ABSTRACT
The tailings of the Shimen realgar mine have unique geochemical features. Arsenite oxidation is one of the major biogeochemical processes that occurs in the tailings. However, little is known about the functional and molecular aspects of the microbial community involved in arsenite oxidation. Here, we fully explored the functional and molecular features of the microbial communities from the tailings of the Shimen realgar mine. We collected six samples of tailings from sites A, B, C, D, E, and F. Microcosm assays indicated that all of the six sites contain both chemoautotrophic and heterotrophic arsenite-oxidizing microorganisms; their activities differed considerably from each other. The microbial arsenite-oxidizing activities show a positive correlation with soluble arsenic concentrations. The microbial communities of the six sites contain 40 phyla of bacteria and 2 phyla of archaea that show extremely high diversity. Soluble arsenic, sulfate, pH, and total organic carbon (TOC) are the key environmental factors that shape the microbial communities. We further identified 114 unique arsenite oxidase genes from the samples; all of them code for new or new-type arsenite oxidases. We also isolated 10 novel arsenite oxidizers from the samples, of which 4 are chemoautotrophic and 6 are heterotrophic. These data highlight the unique diversities of the arsenite-oxidizing microorganisms and their oxidase genes from the tailings of the Shimen realgar mine. To the best of our knowledge, this is the first report describing the functional and molecular features of microbial communities from the tailings of a realgar mine.
IMPORTANCE This study focused on the functional and molecular characterizations of microbial communities from the tailings of the Shimen realgar mine. We fully explored, for the first time, the arsenite-oxidizing activities and the functional gene diversities of microorganisms from the tailings, as well as the correlation of the microbial activities/diversities with environmental factors. The findings of this study help us to better understand the diversities of the arsenite-oxidizing bacteria and the geochemical cycle of arsenic in the tailings of the Shimen realgar mine and gain insights into the microbial mechanisms by which the secondary minerals of the tailings were formed. This work also offers a set of unique arsenite-oxidizing bacteria for basic research of the molecular regulation of arsenite oxidation in bacterial cells and for the environmentally friendly bioremediation of arsenic-contaminated groundwater.
INTRODUCTION
Arsenic is distributed ubiquitously in the earth's crust and is toxic to life in some forms. Levels of arsenic differ considerably from one geographical region to another, depending on the geochemical conditions of the sites and the anthropogenic activities carried out in the vicinity (1, 2). Arsenic occurs naturally in organic and inorganic forms. Arsenic typically exists in one of the following four oxidation states: As(V), As(III), As(−III), and As(0). The dominant forms are As(III) (arsenite) and As(V) (arsenate). Arsenite is the most soluble form of arsenic and is dominant in reducing environments, whereas arsenate, which is less mobile than arsenite, is dominant in oxidizing conditions (3–5). Arsenate and arsenite occur naturally in various soils, minerals, and waters.
Various arsenic-oxidizing and arsenic-reducing microorganisms are extensively involved in the redox reactions of arsenic (4, 6–10). Under anaerobic conditions, As(V) can be directly reduced into As(III) by dissimilatory arsenate-respiring prokaryotes (DARPs) using As(V) as an electron acceptor and other inorganic or organic materials as electron donors (8, 11–13). Many bacteria are also able to reduce arsenate as a means of detoxification using ArsC, an arsenate reductase that is capable of converting intracellular arsenate into arsenite, which is further transported out of the cell by an energy-dependent efflux process (14–16). Arsenite-oxidizing bacteria (AOB) can oxidize As(III) into As(V) under aerobic or anaerobic conditions (17–25). Under aerobic conditions, AOB are able to convert As(III) into As(V) by using As(III) as an electron donor and oxygen as an electron acceptor (19–23); under anaerobic conditions, some AOB can convert As(III) into As(V) by using As(III) as an electron donor and nitrate, selenate, or chlorate as an electron acceptor (18, 24–27). AOB are either chemoautotrophic, using carbon dioxide as the sole carbon source, or heterotrophic, using organic carbon as the sole carbon source.
Aerobic arsenite oxidation is catalyzed by arsenite oxidase, which is generally an α1β1 heterodimer, consisting of a large catalytic subunit (AioA) and a small subunit (AioB) (28, 29, 59). The aioA gene is usually used as a valuable molecular marker for investigations of the diversity, evolution, and potential activity of AOB (22, 29). Recent studies based on metagenomic DNA extracted from soils, sediments, water, minerals, tailings, and geothermal mats with different chemical characteristics and various levels of arsenic contamination suggested that the diversities of AioA proteins differ considerably among these sampling sites (29–37). Until now, the aioA genes have been identified from more than 80 genera of bacteria and archaebacteria, which were isolated from various arsenic-contaminated environments. Most of the aioA genes belong to Alphaproteobacteria, Betaproteobacteria, or Gammaproteobacteria (31–34, 36). However, little is known about the microbial diversity and functional features of AOB from the tailings of a realgar mine that are characteristic of high concentrations of arsenic and sulfate.
The Shimen realgar mine in the Hunan Province of China was the largest realgar mine in Asia for hundreds of years. Due to severe environmental contamination caused by mining and industrial production, the Chinese Government closed the mine in 2011. A previous investigation indicated that different types of As-bearing secondary minerals were present in the tailings of the mine, including arsenic oxides, sulfur-bearing arsenates, As-gypsum, and As-Fe minerals (38). Arsenite is more abundant in the gray-white tailings than in the gray tailings, whereas the arsenate is dominant in the gray tailing. This variation suggests that the formation of the secondary minerals can be divided into the following two stages: the As(III) stage and As(V) stage (38). These investigations strongly suggested that arsenite oxidation may be a major biological process that occurred in the tailings of the Shimen realgar mine (37, 38).
In this work, we collected six samples from the representative sites of the tailings of the Shimen realgar mine. The geochemical features of the six sampling sites differ considerably. We systematically analyzed the arsenite-oxidizing activities, the oxidase gene diversities, and the microorganism compositions of the microbial communities from the tailings. It is interesting to see that the chemoautotrophic and heterotrophic arsenite-oxidizing activities of the samples show a positive correlation with the concentrations of soluble arsenic. Soluble arsenic, sulfate, pH value, and total organic carbon (TOC) were the key environmental factors that shaped the microbial communities. Using an enrichment technique, we also isolated 10 novel AOB strains, which are either chemoautotrophic or heterotrophic and possess high arsenite-oxidizing activities. These data highlight the unique diversities of the arsenite-oxidizing bacteria in the tailings of the Shimen realgar mine. To the best of our knowledge, this is the first report of the functional and molecular characterizations of the microbial communities from the tailings of a realgar mine.
MATERIALS AND METHODS
Sample collection and geochemical analyses.
We collected six samples from the representative sites A, B, C, D, E, and F of the tailings of the Shimen realgar mine in Hunan Province, China. The sampling sites are located at 29°33′55.4′'N, 111°1′45.0′'E (see Fig. S1 in the supplemental material). All samples were kept at 4°C in sterile 50-ml tubes for less than 2 days until the microbial and geochemical analyses were performed. Geochemical analyses were performed as described previously (39–41). Soil sulfate was measured using ion chromatography. Total organic carbon (TOC) content was determined using a TOC analyzer (TOC-VCPH; Shimadzu Corporation, Japan). The concentrations of soluble As(III) and As(V) were measured using high-performance liquid chromatography coupled with inductively coupled plasma mass spectrometry (HPLC-ICP-MS). Total arsenic was extracted from the tailing soils with aqua regia. Bioavailable As(III) and As(V) were extracted from the samples using 1.0% HCl.
