Abstract
Background: Poor nutritional intake can exacerbate the chronic disease burden in childhood cancer survivors, whereas a healthful diet serves a protective function. Few studies have provided detailed evaluations of the diet of childhood cancer survivors.
Objectives: This study aimed to evaluate diet quality and dietary intakes of key food groups and nutrients in a large cohort of childhood cancer survivors and whether cancer and treatment characteristics have an impact on survivors’ long-term intake.
Methods: Diet was assessed in 2570 adult survivors of childhood cancer enrolled in the St. Jude Lifetime cohort (mean age = 32.3 y) by using the Block food-frequency questionnaire. The Healthy Eating Index–2010 (HEI-2010) was calculated to quantify diet quality. Cancer diagnosis and treatment exposure were abstracted from medical records. Differences in HEI-2010 by patient characteristics and treatment exposure were examined by using ANCOVA.
Results: The mean ± SD HEI-2010 in childhood cancer survivors was 57.9 ± 12.4 of a maximum score of 100. Referenced to Dietary Reference Intakes, survivors consumed inadequate amounts of vitamin D, vitamin E, potassium, fiber, magnesium, and calcium (27%, 54%, 58%, 59%, 84%, and 90% of the recommended intakes) but excessive amounts of sodium and saturated fat (155% and 115% of the recommended intakes) from foods. Survivors diagnosed when <5 y of age had a lower diet quality than did those diagnosed when ≥5 y of age (mean HEI-2010 score: 56.9 compared with 58.2; P = 0.046). Survivors who received higher radiation doses to the abdomen had a lower diet quality than those who received lower doses (mean HEI-2010 scores = 58.9, 57.2, 56.7, and 56.1 for doses of 0, 1–19.9, 20–29.9, and ≥30 Gy, respectively; P = 0.02).
Conclusions: Long-term childhood cancer survivors have poor adherence to the 2010 Dietary Guidelines for Americans. Findings reinforce the need to incorporate nutrition into cancer care to improve diet quality and to reduce morbidities.
Keywords: diet quality, nutrition, childhood cancer survivors, cancer survivors, cancer treatment
Introduction
Dramatic improvements in the diagnosis and treatment of cancer in childhood have led to a rapidly growing cohort of survivors, now estimated to exceed 450,000 (1). However, after successful treatment, childhood cancer survivors experience a substantially higher risk of premature mortality and serious morbidity at a young age (2–4). Nutrition is among the few modifiable factors that can prevent or delay the early onset of chronic diseases. There are imperative needs to identify nutritional targets for interventions that can potentially reduce morbidity, improve survival, and increase quality of life.
However, nutritional intake among childhood cancer survivors is not well documented. Although the few existing studies provide some evidence that suggests poor adherence to current dietary guidelines (5–8), this evidence is primarily based on small cohorts of survivors or focused on specific cancer groups, such as pediatric acute lymphoblastic leukemia. We lack a comprehensive evaluation of survivors’ intakes of key nutrients and food groups, which is essential for developing targeted nutritional interventions to address the specific nutritional needs in this high-risk population. Importantly, cancer diagnosis and cancer-directed therapy can affect survivors’ nutritional intake through complex pathways. These changes were originally thought to be short-term adaptive responses to cancer treatment. However, these early adaptive responses may persist beyond treatment completion and last longer through survivorship. In a large and histologically diverse cohort of adult survivors of childhood cancer enrolled in the St. Jude Lifetime (SJLIFE) cohort, we assessed how well childhood cancer survivors’ overall eating patterns adhere to the Dietary Guidelines for Americans, whether survivors’ intakes of key nutrients and food groups meet the recommended levels, and the potential impact of cancer and treatment characteristics on survivors’ long-term intakes.
Methods
Study population.
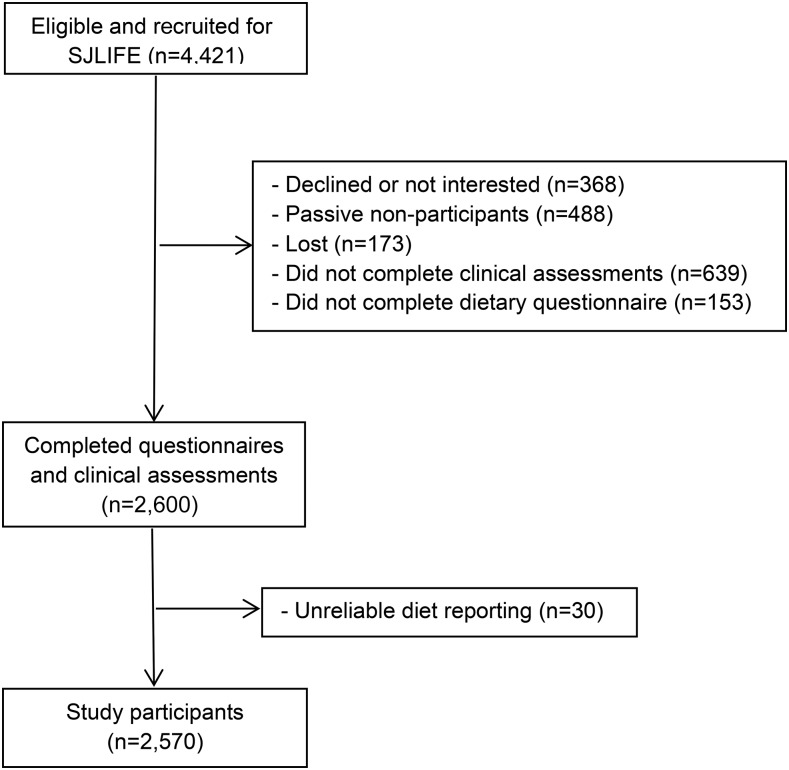
The SJLIFE cohort comprises adult survivors of childhood cancer who were treated at St. Jude Children’s Research Hospital and who survived ≥10 y after cancer diagnosis (2, 9). The primary aim of the SJLIFE cohort is to systematically and prospectively assess health outcomes among childhood cancer survivors as they age. Between October 2007 and October 2013, 4421 survivors were eligible for SJLIFE, of whom 2600 completed comprehensive health questionnaires, including dietary questionnaires followed by clinical assessments on the St. Jude campus, and were included as the study population for the current study (Figure 1). This study was approved by the institutional review board at St. Jude Children’s Research Hospital and Tufts Medical Center/Tufts University, and all participants provided informed consent.
FIGURE 1.
Study flow chart. SJLIFE, St. Jude Lifetime cohort.
Medical records of childhood cancer survivors were abstracted for cancer diagnosis and treatment exposures by using a protocol similar to that used in the Childhood Cancer Survivor Study (9). This includes abstraction of all chemotherapy received, including cumulative doses for 32 specific chemotherapeutic agents, surgical procedures, and radiation treatment fields, dose, and energy source. Participants also completed a series of health questionnaires, including demographic characteristics and self-reported health behaviors such as cigarette smoking, alcohol intake, and physical activity. Height and weight were measured at study evaluations by using a wall-mounted stadiometer and an electronic scale.
