ABSTRACT
Ferredoxin:NAD+ oxidoreductase (NADH-FNOR) catalyzes the transfer of electrons from reduced ferredoxin to NAD+. This enzyme has been hypothesized to be the main enzyme responsible for ferredoxin oxidization in the NADH-based ethanol pathway in Thermoanaerobacterium saccharolyticum; however, the corresponding gene has not yet been identified. Here, we identified the Tsac_1705 protein as a candidate FNOR based on the homology of its functional domains. We then confirmed its activity in vitro with a ferredoxin-based FNOR assay. To determine its role in metabolism, the tsac_1705 gene was deleted in different strains of T. saccharolyticum. In wild-type T. saccharolyticum, deletion of tsac_1705 resulted in a 75% loss of NADH-FNOR activity, which indicated that Tsac_1705 is the main NADH-FNOR in T. saccharolyticum. When both NADH- and NADPH-linked FNOR genes were deleted, the ethanol titer decreased and the ratio of ethanol to acetate approached unity, indicative of the absence of FNOR activity. Finally, we tested the effect of heterologous expression of Tsac_1705 in Clostridium thermocellum and found improvements in both the titer and the yield of ethanol.
IMPORTANCE Redox balance plays a crucial role in many metabolic engineering strategies. Ferredoxins are widely used as electron carriers for anaerobic microorganism and plants. This study identified the gene responsible for electron transfer from ferredoxin to NAD+, a key reaction in the ethanol production pathway of this organism and many other metabolic pathways. Identification of this gene is an important step in transferring the ethanol production ability of this organism to other organisms.
INTRODUCTION
Ferredoxins are iron-sulfur proteins found in many anaerobic bacteria and archaea and mediate electron transfer in various metabolic processes, including photosynthesis (1, 2), alcohol production (3, 4), nitrogen fixation (5, 6), and hydrogen production (7). Lack of knowledge about these ferredoxin-dependent pathways currently limits our ability to incorporate them into metabolic engineering strategies. The ferredoxin:NAD+/NADP+ oxidoreductase (FNOR) enzyme (EC 1.18.1.2/EC 1.18.1.3) forms a key bridge in metabolism between the nicotinamide cofactor (i.e., NAD+, NADH, NADP+, and NADPH)-dependent pathways and ferredoxin-dependent pathways (Fig. 1, equation a) (8–11). Recently, the thioredoxin reductase-like (TrxR-type) FNORs were found, and they are widely distributed among the bacteria and archaea (12–14). Even these types of FNORs are more homologous to bacterial NADPH-TrxRs, but they have catalytic properties similar to those of FNOR (14). FNOR enzymes are widely believed to be of central importance for the bioenergetics of anaerobic bacteria due to their ability to couple electron transport with ion or Na+ gradient generation (15) or transhydrogenation. Furthermore, they are the key enzymes for many biochemical and biofuel pathways, including isopropanol, ethanol, and n-butanol (3, 4, 16). Since ferredoxin has a lower standard reduction potential than nicotinamide cofactors (−420 mV versus −320 mV) (10), this exergonic reaction is frequently coupled to another endergonic reaction for energy conservation. One coupling reaction is the translocation of proton or sodium ions, resulting in the RNF reaction (Fig. 1, equation b) (8, 17, 18). Another coupling reaction is the transhydrogenation reaction, resulting in the NADH-dependent reduced FNOR reaction (Fig. 1, equation c) (10, 19, 20).
FIG 1.
The stoichiometries of three types of FNOR reactions. a, uncoupled FNOR reaction; b, proton- or Na+-translocating FNOR reaction (RNF); c, NADH-dependent FNOR reaction (NFN).
Thermoanaerobacterium saccharolyticum is a thermophilic bacterium with the ability to ferment many components of the hemicellulose fraction of lignocellulosic biomass, including xylan, and can produce ethanol at high yields and titers. Engineered strains of T. saccharolyticum that can produce over 70 g/liter ethanol at near-theoretical yield have been developed (21), and this has inspired us to study the pathway this organism uses to produce ethanol. A key step in the pathway is the transfer of electrons from ferredoxin to NAD+ and/or NADP+, which T. saccharolyticum performs readily (19). One candidate for this activity is the Nfn complex (Tsac_2085 NfnA and Tsac_2086 NfnB). The deletion of these two genes led to the total loss of NADPH-FNOR activity (10, 19); however, NADH-FNOR activity remained and was sufficient for high-yield ethanol production (83% of the theoretical yield) under certain conditions. This suggested the possibility of an ethanol production pathway that used NADH (i.e., not NADPH) for all redox steps; however, the gene responsible for the NADH-FNOR activity was not known (19).
Since T. saccharolyticum is not able to use the cellulose fraction of biomass, we have been working to engineer Clostridium thermocellum, an anaerobic thermophilic bacterium that can solubilize the cellulosic fraction of biomass, for improved ethanol production. For C. thermocellum, the main factor limiting commercialization is the low titer and yield of ethanol. We think this is caused by a limitation in electron transfer from ferredoxin to NAD+ (i.e., NADH-FNOR activity).
In this work, we have identified the NADH-FNOR in T. saccharolyticum and confirmed its function by heterologous expression in Escherichia coli. We then determined its role in T. saccharolyticum metabolism by gene deletion. Finally, we demonstrated the utility of this enzyme for metabolic engineering by expressing it in C. thermocellum, which improved both ethanol yield and titer.
MATERIALS AND METHODS
Media and growth conditions.
All chemicals were reagent grade and obtained from Sigma-Aldrich (St. Louis, MO) or Fisher Scientific (Pittsburgh, PA) unless indicated otherwise. CTFUD rich medium at pH 7.0 and pH 6.0 was used for C. thermocellum and T. saccharolyticum, respectively (22, 23). The growth temperature was 55°C for both strains. For end product analysis, C. thermocellum was grown in chemically defined MTC medium (24), and T. saccharolyticum was grown in modified MTC medium (25). Escherichia coli strains were grown in LB medium Miller (Acros) with the appropriate antibiotic for maintenance (carbenicillin at 100 mg/liter, kanamycin at 50 mg/liter, and tetracycline at 12 mg/liter) and in TB medium for protein expression.
Strains, primers, and plasmids.
The strains, primers, and plasmids used in this study are listed in Table 1, Table 2, and Table 3, respectively.
TABLE 1.
