Abstract
Background: Mortality in children with severe acute malnutrition (SAM) remains high despite standardized rehabilitation protocols. Two forms of SAM are classically distinguished: kwashiorkor and marasmus. Children with kwashiorkor have nutritional edema and metabolic disturbances, including hypoalbuminemia and hepatic steatosis, whereas marasmus is characterized by severe wasting. The metabolic changes underlying these phenotypes have been poorly characterized, and whether homeostasis is achieved during hospital stay is unclear.
Objectives: We aimed to characterize metabolic differences between children with marasmus and kwashiorkor at hospital admission and after clinical stabilization and to compare them with stunted and nonstunted community controls.
Methods: We studied children aged 9–59 mo from Malawi who were hospitalized with SAM (n = 40; 21 with kwashiorkor and 19 with marasmus) or living in the community (n = 157; 78 stunted and 79 nonstunted). Serum from patients with SAM was obtained at hospital admission and 3 d after nutritional stabilization and from community controls. With the use of targeted metabolomics, 141 metabolites, including amino acids, biogenic amines, acylcarnitines, sphingomyelins, and phosphatidylcholines, were measured.
Results: At admission, most metabolites (128 of 141; 91%) were lower in children with kwashiorkor than in those with marasmus, with significant differences in several amino acids and biogenic amines, including those of the kynurenine-tryptophan pathway. Several phosphatidylcholines and some acylcarnitines also differed. Patients with SAM had profiles that were profoundly different from those of stunted and nonstunted controls, even after clinical stabilization. Amino acids and biogenic amines generally improved with nutritional rehabilitation, but most sphingomyelins and phosphatidylcholines did not.
Conclusions: Children with kwashiorkor were metabolically distinct from those with marasmus, and were more prone to severe metabolic disruptions. Children with SAM showed metabolic profiles that were profoundly different from stunted and nonstunted controls, even after clinical stabilization. Therefore, metabolic recovery in children with SAM likely extends beyond discharge, which may explain the poor long-term outcomes in these children. This trial was registered at isrctn.org as ISRCTN13916953.
Keywords: marasmus, kwashiorkor, children, severe acute malnutrition, targeted metabolomics, metabolites, p180 kit
Introduction
Severe acute malnutrition (SAM)17 remains a substantial global health problem. Each year, SAM affects >18 million children, most living in low-income settings. SAM contributes to ∼45% of all deaths in children <5 y of age worldwide (1). Even in nutritional rehabilitation centers that follow WHO-established treatment protocols, in-hospital mortality remains high, ranging from 10% to 30% of all treated cases (2). Postdischarge mortality is also significant, with studies reporting mortality rates of 8.7% within 3 mo of discharge (3), or ≤18% of all treated cases dying within 1 y of discharge (4). Furthermore, even if the children survive, they suffer from long-term adverse effects, showing patterns of thrifty growth and functional deficits such as weak hand grip and impaired cardiovascular capacity (5). Children with SAM also show impaired cognitive function and substantial attention deficits (6, 7).
Two clinical phenotypes of SAM are classically recognized: marasmus and kwashiorkor. Whereas marasmus is characterized by severe wasting, kwashiorkor presents with a range of clinical signs, including nutritionally induced bilateral pitting edema, loss of hair pigmentation, skin lesions, hypoalbuminemia, and hepatic steatosis (8, 9). Historically, kwashiorkor was associated with higher mortality, but case fatalities have shifted toward marasmus (10). This change in mortality rates has been attributed to an increased prevalence of comorbidities, including HIV and pneumonia, in marasmic patients (11).
The pathophysiologic mechanisms underlying the development of SAM and its major clinical phenotypes are poorly understood, and current literature examining the metabolic changes in SAM is limited. However, some studies have shown that SAM is associated with altered protein, glucose, and lipid metabolism (12–15). To our knowledge, the only comprehensive metabolomics analysis of children with SAM was conducted by Bartz et al. (16) in 2014. This analysis showed that SAM induced significant changes in acylcarnitines, inflammatory cytokines, FAs, amino acids, and hormones related to appetite and energy metabolism. However, they lacked a control group, and the number of metabolites analyzed was limited.
Metabolic dysregulations such as hypoglycemia, impaired gluconeogenesis, or disrupted amino acid or lipid metabolism could underlie the different clinical manifestations of SAM and explain the differences in mortality. Our objective was to better understand the metabolic disturbances associated with malnutrition. Therefore, we used large-scale targeted metabolomics to examine the metabolite profiles in the serum of children with either kwashiorkor or marasmus and compared them with community controls.
Methods
Study population
This observational study (ISRCTN 13916953) examined children aged 9–59 mo either undergoing in-patient treatment for SAM (n = 40) at the Queen Elizabeth Central Hospital in Blantyre or living in rural communities (n = 157) in southern Malawi.
In-patients with SAM.
Children with SAM (n = 40; 21 with kwashiorkor and 19 with marasmus) were recruited from the Nutritional Rehabilitation Unit at the Queen Elizabeth Central Hospital in Blantyre, the largest hospital in Malawi. All children admitted to this unit had medical complications or danger signs as defined by the current WHO guidelines (2), including anorexia, vomiting, convulsions, and loss of consciousness. Cases of uncomplicated SAM were treated as outpatients and not included in this study. Children were diagnosed with kwashiorkor if they showed moderate or severe nutritional bilateral pitting edema and other phenotypic changes, such as hair and skin abnormalities. Edema was graded as mild (+) when only in the feet; moderate (++) when in the feet, legs, and lower arms; and severe (+++) when visible on the upper arm and/or face. Marasmus was defined as having a weight-for-height z score calculated from WHO growth standards >3 SD below the median and/or having a midupper arm circumference <115 mm. Five children had a mixed marasmic-kwashiorkor phenotype, with both edema and a midupper arm circumference <115 mm. For analysis, these children were included in the kwashiorkor group, because results did not differ when they were excluded.
Recorded clinical data included anthropometric measurements, appetite, HIV reactivity (or exposure in children <18 mo of age), time-to-nutritional stabilization, and duration of hospital stay. All children with SAM participated in the TranSAM clinical trial, which evaluated 3 common refeeding diets [i.e., F100, ready-to-use food (RUTF), or both RUTF and F100]. These diets were isocaloric but varied in their composition of carbohydrates and fat ratios. The diets were used during the rehabilitation (poststabilization) phase of treatment. Children with SAM were randomly assigned and stratified by HIV status. They were excluded if they died before nutritional stabilization, were diagnosed with kwashiorkor but had only mild edema, had confirmed or suspected malaria and/or tuberculosis, or had insufficient serum for analysis. Informed consent was obtained from legal guardians, and the study was approved by the University of Malawi College of Medicine Research and Ethics Committee, and by the Hospital for Sick Children in Toronto, Canada.
Community controls.
