Abstract
Background
The Biomarker-integrated Approaches of Targeted Therapy for Lung Cancer Elimination trial1 prospectively obtained serum and tumor core biopsies and randomized 255 chemorefractory non–small-cell lung cancer (NSCLC) patients into four phase II trials: erlotinib, erlotinib-bexarotene, vandetanib, or sorafenib. Herein, we report the clinical and biomarker results of the phase II vandetanib trial.
Results
Fifty-four patients received vandetanib. The 8-week disease control rate was 33%, median progression-free survival (PFS) 1.81 months, and median overall survival (OS) 6.5 months. No demographic subgroups had PFS or OS benefit. Eight patients with EGFR mutations had a trend for higher 8-week disease control rate (63% versus 31%; p = 0.12) but worse OS (5.9 months versus 9 months; p = 0.8). Patients with EGFR gene amplification (n = 6) had a worse OS (3.9 months versus 9.5 months; p = 0.04). KRAS mutation patients (3.9 months versus 9.5 months; p = 0.23) also had a worse OS. For the serum biomarker analysis, patients with below the median serum expression of interleukin 9c (p = 0.019) and eotaxin (p = 0.007) had a shorter PFS. A trend toward a shorter PFS was also seen in patients with higher than the median neutrophil gelatinase-associated lipocalin (p = 0.079) and lower than the median TNF-related apoptosis-inducing ligand (p = 0.087).
Conclusion
Our trial results are largely consistent with the literature in unselected pretreated NSCLC patients. Although vandetanib improved median PFS in EGFR mutation patients with epidermal growth factor receptor tyrosine kinase inhibitor–resistance compared with EGFR wild-type, there was no OS advantage. Although vandetanib is no longer in development in NSCLC, identification of a molecular phenotype that responds to dual epidermal growth factor receptor and vascular endothelial growth factor receptor inhibition would contribute to the field.
Keywords: Vandetanib, Non–small-cell lung cancer, EGFR mutation, EGFR gene amplification
The Biomarker-integrated Approaches of Targeted Therapy for Lung Cancer Elimination (BATTLE) trial1 conducted at M.D. Anderson Cancer Center (Houston, Texas) randomized (using 1 of 2 algorithms) 255 chemorefractory non–small-cell lung cancer (NSCLC) patients into four separate phase II targeted therapy trials: erlotinib (OSIP/Genentech, San Francisco, CA), erlotinib plus bexarotene (Eisai, Tokyo, Japan), vandetanib (AstraZeneca, London, UK), or sorafenib (Bayer/Onyx, San Francisco, CA). In this trial, core tumor biopsies were prospectively obtained for biomarker analysis of 11 prespecified markers. Herein, we report the clinical and biomarker results of the phase II vandetanib trial. Vandetanib targets vascular endothelial growth factor receptor (VEGFR) and epidermal growth factor receptor (EGFR). The rationale for this trial was based on prior vandetanib salvage studies that demonstrated improved progression-free survival (PFS) but no overall survival (OS) benefit in NSCLC.2–5 Identifying the molecular phenotype or subgroup of patients that would benefit from vandetanib was a high priority, and it was hypothesized that patients with EGFR tyrosine kinase inhibitor (TKI) resistance would benefit from vandetanib salvage therapy.
