Abstract
Background: Although it is recognized that vitamin D deficiency is associated with cardiovascular disease (CVD) risk factors, and is more common in African Americans (AAs), the pathologic mechanisms by which vitamin D may influence these risk factors are poorly understood.
Objectives: We explored the association between vitamin D status, as reflected by serum 25-hydroxyvitamin D [25(OH)D] concentrations, and CVD risk factors including mean arterial pressure (MAP), fasting plasma glucose (FPG), plasma HDL cholesterol, and waist circumference (WC) in adult AAs. We also tested whether plasma C-reactive protein (CRP), adipokines (adiponectin and leptin), and aldosterone mediated the associations between 25(OH)D and these risk factors.
Methods: Data on 4010 (63.8% women; mean age: 54.0 y) individuals from the Jackson Heart Study were analyzed. Multivariable linear regression models were used to examine the associations of 25(OH)D with CVD risk factors. We used path analysis and bootstrapping methods to quantify and test the share of these associations that was statistically explained by each of the mediators by decomposing the associations into direct and indirect effects.
Results: Serum 25(OH)D concentrations were inversely associated with WC, FPG, and MAP and were positively associated with HDL cholesterol in multivariable analysis. A nearly 20% effect of 25(OH)D on MAP was masked by aldosterone (total indirect effect: β = 0.01, P < 0.05). Approximately 23% of the effect of 25(OH)D on WC (β = −0.03, P < 0.05) and ∼9% of the effect of 25(OH)D on FPG (β = −0.02, P < 0.05) were mediated through CRP, adiponectin, and leptin together. A 23% share of the association between 25(OH)D and HDL cholesterol was mediated by adiponectin alone (β = 0.03, P < 0.05).
Conclusions: Our findings suggest that the associations between vitamin D status and CVD risk factors in AAs are partially mediated through circulating adipokines and CRP. More evidence, however, is required from longitudinal and randomized controlled studies to validate our findings.
Keywords: 25-hydroxyvitamin D, African Americans, cardiovascular disease risk factors, path analysis, adipokine
Introduction
Several studies have shown that vitamin D deficiency, as reflected by concentrations of circulating 25-hydroxyvitamin D [25(OH)D]6, is associated with a significantly increased prevalence of cardiovascular disease (CVD) risk factors, such as diabetes, hypertension, dyslipidemia, and obesity (1–8). Although it is recognized that vitamin D deficiency is associated with several CVD risk factors and is more common and severe in the African-American (AA) population, the pathologic mechanisms by which 25(OH)D may influence CVD risk factors are not fully understood. One of the suggested mechanisms through which 25(OH)D can decrease blood pressure (BP) is by downregulating the renin-angiotensin-aldosterone system. Human and animal studies indicate that 25(OH)D decreases plasma renin production and thus inhibits the conversion of angiotensinogen to angiotensin I and the conversion of angiotensin I to angiotensin II, which, in turn, results in reduced synthesis of aldosterone. Because aldosterone causes the body to retain water and sodium by increasing the reabsorption from kidney tubules, increased 25(OH)D is believed to lower BP through reducing aldosterone concentrations (9, 10). A deficiency of 25(OH)D can also cause secondary increased secretion of parathyroid hormone (PTH) and increase the risk of inflammation and an elevated concentration of inflammatory markers, such as C-reactive protein (CRP) (11). Low-grade inflammation, as indicated by increased plasma concentrations of CRP, may increase the likelihood of developing insulin resistance and subsequent hyperinsulinemia either by influencing insulin signaling in peripheral tissues or by inducing β cell dysfunction (12). Thus, low 25(OH)D may exert its effect on diabetes and obesity through an increased concentration of CRP. The role of 25(OH)D on adipokines, such as leptin and adiponectin, is also a major area of research interest. Evidence suggests that, in humans, leptin secretion is negatively (13), and adiponectin secretion is positively (14), controlled by serum 25(OH)D. Hence, it is also possible that 25(OH)D may affect the pathogenesis of diabetes, obesity, and dyslipidemia through alteration in adipokine concentrations (15, 16). Serum 25(OH)D can potentially affect mechanisms related to type 2 diabetes and obesity pathophysiology by impairing β cell function leading to the development of insulin resistance (17). These mechanisms are interrelated, and studies examining these pathways through which vitamin D directly or indirectly affects CVD risk factors are rare.
We hypothesized that 25(OH)D is inversely associated with CVD risk factors, including BP, fasting plasma glucose (FPG), LDL cholesterol, and waist circumference (WC), and is positively associated with HDL cholesterol. We also hypothesized that CRP, aldosterone, adiponectin, and leptin partially mediate the associations between 25(OH)D and these risk factors. The present study explores the pathways through which vitamin D [i.e., serum 25(OH)D] may exert its beneficial role on different CVD risk factors (BP, FPG, LDL cholesterol, HDL cholesterol, and WC) in AAs with the use of the Jackson Heart Study (JHS) cohort.
Methods
Data source.