The community controls (n = 157) were recruited from 6 villages in Malawi as previously described (17). Children were from families who primarily relied on subsistence farming, which is highly vulnerable to climatic disruptions. The typical diet of these children is mainly composed of cereals (i.e., maize) and starchy fruits (plantain) and roots (cassava and potatoes), and is low in protein, especially from animal sources. Enrolled children were weighed and measured by experienced field workers; stunted children were identified as having a height-for-age z score of 2 SD below the median, as calculated from WHO growth standards. Children were enrolled if they did not have SAM, congenital or chronic disease, or recent diarrhea. Controls were age- and sex-matched with the in-patient SAM cohort with the use of an automated function implemented in the R package MatchIt (18). HIV status was not tested in the community controls, but these children were asymptomatic and thought to be negative. For children recruited from the community, informed consent was obtained from legal guardians, and the study was approved by the University of Malawi College of Medicine Research and Ethics Committee, by the Human Research Protection Office of Washington University in St. Louis, and by the John Hopkins School of Medicine Institutional Review Board.
Serum collection and metabolite measurements
For in-patients with SAM, blood samples were collected at admission within 24 h of enrollment and 3 d after clinical stabilization as defined by WHO criteria (19). Clinically stable children were transitioned from a standard F-75 diet to the F-100 and/or RUTF; thus, the point of clinical stabilization coincided with diet transition. Children did not fast before blood collection, but at admission, children with SAM can suffer from anorexia and may be in a fasting state. After collection, blood was centrifuged at 2180 × g for 10 min at 4°C and the serum obtained was stored at −80°C until further analysis. For metabolomics, samples were analyzed with the use of LC tandem MS and the AbsoluteIDQ p180 Kit (Biocrates Life Sciences). This analytic method conforms with the FDA guideline Guidance for Industry—Bioanalytical Method Validation (May 2001), and is reproducible and comparable across analytic sites (20). Supplemental Tables 1–8 list all metabolites (n = 188) and other markers (n = 5, Supplemental Table 8) that were targeted originally. These include the following compound classes: sugars (i.e., the sum of all hexoses including glucose, Supplemental Table 8); amino acids (n = 21, Supplemental Table 1); biogenic amines (n = 21, Supplemental Table 2); sphingomyelins (n = 15, Supplemental Table 3); acylcarnitines (n = 40, Supplemental Table 4); and phosphatidylcholines (n = 90), including lysophosphatidylcholine (n = 14, Supplemental Table 5), phosphatidylcholine acyl-alkyls (n = 38, Supplemental Table 6), and phosphatidylcholine diacyl (n = 38, Supplemental Table 7). In total, 141 metabolites passed quality control cutoffs (i.e., had a mean CV <25% across different experimental batches, had <10% missing values, and had a median value greater than or equal to the lower limit of quantification in ≥1 study group). Additional markers, including albumin and electrolytes (i.e., potassium, sodium, phosphate, and magnesium, Supplemental Table 8), were measured by routine clinical laboratory platforms in patients with SAM but not in community controls.
Statistical analysis
To assess differences in metabolite profiles, we conducted both univariate and partial least-squares multivariate analyses. Sample outliers were assessed by principal component analysis and removed (n = 2; a marasmic sample at admission and a stunted control). Concentrations below the lower limit of detection were set at 0, and missing values were imputed with the use of bagImpute implemented in the R package caret (21). Values were offset by 1, log transformed, mean centered, and scaled. Partial least-squares discriminative analysis (PLS-DA) was conducted on age- and sex-corrected values with the mixOmics package (22). Multilevel PLS-DA was performed between admission and nutritional stabilization to account for repeated measures. The predictive power of the partial least-squares components to discriminate between groups was assessed by classification error rates on the basis of the maximum distance obtained from 10-fold crossvalidation. This was run iteratively 10 times, and the mean classification error rate for left-out cases was calculated. Sparse PLS-DA was used to select the 10 features that best distinguish patients with kwashiorkor or marasmus at admission. This method can identify the most robust and stable discriminating variables, which were considered to be those selected in the top 10 discriminative features >80% of the time upon crossvalidation, and had a variable importance in projection score ≥1. Variable importance in projection is an estimate of the importance of each variable in the projection of the PLS-DA model and is used for variable selection. Mixed-effects models were developed on log-transformed and scaled variables to assess differences in children with or without edema at admission and after clinical stabilization while accounting for age, sex, and repeated measures (i.e., patient IDs were included as random factors). Linear regression models were used to compare samples that did not include repeated measures (i.e., when not comparing admission with after clinical stabilization). To control for multiple testing, P values were adjusted with the use of false discovery rate (FDR). Dendrograms reflecting Pearson-based hierarchal clustering of all metabolite concentrations obtained from patients with SAM at admission and nutritional stabilization were created to order the presented SEs and SEMs obtained from group comparison. Scaled estimates and SEMs allow for the comparison of effect sizes across different metabolites. R software (version 3.2.3) was used for all analysis, and figures were generated with the use of the ggplot2 package and Inkscape.
Results
The characteristics of the children with SAM who had either marasmus or kwashiorkor, as well as those of the stunted or nonstunted community controls, are presented in Table 1. Marasmic children tended to be younger than those in the other studied groups. Patients with kwashiorkor or marasmus had a similar time to stabilization and length of hospital stay.
TABLE 1.
Characteristics of patients hospitalized for SAM, including marasmus and kwashiorkor, and of stunted and nonstunted community controls1
| SAM |
Community controls |
|||||
| All (n = 39) | Kwashiorkor2 (n = 21) | Marasmus (n = 19) | All (n = 157) | Stunted (n = 78) | Nonstunted (n = 79) | |
| Female | 21 (54) | 12 (57) | 10 (53) | 91 (58) | 45 (58) | 46 (58) |
| HIV reactive | 19 (49) | 9 (43) | 10 (53) | — | — | — |
| Age, mo | 26.8 ± 13.0 | 30.8 ± 13.6 | 21.4 ± 10.9 | 29.4 ± 11.8 | 30.9 ± 11 | 27.8 ± 12.5 |
| Length, cm | 76.7 ± 8.9 | 80.8 ± 7.7 | 71.4 ± 7.7 | 83.3 ± 7.9 | 81.2 ± 6.4 | 85.4 ± 8.7 |
| Height-for-age z score | −3.5 ± 1.9 | −2.9 ± 1.9 | −4.1 ± 1.7 | −2.0 ± 1.3 | −3 ± 0.9 | −1.0 ± 0.9 |
| Weight-for-age z score | −3.9 ± 1.8 | −2.8 ± 1.5 | −5.1 ± 1.0 | −1.0 ± 1.0 | −1.5 ± 1.0 | −0.45 ± 0.8 |
| Weight-for-height z score | −2.9 ± 1.9 | −1.7 ± 1.6 | −4.4 ± 0.9 | 0.2 ± 0.9 | 0.25 ± 1.0 | −0.14 ± 0.7 |
| MUAC, cm | 11.6 ± 1.8 | 12.7 ± 1.6 | 10.2 ± 1.0 | — | — | — |
| Time to clinical stabilization,3 d | 3 [2–4] | 3 [2–4] | 3 [2–4.5] | — | — | — |
| Duration of stay, d | 9 [9–14] | 9 [9–14] | 9 [8–14] | — | — | — |
Values are n (%), means ± SDs, or medians [IQRs]. MUAC, midupper arm circumference; SAM, severe acute malnutrition.
