ABSTRACT
Pendimethalin [N-(1-ethylpropyl)-2,6-dinitro-3,4-xylidine] is a selective preemergence dinitroaniline herbicide. Several fungi and bacteria have been reported to degrade pendimethalin, but the enzymes or genes involved in this process have not been characterized. Nitroreduction is the initial degradation and detoxification step for pendimethalin. In this study, a pendimethalin nitroreductase (PNR), responsible for the nitroreduction of pendimethalin, was purified from the pendimethalin-degrading strain Bacillus subtilis Y3. Based on a comparison of its mass fingerprints with all of the deduced proteins from the draft genome of strain Y3, a protein annotated as a nitroreductase was identified, and its corresponding encoding gene was termed pnr. PNR was a functional homodimer with a subunit molecular mass of approximately 23 kDa. PNR reduced the C-6 nitro group of the aromatic ring of pendimethalin, yielding 2-nitro-6-amino-N-(1-ethylpropyl)-3,4-xylidine. PNR could also catalyze the nitroreduction of three other major varieties of dinitroaniline herbicides, including butralin, oryzalin, and trifluralin. However, the number of reduced nitro groups was two instead of one, which differed from the nitroreduction of pendimethalin by PNR and which may be due to the symmetry in the chemical structures of the two nitro groups. A detoxification assay revealed that 2-nitro-6-amino-N-(1-ethylpropyl)-3,4-xylidine (PNR-reduced pendimethalin) showed no inhibitory effect on the growth of Saccharomyces cerevisiae BY4741, whereas pendimethalin showed an obvious inhibitory effect on its growth, indicating the detoxification effect of pendimethalin by PNR. Therefore, PNR has potential in pendimethalin detoxification applications. This report describes an enzyme (and corresponding gene) involved in the biodegradation of pendimethalin and dinitroaniline herbicides.
IMPORTANCE Pendimethalin [N-(1-ethylpropyl)-2,6-dinitro-3,4-xylidine] is a widely used selective preemergence dinitroaniline herbicide, and its residue has been frequently detected in the environment. The U.S. Environmental Protection Agency (EPA) has classified pendimethalin as a persistent bioaccumulative toxin. To date, no enzymes or genes involved in pendimethalin biodegradation have been reported. In the present study, the gene pnr, which encodes the nitroreductase PNR, responsible for the nitroreduction of pendimethalin, was cloned from the pendimethalin-degrading strain Bacillus subtilis Y3. PNR could also catalyze the nitroreduction of three other major varieties of dinitroaniline herbicides, including butralin, oryzalin, and trifluralin. The reduction of pendimethalin by PNR might eliminate its toxicity against Saccharomyces cerevisiae BY4741, indicating the application potential of PNR in the detoxification of pendimethalin.
INTRODUCTION
Pendimethalin [N-(1-ethylpropyl)-2,6-dinitro-3,4-xylidine], a selective preemergence dinitroaniline herbicide, is widely used to control annual grasses and certain broadleaf weeds in the planting of dryland crops. It is the third most frequently used herbicide behind glyphosate and parquet and the most frequently used selective herbicide in the world. Pendimethalin's herbicidal action lies in its inhibition of cell elongation and cell division. Compared to other dinitroanilines, pendimethalin has a relatively low volatility, so it is lost less rapidly from the surface soil by volatilization (1, 2). Pendimethalin is moderately persistent in soil, with a half-life of approximately 69 days in tropical fields, and its residue has been frequently detected in soil, ground water, and surface water (3). The U.S. Environmental Protection Agency (EPA) has classified pendimethalin as a persistent bioaccumulative toxic agent (4). Although it has low acute toxicity, it is a possible human carcinogen and is also toxic to terrestrial and aquatic invertebrates (5, 6). Therefore, great concern and interest have been raised regarding the environmental behavior and degradation mechanisms of pendimethalin.
To date, several microorganisms capable of degrading pendimethalin have been isolated and characterized, including Azotobacter chroococcum, Fusarium oxysporum, Pyricularia oryzae Cav., Lecanicillium saksenae, Bacillus circulans, and Bacillus subtilis Y3 (7–10). The degradation pathway of pendimethalin has been proposed and summarized based on the structural identification of intermediate metabolites that appear during its degradation by different strains (Fig. 1). In the degradation process, nitroreduction is the key step.
FIG 1.
Proposed degradation pathways of pendimethalin by the reported strains.
Because no genes or enzymes involved in the biodegradation of pendimethalin have been described thus far and because the nitroreduction process is a general strategy for the detoxification of nitroaromatic compounds (7, 11, 12), in this study, we focused on the cloning of a gene encoding the nitroreductase responsible for the conversion of pendimethalin to 2-nitro-6-amino-N-(1-ethylpropyl)-3,4-xylidine in B. subtilis Y3, which was previously isolated by our group (10).
MATERIALS AND METHODS
Chemicals and media.
Pendimethalin (97%) was a generous gift from Rosi Chemical Co. Ltd., Zhejiang Province, China. Butralin (99%), oryzalin (99%), and trifluralin (99%) were purchased from Sigma-Aldrich, France. All other chemical reagents were of the highest analytical purity.
Mineral salts medium (MSM) consisted of 1.0 g/liter NH4NO3, 0.5 g/liter NaCl, 1.5 g/liter K2HPO4, 0.5 g/liter KH2PO4, and 0.2 g/liter MgSO4·7H2O; the carbon source was added as needed. Luria-Bertani (LB) broth contained 10.0 g/liter tryptone, 5.0 g/liter yeast extract, and 10.0 g/liter NaCl. Yeast extract peptone dextrose (YPD) medium contained 20.0 g/liter tryptone, 10.0 g/liter yeast extract, and 20.0 g/liter glucose. For solid medium, 15.0 g agar was added per liter.
Strains, plasmids, and culture conditions.