The JHS is a large, community-based observational study initiated in 2000 to prospectively investigate the epidemiology and determinants of CVD in AAs (18). The baseline JHS comprises 5301 AA participants between the ages of 21 and 94 y residing in the Jackson, Mississippi, metropolitan area. Clinic visits and interviews occur approximately every 3 y. The JHS was approved by the University of Mississippi Medical Center, Jackson State University, and Tougaloo College Institutional Review Board; and the participants provided written informed consent. Details of the study design and data collection methods are described elsewhere (19, 20). Current study data were obtained from baseline data collection during clinic visits from 2000 to 2004.
Assessment of serum vitamin D status.
Serum 25-hydroxyergocalciferol and 25-hydroxycholecalciferol concentrations were quantified with LC–tandem MS and analyzed with isotope dilution–tandem MS (Quattro Micro with a 2795 HPLC; Waters). Serum concentrations were combined to obtain the serum total 25(OH)D concentration. The details are described in the Supplemental Methods 1. The cutoffs of ≥30 ng/mL (optimal), 20–29 ng/mL (insufficient), and <20 ng/mL (deficient) were used for our descriptive analysis (21).
Assessment of clinical outcomes and intervening variables.
Information was collected about each participant’s WC (cm) and resting systolic BP (SBP) and diastolic BP (mm Hg). Two measures of the waist at the level of the umbilicus, in the upright position, were averaged to measure WC for each participant. Sitting BP was measured twice at 5-min intervals, and the average of 2 measurements was used for analysis. We estimated mean arterial pressure (MAP) as 1/3 (SBP) + 2/3 (diastolic BP), which is defined as the average pressure in a patient’s arteries during 1 cardiac cycle (22). Information was also obtained on plasma concentrations of FPG (expressed as mg/dL), fasting lipids (LDL cholesterol and HDL cholesterol; expressed as mg/dL), CRP (expressed as mg/dL), adiponectin ( expressed as μg/mL), leptin (expressed as ng/dL), and aldosterone (expressed as ng/dL). Fasting blood samples were collected according to standardized protocols, and the assessments of all of these variables were processed at the Central Laboratory, University of Minnesota (23). Log-transformed values of CRP, adiponectin, leptin, and aldosterone concentrations were used due to deviation from normal distribution.
Assessment of covariates.
Information on participants’ age, sex, education, physical activity, smoking status, alcohol consumption, BMI, month of blood draw, and medication use (for elevated FPG, BP, and lipids) was obtained. Educational status was self-reported and was divided into 3 categories (less than high school, high school or some college, and college or associate’s degree or higher). A physical activity index composite score was calculated as the sum of 4 different domains of physical activity: active living, work, home and garden, and sports and exercise indexes (24). The score was grouped in tertiles. Smoking status was classified as never, current, or former. Alcohol consumption status was defined as “yes” if participants currently consumed alcoholic beverages and “no” if they had stopped drinking >1 ago or if they never consumed alcohol. BMI (kg/m2) was calculated as weight in kilograms divided by height in meters squared; cutoffs of <25 were defined as normal, 25–29.9 as overweight, and ≥30 as obese (25). Months were grouped into 4 seasons as summer (June–August), fall (September–November), winter (December–February), and spring (March–May) to account for seasonal variation in 25(OH)D (26).
Statistical methods.
We excluded 140 participants with missing information on serum 25(OH)D and 407 participants who were not fasting. Individuals with missing information for any of the mediating variables and covariates were also excluded, resulting in a final sample of 4010 participants. Descriptive statistics were used for summarizing characteristics of the participants. Characteristics across 25(OH)D status (optimal, insufficient, or deficient) were compared by using ANOVA or chi-square test. Next, we examined the associations of 25(OH)D with CVD risk factors (FPG, WC, HDL cholesterol, LDL cholesterol, and MAP) by using multivariable linear regression models. We fitted 2 models for each of the CVD risk factors. Model 1 adjusted for potential confounders (age, sex, education, smoking status, BMI, physical activity level, season, and respective medication use). Model 2 additionally adjusted prespecified intervening or mediator variables (CRP, adiponectin, leptin, and aldosterone).
Supplemental Figure 1 shows the proposed path analysis model through which 25(OH)D may modulate CVD risk factors. Briefly, our model postulated direct and indirect paths from 25(OH)D to 5 different CVD risk factors, including FPG, WC, LDL cholesterol, HDL cholesterol, and MAP through several intervening variables (CRP, aldosterone, adiponectin, and leptin). To investigate the extent to which the intervening variables mediated the association between 25(OH)D and the CVD risk factors, we performed path analysis by using Analysis of Moment Structures (AMOS) (27). Path analysis simultaneously estimated the regression of the outcomes (CVD risk factors) on the respective mediators and the regression of each of those mediators on exposure [25(OH)D]. This method thus allowed us to decompose the total effect of 25(OH)D on each CVD risk factor into a direct effect and an indirect effect that acted via intervening variables as shown in Supplemental Figure 1. All of the regression equations involved in the path analysis model were adjusted for participants’ age, sex, education, smoking status, BMI, physical activity level, season, and respective medication use. Mediation effects were estimated by using bootstrapping methods (sample = 5000), and bias-corrected CI estimates for the indirect effects were obtained (28). The comparative fit index, normal fit index, and the root mean square error of approximation were used to evaluate the model fit of the path analysis. A detailed description of the biological plausibility of the pathways of the path model is presented in Supplemental Methods 2. All of the variables of the model were checked for significant associations with each other, and a correlation matrix was derived by using all of the variables in the model (Supplemental Tables 1 and 2). The details with regard to the derivation of direct, indirect, and total effects are described in Supplemental Methods 3.