Dietary intake.
Dietary intake was collected by using a self-administered 2005 Block FFQ. The Block FFQ asks participants about their usual dietary intake of 110 food items during the past 12 mo. Because study participants were, on average, 24 y postdiagnosis, their diet as assessed by the Block FFQ represented their usual diet or current diet as cancer survivors. For each food item, the FFQ assessed the frequency of consumption in 8 categories (never or hardly ever, 1 time/mo, 2–3 times/mo, 1 time/wk, 2–3 times/wk, 4–6 times/wk, 1 time/d, and ≥2 times/d). Portion size was asked for each food, and pictures were provided to enhance the accuracy of quantification. Completed FFQs were processed by Block Dietary Data Systems, which estimates nutrient intake on the basis of a food list from the NHANES (10) and food-composition values for nutrients from the USDA Food and Nutrient Database for Dietary Studies (11). The intake of nutrients was based on the consumption of food and beverages and did not include intakes from supplements. The Block FFQ was previously validated with three 24-h diet recalls and showed reasonable correlations for most nutrients, ranging between 0.4 and 0.7 (12–14).
Survivors’ diet quality was estimated by using the Healthy Eating Index–2010 (HEI-2010) calculated by Block Dietary Data Systems. The HEI-2010 measures adherence to the 2010 Dietary Guidelines for Americans and has 12 components (15, 16). For each component, intakes of foods and nutrients are represented on a density basis, counted as amount per 1000 kcal. The 9 adequacy components include total fruit, whole fruit, total vegetables, greens and beans, whole grains, dairy, total protein foods, seafood and plant proteins, and FAs, which reflects the ratio of PUFAs and MUFAs to SFAs. Three moderation components include refined grains, sodium, and empty calories, which reflect calories from solid fats, alcohol, and added sugars. For adequacy components, a score of zero is assigned for no intake, and the scores increase proportionately as intake increases up to the recommended level. For moderation components, intake at the recommended level is assigned the maximum score and the score decreases as intake increases. The total HEI-2010 score ranges from zero (nonadherence) to 100 (perfect adherence).
Statistical analysis.
We first identified individuals with potentially unreliable reporting for dietary intake, defined as total caloric intake >3 SDs above and below the mean value of the natural log-transformed caloric intake in the study population. After excluding individuals with unreliable reporting (n = 30), we calculated the mean HEI-2010 total score and component scores and the mean dietary intake of nutrients. To further evaluate whether survivors’ dietary intake of nutrients met the nutrition goals (17), we compared survivors’ mean intake to the recommended intake, estimated on the basis of the age-sex groups of the RDA or, when not available, Adequate Intake (18), weighted by the age and sex distribution of the study population, as follows:
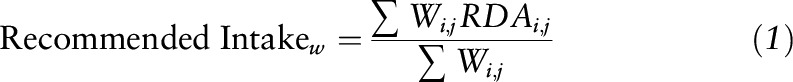 |
where RDAi,j are RDAs for age group i and sex group j and Wi,j are weights calculated on the basis of the number of the survivors in age i and sex group j over the total number of survivors. For example, the RDA for vitamin B-6 is 1.3 mg for women and men aged 18–30 y, 1.5 mg for women aged ≥31 y, and 1.7 mg for men aged ≥31 y. Because the percentages (i.e., weights) of women aged 18–30 y and ≥31 y and men aged 18–30 y and ≥31 y were 47.5%, 0.9%, 50.4%, and 1.2%, respectively, the recommended intake for the study population was estimated to be 1.3 mg (=1.3 mg × 47.5% + 1.5 mg × 0.9% + 1.3 mg × 50.4% + 1.7 mg × 1.2%).
We assessed whether survivors’ diet quality differed by survivors’ demographic (age, sex, race/ethnicity, and education) and lifestyle (cigarette smoking, alcohol intake, physical activity, and weight status) factors with the use of ANCOVA (19) with simultaneous adjustment for demographic and lifestyle factors significantly associated with diet quality. We further evaluated whether cancer diagnosis and treatment exposures were associated with diet quality, after controlling for survivors’ age and sex. Cancer diagnosis was adjusted in all analyses for treatment exposures because it directly affected the treatment regimens patients received. Because education, cigarette smoking, physical activity, and weight status can be affected by cancer diagnosis and treatment exposures (i.e., intermediates on the causal pathway), these covariates were not adjusted in the analysis for cancer diagnosis and treatment exposures to avoid overadjustments (20). SAS version 9.3.1 (SAS Institute) was used for all analyses.
Results
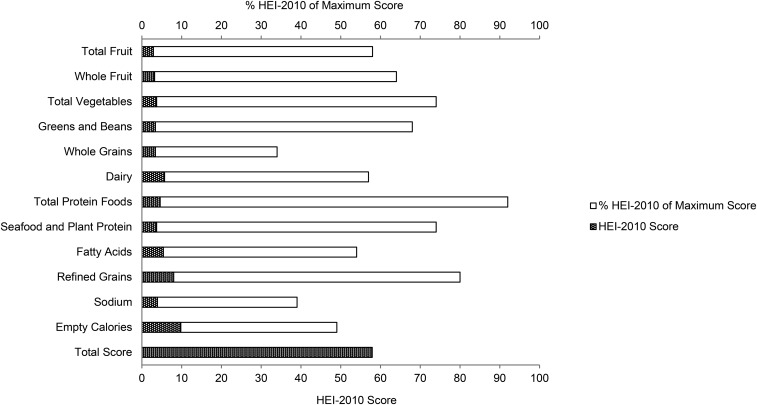
Our study population comprised 2570 adult survivors of childhood cancer, among which leukemia was the most common cancer diagnosis, followed by lymphoma, embryonal tumor, sarcoma, central nervous system tumor, and others (Table 1). The mean HEI-2010 total score was low, 57.9 out of a maximum score of 100 (Table 2). For individual components, whole grains, sodium, and empty calories (34%, 39%, and 49%, respectively) had component scores <50% of the maximum score (Figure 2). The mean intake of whole grains was only 0.6 ounce equivalents · d−1 · 1000 kcal−1 (1 ounce equivalent = 16 g), which did not meet half of the recommended intake (i.e., ≥1.5 ounce equivalents · d−1 · 1000 kcal−1), whereas the mean intake of sodium was substantially higher than the Tolerable Upper Intake Level (UL) (i.e., 3566 compared with 2300 mg/d). Childhood cancer survivors also overconsumed empty calories. Calories from added sugars and solid fats each contributed to 14% and 20% of total calories, respectively, which well exceeded the HEI-2010 limit (i.e., <19% of total calories from empty calories).
TABLE 1.