Functional analysis of the microbial communities.
To detect the arsenite-oxidizing activity of the microbial community from a sample, active microcosm was prepared in triplicate by inoculating 10.0 g of soils into 90.0 ml of modified minimal salts (MMS) medium amended with 5.0 mM As(III) and 0.2% yeast extract or 10.0 mM NaHCO3 in a 250-ml flask as described previously (42). Duplicate controls for each set of microcosms were prepared by inoculating 10.0 g of soils into 90.0 ml of the same medium without supplementing any carbon source. All of the flasks were incubated at 30°C with shaking for 6 days. At an interval of 24 h, 1.0 ml of the culture suspension was taken out from each of the flasks to measure the concentrations of As(III) and As(V) using HPLC-ICP-MS.
Sequencing and analysis of microbial 16S rRNA genes.
Microbial genomic DNA from soils was prepared using the E.Z.N.A. soil DNA kit (OMEGA Bio-tec, Inc., USA) according to manufacturer recommendations. The barcoded primers flanking the V4 region of the microbial 16S rRNA gene were used for PCR amplification (Table 1) (43). The PCR products were gel purified. Barcoded V4 amplicons were sequenced using an Illumina MiSeq sequencing platform.
TABLE 1.
PCR primers employed in this study
| Target gene | Primer sequence | Reference |
|---|---|---|
| aioA | TGYATYGTNGGNTGYGGNTAYMA (forward) | 33 |
| TANCCYTCYTGRTGNCCNCC (reverse) | 33 | |
| CCACTTCTGCATCGTGGGNTGYGGNTA (forward) | 48 | |
| TGCCCCAGATGATGCCYTTYTCRWA (reverse) | 48 | |
| TGTCGTTGCCCCAGATGADNCCYTTYTC (reverse) | 48 | |
| T vector | CGCCAGGGTTTTCCCAGTCACGAC (forward) | |
| GAGCGGATAACAATTTCACACAGG (reverse) | ||
| 16S rRNA V4 | AYTGGGYDTAAAGNG (forward) | 43 |
| TACNVGGGTATCTAATCC (reverse) | 43 | |
| 16S rRNA | AGAGTTTGATCCTGGCTCAG (forward) | 58 |
| TACGGCTACCTTGTTACGACTT (reverse) | 58 |
Raw reads were processed and assembled as described previously (44, 45). Sequences that were shorter than 110 bp or with an average Phred Q value of less than 25 were discarded. The assembled sequences were uploaded into MG-RAST for taxonomy analysis. Sequences were assigned to operational taxonomic units (OTUs) based on a threshold of 97% similarity (46). Rarefaction analysis was performed using Qiime software (44). The ACE, Chao1, Shannon, and Simpson indexes were calculated using mothur (47).
Alpha diversity indices were further tested for their correlations with geochemical data using the Mantel test. Analysis of similarity (ANOSIM) was performed using the vegan package to test for the existence of differences in microbial community compositions among the six samples. The unweighted pair group method with arithmetic means (UPGMA) clustering was used to analyze the microbial community similarity among the samples. Principal-coordinate analysis (PCoA) was performed in mothur software using Bray-Curtis as the distance index. Biota-environment matching analysis (BIO-ENV; vegan package) was used to evaluate the relationships between microbial community compositions and environment factors.
Enrichment and isolation of cultivable arsenite-oxidizing bacteria.
Approximately 5.0 g of soils was inoculated into 95.0 ml of MMS medium amended with 5.0 mM As(III) and 10.0 mM NaHCO3 or 0.2% yeast extract. The mixture was incubated at 30°C with shaking until As(III) was completely oxidized into As(V). About 5.0 ml of the culture was transferred into 95.0 ml of the same medium for further enrichment. After As(III) was completely oxidized, 1.0 ml of the culture was serially diluted and plated onto MMS agar plates amended with 5.0 mM As(III) and 10.0 mM NaHCO3 or 0.2% yeast extract. The plates were incubated at 30°C until visible colonies appeared. Individual colonies were purified for further analysis.
Cloning and analyses of arsenite oxidases from the microbial communities.
To detect the diversity of the arsenite oxidases in microbial communities from the tailings of the Shimen realgar mine, three pairs of primers were used to amplify the aioA genes from the metagenomic DNA of the samples as described previously (Table 1) (33, 48). PCR products were gel purified using the E.Z.N.A. gel extraction kit (Omega, USA). Purified DNA was ligated into the pMD19-T vector (TaKaRa, Japan). An aioA gene library was generated by transforming the ligated vectors into Escherichia coli DH5α-competent cells. The DNA library was screened by PCR amplification. Positive clones were selected for sequencing.
Phylogenetic analysis.
Protein sequence homology searches against the GenBank database were performed using the BLAST server (http://blast.ncbi.nlm.nih.gov/). Multiple sequence alignment was conducted using ClustalW2 software. Phylogenetic trees of the AioA amino acid sequences were constructed using the maximum likelihood method implemented in MEGA 6.0. The neighbor-joining (NJ) method was used for comparison between the phylogenetic trees of the aioA genes and the 16S rRNA genes.
Accession number(s).
The aioA sequences from the tailings of the Shimen realgar mine have been deposited into the GenBank database under the accession numbers KT992226 to KT992324 and KT992340 to KT992354. The 16S rRNA gene sequences of the cultivable arsenite-oxidizing strains also have been deposited into the database under the accession numbers of KT992325 to KT992339.
RESULTS
Characteristics of the sampling sites.
We collected six samples (A, B, C, D, E, and F) from the representative points of the tailings on 10 July 2014 (see Fig. S1 in the supplemental material). As shown in Table 2, geochemical analysis indicated that the tailings had a high content of arsenic (ranging from 0.06 to 117.47 g/kg) and SO42− (ranging from 0.30 to 3.05 mmol/kg). The concentrations of soluble arsenic and arsenite from the six sites varied from 0.07 to 6.37 g/kg and from 0.003 to 0.935 g/kg, respectively. The concentrations of TOC varied from 5.6 to 59.0 g/kg. These data suggest that the geochemical features of the six sampling points differ from each other and differ considerably from other arsenic-contaminated sites (40, 49, 50).
TABLE 2.
Geochemical features of the six samples from the tailings of the realgar mine
| Parameter | Tailing sample |
|||||
|---|---|---|---|---|---|---|
| A | B | C | D | E | F | |
| Soil matrix | Fill | Fill | Fill | Clay | Clay | Clay |
| Moisture content (%) | 9.50 | 2.63 | 6.82 | 18.52 | 22.79 | 8.29 |
| pH | 7.46 | 8.3 | 8.05 | 8.35 | 8.41 | 4.12 |
| ECa (μS cm−1) | 521 | 830 | 631 | 162 | 98 | 58 |
| TOC (g/kg) | 59.0 | 13.6 | 11.3 | 9.4 | 6.7 | 5.6 |
| SO4 2− (mmol/kg) | 3.05 | 2.33 | 1.27 | 0.30 | 0.81 | 0.65 |
| Total As (g/kg) | 117.47 | 14.49 | 8.26 | 0.32 | 0.28 | 0.06 |
| Aqueous As (g/kg) | 6.374 | 5.99 | 2.433 | 0.193 | 0.126 | 0.07 |
| Aqueous As(III) (g/kg) | 0.935 | 0.775 | 0.238 | 0.012 | 0.018 | 0.003 |
EC, electrical conductivity.