Because vitamin D is known to be affected by seasonal variation, we also performed sensitivity analysis accounting for the effects of seasonal variation of 25(OH)D. We estimated the annual mean 25(OH)D by fitting a parametric seasonal cosinor model to the observed 25(OH)D data, which follows a sinusoidal pattern (29). A detailed description of the cosinor model is presented in Supplemental Methods 4. A parallel regression and path analysis were conducted by using the annual mean 25(OH)D. We also repeated a separate path analysis for nonobese participants (BMI <30; n = 1909). All of the tests were 2-sided, and P < 0.05 was considered to indicate significant results.
Results
The mean ± SD serum 25(OH)D concentration of 4010 participants was 14.4 ± 6.70 ng/mL. Overall, 3207 (80%) were 25(OH)D deficient and only 93 (2.30%) had optimal 25(OH)D concentrations. Participants’ mean age was 54.0 ± 12.8 y; 63.8% were women. Unadjusted associations of 25(OH)D categories with lifestyle, clinical, and metabolic traits are summarized in Table 1. Deficiency of 25(OH)D in general was more prevalent among women, smokers, participants with low physical activity, and obese individuals (P < 0.05 for all). In addition, greater 25(OH)D deficiency in older-aged participants and during cold seasons was observed. MAP and LDL cholesterol were not significantly different across the 25(OH)D categories, but mean FPG and WC were higher and mean HDL cholesterol was lower among 25(OH)D-deficient participants than among participants with optimal 25(OH)D concentrations (P < 0.05 for all).
TABLE 1.
Characteristics of Jackson Heart Study participants according to serum 25(OH)D status1
| Serum 25(OH)D |
|||||
| Characteristics | Full sample (n = 4010) | Deficient, <20 ng/mL (n = 3207) | Insufficient, 20–29 ng/mL (n = 710) | Optimal, ≥30 ng/mL (n = 93) | P2 |
| Age, y | 54.0 ± 12.8 | 53.1 ± 12.7 | 58.0 ± 12.6 | 58.2 ± 12.6 | <0.01 |
| Sex, % | <0.01 | ||||
| Male | 36.2 | 76.8 | 21.0 | 2.2 | |
| Female | 63.8 | 81.8 | 15.9 | 2.4 | |
| Educational level, % | 0.01 | ||||
| Less than high school | 16.7 | 75.9 | 21.3 | 2.8 | |
| High school or some college | 42.3 | 82.3 | 15.5 | 2.1 | |
| College: associate’s degree or higher | 41.0 | 79.2 | 18.5 | 2.3 | |
| Smoking status, % | <0.01 | ||||
| Never | 68.9 | 80.2 | 17.7 | 2.1 | |
| Former | 18.6 | 74.7 | 21.7 | 3.6 | |
| Current | 12.4 | 86.4 | 12.0 | 1.6 | |
| Alcohol drinking status, % | 0.33 | ||||
| Yes | 46.8 | 80.8 | 16.8 | 2.5 | |
| No | 53.2 | 79.3 | 18.5 | 2.2 | |
| Physical activity,3 % | <0.01 | ||||
| Low | 33.3 | 82.6 | 15.6 | 1.8 | |
| Medium | 33.3 | 80.6 | 17.7 | 2.3 | |
| High | 33.3 | 76.7 | 20.6 | 2.3 | |
| BMI (in kg/m2), % | <0.01 | ||||
| Normal (<25) | 14.5 | 74.1 | 21.4 | 4.5 | <0.01 |
| Overweight (25–29.9) | 33.1 | 75.4 | 21.8 | 2.8 | |
| Obese (≥30) | 52.4 | 84.5 | 14.1 | 1.4 | 0.62 |
| MAP,4 mm Hg | 95.0 ± 11.4 | 95.0 ± 11.5 | 94.9 ± 10.9 | 96.1 ± 11.2 | 0.01 |
| Fasting plasma glucose, mg/dL | 99.4 ± 32.1 | 100 ± 34.1 | 96.9 ± 23.7 | 93.3 ± 14.3 | 0.04 |
| Waist circumference, cm | 100 ± 16.2 | 101 ± 16.5 | 97.9 ± 14.6 | 92.7 ± 13.6 | 0.03 |
| Plasma | |||||
| LDL cholesterol, mg/dL | 126 ± 36.8 | 126 ± 37.3 | 127 ± 34.5 | 118 ± 34.4 | 0.09 |
| HDL cholesterol, mg/dL | 51.6 ± 14.4 | 51.1 ± 14.2 | 53.3 ± 14.9 | 56.3 ± 15.8 | 0.02 |
| CRP, mg/dL | 0.51 ± 0.92 | 0.53 ± 0.95 | 0.44 ± 0.74 | 0.43 ± 0.57 | 0.04 |
| Leptin, ng/dL | 27.7 ± 23.0 | 28.7 ± 23.5 | 23.4 ± 20.2 | 24.1 ± 21.1 | <0.01 |
| Adiponectin, μg/mL | 5.38 ± 4.33 | 5.23 ± 4.26 | 5.89 ± 4.57 | 6.70 ± 4.31 | <0.01 |
| Aldosterone, ng/dL | 5.71 ± 5.5 | 5.62 ± 5.77 | 6.01 ± 5.06 | 6.17 ± 4.23 | 0.19 |
| Season of blood collection5 | <0.01 | ||||
| Summer | 27.4 | 74.7 | 22.4 | 2.8 | |
| Spring | 30.0 | 86.5 | 11.9 | 1.7 | |
| Fall | 20.4 | 74.4 | 22.6 | 3.0 | |
| Winter | 22.2 | 83.0 | 15.2 | 1.8 | |
Values are means ± SDs unless otherwise indicated, n = 4010. CRP, aldosterone, leptin, and adiponectin had skewed distributions and their medians were 0.26, 4.30, 20.1, and 4.2, respectively. CRP, C-reactive protein; MAP, mean arterial pressure; 25(OH)D, 25-hydroxyvitamin D.