Strains used in this study
| Organism | Strain | Description | Source or reference |
|---|---|---|---|
| T. saccharolyticum | LL1025 | Wild-type T. saccharolyticum JW/SL-YS485 | 40 |
| LL1025-0705 | LL1025 ΔxynA::0705 Eryr | This work | |
| LL1025-1705 | LL1025 ΔxynA::1705 Eryr | This work | |
| LL1049 | LL1025 Δpta Δack Δldh adhEG544D | 41 | |
| LL1144 | LL1025 ΔnfnAB::Kanr | 19 | |
| LL1145 | LL1025 Δpta Δack Δldh ΔpyrF ΔnfnAB::Kanr | 19 | |
| LL1305 | LL1025 Δtdk | This work | |
| LL1306 | LL1025 Δtdk Δ1705 | This work | |
| LL1316 | LL1025 Δtdk Δ1705 ΔnfnAB::Kanr | This work | |
| LL1317 | LL1025 Δtdk ΔnfnAB::Kanr | This work | |
| C. thermocellum | LL1004 | Wild-type C. thermocellum strain DSM 1313 | DSMZ |
| LL1087 | LL1004 Δhpt Δrnf | This work | |
| LL1087-1705 | LL1004 Δhpt Δrnf(pDGO126-1705) | This work | |
| E. coli | T7 Express lysY/lq | Used for heterologous protein expression | New England Biolabs |
| DH5α | Used for plasmid screening and propagation | New England Biolabs |
TABLE 2.
Primers used in this study
| Primer | Sequencea | Comment |
|---|---|---|
| LT_01 | CTTTTCCTCCCTCGTCTTC | T.sac_tdk deletion |
| LT_02 | ACTTTTTGTGGTTTTAAACTATTTTCTAAGAGGTGGATTATGGCGGATTTTTAAGGAGGT | |
| LT_03 | TCTTCTTCATTGCTGCACCTCCTTAAAAATCCGCCATAATCCACCTCTTAGAAAATAGTT | |
| LT_04 | GGAATACGCAAAAAGATTG | |
| LT_15 | TACACGTACTTAGTCGCTGAAGCTCTTCTATGAGATACGTTGTTAGAGAAAATAGAG | Amplification of tsac_1705 for plasmid pD861-tsac1705 |
| LT_16 | TAGGTACGAACTCGATTGACGGCTCTTCTACCTCAAAATACTACCTCCCTTGACC | |
| LT_23 | CCACCACAATTCAGCAAA | pD861-tsac1705 sequencing |
| LT_24 | AAAAAACCCCTCAAGACCC | |
| LT_28 | CGATCTCGAGAGATACGTTGTTAGAGAAAATAGAG | Amplification of tsac_1705 for plasmid pDGO126′ins′_p2638-1705 |
| LT_29 | GTCACTCGAGCCGGGTTGAACTACTCTTTAATA | |
| LT_36 | GCAGGCATGCAAGCTTTAATAG | Amplification of vector backbone for plasmid pDGO126′ins′_p2638-1705 |
| LT_37 | AGGTCGACTCTAGAGGATCC | |
| LT_44 | TGATGACGAAAAAGCCGA | pDGO126′ins′_p2638-1705 sequencing |
| LT_45 | ATCCCAATAACCTAACTCTCC | |
| LT_153 | ACGGGAACAATACAAAAGGA | tsac_1705 deletion verification |
| LT_154 | AATTCCTCCCATCCCTATC | |
| LT_155 | TGTGCTGTTGCATGTTGT | tsac_nfnAB deletion |
| LT_156 | GGTGGAGTAATAATTGGTGGT | |
| 0705 F | ATAAATGTGTACATGCCAAAAAAAGTAGAAATATTG | Amplification of tsac_0705 for plasmid pTOPO-0705 |
| 0705 R | CGACCTGCATTAATCGAGAAGTTGCTTTGATTTTGTG | |
| Histag 0705 F | CTGGTTCTCATCATCATCATCATCATGGTATAAATGTGTACATGCCAAAAAAAGTAGAAATATTG | |
| 1705 F | AGATACGTTGTTAGAGAAAATAGAGAAATTAGCAATGG | Amplification of tsac_1705 for plasmid pTOPO-0705 |
| 1705 R | CGACCTGCATCAAAATACTACCTCCCTTGACCAAAATACAGG | |
| Histag 1705 F | CTGGTTCTCATCATCATCATCATCATGGTAGATACGTTGTTAGAGAAAATAGAGAAATTAGCAATGG | |
| pkan 1705 F | GGTCAAGGGAGGTAGTATTTTGATGCAGGTCGATAAACCCAGCG | |
| xynA up F | ATCTTTTCTGGCCTTTAATGGCGC | Amplification of xynA operon and Erm resistance gene for plasmid pTOPO-0705 |
| xynA up R | TGATGATGATGATGATGAGAACCAGACATTCTTACTTCCTCCCTCAGTAAATTTAATTTATTG | |
| pkan 0705 F | CAAAGCAACTTCTCGATTAATGCAGGTCGATAAACCCAGCG | |
| xynA down R | AGTCAAATGCGACAAAAAAACGCC | |
| xynA up R-2 | TCTTACTTCCTCCCTCAGTAAATTTAATTTATTG | |
| pkan F-2 | TGCAGGTCGATAAACCCAGCG | |
| xynA SQ F | GAAATAATTCTAATTCAGTTACCCCG | tsac_0705 or tsac_1705 xynA replacement verification |
| xynA SQ R | GGTGAATTCGAATTTACAGGC |
Underlined sequences indicate binding regions.
TABLE 3.
Plasmids used in this study
| Plasmid | Description | Accession no. |
|---|---|---|
| pTOPO-0705 | Insertion of tsac_0705 at the xynA locus of T. saccharolyticum | KX272607 |
| pTOPO-1705 | Insertion of tsac_0705 at the xynA locus of T. saccharolyticum | KX272606 |
| pD861-tsac1705 | Overexpression of tsac_1705 gene in E. coli | KX272605 |
| pLT-26 | Markerless deletion of tsac_1705 gene in T. saccharolyticum | KX272604 |
| pDGO126′ins′_p2638-1705 | Overexpression of tsac_1705 gene in C. thermocellum | KX272603 |
| pLT-18 | Overexpression of Clo1313_2761 gene in E. coli | KX925513 |
Markerless gene deletion in T. saccharolyticum.