Patients with kwashiorkor had either moderate or severe edema as detailed in methods.
Clinical stabilization coincides with diet transition (i.e., when children are transitioned from the F75 diet to the F100 diet and/or ready-to-use food).
Children with different forms of SAM were metabolically distinct from community controls.
Metabolic profiles differed between children with kwashiorkor and those with marasmus at admission, and both SAM phenotypes were profoundly different metabolically from community controls. Mean concentrations and SDs of all 141 metabolites measured with targeted LC tandem MS are presented stratified by group; differences in metabolite concentrations between groups are detailed in Supplemental Tables 9–26. These include amino acids (Supplemental Tables 9 and 10), biogenic amines (Supplemental Tables 11 and 12), sphingomyelins (Supplemental Tables 13 and 14), acylcarnitines (Supplemental Tables 15 and 16), lysophosphatidylcholines (Supplemental Tables 17 and 18), phosphatidylcholine acyl alkyls (Supplemental Tables 19 and 20), phosphatidylcholine diacyls (Supplemental Tables 21 and 22), other markers (Supplemental Tables 23 and 24), and summary values and metabolite ratios (Supplemental Tables 25 and 26).
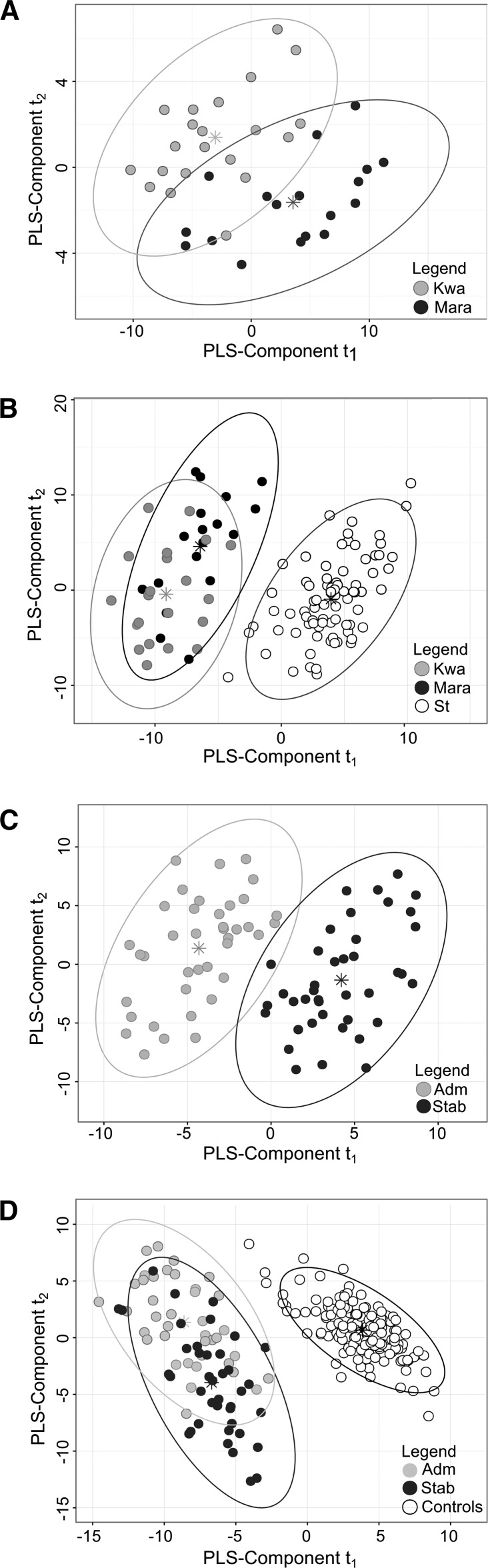
PLS-DA was used to distinguish children with kwashiorkor from those with marasmus on the basis of metabolites measured in serum obtained at hospital admission (Figure 1A). Although children with kwashiorkor or marasmus formed distinct clusters, the classification error rate based on maximum distance was still 30%. After nutritional stabilization, these children were indistinguishable on the basis of their circulating metabolites, with the mean classification error rate being 44%, which is not significantly better than chance (Supplemental Figure 1). Therefore, after nutritional stabilization, children with kwashiorkor and marasmus had similar metabolic profiles (Supplemental Figure 2). We then compared groups of children with either kwashiorkor or marasmus at admission to stunted community controls. As expected, these children were profoundly different metabolically from stunted children (Figure 1B). To assess metabolic recovery in children with SAM, we compared their metabolic profiles at admission with their profiles after nutritional stabilization (Figure 1C). Samples obtained before and after nutritional stabilization could be readily distinguished with the use of PLS-DA with a mean classification error rate of 6.9%. Finally, we found that patients with SAM had metabolic profiles that were significantly different from those of community controls both at admission and after clinical stabilization (Figure 1D). Thus, metabolic disturbances lingered in the children with SAM even after achieving nutritional stabilization.
FIGURE 1.
PLS-DA t1 compared with t2 score plots derived from the analysis to distinguish children with kwashiorkor from those with marasmus at admission (A), children with kwashiorkor or marasmus at admission from stunted community controls (B), patients with SAM at admission from patients after clinical stabilization (C), and patients with SAM at admission and after nutritional stabilization from community controls (including both stunted and nonstunted children) (D). PLS-DA analysis was performed on age- and sex-adjusted concentrations of metabolites and additional markers. These were measured in the serum of children with SAM at hospital admission and after nutritional stabilization or in the serum of stunted and nonstunted community controls. Multilevel PLS-DA analysis was used to compare children at admission with those at discharge, which accounts for repeated measures within patients. Asterisks are the centroids of each group. The 2 main PLS-DA components that capture the variability relating to group classification are represented by t1 and t2 in each panel. Adm, admission; Kwa, kwashiorkor; Mara, marasmus; PLS, partial least-squares; PLS-DA, partial least-squares discriminative analysis; SAM, severe acute malnutrition; Stab, stabilization; St, stunted.
Specific metabolite differences between children with SAM and community controls.
We then evaluated the specific metabolites that differed between 1) children with SAM who had either kwashiorkor or marasmus, 2) children with SAM either at admission or after nutritional stabilization, and 3) children with SAM after stabilization and community controls (Figures 2 and 3 and Supplemental Tables 9–26).