Derived by using 1-factor ANOVA or chi-square test.
Sum of the 4 different domains of physical activity: active living, work, home and garden, and sports and exercise indexes. Scores were categorized in tertiles.
MAP was calculated as 1/3 (systolic blood pressure) + 2/3 (diastolic blood pressure).
Summer (June–August), fall (September–November), winter (December–February), and spring (March–May).
Results of multivariable regressions are shown in Table 2. After controlling for age, sex, BMI, physical activity, smoking status, season, education, and respective medication use, 25(OH)D was positively associated with HDL cholesterol (regression coefficient β: 0.13; 95% CI: 0.06, 0.19) and was inversely associated with FPG (β: −0.22; 95% CI: −0.36, −0.09), WC (β:−0.13; 95% CI: −0.19, −0.08), and MAP (β:−0.06; 95% CI: −0.11, −0.01). No significant association with LDL cholesterol was found. Once mediator variables (CRP, adiponectin, and leptin for FPG and WC; adiponectin and leptin for HDL cholesterol; and aldosterone for MAP) were introduced in the model, with the exception of MAP, all of the estimates were reduced but remained significant. As expected, the associations between mediator variables and each CVD risk factor also remained significant (except for leptin and HDL cholesterol), suggesting partial mediation by these factors.
TABLE 2.
Associations between serum 25(OH)D concentrations and different cardiovascular disease risk factors without (model 1) and with (model 2) additional adjustment for mediator variables, estimated from multivariable regression models: the Jackson Heart Study1
| FPG, mg/dL | WC, cm | HDL cholesterol, mg/dL | MAP,2 mm Hg | LDL cholesterol, mg/dL | |
| Model 13 | |||||
| 25(OH)D | −0.22 (−0.36, −0.09) | −0.13 (−0.19, −0.08) | 0.13 (0.06, 0.19) | −0.06 (−0.11, −0.01) | −0.07 (−0.24, 0.11) |
| Model 24 | |||||
| 25(OH)D | −0.20 (−0.33, −0.07) | −0.11 (−0.16, −0.06) | 0.10 (0.04, 0.16) | −0.06 (−0.12, −0.01) | −0.09 (−0.26, 0.09) |
| Mediator variables5 | |||||
| Log adiponectin | −10.1 (−13.9, −6.93) | −4.11 (−5.31, −2.91) | 15.2 (13.2, 16.7) | — | — |
| Log leptin | −4.9 (−8.87, −1.08) | 17.9 (16.5, 19.4) | — | — | — |
| Log CRP | 3.65 (1.84, 5.44) | 2.40 (1.71, 3.08) | — | — | — |
| Log aldosterone | — | — | — | 2.33 (1.05, 3.56) | — |
Values are multivariable-adjusted β coefficients (95% CIs), n = 4010. CRP, C-reactive protein; FPG, fasting plasma glucose; MAP, mean arterial pressure; WC, waist circumference; 25(OH)D, 25-hydroxyvitamin D.
MAP was calculated as 1/3 (systolic blood pressure) + 2/3 (diastolic blood pressure).
Model 1 adjusted for age, sex, BMI, physical activity, smoking status, season, education, and respective medication use (if applicable).
Model 2 adjusted for the covariates of model 1 plus the specified mediator variables.
Mediator variables: adiponectin, leptin and CRP for FPG and WC; adiponectin for HDL cholesterol; and aldosterone for MAP.