Arsenite-oxidizing activities of the microbial communities and their correlations with geochemistry.
The microcosm assays showed that arsenite-oxidizing activities were detectable in all of the six samples that were supplemented with 10.0 mM NaHCO3 or 0.2% yeast extract as a carbon source, and no significant arsenite-oxidizing activities were detectable when no external carbon source was added to the microcosms (Fig. 1).
FIG 1.
Arsenite-oxidizing activities of the microbial communities from the tailings of the Shimen realgar mine. (a) Arsenite oxidation curve of each microcosm supplemented with 0.2% yeast extract or 10.0 mM NaHCO3 as a carbon source. Active microcosms of the six samples A, B, C, D, E, and F were each inoculated into MMS medium. The medium was amended with 5.0 mM NaAsO2 and supplemented with 0.2% yeast extract or 10.0 mM NaHCO3 as a carbon source. Duplicate controls were the same microcosm without the addition of any carbon sources. (b) Comparison of the arsenite-oxidizing rates of the microbial communities from the six samples. **, P < 0.01.
The microbial arsenite-oxidizing activities of the six samples differ considerably from each other. When no external carbon source was added to the microcosms of sites A and B, the concentrations of soluble As(III) increased from 5.0 to 8.3 mM in 4.0 days and from 5.0 to 7.9 mM in 5.0 days, respectively (Fig. 1a). This suggests that approximately 3.3 mM and 2.9 mM As(III) were released from the microcosms A and B into the aqueous phase, respectively. In comparison, when no external carbon source was added to the microcosms of samples C, D, E, and F, no arsenic was released. For all of the six microcosms, no As(III) oxidation occurred without the addition of any external carbon source (Fig. 1a).
As shown in Fig. 1a, when 10.0 mM NaHCO3 was added to the microcosms of A, B, C, D, E, and F, As(III) was completely oxidized into As(V) in 3.0, 2.0, 7.0, 9.0, 9.0, and >8.0 days, respectively. When 0.2% yeast extract was supplemented to the six microcosms, 3.0, 3.0, 3.0, 5.0, 3.0, and 8.0 days were needed to completely oxidize all As(III), respectively.
Taken together, these data suggest that both chemoautotrophic and heterotrophic AOB are present in all of the sampling sites. The arsenite-oxidizing activities of the six samples, expressed as the oxidized concentration of As(III) per hour, differ considerably from each other (Fig. 1b).
Correlation analysis indicated that the chemoautotrophic arsenite-oxidizing activities of the six samples show a positive correlation with their soluble arsenic concentrations (r = 0.7688; P = 0.011). With the exception of site C, the heterotrophic arsenite-oxidizing activities are moderately correlated with the soluble arsenic concentrations of the samples (r = 0.4406; P = 0.033).
Unique diversity of the microbial communities and their correlations with geochemistry.
We used Illumina MiSeq high-throughput sequencing techniques to investigate the microbial community compositions of the tailings of the Shimen realgar mine. The results showed that a total of 354,797 high-quality 16S rRNA gene sequences were obtained, among which 45,254, 91,218, 39,480, 54,718, 44,224, and 79,903 sequences were assigned to samples A, B, C, D, E, and F, respectively. About 99% of the sequences are 225 bp long. These sequences were clustered into OTUs at a similarity threshold of 97%, yielding 15,186 OTUs. The numbers of OTUs that correspond to the sampling sites are 3,730 from the site A, 5,276 from B, 4,285 from C, 4,569 from D, 3,686 from E, and 1,447 from F. The rarefaction analysis using Qiime indicated that these sequences cover 99.1% (site A), 99.5% (B), 98.4% (C), 99.2% (D), 98.8% (E), and 99.9% (F) of the microbial diversities of the corresponding sites (Table 3; Fig. 2a).
TABLE 3.
Diversity and richness of the microbial communities
| Sample | Index |
Coverage (%) | |||
|---|---|---|---|---|---|
| Chao1 | ACE | Simpson | Shannon | ||
| A | 2,112.5882 | 2,011.4302 | 0.015205 | 5.46897 | 99.08 |
| B | 3,085.1402 | 2,988.8199 | 0.017719 | 5.702957 | 99.52 |
| C | 2,951.8764 | 2,762.7773 | 0.006797 | 6.240306 | 98.43 |
| D | 3,026.5869 | 2,918.4281 | 0.003691 | 6.556586 | 99.16 |
| E | 2,809.125 | 2,722.6293 | 0.007258 | 6.165388 | 98.78 |
| F | 958.1176 | 932.6597 | 0.037507 | 4.504256 | 99.91 |
FIG 2.
Microbial communities in the tailings from the Shimen realgar mine. (a) Rarefaction curves of the microbial communities of the tailing samples. (b) Relative abundances of the dominant phyla in the microbial communities of the tailing samples from the Shimen realgar mine and UPGMA analysis of the microbial communities. (c) Principal-coordinate analysis (PCoA) of the microbial communities of the tailing samples.
Representative sequences of each OTU were classified into phyla using RDP Classifier with a confidence threshold of 80%. Only a small proportion of the sequences (2.0%) cannot be classified at the domain level. From the six samples, we identified 40 phyla of bacteria and 2 phyla of archaea. Figure S2 in the supplemental material shows the structures of the microbial communities from the six sampling sites. The dominant microbial phyla on average are Proteobacteria (30.0% of the total microorganisms), Actinobacteria (14.9%), Bacteroidetes (10.7%), Chloroflexi (10.5%), Acidobacteria (9.9%), and Planctomycetes (8.7%); other microorganisms are less abundant, including Verrucomicrobia (3.5%), candidate division WS3 (2.8%), Gemmatimonadetes (2.8%), candidate division OD1 (2%), Nitrospirae (1.1%), Chlorobi (0.9%), and Firmicutes (0.5%) (Fig. 2b). This microbial community composition considerably differs from those of other described arsenic-contaminated sites (38, 47, 48). Although the dominant phyla are present in all of the six sampling sites, their abundances differ considerably from each other (Fig. 2b; see also Fig. S2).
Many investigations indicated that Deltaproteobacteria were always associated with the sulfate-reducing ability of the microbial community, and almost all of the cultivable sulfate-reducing bacteria belong to this group (51). The bacteria of Deltaproteobacteria were dominant in all six sampling points, suggesting that microbial reduction of sulfate may naturally occur in the sampling sites.
Among the total OTUs (15,186) obtained from the sampling sites, only 78 OTUs or 0.5% were shared by the six sites. The shared phyla are Proteobacteria (52.7% of the total shared microorganisms), Actinobacteria (29.0%), Chloroflexi (8.3%), Bacteroidetes (3.7%), Planctomycetes (3.2%), Acidobacteria (2.0%), Gemmatimonadetes (0.6%), Nitrospirae (0.4%), and Firmicutes (0.1%) (see Fig. S3 in the supplemental material); this highlights the significant differences in the microbial compositions of the six sampling sites.