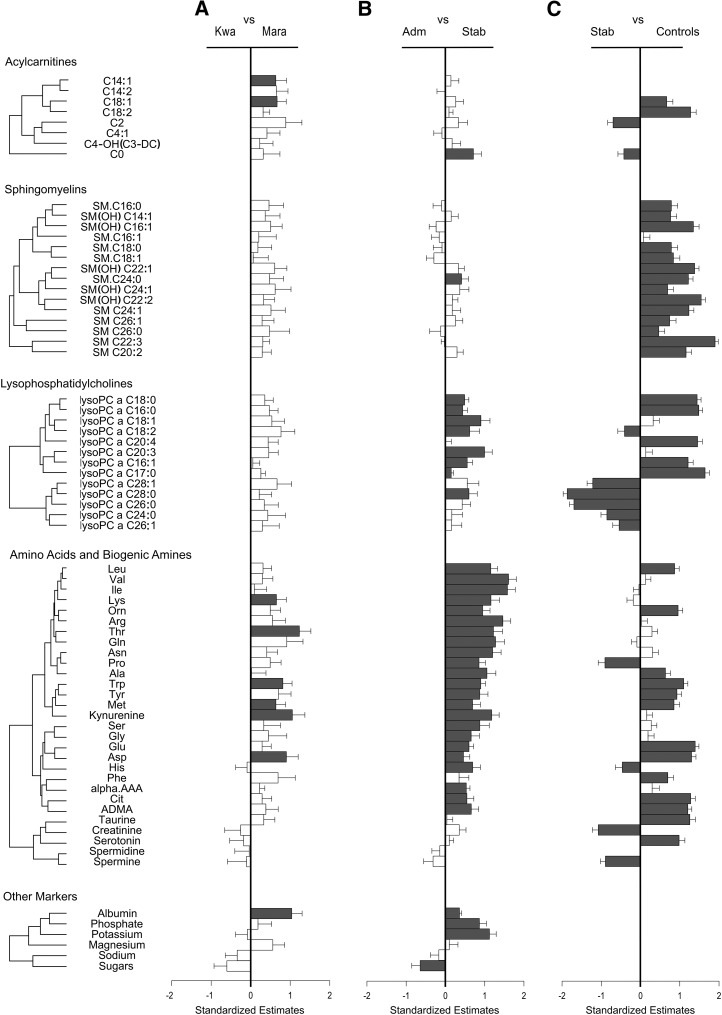
FIGURE 2.
Serum metabolites of acylcarnitines, sphingomyelins, lysophosphatidylcholines, amino acids, and biogenic amines, as well as other markers, were compared between children with kwashiorkor compared with marasmus (A), children with SAM at admission compared with after clinical stabilization (B), and children after nutritional stabilization compared with community controls (including both stunted and nonstunted children) (C). SEs and SEMs were obtained from either mixed linear regression models that accounted for age, sex, and repeated measures (i.e., when comparing children at admission with after nutritional stabilization) or linear regression models adjusted for age and sex. Group numbers are as follows: kwashiorkor, n = 21; marasmus, n = 18; admission, n = 39; stabilization, n = 40; community controls, n = 157. Metabolites are ordered on the basis of Pearson hierarchal clustering of all metabolites within each compound class from patients with SAM at admission and after nutritional stabilization, and this clustering is represented by the dendrograms. Scaled estimates and SEMs allowed for the comparison of effect sizes across different metabolites. The direction of the bar indicates which diagnostic group had higher metabolite concentrations, and the metabolites that reached false discovery rate–adjusted significance are labeled in dark gray. All metabolite abbreviations are detailed in Supplemental Tables 1–8. Adm, admission; Kwa, kwashiorkor; Mara, marasmus; Stab, stabilization.
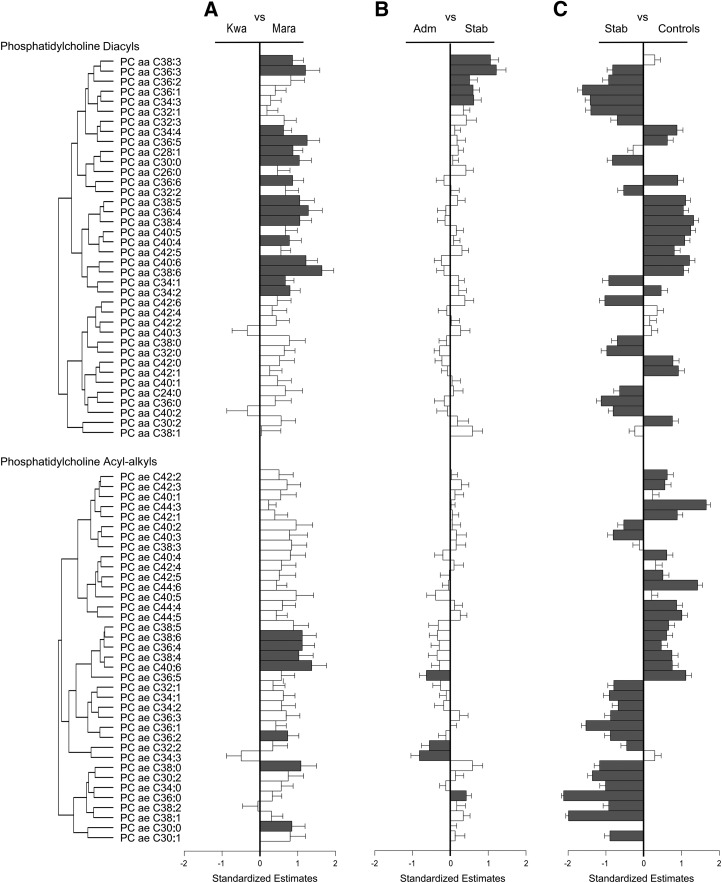
FIGURE 3.
Serum metabolites of phosphatidylcholine diacyls and acyl-alkyls were compared between children with kwashiorkor and those with marasmus (A), children with SAM at admission and children after clinical stabilization (B) and children after nutritional stabilization and community controls (including both stunted and nonstunted children) (C). Standardized estimates and SEs were obtained from either mixed linear regression models that accounted for age, sex, and repeated measures (i.e., when comparing patients at admission and after nutritional stabilization) or linear regression models adjusted for age and sex. Group numbers are as follows: kwashiorkor, n = 21; marasmus, n = 18; admission, n = 39; stabilization, n = 40; community controls, n = 157. Metabolites are ordered on the basis of Pearson hierarchal clustering of all metabolites within each compound class from patients with SAM at admission and after nutritional stabilization, and this clustering is represented by the dendrograms. Scaled estimates and SEMs allowed for the comparison of effect sizes across different metabolites. The direction of the bar indicates which diagnostic group had higher metabolite concentrations, and the metabolites that reached false discovery rate–adjusted significance are labeled in dark gray. All metabolite abbreviations are detailed in Supplemental Tables 1–8. Adm, admission; Kwa, kwashiorkor; Mara, marasmus; SAM, severe acute malnutrition; Stab, stabilization.