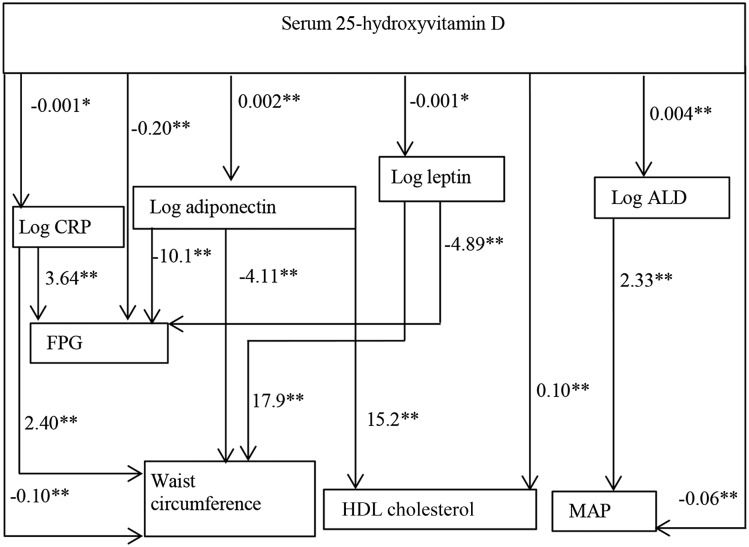
The path analysis model assessed the role played by potential mediators, which explained the associations between 25(OH)D and the CVD risk factors (Figure 1). LDL cholesterol was not included in path analysis because no significant association between 25(OH)D and LDL cholesterol was found. The upper half of the path model shows the relation between 25(OH)D and the mediators and the lower half shows the effects of each individual mediator on the CVD risk factors. According to the model, a 1-ng/mL increase in 25(OH)D was associated with a 0.2% and a 0.4% increase in adiponectin and aldosterone concentrations, respectively (P < 0.05). A 1-ng/mL increase in 25(OH)D was also associated with a 0.1% decrease in leptin and CRP concentrations (P < 0.10 for both). As shown in Figure 1, if the adiponectin concentration increased by 1%, we would expect FPG to decrease by 0.10 mg/dL, WC to decrease by 0.04 cm, and HDL cholesterol to increase by 0.15 mg/dL. Similarly, if leptin concentration increased by 1%, we would expect WC to increase by 0.17 cm and FPG to decrease by 0.04 mg/dL; and if aldosterone concentration increased by 1%, MAP would increase 0.02 mm Hg.
FIGURE 1.
Estimated path analysis model showing the associations between serum 25-hydroxyvitamin D concentrations, potential mediators, and cardiovascular risk factors: the Jackson Heart Study (n = 4010). HDL cholesterol is in milligrams per deciliter, and waist circumference is in centimeters. Values of ALD, CRP, adiponectin, and leptin were log-transformed for normalization. The regression equations, with the potential mediators and cardiovascular risk factors as the outcomes, are represented by single-headed arrows. Coefficients with significance levels are presented beside each arrow (*P < 0.10, **P < 0.05). The product of the coefficients along a compound path reflects the total weight of that path. All of the regression equations involved in the path analysis model (for cardiovascular risk factors and all of the mediating risk factors) were adjusted for individual participant’s age, sex, education, smoking status, BMI, physical activity level, medication use (if applicable), and season. ALD, aldosterone; CRP, C-reactive protein; FPG, fasting plasma glucose (in milligrams per deciliter); MAP, mean arterial pressure [mm Hg; calculated as 1/3 (systolic blood pressure) + 2/3 (diastolic blood pressure)].
Table 3 presents the estimated direct, indirect, and total effects of 25(OH)D on CVD risk factors with 95% CI from the path analysis. Table 3 also shows the percentage of mediation [i.e., the proportion of the associations between 25(OH)D and each CVD risk factor attributable to the mediator variables]. The mediator that had appreciable shares of the associations between 25(OH)D and HDL cholesterol was adiponectin (23.1% of the association; total indirect effect: β = 0.03; 95% CI: 0.01, 0.05); mediators that had appreciable shares of the associations between 25(OH)D and FPG were CRP, adiponectin, and leptin (9.09% of the association; β = −0.02; 95% CI: −0.04, −0.01) and between 25(OH)D and WC were CRP, adiponectin, and leptin (23.1% of the association; β = −0.03; 95% CI: −0.05, −0.01). Notably, in Figure 1 and in Table 3, aldosterone concentration increased with higher 25(OH)D. Therefore, instead of explaining, aldosterone rather masked the beneficial effect of 25(OH)D on MAP (indirect effect: β = 0.01; 95% CI: 0.005, 0.02). For the comparative fit index, normal fit index, and the root mean square error of approximation of our path model, values were 0.82, 0.81, and 0.09, respectively, reflecting an acceptable fit. As shown in Supplemental Tables 3 and 4 and Supplemental Figure 2, the estimates and patterns of association remained fairly similar when annualized mean 25(OH)D was used. Regression and path models conducted with nonobese individuals also had similar patterns of association, except that leptin did not significantly mediate the association between 25(OH)D and FPG. Its mediation effect on WC, however, remained the same (Supplemental Figure 3).
TABLE 3.
Estimation of indirect, direct, and total effects of 25(OH)D status on different cardiovascular risk factors and the proportion of the effects attributable to mediators from the path analysis: the Jackson Heart Study1
| Total effect | Total indirect effect | Direct effect | Mediation attributable to mediator, % | |
| Fasting plasma glucose2 | −0.22 (−0.36, −0.09) | −0.02 (−0.04, −0.01) | −0.20 (−0.32, −0.09) | 9.09 |
| Waist circumference2 | −0.13 (−0.19, −0.08) | −0.03 (−0.05, −0.01) | −0.10 (−0.15, −0.06) | 23.1 |
| HDL cholesterol3 | 0.13 (0.064, 0.192) | 0.03 (0.01, 0.05) | 0.10 (0.03, 0.16) | 23.1 |
| MAP4,5 | −0.05 (−0.11, −0.001) | 0.01 (0.01, 0.02) | −0.06 (−0.12, −0.01) | −20.0 |
Values are β coefficients (95% CIs) derived from path analysis, n = 4010. Mediation analyses were tested by using bootstrapping methods with bias-corrected confidence estimates. MAP, mean arterial pressure; 25(OH)D, 25-hydroxyvitamin D.