Characteristics of the 2570 adult survivors of childhood cancer in the SJLIFE cohort1
| Value | |
| Age at study, y | 32.3 ± 8.3 |
| Sex, n (%) | |
| Male | 1326 (51.6) |
| Female | 1244 (48.4) |
| Race/ethnicity, n (%) | |
| Non-Hispanic white | 2141 (83.3) |
| Non-Hispanic black | 340 (13.2) |
| Other | 89 (3.5) |
| Education, n (%) | |
| Grades 0–12 | 692 (26.9) |
| Some post–high school | 805 (31.3) |
| College graduate | 923 (35.9) |
| Smoking,2 n (%) | |
| Nonsmoker | 1665 (64.8) |
| Former smoker | 292 (11.4) |
| Current smoker | 585 (22.8) |
| Alcohol consumption, n (%) | |
| Nondrinker | 1188 (46.2) |
| <14 g ethanol/d | 932 (36.3) |
| ≥14 g ethanol/d | 406 (15.8) |
| Physical activity,3 n (%) | |
| Active | 1270 (49.4) |
| Inactive | 1253 (48.8) |
| BMI,4 kg/m2 | 28.5 ± 7.4 |
| Weight status,4 n (%) | |
| Underweight (BMI <18.5) | 89 (3.5) |
| Normal weight (BMI = 18.5–24.9) | 837 (32.6) |
| Overweight (BMI = 25–29.9) | 730 (28.4) |
| Obese (BMI ≥30) | 896 (34.9) |
| Height,5 SDS | −0.25 (1.17) |
| Primary diagnosis, n (%) | |
| Leukemia | 986 (38.4) |
| Lymphoma | 497 (19.4) |
| Embryonal tumor | 335 (13.1) |
| Sarcoma | 328 (12.8) |
| Central nervous system tumor | 239 (9.3) |
| Other | 181 (7.1) |
| Age at diagnosis, y | 8.3 ± 5.6 |
| Time from diagnosis, y | 24.1 ± 8.1 |
| Treatment exposures, n (%) | |
| Radiation (any) | 1532 (59.6) |
| Brain | 916 (35.6) |
| Abdomen | 588 (22.9) |
| Alkylating agents | 1627 (63.3) |
| Anthracyclines | 1499 (58.3) |
| Antimetabolites | 1369 (53.3) |
| Glucocorticoids | 1260 (49.0) |
Values are means ± SDs unless otherwise indicated. MVPA, moderate-to-vigorous physical activity; SDS, SD score; SJLIFE, St. Jude Lifetime.
Smokers were defined as individuals who reported smoking ≥100 cigarettes during their lifetime, with former smokers defined as not currently smoking and current smokers defined as currently smoking.
Minutes per week of MVPA was calculated by summarizing minutes, with vigorous physical activities weighted at 1.67 min for each minute of vigorous physical activity. Participants were classified as physically active if MVPA met or exceeded 150 min/wk, according to the CDC Physical Activity Guidelines for Americans (21).
BMI was calculated by dividing weight in kilograms by height in meters squared, adjusted for amputation status. Participants were classified as underweight (BMI <18.5), normal weight (BMI = 18.5–24.9), overweight (BMI = 25–29.9), or obese (BMI ≥30).
Height was assessed by SDS. Raw height was converted to the SDS by comparing the height of the survivors with the mean height of a reference population (i.e., the 2000 CDC growth chart).
TABLE 2.
Diet quality in adult survivors of childhood cancer: the SJLIFE cohort1
| HEI-2010 component | Maximum score | Standard for maximum score | Standard for minimum score of 0 | Score2 |
| Total fruit | 5 | ≥0.8 cup equiv./1000 kcal | No fruit | 2.9 ± 1.6 |
| Whole fruit | 5 | ≥0.4 cup equiv./1000 kcal | No whole fruit | 3.2 ± 1.6 |
| Total vegetables | 5 | ≥1.1 cup equiv./1000 kcal | No vegetables | 3.7 ± 1.2 |
| Greens and beans | 5 | ≥0.2 cup equiv./1000 kcal | No dark-green vegetables, beans, or peas | 3.4 ± 1.7 |
| Whole grains | 10 | ≥1.5 ounce equiv./1000 kcal | No whole grains | 3.4 ± 2.5 |
| Dairy | 10 | ≥1.3 cup equiv./1000 kcal | No dairy | 5.7 ± 2.6 |
| Total protein foods | 5 | ≥2.5 cup equiv./1000 kcal | No protein foods | 4.6 ± 0.7 |
| Seafood and plant protein | 5 | ≥0.8 cup equiv./1000 kcal | No seafood or plant proteins | 3.7 ± 1.4 |
| FAs | 10 | (PUFAs + MUFAs):SFAs ≥2.5 | (PUFAs + MUFAs):SFAs <1.2 | 5.4 ± 2.4 |
| Refined grains | 10 | ≤1.8 ounce equiv./1000 kcal | ≥4.3 ounce equiv./1000 kcal | 8.0 ± 2.1 |
| Sodium | 10 | ≤1.1 g/1000 kcal | ≥2.0 g/1000 kcal | 3.9 ± 2.5 |
| Empty calories3 | 20 | ≤19% of calories | ≥50% of calories | 9.8 ± 5.1 |
| Total HEI-2010 score | 100 | — | — | 57.9 ± 12.4 |
equiv., equivalents; HEI-2010, Healthy Eating Index–2010; SJLIFE, St. Jude Lifetime.
Values are means ± SDs.
“Empty calories” refers to calories from solid fats, alcohol, and added sugars. The threshold for counting alcohol is >13 g/1000 kcal.
FIGURE 2.
HEI-2010 mean scores and percentage of mean scores to the maximum scores in adult survivors of childhood cancer. HEI-2010, Healthy Eating Index–2010.