To delete the target genes in T. saccharolyticum, the markerless gene deletion system reported for Thermoanaerobacter ethanolicus was used (26). The thymidine kinase (tdk) gene was deleted in T. saccharolyticum to create a background strain LL1305. The high-temperature kanamycin (htk) marker was used for positive selection. 5-Fluoro-2′-deoxyuridine (FUDR) was used in the subsequent negative selection step to remove the htk marker. Transformation of T. saccharolyticum was performed as described previously (27). The primers and plasmids used for genes deletions are listed in Tables 2 and 3, respectively.
Preparation of cell extracts.
For C. thermocellum and T. saccharolyticum, CTFUD rich medium was used (23), and cells were harvested by centrifugation when the optical density at 600 nm (OD600) reached a value of 0.6. The cell pellet was resuspended in lysis buffer (1× BugBuster reagent [EMD Millipore, Darmstadt, Germany] with 0.2 mM dithiothreitol [DTT]). The cells were lysed with Ready-Lyse lysozyme (Epicentre, Madison, WI, USA), and DNase I (New England Biolabs, Ipswich, MA, USA) was added to reduce the viscosity. After incubation for 30 min at room temperature, the resulting solution was centrifuged at 10,000 × g for 5 min. The supernatant was used as cell extract for enzyme assays.
Heterologous expression protein in E. coli.
Target genes were amplified by PCR with Q5 DNA polymerase (New England Biolabs, Ipswich, MA USA). T. saccharolyticum or C. thermocellum genomic DNA was used as the template. The primers used for each gene are listed in Table 2. The target genes were inserted into plasmid pD861-NH (DNA2.0 Inc., Menlo Park, CA, USA) and tagged with a 5′ His6 cassette. The vector was transformed into E. coli BL21(DE3) harboring the pRKISC plasmid (28). This pRKISC plasmid contained the E. coli isc (iron-sulfur cluster) locus (28), which has previously been shown to improve the expression of iron-sulfur proteins (29, 30).
Cells were aerobically grown in TB medium at 37°C with a stirring speed of 225 rpm. When the OD600 reached 0.6, 4 mM rhamnose and 0.2 mM IPTG (isopropyl-β-d-thiogalactopyranoside) were add to induce the expression of the target gene and isc operon, respectively. Cysteine (0.12 g/liter), ferrous sulfate (0.1 g/liter), ferric citrate (0.1 g/liter), and ferric ammonium citrate (0.1 g/liter) were supplemented to enhance the iron-sulfur cluster synthesis. The cells were then grown aerobically for 4 h before harvesting by centrifugation. The cell pellets were washed with 50 mM Tris-HCl–0.5 mM DTT (pH 7.5) and stored at −80°C.
The cell extracts were prepared as described above, and E. coli proteins were denatured by incubating at 55°C for 30 min. The denatured proteins were removed by centrifugation at 10,000 × g for 5 min. All steps of purification were performed at room temperature in a Coy anaerobic chamber (Coy Labs, Grass Lake, MI) with an atmosphere of 85% N2, 10% CO2, and 5% H2. His tag affinity spin columns (His SpinTrap; GE Healthcare Bio-Sciences, Pittsburgh, PA, USA) were used to purify the protein. The column was first equilibrated with binding buffer (50 mM sodium phosphate, 500 mM NaCl, 20 mM imidazole, pH 7.5). Cell extracts were applied to the column, and then the column was washed twice with wash buffer (50 mM sodium phosphate, 500 mM NaCl, 50 mM imidazole, 20% ethanol, pH 7.5). The His-tagged protein was eluted with elution buffer (50 mM sodium phosphate, 500 mM NaCl, 500 mM imidazole, pH 7.5).
Enzyme assay.
Except where indicated, all the enzyme assays were performed at 55°C in an anaerobic chamber (85% N2, 10% CO2, 5% H2). When carbon monoxide (CO) was used as a substrate, the cuvettes (still inside the anaerobic chamber) were closed with a rubber stopper and purged with CO gas.
Assay of carbon monoxide dehydrogenase.
The plasmid for overexpression of Carboxydothermus hydrogenoformans carbon monoxide dehydrogenase (CODH) in E. coli was a gift from Holger Dobbek. Expression and purification of this enzyme were performed as previously described (31). To measure CODH activity, a cuvette was filled with buffer containing 20 mM morpholinepropanesulfonic acid (MOPS)-NaOH (pH 7.5), 2 mM DTT, and 2 mM benzyl viologen (BV). The reaction was started by purging with 60% (vol/vol) carbon monoxide (CO balanced with 40% N2). The activity was measured at 578 nm (ε = 7.8 mM−1 cm−1) (32). Reduction of ferredoxin (20 μM) was assayed at 430 nm (ε = 13.1 mM−1 cm−1) (10).
Assay of BV-based FNOR (NADH:BV FNOR).
The reaction mixture contained 20 mM MOPS-NaOH (pH 7.5), 0.2 mM NADH or NADPH, and 1 mM benzyl viologen (BV). The reaction was started with enzyme, and the benzyl viologen reduction was followed by photometric observations at 578 nm (ε = 7.8 mM−1 cm−1) (32).
Assay of ferredoxin-based FNOR (Fd:NAD+ FNOR).
Since benzyl viologen is a promiscuous electron acceptor, we also performed FNOR assays with NAD+ as the electron acceptor and reduced ferredoxin as the electron donor. Ferredoxin from C. thermocellum (Clo1313_2761) was expressed in E. coli by using the pLT-18 plasmid. The expression plasmid was transformed into E. coli strain BL21 harboring the pRKISC plasmid (for correct assembly of iron-sulfur clusters in ferredoxin). The proteins were purified using His tag affinity columns in an anaerobic chamber.
The reaction mixture contained 20 mM MOPS-NaOH (pH 7.5), 4 mM NAD+, 3 μM ferredoxin, and 0.5 μM CODH. The cuvette was purged with CO for 30 s to saturate the liquid with CO. The reaction was then started with the FNOR enzyme (purified from E. coli), and the formation of NADH was followed by photometric observation at 340 nm (ε = 6.2 mM−1 cm−1) (11).
Analytical methods.
Acetate, formate, ethanol, glucose, and residual cellobiose were determined by high-pressure liquid chromatography (HPLC) (Waters, Milford, MA) with refractive index detection using an Aminex HPX-87H column (Bio-Rad, Hercules CA) with a 5 mM sulfuric acid solution eluent. The column was incubated at 55°C, and the mobile-phase flow rate was 0.6 ml/min. H2 was determined by measuring the total pressure and the H2 percentage in the headspace. For a 100-ml serum bottle, the culture volume is 50 ml, so the headspace is also 50 ml. The headspace gas pressure in bottles was measured using a digital pressure gauge (Ashcroft, Stratford, CT). The headspace H2 percentage was measured using a gas chromatograph (model 310; SRI Instruments, Torrance, CA) with a HayeSep D packed column using a thermal conductivity detector with nitrogen carrier gas.
