Abstract
Aim:
The aim of this study is to investigate the Protein-Protein Interaction Network of Celiac Disease.
Background:
Celiac disease (CD) is an autoimmune disease with susceptibility of individuals to gluten of wheat, rye and barley. Understanding the molecular mechanisms and involved pathway may lead to the development of drug target discovery. The protein interaction network is one of the supportive fields to discover the pathogenesis biomarkers for celiac disease.
Material and methods:
In the present study, we collected the articles that focused on the proteomic data in celiac disease. According to the gene expression investigations of these articles, 31 candidate proteins were selected for this study. The networks of related differentially expressed protein were explored using Cytoscape 3.3 and the PPI analysis methods such as MCODE and ClueGO.
Results:
According to the network analysis Ubiquitin C, Heat shock protein 90kDa alpha (cytosolic and Grp94); class A, B and 1 member, Heat shock 70kDa protein, and protein 5 (glucose-regulated protein, 78kDa), T-complex, Chaperon in containing TCP1; subunit 7 (beta) and subunit 4 (delta) and subunit 2 (beta), have been introduced as hub-bottlnecks proteins. HSP90AA1, MKKS, EZR, HSPA14, APOB and CAD have been determined as seed proteins.
Conclusion:
Chaperons have a bold presentation in curtail area in network therefore these key proteins beside the other hub-bottlneck proteins may be a suitable candidates biomarker panel for diagnosis, prognosis and treatment processes in celiac disease.
Key Words: Protein-protein interaction, Network, celiac disease, hub-bottleneck
Introduction
Celiac disease (CD) is an autoimmune disease in susceptible individuals sensitive to gluten component of wheat, rye and barley (1, 2), which is responsible for immune reaction response (3). During initiation and development of CD, both genetic (human leukocyte antigen (HLA) genes (DQ2 or DQ8)) and environmental factors (gluten) are implicated participated (4, 5). Celiac patients are at the risk of nutritional deficiency leading to conditions like osteoporosis and iron deficiency anemia (6).
The proteomics study has been used to identify proteins or changes in protein expression of CD to discover a biomarkers using several techniques, such as antibody and tissue arrays, two-dimensional (2D) gel electrophoresis, mass spectrometry. Results of these techniques are suggesting that we may approached new insight in some diagnostic biomarkers by observing body fluids with posttranslational modifications during signaling (7) which will provide an understanding of the pathogenesis of CD. One of the logical fields to biomarker discovery is to study protein interaction networks, which can be useful because of making scientific abstraction based on principle roles of proteins in the biological function. However, the analysis of protein –protein interactions (PPIs) enable us to better understanding of biological processes, organization, and functions of proteins. In addition, it provides protein complex identification, (8) domain-domain interactions, (9) detection of proteins involved in disease pathways, (10) comparison between model organisms and humans (11) and introducing drug targets from network (12). Consequently, this knowledge can be translated and applied into effective diagnostic and therapeutic strategies (13) by identification of drug targets and hubs (proteins with larger number of interactions) (14, 15). Meanwhile proteomics and PPI networks analysis together are powerful tools to determine associated biomarkers with specific pathways and biological functions (16-23). In this study, the enrichment analysis of selected proteins based on the GO and PPI is investigated to introduce some related molecular biomarkers (as a panel) for celiac disease.
Material and Methods
PubMed (PubMed Central), ISI of web knowledge, and Google scholar were searched for full text articles on the associated keywords "proteomic" and "celiac disease" published until April 2016 (24, 25). Based on results from previous gene expression studies, 31 protein candidates were selected for celiac disease and the PPI network was visualized using the Cytoscape 3.3 software (26). "Cytoscape is an open source software project for integrating bimolecular interaction networks with high-throughput expression data and other molecular entity into a unified conceptual state (27). In this study, MINT and Reactome-FLs databases were used for topology visualization. Also, we used Molecular Complex Detection (MCODE) based on topology to find densely connected region to analyze the characteristics of the networks. These include Kappa statistic ≥ 0.5, enrichment and Bonferroni step down method for probability value correction (28). Gene ontology categories were analyzed to identify the function of each highly connected region that was generated by the MCODE. It visualizes the biological terms for large clusters of proteins in a functionally grouped network non-redundantly (29). Functional enrichment for a given cluster was assessed (P-value) quantitatively, using the ClueGO tool (30).