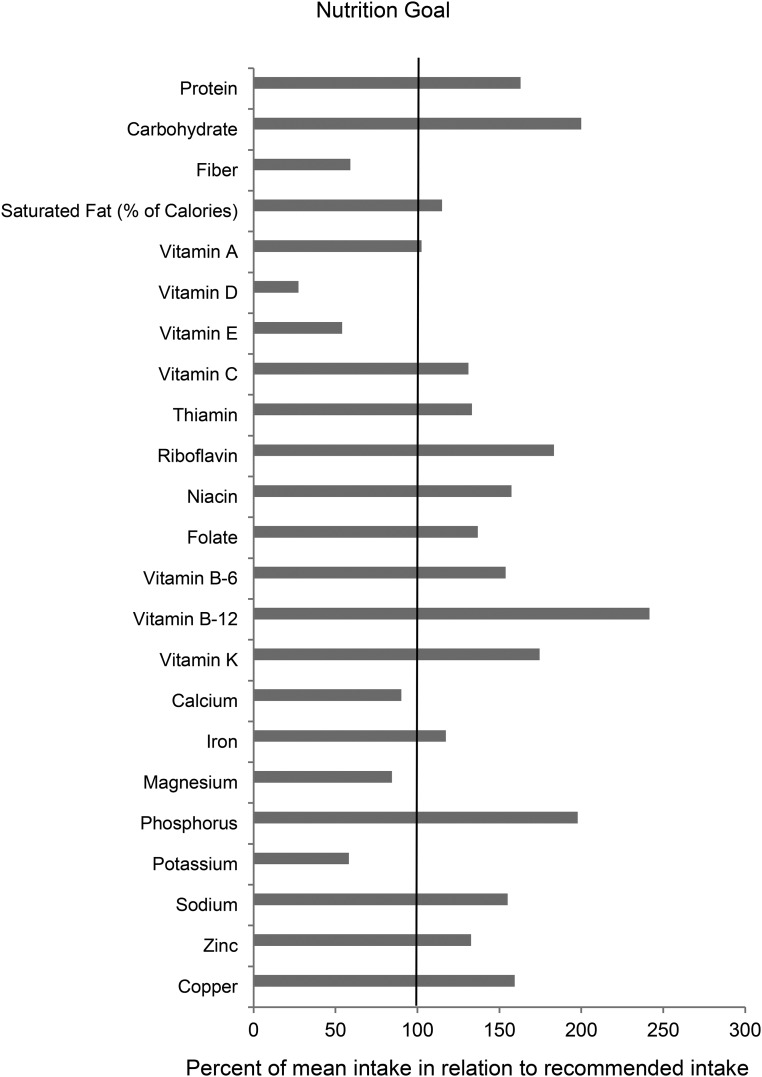
Survivors’ nutrient intakes from foods are presented in Table 3. When survivors’ intakes were compared with the age- and sex-specific DRIs, only 15% of survivors met the recommended intake for fiber and 25% met the recommendation of consuming <10% energy from saturated fat. The mean intake of fiber was 59% of the recommended intake, whereas the mean percentage of calories from saturated fat was 15% above the recommended level (Figure 3). For dietary intakes of minerals and vitamins, only 8% of survivors met the recommended intake for potassium and 31% and 34% of survivors met the recommended intakes for magnesium and calcium, respectively. However, 73% of survivors’ sodium intake exceeded the UL. The mean intakes of potassium, magnesium, and calcium were 58%, 84%, and 90% of the recommended levels, respectively, whereas the mean sodium intake was 55% above the UL. Only 1% and 8% of the survivors met the recommended intakes for vitamin D and vitamin E, respectively. The mean intakes of vitamins D and E were 27% and 54% of the recommended intakes.
TABLE 3.
Dietary intake of nutrients from foods in adult survivors of childhood cancer: the SJLIFE cohort1
| Nutrients | Amount of intake |
| Macronutrients | |
| Protein, g/d | 83.4 ± 49.9 |
| % of calories | 15.5 ± 3.1 |
| Carbohydrate, g/d | 260 ± 143 |
| % of calories | 48.5 ± 7.8 |
| Total fiber, g/d | 17.4 ± 9.7 |
| Total fat, % of calories | 36.1 ± 5.9 |
| Saturated fat, % of calories | 11.5 ± 2.3 |
| Cholesterol, mg/d | 294 ± 208 |
| Minerals | |
| Calcium, mg/d | 906 ± 488 |
| Iron, mg/d | 14.9 ± 8.4 |
| Magnesium, mg/d | 308 ± 162 |
| Phosphorus, mg/d | 1385 ± 744 |
| Potassium, mg/d | 2735 ± 1407 |
| Sodium, mg/d | 3566 ± 2044 |
| Zinc, mg/d | 12.6 ± 8.3 |
| Copper, g/d | 1435 ± 824 |
| Selenium, μg/d | 109 ± 66.1 |
| Micronutrients | |
| Vitamin A, μg RAEs/d | 823 ± 531 |
| Vitamin D, μg/d | 4.1 ± 3.3 |
| Vitamin E, mg AT/d | 8.1 ± 4.6 |
| Vitamin C, mg/d | 109 ± 80.7 |
| Thiamin, mg/d | 1.6 ± 1.0 |
| Riboflavin, mg/d | 2.2 ± 1.2 |
| Niacin, mg/d | 23.6 ± 14.2 |
| Folate, μg/d | 548 ± 316 |
| Vitamin B-6, mg/d | 2.0 ± 1.2 |
| Vitamin B-12, μg/d | 5.8 ± 4.1 |
| Vitamin K, μg/d | 184 ± 168 |
Values are means ± SDs. Nutrient intake amounts were assessed from food sources only. Supplement intake was not included. AT, α-tocopherol; RAE, retinol activity equivalent; SJLIFE, St. Jude Lifetime.
FIGURE 3.
Dietary intakes of nutrients from foods compared with recommended intakes or limits in adult survivors of childhood cancer. The length the gray bar for each nutrient corresponds to the percentage of mean intake to the recommended intake level × 100. Recommended intake is estimated on the basis of the age-sex groups of RDAs published by Institute of Medicine (18), weighted by the age and sex distribution of the childhood cancer survivors. For sodium, the limit is estimated on the basis of the Tolerable Upper Intake Level. For the percentage of calories from saturated fat with no quantitative DRI, dietary guidelines recommendations were used (17). Nutritional goals are set at 100 when the mean intake meets the recommended intake or limit.
The mean HEI-2010 total score increased with age. Older survivors (aged ≥40 y) had a higher score than younger survivors (aged 18–29 y). Female survivors had a higher score than male survivors, and survivors who were college graduates had a higher score than those who had a high school education or less. Current smokers had a lower score than former or nonsmokers. Survivors who were physically inactive had a lower score than those who were physically active. Mean HEI-2010 total score was lower in survivors who were underweight or obese than in survivors who had a normal weight or were overweight (Table 4).
TABLE 4.