Accession number(s).
Newly determined sequences were deposited in GenBank and are listed in Table 3.
RESULTS AND DISCUSSION
Identification of ferredoxin:NAD+ oxidoreductase candidates from T. saccharolyticum.
To identify candidate genes with FNOR activity, we searched for the presence of both iron-sulfur (Fe-S) binding domains and NAD cofactor binding domains in the T. saccharolyticum genome using the Pfam online database (33). The two best candidates were tsac_0705 and tsac_1705, which are the only candidates (besides nfnA) that have both the oxidoreductase NAD binding domain and the iron-sulfur cluster binding domain (Fig. 2).
FIG 2.
Protein functional domain alignment. PF00175 is the hidden Markov model (HMM) consensus sequence of the oxidoreductase NAD binding domain. PF10418 is the HMM consensus sequence of the iron-sulfur cluster binding domain. Uppercase letters indicate conserved residues in the HMM consensus sequence.
These two candidates were overexpressed in T. saccharolyticum by insertion of an additional copy at the xynA locus. This locus had previously been used for xylose-inducible expression of genes (34). Xylose was added at 5 g/liter as a carbon source and to induce expression of target genes, and the benzyl viologen-based NADH-FNOR (NADH:BV FNOR) was used to measure activity of the cell extract. A 5-fold increase in activity was found when the tsac_1705 gene was overexpressed (Table 4, strains LL1025, LL1352, and LL1353), suggesting that Tsac_1705 is responsible for NADH-FNOR activity.
TABLE 4.
Fermentation products and enzyme assay result of T. saccharolyticum and C. thermocellum
| Strain | Description | Amt of fermentation product (mmol)a |
Ethanol/acetate ratio (mmol/mmol)b | Ethanol yield (%)c | FNOR sp act (U/mg)d |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lactate | Formate | Ethanol | Acetate | H2 | NADH | NADPH | ||||
| LL1025 | Wild-type T. saccharolyticum JW/SL-YS485 | 0.16 ± 0.02 | 0.02 ± 0.00 | 1.26 ± 0.02 | 0.76 ± 0.01 | 1.72 ± 0.05 | 1.66 ± 0.04 | 23 | 0.56 ± 0.13 | 0.29 ± 0.12 |
| LL1049 | LL1025 Δpta Δack Δldh adhEG544D | 42 (19) | 0.18 ± 0.03 | 0.74 ± 0.15 | ||||||
| LL1145 | LL1025 Δpta Δack Δldh ΔpyrF ΔnfnAB::Kanr | 42 (19) | 0.48 ± 0.04 | 0.04 ± 0.01 | ||||||
| LL1352 | LL1025 ΔxynA::0705 Eryr | 0.45 ± 0.18 | 0.27 ± 0.10 | |||||||
| LL1353 | LL1025 ΔxynA::1705 Eryr | 2.51 ± 0.44 | 0.15 ± 0.07 | |||||||
| LL1305 | LL1025 Δtdk | 0.17 ± 0.02 | 0.02 ± 0.01 | 1.28 ± 0.01 | 0.77 ± 0.01 | 1.7 ± 0.1 | 1.67 ± 0.02 | 23 | 0.57 ± 0.08 | 0.32 ± 0.10 |
| LL1306 | LL1305 Δ1705 | 0.23 ± 0.01 | 0.02 ± 0.02 | 1.30 ± 0.02 | 0.88 ± 0.01 | 1.9 ± 0.2 | 1.57 ± 0.03 | 24 | 0.18 ± 0.07 | 0.35 ± 0.11 |
| LL1317 | LL1305 ΔnfnAB::Kanr | 0.22 ± 0.03 | 0.02 ± 0.00 | 1.31 ± 0.01 | 0.91 ± 0.01 | 2.4 ± 0.2 | 1.44 ± 0.02 | 24 | 0.48 ± 0.10 | 0.03 ± 0.01 |
| LL1316 | LL1305 Δ1705 ΔnfnAB::Kanr | 0.26 ± 0.02 | 0.02 ± 0.01 | 1.06 ± 0.02 | 0.99 ± 0.2 | 2.7 ± 0.1 | 1.07 ± 0.03 | 19 | 0.15 ± 0.04 | 0.04 ± 0.01 |
| LL1004 | Wild-type C. thermocellum strain DSM 1313 | 16 (22) | 0.21 ± 0.04 | NDe | ||||||
| LL1087 | LL1004 Δhpt Δrnf | 0.72 ± 0.04 | 1.23 ± 0.03 | 1.42 ± 0.02 | 2.11 ± 0.03 | 0.67 ± 0.02 | 14 | 0.27 ± 0.04 | ND | |
| LL1338 | LL1087(pDGO126-1705) | 0.47 ± 0.02 | 1.14 ± 0.03 | 1.83 ± 0.03 | 1.82 ± 0.02 | 1.00 ± 0.02 | 18 | 0.34 ± 0.06 | ND | |
For quantification of all the fermentation products, the working volume was 50 ml on 0.72 mmol cellobiose for T. saccharolyticum and 14.4 mmol for C. thermocellum, and the headspace volume are 50 ml for a 100-ml serum bottle. Values are means ± standard deviations; n = 3.
Values are means ± standard deviations; n = 3.
Ethanol yield is in grams per gram of glucose produced from cellobiose. Values are means (reference numbers are given in parentheses); n = 3.
Specific activity determined from cell extracts. FNOR activity was determined using the NADH:BV assay. Values are means ± standard deviations; n = 3.
ND, not detected.
To further determine whether the tsac_1705 gene encodes the enzyme catalyzing NAD+-linked reduction of ferredoxin, we expressed it in E. coli, purified the resulting protein, and measured FNOR activity. We found that the specific enzyme activities were identical for aerobic and anaerobic cultures; Tsac_1705 is strictly NADH linked, and no activity was found with NADPH. Based on the assay of NADH:BV FNOR, the Km for benzyl viologen was 0.07 ± 0.01 mM and the Vmax was 23 ± 0.3 U/mg. Since the benzyl viologen assay is somewhat promiscuous, we also used a ferredoxin-based assay (Fd:NAD+ FNOR). For this assay, the apparent Km for NAD+ was found to be approximately 0.5 mM and the apparent Vmax was found to be 3.28 U per mg of protein at 55°C and pH 7.5.