PATIENTS AND METHODS
BATTLE was a phase II trial that enrolled patients with chemorefractory NSCLC at M.D. Anderson Cancer Center, who had Eastern Cooperative Oncology Group (ECOG) performance status 0 to 2, tumors amenable to core biopsy, any line of prior therapy, and adequate organ function. Patients with stable treated brain metastases more than 4 weeks before were allowed on study. After molecular tumor biomarker evaluation, patients were randomized to oral therapy with erlotinib (150 mg daily), erlotinib (150 mg daily) plus bexarotene (400 mg/m2 daily), vandetanib (300 mg daily), or sorafenib (400 mg twice daily). Radiographic assessment for response was obtained every 8 weeks. Adverse events were assessed by National Cancer Institute Common Toxicity Criteria v. 3.0. every 4 weeks while on therapy. Clinical outcomes evaluated included disease control rate (DCR = stable disease [SD] + partial response [PR] + complete response [CR]), response rate (PR + CR), PFS, OS, and toxicity. PFS was defined as time from randomization to disease progression or death without progression. PFS, OS, and response duration were estimated using Kaplan–Meier method. Log-rank tests were used to conduct univariate analyses, and Cox proportional hazards models were used to adjust for multivariables. The molecular biomarkers evaluated include: EGFR mutation, EGFR gene amplification, EGFR high polysomy, KRAS mutation, BRAF mutation, VEGF immunohistochemistry (IHC), VEGFR2 IHC, retinoid × receptor α cytoplasmic and nucleic IHC, retinoid × receptor β cytoplasmic and nucleic IHC, retinoid × receptor γ cytoplasmic IHC, cyclin D1 IHC, cyclin D1 gene amplification. The IHC biomarkers were assessed as continuous values and also as discrete markers with the cutoff detailed in the original article.1 The other biomarkers were all assessed as discrete biomarkers. Previously, we had identified that levels of circulating protein biomarkers may be associated with outcome in patients treated with vandetanib and other VEGFR inhibitors.6–8 In this study, we assessed 58 factors in serum from patients before treatment, using multiplex bead analysis.6,7,9
RESULTS
Of the 255 patients randomized in the BATTLE-1 clinical trial, 54 patients received vandetanib. Patient characteristics are included in Table 1. Average overall compliance was high at 99%, with only two patients (4%) requiring a dose reduction. Table 2 summarizes the adverse events encountered during the trial. The main toxicities experienced on the trial (any grade) included fatigue, diarrhea, elevated alkaline phosphatase, elevated liver function tests, hypertension, and rash. There was an 11% rate of grade 3 to 5 nonhematologic toxicity. One patient had a possible treatment-related death after developing a pulmonary embolus, and one squamous cell carcinoma patient experienced grade two hemoptysis; there were no other incidences of bleeding.
TABLE 1.
Patient Characteristics
| Characteristic | N Patients (%) | |
|---|---|---|
| Age (yr) | <50 | 11 (20) |
| Mean = 61 | 51–60 | 15 (28) |
| Range, 34–80 | 61–70 | 19 (35) |
| >70 | 9 (17) | |
| Sex | ||
| Female | 29 (54) | |
| Male | 25 (46) | |
| Ethnicity | ||
| White | 41 (76) | |
| Hispanic | 7 (13) | |
| African American | 2 (4) | |
| Asian | 4 (7) | |
| Smoker | ||
| Current | 5 (9) | |
| Former | 31 (57) | |
| Never | 18 (33) | |
| Histology | ||
| Adenocarcinoma | 35 (65) | |
| Squamous cell | 7 (13) | |
| Other | 12 (22) | |
| Prior therapy | Erlotinib | 45 (83) |
| Median 2 | 1 Chemotherapy | 21 (39) |
| Range 1–6 | 2 Chemotherapy | 17 (31) |
| 3 Chemotherapy | 7 (13) | |
| 4 Chemotherapy | 6 (11) | |
| 5 Chemotherapy | 2 (4) | |
| 6 Chemotherapy | 1 (2) | |
| ECOG performance status | ||
| 0 | 9 (17) | |
| 1 | 36 (67) | |
| 2 | 9 (17) |
ECOG, Eastern Cooperative Oncology Group.
TABLE 2.
