Abstract
Ewing sarcoma/primitive neuroectodermal tumor (ES/PNET) is a tumor of small round cells arising in skeletal tissues. These tumors rarely arise in the stomach. We present a 31-year-old healthy female patient who was admitted to our surgical ward due to upper gastrointestinal hemorrhage. Upper endoscopy revealed a large ulcerated bleeding mass originating from the lesser curvature. Biopsy revealed tumor cell immunoreactivity positive for CD99, vimentin, and Ki67 (an index of proliferation). These findings were compatible with gastric ES/PNET. The fluorescence in situ hybridization analysis result for the EWSR1 gene rearrangement (11: 22 translocation) was positive. The patient refused neoadjuvant treatment and thus underwent an operation during which a mass at the lesser curvature of the stomach was found. The mass was adhering to the pancreatic tail and to the mesentery of the transverse and descending colon. Total gastrectomy, distal pancreatectomy, splenectomy, and left adrenalectomy were done. The patient refused adjuvant treatment. She is free of disease 3 years after surgery.
Keywords: Primary Ewing sarcoma, Primitive neuroectodermal tumor of the stomach, Rare tumor, CD99, Surgical management
Introduction
Ewing sarcoma/primitive neuroectodermal tumor (ES/PNET), previously thought to be separate tumors, is now treated as the same tumor; both have similar immunohistochemical characteristics and chromosomal translocation [1]. They are malignant tumors composed of undifferentiated small round cells, usually affecting children, adolescents, and young adults [2]. Generally ES/PNET affects the bones and deep soft tissues [3], although other organs such as the pancreas, small bowel, esophagus, kidneys, prostate, ovaries, vagina and rectovaginal septum have been reported; this is termed as extraskeletal ES/PNET [4]. To the best of our knowledge, only 5 cases of gastric ES/PNET have been reported in the English language literature.
Case Report
A 31-year-old healthy female patient was admitted to the surgical ward due to upper abdominal pain and coffee ground vomiting of 3 days’ duration. The patient had no other complaints and was hemodynamically stable.
No pathology was encountered on physical examination. Rectal examination revealed melena. A nasogastric tube was inserted and revealed coffee ground secretions. Complete blood count showed a hemoglobin level of 4.5 gr%. Liver and kidney function tests were within normal limits. Upper endoscopic examination revealed a large ulcerated mass located at the lesser curvature of the stomach, with oozing of blood. The bleeding was arrested by cautery and the mass was biopsied. Biopsy revealed tumor cells showing positive immunoreactivity for CD99 (fig 1), FLI1, vimentin, and Ki67, and negative immunoreactivity for cytokeratin, S100, CD20, CD3, CD79A, PAX5, CD30, CD43, DOG-1, CD68, CD163, CD33, MPOX, and desmin. ES/PNET was suspected and fluorescence in situ hybridization (FISH) analysis was ordered, which was positive for the EWSR1 gene rearrangement (11: 22 translocation). The patient was thus diagnosed with ES/PNET of the stomach.
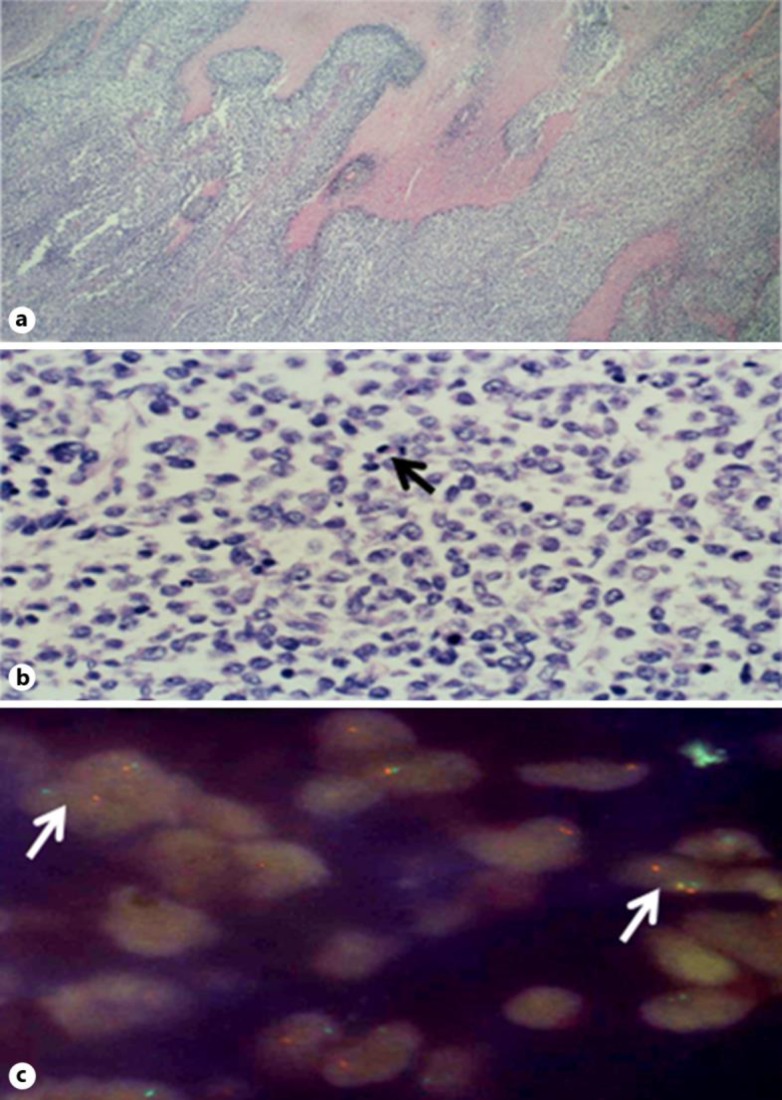
Fig. 1.