Results
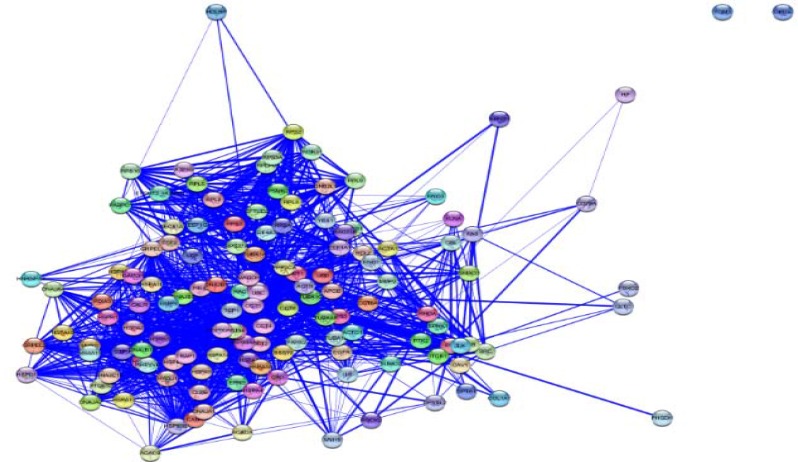
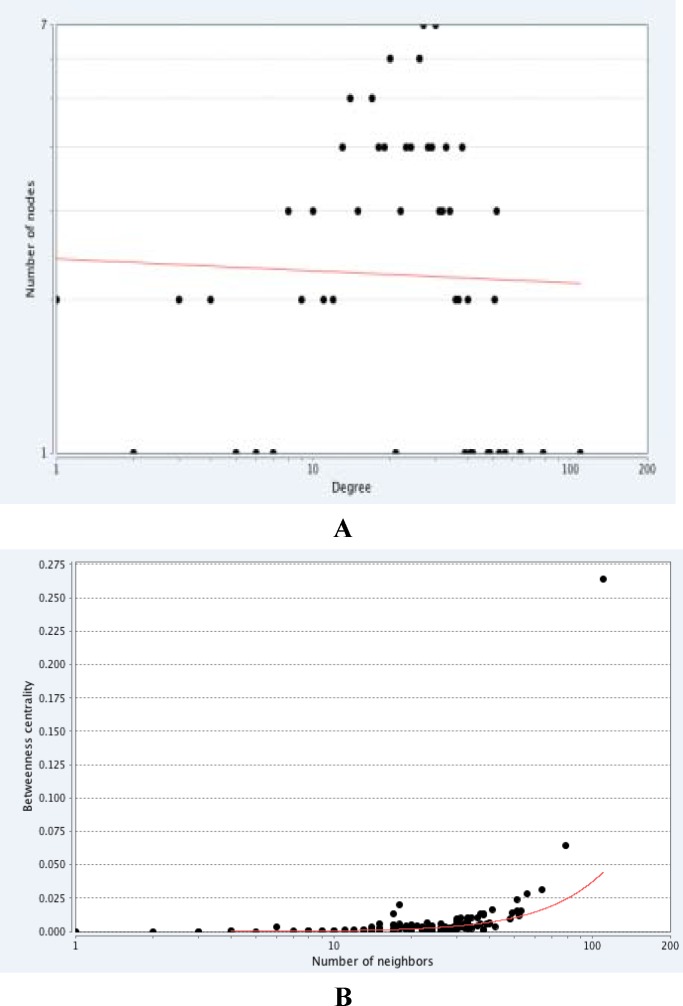
The topological analyses were carried out via algorithms such as centrality measures. Graph centrality measures like degree, betweenness and closeness centrality are so useful in the identification of nodes that are functionally crucial in the network (31). In the PPI network the nodes with high degree defined as hub proteins and the nodes with high betweenness defined as bottleneck proteins, which both paly a fundumental role in the network (32). Cytoscape analysis revealed a great number of close interconnections that can be seen in Figure 1. The power law of node degree distribution is one of the most important criteria of biological networks which indicated that the PPI networks were scale-free (figure 2). This scale-free distribution network implies on the presence of proteins with high centrality values computed by Network Analyzer in celiac patients. The red line indicates the power law. The R-squared value is computed on logarithmized values, which are equal to 0. 60, - 0.02 and the correlation= 0.86, 0.001 for betweenness centrality and degree, respectively.
Figure 1.
PPI network of celiac disease consists of 134 nodes and 1686 edges
Figure 2.
Th power law distribution of nod's centrality measures. (A) Degree distribution of the PPI network in celiac. (B) Betweenness centrality distribution of PPI network in celiac
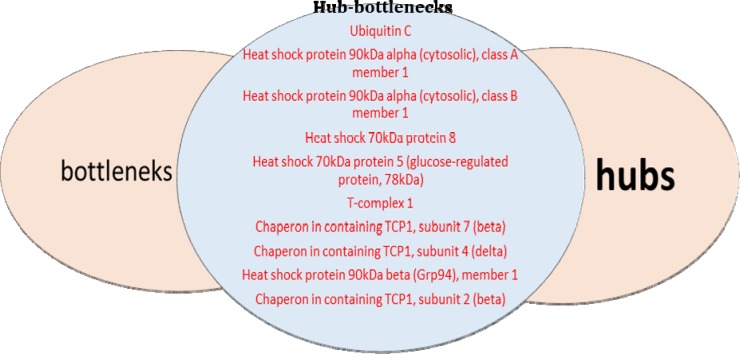
To identify crutial nodes in PPI networks, two centrality criteria, including degree and betweenness have been used in this study. First, we prepared the list of proteins, which were hubs in PPI network. The results indicate that some nodes have a large number of links to other protein nodes and act as hubs. Then we determine the bottlenecks (highest betweeness nodes) in the network (table 1). It was shown that there are common proteins between hubs and bottleneck (figure 3).