Summary of Adverse Events (>10% in All Patients, Based on 54 pts)
| Total | ||||
|---|---|---|---|---|
| (N = 54) | ||||
| All Grade | Grade 3, 4, or 5 | |||
| Event | n (%) | n (%) | ||
| Abnormal electrolytes | 25 | 46.3 | 3 | 5.6 |
| Constitutional symptoms | 24 | 44.4 | 3 | 5.6 |
| Diarrhea | 23 | 42.6 | 0 | 0 |
| Elevated Alk phos | 21 | 38.9 | 2 | 3.7 |
| Abnormal liver enzymes | 19 | 35.2 | 2 | 3.7 |
| Hypertension | 18 | 33.3 | 7 | 13 |
| Rash | 17 | 31.5 | 1 | 1.9 |
| Anorexia | 15 | 27.8 | 2 | 3.7 |
| Hyperglycemia | 15 | 27.8 | 0 | 0 |
| Pain | 15 | 27.8 | 1 | 1.9 |
| Proteinuria | 14 | 25.9 | 0 | 0 |
| GI complaint | 13 | 24.1 | 1 | 1.9 |
| Infection | 13 | 24.1 | 2 | 3.7 |
| Renal insufficiency | 12 | 22.2 | 0 | 0 |
| Anemia | 11 | 20.4 | 0 | 0 |
| Pulmonary | 11 | 20.4 | 3 | 5.6 |
| Bleed | 10 | 18.5 | 0 | 0 |
| Hypoalbuminemia | 7 | 13 | 1 | 1.9 |
The DCR at 8 weeks for all patients treated with vandetanib was 33%. With a median follow-up time of 7.42 months, the median PFS was 1.81 months and 1-year PFS rate was 5%. After a median follow-up time of 17.5 months, the median OS was 6.5 months with a 1-year OS rate of 26%. No demographic subgroups had a PFS or OS benefit; patients with ECOG performance status 2 had a worse OS (hazard ratio [HR] 2.42; p = 0.02) compared with patients with ECOG 0 to 1.
Eighteen patients (11 grade 2 and 7 grade 3) experienced hypertension and had a higher 8-week DCR (64.71% versus 17.14%; p = 0.0006), better PFS (HR = 0.31; p = 0.0006), and better OS (HR = 0.42; p = 0.0096). Seventeen patients developed a rash (11 grade 1, 5 grade 2, and 1 grade 3) and had an improved 8-week DCR (52.94% versus 22.86%; p = 0.03), better PFS (HR = 0.45; p = 0.01), and improved OS though it did not reach statistical significance (HR = 0.62; p = 0.14).
In the tissue biomarker analysis, when the angiogenesis biomarkers were analyzed as a continuous variable, higher expression of VEGFR2 IHC correlated with an improved DCR (p = 0.05). However, this was not predictive of a PFS benefit. A marginally significant trend associated higher VEGFR2 IHC expression (HR = 0.69 [95% CI: 0.47, 1.02] for every 100 units increase of VEGFR2 IHC; p = 0.06) with an improved OS.
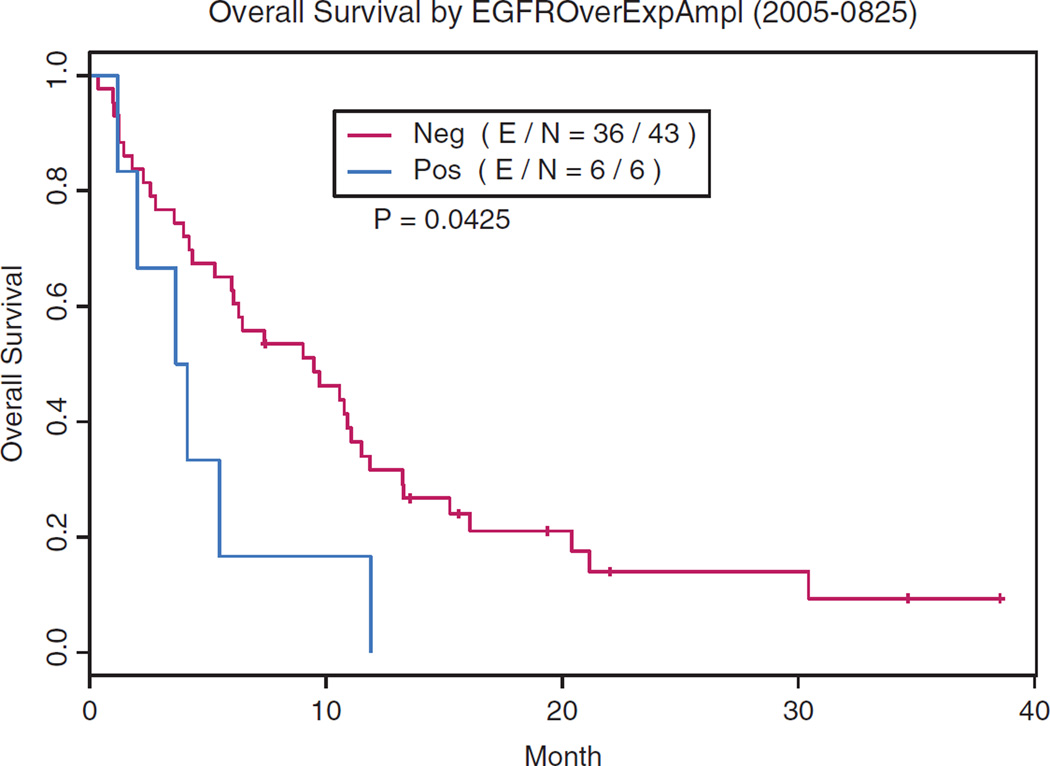
Eight patients with EGFR mutations (Table 3) trended with higher 8-week DCR (63% versus 31%; p = 0.12) but worse OS (5.9 months versus 9 months; p = 0.8) compared with EGFR wild-type patients. Seven of these eight EGFR-mutated patients were resistant to erlotinib and had two or more lines of prior chemotherapy. Patients with EGFR gene amplification (n = 6) had a worse OS (3.9 months versus 9.5 months; p = 0.04)(Fig. 1). KRAS mutation patients (3.9 months versus 9.5 months; p = 0.23) also had a worse OS.