a Photomicrograph of the mass in the stomach wall showing a solid tumor with areas of necrosis. b Higher magnification shows cells of intermediate size, irregular nuclei, and clear cytoplasm with numerous mitoses (arrow). Immunohistochemistry stains for FLI1 and CD99 were positive. c FISH for 11: 22 translocation EWSR1 rearrangement was positive in 37% of cells. Positive cells show dissociation of green and orange probes (arrows).
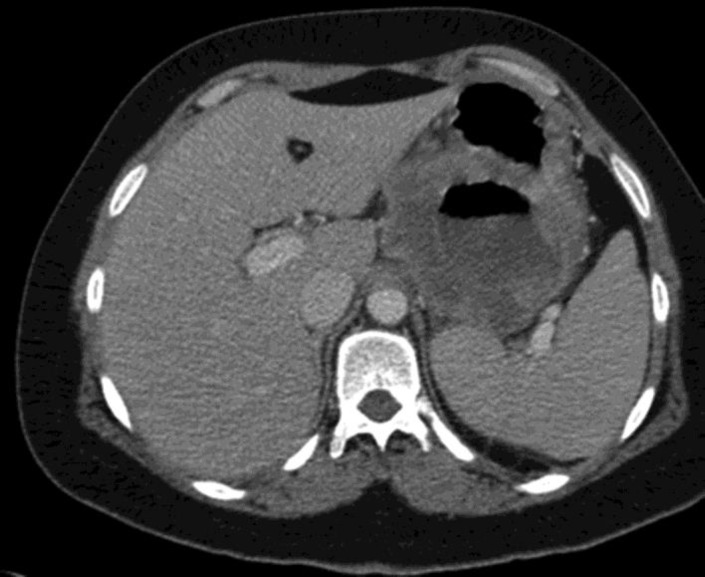
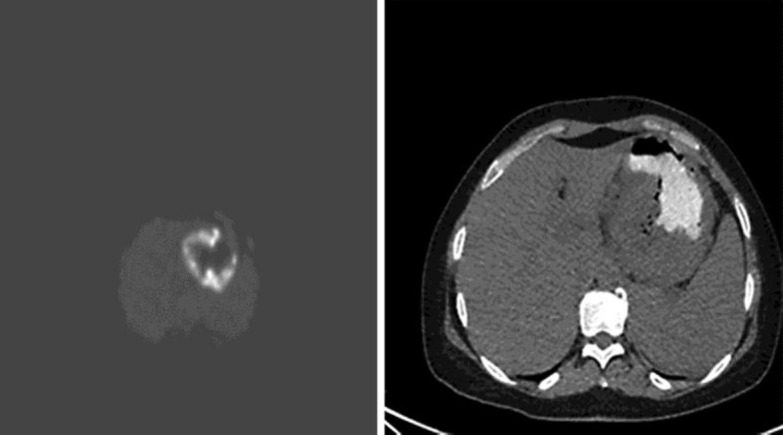
Total body computed tomography (CT) showed a hypodense mass measuring 9 cm at the lesser curvature of the stomach, with compression on the splenic vein (fig 2). Positron emission tomography-CT (PET-CT) revealed pathological uptake of fluorodeoxyglucose at the gastric mass and lymph nodes at the gastrohepatic ligament (fig 3). Bone marrow biopsy was negative for tumor cells. The patient refused neoadjuvant treatment, and thus surgery was performed.