Table 1.
The proteins with highest degree in celiac that introduce as hub sorted by degree number
| stringdb | Gene name | Degree | Betweenness centrally |
|---|---|---|---|
| 9606.ENSP00000344818 | ubiquitin C | 110 | 0.26405012 |
| 9606.ENSP00000335153 | heat shock protein 90kDa alpha (cytosolic), class A member 1 | 79 | 0.06416467 |
| 9606.ENSP00000325875 | heat shock protein 90kDa alpha (cytosolic), class B member 1 | 64 | 0.03145362 |
| 9606.ENSP00000227378 | heat shock 70kDa protein 8 | 56 | 0.02820046 |
| 9606.ENSP00000317334 | t-complex 1 | 53 | 0.01534998 |
| 9606.ENSP00000377958 | chaperonin containing TCP1, subunit 4 (delta) | 52 | 0.01440804 |
| 9606.ENSP00000299300 | chaperonin containing TCP1, subunit 2 (beta) | 52 | 0.01366942 |
| 9606.ENSP00000324173 | heat shock 70kDa protein 5 (glucose-regulated protein, 78kDa) | 51 | 0.02409187 |
| 606.ENSP00000258091 | chaperonin containing TCP1, subunit 7 (beta) | 51 | 0.01527081 |
| 9606.ENSP00000299767 | heat shock protein 90kDa beta (Grp94), member 1 | 49 | 0.01383266 |
Figure 3.
The proteins with high scores of degree as hub and high scores of betweeness in celiac that introduce as bottleneck. The common proteins in hub and bottleneck group introduce as hub- bottleneck
As represented in table 1, Ubiquitin C, Heat shock protein 90kDa alpha (cytosolic and Grp94); class A, B and 1 member, Heat shock 70kDa protein, and protein 5 (glucose-regulated protein, 78kDa), T-complex, Chaperon in containing TCP1; subunit 7 (beta) and subunit 4 (delta) and subunit 2 (beta), have been introduced as hub – bottleneck. Chaperon in containing TCP1, subunit 2 (beta) have been introduced as hub – bottlenecks. In this study hubs and bottlenecks were the same as hub-bottlenecks in which bold the importance of these proteins.
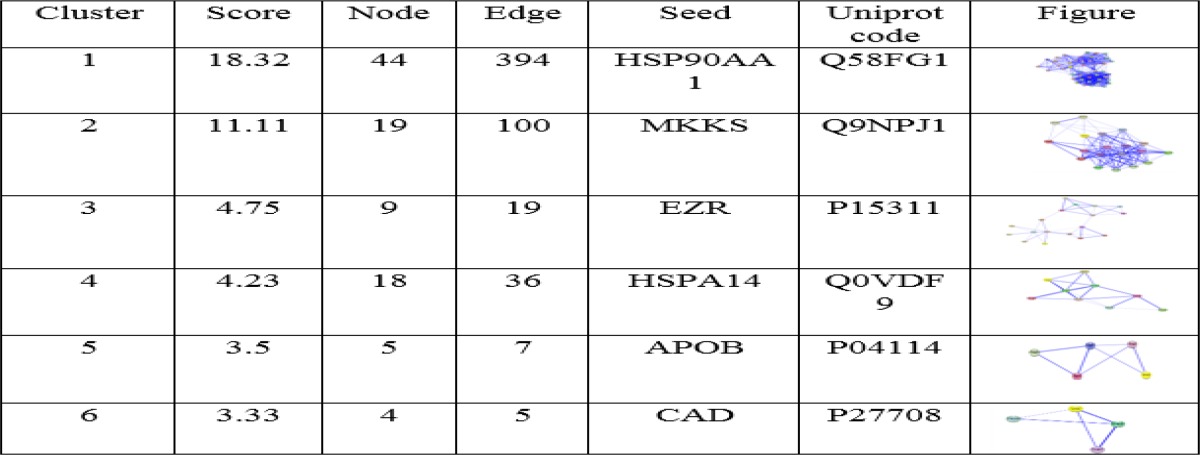
The results of MCODE analysis were shown in table 2. Bonferroni step down was applied for p-value adjustment and pathways with adjusted p-value<0.05 were selected. Further analysis of complex by MCODE revealed six sub networks for the network (Table 3). HSP90AA1, MKKS, EZR, HSPA14, APOB and CAD are proteins that involved in protein networks of celiac disease and have been determined as seeds.
Table 2.