We further analyzed the correlation between the microbial community compositions and the environmental factors. Based on the Bray-Curtis dissimilarity analysis using UPGMA, the six samples can be grouped into four groups: I (A and B), II (C), III (D and E), and IV (F) (Fig. 2b). PCoA showed that the microbial communities of the tailing samples were divided into four distinct groups as well (Fig. 2c). Pairwise comparison using ANOSIM indicated that there are significant differences in the microbial community compositions among the six samples (r = 0.9231; P = 0.022). Mantel tests suggested that the diversity indices of the microbial communities showed a highly positive correlation with the pH value of the samples (pH and Chao1, r = 0.9459 and P = 0.018; pH and ACE, r = 0.9426 and P = 0.017; pH and Shannon, r = 0.8480 and P = 0.019). With the exception of site C, the Shannon diversity was positively correlated with SO42− and TOC concentrations (SO42− and Shannon, r = 0.9253 and r = 0.017; TOC and Shannon, r = 0.454 and P = 0.042). BIO-ENV confirmed that soluble arsenic, sulfate, pH value, and TOC were the key environmental factors that shaped the observed microbial communities from the tailings of the realgar mine.
Enrichment of arsenite-oxidizing bacteria from the samples.
Ten arsenite-oxidizing bacterial strains were isolated from the six samples using an enrichment strategy (Table 4). Their 16S rRNA genes were cloned and sequenced. Phylogenetic analysis indicated that the strains SM1, SM2, SM3, SM5, SM6, SM7, and SM8 are affiliated with Alphaproteobacteria, SM11 belongs to Betaproteobacteria, and SM13 and SM15 belong to Gammaproteobacteria (Table 4).
TABLE 4.
Cultivable arsenite oxidizers from the tailings of Shimen realgar mine
| Strain | GenBank accession no. | Class | Heterotrophic/autotrophic | Closest relative | 16S rRNA identity (%) |
|---|---|---|---|---|---|
| SM1 | KT992325 | Alphaproteobacteria | Autotrophic | Gemmobacter aquatilis DSM 3857(T) | 98.37 |
| SM2 | KT992335 | Alphaproteobacteria | Autotrophic | Ensifer adhaerens LMG 20216(T) | 99.56 |
| SM3 | KT992327 | Alphaproteobacteria | Autotrophic | Shinella zoogloeoides ATCC 19623(T) | 98.98 |
| SM5 | KT992326 | Alphaproteobacteria | Autotrophic | Rhizobium nepotum 39/7(T) | 98.30 |
| SM6 | KT992328 | Alphaproteobacteria | Heterotrophic | Rhizobium daejeonense KCTC12121 | 98.24 |
| SM7 | KT992333 | Alphaproteobacteria | Heterotrophic | Rhizobium leguminosarum USDA 2370(T) | 98.91 |
| SM8 | KT992334 | Alphaproteobacteria | Heterotrophic | Ochrobactrum pseudintermedium ADV31(T) | 99.56 |
| SM11 | KT992332 | Betaproteobacteria | Heterotrophic | Variovorax boronicumulans BAM-48(T) | 99.71 |
| SM13 | KT992337 | Gammaproteobacteria | Heterotrophic | Pseudomonas vancouverensis ATCC 700688(T) | 99.51 |
| SM15 | KT992339 | Gammaproteobacteria | Heterotrophic | Pseudomonas koreensis Ps 9-14(T) | 99.33 |
Arsenite-oxidizing assays indicated that strains SM6, SM7, SM8, SM11, SM13, and SM15 are heterotrophic; they are able to completely oxidize As(III) into As(V) in 17.5, 17.5, 17.5, 21.5, 45.5, and 57.5 h, respectively (Fig. 3a). Strains SM1, SM2, SM3, and SM5 are chemoautotrophic; they are able to completely oxidize As(III) into As(V) in 94.5, 118.5, 34.5, and 142.5 h, respectively (Fig. 3b).
FIG 3.
Arsenite-oxidizing activities of the cultivable isolates from the tailings of the Shimen realgar mine. (a) Arsenite-oxidizing curves of the new heterotrophic strains. (b) Arsenite-oxidizing curves of the new chemoautotrophic strains. The chemoautotrophic strains SM1, SM2, SM3, and SM5 belong to the genera Gemmobacter, Ensifer, Shinella, and Rhizobium, respectively; the heterotrophic strains SM6, SM7, SM8, SM11, SM13, and SM15 are affiliated with the genera Rhizobium, Rhizobium, Ochrobactrum, Variovorax, Pseudomonas, and Pseudomonas, respectively.
As shown in Fig. 3a, the heterotrophic strains SM6, SM7, SM8, SM11, SM13, and SM15 began to oxidize As(III) at the middle or early stage of the log phase of the bacterial growth cycle. In contrast, SM13 started to oxidize As(III) at the decline phase of the bacterial growth cycle. This observation suggests that about 10 to 21 h were required to induce the expression of arsenite oxidase genes in the heterotrophic bacterial cells. Thus, the expression of the arsenite oxidase gene is inducible in the cells of all of the heterotrophic bacterial strains.
Figure 3b illustrates the arsenite-oxidizing curves of the chemoautotrophic bacterial strains, which differ considerably from those of the heterotrophic bacterial strains. It is interesting to see that all of the chemoautotrophic strains started to oxidize As(III) as the cells began to proliferate, and the bacterial growth occurred without a lag phase. This strongly suggests that the As(III) oxidation was linked to bacterial growth for all four chemoautotrophic strains. It is likely that the As(III) oxidation may be required for the chemoautotrophic growth of these strains.
Unique diversity of arsenite oxidases from the microbial communities.
To understand the molecular basis of the arsenite-oxidizing activities of the microbial communities from the tailings of the Shimen realgar mine, we explored the diversity of the arsenite oxidase genes present in the microbial communities by cloning, sequencing, and analyzing the aioA sequences encoding the catalytic subunits (AioAs) of the arsenite oxidases. The results showed that we identified 114 novel AioA proteins, including 99 from the metagenomic DNA and 15 from the cultivable arsenite-oxidizing bacteria. The AioA proteins identified from the metagenomic DNA were referred to as A1 to A13, B1 to B9, C1 to C19, D1 to D15, E1 to E18, and F1 to F25 (A, B, C, D, E, and F refer to the sampling sites).
The amino acid sequences of these AioA proteins were used as queries to search against the GenBank database using the BLAST server. We found that the AioA proteins from the tailings share 34 to 99% sequence identities with other known AioAs from bacteria and archaea. We constructed a phylogenetic tree based on the multiple sequence alignment of the AioA proteins from the tailings and their closely related AioAs from bacteria and archaea. An AioA sequence (BAB67500) from the archaeon Sulfolobus tokodaii was chosen as an outgroup in the tree (Fig. 4).
