS, Phillips BE, Wilkinson DJ, Limb MC, Rankin D, Mitchell WK, Kobayashi H, Greenhaff PL, Smith K, Atherton PJ. Intake of low-dose leucine-rich essential amino acids stimulates muscle anabolism equivalently to bolus whey protein in older women, at rest and after exercise. Am J Physiol Endocrinol Metab 2015;308:E1056–65. [DOI] [PubMed] [Google Scholar]
- 44.Mitchell CJ, McGregor RA, D’Souza RF, Thorstensen Eric B, Markworth JF, Fanning AC, Poppitt SD, Cameron-Smith D. Consumption of milk protein or whey protein results in a similar increase in muscle protein synthesis in middle aged men. Nutrients 2015;7:8685–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paddon-Jones D, Sheffield-Moore M, Zhang XJ, Volpi E, Wolf SE, Aarsland A, Ferrando AA, Wolfe RR. Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am J Physiol Endocrinol Metab 2004;286:E321–8. [DOI] [PubMed] [Google Scholar]
- 46.Kumar V, Atherton P, Smith K, Rennie MJ. Human muscle protein synthesis and breakdown during and after exercise. J Appl Physiol 2009;106:2026–39. [DOI] [PubMed] [Google Scholar]
- 47.Dreyer HC, Fujita S, Glynn EL, Drummond MJ, Volpi E, Rasmussen BB. Resistance exercise increases leg muscle protein synthesis and mTOR signalling independent of sex. Acta Physiol (Oxf) 2010;199:71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]