The strains and plasmids used in the study are presented in Table 1. Strain Y3 (deposit number Canadian Clinical Trials Coordinating Centre [CCTCC] AB 2015029) was grown in LB broth or MSM supplemented with 0.36 mM pendimethalin at 30°C aerobically unless otherwise stated. Escherichia coli BL21(DE3) and Staphylococcus aureus ATCC 25923 were incubated aerobically at 37°C in LB broth. Saccharomyces cerevisiae BY4741 was grown on YPD medium at 30°C.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| Strains | ||
| Bacillus subtilis Y3 | Degrades pendimethalin | 10 |
| Escherichia coli BL21(DE3) | F− ompT hsdS(rB− mB−) gal dcm lacY1 (DE3) | Invitrogen |
| E. coli BL21(DE3)-pnr | E. coli BL21(DE3) harboring the plasmid pET29a-pnr | This study |
| Saccharomyces cerevisiae BY4741 | MATα his3Δ1 leu2Δ met15Δ ura3Δ | This lab |
| Staphylococcus aureus ATCC 25923 | Standard strain of Gram-positive bacteria | This lab |
| Plasmids | ||
| pET29a(+) | Expression vector, Kmra | Novagen |
| pET29a-pnr | pET29a(+) derivative carrying pnr, Kmr | This study |
Kmr, kanamycin resistant.
Sequencing, assembly, and annotation.
DNA extraction was performed according to the method described by Sambrook and Russell (13). The sequencing of the strain Y3 draft genome was performed by Shanghai Majorbio Bio-pharm Technology Co. Ltd. (Shanghai, China) using the Illumina HiSeq 2000 sequencing system (14–16). Shotgun libraries consisting of 500-bp paired-read fragments were sequenced and assembled using SOAPdenovo software (version 2; http://soapdenovo2.sourceforge.net/). Gene prediction and functional annotation were performed using Glimmer 3.02 (17), tRNAscan-SE version 1.3.1 (18), and Barrnap 0.4.2 (19).
Purification of pendimethalin nitroreductase.
Strain Y3 cells were grown in LB broth, harvested by centrifugation at 8,000 × g for 10 min at 4°C, washed twice with 20 mM Tris-HCl buffer (pH 7.5), and lysed by sonication (Auto Science, UH-650B ultrasonic processor, 40% intensity) for 15 min. After centrifugation at 12,000 × g for 30 min at 4°C to remove unbroken cells, the supernatant obtained, referred to as the cell extract, was precipitated with ammonium sulfate. The 45 to 70% fraction was dissolved in 20 ml of 20 mM Tris-HCl buffer (pH 7.5) and desalted overnight in a Slide-A-Lyzer dialysis membrane (10 kDa) (Pierce, USA) against 20 mM Tris-HCl buffer (pH 7.5) at 4°C. After dialysis, the resulting mixture was subjected to DEAE-Sepharose chromatography, and proteins were eluted with a 0 to 1 M linear gradient of NaCl in 20 mM Tris-HCl buffer (pH 7.5). Active fractions were combined, dialyzed, and subjected to ammonium sulfate precipitation to a final ammonium sulfate concentration of 45%. The fraction was collected by centrifugation at 12,000 × g for 30 min at 4°C, dissolved in 20 mM Tris-HCl buffer (pH 7.5), subjected to a hydrophobic interaction chromatography column, and eluted with a 45% to 0% ammonium sulfate gradient in 20 mM Tris-HCl buffer (pH 7.5). Pooled active fractions were collected, concentrated using Microcon centrifugal filters (10-kDa cutoff), subjected to Sephadex G-75 Gel filtration, and eluted with 20 mM Tris-HCl buffer (pH 7.5). All purification steps were performed at 4°C. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed to determine the molecular weight of the denatured protein (20), and the Bradford method (21) was used to quantify the protein concentration.
Pendimethalin is orange-yellow in color; the fading or disappearance of this specific color could be used as a method to determine its degradation (see Fig. S1 in the supplemental material). Native polyacrylamide gel electrophoresis (native PAGE) was performed according to the description of Wittig et al. (22) with some modifications. Pendimethalin (0.18 mM) was added to the gel as an indicator. The purified proteins of strain Y3 in the native PAGE were assayed for nitroreductase activity against pendimethalin after electrophoresis, which would result in a transparent zone in the native PAGE gel if the protein mixture exhibited nitroreductase activity.
Protein assay, sequencing, mass spectroscopy analysis, and genome comparison.
The transparent zone on the native PAGE gel was cut out and sent to Bo-Yuan Biological Technology Co. Ltd. (Shanghai, China) for peptide mass fingerprint analysis. The resulting peptide fragments were compared with the amino acid sequences of the annotated open reading frames (ORFs) from the draft genome of strain Y3 to identify the sequences with high similarity.
For the phylogenetic analysis of pendimethalin nitroreductase (PNR), all protein sequences were aligned in Clustal X (version 2.1) (23) and then imported into MEGA software (version 5.0) (24) for phylogenetic tree construction using the neighbor-joining method (25). Distances were calculated with a Kimura two-parameter distance model (26).
Expression of pnr and purification of recombinant PNR.
The pnr gene was amplified from the genome DNA of strain Y3 with primers pnr-F (5′-GGAATTCCATATGATCAAAACAAACGATTTTATGG-3′) (the NdeI digestion site is underlined) and pnr-R (5′-CCGCTCGAGTTTCCATTCTGCAATTGTATCAATC-3′) (the XhoI digestion site is underlined). The products were then digested with NdeI and XhoI and introduced into the corresponding sites of pET29a(+), yielding pET29a-pnr, which was transformed into E. coli BL21(DE3) and sequenced for validating the correct amplification and insertion into pET29a(+). E. coli BL21(DE3) cells harboring pET29a-pnr were incubated in 100 ml of LB broth at 37°C to an optical density at 600 nm (OD600) of 0.6 to 0.8, after which 0.3 mM isopropyl-β-d-thiogalactopyranoside (IPTG) was added. After another incubation at 16°C for 16 h, cells were harvested by centrifugation and subjected to ultrasonic disruption as described above. Nickel-nitrilotriacetic acid (Ni2+-NTA) resin was used for the purification of the recombinant PNR (27). A series concentration of imidazole in 20 mM Tris-HCl buffer (pH 7.5) was used to elute the recombinant PNR. Sephadex G-75 gel filtration was used to determine the molecular weight of the native protein.
Enzyme activity assay.