Mediators: C-reactive protein, adiponectin, and leptin.
Mediator: adiponectin.
Mediator: aldosterone.
MAP was calculated as 1/3 (systolic blood pressure) + 2/3 (diastolic blood pressure).
Discussion
We found that 25(OH)D was independently and positively associated with HDL cholesterol and was independently and inversely associated with FPG, WC, and MAP. No association with LDL cholesterol was observed. According to our model, 25(OH)D exerted its effect mainly through adiponectin and leptin, and modestly through CRP. We also found that some of the beneficial effect of 25(OH)D on MAP was masked by aldosterone. Approximately 23% of the effect of 25(OH)D on WC and ∼9% of the effect of 25(OH)D on FPG were mediated through CRP, adiponectin, and leptin together; and a 23% association between 25(OH)D and HDL cholesterol was mediated through adiponectin only.
Although a number of observational studies have shown 25(OH)D to be related to CVD risk factors (1–8, 17), very few studies reported the consequences of 25(OH)D deficiency with respect to CVD risk in AAs (2–4). One cross-sectional analysis reported a positive association between 25(OH)D deficiency and obesity. However, the association did not remain significant when the follow-up data were analyzed (4). Studies conducted in national-level samples reported 25(OH)D deficiency to be associated with increased SBP (3) but found no association with diabetes (2). By using the data of a large AA cohort, we found beneficial associations of 25(OH)D on several CVD risk factors, including FPG, MAP, WC, and HDL cholesterol. However, further evidence from prospective or interventional studies is required on the far-reaching cardiometabolic consequences of 25(OH)D deficiency in AAs to confirm these relations.
Adiponectin appeared to have a mediating role between the association of 25(OH)D and several CVD risk factors, such as FPG, WC, and HDL cholesterol, in our path analysis. One of the plausible explanations underlying this mediating effect is the direct role that 25(OH)D has in modulating adipocyte signaling, which results in increased secretion of adiponectin (30, 31). Adiponectin consequently could manifest its beneficial influence on metabolic health by increasing the rate of FA oxidation and FA uptake, and by slowing FA synthesis and gluconeogenesis (15, 16). Similar to adiponectin, leptin is also produced mainly by adipocytes (32, 33). Typically, leptin signals the body through the brain to restrain food intake and induce energy expenditure; leptin thereby has the ability to decrease blood sugar and regulate body weight (32, 33). In our analysis, although the individual effect of leptin on FPG was expectedly inverse, there was a significant positive or same-directional association between leptin and WC. This positive pattern of association may reflect a state of leptin resistance in our participants, in whom chronically elevated leptin concentrations may result in impaired leptin signaling (34). Impaired signaling can compromise leptin’s action and it may no longer inhibit food intake and reduce weight (35). The role of leptin on body weight or obesity thus remains complex. Additional studies investigating the integrated actions of leptin could provide an understanding of how altered leptin signaling contributes to obesity-related CVD.
The overall effect of 25(OH)D on MAP was beneficial among our study participants. However, we also identified a possible unfavorable influence of 25(OH)D on MAP through aldosterone, because of the positive association between 25(OH)D and aldosterone that we observed. This finding is inconsistent with the current literature, in which it is generally recognized that, instead of a positive relation, 25(OH)D rather reduces the synthesis of aldosterone by downregulating the renin-angiotensin-aldosterone system (7, 9, 10, 36). To what extent and under which circumstances the relation between 25(OH)D and aldosterone is reversed or influenced should be assessed through further research. This assessment is important from a clinical perspective, because widely used antihypertensive drugs, such as angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and even some diuretic drugs, reduce BP partly by decreasing aldosterone concentrations. If the relation between 25(OH)D and aldosterone is reversed in AAs for any biological or environmental factors, these drugs may not have the desired optimal effect on BP reduction.
We acknowledge that, given the cross-sectional observational design, our postulated pathways were not motivated by temporal order and cannot prove causality. However, this analysis provides an assessment of the relation between CVD risk factors and 25(OH)D concentrations and a quantification of probable mediation proportions of this relation from a large community-based AA cohort. One of the assumptions of path models to reliably decompose an association into direct and indirect effects is additivity of effects and linear contrasts. By using continuous measures for our exposure, mediators, and outcome variables, this assumption was met. Evidence indicates that chronic vitamin D deficiency causes an increase in PTH concentrations and PTH can elevate the risk of different CVDs through enhancing inflammation (37). We used a surrogate indicator—CRP, instead of PTH, and proinflammatory cytokines—and thus could not delineate if the use of PTH or the cytokines would have yielded a different result. It can be argued that a single measurement of 25(OH)D is an imperfect reflection of vitamin D status over a long period and does not capture seasonal variation. To address this, we repeated the analysis with the use of mean annualized 25(OH)D concentrations. However, the pattern of associations remained similar. Adipokines are produced mainly by adipocytes, and we recognize that due to reverse causal effects between adipokines and obesity, obese persons might produce increased concentrations of adiponectin and leptin and the directionality of those paths could be reversed (38, 39). To address this concern, we carried out a sensitivity analysis exclusively in nonobese participants. Similar findings from them added validity to our main results.