TABLE 3.
Patient EGFR Mutations and Associated Clinical Outcome
| Patient |
EGFR Exon Mutation |
Altered Nucleotide | Altered Amino Acid | Patient History of Resistance to EGFR TKI |
Best Response |
8-Week DCR |
PFS (mo) |
OS (mo) |
|---|---|---|---|---|---|---|---|---|
| 1 | E21 | CTG858CGG | Leu858Arg | Yes | SD | Yes | 6.1 | 20.4 |
| 2 | E21 | CTG858CGG | Leu858Arg | Yes | SD | Yes | 5.7 | 38.5 |
| 3 | E19/E20 | del15bp (746E-750A) /ACG790ATG | del746Glu750Ala/Thr790Met | Yes | SD | Yes | 3.8 | 5.5 |
| 4 | E19 | del15bp (746E-750A) | del746Glu-750Ala | Yes | PD | No | 0.9 | 1.2 |
| 5 | E19/E20 | del15bp (746E-750A) /TGC797TAC | del746Glu-750Ala/Cys797Tyr | No | PD | No | 0.9 | 1.0 |
| 6 | E20 | CAC773CGC | His773Arg | Yes | PR | Yes | 7.4 | 7.4 |
| 7 | E20/E21 | ACG790ATG/CTG858CGG | Thr790Met/Leu858Arg | Yes | PD | No | 1.9 | 4.1 |
| 8 | E20/E21 | GTC802ATC/AAG852AGG | Val802Ile/Lys852Arg | Yes | SD | Yes | 2.6 | 6.3 |
EGFR TKI, epidermal growth factor receptor tyrosine kinase inhibitor; DCR, disease control rate; PFS, progression-free survival; OS, overall survival.
FIGURE 1.
Non–small-cell lung cancer patients with EGFR gene amplification have a worse overall survival (p = 0.04) when treated with vandetanib.
For the serum biomarker analysis, patients with below the median serum expression of interleukin 9c (p = 0.019) and eotaxin (p = 0.007) had a shorter PFS. A trend toward a shorter PFS was also seen in patients with higher than the median neutrophil gelatinase-associated lipocalin (p = 0.079) and lower than the median TNF-related apoptosis-inducing ligand (p = 0.087).
DISCUSSION
The clinical results of our vandetanib study were largely consistent with the literature compared with prior published salvage vandetanib monotherapy arm randomized trials (Table 4).2,10,11 There were no new safety signals observed and the prognostic correlation of rash and hypertension development with improved clinical outcomes was noted. It was evident that certain NSCLC patients benefit from vandetanib but are yet to be defined molecularly.12
TABLE 4.