MCODE algorithm analysis demonstrate clusters and have been sorted by score
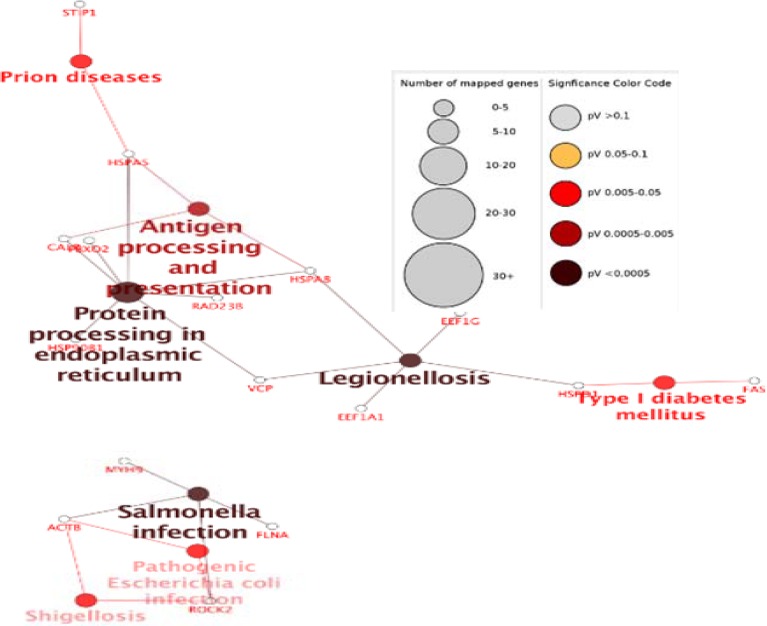
Functional distribution of the biological process of celiac modules was shown in figure 4. According to information presented in figure 3 some of the important proteins as hub – bottleneck are involved in antigen processing and presentation (HSPA5, HSPA8), protein processing in the endoplasmic reticulum (HSP90B1, HSPA5, HSPA8) and legionellosis (HSPA8).
Figure 4.
Functional distribution of biological process of modules of celiac disease
Discussion
Since proteins act as complex and corporate with each other in cellular events and pathways, their deregulation are resulted in disorders. One way to evaluate and analyze the pathways is molecular mapping (33). Recently, quantitative tools have been developed for analyzing the molecular mapping and networks. Analyzing the network attributes of gene-expression data might reveal the pattern of gene expression in diseases, which might be helpful to identify new potential drug targets (34). Therefore, in this study, the network is analyzed to extract celiac disease markers by Cytoscape software. As represented in table 1; Heat shock proteins and chaperonines have been introduced as a hub – bottleneck. In this study, hubs and bottlenecks were the same as hub-bottlenecks. Therefore, it can be concluded that these proteins play important roles in the pathology of disease. The highest score of degree and centrality belong to Ubiquitin C (UbC) which is one of the four genes encoding ubiquitin in the mammalian genome. The role of this gene in several processes such as oxidative stress, UV irradiation, heat shock, and translational impairment is discussed (35). The 6q21-22 region of ubiquitin-pathways components was confirmed as a celiac disease susceptibility locus (36). Heat shock proteins (HSPs) have been introduced as hub-bottleneck such as Heat shock protein 90kDa alpha (cytosolic), class A and B member, Heat shock 70kDa protein, Heat shock protein 90kDa beta (Grp94), member 1 and Heat shock 70kDa protein 5 (glucose-regulated protein, 78kDa). HSPs are molecular chaperones that increase survival by allowing cells to resist stress through cyto-protective mechanisms (37). Heat shock proteins (HSPs) are classes of proteins that have already appeared as drug targets for autoimmune diseases that protect cells against harmful extracellular factors. HSPs play a key role in the maintenance of epithelial cell structure and function and also put immunomodulatory effects. They are responsible for cell repair processes after damage and adequate protein folding, proliferation and apoptosis, influence the degradation of proteins and modulate cell signaling (38). In this study, Hsp90AA1 has also been introduced as another hub-bottleneck and seed with the highest score. The expression changes of HSP90AA1 in celiac disease is poorly documented, but in cancer research the 90-kDa heat shock protein HSP90AA1 is critical for the stability of several proteins that are important for tumor progression and introduced as a promising target for cancer therapy (39). Introducing HSP 90 AA1 as a drug target and curtail protein in our network is not unexpected because in CD nitric oxide levels increases via gluten consumption and then increases ROS level (40, 41). Interestingly, these oxygen-free radicals induce the expression of various HSPs such HSP 90, which take part in the defense against oxidative stress (42). Another chaperon that in this study introduced as hub-bottelnek was T-complex 1. As mentioned, the chaperonins are key molecular complexes, which are essential in the folding of proteins to stabilization. One member of the chaperonin group of proteins is TCP1, but little is known about this protein in diseases. Studies were shown that both TCP1 beta and TCP1 epsilon are over-expressed in colorectal cancer and indicate a role in colorectal cancer progression (43). It has also been represented alteration in protein expression with chaperone activities such as T-complex protein-1 in a human intestinal epithelial cell line for celiac disease (24). Chaperon in containing TCP1, subunit 7 (beta), Chaperon in containing TCP1, subunit 4 (delta) as well as chaperon in containing TCP1, subunit 2 (beta) are molecular chaperones that are another