Fig. 2.
Abdominopelvic CT scan showing a hypodense mass at the lesser curvature of the stomach.
Fig. 3.
PET-CT scan showing pathological uptake of fluorodeoxyglucose at the gastric mass.
On exploration of the abdomen, a large mass at the lesser curvature of the stomach was seen. The mass was adhering to the pancreatic tail and mesentery of the transverse and descending colon, along with abnormal pathological lymph nodes at the greater curvature. The celiac and superior mesenteric arteries were free of tumor. Total gastrectomy, distal pancreatectomy, splenectomy, and left adrenalectomy were done. The patient was discharged home on postoperative day 13.
Histopathological examination revealed the mass measuring 11 cm in diameter to be an ES/PNET invading the gastric wall, pancreas, and splenic hilum, without involvement of the left adrenal. Surgical margins along with 19 lymph nodes were free of disease. The patient refused postoperative adjuvant treatment.
Three years postoperatively, the patient is doing well, with no evidence of disease recurrence.
Discussion
ES, a term used to describe tumors that lack neuroectodermal differentiation, and PNET, used to describe tumors that exhibit neuroectodermal features, are now treated as a single entity [1]. Tumor cells are rich in glycogen, and pseudorosette formation characterizes the tumor's morphological differentiation [3].
Primary gastric ES/PNET is a very rare disease. The diagnosis can be made by immunohistochemical staining for a monoclonal antibody to CD99 (HBA/71, 12E7, and 013), which is positive in almost all cases of ES/PNET [5, 6, 7]. Another immunohistological reagent that can be used for diagnosis is the intermediate filament vimentin, which is usually positive [5]. Markers showing variable immunohistochemical staining include S100, chromogranin A, synaptophysin, and neuron-specific enolase [5].
For tumors occurring in older patients or at unusual sites, FISH or reverse transcription polymerase chain reaction can be used for diagnosis [3, 8]. These are used to test for the presence of genetic mutation (11: 22)(q24:q12) translocation (EWS/FLI1 fusion), which is an essential criterion for the diagnosis of ES/PNET, although sometimes these tests are negative [9, 10].
Due to the poor results of surgery alone as a treatment modality for extraskeletal ES/PNET, the current recommendation is multimodal treatment including surgery, chemotherapy, and radiotherapy [11]. The chemotherapeutical agents used as standard therapy for ES/PNET include vincristine, doxorubicin, cyclophosphamide, ifosfamide, and etoposide [12]. These multimodal treatments improve survival from 10 to 50–60% or more [13].
Only 5 cases of gastric ES/PNET have been reported. Of these 5 cases, 3 were females and 2 males, with an average age of 46.6 years (range 14–68 years) and an average tumor size of 8.5 cm. Most of these patients presented with abdominal pain, and 1 presented with an abdominal mass. Two cases had hepatic metastasis, 1 had lymph node and peritoneal metastasis, 1 no metastasis, and 1 was not reported. Only 2 patients (of the 3 with metastasis) were treated with systemic chemotherapy [14].
Herein, we describe the 6th case of ES/PNET of the stomach. Due to the patient's refusal of chemotherapeutical treatment, she eventually underwent a radical operation (R0 resection). Three years postoperatively, the patient is doing well, with no evidence of disease recurrence.
Conclusion
Primary gastric ES/PNET is a very rare tumor, and few cases are reported in the literature. Due to its rarity, the management algorithm is ill defined. Surgical resection of the tumor along with adjuvant chemoradiotherapy is recommended.
Statement of Ethics
Written informed consent was obtained from the patient for publication of this case report and accompanying images; it is available for consultation.
Disclosure Statement
The authors declare that they have no competing interests. They confirm that the manuscript has not been published elsewhere and is not under consideration by another journal.