Predicted structure of the Tsac_1705 protein.
Although the Tsac_1705 protein was annotated as a dihydroorotate dehydrogenase (DodH) electron transfer subunit and is part of a putative operon for dihydroorotate dehydrogenase, it is similar (32% amino acid sequence identity) to the NfnA protein, one of the subunits of the NfnAB complex from Thermotoga maritima with known NFN activity (20). The most similar protein with an available crystal structure is DodH from Lactococcus lactis, with a sequence identity of 39% (35). We constructed a protein homology model of Tsac_1705 using the crystal structure of L. lactis DodH (Protein Data Bank code 1EP2; root mean square deviation [RMSD] = 9.515; 98 to 98 atoms). The model was aligned to the NfnA of T. maritima (Protein Data Bank code 4YRY) to superimpose the NADH, FAD, and [2Fe-2S] cluster from 4YRY to our homology model (Fig. 3A). Tsac_1705 has similar NADH, FAD, and [2Fe-2S] binding domains. Specially, the four residues which bind to the [2Fe-2S] cluster are conserved with NfnA from T. maritima (Fig. 3B and C) (35). Interestingly, although we did not find activity with this protein, Tsac_0705 is also very similar to NfnA from T. maritima, with a sequence identity at the amino acid level of 28%.
FIG 3.
Homology modeling and docking analysis of Tsac_1705. (A) Substrate binding domain of Tsac_1705 (blue). T. maritima NfnA (green) was used to superimpose the NADH, FAD, and [2Fe-2S] cluster. (B) The [2Fe-2S] cluster in T. maritima NfnA. (C) The predicted [2Fe-2S] cluster in Tsac_1705.
Role of Tsac_1705 in the NADH-linked ethanol production pathway.
To further confirm the role of tsac_1705, it was deleted individually and in combination with nfnAB (Table 4, strains LL1306, LL1317, and LL1316). Note that for the nfnAB and tsac_1705 deletion strains, an additional deletion of tdk was introduced for purposes of strain construction; however, the tdk deletion did not have an effect on fermentation products or FNOR activity (Table 4, strain LL1305). In comparison with that of the parent strain (Table 4, strain LL1305), nearly 70% of NADH-FNOR activity was lost when tsac_1705 was deleted (Table 4, strain LL1306), which indicated that Tsac_1705 is the main NADH-FNOR in T. saccharolyticum. A small amount of NADH-FNOR activity (∼30%) remained even after deletion of tsac_1705. Although we have identified the primary gene responsible for NADH-FNOR activity, there may still be other cryptic FNOR enzymes in T. saccharolyticum. Another possibility is the presence of a set of enzymes whose net activity is equivalent to FNOR activity.
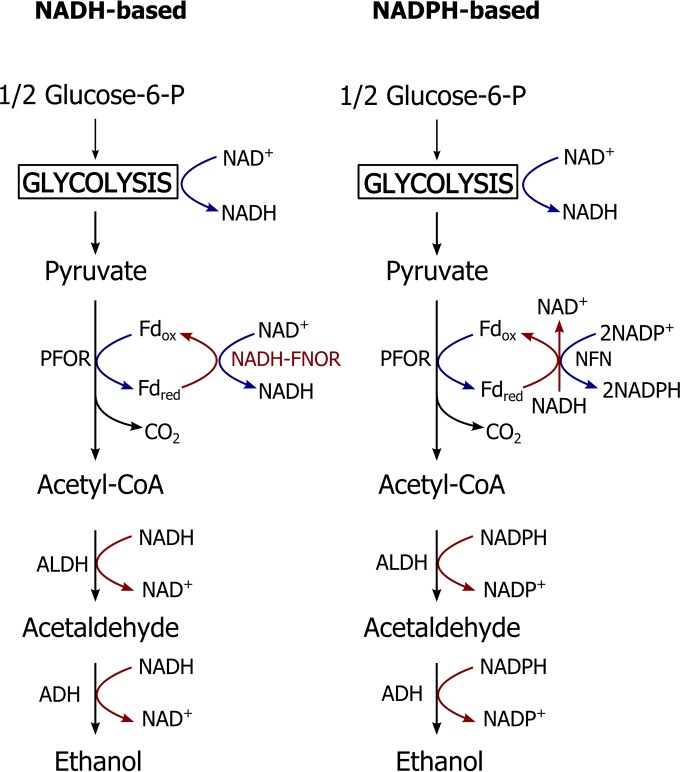
As described in a previous study (19), there are two ethanol production pathways in T. saccharolyticum, one NADH based and one NADPH based (Fig. 4). Strains with the NADH-based (LL1145) or NADPH-based (LL1049) ethanol pathway can produce ethanol at high yield (Table 4). According to the NADH:BV FNOR assay result, these two strains have different cofactor preferences. We tried to delete the tsac_1705 gene in strain LL1145. However, no colonies were obtained, which is consistent with our understanding of Tsac_1705 as the primary FNOR in this strain.
FIG 4.
Cofactor-based models for stoichiometric ethanol production in T. saccharolyticum. NADH-based ethanol production relies on a ferredoxin:NAD oxidoreductase (NADH-FNOR) to transfer electrons from reduced ferredoxin to NAD+. NADPH-based ethanol formation relies on the electron transfer from NADH and reduced ferredoxin to 2 NADP+. NADH- or NADPH-linked aldehyde dehydrogenase (ALDH) and alcohol dehydrogenase (ADH) were used for different pathway. Blue arrows indicate that cofactor is reduced, while red arrows indicate that cofactor is oxidized. PFOR, pyruvate:ferredoxin oxidoreductase; FNOR, ferredoxin:NADH oxidoreductase; Fdox, oxidized ferredoxin; Fdred, reduced ferredoxin.