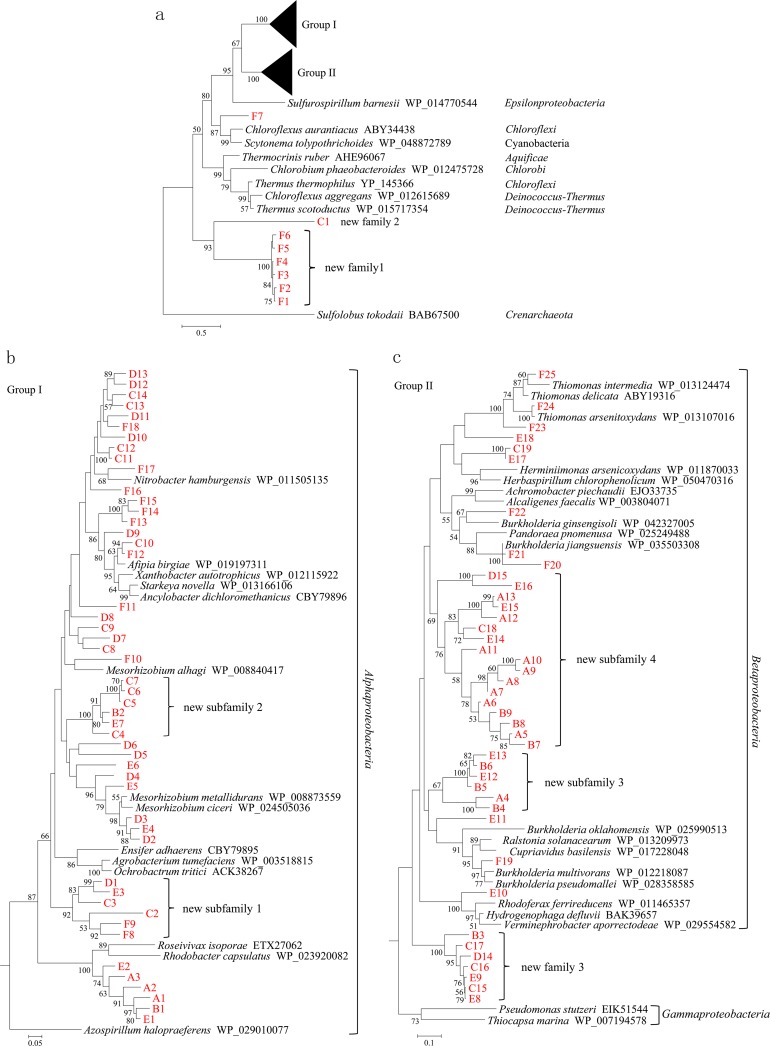
FIG 4.
Phylogenetic analysis of arsenite oxidases from the tailings of the Shimen realgar mine using the catalytic subunits (AioA subunits) as molecular markers. (a) Maximum likelihood tree constructed from the amino acid sequences of the bacterial and archaeal AioA proteins from the microbial communities of the tailings and those of their closely related AioA proteins from the GenBank database, where groups I and II stand for two large independent branches. (b) Unrooted trees of the group I. (c) Unrooted tree of group II. The AioA amino acid sequence from Sulfolobus tokodaii was chosen as an outgroup. All of the AioA sequences in this study are shown in red. Numbers on the branches are bootstrap values based on 1,000 replicates. Only the bootstrap values larger than 50% are shown. The scale bar represents the average number of substitutions per site.
The AioA proteins F1, F2, F3, F4, F5, and F6 from the realgar tailings formed an independent basal branch in the phylogenetic tree, which was located between the AioA protein of the outgroup (archaeon) and those from the phyla Deinococcus-Thermus, Chloroflexi, and Chlorobi (Fig. 4a). This suggests that the six new AioAs constitute a novel family of AioA proteins that represent an evolutionary intermediate between the AioAs of archaea and bacteria.
The AioA C1 shares the highest sequence homology (34% identity) with the AioA from Thermus scotoductus, and it formed an independent branch in the tree (Fig. 4a). This suggests that C1 is most likely the first member of a new family of bacterial AioA proteins. F7 shows 62% sequence identity to the AioA from Scytonema tolypothrichoides, suggesting that F7 is a new member of the AioA family affiliated with the phylum Cyanobacteria.
The AioA proteins E1, B1, A1, A2, A3, and E2 from the tailings share 71 to 76% and 73 to 75% sequence identities with the AioAs from Rhodobacter capsulatus and Roceivivax isoporae, respectively. All of these AioA proteins fell into the same branch in the tree (Fig. 4b); this suggests that E1, B1, A1, A2, A3, and E2 are six new members of the AioA family affiliated with Alphaproteobacteria. Two subgroups of novel AioA proteins, one including F8, F9, C2, C3, E3, and D1 and the other comprising C4, E7, B2, C5, C6, and C7, share 75 to 78% and 77 to 81% sequence identities, respectively, with other known AioAs from the bacterial species of Alphaproteobacteria; moreover, they formed two independent branches in the tree (Fig. 4b). This suggests that the two subgroups of AioA proteins constitute two novel subfamilies of AioA proteins affiliated with Alphaproteobacteria. Another two subgroups of new AioA proteins from the tailings, one comprising D2, E4, D3, E5, D4, E6, D5, and D6 and the other consisting of F10, C8, D7, C9, D8, F11, F12, C10, D9, F13, F14, F15, F16, F17, C11, C12, D10, F18, D11, C13, C14, D12, and D13, share 74 to 94% and 80 to 95% sequence identities, respectively, with other known AioAs from Alphaproteobacteria. All of these new AioA proteins clustered together with the known AioAs; this suggests that these AioAs from the tailings are new members of the AioA family affiliated with Alphaproteobacteria (Fig. 4b).
As shown in Fig. 4c, E8, C15, E9, C16, D14, C17, and B3 formed an independent branch that was located between the known AioA proteins from the bacterial species of Gammaproteobacteria and those from the species of Betaproteobacteria. This indicated that the seven new AioA proteins constitute a novel family of AioAs that represents an evolutionary intermediate between the AioAs from Gammaproteobacteria and Betaproteobacteria. We also identified two novel subfamilies of AioA proteins affiliated with Betaproteobacteria, one comprising B4, A4, B5, E12, B6, and E13 and the other comprising B7, A5, B8, B9, A6, A7, A8, A9, A10, A11, E14, C18, A12, E15, A13, E16, and D15 (Fig. 4c). They share 75 to 77% and 72 to 77% sequence identities, respectively, with other known AioA proteins from Betaproteobacteria and formed two independent branches in the tree (Fig. 4c). Another two subgroups of AioA proteins, one comprising E10, F19, and E11 and the other comprising F20, F21, F22, E17, C19, E18, F23, F24, and F25, share 76 to 93% and 75 to 99% sequence identities, respectively, with other known AioAs from Betaproteobacteria. All of these new AioA proteins clustered together with the known AioAs in the tree. This suggests that they are new members of the AioA family affiliated with Betaproteobacteria (Fig. 4c).
Taken together, the new families/subfamilies of AioA proteins are largely dominant in the total AioAs of the microbial communities from the tailings; this highlights the unique diversity of the arsenite-oxidizing bacteria from the tailings of the Shimen realgar mine.
Horizontal transfer of the aioA genes.
To determine whether horizontal and vertical transfers of the aioA genes occurred between different genera of bacteria and archaea, we compared the phylogeny of the aioA genes with that of the corresponding bacterial 16S rRNA genes. The phylogenetic tree of the aioA genes was constructed from the multiple sequence alignment of aioA genes from the tailings of the Shimen realgar mine and closely related known aioA genes from bacteria. The tree of 16S rRNA genes was constructed from the multiple sequence alignment of the 16S rRNA genes of the microorganisms bearing the aioA genes. We found that horizontal transfer of the aioA genes may occur among the microorganisms from the tailings of the Shimen realgar mine.