The enzyme reaction was performed at 35°C for 10 min in 3 ml of 20 mM Tris-HCl buffer (pH 7.5) containing 0.36 mM pendimethalin, a suitable amount of enzyme (cell extract or recombinant PNR), 0.8 mM NADH, and 1 mM Mg2+. One unit of enzyme activity was defined as the amount of enzyme required to catalyze the consumption of 1 nmol pendimethalin per min.
Different concentrations of pendimethalin (0.09 to 0.72 mM) or NADH (0.2 to 1 mM) were added into the enzyme reaction mixture, which was then incubated at 35°C for 10 min. The kinetic parameters Km and Vmax of PNR were calculated using the Lineweaver-Burk plot method (28).
Preparation of the reduced product of pendimethalin by PNR.
Enzyme assay samples were extracted with equal volumes of dichloromethane. The organic phase was dehydrated with anhydrous sodium sulfate, and 3 ml of the dehydrated organic phase was drawn, evaporated to dryness with nitrogen gas, and concentrated to 0.5 ml with a mixture of chloroform and acetonitrile (1:1 [vol/vol]). The concentrated samples were further purified by thin-layer chromatography (TLC). The developing solvent for TLC was a mixture of petroleum ether, chloroform, and acetonitrile in proportions of 25:1:1 (vol/vol/vol), and the flow rate was 2 ml/min. Eluted fractions were collected every 10 ml and subjected to high-performance liquid chromatography (HPLC) analysis to identify the fractions containing the reduced product of pendimethalin. These fractions were then pooled and dried with nitrogen gas to prepare them for the following analysis.
Identification of PNR-reduced pendimethalin by UHPLC-MS/MS and NMR.
For ultrahigh-performance liquid chromatography-tandem mass spectroscopy (UHPLC-MS/MS) analysis, the dried fraction was dissolved in methanol and then filtered through a 0.22-μm Millipore membrane filter before use. The UHPLC column utilized was a Hypersil Gold C18 column (100 mm by 2.10 mm, 3-μm particle sizes; Thermo Fisher Scientific). The mobile phase consisted of 0.02% formic acid in water (solvent A) and 100% acetonitrile (solvent B), with a flow rate of 0.2 ml/min. The injection volume was 5 μl. For mass spectrometry analysis, an LTQ Orbitrap XL mass spectrometer (Thermo Fisher Scientific) equipped with an electrospray ionization (ESI) probe was used. Data analysis was under the positive mode (29).
To confirm which nitro group of pendimethalin was reduced, the dried fraction was dissolved in dimethyl sulfoxide (DSMO) and subjected to 1H nuclear magnetic resonance (NMR) analysis using a Bruker Avance 500-MHz spectrometer (Bruker Biospin, France). The probe was a 5-mm PABBO BB-1H, the pulse program was zgpr, and the sweep width was 10,000,000 Hz.
RNA isolation and quantitative real-time PCR.
An aliquot of the cells of strain Y3 was inoculated at the level of 2% (vol/vol) into 20 ml of MSM supplemented with 0.36 mM pendimethalin or 0.56 mM glucose, respectively. The cultures were incubated at 30°C for 12 h (about 50% of pendimethalin was transformed), and the cells were harvested by centrifugation (3,770 × g, 10 min at 4°C). Total RNA was extracted using a MiniBEST universal RNA extraction kit (TaKaRa, China) and treated with genomic DNA (gDNA) eraser (TaKaRa), according to the manufacturer's instructions. A reverse transcription (RT) reaction was performed using a PrimeScript RT reagent kit (TaKaRa). Expression of pnr in strain Y3 was analyzed by quantitative real-time PCR in an Applied Biosystems 7300 real-time PCR system (Applied Biosystems, USA) using an SYBR Premix Ex Taq RT-PCR kit (TaKaRa). The 16S rRNA gene was used as the internal control gene since it was transcribed both in the presence and absence of pendimethalin as demonstrated in reverse transcription-PCR (data not shown). Gene-specific primers RT-16SF/16SR and RT-PF/PR used for quantitative real-time PCR are listed in Table 2. Relative changes in pnr expression were calculated using the 2−ΔΔCT threshold cycle number (CT) method (30).
TABLE 2.
Primers used in this study
| Primer | DNA sequence (5′ to 3′)a | Description |
|---|---|---|
| pnr-F | GGAATTCCATATGATCAAAACAAACGATTTTATGG | Forward primer to amplify pnr with a NdeI site |
| pnr-R | CCGCTCGAGTTTCCATTCTGCAATTGTATCAATC | Reverse primer to amplify pnr with a XhoI site |
| RT-16SF | CCAGCATTCAGTTGGGCACTCTAAG | Forward primer of quantitative real-time PCR to amplify a 173-bp fragment of 16S rRNA sequence |
| RT-16SR | ACTGAGAACAGATTTGTGGGATTGG | Reverse primer of quantitative real-time PCR to amplify a 173-bp fragment of 16S rRNA sequence |
| RT-PF | GCCGCCGTTCTATTCGCAACTATG | Forward primer of quantitative real-time PCR to amplify a 113-bp fragment of pnr sequence |
| RT-PR | CCATGGCTGCGCGTTAACAGAAGAT | Reverse primer of quantitative real-time PCR to amplify a 113-bp fragment of pnr sequence |
Underlined sequences refer to the restriction sites.
Biochemical properties of the recombinant PNR.
The effects of pH and temperature on the activity and stability of PNR were determined. Three different buffers (20 mM citrate buffer [pH 3.0 to 6.0], 20 mM phosphate-buffered saline [PBS] [pH 6.0 to 7.5], and 20 mM Tris-HCl [pH 7.0 to 8.8]) were used to assess the optimal reaction pH. To determine the optimal reaction temperature, a range of 4°C to 65°C was investigated. The PNR activity observed at Tris-HCl (pH 7.0) and 35°C was defined as 100%, and the relative activities of each reaction were calculated. To determine pH stability, PNR in different pH buffers was incubated at 4°C for 12 h, and the residual activity was measured. To evaluate thermal stability, the enzyme assay was performed every 2 h at different temperatures.