Next, we analyzed the effect of the tsac_1705 gene deletion on the distribution of fermentation products, particularly ethanol and acetate (Table 4, strains LL1025, LL1305, LL1306, LL1317, and LL1316). Assuming that glucose is converted to pyruvate by glycolysis and pyruvate is converted to acetyl coenzyme A (acetyl-CoA) by pyruvate ferredoxin oxidoreductase, central metabolism can be described by equation 1 below. Note that only trivial amounts of formate (less than 1 mM) were produced for all 5 strains, so flux from pyruvate formate lyase was omitted from the equations. Bifurcating hydrogenase activity was not considered in the equations either, because in T. saccharolyticum, the ferredoxin-dependent [FeFe]-hydrogenase is responsible for hydrogen generation and is not thought to be bifurcating (36). The resulting reduced ferredoxin (Fdred) is either used to produce H2 or NAD(P)H (equations 2 and 3).
| (1) |
When FNOR converts 100% Fdred to NAD(P)H:
| (2) |
When FNOR is eliminated:
| (3) |
In wild-type strain LL1025, the ethanol-to-acetate ratio was about 1.66, which means FNOR converts only about 25% of Fdred to NAD(P)H (Table 4 and equation 2). The single deletion of either tsac_1705 or nfnAB only slightly influenced this ratio, which means that these two FNOR enzymes can complement the deletion of each other (Table 4, strains LL1306 and LL1317). Meanwhile, the H2 production was increased in both of these strains. In strain LL1316, which had both the nfnAB and tsac_1705 genes deleted, this ratio decreased to 1.07, which is close to 1, the value we would expect for a strain that does not have FNOR activity (i.e., its metabolism is described by equation 3). The H2 production in this strain was further increased in comparison with that caused by the single deletion of either tsac_1705 or nfnAB. This provides further confirmation that tsac_1705 is the gene responsible for NADH-FNOR activity in T. saccharolyticum.
Heterologous overexpression of tsac_1705 in C. thermocellum.
The best engineered strains of T. saccharolyticum can produce up to 70 g/liter ethanol (21); however, the highest ethanol titer of C. thermocellum is still less than 30 g/liter (16). FNOR activity is necessary for high-yield production of ethanol (37). The NADH-FNOR activity was found to be 2-fold higher in wild-type T. saccharolyticum than in wild-type C. thermocellum (Table 4, strains LL1025 and LL1004). Furthermore, wild-type T. saccharolyticum has NADPH-FNOR activity, which was not found in wild-type C. thermocellum (which has only NADH-FNOR activity). Therefore, it is possible that NADH-FNOR activity is currently the limiting step for ethanol production. Since the alcohol dehydrogenase and acetaldehyde dehydrogenase reactions in C. thermocellum are NADH linked (Fig. 4, NADH based) (22), the NADH-FNOR is more suitable for cofactor balance in C. thermocellum (as opposed to the NADPH-linked NfnAB complex). To test this hypothesis, the tsac_1705 gene was inserted into plasmid pDGO126 (38), and the resulting plasmid was transformed into C. thermocellum. Although transformation was attempted in several strains (Table 5), we obtained colonies only in strains where the native proton-translocating FNOR operon (i.e., rnf) was deleted. One possible explanation is that Rnf and Tsac_1705 create a futile cycle. The equation shown in Fig. 5 describes this reaction, which could occur when the Rnf complex and Tsac_1705 are both present. This cycle would result in a net transfer of protons across the cell membrane, eliminating the proton gradient, which would likely be lethal.
TABLE 5.
Transformation results for plasmid pDGO126′ins′_p2638-1705
| Strain | Description | Transformants |
|---|---|---|
| LL1004 | Wild-type C. thermocellum strain DSM 1313 | No |
| LL345 | LL1004 Δhpt | No |
| LL350 | LL1004 Δhpt ΔhydG | No |
| LL1147 | LL1004 Δhpt ΔhydG Δech | No |
| LL1210 | LL1004 Δhpt ΔhydG Δpfl Δpta Δldh | No |
| LL1087 | LL1004 Δhpt ΔrnfDG | Yes |
| LL1083 | LL1004 Δhpt ΔhydG ΔrnfDG | Yes |
| LL1152 | LL1004 Δhpt ΔrnfABCDEG | Yes |
FIG 5.

The cycling reaction between RNF and FNOR.
In the rnf deletion strain, overexpression of tsac_1705 gene increased the titer of ethanol by 28% (Table 4, strains LL1087 and LL1338). The production of lactate and acetate both decreased, although formate production was unchanged. If we ignore lactate and formate production, C. thermocellum metabolism can be described as follows: glucose → 2 acetyl-CoA + 2 CO2 + 2 NADH + 2 Fdred (identical to the case for T. saccharolyticum [see equation 1]). When the bifurcating hydrogenase converts 100% Fdred to H2, the process is as follows: glucose → 2 acetyl-CoA + 2 CO2 + 2 NADH + 2 Fdred → 2 acetate + 2 CO2 + 4 H2. When the bifurcating hydrogenase and FNOR contribute equally to ferredoxin oxidization, the process is as follows: glucose → 2 acetyl-CoA + 2 CO2 + 2 NADH + 2 Fdred → ethanol + acetate + 2 CO2 +2 H2.
Thus, in T. saccharolyticum, a 1:1 ethanol-to-acetate ratio was indicative of a lack of FNOR activity, and in C. thermocellum, a 1:1 ethanol-to-acetate ratio can exist even in the presence of substantial flux through FNOR. This analysis is further complicated by the fact that C. thermocellum has both bifurcating and nonbifurcating hydrogenases (39). Regardless of the type of hydrogenase, an increase in ethanol production at the expense of acetate production generally indicates an increase in FNOR flux. It is, of course, true that a reduction in bifurcating hydrogenase activity and a corresponding increase in nonbifurcating hydrogenase activity would have the same effect on the ethanol/acetate ratio; however, since we observe increased FNOR activity that corresponded to the introduction of the tsac_1705 gene, we believe that increased ethanol production caused by FNOR activity from Tsac_1705 is the simplest explanation. The ability to use the tsac_1705 gene to improve ethanol production in C. thermocellum demonstrates its utility for metabolic engineering.
ACKNOWLEDGMENTS
We thank Holger Dobbek for his gift of the CODH plasmid, Yasuhiro Takahashi for plasmid pRKISC, and Johannes P. van Dijken for useful discussions regarding metabolism.
The BioEnergy Science Center is a U.S. Department of Energy Bioenergy Research Center supported by the Office of Biological and Environmental Research in the DOE Office of Science. This paper has been authored by Dartmouth College under contract no. DE-AC05-00OR22725 with the U.S. Department of Energy.
Lee R. Lynd is a founder of the Enchi Corporation, which has a financial interest in T. saccharolyticum and C. thermocellum. No nonfinancial competing interests exist for any of the authors.
Funding Statement
The BioEnergy Science Center is a U.S. Department of Energy Bioenergy Research Center supported by the Office of Biological and Environmental Research in the DOE Office of Science. This paper has been authored by Dartmouth College under contract no. DE-AC05-00OR22725 with the U.S. Department of Energy.