As shown in Fig. S4 in the supplemental material, comparison of the phylogenetic tree of the 16S rRNA genes and that of the aioA genes revealed apparent inconsistency between the microorganism and gene evolutionary histories. According to the horizontal gene transfer (HGT) detection method described previously (52, 53), many HGT events likely occurred among the distant lineages. For example, in the 16S rRNA gene-based tree, Rhizobium sp. SM4 fell into the branch of the genus Rhizobium; however, in the aioA gene-based tree, it clustered together with a species from the genus Gemmobacter. This inconsistency strongly suggests that Rhizobium sp. SM4 may acquire its aioA via HGT. Similarly, the analyses indicated that Variovorax sp. SM11, Shinella sp. SM3, Stenotrophomonas sp. SM14, Pseudomonas sp. SM13, and Pseudomonas sp. SM15 may also acquire their aioA genes via HGT (see Fig. S4).
DISCUSSION
Realgar, α-As4S4, is an arsenic sulfide mineral. The Shimen realgar mine was the largest in Asia and the third largest in the world. The tailings of the mine have unique geochemical features that are characteristic of high contents of arsenic and sulfate. It was found that arsenite oxidation was one of the dominant biogeochemical processes that occurred in the tailings of the realgar mine (38). In this study, we fully explored the arsenite-oxidizing activities and the functional basis of the microbial communities from the tailings of the Shimen realgar mine. To the best of our knowledge, this is the first work that described the functional and molecular features of the microbial communities from the tailings of a realgar mine.
In recent years, the microcosm strategy was used to investigate the functions or activities of microbial communities (54–57). Using this technique, we detected the arsenite-oxidizing activities of the microbial communities from the six sites of the tailings. This raised an interesting issue: do the microbial communities affect arsenic mobilization? Our observations suggested that the microbial communities of sites A and B, rather than other sampling sites, can promote arsenic mobilization under aerobic condition (Fig. 1a and b).
In general, most of the autotrophic oxidizers tend to be members of Alphaproteobacteria and the heterotrophic strains belong to Betaproteobacteria (22, 24). Consistent with this observation, all of the four chemoautotrophic arsenite-oxidizing strains isolated from the tailings belong to Alphaproteobacteria. However, among the six heterotrophic oxidizers, three strains are members of Alphaproteobacteria, one strain belongs to Betaproteobacteria, and two strains belong to Gammaproteobacteria. This finding expanded the taxonomy of heterotrophic AOB.
We identified 114 new AioA proteins from the microbial communities of the tailings, including a unique group of AioAs that represents an evolutionary intermediate between the AioAs of bacteria and archaea, seven novel families/subfamilies of AioAs, and other novel AioAs affiliated with Chloroflexi, Alphaproteobacteria, and Betaproteobacteria. These results highlight the unique diversities of the arsenite oxidase genes and the arsenite-oxidizing microorganisms from the tailings of the Shimen realgar mine.
Using high-throughput sequencing, we identified 40 phyla of bacteria and 2 phyla of archaea from the tailings. Bioinformatical analysis suggested that a unique diversity of microorganisms was present in the tailings. ANOSIM data indicated that there are significant differences among the microbial community compositions of the six samples. Remarkably, we found that soluble arsenic, sulfate, pH, and TOC were the key environmental factors that shaped the microbial communities.
These data help us to better understand the interactions between the arsenite-oxidizing microorganisms and the environment and gain insights into the molecular mechanisms by which the secondary minerals were formed in the tailings of the Shimen realgar mine.
Supplementary Material
Funding Statement
This work was financially supported by the General Programs of the National Natural Science Foundation of China (no. 41272257, 41072181, and 41472219) and the Foundation for Innovative Research Groups of the National Natural Science Foundation of China (no. 41521001).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.02190-16.
REFERENCES
- 1.Colbourn P, Alloway BJ, Thornton I. 1975. Arsenic and heavy metals in soils associated with regional geochemical anomalies in south-west England. Sci Total Environ 4:359–363. doi: 10.1016/0048-9697(75)90027-3. [DOI] [Google Scholar]
- 2.Bhowmick S, Nath B, Halder D, Biswas A, Majumder S, Mondal P, Chakraborty S, Nriagu J, Bhattacharya P, Iglesias M, Roman-Ross G, Mazumder DG, Bundschuh J, Chatterjee D. 2013. Arsenic mobilization in the aquifers of three physiographic settings of West Bengal, India: understanding geogenic and anthropogenic influences. J Hazard Mater 262:915–923. doi: 10.1016/j.jhazmat.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 3.Zhu J, Pigna M, Cozzolino V, Caporale AG, Violante A. 2013. Higher sorption of arsenate versus arsenite on amorphous Al-oxide, effect of ligands. Environ Chem Lett 11:289–294. doi: 10.1007/s10311-013-0405-7. [DOI] [Google Scholar]
- 4.Oremland RS, Stolz JF. 2003. The ecology of arsenic. Science 300:939–944. doi: 10.1126/science.1081903. [DOI] [PubMed] [Google Scholar]
- 5.Dixit S, Hering JG. 2003. Comparison of arsenic(V) and arsenic(III) sorption onto iron oxide minerals: implications for arsenic mobility. Environ Sci Technol 37:4182–4189. doi: 10.1021/es030309t. [DOI] [PubMed] [Google Scholar]
- 6.Islam ABMR, Maity JP, Bundschuh J, Chen CY, Bhowmik BK, Tazaki K. 2013. Arsenic mineral dissolution and possible mobilization in mineral-microbe-groundwater environment. J Hazard Mater 262:989–996. doi: 10.1016/j.jhazmat.2012.07.022. [DOI] [PubMed] [Google Scholar]
- 7.Rhine ED, Garcia-Dominguez E, Phelps CD, Young LY. 2005. Environmental microbes can speciate and cycle arsenic. Environ Sci Technol 39:9569–9573. doi: 10.1021/es051047t. [DOI] [PubMed] [Google Scholar]
- 8.Sierra-Alvarez R, Field JA, Cortinas I, Feijoo G, Moreira MT, Kopplin M, Gandolfi AJ. 2005. Anaerobic microbial mobilization and biotransformation of arsenate adsorbed onto activated alumina. Water Res 39:199–209. doi: 10.1016/j.watres.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 9.Kulp TR. 2014. Early earth: arsenic and primordial life. Nat Geosci 7:785–786. doi: 10.1038/ngeo2275. [DOI] [Google Scholar]