In summary, our results suggest an overall beneficial association between vitamin D status, as assessed by serum 25(OH)D concentration, and CVD risk factors. These associations were partially mediated through circulating adipokines and CRP. We think that our study is an important addition to the limited existing literature on AAs, because this population is vastly understudied despite having a high prevalence of vitamin D deficiency and CVD. We recommend further clinical and experimental studies on the influence of vitamin D status on cardiovascular physiology and pathology at a cellular level, particularly with respect to ethnicity, to validate our findings and to understand the underlying mechanisms through which vitamin D affects CVD risk factors.
Acknowledgments
We thank Cindy Clark, NIH Library Editing Service, for reviewing the manuscript. RJK conceptualized the study and completed the main data analysis; RJK and SKD drafted the manuscript; and SYG, PR, MS, AG, RX, and SKD contributed to the study design, interpretation of data, and the preparation of the final manuscript. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: AA, African American; BP, blood pressure; CRP, C-reactive protein; CVD, cardiovascular disease; FPG, fasting plasma glucose; JHS, Jackson Heart Study; MAP, mean arterial pressure; PTH, parathyroid hormone; SBP, systolic blood pressure; WC, waist circumference; 25(OH)D, 25-hydroxyvitamin D.
References
- 1.Martins D, Wolf M, Pan D, Zadshir A, Tareen N, Thadhani R, Felsenfeld A, Levine B, Mehrotra R, Norris K. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med 2007;167:1159–65. [DOI] [PubMed] [Google Scholar]
- 2.Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care 2004;27:2813–8. [DOI] [PubMed] [Google Scholar]
- 3.Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, ethnicity, and blood pressure in the Third National Health and Nutrition Examination Survey. Am J Hypertens 2007;20:713–9. [DOI] [PubMed] [Google Scholar]
- 4.Young KA, Engelman CD, Langefeld CD, Hairston KG, Haffner SM, Bryer-Ash M, Norris JM. Association of plasma vitamin D levels with adiposity in Hispanic and African Americans. J Clin Endocrinol Metab 2009;94:3306–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hyppönen E, Laara E, Reunanen A, Jarvelin MR, Virtanen SM. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet 2001;358:1500–3. [DOI] [PubMed] [Google Scholar]
- 6.Afzal S, Bojesen SE, Nordestgaard BG. Low 25-hydroxyvitamin D and risk of type 2 diabetes: a prospective cohort study and metaanalysis. Clin Chem 2013;59:381–91. [DOI] [PubMed] [Google Scholar]
- 7.Pilz S, Tomaschitz A, Ritz E, Pieber TR. Vitamin D status and arterial hypertension: a systematic review. Nat Rev Cardiol 2009;6:621–30. [DOI] [PubMed] [Google Scholar]
- 8.Jorde R, Grimnes G. Vitamin D and metabolic health with special reference to the effect of vitamin D on serum lipids. Prog Lipid Res 2011;50:303–12. [DOI] [PubMed] [Google Scholar]
- 9.Li YC. Vitamin D regulation of the renin-angiotensin system. J Cell Biochem 2003;88:327–31. [DOI] [PubMed] [Google Scholar]
- 10.Vaidya A, Forman JP, Hopkins PN, Seely EW, Williams JS. 25-Hydroxyvitamin D is associated with plasma renin activity and the pressor response to dietary sodium intake in Caucasians. J Renin Angiotensin Aldosterone Syst 2011;12:311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valiña-Tóth AL, Lai Z, Yoo W, Abou-Samra A, Gadegbeku CA, Flack JM. Relationship of vitamin D and parathyroid hormone with obesity and body composition in African Americans. Clin Endocrinol (Oxf) 2010;72:595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract 2014;105:141–50. [DOI] [PubMed] [Google Scholar]
- 13.Menendez C, Lage M, Peino R, Baldelli R, Concheiro P, Dieguez C, Casanueva FF. Retinoic acid and vitamin D(3) powerfully inhibit in vitro leptin secretion by human adipose tissue. J Endocrinol 2001;170:425–31. [DOI] [PubMed] [Google Scholar]
- 14.Lorente-Cebrián S, Eriksson A, Dunlop T, Mejhert N, Dahlman I, Astrom G, Sjolin E, Wahlen K, Carlberg C, Laurencikiene J, et al. Differential effects of 1alpha,25-dihydroxycholecalciferol on MCP-1 and adiponectin production in human white adipocytes. Eur J Nutr 2012;51:335–42. [DOI] [PubMed] [Google Scholar]
- 15.Cnop M, Havel PJ, Utzschneider KM, Carr DB, Sinha MK, Boyko EJ, Retzlaff BM, Knopp RH, Brunzell JD, Kahn SE. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia 2003;46:459–69. [DOI] [PubMed] [Google Scholar]