The effects of potential activators or inhibitors on PNR activity were determined by the addition of 1 mM different metal cations (Mg2+, Ca2+, Fe2+, Fe3+, Cd2+, Hg2+, Li+, K+, Cu2+, Co2+, Mn2+, Zn2+, Ni2+, Ag+), 100 mM metal-chelating agent EDTA, and 100 mM chemical agents dithiothreitol (DTT) and SDS into the PNR reaction mixture. PNR activity with no additive was referred to as the blank control and was defined as 100%, and the relative activity of PNR with different treatments was calculated. Potential enhancers and inhibitors of PNR activity were distinguished by the threshold value 100% ± 10%. Butralin, oryzalin, and trifluralin were used to determine the substrate spectrum of PNR.
Detoxification assay.
The effects of pendimethalin and its PNR-catalyzed product 2-nitro-6-amino-N-(1-ethylpropyl)-3,4-xylidine on the growth of strains BY4741, BL21(DE3), and ATCC 25923 were investigated. Pendimethalin was dissolved in methanol. 2-Nitro-6-amino-N-(1-ethylpropyl)-3,4-xylidine was produced from the transformation of pendimethalin by PNR and was redissolved in methanol.
All of the strains were grown to the exponential phase, after which they were washed three times and used as inoculants. Strain BY4714 was then inoculated in 5-fold-diluted YPD medium. Strain BL21(DE3) was inoculated in MSM supplemented with 0.56 mM glucose. Strain ATCC 25923 was cultured in 10-fold-diluted LB. Pendimethalin or 2-nitro-6-amino-N-(1-ethylpropyl)-3,4-xylidine was added into the medium at a final concentration of 0.36 mM or 0.4 mM, respectively. The strain growth was measured every 4 h by the spreading plate method and by counting the CFU per milliliter.
Accession number(s).
The GenBank accession numbers for the draft genome of strain Y3 and the nucleotide sequence of pnr are LRFK00000000 and KU565870, respectively.
RESULTS AND DISCUSSION
Purification of PNR from strain Y3.
PNR was purified from the strain Y3 cell extract to obtain the protein sequence and subsequently identify the corresponding gene. The PNR purification process was summarized in Table 3. The PNR activity of the cell extract of strain Y3 was only 0.38 U/mg protein. However, after four steps of purification, the specific activity of the purified enzyme had increased to 18.5 U/mg protein, with a purification factor of 48.7-fold. The purified protein yielded a band of approximately 23 kDa on an SDS-PAGE gel (Fig. 2A). The purified protein was also run on a native PAGE gel supplemented with 0.18 mM pendimethalin, after which the gel was placed into 20 mM Tris-HCl buffer (pH 7.5) with the addition of 0.5 mM NADH and 1 mM Mg2+ for 10 min to test the pendimethalin nitroreductase activity. The purified protein yielded an obvious transparent zone on the native PAGE gel (Fig. 2B), suggesting that the purified protein from strain Y3 displayed nitroreductase activity against pendimethalin. The gel's transparent zone was then excised for matrix-assisted laser desorption ionization−time of flight (MALDI-TOF) mass spectrometry analysis.
TABLE 3.
Purification of PNR from B. subtilis Y3
| Purification step | Total protein (mg) | Total activity (U) | Specific activity (U/mg protein) | Purification (fold) | Yield (%) |
|---|---|---|---|---|---|
| Cell extract | 231.62 | 609.53 | 0.38 | 100 | |
| Ammonium sulfate precipitation | 121.82 | 348.41 | 2.86 | 7.53 | 56.56 |
| DEAE-Sepharose chromatography | 37.29 | 241.27 | 6.47 | 17.03 | 39.58 |
| Hydrophobic interaction chromatography | 5.46 | 58.91 | 10.79 | 28.39 | 9.67 |
| Sephadex G-75 gel filtration | 0.12 | 2.22 | 18.5 | 48.68 | 0.36 |
FIG 2.
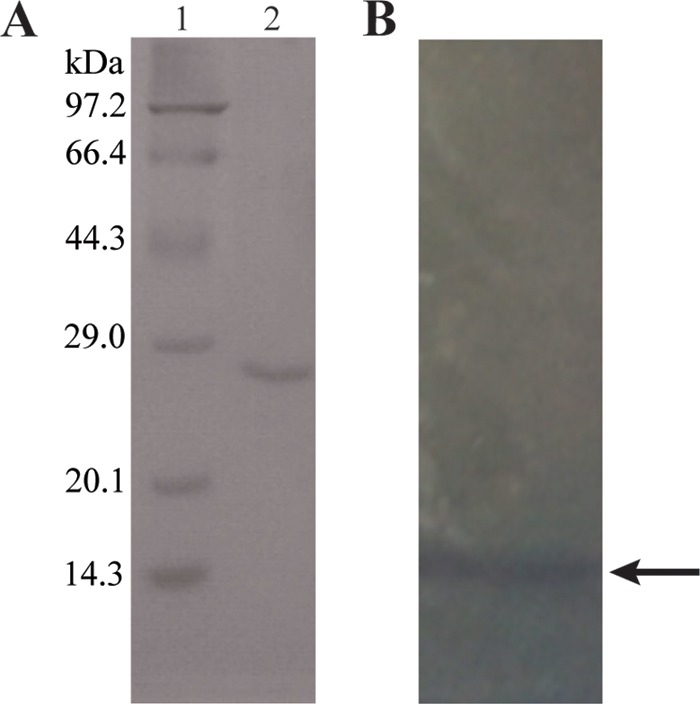
Purification of pendimethalin nitroreductase from B. subtilis Y3. (A) SDS-PAGE spectrum of PNR purified from cell extract of strain Y3. Lane 1, protein marker (kilodaltons); lane 2, purified protein after four steps of purification. (B) Native PAGE analysis of PNR purified from strain Y3; the arrow points to the transparent zone.
Sequences of several peptide fragments were obtained and compared to those of all of the annotated proteins from the draft genome of strain Y3, and they matched a nitroreductase encoded by open reading frame orf03879 (630 bp in length) (see Fig. S2 in the supplemental material). orf03879 was subsequently designated pnr and chosen for the following study.
Sequence analysis of PNR.