When children with kwashiorkor were compared with those with marasmus, we found that at admission most metabolites (128 of 141; 91%) were lower in those with kwashiorkor; although only 31 of these achieved statistical significance (Figure 2A and 3A). Almost all amino acids were lower in those with kwashiorkor at admission, and essential amino acids were significantly more depleted (mean ± SD: 405 ± 133 μmol/L in those with kwashiorkor compared with 521 ± 148 μmol/L in those with marasmus; FDR-adjusted P < 0.02). Specific differences were found in lysine (mean ± SD: 97 ± 26 μmol/L in those with kwashiorkor compared with 132 ± 48 μmol/L in those with marasmus; FDR-adjusted P < 0.03), threonine (mean ± SD: 36 ± 16 μmol/L in those with kwashiorkor compared with 70 ± 34 μmol/L in those with marasmus; FDR-adjusted P < 0.0004), methionine (mean ± SD: 10.7 ± 3.6 μmol/L in those with kwashiorkor compared with 15.2 ± 5.1 μmol/L in those with marasmus; FDR-adjusted P < 0.03), aspartic acid (mean ± SD: 15.6 ± 10.2 μmol/L in those with kwashiorkor compared with 25.6 ± 14.1 μmol/L in those with marasmus; FDR-adjusted P < 0.009) and tryptophan (mean ± SD: 4.9 ± 3.7 μmol/L in those with kwashiorkor compared with 12.4 ± 11.0 μmol/L in those with marasmus; FDR-adjusted P < 0.003), along with its derivative, the biogenic amine kynurenine (0.91 ± 0.81 μmol/L in those with kwashiorkor compared with 2.21 ± 1.58 μmol/L in those with marasmus; FDR-adjusted P = 0.004) (Figure 2A). However, the kynurenine-to-tryptophan ratio did not differ, with both metabolites proportionally lower in patients with kwashiorkor. Also, serotonin was not different between groups, nor was the serotonin-to-tryptophan ratio (Supplemental Tables 25 and 26). Electrolyte concentrations were similar between groups. However, those with kwashiorkor did have lower circulating albumin (Figure 2A and Supplemental Tables 23 and 24).
Apart from these differences in amino acids and biogenic amines, many other metabolites were significantly lower in children with kwashiorkor. These included 7 phosphatidylcholine acyl-alkyls (PC ae C40:6, PC ae 38:6, PC ae C38:4, PC ae C38:0, PC ae C36:4, PC ae C36:2, and PC ae C30:0), 15 phosphatidylcholine diacyls (PC aa C38:3, PC aa C36:3, PC aa C34:4, PC aa C36:5, PC aa C28:1, PC aa C30:0, PC aa C36:6, PC aa C38:5, PC aa C36:4, PC aa C38:4, PC aa C40:4, PC aa C40:6, PC aa C38:6, PC aa C34:1, and PC aa C34:2), and 2 acylcarnitines (C14:1 and C18:1) (Figures 2A and 3A and Supplemental Tables 15 and 16 and 19–22). The ratio of acetylcarnitine to free carnitine can be used as a measure of β oxidation of even-numbered FAs and was found to be lower in kwashiorkor patients (mean ± SD: 0.61 ± 0.57 in children with marasmus compared with 0.27 ± 0.18 in children with kwashiorkor) (Supplemental Tables 23 and 24).
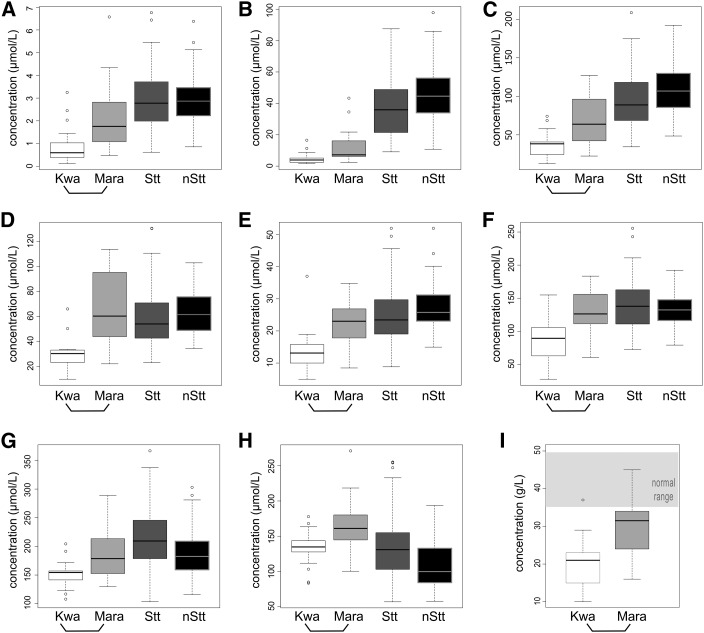
Finally, we used sparse PLS-DA to select the top discriminating metabolites that best distinguish between patients with kwashiorkor and marasmus at admission. With the use of 10-fold crossvalidation, 9 metabolites were robustly and stably (>80%) selected as being in the top 10 distinguishing features. The metabolites were tryptophan, kynurenine, threonine, PC aa C36:4, PC aa C38:6, PC aa C40:6, PC aa C34:1, PC aa C34:2, and albumin (Figure 4 and Supplemental Table 27). These metabolites were specifically lower in kwashiorkor patients, whereas marasmus patients had concentrations that were either intermediate or similar to those of controls.
FIGURE 4.
Concentrations of serum metabolites that most robustly discriminate between children with kwashiorkor and those with marasmus at admission as identified by sparse partial least-squares discriminative analysis. These include the biogenic amines and amino acids kynurenine (A), tryptophan (B) and threonine (C), with the 5 phosphatidylcholine diacyls PC aa C38:6 (D), PC aa C40:6 (E), PC aa C36:4 (F), PC aa C34:2 (G), and PC aa C34:1 (H), in addition to albumin (I). Boxplots summarize the median (midline) and IQR (upper and lower box). Open circles beyond boxplot whiskers indicate outlying data points. Numbers in groups are as follows: kwashiorkor, n = 21; marasmus, n = 18; stunted, n = 78 community controls; and nonstunted, n = 79 community controls. The bottom bracket indicates significant differences between kwashiorkor and marasmus at admission as obtained by both partial least-squares discriminative analysis (variable importance in projection score ≥1 and feature stability ≥80%) and linear regression (false discovery rate–adjusted P values < 0.05) models. Albumin was not measured in community controls; the light-gray zone indicates the normal clinical range of circulating albumin in children. All metabolite abbreviations are detailed in Supplemental Tables 1–8. Kwa, kwashiorkor; Mara, marasmus; nStt, nonstunted; Stt, stunted.
Metabolite differences in children with SAM at admission compared with after nutritional stabilization.
Two main clusters of correlated metabolites increased with nutritional stabilization (Figures 2B and 3B). The first cluster (n = 23) mainly was composed of amino acids (leucine, valine, isoleucine, lysine, ornithine, arginine, threonine, glutamine, asparagine, proline, alanine, tryptophan, tyrosine, methionine, serine, glycine, glutamic acid, aspartic acid, histidine, and citrulline) and the biogenic amines kynurenine, α-aminoadipic acid, and asymmetric dimethylarginine. The sum of all amino acids, including that of essential amino acids, was increased in patients with SAM after stabilization (mean ± SD: 2000 ± 740 μmol/L at admission compared with 3190 ± 1160 μmol/L after stabilization; FDR-adjusted P < 0.0001) (Supplemental Figure 3 and Supplemental Tables 25 and 26). This increase in circulating amino acids and biogenic amines could suggest the restoration of proteogenic capacity and nitrogen homeostasis, but may also reflect other processes (e.g., this could indicate increased demand for these metabolites). However, the achieved concentrations of amino acids and biogenic amines were significantly lower than those of controls. The kynurenine-to-tryptophan ratio did not differ between admission and after stabilization, with both metabolites increasing proportionally, although this ratio was higher than in controls (Supplemental Tables 25 and 26). The serotonin-to-tryptophan ratio was significantly decreased after stabilization, because serotonin was low at admission and stayed low after stabilization (Supplemental Tables 25 and 26). There was also an increase in circulating albumin, phosphate, and potassium, whereas the total sum of hexoses (i.e., total sugars) decreased after nutritional stabilization (Figure 2B). Furthermore, several phosphatidylcholines were significantly higher in patients with SAM after nutritional stabilization (Figure 3B). These included lysophosphatidylcholines (lysoPC a C18:0, lysoPC a C16:0, lysoPC a C18:1, lysoPC a C18:2, lysoPC a C20:3, lysoPC a C16:1, lysoPC a C17:0, and lysoPC a C28:0) and phosphatidylcholine diacyls (PC aa C38:3, PC aa C36:3, PC aa C36:2, PC aa C36:1, and PC aa C34:3). Of the acylcarnitines and sphingomyelins, only C0 and SM C24:0 were significantly higher.