Summary of Selected Phase II/Phase III Trials Containing Vandetanib Monotherapy Arms and Clinical Outcome
| Trials with Vandetanib Monotherapy Arms |
Trial Phase |
Median PFS (mo) |
HR p |
Median OS (mo) |
HR p |
|---|---|---|---|---|---|
| Current trial | II | 1.81 | N/A | 6.5 | N/A |
| EGFR mutation | 3.2 | 5.9 | |||
| ZEPHYR | III | ||||
| Lee et al.11 | |||||
| Vandetanib arm | 1.9 | 0.63 | 8.5 | 0.95 | |
| Placebo arm | 1.8 | p < 0.001 | 7.8 | p = 0.527 | |
| Natale et al.2 | III | ||||
| Vandetanib arm | 2.6 | 0.98 | 6.9 | 1.01 | |
| Erlotinib arm | 2.0 | p = 0.72 | 7.8 | p = 0.83 | |
| Natale et al.10 | II | ||||
| Vandetanib arm | 2.75a | 0.69a | 6.1 | 1.19 | |
| Gefitinib arm | 2.03a | p = 0.025 | 7.4 | p = 0.34 |
Results reported are before cross-over.
PFS, progression-free survival; HR, hazard ratio; OS, overall survival; N/A, not applicable; ZEPHYR, International, Randomised, Double-Blind Parallel Group, Multicenter Study to Assess the Efficacy of ZD6474 (ZACTIMA) plus Best Supportive Care Versus Placebo Plus Best Supportive Care in Patients With Locally Advanced or Metastatic (Stage IIIB–IV) Non-Small Cell Lung cancer (NSCLC) after Prior Therapy with an Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor (EGFR-TKI).
Whether predictive biomarkers will ultimately be identified in serum or tissue remains unclear. Prior reports have suggested that lower baseline plasma VEGF levels seem predictive of clinical benefit from vandetanib relative to erlotinib;6,12 and other trials have shown distinct cytokine and angiogenic factor modulation in patients treated with either vandetanib or chemotherapy.9 In our trial, lower median serum expression of interleukin 9 and eotaxin correlated with a worse PFS. However, the significance of this will require additional study.
In our tumor tissue studies, we were unable to identify a reliable predictive biomarker to vandetanib treatment. However, our patients with increased EGFR gene copy number experienced a worse OS. A similar finding has been reported in a Japanese study, where one of 27 Japanese patients treated with vandetanib had increased EGFR gene copy number and had progression of disease as their best response.12 The mechanism by which this occurs remains unknown and stands in contrast to other EGFR TKI trials with erlotinib where EGFR gene copy number was predictive of a clinical benefit to EGFR TKI.13,14
When our trial was designed from preclinical studies,15 it was hypothesized that patients with EGFR TKI resistance (including T790M) would benefit from vandetanib salvage. In our trial, the eight EGFR-mutated patients had a higher median PFS (3.2 months versus 1.8 months) compared with EGFR wild-type patients; but, unlike other studies,11 in our study the mutated patients had a trend toward a worse OS with vandetanib. This finding may be because the majority of our EGFR-mutated patients had prior EGFR TKI resistance and two had the T790M resistance mutation. Also, patients with less common EGFR mutations did not seem to gain any significant OS benefit from vandetanib.
In conclusion, our phase II vandetanib was consistent with prior reported clinical outcomes in unselected pretreated NSCLC patients. Although vandetanib slightly improved median PFS in patients with EGFR mutations with demonstrated EGFR TKI resistance (with or without T790M) compared with EGFR wild-type patients, no OS advantage was seen. Although vandetanib is no longer in development in NSCLC, identification of the patient molecular phenotype that responds to dual EGFR and VEGFR inhibition would contribute to the field.
Footnotes
Disclosure: The authors declare no conflict of interest.