Diet quality in adult survivors of childhood cancer by patient characteristics and treatment exposure: the SJLIFE cohort1
| HEI-2010 score | P | |
| Patient characteristics | ||
| Age at study, y | <0.0001 | |
| 18–29 | 55.1 (54.0, 56.2) | |
| 30–39 | 56.3 (55.2, 57.4) | |
| 40–64 | 58.0 (56.7, 59.3) | |
| Sex | <0.0001 | |
| Male | 53.6 (52.6, 54.7) | |
| Female | 59.3 (58.3, 60.4) | |
| Race/ethnicity | 0.06 | |
| Non-Hispanic white | 57.1 (56.2, 57.9) | |
| Other | 55.9 (54.5, 57.2) | |
| Education | <0.0001 | |
| Grades 0–12 | 53.2 (52.0, 54.4) | |
| Some post–high school | 55.7 (54.6, 56.8) | |
| College graduate | 60.1 (59.4, 61.7) | |
| Smoking status | <0.0001 | |
| Nonsmoker | 57.9 (57.0, 58.9) | |
| Former smoker | 57.7 (56.1, 59.2) | |
| Current smoker | 53.9 (52.7, 55.1) | |
| Alcohol consumption | 0.07 | |
| Nondrinker | 56.0 (54.9, 57.1) | |
| <14 g ethanol/d | 57.1 (56.1, 58.2) | |
| ≥14 g ethanol/d | 56.3 (55.9, 57.7) | |
| Physical activity2 | <0.0001 | |
| Active | 58.5 (57.5, 59.6) | |
| Inactive | 54.5 (53.4, 55.5) | |
| Weight status3 | 0.006 | |
| Underweight | 54.1 (51.6, 56.7) | |
| Normal weight | 57.3 (56.3, 58.3) | |
| Overweight | 58.0 (57.0, 59.0) | |
| Obese | 56.5 (55.5, 57.5) | |
| Height,4 SDS | 0.38 | |
| <2 | 57.1 (55.4, 58.8) | |
| ≥2 | 57.9 (57.4, 58.4) | |
| Primary diagnosis | 0.01 | |
| Leukemia | 58.7 (57.9, 59.5) | |
| Lymphoma | 59.4 (58.3, 60.4) | |
| Embryonal tumor | 56.9 (55.5, 58.2) | |
| Sarcoma | 57.3 (56.0, 58.6) | |
| Central nervous system tumor | 57.7 (56.1, 59.3) | |
| Other | 57.0 (55.2, 58.8) | |
| Age at diagnosis, y | 0.045 | |
| <5 | 56.9 (56.0, 57.8) | |
| 5–9 | 58.2 (57.1, 59.2) | |
| 10–14 | 58.5 (57.4, 59.5) | |
| ≥15 | 58.2 (56.9, 59.5) | |
| Treatment exposures | ||
| Any radiation | 0.83 | |
| No | 57.7 (56.9, 58.6) | |
| Yes | 57.8 (57.2, 58.5) | |
| Brain radiation dose,5 Gy | 0.95 | |
| 0 | 58.2 (57.1, 59.3) | |
| 1–19.9 | 57.6 (55.5, 59.7) | |
| 20–29.9 | 57.7 (55.9, 59.6) | |
| ≥30 | 58.1 (55.9, 60.5) | |
| Abdomen radiation dose, Gy | ||
| 0 | 58.9 (58.0, 59.7) | 0.02 |
| 1–19.9 | 57.2 (55.0, 59.4) | |
| 20–29.9 | 56.7 (54.8, 58.5) | |
| ≥30 | 56.1 (54.2, 58.0) | |
| Cumulative alkylating agent dose,6 mg/m2 | 0.85 | |
| 0 | 58.0 (57.2, 58.9) | |
| 1–7999 | 57.6 (56.5, 58.6) | |
| 8000–11,999 | 57.5 (56.2, 58.8) | |
| ≥12,000 | 57.7 (56.5, 58.9) | |
| Cumulative anthracycline dose, mg/m2 | 0.43 | |
| 0 | 58.1 (57.4, 58.9) | |
| 1–99 | 57.0 (55.5, 58.5) | |
| 100–299 | 57.6 (56.5, 58.7) | |
| ≥300 | 57.0 (55.5, 58.5) | |
| Cumulative glucocorticoid dose,7 mg/m2 | 0.03 | |
| 0 | 57.7 (56.9, 58.4) | |
| 1–1499 | 57.1 (55.4, 58.7) | |
| 1500–8999 | 59.7 (57.9, 61.5) | |
| ≥9000 | 56.9 (55.0, 58.5) |
Values are means (95% CIs). For patient characteristics, means, SEs, and P values were adjusted for age at study, sex, race/ethnicity, education, smoking status, alcohol consumption, physical activity, and weight status by using ANCOVA; for cancer and treatment characteristics, means, SEs, and P values were adjusted for age at study, sex, and cancer diagnosis by using ANCOVA. SDS, SD score; SJLIFE, St. Jude Lifetime.
Physical activity was defined as active if engaging in moderate-to-vigorous physical activity ≥150 min/wk and inactive otherwise.
Weight status was defined as underweight if BMI (kg/m2) <18.5, normal weight if BMI = 18.5–24.9, overweight if BMI = 25–29.9, and obese if BMI ≥30.
Height was assessed by SDS. Raw height was converted to SDS by comparing the height of the survivors with the mean height of a reference population (i.e., the 2000 CDC growth chart).
Brain radiation dose was based on the maximum brain segment dose.
Cumulative dose of alkylating agents was calculated as the cyclophosphamide equivalent dose by using the following equation: cyclophosphamide equivalent dose (mg/m2) = 1.0 [cumulative cyclophosphamide dose (mg/m2)] + 0.244 [cumulative ifosfamide dose (mg/m2)] + 0.857 [cumulative procarbazine dose (mg/m2)] + 14.286 [cumulative chlorambucil dose (mg/m2)] + 15.0 [cumulative carmustine (BCNU) dose (mg/m2)] +16.0 [cumulative Lomustine (CCNU) dose (mg/m2)] + 40 [cumulative melphalan dose (mg/m2)] +50 [cumulative Thio-TEPA dose (mg/m2)] + 100 [cumulative nitrogen mustard dose (mg/m2)] + 8.823 [cumulative busulfan dose (mg/m2)].
Cumulative dose of glucocorticoids was calculated by converting the dexamethasone dose to a prednisone equivalent dose (1 mg dexamethasone = 6.67 mg prednisone) and summing across the 2 medications.
For common cancer diagnoses in children, survivors of lymphoma had the highest HEI-2010 total score (59.4) followed by survivors of leukemia (58.7), whereas survivors of sarcoma had the lowest score (56.9) (P = 0.01). Survivors diagnosed at <5 y of age had a lower score (56.9) than did those diagnosed at older ages (58.2, 58.5, and 58.2 for diagnosed at 5–9, 10–14, and ≥15 y of age, respectively; P = 0.045). Because similar diet quality scores were observed in those diagnosed at ≥5 y of age, they were later combined into 1 group. Survivors diagnosed at <5 y of age had a lower score than those diagnosed at ≥5 y of age (56.9 compared with 58.2; P = 0.046).
For treatment exposures, exposure to any radiation or cumulative radiation dose to the brain was associated with similar diet quality. However, a higher radiation dose to the abdomen was associated with a lower diet quality in childhood cancer survivors: the higher the radiation dose the worse the diet quality (HEI-2010 total scores = 58.9, 57.2, 56.7, and 56.1 for radiation doses of 0, 1–19.9, 20–29.9, and ≥30 Gy, respectively; P = 0.02). For chemotherapeutic agents, survivors who received ≥9000 mg/m2 cumulative doses of glucocorticoids had a lower HEI-2010 total score (56.9), whereas those treated with cumulative doses between 1500 and 8999 mg/m2 had a higher score (59.7) than those not treated with glucocorticoids (57.7) (P = 0.03). No clear pattern was observed for treatment with glucocorticoids. Exposure to alkylating agents and anthracyclines and cumulative dose were not associated with diet quality in childhood cancer survivors (Table 4).