However, other metabolites were found to be significantly higher in patients after nutritional stabilization than in community controls (Figure 2A and B). These included proline; histidine; creatinine; spermine; the acylcarnitines C2 and C0; the lysophosphatidylcholines lysoPC a C18:2, lysoPC a C24:0, lysoPC a C26:0, lysoPC a C26:1, lysoPC a C28:0, and lysoPC a C28:1; and several phosphatidylcholine diacyls (15 of 38) and acyl-alkyls (16 of 38).
In fact, after nutritional stabilization, 81% of metabolites in children with SAM were still significantly different from those of controls (i.e., 107 of the 132 metabolites available in controls) (Figures 2C and 3C and Supplemental Tables 9–26). These included amino acids (12 of 21), biogenic amines (5 of 7), acylcarnitines (4 of 4), and sphingomyelins (14 of 15), as well as lysophosphatidylcholine (11 of 13), phosphatidylcholine diacyls (30 of 36) and acyl-alkyls (31 of 37). The large number of metabolites that did not return to concentrations seen in community controls is especially striking. Therefore, children with SAM that were stabilized were still metabolically very different from community controls, even when they were compared exclusively to stunted children.
Discussion
Despite the fact that SAM is a major contributor to childhood mortality in low-income countries, the etiology and progression of the metabolic disturbances associated with acute malnutrition are not well understood. Here, we used targeted metabolomics to measure circulating metabolites in the serum of severely malnourished children treated in a nutritional rehabilitation center. Our aims were as follows: 1) to identify metabolic signatures that could distinguish patients with kwashiorkor from those with marasmus, 2) to compare their profiles with those of community controls, and 3) to identify the metabolic pathways that respond to nutritional rehabilitation.
Our analysis suggests that metabolic profiling can distinguish between kwashiorkor and marasmus patients, although there is considerable overlap between groups. The availability of amino acids, especially that of essential amino acids, was more reduced in those with kwashiorkor, and this may relate to differences in metabolic flux of amino acids that was previously demonstrated in those with kwashiorkor (14, 23). Also, aspartic acid, tryptophan, and its derivative kynurenine were more depleted in patients with kwashiorkor. However, the kynurenine-to-tryptophan ratio, a surrogate measure of the activity of indoleamine 2,3-dioxygenase (i.e., the rate-limiting enzyme in the synthesis of kynurenine from tryptophan), did not change. Tryptophan may be the most limiting essential amino acid, because once it is metabolized into either serotonin or kynurenine, it is no longer available for protein synthesis (24). Also, reductions in tryptophan to kynurenine catabolism because of defective indoleamine 2,3-dioxygenase activity have been associated with liver inflammation and hepatic fibrosis (25), as seen in nonalcoholic fatty liver disease. It is tempting to speculate that low kynurenine could be associated with the hepatic steatosis seen in patients with kwashiorkor. Tryptophan and kynurenine are also precursors for de novo biosynthesis of NAD in humans via quinolinic acid (26), which is itself derived from aspartate. NAD is an important coenzyme linked to the generation of energy in the tricarboxylic acid cycle. The tryptophan-kynurenine pathway also has been implicated in mood disorders and major depression (27, 28), and children with kwashiorkor are clinically more irritable, apathetic, and depressed than those with marasmus (29). This pathway also was shown to be modulated by inflammation, HIV, and antiretroviral treatment in an HIV-positive South African population (30). It would be interesting to study how the kynurenine-tryptophan pathway is modified by HIV infection in patients with kwashiorkor and marasmus. Low tryptophan availability also may be related to the depletion of albumin in patients with kwashiorkor, because it normally binds 80–90% of tryptophan in circulation (24). Finally, children with kwashiorkor had lower circulating threonine. This amino acid is an important component of mucin-2 that is secreted by goblet cells to maintain the intestinal barrier (31). The lower threonine in those with kwashiorkor may reflect a more severe barrier dysfunction, which is known to be severely compromised in children with SAM (32).
Compared with those with marasmus, children with kwashiorkor had lower concentrations of 2 acylcarnitines and of the ratio of acetylcarnitine to free carnitine, a marker of β oxidation. Others have also reported lower concentrations of acylcarnitines in children with kwashiorkor (33). This may indicate a reduction in FA transport into the mitochondria via the carnitine shuttle, thus limiting β oxidation (34). Interestingly, carnitine biosynthesis is a NAD-dependent process that requires the amino acids lysine and methionine (35), which also are more depleted in those with kwashiorkor. Taken together, these interconnected pathways may provide a link between the depletion of acylcarnitine, lysine, methionine, aspartate, tryptophan, kynurenine, and albumin, which were all significantly lower in children with kwashiorkor than in those with marasmus.
The other main cluster of metabolites that differed between kwashiorkor and marasmus patients were the phosphatidylcholine diacyls PC aa C36:4, PC aa C38:6, and PC aa C40:6, all of which were lower in kwashiorkor patients, whereas concentrations in marasmus patients were similar to those of controls. A piglet model of protein energy malnutrition (created to mimic kwashiorkor) also had lower phospholipid concentrations, which modified the composition of the intestinal lipid membrane (36). Phosphatidylcholines are the main component of the phospholipid layer of plasma membranes. Their depletion in those with kwashiorkor could constrain cellular turnover in tissues such as the skin and the intestinal lining, which need constant replenishing.
A large number of metabolites differed between children with SAM at admission and after nutritional stabilization. Many were increased, especially amino acids, biogenic amines, and some lysophosphatidylcholines. However, most sphingomyelins and phosphatidylcholines did not recover, which may be a reflection of hepatic dysfunction or lipid malabsorption that has been previously associated with SAM (37). Furthermore, increased gut permeability has been linked to low circulating sphingomyelins and phosphatidylcholines (38). Considering this, our results suggest that gut barrier function is slow to recover in children with SAM.