hub-bottlenecks which also known as members of the chaperonin containing TCP1 complex (CCT). This ATP-dependent complex folds various proteins, including actin and tubulin (44, 45) introduced as a curtail protein in cancer development (46) are considered as novel therapeutic targets in breast cancer (47). Another seed is McKusick-Kaufman/Bardet-Biedl syndromes putative chaperonin (MKKS) as a molecular chaperone that assists the folding of proteins upon ATP hydrolysis. As part of the BBS/CCT complex may play a role in the assembly of BBSome (complex involved in ciliogenesis ) and cytokinesis (48), but there are no obvious documents of its relation with celiac disease up to now. Another seed named Ezrin is the only ERM family member expressed in the intestinal epithelium (49) and interacts with F-actin (50). It has been suggested phosphorylation of Ezrin will regulate the early events in brush border induction (51) and loss of microvilli and brush border lead to common features of several intestinal diseases (52, 53). Another chaperonin that has been suggested as seed is HSPA14. Increased HSP expression in tumour tissues is a common phenomenon (54). The HSPA14 was one of overexpressed proteins in HCC tumour tissues (55). Apolipoprotein B is another seed and a major protein constituent of VLDL (apo B-100), LDL (apo B-100) and chylomicrons (apo B-48). In CD, The basal condition was characterized by low cholesterol absorption, enhanced cholesterol synthesis, and high removal rate of LDL apo B (56). It has also been reported that low high-density lipoprotein-cholesterol concentration associated with CD (57-59). In the celiac disease, protein- protein interaction network the lowest score of seeds was belonged to CAD which encoding four enzymatic activities of the pyrimidine pathway (60). The association of CAD activation and uncontrolled cell proliferation in cancer has been reported(61), but no clear and directed relation has been reported with celiac disease yet. Our major finding pathways- based network analysis between the patients and a normal one, include antigen processing and presentation (HSPA5, HSPA8), protein processing in the endoplasmic reticulum (HSP90B1, HSPA5, HSPA8) and legionellosis (HSPA8). Understanding the molecular mechanisms and involved pathway may lead to the development of drugs target discovery. Through our analysis, it has been provided new insight into celiac pathogenesis by analyzing the networks and consequently presenting the specified pattern of gene expression, which might identify new biomarkers and targets for potential treatment. Introducing chaperons (HSPs &TCP) as powerful biomarkers and drug targets lead to emphasize a possible close molecular relationship between celiac and oxidative stress, repair processes after damage and protein folding. This information can suggest a strong possibility to design drug through suppressing ROS and free radicals. To reveal the possible role(s) of these proteins in celiac disease, further investigations are needed.
Acknowledgments
This paper is derived from the PhD thesis of Mrs Akram Safaei.
References
- 1.Farrell RJ, Kelly CP. Celiac sprue. N Eng J Med. 2002;346:180–88. doi: 10.1056/NEJMra010852. [DOI] [PubMed] [Google Scholar]
- 2.Green PH, Jabri B. Celiac disease. Annu Rev Med. 2006;57:207–21. doi: 10.1146/annurev.med.57.051804.122404. [DOI] [PubMed] [Google Scholar]
- 3.Koning F. Celiac disease: caught between a rock and a hard place. Gastroenterology. 2005;129:1294–301. doi: 10.1053/j.gastro.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 4.Godkin A, Jewell D. The pathogenesis of celiac disease. Gastroenterology. 1998;115:206–10. doi: 10.1016/s0016-5085(98)70382-8. [DOI] [PubMed] [Google Scholar]
- 5.Green PH, Cellier C. Celiac disease. N Eng J Med. 2007;357:1731–43. doi: 10.1056/NEJMra071600. [DOI] [PubMed] [Google Scholar]
- 6.Nejad MR, Rostami K, Pourhoseingholi MA, Mojarad EN. Atypical presentation is dominant and typical for coeliac. J Gastrointestin Liver Dis. 2009;18:285–91. [PubMed] [Google Scholar]
- 7.De Re V, Simula MP, Canzonieri V, Cannizzaro R. Proteomic analyses lead to a better understanding of celiac disease: focus on epitope recognition and autoantibodies. Dig Dis Sci. 2010;55:3041–46. doi: 10.1007/s10620-010-1323-1. [DOI] [PubMed] [Google Scholar]