Discussion
In a large cohort of adult survivors of childhood cancer, we found evidence of poor adherence of survivors’ diet to the 2010 Dietary Guidelines for Americans. Childhood cancer survivors are especially burdened by low dietary intakes of whole grains and high intakes of sodium and empty calories. These intake patterns are established risk factors for chronic diseases such as cardiovascular disease, diabetes, and obesity. The remarkably high chronic disease burden and early onset of chronic diseases in this population (2, 22, 23) reinforce the need to incorporate nutrition into cancer care to improve diet quality and to reduce morbidities in the growing number of children who have survived cancer.
The mean HEI-2010 score identified in this large cohort of childhood cancer survivors is consistent with findings from previous studies in smaller cohorts of survivors that reported diet quality scores ranging from 33% to 56% of the maximum score (5–8, 24). Although dietary assessment methods or indexes to measure diet quality varied between studies, the collective evidence consistently points to poor diet quality in childhood cancer survivors. Older survivors had better diet quality than did younger survivors. Such an age effect was previously reported in cancer survivors (25–28)and may partly reflect better dietary habits in older than in younger generations (i.e., cohort effect) or overall better health in survivors who survived longer (i.e., survivor bias) (29). Consistent with previous findings (25, 28, 30–32), diet quality for male survivors and survivors with low educational attainment, who currently smoke, or who are obese was poor and these individuals may be viable targets for dietary interventions. Survivors who were underweight, although they represented a small proportion (3.5%), also had a poor diet quality, which could be due to poor health status or unintentional weight loss associated with poor intake.
Because cancer diagnosis directly affected cancer treatments that patients received, we focused our analyses on cancer treatments that potentially affect dietary intake. For example, glucocorticoids are known to be critically involved in regulating energy intake, storage, and mobilization (33, 34). Although we saw some indications of lower diet quality in survivors who received cumulative doses of glucocorticoids >9000 mg/m2, there was no clear pattern or trend. Radiation to the brain can directly damage the hypothalamic-pituitary region, impairing signaling reception from hormones such as ghrelin and leptin that regulate hunger and appetite (35–38). We assessed survivors’ diet quality in relation to radiation to different segments of the brain, as well as radiation dose to each segment, and found no associations overall with different brain segments and across different cancer diagnoses (e.g., central nervous system tumor, leukemia, and lymphoma). However, abdominal radiation was associated with a suboptimal diet quality, and poorer diet quality was associated with a higher radiation dose in a dose-response fashion. It has been increasingly recognized that the gastrointestinal tract acts as the body’s largest endocrine organ and releases important hormones that activate brain regions to regulate hunger and satiety (39). Some gut hormones may also affect taste preference and food craving (39). High doses of abdominal radiation can disrupt gut hormone secretion, causing changes in appetite, taste preference, and food craving over the long term (40, 41). Abdominal radiation has also been associated with an increased risk of insulin resistance in childhood cancer survivors (42), which can cause subsequent changes in energy regulation. In addition, radiation damage to small bowel tissue can result in chronic radiation enteritis, producing symptoms such as pain, bloating, nausea, and diarrhea and causing nutrient malabsorption (43). Radiation-induced injury may also disrupt the gut’s resident bacteria (44, 45). Current studies have shed light on the dynamic interactions between the gut and its microbiota composition (46) and subsequent impact on survival (47). In addition, high doses of abdominal radiation can negatively affect dietary intake patterns by altering the intestinal microbiota (48). All of these changes can be long-lasting, preventing survivors from adhering to a healthful diet after treatment completion and negatively affecting their long-term health.
We observed that children diagnosed at a younger age (<5 y) had a worse diet quality than those diagnosed at older ages. We speculate that psychosocial and behavioral factors may explain this association. For example, family environment is critical in shaping children’s dietary behaviors (49), particularly for children diagnosed with cancer at a young age. Parents may practice permissive parenting, which is often associated with unhealthy eating, while the young child is undergoing cancer therapy. After therapy completion, parents may have challenges in reversing the unhealthy eating habits that were established during cancer treatment (50). Adaptive behaviors formed during childhood may persist into adulthood. Therefore, early intervention (e.g., during treatment) and ongoing evaluation of survivors and families may be necessary to assess intake patterns and promote healthy consumption.
Some limitations of our study should be considered. First, we were not able to directly compare whether the diets of childhood cancer survivors differ from those without cancer because of the lack of a comparison group for whom diet was assessed by using the same FFQ. However, we recently reported that adult cancer survivors had worse overall diet quality than age- and sex-matched controls in the NHANES, and survivors’ intake patterns were particularly worse for fiber and empty calories than those in the general population (28). We need to keep in mind that childhood cancer survivors experience a substantially higher chronic disease burden than the general population (2, 22, 23). Even when diet quality in the survivors and general population is similarly poor, poor diet can have a much larger impact on overall health in long-term childhood cancer survivors. In addition to describing the overall diet quality, we attempted to evaluate the susceptibility of the survivors, especially the potential impact of treatment exposure (e.g., radiotherapy, chemotherapy) and cancer characteristics (e.g., cancer type, age at diagnosis) on survivors’ intake. Second, survivors’ diet was assessed by using an FFQ, which is subject to measurement error in determining absolute intake. Previous validation studies, however, have shown reasonable validity coefficients for most nutrients assessed by using the Block FFQ (12–14), and we excluded participants with potential unreliable reporting for dietary intake. In addition, diet quality index was constructed in proportion to energy intake (i.e., energy adjustment), which reduces confounding and improves validity by removing correlated errors (51). We did not include supplemental intakes of vitamins and minerals. Thus, it is plausible that the percentages of the survivors meeting the recommended intakes for vitamins and minerals could be higher if both dietary and supplemental sources were included in the evaluation. Third, our analyses were based on a cross-sectional assessment of dietary intake, which precludes assessing the potential timing of poor dietary intake before diagnosis, during treatment, and after treatment. Future longitudinal studies are needed to further identify the sensitive window at which cancer treatment affects dietary intake as well as psychosocial factors that affect survivors’ eating patterns over the long term. Fourth, our results may be sensitive to bias from nonparticipation and nonresponse given that not all eligible childhood cancer survivors participated and completed questionnaires and clinical assessments. An analysis of the overall SJLIFE cohort suggested no substantial differences between participants and nonparticipants (52).
In addition, we compared characteristics between participants and nonparticipants for survivors eligible for the current study. Our findings suggest no difference in survivors’ age, race/ethnicity, cancer type, and age at diagnosis, except for a modest overrepresentation of females in participants of the current study (48.3% compared with 41.6% of participants and nonparticipants, respectively) (Supplemental Table 1). Because female survivors tend to have a better diet quality than male survivors, the overall diet quality in childhood cancer survivors could be worse than what was found in participants. Last, our study did not assess diet quality in association with clinical endpoints such as occurrence of chronic health conditions, cancer recurrence, and mortality. Prospective longitudinal studies are required to evaluate if differences in mean HEI-2010 are associated with these clinically meaningful endpoints.