Sequence analysis indicated that PNR consisted of 209 amino acids. The result of a BLASTP search in the NCBI protein databases (the UniProt Knowledge Base/Swiss-Prot databases) revealed that PNR showed 100% identity to the uncharacterized NAD(P)H nitroreductase YdgI from Bacillus subtilis subsp. subtilis strain 168 (GenBank accession number NP388447). Among the characterized nitroreductases, PNR showed the highest identity (35%) to nitrobenzoate nitroreductase PnbA of Lactobacillus plantarum WCFS1; it also shared 31%, 29%, and 28% amino acid sequence identities with the quinone reductase DrgA of Synechocystis sp., the NAD(P)H-flavin oxidoreductase FRase I of Vibrio fischeri, and the NAD(P)H-dependent oxidoreductase PnrB of Pseudomonas putida, respectively (Fig. 3). Bacterial nitroreductases can be divided into two types according to the one- or two-electron mechanism of nitroreduction of polynitroaromatic compounds. Type I nitroreductases are oxygen-insensitive and require NAD(P)H as electron donors in an obligatory two-electron transfer. Type II nitroreductases are oxygen-sensitive and catalyze the nitro group through a single-electron reduction, bearing a nitro anion radical, which can be reoxidized to the parent structure in aerobic conditions. Therefore, as the reduction of nitro groups by type II nitroreductases results in a futile cycle, studies on type II nitroreductases have been very limited. Type I nitroreductases catalyze the sequential reduction of the nitro groups to generate nitroso, hydroxyamino, and amino derivatives (31, 32). Type I nitroreductases can be further divided into two main groups, which are represented by the E. coli nitroreductases NfsA (group A in Fig. 3) and NfsB (group B in Fig. 3), respectively. Sequence alignment revealed that PNR was assigned to the NfsB-like nitroreductase, which is classified as type I group B. Group B nitroreductases are divided into two subfamilies. Subfamily B1 includes the minor nitroreductase NfsB of E. coli (33), the retro-nitroreductase (RNR) of Enterobacter cloacae (34), the nitroreductase PnrB of P. putida JLR11 (35), the NAD(P)H-flavin oxidoreductase FRase I of V. fischeri (36), the quinone reductase DrgA of Synechocystis sp. (37), the nitrobenzoate nitroreductase PnbA of L. plantarum WCFS1 (38), and others. Subfamily B2 contains the YdjA of E. coli and Salmonella enterica and the nitrobenzene nitroreductase CnbA encoded by the cnbA gene located on plasmid pCNB1 of Comamonas testosteroni CNB-1 (39). A phylogenetic analysis revealed that PNR was a member of the B1 subfamily and clustered into a single branch on the phylogenetic tree (Fig. 3).
FIG 3.
Neighbor-joining phylogenetic tree constructed based on the alignment of PNR with related type I nitroreductases. Confidence values for branches were determined using bootstrap analyses based on 1,000 resamplings. The subgroups (A, B1, and B2) of type I nitroreductases are marked on the right.
Heterogeneous gene expression of pnr.
To further identify whether PNR was responsible for the nitroreduction of pendimethalin, the pnr gene was amplified using a specific primer pair pnr-F/R and introduced into pET29a(+) to produce pET29a-pnr. A whole-cell transformation assay revealed that E. coli BL21(DE3)-pnr gained the capacity to transform pendimethalin (data not shown). Recombinant PNR was purified using Ni2+-NTA resin, and SDS-PAGE analysis showed that the recombinant PNR possessed a monomer size of approximately 23 kDa (see Fig. S3 in the supplemental material), which was in agreement with its theoretical molecular mass. The PNR activity was as high as 25.1 U/mg protein. Sephadex G-75 gel filtration analysis of native PNR indicated a molecular mass of approximately 48 kDa, indicating that the protein acted functionally as a homodimer.
Transcriptional level of pnr of strain Y3 under the induction of pendimethalin.
The relative changes in the transcription of pnr of strain Y3 under pendimethalin-induced and uninduced conditions were investigated by quantitative real-time PCR. The data showed that there was no significant difference between the cells grown under induced and uninduced conditions (see Fig. S4 in the supplemental material), indicating that the expression of pnr in strain Y3 was conservative. This also explained the purification of PNR from the cell extract of strain Y3 cultured in LB broth without the addition of pendimethalin.
Identification of the pendimethalin-nitroreduced product by PNR.
The nitroreduction product of pendimethalin by PNR was first identified by UHPLC-MS/MS (Fig. 4A), which was consistent with a previously reported result (10). Because the previous report focused on the structural identification of metabolites and the proposed degradation pathway of pendimethalin, there was no strong evidence identifying which nitro group of pendimethalin was reduced (7–10). For further confirmation, the nitroreduction product of pendimethalin was subjected to 1H NMR analysis (Fig. 4B). The 1H NMR spectrum displayed an aromatic ring proton (C-5 proton) at δ 6.6 ppm, two aromatic methyl groups at δ 1.9 ppm and δ 2.1 ppm, and an amino proton at δ 4.9 ppm. The proton signals for the N-(1-ethylpropyl) group were present at δ 3.5 ppm, δ 2.75 ppm, δ 1.3 ppm, and δ 0.8 ppm. The discrepancy between 2-nitro-6-amino-N-(1-ethylpropyl)-3,4-xylidine and 2-amino-6-nitro-N-(1-ethylpropyl)-3,4-xylidine in the 1H NMR spectrum was the chemical shift of the C-5 proton, which was observed at δ 6.6 ppm in 2-nitro-6-amino-N-(1-ethylpropyl)-3,4-xylidine and at δ 8.0 ppm in 2-amino-6-nitro-N-(1-ethylpropyl)-3,4-xylidine (7). The reduction of the C-6 nitro group to an amino group might result in the upfield shift of the C-5 proton signal in 2-nitro-6-amino-N-(1-ethylpropyl)-3,4-xylidine. Therefore, PNR reduced the C-6 nitro group of the aromatic ring of pendimethalin, resulting in the production of 2-nitro-6-amino-N-(1-ethylpropyl)-3,4-xylidine (Fig. 5).
FIG 4.
Identification of pendimethalin-reduced product by PNR. (A) UHPLC-MS/MS analysis of the pendimethalin-reduced product by PNR, whose retention time (RT) was 16.16 min. Data analysis of the product's MS/MS spectrum was under the positive mode. (B) 1H NMR analysis of pendimethalin-reduced product by PNR.
FIG 5.
Nitroreduction of pendimethalin, trifluralin, butralin, and oryzalin by PNR.