REFERENCES
- 1.Hanke G, Mulo P. 2013. Plant type ferredoxins and ferredoxin-dependent metabolism. Plant Cell Environ 36:1071–1084. doi: 10.1111/pce.12046. [DOI] [PubMed] [Google Scholar]
- 2.Tagawa K, Arnon DI. 1962. Ferredoxins as electrond carriers in photosynthesis and in the biological production and consumption of hydrogen gas. Nature 195:537–543. doi: 10.1038/195537a0. [DOI] [PubMed] [Google Scholar]
- 3.Dusséaux S, Croux C, Soucaille P, Meynial-Salles I. 2013. Metabolic engineering of Clostridium acetobutylicum ATCC 824 for the high-yield production of a biofuel composed of an isopropanol/butanol/ethanol mixture. Metab Eng 18:1–8. doi: 10.1016/j.ymben.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Gheshlaghi R, Scharer JM, Moo-Young M, Chou CP. 2009. Metabolic pathways of clostridia for producing butanol. Biotechnol Adv 27:764–781. doi: 10.1016/j.biotechadv.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Miflin BJ, Habash DZ. 2002. The role of glutamine synthetase and glutamate dehydrogenase in nitrogen assimilation and possibilities for improvement in the nitrogen utilization of crops. J Exp Bot 53:979–987. doi: 10.1093/jexbot/53.370.979. [DOI] [PubMed] [Google Scholar]
- 6.Egener T, Martin DE, Sarkar A, Reinhold-Hurek B. 2001. Role of a ferredoxin gene cotranscribed with the nifHDK operon in N2 fixation and nitrogenase “switch-off” of Azoarcus sp. strain BH72. J Bacteriol 183:3752–3760. doi: 10.1128/JB.183.12.3752-3760.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu F, Smith PR, Mehta K, Swartz JR. 2015. Development of a synthetic pathway to convert glucose to hydrogen using cell free extracts. Int J Hydrogen Energy 40:9113–9124. doi: 10.1016/j.ijhydene.2015.05.121. [DOI] [Google Scholar]
- 8.Tremblay P, Zhang T, Dar SA. 2012. The Rnf complex of Clostridium ljungdahlii is a proton-translocating ferredoxin:NAD+ oxidoreductase essential for autotrophic growth. mBio 4:e0040612. doi: 10.1128/mBio.00406-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hess V, Schuchmann K, Muller V. 2013. The ferredoxin:NAD+ oxidoreductase (Rnf) from the acetogen Acetobacterium woodii requires Na+ and is reversibly coupled to the membrane potential. J Biol Chem 288:31496–31502. doi: 10.1074/jbc.M113.510255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang S, Huang H, Moll J, Thauer RK. 2010. NADP+ reduction with reduced ferredoxin and NADP+ reduction with NADH are coupled via an electron-bifurcating enzyme complex in Clostridium kluyveri. J Bacteriol 192:5115–5123. doi: 10.1128/JB.00612-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma K, Adams MW. 2001. Ferredoxin:NADP oxidoreductase from Pyrococcus furiosus. Methods Enzymol 334:40–45. doi: 10.1016/S0076-6879(01)34456-7. [DOI] [PubMed] [Google Scholar]
- 12.Skråmo S, Hersleth H-P, Hammerstad M, Andersson KK, Røhr ÅK. 2014. Cloning, expression, purification, crystallization and preliminary X-ray diffraction analysis of a ferredoxin/flavodoxin-NADP(H) oxidoreductase (Bc0385) from Bacillus cereus. Acta Crystallogr F Struct Biol Commun 70:777–780. doi: 10.1107/S2053230X14008334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Komori H, Seo D, Sakurai T, Higuchi Y. 2010. Crystal structure analysis of Bacillus subtilis ferredoxin-NADP+ oxidoreductase and the structural basis for its substrate selectivity. Protein Sci 19:2279–2290. doi: 10.1002/pro.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muraki N, Seo D, Shiba T, Sakurai T, Kurisu G. 2010. Asymmetric dimeric structure of ferredoxin-NAD(P)+ oxidoreductase from the green sulfur bacterium Chlorobaculum tepidum: implications for binding ferredoxin and NADP+. J Mol Biol 401:403–414. doi: 10.1016/j.jmb.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 15.Buckel W, Thauer RK. 2013. Energy conservation via electron bifurcating ferredoxin reduction and proton/Na+ translocating ferredoxin oxidation. Biochim Biophys Acta 1827:94–113. doi: 10.1016/j.bbabio.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Tian L, Papanek B, Olson DG, Rydzak T, Holwerda EK, Zheng T, Zhou J, Maloney M, Jiang N, Giannone R, Hettich R, Guss A, Lynd L. 2016. Simultaneous achievement of high ethanol yield and titer in Clostridium thermocellum. Biotechnol Biofuels 9:116. doi: 10.1186/s13068-016-0528-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Biegel E, Schmidt S, González JM, Müller V. 2011. Biochemistry, evolution and physiological function of the Rnf complex, a novel ion-motive electron transport complex in prokaryotes. Cell Mol Life Sci 68:613–634. doi: 10.1007/s00018-010-0555-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biegel E, Müller V. 2010. Bacterial Na+-translocating ferredoxin:NAD+ oxidoreductase. Proc Natl Acad Sci U S A 107:18138–18142. doi: 10.1073/pnas.1010318107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lo J, Zheng T, Olson DG, Ruppertsberger N, Tripathi SA, Tian L, Guss AM, Lynd LR. 2015. Deletion of nfnAB in Thermoanaerobacterium saccharolyticum and its effect on metabolism. J Bacteriol 197:2920–2929. doi: 10.1128/JB.00347-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Demmer JK, Huang H, Wang S, Demmer U, Thauer RK, Ermler U. 2015. Insights into flavin-based electron bifurcation via the NADH-dependent reduced ferredoxin:NADP oxidoreductase structure. J Biol Chem 290:21985–21995. doi: 10.1074/jbc.M115.656520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herring CD, Kenealy WR, Joe Shaw A, Covalla SF, Olson DG, Zhang J, Ryan Sillers W, Tsakraklides V, Bardsley JS, Rogers SR, Thorne PG, Johnson JP, Foster A, Shikhare ID, Klingeman DM, Brown SD, Davison BH, Lynd LR, Hogsett DA. 2016. Strain and bioprocess improvement of a thermophilic anaerobe for the production of ethanol from wood. Biotechnol Biofuels 9:125. doi: 10.1186/s13068-016-0536-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng T, Olson DG, Tian L, Bomble YJ, Himmel ME, Lo J, Hon S, Shaw AJ, van Dijken JP, Lynd LR. 2015. Cofactor specificity of the bifunctional alcohol and aldehyde dehydrogenase (AdhE) in wild-type and mutants of Clostridium thermocellum and Thermoanaerobacterium saccharolyticum. J Bacteriol 197:2610–2619. doi: 10.1128/JB.00232-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olson DG, Lynd LR. 2012. Transformation of Clostridium thermocellum by electroporation. Methods Enzymol 510:317–330. doi: 10.1016/B978-0-12-415931-0.00017-3. [DOI] [PubMed] [Google Scholar]