- 10.Rodriguez-Freire L, Sierra-Alvarez R, Root R, Chorover J, Field JA. 2014. Biomineralization of arsenate to arsenic sulfides is greatly enhanced at mildly acidic conditions. Water Res 66:242–253. doi: 10.1016/j.watres.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohtsuka T, Yamaguchi N, Makino T, Sakurai K, Kimura K, Kudo K, Homma E, Dong DT, Amachi S. 2013. Arsenic dissolution from Japanese paddy soil by a dissimilatory arsenate-reducing bacterium Geobacter sp. OR-1. Environ Sci Technol 47:6263–6271. [DOI] [PubMed] [Google Scholar]
- 12.Kudo K, Yamaguchi N, Makino T, Ohtsuka T, Kimura K, Dong DT, Amachi S. 2013. Release of arsenic from soil by a novel dissimilatory arsenate-reducing bacterium, Anaeromyxobacter sp. strain PSR-1. Appl Environ Microbiol 79:4635–4642. doi: 10.1128/AEM.00693-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osborne TH, McArthur JM, Sikdar PK, Santini JM. 2015. Isolation of an arsenate-respiring bacterium from a redox front in an arsenic-polluted aquifer in West Bengal, Bengal Basin. Environ Sci Technol 49:4193–4199. doi: 10.1021/es504707x. [DOI] [PubMed] [Google Scholar]
- 14.Ji G, Silver S. 1992. Reduction of arsenate to arsenite by the ArsC protein of the arsenic resistance operon of Staphylococcus aureus plasmid pI258. Proc Natl Acad Sci U S A 89:9474–9478. doi: 10.1073/pnas.89.20.9474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin P, DeMel S, Shi J, Gladysheva T, Gatti DL, Rosen BP, Edwards BF. 2001. Insights into the structure, solvation, and mechanism of ArsC arsenate reductase, a novel arsenic detoxification enzyme. Structure 9:1071–1081. doi: 10.1016/S0969-2126(01)00672-4. [DOI] [PubMed] [Google Scholar]
- 16.Saltikov CW, Olson BH. 2002. Homology of Escherichia coli R773 arsA, arsB, and arsC genes in arsenic-resistant bacteria isolated from raw sewage and arsenic-enriched creek waters. Appl Environ Microbiol 68:280–288. doi: 10.1128/AEM.68.1.280-288.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rhine ED, Phelps CD, Young LY. 2006. Anaerobic arsenite oxidation by novel denitrifying isolates. Environ Microbiol 8:899–908. doi: 10.1111/j.1462-2920.2005.00977.x. [DOI] [PubMed] [Google Scholar]
- 18.Oremland RS, Hoeft SE, Santini JM, Bano N, Hollibaugh RA, Hollibaugh JT. 2002. Anaerobic oxidation of arsenite in Mono Lake water and by a facultative, arsenite-oxidizing chemoautotroph, strain MLHE-1. Appl Environ Microbiol 68:4795–4802. doi: 10.1128/AEM.68.10.4795-4802.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Casiot C, Morin G, Juillot F, Bruneel O, Personné JC, Leblanc M, Duquesne K, Bonnefoy V, Elbaz-Poulichet F. 2003. Bacterial immobilization and oxidation of arsenic in acid mine drainage (Carnoulès creek, France). Water Res 37:2929–2936. doi: 10.1016/S0043-1354(03)00080-0. [DOI] [PubMed] [Google Scholar]
- 20.Donahoe-Christiansen J, D'Imperio S, Jackson CR, Inskeep WP, McDermott TR. 2004. Arsenite-oxidizing Hydrogenobaculum strain isolated from an acid-sulfate-chloride geothermal spring in Yellowstone National Park. Appl Environ Microbiol 70:1865–1868. doi: 10.1128/AEM.70.3.1865-1868.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Casiot C, Pedron V, Bruneel O, Duran R, Personné JC, Grapin G, Drakidès C, Elbaz-Poulichet F. 2006. A new bacterial strain mediating As oxidation in the Fe-rich biofilm naturally growing in a groundwater Fe treatment pilot unit. Chemosphere 64:492–496. doi: 10.1016/j.chemosphere.2005.11.072. [DOI] [PubMed] [Google Scholar]
- 22.Quéméneur M, Heinrich-Salmeron A, Muller D, Lièvremont D, Jauzein M, Bertin PN, Garrido F, Joulian C. 2008. Diversity surveys and evolutionary relationships of aoxB genes in aerobic arsenite-oxidizing bacteria. Appl Environ Microbiol 74:4567–4573. doi: 10.1128/AEM.02851-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang JS, Yoon IH, Lee JH, Kim KR, An J, Kim KW. 2010. Arsenic detoxification potential of aox genes in arsenite-oxidizing bacteria isolated from natural and constructed wetlands in the Republic of Korea. Environ Geochem Health 32:95–105. doi: 10.1007/s10653-009-9268-z. [DOI] [PubMed] [Google Scholar]
- 24.Garcia-Dominguez E, Mumford A, Rhine ED, Paschal A, Young LY. 2008. Novel autotrophic arsenite-oxidizing bacteria isolated from soil and sediments. FEMS Microbiol Ecol 66:401–410. doi: 10.1111/j.1574-6941.2008.00569.x. [DOI] [PubMed] [Google Scholar]
- 25.Santini JM, Sly LI, Schnagl RD, Macy JM. 2000. A new chemolithoautotrophic arsenite-oxidizing bacterium isolated from a gold mine: phylogenetic, physiological, and preliminary biochemical studies. Appl Environ Microbiol 66:92–97. doi: 10.1128/AEM.66.1.92-97.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun W, Sierra-Alvarez R, Milner L, Field JA. 2010. Anaerobic oxidation of arsenite linked to chlorate reduction. Appl Environ Microbiol 76:6804–6811. doi: 10.1128/AEM.00734-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fisher JC, Hollibaugh JT. 2008. Selenate-dependent anaerobic arsenite oxidation by a bacterium from Mono Lake, California. Appl Environ Microbiol 74:2588–2594. doi: 10.1128/AEM.01995-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderson GL, Williams J, Hille R. 1992. The purification and characterization of arsenite oxidase from Alcaligenes faecalis, a molybdenum-containing hydroxylase. J Biol Chem 267:23674–23682. [PubMed] [Google Scholar]
- 29.Inskeep WP, Macur RE, Hamamura N, Warelow TP, Ward SA, Santini JM. 2007. Detection, diversity and expression of aerobic bacterial arsenite oxidase genes. Environ Microbiol 9:934–943. doi: 10.1111/j.1462-2920.2006.01215.x. [DOI] [PubMed] [Google Scholar]
- 30.Hamamura N, Macur RE, Korf S, Ackerman G, Taylor WP, Kozubal M, Reysenbach AL, Inskeep WP. 2009. Linking microbial oxidation of arsenic with detection and phylogenetic analysis of arsenite oxidase genes in diverse geothermal environments. Environ Microbiol 11:421–431. doi: 10.1111/j.1462-2920.2008.01781.x. [DOI] [PubMed] [Google Scholar]
- 31.Ghosh D, Bhadury P, Routh J. 2014. Diversity of arsenite oxidizing bacterial communities in arsenic-rich deltaic aquifers in West Bengal, India. Front Microbiol 5:602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quéméneur M, Cébron A, Billard P, Battaglia-Brunet F, Garrido F, Leyval C, Joulian C. 2010. Population structure and abundance of arsenite-oxidizing bacteria along an arsenic pollution gradient in waters of the Upper Isle River Basin, France. Appl Environ Microbiol 76:4566–4570. doi: 10.1128/AEM.03104-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heinrich-Salmeron A, Cordi A, Brochier-Armanet C, Halter D, Pagnout C, Abbaszadeh-Fard E, Montaut D, Seby F, Bertin PN, Bauda P, Arsène-Ploetze F. 2011. Unsuspected diversity of arsenite-oxidizing bacteria revealed by a widespread distribution of the aoxB gene in prokaryotes. Appl Environ Microbiol 77:4685–4692. doi: 10.1128/AEM.02884-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sultana M, Vogler S, Zargar K, Schmidt AC, Saltikov C, Seifert J, Schlömann M. 2012. New clusters of arsenite oxidase and unusual bacterial groups in enrichments from arsenic-contaminated soil. Arch Microbiol 194:623–635. doi: 10.1007/s00203-011-0777-7. [DOI] [PubMed] [Google Scholar]