Characterization of PNR.
The highest PNR activity was observed at pH 7.0 and 35°C (see Fig. S5A and C in the supplemental material). PNR retained greater than 80% activity after incubation at pH values ranging from 6.0 to 8.0 at 4°C for 12 h (see Fig. S5B) and might also retain greater than 80% activity for 6 h in temperatures ranging from 4 to 35°C (see Fig. S5D); however, in high-temperature conditions (50 to 60°C), the residual activity of PNR fell below 10% in 2.5 h.
PNR activity was enhanced by Mg2+, Fe2+, and Fe3+, while it was inhibited by Cd2+, Hg2+, Cu2+, Ag+, and Zn2+ (see Table S1 in the supplemental material). Additionally, Li+, K+, Ca2+, Co2+, Mn2+, and Ni2+ had no obvious effects on PNR activity. EDTA also had no clear effect on PNR activity, whereas SDS inhibited it. Surprisingly, DTT strongly enhanced PNR activity by 1.3-fold.
Butralin, oryzalin, and trifluralin are three prominent members of the selective preemergence dinitroaniline herbicides. These compounds have many physical and chemical properties in common with pendimethalin, but they have two symmetrical nitro groups, in contrast to pendimethalin (Fig. 5). They show relatively high toxicity toward aquatic organisms and rats (40–43); in addition, trifluralin is suspected to be an endocrine disruptor (44) and has been classified as a group C possible human carcinogen (45). Until now, there has been no report on the genes or enzymes that are responsible for the degradation of butralin, oryzalin, and trifluralin. Thus, a comparison of the transformations of the three dinitroanilines with pendimethalin by PNR was conducted. PNR displayed nitroreduction activity against butralin, oryzalin, and trifluralin. Moreover, UHPLC-MS analysis of their PNR-reduced metabolites revealed that both nitro groups on the aromatic ring could be reduced by PNR (see Fig. S6 to S8 in the supplemental material). Although PNR reduced both nitro groups of butralin, oryzalin, and trifluralin, it only reduced the C-6 nitro group of the aromatic ring of pendimethalin (Fig. 5), which may have been due to structural differences in these compounds. For butralin, oryzalin, and trifluralin, the two nitro groups are symmetrical, whereas pendimethalin contains a substituent methyl group at C-3 of the aromatic ring, distinct from the structures of butralin, oryzalin, and trifluralin. This substituent group may make it difficult for PNR to catalyze the second nitroreduction reaction.
The kinetic constants for PNR against the four dinitroaniline herbicides are shown in Table 4. The Kcat/Km values of PNR against pendimethalin, butralin, and oryzalin showed no significant differences, indicating that the catalytic efficiency of PNR toward pendimethalin, butralin, and oryzalin was almost at the same level, while it was 10.89 μM−1 · s−1 against trifluralin and a little bit lower. The Kcat/Km value of NADH was observed at 10.68 μM−1 · s−1 with pendimethalin as the electron acceptor.
TABLE 4.
Kinetic constants for PNRa
| Drug | Specific activity (U/mg protein) | Vmax (nmol/min/mg protein) | Km (μM) | Kcat (s−1) | Kcat/Km (μM−1 · s−1) |
|---|---|---|---|---|---|
| NADH | 49.98 | 1.06 × 103 | 35.13 | 375.22 | 10.68 |
| Pendimethalin | 25.11 | 4.39 × 102 | 13.05 | 155.40 | 11.91 |
| Butralin | 23.71 | 3.87 × 102 | 11.54 | 136.99 | 11.61 |
| Oryzalin | 20.22 | 3.76 × 102 | 11.19 | 133.10 | 11.89 |
| Trifluralin | 17.85 | 56.60 | 1.82 | 20.04 | 10.89 |
PNR constants for pendimethalin, butralin, oryzalin, and trifluralin calculated with NADH served as electron donors. NADH constants were determined with pendimethalin as the electron acceptor.
Detoxification assay.
Studies were performed to measure the toxic effects of pendimethalin and its nitroreduced metabolite 2-nitro-6-amino-N-(1-ethylpropyl)-3,4-xylidine on the growth of strains BY4741, BL21(DE3), and ATCC 25923. Cell growth in the presence of different treatments was evaluated, and the results are presented in Fig. 6. Compared with the control settings (blank control and solvent treatment), strain BY4741 treated with 0.4 mM PNR-reduced product 2-nitro-6-amino-N-(1-ethylpropyl)-3,4-xylidine showed no obvious differences in growth, whereas strain BY4741 treated with 0.36 mM pendimethalin for 8 h displayed a lower cell concentration. Furthermore, an obvious inhibitory effect on its cell growth was observed after 12 h of treatment, indicating that pendimethalin had a toxic effect on the cell growth of strain BY4741. However, the cell growth of strains BL21(DE3) and ATCC 25923 treated with pendimethalin or 2-nitro-6-amino-N-(1-ethylpropyl)-3,4-xylidine showed no significant difference compared to the control conditions after 12 h of treatment, suggesting that neither pendimethalin or 2-nitro-6-amino-N-(1-ethylpropyl)-3,4-xylidine had no effects on the cell growth of strains BL21(DE3) and ATCC 25923.
FIG 6.
Effects of pendimethalin and 2-nitro-6-amino-N-(1-ethylpropyl)-3,4-xylidine on the growth of S. cerevisiae BY4741 (A), E. coli BL21(DE3) (B), and S. aureus ATCC 25923 (C). Strain growth was determined at 4 h, 8 h, and 12 h based on the CFU per milliliter. Blank control, inoculation alone; solvent treatment, methanol; pendimethalin with solvent, pendimethalin and methanol; 2-nitro-6-amino-N-(1-ethylpropyl)-3,4-xylidine with solvent, 2-nitro-6-amino-N-(1-ethylpropyl)-3,4-xylidine and methanol.
The herbicidal mechanism of pendimethalin involves inhibiting chromosome separation and cell wall formation during cell division, causing weed death (46). Strain BY4741 is a eukaryotic organism; therefore, its manner of cell division is similar to that of plant cells. Thus, pendimethalin was capable of inhibiting its cell division and cell growth. In contrast, strains BL21(DE3) and ATCC 25923 are prokaryotic organisms; therefore, pendimethalin could not inhibit their cell division and cell growth. The nitroreduction of pendimethalin may eliminate its toxicity and inhibitory effect on eukaryotic and plant cells, suggesting that nitroreduction is the critical detoxification step for pendimethalin. These findings imply that PNR has potential in the elimination of toxicity caused by pendimethalin.