Overall, metabolite concentrations in the SAM cohort remained remarkably different from both stunted and nonstunted community controls, even after stabilization. Several metabolites were actually higher in patients with SAM than in community controls, which probably does not reflect better nutritional status, but indicates persistent metabolic dysregulations. Thus, metabolic homeostasis likely is not restored in children with SAM before they are discharged, even though they appear to be clinically recovered. Many children die after hospital discharge (11), and persistent metabolic derangements could contribute to postdischarge mortality. Some metabolites may be useful biomarkers to predict recovery or poor outcome in children before their discharge; this could be addressed by future studies. Also, it is unknown whether metabolic recovery could be accelerated with longer clinical management or specific nutritional supplements (e.g., niacin for NAD production or eggs, which have the highest content of both tryptophan and phosphatidylcholines).
Finally, if the metabolic changes induced by SAM persist, this may lead to long-term consequences that extend into adulthood. For example, sphingomyelins, which are essential for neural myelination, do not recover after nutritional stabilization. Their synthesis depends on phosphatidylcholines, which also show a delayed recovery. Therefore, low concentrations of sphingomyelins may extend well beyond discharge. In fact, stunted children living in rural villages in Malawi have lower concentrations of circulating sphingomyelin than do nonstunted children (17). Considering the important developmental stage of these children, persistently low sphingomyelins could, at least in part, explain the long-term developmental consequences of childhood malnutrition (39). Also, many low-income countries are increasingly faced with a nutritional double burden, with people who developed SAM in childhood tending to become obese and develop type 2 diabetes when challenged by an obesogenic environment in adulthood (40, 41). It is unclear whether the metabolic derangements associated with childhood SAM persist into adulthood and induce adverse metabolic responses to obesogenic environments.
There are a number of limitations in this study, the most important being the difficulty of relating metabolite changes to specific physiologic processes. In normal basal conditions, a metabolite change may indicate a certain physiologic state, but be associated with a different phenomenon in a severely metabolically deranged population, such as children with SAM. Also, the number of studied patients was relatively low, and we did not have specific data on the timing of blood draws in relation to the last meal, which adds variability to the metabolic profiles. Given the changes found in amino acid and lipid metabolism, it would have been valuable to measure more metabolites of the kynurenine-tryptophan pathway and of the tricarboxylic acid cycle. Also, having a longer follow-up would have helped to establish the duration of the metabolic changes. Finally, we only investigated cases of complicated SAM, which represent a relatively small proportion of the global burden of SAM. Children with moderate acute malnutrition should also be investigated. The metabolic profile of children with uncomplicated SAM may be more similar to that of community controls.
In conclusion, we used targeted metabolomics to identify metabolic disturbances in children treated for SAM. We found that most metabolites were lower in those with kwashiorkor than in those with marasmus. These different clinical phenotypes may relate to specific dysregulations of amino acid metabolism, especially that of the kynurenine-tryptophan pathway; acylcarnitines, which regulate FA oxidation; and phosphatidylcholines, which are major components of the plasma membranes. Nutritional rehabilitation of children with SAM improved several metabolites, mainly amino acids, biogenic amines, and lysophosphatidylcholines. However, most lipid metabolites, such as sphingomyelins, phosphatidylcholine diacyls, and acyl-alkyls, were not improved. In fact, even after nutritional stabilization, the metabolic profiles of children with SAM were still very different from those of stunted and nonstunted community controls. These results clearly indicate that, although metabolic recovery may be headed in the right direction, severe metabolic derangements still impair the physiologic functioning of children who appear to be clinically stabilized for SAM, and these may lead to long-term consequences.
Acknowledgments
We thank Hilda Khengere, Alice Tsokonombwe, Agatha Gaussi, Patricia Banda, and Agnes Malamula for their hard work at Queen Elizabeth Central Hospital in Blantyre, Malawi, and Horris Chikwiri, Eleanor Chipofya, Rosemary Godwa, Lydia Kamenya, Jackson Makwinja, Jeanne Mbawa, Nester Mwase, and Vegas Riscado from the St. Louis Nutrition Project. Also, we thank Martin Post, Michael Leadley, and Denis Reynaud from the Analytical Facility for Bioactive Molecules at the Hospital for Sick Children. VDG, CB, RDS, IT, RM, MJM, and RHJB conceptualized and designed the study; VDG, CB, RDS, IT, MIO, MJM, and RHJB coordinated data collection and transfer; VDG, CB, DXW, and RHJB analyzed the data and drafted the final manuscript; ES assisted with the literature review and drafting of the manuscript; CJV, IT, MIO, and LZ assisted in analyzing and coordinating clinical samples; CJV and MIO created and managed the original database; SS, RM, and JP assisted in pathway analysis and contributed to the initial manuscript; WV coordinated and participated in patient recruitment and data collection; and RHJB had primary responsibility for the final content. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: FDR, false discovery rate; PLS-DA, partial least-squares discriminative analysis; RUTF, ready-to-use food; SAM, severe acute malnutrition.
References
- 1.Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, de Onis M, Ezzati M, Grantham-McGregor S, Katz J, Martorell R, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013;382:427–51. [DOI] [PubMed] [Google Scholar]
- 2.Guideline WHO. Updates on the management of severe acute malnutrition in infants and children. Geneva (Switzerland): WHO; 2013. [PubMed] [Google Scholar]
- 3.Chisti MJ, Graham SM, Duke T, Ahmed T, Faruque ASG, Ashraf H, Bardhan PK, Shahid ASMSB, Shahunja KM, Salam MA. Post-discharge mortality in children with severe malnutrition and pneumonia in Bangladesh. PLoS One 2014;9:e107663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kerac M, Bunn J, Chagaluka G, Bahwere P, Tomkins A, Collins S, Seal A. Follow-up of post-discharge growth and mortality after treatment for severe acute malnutrition (FuSAM study): A prospective cohort study. PLoS One 2014;9:e96030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lelijveld N, Seal A, Wells JC, Kirkby J, Opondo C, Chimwezi E, Bunn J, Bandsma R, Heyderman RS, Nyirenda MJ, et al. Chronic disease outcomes after severe acute malnutrition in Malawian children (ChroSAM): a cohort study. Lancet Glob Health 2016;4:e654–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waber DP, Bryce CP, Girard JM, Zichlin M, Fitzmaurice GM, Galler JR. Impaired IQ and academic skills in adults who experienced moderate to severe infantile malnutrition: a 40-year study. Nutr Neurosci 2014;17:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galler JR, Bryce CP, Zichlin ML, Fitzmaurice G, Eaglesfield GD, Waber DP. Infant malnutrition is associated with persisting attention deficits in middle adulthood. J Nutr 2012;142:788–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Golden MH. Oedematous malnutrition. Br Med Bull 1998;54:433–44. [DOI] [PubMed] [Google Scholar]