- 35.Jackson CR, Langner HW, Donahoe-Christiansen J, Inskeep WP, McDermott TR. 2001. Molecular analysis of microbial community structure in an arsenite-oxidizing acidic thermal spring. Environ Microbiol 3:532–542. doi: 10.1046/j.1462-2920.2001.00221.x. [DOI] [PubMed] [Google Scholar]
- 36.Engel AS, Johnson LR, Porter ML. 2013. Arsenite oxidase gene diversity among Chloroflexi and Proteobacteria from El Tatio Geyser Field, Chile. FEMS Microbiol Ecol 83:745–756. doi: 10.1111/1574-6941.12030. [DOI] [PubMed] [Google Scholar]
- 37.Li H, Zeng XC, He Z, Chen X, E G, Han Y, Wang Y. 2016. Long-term performance of rapid oxidation of arsenite in simulated groundwater using a population of arsenite-oxidizing microorganisms in a bioreactor. Water Res 101:393–401. doi: 10.1016/j.watres.2016.05.058. [DOI] [PubMed] [Google Scholar]
- 38.Zhu X, Wang R, Lu X, Liu H, Li J, Ouyang B, Lu J. 2015. Secondary minerals of weathered orpiment-realgar-bearing tailings in Shimen carbonate-type realgar mine, Changde, Central China. Mineral Petrol 109:1–15. doi: 10.1007/s00710-014-0344-4. [DOI] [Google Scholar]
- 39.Chen LX, Li JT, Chen YT, Huang LN, Hua ZS, Hu M, Shu WS. 2013. Shifts in microbial community composition and function in the acidification of a lead/zinc mine tailings. Environ Microbiol 15:2431–2444. doi: 10.1111/1462-2920.12114. [DOI] [PubMed] [Google Scholar]
- 40.Li P, Wang Y, Dai X, Zhang R, Jiang Z, Jiang D, Wang S, Jiang HC, Wang YX, Dong H. 2015. Microbial community in high arsenic shallow groundwater aquifers in Hetao Basin of Inner Mongolia, China. PLoS One 10(5):e0125844. doi: 10.1371/journal.pone.0125844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Miguel E, Mingot J, Chacón E, Charlesworth S. 2012. The relationship between soil geochemistry and the bioaccessibility of trace elements in playground soil. Environ Geochem Health 34:677–687. doi: 10.1007/s10653-012-9486-7. [DOI] [PubMed] [Google Scholar]
- 42.Bahar MM, Megharaj M, Naidu R. 2012. Arsenic bioremediation potential of a new arsenite-oxidizing bacterium Stenotrophomonas sp. MM-7 isolated from soil. Biodegradation 23:803–812. [DOI] [PubMed] [Google Scholar]
- 43.Claesson MJ, O'Sullivan O, Wang Q, Nikkila J, Marchesi JR, Smidt H, de Vos WM, Ross RP, O'Toole PW. 2009. Comparative analysis of pyrosequencing and a phylogenetic microarray for exploring microbial community structures in the human distal intestine. PLoS One 4:e6669. doi: 10.1371/journal.pone.0006669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. 2010. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Edgar RC. 2013. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods 10:996–998. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- 46.Blaxter M, Mann J, Chapman T, Thomas F, Whitton C, Floyd R, Abebe E. 2005. Defining operational taxonomic units using DNA barcode data. Philos Trans R Soc Lond B Biol Sci 360:1935–1943. doi: 10.1098/rstb.2005.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, Weber CF. 2009. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rhine ED, Chadhain SN, Zylstra GJ, Young LY. 2007. The arsenite oxidase genes (aroAB) in novel chemoautotrophic arsenite oxidizers. Biochem Biophys Res Commun 354:662–667. doi: 10.1016/j.bbrc.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 49.Sheik CS, Mitchell TW, Rizvi FZ, Rehman Y, Faisal M, Hasnain S, Mclnerney MJ, Krumholz LR. 2012. Exposure of soil microbial communities to chromium and arsenic alters their diversity and structure. PLoS One 7:e40059. doi: 10.1371/journal.pone.0040059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luo J, Bai Y, Liang J, Qu J. 2014. Metagenomic approach reveals variation of microbes with arsenic and antimony metabolism genes from highly contaminated soil. PLoS One 9(10):e108185. doi: 10.1371/journal.pone.0108185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Karlin S, Brocchieri L, Mrázek J, Kaiser D. 2006. Distinguishing features of δ-proteobacterial genomes. Proc Natl Acad Sci U S A 103:11352–11357. doi: 10.1073/pnas.0604311103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Philippe H, Douady CJ. 2003. Horizontal gene transfer and phylogenetics. Curr Opin Microbiol 6:498–505. doi: 10.1016/j.mib.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 53.Koonin EV, Makarova KS, Aravind L. 2001. Horizontal gene transfer in prokaryotes: quantification and classification. Annu Rev Microbiol 55:709–742. doi: 10.1146/annurev.micro.55.1.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kneebone PE, O'Day PA, Jones N, Hering JG. 2002. Deposition and fate of arsenic in iron- and arsenic-enriched reservoir sediments. Environ Sci Technol 36:381–386. doi: 10.1021/es010922h. [DOI] [PubMed] [Google Scholar]
- 55.Islam FS, Gault AG, Boothman C, Polya DA, Charnock JM, Chatterjee D, Lloyd JR. 2004. Role of metal-reducing bacteria in arsenic release from Bengal delta sediments. Nature 430:68–71. doi: 10.1038/nature02638. [DOI] [PubMed] [Google Scholar]
- 56.Héry M, Casiot C, Resongles E, Gallice Z, Bruneel O, Desoeuvre A, Delpoux S. 2014. Release of arsenite, arsenate and methyl-arsenic species from streambed sediment affected by acid mine drainage: a microcosm study. Environ Chem 11:514–524. doi: 10.1071/EN13225. [DOI] [Google Scholar]
- 57.Nicol GW, Leininger S, Schleper C, Prosser JI. 2008. The influence of soil pH on the diversity, abundance and transcriptional activity of ammonia oxidizing archaea and bacteria. Environ Microbiol 10:2966–2978. doi: 10.1111/j.1462-2920.2008.01701.x. [DOI] [PubMed] [Google Scholar]
- 58.Lane DJ. 1991. 16S/23S rRNA sequencing, p 115–175. In Stackebrandt E, Goodfellow M (ed), Nucleic acid techniques in bacterial systematics. Wiley, Hoboken, NJ. [Google Scholar]
- 59.Ellis PJ, Conrads T, Hille R, Kuhn P. 2001. Crystal structure of the 100 kDa arsenite oxidase from Alcaligenes faecalis in two crystal forms at 1.64 Å and 2.03 Å. Structure 9:125–132. doi: 10.1016/S0969-2126(01)00566-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.