- 8.Srihari S, Leong HW. A survey of computational methods for protein complex prediction from protein interaction networks. J Bioinform Comput Biol. 2013;11:1230002. doi: 10.1142/S021972001230002X. [DOI] [PubMed] [Google Scholar]
- 9.Guimarães KS, Jothi R, Zotenko E, Przytycka TM. Predicting domain-domain interactions using a parsimony approach. Genome Biol. 2006;7:R104.1–14. doi: 10.1186/gb-2006-7-11-r104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rhodes DR, Tomlins SA, Varambally S, Mahavisno V, Barrette T, Kalyana-Sundaram S, et al. Probabilistic model of the human protein-protein interaction network. Nat Biotechnol. 2005;23:951–59. doi: 10.1038/nbt1103. [DOI] [PubMed] [Google Scholar]
- 11.Gandhi T, Zhong J, Mathivanan S, Karthick L, Chandrika K, Mohan SS, et al. Analysis of the human protein interactome and comparison with yeast, worm and fly interaction datasets. Nat Genet. 2006;38:285–93. doi: 10.1038/ng1747. [DOI] [PubMed] [Google Scholar]
- 12.Safaei A, Tavirani MR, Oskouei AA, Azodi MZ, Mohebbi SR, Nikzamir AR. Protein-protein interaction network analysis of cirrhosis liver disease. Gastroenterol Hepatol Bed Bench. 2016;9:114. [PMC free article] [PubMed] [Google Scholar]
- 13.Safari-Alighiarloo N, Taghizadeh M, Rezaei-Tavirani M, Goliaei B, Peyvandi AA. Protein-protein interaction networks (PPI) and complex diseases. Gastroenterol Hepatol Bed Bench. 2014;7:17–31. [PMC free article] [PubMed] [Google Scholar]
- 14.Singh GP, Ganapathi M, Dash D. Role of intrinsic disorder in transient interactions of hub proteins. Proteins. 2007;66:761–65. doi: 10.1002/prot.21281. [DOI] [PubMed] [Google Scholar]
- 15.Sarmady M, Dampier W, Tozeren A. HIV protein sequence hotspots for crosstalk with host hub proteins. PLoS One. 2011;6:e23293. doi: 10.1371/journal.pone.0023293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee S-A, Tsao TT, Yang K-C, Lin H, Kuo Y-L, Hsu C-H, et al. Construction and analysis of the protein-protein interaction networks for schizophrenia, bipolar disorder, and major depression. BMC Bioinformatics. 2011;12 doi: 10.1186/1471-2105-12-S13-S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ran J, Li H, Fu J, Liu L, Xing Y, Li X, et al. Construction and analysis of the protein-protein interaction network related to essential hypertension. BMC Syst Biol. 2013;7:32. doi: 10.1186/1752-0509-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rakshit H, Rathi N, Roy D. Construction and analysis of the protein-protein interaction networks based on gene expression profiles of Parkinson's disease. PLoS One. 2014;9:e103047. doi: 10.1371/journal.pone.0103047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zali H, Rezaei-Tavirani M, Azodi M. Gastric cancer: prevention, risk factors and treatment. Gastroenterol Hepatol Bed Bench. 2011;4:175–85. [PMC free article] [PubMed] [Google Scholar]
- 20.Zamanian-Azodi M, Rezaei-Tavirani M, Hasanzadeh H, Rahmati Rad S, Dalilan S. Introducing biomarker panel in esophageal, gastric, and colon cancers; a proteomic approach. Gastroenterol Hepatol Bed Bench. 2015;8:6–18. [PMC free article] [PubMed] [Google Scholar]
- 21.Zali H, Zamanian-Azodi M, Rezaei Tavirani M, Akbar-Zadeh Baghban A. Protein drug targets of lavandula angustifolia on treatment of rat Alzheimer's disease. Iran J Pharm Res. 2015;14:291–302. [PMC free article] [PubMed] [Google Scholar]
- 22.Rezaei-Tavirani M, Zamanian-Azodi M, Rajabi S, Masoudi-Nejad A, Rostami-Nejad M, Rahmatirad S. Protein clustering and interactome analysis in Parkinson and Alzheimer's diseases. Arch Iran Med. 2016;19:101–09. [PubMed] [Google Scholar]
- 23.Rezaei-Tavirani M, Rahmati-Rad S, Rezaei-Tavirani M. Ethanol and cancer induce similar changes on protein expression pattern of human fibroblast cell. Iran J Pharm Res. 2016;15:175–84. [PMC free article] [PubMed] [Google Scholar]
- 24.Orrù S, Caputo I, D'Amato A, Ruoppolo M, Esposito C. Proteomics identification of acyl-acceptor and acyl-donor substrates for transglutaminase in a human intestinal epithelial cell line Implications for celiac disease. J Biol Chem. 2003;278:31766–73. doi: 10.1074/jbc.M305080200. [DOI] [PubMed] [Google Scholar]
- 25.Stulík J, Hernychová L, Porkertová S, Pozler O, Tučková L, Sánchez D, et al. Identification of new celiac disease autoantigens using proteomic analysis. Proteomics. 2003;3:951–56. doi: 10.1002/pmic.200300370. [DOI] [PubMed] [Google Scholar]