- 9.Buchanan N, Moodley G, Eyberg C, Bloom HJ Sr. Hypoglycaemia associated with severe kwashiorkor. S Afr Med J 1976;50:1442–6. [PubMed] [Google Scholar]
- 10.Trehan I, Goldbach HS, LaGrone LN, Meuli GJ, Wang RJ, Maleta KM, Manary MJ. Antibiotics as part of the management of severe acute malnutrition. N Engl J Med 2013;368:425–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Munthali T, Jacobs C, Sitali L, Dambe R, Michelo C. Mortality and morbidity patterns in under-five children with severe acute malnutrition (SAM) in Zambia: a five-year retrospective review of hospital-based records (2009–2013). Arch Public Health 2015;73:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spoelstra MN, Mari A, Mendel M, Senga E, Van Rheenen P, Van Dijk TH, Reijngoud DJ, Zegers RGT, Heikens GT, Bandsma RHJ. Kwashiorkor and marasmus are both associated with impaired glucose clearance related to pancreatic β-cell dysfunction. Metabolism 2012;61:1224–30. [DOI] [PubMed] [Google Scholar]
- 13.Bandsma RHJ, Mendel M, Spoelstra MN, Reijngoud DJ, Boer T, Stellaard F, Brabin B, Schellekens R, Senga E, Tom Heikens G. Mechanisms behind decreased endogenous glucose production in malnourished children. Pediatr Res 2010;68:423–8. [DOI] [PubMed] [Google Scholar]
- 14.Jahoor F, Badaloo A, Reid M, Forrester T. Protein kinetic differences between children with edematous and nonedematous severe childhood undernutrition in the fed and postabsorptive states. Am J Clin Nutr 2005;82:792–800. [DOI] [PubMed] [Google Scholar]
- 15.Manary MJ. Hart C a, Whyte MP. Severe hypophosphatemia in children with kwashiorkor is associated with increased mortality. J Pediatr 1998;133:789–91. [DOI] [PubMed] [Google Scholar]
- 16.Bartz S, Mody A, Hornik C, Bain J, Muehlbauer M, Kiyimba T, Kiboneka E, Stevens R, Bartlett J, St Peter JV, et al. Severe acute malnutrition in childhood: hormonal and metabolic status at presentation, response to treatment, and predictors of mortality. J Clin Endocrinol Metab 2014;99:2128–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Semba RD, Shardell M, Sakr FA, Moaddel R, Trehan I, Maleta KM, Ordiz MI, Kraemer K, Khadeer MA, Ferrucci L, et al. Child stunting is associated with low circulating essential amino acids. EBioMedicine 2016;6:246–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ho DE, Imai K, King G, Stuart EA. MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw 2011;42:1–28. [Google Scholar]
- 19.WHO. Management of severe malnutrition: a manual for physicians and other senior health workers. Geneva (Switzerland): WHO; 1999. [Google Scholar]
- 20.FDA. Guidance for industry—bioanalytical method validation. Silver Spring (MD): FDA; 2001. [Google Scholar]
- 21.Kuhn M, Wing J, Weston S, Williams A, Keefer C, Engelhardt AT, Cooper T, Mayer Z, Kenkel B, Benesty M, et al. caret: classification and regression training 2016 [cited 2016 Jun 13]. Available from: https://cran.r-project.org/package=caret.
- 22.Le Cao KA, Gonzalez I, Dejean S, Rohart F, Gautier B, Monget P, Coquery J, Yao FZ, Liquetey BB. CRAN - Package mixOmics . 2015 [cited 2016 Jun 26]. Available from: http://www.mixomics.org.
- 23.Manary MJ, Broadhead RL, Yarasheski KE. Whole-body protein kinetics in marasmus and kwashiorkor during acute infection. Am J Clin Nutr 1998;67:1205–9. [DOI] [PubMed] [Google Scholar]
- 24.Le Floc’h N, Otten W, Merlot E. Tryptophan metabolism, from nutrition to potential therapeutic applications. Amino Acids 2011;41:1195–205. [DOI] [PubMed] [Google Scholar]
- 25.Nagano J, Shimizu M, Hara T, Shirakami Y, Kochi T, Nakamura N, Ohtaki H, Ito H, Tanaka T, Tsurumi H, et al. Effects of indoleamine 2,3-dioxygenase deficiency on high-fat diet- induced hepatic inflammation. PLoS One 2013;8:e73404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di Stefano M, Conforti L. Diversification of NAD biological role: the importance of location. FEBS J 2013;280:4711–28. [DOI] [PubMed] [Google Scholar]
- 27.Oxenkrug GF. Tryptophan kynurenine metabolism as a common mediator of genetic and environmental impacts in major depressive disorder: the serotonin hypothesis revisited 40 years later. Isr J Psychiatry Relat Sci 2010;47:56–63. [PMC free article] [PubMed] [Google Scholar]
- 28.Myint AM. Kynurenines: from the perspective of major psychiatric disorders. FEBS J 2012;279:1375–85. [DOI] [PubMed] [Google Scholar]
- 29.Williams CD. A nutritional disease of childhood associated with a maize diet. Arch Dis Child 1933;8:423–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bipath P, Levay PF, Viljoen M. The kynurenine pathway activities in a sub-Saharan HIV/AIDS population. BMC Infect Dis 2015;15:346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu G. Amino acids: metabolism, functions, and nutrition. Amino Acids 2009;37:1–17. [DOI] [PubMed] [Google Scholar]
- 32.Hossain MI, Nahar B, Hamadani JD, Ahmed T, Roy AK, Brown KH. Intestinal mucosal permeability of severely underweight and nonmalnourished Bangladeshi children and effects of nutritional rehabilitation. J Pediatr Gastroenterol Nutr 2010;51:638–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanzer F, Uzunsel S, Atalay A. Plasma free carnitine levels in children with malnutrition. Turk J Pediatr 1994;36:133–7. [PubMed]
- 34.Hammond KD, Tobiansky R, Abrahams OL. Serum carnitine in children with kwashiorkor. Ann Trop Paediatr 1987;7:214–6. [DOI] [PubMed] [Google Scholar]
- 35.Vaz FM, Wanders RJA. Carnitine biosynthesis in mammals. Biochem J 2002;361:417–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lopez-Pedrosa JM, Torres MI, Fernández MI, Rıos A, Gil A. Nutrient metabolism severe malnutrition alters lipid composition and fatty acid profile of small intestine in newborn piglets. J Nutr 1998;128:224–33. [DOI] [PubMed] [Google Scholar]
- 37.Murphy JL, Badaloo AV, Chambers B, Forrester TE, Wootton SA, Jackson AA. Maldigestion and malabsorption of dietary lipid during severe childhood malnutrition. Arch Dis Child 2002;87:522–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Semba RD, Shardell M, Trehan I, Moaddel R, Maleta KM, Ordiz MI, Kraemer K, Khadeer M, Ferrucci L, Manary MJ. Metabolic alterations in children with environmental enteric dysfunction. Sci Rep 2016;6:28009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kerac M, Postels DG, Mallewa M, Alusine Jalloh A, Voskuijl WP, Groce N, Gladstone M, Molyneux E. The interaction of malnutrition and neurologic disability in Africa. Semin Pediatr Neurol 2014;21:42–9. [DOI] [PubMed] [Google Scholar]
- 40.Tzioumis E, Adair LS. Childhood dual burden of under- and overnutrition in low- and middle-income countries: a critical review. Food Nutr Bull 2014;35:230–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kolčić I. Double burden of malnutrition: a silent driver of double burden of disease in low- and middle-income countries. J Glob Health 2012;2:020303. [DOI] [PMC free article] [PubMed] [Google Scholar]