- 24.Zhou J, Olson DG, Argyros DA, Deng Y, van Gulik WM, van Dijken JP, Lynd LR. 2013. Atypical glycolysis in Clostridium thermocellum. Appl Environ Microbiol 79:3000–3008. doi: 10.1128/AEM.04037-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lo J, Zheng T, Hon S, Olson DG, Lynd LR. 2015. The bifunctional alcohol and aldehyde dehydrogenase gene, adhE, is necessary for ethanol production in Clostridium thermocellum and Thermoanaerobacterium saccharolyticum. J Bacteriol 197:1386–1393. doi: 10.1128/JB.02450-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shao X, Zhou J, Olson DG, Lynd LR. 2016. A markerless gene deletion and integration system for Thermoanaerobacter ethanolicus. Biotechnol Biofuels 9:100. doi: 10.1186/s13068-016-0514-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaw AJ, Hogsett DA, Lynd LR. 2010. Natural competence in Thermoanaerobacter and Thermoanaerobacterium species. Appl Environ Microbiol 76:4713–4719. doi: 10.1128/AEM.00402-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takahashi Y, Nakamura M. 1999. Functional assignment of the ORF2-iscS-iscU-iscA-hscB-hscA-fdx-0RF3 gene cluster involved in the assembly of Fe-S clusters in Escherichia coli. J Biochem 126:917–926. doi: 10.1093/oxfordjournals.jbchem.a022535. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura M, Saeki K, Takahashi Y. 1999. Hyperproduction of recombinant ferredoxins in Escherichia coli by coexpression of the ORF1-ORF2-iscS-iscU-iscA-hscB-hs cA-fdx-ORF3 gene cluster. J Biochem 126:10–18. doi: 10.1093/oxfordjournals.jbchem.a022409. [DOI] [PubMed] [Google Scholar]
- 30.Huang H, Hu L, Yu W, Li H, Tao F, Xie H, Wang S. 2016. Heterologous overproduction of 2[4Fe4S]- and [2Fe2S]-type clostridial ferredoxins and [2Fe2S]-type agrobacterial ferredoxin. Protein Expr Purif 121:1–8. doi: 10.1016/j.pep.2015.12.019. [DOI] [PubMed] [Google Scholar]
- 31.Jeoung J-H, Dobbek H. 2007. Carbon dioxide activation at the Ni,Fe-cluster of anaerobic carbon monoxide dehydrogenase. Science 318:1461–1464. doi: 10.1126/science.1148481. [DOI] [PubMed] [Google Scholar]
- 32.Zhou J, Olson DG, Lanahan AA, Tian L, Murphy SJ-L, Lo J, Lynd LR. 2015. Physiological roles of pyruvate ferredoxin oxidoreductase and pyruvate formate-lyase in Thermoanaerobacterium saccharolyticum JW/SL-YS485. Biotechnol Biofuels 8:138. doi: 10.1186/s13068-015-0304-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Finn RD, Coggill P, Eberhardt RY, Eddy SR, Mistry J, Mitchell AL, Potter SC, Punta M, Qureshi M, Sangrador-Vegas A, Salazar GA, Tate J, Bateman A. 2015. The Pfam protein families database: towards a more sustainable future. Nucleic Acids Res 44:gkv1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Currie DH, Herring CD, Guss AM, Olson DG, Hogsett DA, Lynd LR. 2013. Functional heterologous expression of an engineered full length CipA from Clostridium thermocellum in Thermoanaerobacterium saccharolyticum. Biotechnol Biofuels 6:32. doi: 10.1186/1754-6834-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rowland P, Nørager S, Jensen KF, Larsen S. 2000. Structure of dihydroorotate dehydrogenase B: electron transfer between two flavin groups bridged by an iron-sulphur cluster. Structure 8:1227–1238. doi: 10.1016/S0969-2126(00)00530-X. [DOI] [PubMed] [Google Scholar]
- 36.Shaw AJ, Hogsett DA, Lynd LR. 2009. Identification of the [FeFe]-hydrogenase responsible for hydrogen generation in Thermoanaerobacterium saccharolyticum and demonstration of increased ethanol yield via hydrogenase knockout. J Bacteriol 191:6457–6464. doi: 10.1128/JB.00497-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olson DG, Sparling R, Lynd LR. 2015. Ethanol production by engineered thermophiles. Curr Opin Biotechnol 33:130–141. doi: 10.1016/j.copbio.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 38.Hon S, Lanahan AA, Tian L, Giannone RJ, Hettich RL, Olson DG, Lynd LR. 2016. Development of a plasmid-based expression system in Clostridium thermocellum and its use to screen heterologous expression of bifunctional alcohol dehydrogenases (adhEs). Metab Eng Commun 3:120–129. doi: 10.1016/j.meteno.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rydzak T, McQueen PD, Krokhin OV, Spicer V, Ezzati P, Dwivedi RC, Shamshurin D, Levin DB, Wilkins JA, Sparling R. 2012. Proteomic analysis of Clostridium thermocellum core metabolism: relative protein expression profiles and growth phase-dependent changes in protein expression. BMC Microbiol 12:214. doi: 10.1186/1471-2180-12-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mai V, Lorenz WW, Wiegel J. 2006. Transformation of Thermoanaerobacterium sp. strain JW/SL-YS485 with plasmid pIKM1 conferring kanamycin resistance. FEMS Microbiol Lett 148:163–167. doi: 10.1111/j.1574-6968.1997.tb10283.x. [DOI] [Google Scholar]
- 41.Shaw AJ, Miller BB, Rogers SR, Kenealy WR, Meola A, Bhandiwad A, Sillers WR, Shikhare I, Hogsett DA, Herring CD. 2015. Anaerobic detoxification of acetic acid in a thermophilic ethanologen. Biotechnol Biofuels 8:75. doi: 10.1186/s13068-014-0179-6. [DOI] [PMC free article] [PubMed] [Google Scholar]