- 26.Huang DW, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Gen Res. 2003;13:2498–504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rivera CG, Vakil R, Bader JS. NeMo: Network Module identification in Cytoscape. BMC Bioinformatics. 2010;11:S61. doi: 10.1186/1471-2105-11-S1-S61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bindea G, Mlecnik B, Hackl H, Charoentong P, Tosolini M, Kirilovsky A, et al. ClueGO: a Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics. 2009;25:1091–93. doi: 10.1093/bioinformatics/btp101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhong S, Xie D. Gene Ontology analysis in multiple gene clusters under multiple hypothesis testing framework. Artif Intell Med. 2007;41:105–15. doi: 10.1016/j.artmed.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 31.Hindumathi V, Kranthi T, Rao S, Manimaran P. The prediction of candidate genes for cervix related cancer through gene ontology and graph theoretical approach. Mol Biosyst. 2014;10:1450–60. doi: 10.1039/c4mb00004h. [DOI] [PubMed] [Google Scholar]
- 32.Yu H, Kim PM, Sprecher E, Trifonov V, Gerstein M. The importance of bottlenecks in protein networks: correlation with gene essentiality and expression dynamics. PLoS Comput Biol. 2007;3:e59. doi: 10.1371/journal.pcbi.0030059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ficenec D, Osborne M, Pradines J, Richards D, Felciano R, Cho RJ, et al. Computational knowledge integration in biopharmaceutical research. Brief Bioinform. 2003;4:260–78. doi: 10.1093/bib/4.3.260. [DOI] [PubMed] [Google Scholar]
- 34.Tavirani MR. Meningioma protein-protein interaction network. Arch Iran Med. 2014;17:262–72. [PubMed] [Google Scholar]
- 35.Radici L, Bianchi M, Crinelli R, Magnani M. Ubiquitin C gene: structure, function, and transcriptional regulation. Adv Biosci Biotech. 2013;4:1057–62. [Google Scholar]
- 36.Einarsdottir E, Bevova MR, Zhernakova A, Monsuur A, Koskinen LL, van't Slot R, et al. Multiple independent variants in 6q21-22 associated with susceptibility to celiac disease in the Dutch, Finnish and Hungarian populations. Eur J Hum Gen. 2011;19:682–86. doi: 10.1038/ejhg.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Almeida MB, do Nascimento JLM, Herculano AM, Crespo-López ME. Molecular chaperones: toward new therapeutic tools. Biomed Pharmacother. 2011;65:239–43. doi: 10.1016/j.biopha.2011.04.025. [DOI] [PubMed] [Google Scholar]
- 38.Sziksz E, Pap D, Veres G, Fekete A, Tulassay T, Vannay Á. Involvement of heat shock proteins in gluten-sensitive enteropathy. World J Gastroenterol. 2014;20:6495–503. doi: 10.3748/wjg.v20.i21.6495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shen H, Zhu H, Song M, Tian Y, Huang Y, Zheng H, et al. A selenosemicarbazone complex with copper efficiently down-regulates the 90-kDa heat shock protein HSP90AA1 and its client proteins in cancer cells. BMC Cancer. 2014;14:629. doi: 10.1186/1471-2407-14-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Straaten EV, Koster‐Kamphuis L, Bovee‐Oudenhoven I, Meer Rvd, Forget PP. Increased urinary nitric oxide oxidation products in children with active coeliac disease. Acta Paediatrica. 1999;88:528–31. doi: 10.1080/08035259950169530. [DOI] [PubMed] [Google Scholar]
- 41.Diosdado B, van Oort E, Wijmenga C. “Coelionomics”: towards understanding the molecular pathology of coeliac disease. Clin Chem Lab Med. 2005;43:685–95. doi: 10.1515/CCLM.2005.117. [DOI] [PubMed] [Google Scholar]
- 42.Omar R, Pappolla M. Oxygen free radicals as inducers of heat shock protein synthesis in cultured human neuroblastoma cells: relevance to neurodegenerative disease. Eur Arch Psychiatry Clin Neurosci. 1993;242:262–67. doi: 10.1007/BF02190384. [DOI] [PubMed] [Google Scholar]
- 43.Coghlin C, Carpenter B, Dundas S, Lawrie L, Telfer C, Murray G. Characterization and over‐expression of chaperonin t‐complex proteins in colorectal cancer. J Pathol. 2006;210:351–57. doi: 10.1002/path.2056. [DOI] [PubMed] [Google Scholar]
- 44.Yaffe MB, Farr GW, Miklos D, Horwich AL, Sternlicht ML, Sternlicht H. TCP1 complex is a molecular chaperone in tubulin biogenesis. Nature. 1992;358:245–48. doi: 10.1038/358245a0. [DOI] [PubMed] [Google Scholar]