In summary, the present work reports a nitroreductase PNR with its encoding gene pnr, which was responsible for the initial degradation step of pendimethalin in strain Y3. PNR catalyzed the C-6 nitro group reduction of pendimethalin. Additionally, PNR could also functionally reduce three other major varieties of dinitroaniline herbicides, including butralin, oryzalin, and trifluralin, in which both aromatic nitro groups were reduced. The reduction of pendimethalin by PNR could eliminate its toxicity against Saccharomyces cerevisiae BY4741, indicating the application potential of PNR in the detoxification of pendimethalin.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (31570105, 31560031, 31370155), the Program for New Century Excellent Talents in University (NCET-13-0861), the Project of University-Industry Collaboration of Guangdong Province-Ministry (2013B090500017), and the Jiangsu Agriculture Science and Technology Innovation Fund CX (15)1004.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.01771-16.
REFERENCES
- 1.Tomlin C. 1994. The pesticide manual. British Crop Protection Council, Surrey, United Kingdom. [Google Scholar]
- 2.Walker A, Bond W. 1977. Persistence of the herbicide AC 92,553, N-(1-ethylpropyl)-2,6-dinitro-3,4-xylidine, in soils. Pesticide Sci 8:359–365. doi: 10.1002/ps.2780080409. [DOI] [Google Scholar]
- 3.Pinto AP, Serrano C, Pires T, Mestrinho E, Dias L, Teixeira DM, Caldeira AT. 2012. Degradation of terbuthylazine, difenoconazole and pendimethalin pesticides by selected fungi cultures. Sci Total Environ 435–436:402–410. doi: 10.1016/j.scitotenv.2012.07.027. [DOI] [PubMed] [Google Scholar]
- 4.Roca E, D'Errico E, Izzo A, Strumia S, Esposito A, Fiorentino A. 2009. In vitro saprotrophic basidiomycetes tolerance to pendimethalin. Int Biodeterior Biodegrad 63:182–186. doi: 10.1016/j.ibiod.2008.08.004. [DOI] [Google Scholar]
- 5.Das N, Ray S, Jena S, Mohanty P. 1998. Effect of certain herbicides on weeds and population of root-knot nematode (Meloidogyne incognita) in mustard. Crop Res (Hisar) 16:156–158. [Google Scholar]
- 6.Fliedner A. 1997. Ecotoxicity of poorly water-soluble substances. Chemosphere 35:295–305. doi: 10.1016/S0045-6535(97)00156-2. [DOI] [Google Scholar]
- 7.Kole RK, Saha J, Pal S, Chaudhuri S, Chowdhury A. 1994. Bacterial degradation of the herbicide pendimethalin and activity evaluation of its metabolites. Bull Environ Contam Toxicol 52:779–786. [DOI] [PubMed] [Google Scholar]
- 8.Kulshrestha G, Singh SB, Lal SP, Yaduraju NT. 2000. Effect of long-term field application of pendimethalin: enhanced degradation in soil. Pest Manag Sci 56:202–206. doi:. [DOI] [Google Scholar]
- 9.Megadi VB, Tallur PN, Hoskeri RS, Mulla SI, Ninnekar HZ. 2010. Biodegradation of pendimethalin by Bacillus circulans. Indian J Biotechnol 9:173–177. [Google Scholar]
- 10.Ni H, Yao L, Li N, Cao Q, Dai C, Zhang J, He Q, He J. 2016. Biodegradation of pendimethalin by Bacillus subtilis Y3. J Environ Sci (China) 41:121–127. doi: 10.1016/j.jes.2015.04.035. [DOI] [PubMed] [Google Scholar]
- 11.Kulkarni M, Chaudhari A. 2007. Microbial remediation of nitro-aromatic compounds: an overview. J Environ Manage 85:496–512. doi: 10.1016/j.jenvman.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 12.Zayed SM, Mostafa IY, Farghaly MM, Attaby HS, Adam YM, Mahdy FM. 1983. Microbial degradation of trifluralin by Aspergillus carneus, Fusarium oxysporum and Trichoderma viride. J Environ Sci Health 18:253–267. doi: 10.1080/03601238309372367. [DOI] [PubMed] [Google Scholar]
- 13.Sambrook J, Russell DW. 1989. Molecular cloning: a laboratory manual, vol 3 Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 14.Chin CS, Alexander DH, Marks P, Klammer AA, Drake J, Heiner C, Clum A, Copeland A, Huddleston J, Eichler EE, Turner SW, Korlach J. 2013. Nonhybrid, finished microbial genome assemblies from long-read SMRT sequencing data. Nat Methods 10:563–569. doi: 10.1038/nmeth.2474. [DOI] [PubMed] [Google Scholar]
- 15.Koren S, Schatz MC, Walenz BP, Martin J, Howard JT, Ganapathy G, Wang Z, Rasko DA, McCombie WR, Jarvis ED, Phillippy AM. 2012. Hybrid error correction and de novo assembly of single-molecule sequencing reads. Nat Biotechnol 30:693–700. doi: 10.1038/nbt.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo R, Liu B, Xie Y, Li Z, Huang W, Yuan J, He G, Chen Y, Pan Q, Liu Y, Tang J, Wu G, Zhang H, Shi Y, Liu Y, Yu C, Wang B, Lu Y, Han C, Cheung DW. 2012. SOAPdenovo2: an empirically improved memory-efficient short-read de novo assembler. GigaScience 1:18. doi: 10.1186/2047-217X-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delcher AL, Bratke KA, Powers EC, Salzberg SL. 2007. Identifying bacterial genes and endosymbiont DNA with glimmer. BioInformatics 23:673–679. doi: 10.1093/bioinformatics/btm009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lowe TM, Eddy SR. 1997. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res 25:955–964. doi: 10.1093/nar/25.5.0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lagesen K, Hallin P, Rødland EA, Staerfeldt HH, Rognes T, Ussery DW. 2007. RNAmmer: consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res 35:3100–3108. doi: 10.1093/nar/gkm160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laemmli UK. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 21.Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 22.Wittig I, Braun HP, Schägger H. 2006. Blue native PAGE. Nat Protoc 1:418–428. doi: 10.1038/nprot.2006.62. [DOI] [PubMed] [Google Scholar]