- 16.Tschritter O, Fritsche A, Thamer C, Haap M, Shirkavand F, Rahe S, Staiger H, Maerker E, Haring H, Stumvoll M. Plasma adiponectin concentrations predict insulin sensitivity of both glucose and lipid metabolism. Diabetes 2003;52:239–43. [DOI] [PubMed] [Google Scholar]
- 17.Pittas AG, Chung M, Trikalinos T, Mitri J, Brendel M, Patel K, Lichtenstein AH, Lau J, Balk EM. Systematic review: vitamin D and cardiometabolic outcomes. Ann Intern Med 2010;152:307–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor HA Jr, Wilson JG, Jones DW, Sarpong DF, Srinivasan A, Garrison RJ, Nelson C, Wyatt SB. Toward resolution of cardiovascular health disparities in African Americans: design and methods of the Jackson Heart Study. Ethn Dis 2005;15(4 Suppl 6):S6-4–17. [PubMed] [Google Scholar]
- 19.Taylor HA., Jr The Jackson Heart Study: an overview. Ethn Dis 2005;15(4 Suppl 6):S6-1–3. [PubMed] [Google Scholar]
- 20.Wilson JG, Rotimi CN, Ekunwe L, Royal CD, Crump ME, Wyatt SB, Steffes MW, Adeyemo A, Zhou J, Taylor HA Jr, et al. Study design for genetic analysis in the Jackson Heart Study. Ethn Dis 2005;15:S6–30. [PubMed] [Google Scholar]
- 21.Holick MF. Vitamin D deficiency. N Engl J Med 2007;357:266–81. [DOI] [PubMed] [Google Scholar]
- 22.Swan HJC, Ganz W. Hemodynamic measurements in clinical practice: a decade in review. J Am Coll Cardiol 1983;1:103–13. [DOI] [PubMed] [Google Scholar]
- 23.Carpenter MA, Crow R, Steffes M, Rock W, Heilbraun J, Evans G, Skelton T, Jensen R, Sarpong D. Laboratory, reading center, and coordinating center data management methods in the Jackson Heart Study. Am J Med Sci 2004;328:131–44. [DOI] [PubMed] [Google Scholar]
- 24.Dubbert PM, Carithers T, Ainsworth BE, Taylor HA Jr, Wilson G, Wyatt SB. Physical activity assessment methods in the Jackson Heart Study. Ethn Dis 2005;15:S6–56. [PubMed] [Google Scholar]
- 25.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157–63. [DOI] [PubMed] [Google Scholar]
- 26.Trenberth KE. What are the seasons? Bull Am Meteorol Soc 1983;64:1276–82. [Google Scholar]
- 27.Amos [computer program]. Version 19. Chicago: SPSS; 2006.
- 28.Hayes A. Beyond Baron and Kenny: statistical mediation analysis in the new millennium. Commun Monogr 2009;76:408–20. [Google Scholar]
- 29.Sachs MC, Shoben A, Levin GP, Robinson-Cohen C, Hoofnagle AN, Swords-Jenny N, Ix JH, Budoff M, Lutsey PL, Siscovick DS, et al. Estimating mean annual 25-hydroxyvitamin D concentrations from single measurements: the Multi-Ethnic Study of Atherosclerosis. Am J Clin Nutr 2013;97:1243–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun X, Zemel MB. Calcium and 1,25-dihydroxyvitamin D3 regulation of adipokine expression. Obesity (Silver Spring) 2007;15:340–8. [DOI] [PubMed] [Google Scholar]
- 31.Shi H, Norman AW, Okamura WH, Sen A, Zemel MB. 1alpha,25-Dihydroxyvitamin D3 modulates human adipocyte metabolism via nongenomic action. FASEB J 2001;15:2751–3. [DOI] [PubMed] [Google Scholar]
- 32.Friedman JM. The function of leptin in nutrition, weight, and physiology. Nutr Rev 2002;60:S1–14. [DOI] [PubMed] [Google Scholar]
- 33.Farooqi IS, O’Rahilly S. Leptin: a pivotal regulator of human energy homeostasis. Am J Clin Nutr 2009;89(Suppl):908S. [DOI] [PubMed] [Google Scholar]
- 34.Park KG, Park KS, Kim MJ, Kim HS, Suh YS, Ahn JD, Park KK, Chang YC, Lee IK. Relationship between serum adiponectin and leptin concentrations and body fat distribution. Diabetes Res Clin Pract 2004;63:135–42. [DOI] [PubMed] [Google Scholar]
- 35.Flier JS. Clinical review 94: what’s in a name? In search of leptin’s physiologic role. J Clin Endocrinol Metab 1998;83:1407–13. [DOI] [PubMed] [Google Scholar]
- 36.Zhou C, Lu F, Cao K, Xu D, Goltzman D, Miao D. Calcium-independent and 1,25(OH)2D3-dependent regulation of the renin-angiotensin system in 1alpha-hydroxylase knockout mice. Kidney Int 2008;74:170–9. [DOI] [PubMed] [Google Scholar]
- 37.Lee JH, O’Keefe JH, Bell D, Hensrud DD, Holick MF. Vitamin D deficiency an important, common, and easily treatable cardiovascular risk factor? J Am Coll Cardiol 2008;52:1949–56. [DOI] [PubMed] [Google Scholar]
- 38.Lee JM, Kim SR, Yoo SJ, Hong OK, Son HS, Chang SA. The relationship between adipokines, metabolic parameters and insulin resistance in patients with metabolic syndrome and type 2 diabetes. J Int Med Res 2009;37:1803–12. [DOI] [PubMed] [Google Scholar]
- 39.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med 1996;334:292–5. [DOI] [PubMed] [Google Scholar]