References
- 1.Ushigome S, Machinami R, Sorensen PH. Ewing sarcoma/primitive neuroectodermal tumour (PNET) In: Fletcher CDM, Unni KK, Mertens F, editors. World Health Organization Classification of Tumours. Pathology & Genetics of Tumours of Soft Tissue and Bone. Lyon: IARC Press; 2002. pp. 298–300. [Google Scholar]
- 2.Kondo S, Yamaguchi U, Sakurai S, Ikezawa Y, Chuman H, Tateishi U, Furuta K, Hasegawa T. Cytogenetic confirmation of a gastrointestinal stromal tumor and Ewing sarcoma/primitive neuroectodermal tumor in a single patient. Jpn J Clin Oncol. 2005;35:753–756. doi: 10.1093/jjco/hyi197. [DOI] [PubMed] [Google Scholar]
- 3.Soulard R, Claude V, Camparo P, Dufau JP, Saint-Blancard P, Gros P. Primitive neuroectodermal tumor of the stomach. Arch Pathol Lab Med. 2005;129:107–110. doi: 10.5858/2005-129-107-PNTOTS. [DOI] [PubMed] [Google Scholar]
- 4.Bloom C, Lisbona A, Begin LR, Pollak M. Extraosseous Ewing's sarcoma. Can Assoc Radiol J. 1995;46:131–133. [PubMed] [Google Scholar]
- 5.Kempson RL, Fletcher CDM, Evans HL, et al. Tumors of the Soft Tissues. 3rd edition. Washington, DC: The Armed Forces Institute of Pathology; 2001. pp. 444–452. [Google Scholar]
- 6.Ambros IM, Ambros PF, Strehl S, Kovar H, Gadner H, Salzer-Kuntschik M. MIC2 is a specific marker for Ewing's sarcoma and peripheral primitive neuroectodermal tumors. Evidence for a common histogenesis of Ewing's sarcoma and peripheral primitive neuroectodermal tumors from MIC2 expression and specific chromosome aberration. Cancer. 1991;67:1886–1893. doi: 10.1002/1097-0142(19910401)67:7<1886::aid-cncr2820670712>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 7.Ramani P, Rampling D, Link M. Immunocytochemical study of 12E7 in small round-cell tumours of childhood: an assessment of its sensitivity and specificity. Histopathology. 1993;23:557–561. doi: 10.1111/j.1365-2559.1993.tb01243.x. [DOI] [PubMed] [Google Scholar]
- 8.Gardner LJ, Ayala AG, Monforte HL, Dunphy CH. Ewing sarcoma/peripheral primitive neuroectodermal tumor: adult abdominal tumors with an Ewing sarcoma gene rearrangement demonstrated by fluorescence in situ hybridization in paraffin sections. Appl Immunohistochem Mol Morphol. 2004;12:160–165. doi: 10.1097/00129039-200406000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Lewis TB, Coffin CM, Bernard PS. Differentiating Ewing's sarcoma from other round blue cell tumors using a RT-PCR translocation panel on formalin-fixed paraffin-embedded tissues. Mod Pathol. 2007;20:397–404. doi: 10.1038/modpathol.3800755. [DOI] [PubMed] [Google Scholar]
- 10.Tanas MR, Rubin BP, Tubbs RR, Billings SD, Downs-Kelly E, Goldblum JR. Utilization of fluorescence in situ hybridization in the diagnosis of 230 mesenchymal neoplasms: an institutional experience. Arch Pathol Lab Med. 2010;134:1797–1803. doi: 10.5858/2009-0571-OAR.1. [DOI] [PubMed] [Google Scholar]
- 11.Kennedy JG, Eustace S, Caulfield R, Fennelly DJ, Hurson B, O'Rourke KS. Extraskeletal Ewing's sarcoma: a case report and review of the literature. Spine (Phila Pa 1976) 2000;25:1996–1999. doi: 10.1097/00007632-200008010-00022. [DOI] [PubMed] [Google Scholar]
- 12.Leavey PJ, Collier AB. Ewing sarcoma: prognostic criteria, outcomes and future treatment. Expert Rev Anticancer Ther. 2008;8:617–624. doi: 10.1586/14737140.8.4.617. [DOI] [PubMed] [Google Scholar]
- 13.Grier HE, Krailo MD, Tarbell NJ, Link MP, Fryer CJ, Pritchard DJ, Gebhardt MC, Dickman PS, Perlman EJ, Meyers PA, Donaldson SS, Moore S, Rausen AR, Vietti TJ, Miser JS. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing's sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med. 2003;348:694–701. doi: 10.1056/NEJMoa020890. [DOI] [PubMed] [Google Scholar]
- 14.Inoue M, Wakai T, Korita PV, Sakata J, Kurosaki R, Ogose A, Kawashima H, Shirai Y, Ajioka Y, Hatakeyama K. Gastric Ewing sarcoma/primitive neuroectodermal tumor: a case report. Oncol Lett. 2011;2:207–210. doi: 10.3892/ol.2011.246. [DOI] [PMC free article] [PubMed] [Google Scholar]