Despite these limitations, to our knowledge, our study provides one of the first detailed evaluations on nutritional intake of a large histologically diverse group of adult survivors of childhood cancer. Nutrition plays an important role in chronic disease prevention. Evidence of poor dietary intake in childhood cancer survivors calls for actions to be taken to incorporate nutrition support as an integral part of cancer care for survivors and families. The early onset of chronic health conditions in childhood cancer survivors reinforces the need for dietary interventions early in survivorship care to avoid long-term morbidity in this vulnerable population.
Acknowledgments
FFZ, RPO, KRK, and MMH designed the research and wrote the manuscript; MMH designed and conducted the original study (the SJLIFE cohort); FFZ performed statistical analysis and had primary responsibility for final content; LL prepared the data set and assisted with the statistical analysis; and TMG, JL, WC, and LLR critically reviewed and revised the manuscript. All authors read and approved the final manuscript.
References
- 1.Robison LL, Hudson MM. Survivors of childhood and adolescent cancer: life-long risks and responsibilities. Nat Rev Cancer 2014;14:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hudson MM, Ness KK, Gurney JG, Mulrooney DA, Chemaitilly W, Krull KR, Green DM, Armstrong GT, Nottage KA, Jones KE, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA 2013;309:2371–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstrong GT, Liu Q, Yasui Y, Neglia JP, Leisenring W, Robison LL, Mertens AC. Late mortality among 5-year survivors of childhood cancer: a summary from the Childhood Cancer Survivor Study. J Clin Oncol 2009;27:2328–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armstrong GT, Chen Y, Yasui Y, Leisenring W, Gibson TM, Mertens AC, Stovall M, Oeffinger KC, Bhatia S, Krull KR, et al. Reduction in late mortality among 5-year survivors of childhood cancer. N Engl J Med 2016;374:833–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Landy DC, Lipsitz SR, Kurtz JM, Hinkle AS, Constine LS, Adams MJ, Lipshultz SE, Miller TL. Dietary quality, caloric intake, and adiposity of childhood cancer survivors and their siblings: an analysis from the cardiac risk factors in childhood cancer survivors study. Nutr Cancer 2013;65:547–55. [DOI] [PubMed] [Google Scholar]
- 6.Robien K, Ness KK, Klesges LM, Baker KS, Gurney JG. Poor adherence to dietary guidelines among adult survivors of childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol 2008;30:815–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tonorezos ES, Robien K, Eshelman-Kent D, Moskowitz CS, Church TS, Ross R, Oeffinger KC. Contribution of diet and physical activity to metabolic parameters among survivors of childhood leukemia. Cancer Causes Control 2013;24:313–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith WA, Li C, Nottage KA, Mulrooney DA, Armstrong GT, Lanctot JQ, Chemaitilly W, Laver JH, Srivastava DK, Robison LL, et al. Lifestyle and metabolic syndrome in adult survivors of childhood cancer: a report from the St. Jude Lifetime Cohort Study. Cancer 2014;120:2742–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hudson MM, Ness KK, Nolan VG, Armstrong GT, Green DM, Morris EB, Spunt SL, Metzger ML, Krull KR, Klosky JL, et al. Prospective medical assessment of adults surviving childhood cancer: study design, cohort characteristics, and feasibility of the St. Jude Lifetime Cohort Study. Pediatr Blood Cancer 2011;56:825–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. Am J Epidemiol 1986;124:453–69. [DOI] [PubMed] [Google Scholar]
- 11.US Department of Agriculture. Composition of foods raw, processed, prepared. USDA National Nutrient Database for Standard Reference. Beltsville (MD): USDA; 2013. [Google Scholar]
- 12.Subar AF, Thompson FE, Kipnis V, Midthune D, Hurwitz P, McNutt S, McIntosh A, Rosenfeld S. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: the Eating at America’s Table Study. Am J Epidemiol 2001;154:1089–99. [DOI] [PubMed] [Google Scholar]
- 13.Block G, Thompson FE, Hartman AM, Larkin FA, Guire KE. Comparison of two dietary questionnaires validated against multiple dietary records collected during a 1-year period. J Am Diet Assoc 1992;92:686–93. [PubMed] [Google Scholar]
- 14.Block G, Woods M, Potosky A, Clifford C. Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epidemiol 1990;43:1327–35. [DOI] [PubMed] [Google Scholar]
- 15.National Cancer Institute. Developing the Healthy Eating Index-2010. [cited 2016 Aug 11]. Available from: http://appliedresearch.cancer.gov/hei/developing.html?&url=/tools/hei/developing.html.
- 16.Guenther PM, Kirkpatrick SI, Reedy J, Krebs-Smith SM, Buckman DW, Dodd KW, Casavale KO, Carroll RJ. The Healthy Eating Index-2010 is a valid and reliable measure of diet quality according to the 2010 Dietary Guidelines for Americans. J Nutr 2014;144:399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.USDA and U.S. Department of Health and Human Services. Appendix 5: Nutritional goals for age-gender groups based on Dietary Reference Intakes and dietary guidelines recommendations. U.S. Government Printing Office. Washington (DC): USDA; 2010. [Google Scholar]
- 18.Institute of Medicine. Dietary Reference Intakes: Estimated Average Requirements and Recommended Intake. Washington (DC): National Academies Press; 2002. [Google Scholar]
- 19.Vittinghoff E, Shiboski SC, Glidden DV, McCulloch CE. Regression methods in biostatistics. New York: Springer; 2005. [Google Scholar]
- 20.Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology 2009;20:488–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.CDC. Physical activity guidelines [cited 2014 Oct 1]. Available from: http://www.health.gov/paguidelines/.