- 23.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. 2007. Clustal W and Clustal X version 2.0. BioInformatics 23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 24.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saitou N, Nei M. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425. [DOI] [PubMed] [Google Scholar]
- 26.Kimura M. 1980. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- 27.Janknecht R, de Martynoff G, Lou J, Hipskind RA, Nordheim A, Stunnenberg HG. 1991. Rapid and efficient purification of native histidine-tagged protein expressed by recombinant vaccinia virus. Proc Natl Acad Sci U S A 88:8972–8976. doi: 10.1073/pnas.88.20.8972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dowd JE, Riggs DS. 1965. A comparison of estimates of Michaelis-Menten kinetic constants from various linear transformations. J Biol Chem 240:863–869. [PubMed] [Google Scholar]
- 29.Ramakrishna M, Venkata Mohan S, Shailaja S, Narashima R, Sarma PN. 2008. Identification of metabolites during biodegradation of pendimethalin in bioslurry reactor. J Hazard Mater 151:658–661. doi: 10.1016/j.jhazmat.2007.06.039. [DOI] [PubMed] [Google Scholar]
- 30.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 31.Bryant DW, McCalla DR, Leeksma M, Laneuville P. 1981. Type I nitroreductases of Escherichia coli. Can J Microbiol 27:81–86. doi: 10.1139/m81-013. [DOI] [PubMed] [Google Scholar]
- 32.Peterson FJ, Mason RP, Hovsepian J, Holtzman JL. 1979. Oxygen-sensitive and -insensitive nitroreduction by Escherichia coli and rat hepatic microsomes. J Biol Chem 254:4009–4014. [PubMed] [Google Scholar]
- 33.Zenno S, Koike H, Tanokura M, Saigo K. 1996. Gene cloning, purification, and characterization of NfsB, a minor oxygen-insensitive nitroreductase from Escherichia coli, similar in biochemical properties to FRase I, the major flavin reductase in Vibrio fischeri. J Biochem 120:736–744. doi: 10.1093/oxfordjournals.jbchem.a021473. [DOI] [PubMed] [Google Scholar]
- 34.Koder RL, Oyedele O, Miller AF. 2001. Retro-nitroreductase, a putative evolutionary precursor to Enterobacter cloacae strain 96-3 nitroreductase. Antioxid Redox Signal 3:747–755. doi: 10.1089/15230860152664957. [DOI] [PubMed] [Google Scholar]
- 35.Caballero A, Lázaro JJ, Ramos JL, Esteve-Núñez A. 2005. PnrA, a new nitroreductase-family enzyme in the TNT-degrading strain Pseudomonas putida JLR11. Environ Microbiol 7:1211–1219. doi: 10.1111/j.1462-2920.2005.00801.x. [DOI] [PubMed] [Google Scholar]
- 36.Zenno S, Saigo K, Kanoh H, Inouye S. 1994. Identification of the gene encoding the major NAD(P)H-flavin oxidoreductase of the bioluminescent bacterium Vibrio fischeri ATCC 7744. J Bacteriol 176:3536–3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takeda K, Iizuka M, Watanabe T, Nakagawa J, Kawasaki S, Niimura Y. 2007. Synechocystis DrgA protein functioning as nitroreductase and ferric reductase is capable of catalyzing the Fenton reaction. FEBS J 274:1318–1327. doi: 10.1111/j.1742-4658.2007.05680.x. [DOI] [PubMed] [Google Scholar]
- 38.Guillén H, Curiel JA, Landete JM, Guillén H, Curiel JA, Landete JM, Muñoz R, Herraiz T. 2009. Characterization of a nitroreductase with selective nitroreduction properties in the food and intestinal lactic acid bacterium Lactobacillus plantarum WCFS1. J Agric Food Chem 57:10457–10465. doi: 10.1021/jf9024135. [DOI] [PubMed] [Google Scholar]
- 39.Wu JF, Jiang CY, Wang BJ, Ma YF, Liu ZP, Liu SJ. 2006. Novel partial reductive pathway for 4-chloronitrobenzene and nitrobenzene degradation in Comamonas sp. strain CNB-1. Appl Environ Microbiol 72:1759–1765. doi: 10.1128/AEM.72.3.1759-1765.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Emmerson J, Pierce E, McGrath J. 1980. The chronic toxicity of compound 36352 (trifluralin) given as a component of the diet to Fischer 344 rats for two years. Studies R-87 and R-97 Elanco Products Co., Eli Lilly and Co., Indianapolis, IN. [Google Scholar]
- 41.Helling CS. 1976. Dinitroaniline herbicides in soils. J Environ Qual 5:1–15. doi: 10.2134/jeq1976.0511. [DOI] [Google Scholar]
- 42.Könen S, Çavaş T. 2008. Genotoxicity testing of the herbicide trifluralin and its commercial formulation Treflan using the piscine micronucleus test. Environ Mol Mutagen 49:434–438. doi: 10.1002/em.20401. [DOI] [PubMed] [Google Scholar]
- 43.Naqvi SM, Leung TS. 1983. Trifluralin and oryzalin herbicides toxicities to juvenile crawfish (Procambarus clarkii) and mosquitofish (Gambusia affinis). Bull Environ Contam Toxicol 31:304–308. doi: 10.1007/BF01608703. [DOI] [PubMed] [Google Scholar]
- 44.US Environmental Protection Agency. 1998. Registration eligibility decision (RED): bromoxynil-738-R-98-013. US Environmental Protection Agency, Washington, DC. [Google Scholar]
- 45.Greene SA, Pohanish RP. 2005. Sittig's handbook of pesticides and agricultural chemicals. William Andrew Publishing, New York, NY. [Google Scholar]
- 46.Strandberg M, Scott-Fordsmand JJ. 2004. Effects of pendimethalin at lower trophic levels—a review. Ecotoxicol Environ Saf 57:190–201. doi: 10.1016/j.ecoenv.2003.07.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.