- 45.Melki R, Vainberg IE, Chow RL, Cowan NJ. Chaperonin-mediated folding of vertebrate actin-related protein and gamma-tubulin. J Cell Biol. 1993;122:1301–10. doi: 10.1083/jcb.122.6.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boudiaf-Benmammar C, Cresteil T, Melki R. The cytosolic chaperonin CCT/TRiC and cancer cell proliferation. PLoS One. 2013;8:e60895. doi: 10.1371/journal.pone.0060895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guest ST, Kratche ZR, Bollig-Fischer A, Haddad R, Ethier SP. Two members of the TRiC chaperonin complex, CCT2 and TCP1 are essential for survival of breast cancer cells and are linked to driving oncogenes. Exp Cell Res. 2015;332:223–35. doi: 10.1016/j.yexcr.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 48.Seo S, Baye LM, Schulz NP, Beck JS, Zhang Q, Slusarski DC, et al. BBS6, BBS10, and BBS12 form a complex with CCT/TRiC family chaperonins and mediate BBSome assembly. Proc Natl Acad Sci U S A. 2010;107:1488–93. doi: 10.1073/pnas.0910268107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bretscher A. Purification of an 80,000-dalton protein that is a component of the isolated microvillus cytoskeleton, and its localization in nonmuscle cells. J Cell Biol. 1983;97:425–32. doi: 10.1083/jcb.97.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Algrain M, Turunen O, Vaheri A, Louvard D, Arpin M. Ezrin contains cytoskeleton and membrane binding domains accounting for its proposed role as a membrane-cytoskeletal linker. J Cell Biol. 1993;120:129–39. doi: 10.1083/jcb.120.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.ten Klooster JP, Jansen M, Yuan J, Oorschot V, Begthel H, Di Giacomo V, et al. Mst4 and Ezrin induce brush borders downstream of the Lkb1/Strad/Mo25 polarization complex. Dev Cell. 2009;16:551–62. doi: 10.1016/j.devcel.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 52.Bailey D, Freedman A, Price S, Chescoe D, Ciclitira P. Early biochemical responses of the small intestine of coeliac patients to wheat gluten. Gut. 1989;30:78–85. doi: 10.1136/gut.30.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bartles JR, Zheng L, Li A, Wierda A, Chen B. Small espin: a third actin-bundling protein and potential forked protein ortholog in brush border microvilli. J Cell Biol. 1998;143:107–19. doi: 10.1083/jcb.143.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Calderwood SK, Khaleque MA, Sawyer DB, Ciocca DR. Heat shock proteins in cancer: chaperones of tumorigenesis. Trends in Biochem Sci. 2006;31:164–72. doi: 10.1016/j.tibs.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 55.Yang Z, Zhuang L, Szatmary P, Wen L, Sun H, Lu Y, et al. Upregulation of heat shock proteins (HSPA12A, HSP90B1, HSPA4, HSPA5 and HSPA6) in tumour tissues is associated with poor outcomes from HBV-related early-stage hepatocellular carcinoma. Int J Med Sci. 2015;12:256–63. doi: 10.7150/ijms.10735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vuoristo M, Kesäniemi Y, Gylling H, Miettinen T. Metabolism of cholesterol and apolipoprotein B in celiac disease. Metabolism. 1993;42:1386–91. doi: 10.1016/0026-0495(93)90187-s. [DOI] [PubMed] [Google Scholar]
- 57.Capristo E, Addolorato G, Mingrone G, Scarfone A, Greco AV, Gasbarrini G. Low-serum high-density lipoprotein-cholesterol concentration as a sign of celiac disease. Am J Gastroenterol. 2000;95:3331–32. doi: 10.1111/j.1572-0241.2000.03329.x. [DOI] [PubMed] [Google Scholar]
- 58.Mediene S, Hakem S, Bard J, Medjaoui I, Benhamamouch S, Lebel P, et al. Serum lipoprotein profile in Algerian patients with celiac disease. Clinica Chimica Acta. 1995;235:189–96. doi: 10.1016/0009-8981(95)06028-1. [DOI] [PubMed] [Google Scholar]
- 59.Brar P, Kwon GY, Holleran S, Bai D, Tall AR, Ramakrishnan R, et al. Change in lipid profile in celiac disease: beneficial effect of gluten-free diet. Am J Med. 2006;119:786–90. doi: 10.1016/j.amjmed.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 60.Grande-García A, Lallous N, Díaz-Tejada C, Ramón-Maiques S. Structure, functional characterization, and evolution of the dihydroorotase domain of human CAD. Structure. 2014;22:185–98. doi: 10.1016/j.str.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 61.Masko E, Mhaskar M, Heyl-Clegg D, Guy-Evans H, Evans D. Protein-protein interactions between the multifunctional protein CAD and protein phosphatase 1 in breast cancer. San Diego, CA: AACR Annual Meeting; 2008. Apr . [Google Scholar]