- 22.Zhang FF, Rodday AM, Kelly MJ, Must A, Macpherson C, Roberts SB, Saltzman E, Parsons SK. Predictors of being overweight or obese in survivors of pediatric acute lymphoblastic leukemia (ALL). Pediatr Blood Cancer 2014;61:1263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Armstrong GT, Kawashima T, Leisenring W, Stratton K, Stovall M, Hudson MM, Sklar CA, Robison LL, Oeffinger KC. Aging and risk of severe, disabling, life-threatening, and fatal events in the Childhood Cancer Survivor Study. J Clin Oncol 2014;32:1218–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang FF, Saltzman E, Kelly MJ, Liu S, Must A, Parsons SK, Roberts SB. Comparison of childhood cancer survivors’ nutritional intake with US dietary guidelines. Pediatr Blood Cancer 2015;62:1461–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.George SM, Irwin ML, Smith AW, Neuhouser ML, Reedy J, McTiernan A, Alfano CM, Bernstein L, Ulrich CM, Baumgartner KB, et al. Postdiagnosis diet quality, the combination of diet quality and recreational physical activity, and prognosis after early-stage breast cancer. Cancer Causes Control 2011;22:589–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Humpel N, Magee C, Jones SC. The impact of a cancer diagnosis on the health behaviors of cancer survivors and their family and friends. Support Care Cancer 2007;15:621–30. [DOI] [PubMed] [Google Scholar]
- 27.Thomson CA, Flatt SW, Rock CL, Ritenbaugh C, Newman V, Pierce JP. Increased fruit, vegetable and fiber intake and lower fat intake reported among women previously treated for invasive breast cancer. J Am Diet Assoc 2002;102:801–8. [DOI] [PubMed] [Google Scholar]
- 28.Zhang FF, Liu S, John EM, Must A, Demark-Wahnefried W. Diet quality of cancer survivors and noncancer individuals: results from a national survey. Cancer 2015;121:4212–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rothman KJ, Greeland S, Lash TL. Modern epidemiology. 3rd ed. Philadelphia: Lippincott Williams, & Wilkins; 2008. [Google Scholar]
- 30.George SM, Alfano CM, Neuhouser ML, Smith AW, Baumgartner RN, Baumgartner KB, Bernstein L, Ballard-Barbash R. Better postdiagnosis diet quality is associated with less cancer-related fatigue in breast cancer survivors. J Cancer Surviv 2014;8:680–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patterson RE, Neuhouser ML, Hedderson MM, Schwartz SM, Standish LJ, Bowen DJ. Changes in diet, physical activity, and supplement use among adults diagnosed with cancer. J Am Diet Assoc 2003;103:323–8. [DOI] [PubMed] [Google Scholar]
- 32.Coups EJ, Ostroff JS. A population-based estimate of the prevalence of behavioral risk factors among adult cancer survivors and noncancer controls. Prev Med 2005;40:702–11. [DOI] [PubMed] [Google Scholar]
- 33.Jansen H, Postma A, Stolk RP, Kamps WA. Acute lymphoblastic leukemia and obesity: increased energy intake or decreased physical activity? Support Care Cancer 2009;17:103–6. [DOI] [PubMed] [Google Scholar]
- 34.Reilly JJ, Brougham M, Montgomery C, Richardson F, Kelly A, Gibson BE. Effect of glucocorticoid therapy on energy intake in children treated for acute lymphoblastic leukemia. J Clin Endocrinol Metab 2001;86:3742–5. [DOI] [PubMed] [Google Scholar]
- 35.Oeffinger KC, Mertens AC, Sklar CA, Yasui Y, Fears T, Stovall M, Vik TA, Inskip PD, Robison LL. Obesity in adult survivors of childhood acute lymphoblastic leukemia: a report from the Childhood Cancer Survivor Study. J Clin Oncol 2003;21:1359–65. [DOI] [PubMed] [Google Scholar]
- 36.Samaan MC, Thabane L, Burrow S, Dillenburg RF, Scheinemann K. Canadian Study of Determinants of Endometabolic Health in ChIlDrEn (CanDECIDE study): a cohort study protocol examining the mechanisms of obesity in survivors of childhood brain tumours. BMJ Open 2013;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sklar CA, Mertens AC, Walter A, Mitchell D, Nesbit ME, O’Leary M, Hutchinson R, Meadows AT, Robison LL. Changes in body mass index and prevalence of overweight in survivors of childhood acute lymphoblastic leukemia: role of cranial irradiation. Med Pediatr Oncol 2000;35:91–5. [DOI] [PubMed] [Google Scholar]
- 38.von Deneen KM, Liu Y. Obesity as an addiction: why do the obese eat more? Maturitas 2011;68:342–5. [DOI] [PubMed] [Google Scholar]
- 39.Murphy KG, Bloom SR. Gut hormones and the regulation of energy homeostasis. Nature 2006;444:854–9. [DOI] [PubMed] [Google Scholar]
- 40.Cohen J, Laing DG, Wilkes FJ, Chan A, Gabriel M, Cohn RJ. Taste and smell dysfunction in childhood cancer survivors. Appetite 2014;75:135–40. [DOI] [PubMed] [Google Scholar]
- 41.Skolin I, Wahlin YB, Broman DA, Koivisto Hursti UK, Vikstrom Larsson M, Hernell O. Altered food intake and taste perception in children with cancer after start of chemotherapy: perspectives of children, parents and nurses. Support Care Cancer . 2006;14:369–78. [DOI] [PubMed] [Google Scholar]
- 42.Meacham LR, Sklar CA, Li S, Liu Q, Gimpel N, Yasui Y, Whitton JA, Stovall M, Robison LL, Oeffinger KC. Diabetes mellitus in long-term survivors of childhood cancer—ncreased risk associated with radiation therapy: a report for the Childhood Cancer Survivor Study. Arch Intern Med 2009;169:1381–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stacey R, Green JT. Radiation-induced small bowel disease: latest developments and clinical guidance. Ther Adv Chronic Dis 2014;5:15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ciorba MA, Stenson WF. Probiotic therapy in radiation-induced intestinal injury and repair. Ann N Y Acad Sci 2009;1165:190–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Packey CD, Ciorba MA. Microbial influences on the small intestinal response to radiation injury. Curr Opin Gastroenterol 2010;26:88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guarner F, Malagelada JR. Gut flora in health and disease. Lancet 2003;361:512–9. [DOI] [PubMed] [Google Scholar]
- 47.Taur Y, Jenq RR, Perales MA, Littmann ER, Morjaria S, Ling L, No D, Gobourne A, Viale A, Dahi PB, et al. The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood 2014;124:1174–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alcock J, Maley CC, Aktipis CA. Is eating behavior manipulated by the gastrointestinal microbiota? Evolutionary pressures and potential mechanisms. BioEssays 2014;36:940–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patrick H, Nicklas TA. A review of family and social determinants of children’s eating patterns and diet quality. J Am Coll Nutr 2005;24:83–92. [DOI] [PubMed] [Google Scholar]
- 50.Stern M, Lamana L, Russell C, Edwin L, Thompson A, Trapp S, Bitsko M, Mazzeo S. Adaptation of an obesity intervention program for pediatric cancer survivors (NOURISH-T). Clin Pract Pediatr Psychol 2013;1:264–75. [Google Scholar]
- 51.Willett WC. Nutritional epidemiology. New York: Oxford University Press; 1998. [Google Scholar]
- 52.Ojha RP, Oancea SC, Ness KK, Lanctot JQ, Srivastava DK, Robison LL, Hudson MM, Gurney JG. Assessment of potential bias from non-participation in a dynamic clinical cohort of long-term childhood cancer survivors: results from the St. Jude Lifetime Cohort Study. Pediatr Blood Cancer 2013;60:856–64. [DOI] [PMC free article] [PubMed] [Google Scholar]