REFERENCES
- 1.Kim ES, Herbst RS, Wistuba II, et al. The BATTLE trial: personalizing therapy for lung cancer. Cancer Discov. 2011;1:44–53. doi: 10.1158/2159-8274.CD-10-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Natale RB, Thongprasert S, Greco FA, et al. Phase III trial of vandetanib compared with erlotinib in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2011;29:1059–1066. doi: 10.1200/JCO.2010.28.5981. [DOI] [PubMed] [Google Scholar]
- 3.Herbst RS, Sun Y, Eberhardt WE, et al. Vandetanib plus docetaxel versus docetaxel as second-line treatment for patients with advanced non-small-cell lung cancer (ZODIAC): a double-blind, randomised, phase 3 trial. Lancet Oncol. 2010;11:619–626. doi: 10.1016/S1470-2045(10)70132-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heymach JV, Paz-Ares L, De Braud F, et al. Randomized phase II study of vandetanib alone or with paclitaxel and carboplatin as first-line treatment for advanced non-small-cell lung cancer. J Clin Oncol. 2008;26:5407–5415. doi: 10.1200/JCO.2008.17.3138. [DOI] [PubMed] [Google Scholar]
- 5.Qi WX, Tang LN, He AN, et al. The role of vandetanib in the second-line treatment for advanced non-small-cell-lung cancer: a meta-analysis of four randomized controlled trials. Lung. 2011;189:437–443. doi: 10.1007/s00408-011-9332-1. [DOI] [PubMed] [Google Scholar]
- 6.Hanrahan EO, Ryan AJ, Mann H, et al. Baseline vascular endothelial growth factor concentration as a potential predictive marker of benefit from vandetanib in non-small cell lung cancer. Clin Cancer Res. 2009;15:3600–3609. doi: 10.1158/1078-0432.CCR-08-2568. [DOI] [PubMed] [Google Scholar]
- 7.Nikolinakos PG, Altorki N, Yankelevitz D, et al. Plasma cytokine and angiogenic factor profiling identifies markers associated with tumor shrinkage in early-stage non-small cell lung cancer patients treated with pazopanib. Cancer Res. 2010;70:2171–2179. doi: 10.1158/0008-5472.CAN-09-2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tran HT, Liu Y, Zurita AJ, et al. Prognostic or predictive plasma cytokines and angiogenic factors for patients treated with pazopanib for metastatic renal-cell cancer: a retrospective analysis of phase 2 and phase 3 trials. Lancet Oncol. 2012;13:827–837. doi: 10.1016/S1470-2045(12)70241-3. [DOI] [PubMed] [Google Scholar]
- 9.Hanrahan EO, Lin HY, Kim ES, et al. Distinct patterns of cytokine and angiogenic factor modulation and markers of benefit for vandetanib and/or chemotherapy in patients with non-small-cell lung cancer. J Clin Oncol. 2010;28:193–201. doi: 10.1200/JCO.2009.22.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Natale RB, Bodkin D, Govindan R, et al. Vandetanib versus gefitinib in patients with advanced non-small-cell lung cancer: results from a two-part, double-blind, randomized phase ii study. J Clin Oncol. 2009;27:2523–2529. doi: 10.1200/JCO.2008.18.6015. [DOI] [PubMed] [Google Scholar]
- 11.Lee JS, Hirsh V, Park K, et al. Vandetanib versus placebo in patients with advanced non-small-cell lung cancer after prior therapy with an epidermal growth factor receptor tyrosine kinase inhibitor: a randomized, double- blind phase III trial (ZEPHYR) J Clin Oncol. 2012;30:1114–1121. doi: 10.1200/JCO.2011.36.1709. [DOI] [PubMed] [Google Scholar]
- 12.Kiura K, Nakagawa K, Shinkai T, et al. A randomized, double-blind, phase IIa dose-finding study of Vandetanib (ZD6474) in Japanese patients with non-small cell lung cancer. J Thorac Oncol. 2008;3:386–393. doi: 10.1097/JTO.0b013e318168d228. [DOI] [PubMed] [Google Scholar]
- 13.Tsao MS, Sakurada A, Cutz JC, et al. Erlotinib in lung cancer—molecular and clinical predictors of outcome. N Engl J Med. 2005;353:133–144. doi: 10.1056/NEJMoa050736. [DOI] [PubMed] [Google Scholar]
- 14.Zhu CQ, da Cunha Santos G, Ding K, et al. National Cancer Institute of Canada Clinical Trials Group Study BR.21. Role of KRAS and EGFR as biomarkers of response to erlotinib in National Cancer Institute of Canada Clinical Trials Group Study BR.21. J Clin Oncol. 2008;26:4268–4275. doi: 10.1200/JCO.2007.14.8924. [DOI] [PubMed] [Google Scholar]
- 15.Ichihara E, Ohashi K, Takigawa N, et al. Effects of vandetanib on lung adenocarcinoma cells harboring epidermal growth factor receptor T790M mutation in vivo. Cancer Res. 2009;69:5091–5098. doi: 10.1158/0008-5472.CAN-08-4204. [DOI] [PubMed] [Google Scholar]