Abstract
Protein thiyl radicals are important intermediates generated in redox processes of thiols and disulfides. Thiyl radicals efficiently react with glutathione and ascorbate, and the common notion is that these reactions serve to eliminate thiyl radicals before they can enter potentially hazardous processes. However, over the past years increasing evidence has been provided for rather efficient intramolecular hydrogen transfer processes of thiyl radicals in proteins and peptides. Based on rate constants published for these processes, we have performed kinetic simulations of protein thiyl radical reactivity. Our simulations suggest that protein thiyl radicals enter intramolecular hydrogen transfer reactions to a significant extent even under physiologic conditions, i.e. in the presence of 30 μM oxygen, 1 mM ascorbate and 10 mM glutathione. At lower concentrations of ascorbate and glutathione, frequently observed when tissue is exposed to oxidative stress, the extent of irreversible protein thiyl radical-dependent protein modification increases.
Keywords: Thiyl radicals, hydrogen atom transfer, carbon-centered radicals, D-amino acids, glutathione
Graphical abstract
Introduction
Protein thiols play an important role in signaling pathways, enzyme activation and inactivation, and the detoxification of reactive oxygen species[1–4]. Key to these pathways are redox reactions, where thiol oxidation proceeds via two-electron and one-electron oxidation, yielding sulfenic acid (RSOH) and thiyl radical (RS•), respectively. The redox chemistry of thiols, and pathways for sulfenic acid and thiyl radical formation has been covered in many articles[1–10] and shall not be reviewed in detail here. Instead, the current article will focus on reaction pathways of protein thiyl radicals and include several novel reactions of thiyl radicals for which experimental evidence has been provided in recent years. We will provide kinetic simulations, based on recent experimental data, which predict the fate of protein thiyl radicals under biologic conditions. These calculations will provide estimates for product yields of thiyl radical reactions, which may need to be taken into account when considering the role of protein thiyl radicals in biologic systems. Our kinetic simulations were motivated by several recent discussions on the potential fate of protein thiyl radicals under conditions of oxidative stress. In this regard, it is important to note that thiyl radicals, via reaction with nitric oxide (NO), have been implicated in the formation of S-nitrosocysteine (S-NO-Cys)[11], i.e., an important product of thiol-dependent signaling. However, further research is required in order to evaluate to what extent this mechanism is physiologically significant[12]. Moreover, thiyl radical-dependent regulation of nucleotide exchange has been documented for a series GTPases[13–17]. While it is commonly assumed that antioxidants such as glutathione (GSH) and ascorbate (HAsc−) prevent deleterious intra- and intermolecular reactions of protein thiyl radicals, our kinetic simulations will show that, due in part to chain reactions, quite significant yields of reaction products other than thiols or disulfides may form from these protein thiyl radicals. This would be especially the case at decreased, suboptimal levels of GSH and/or ascorbate, and under conditions where protein thiyl radicals may not be fully accessible to these antioxidants.
Chemically, protein thiyl radicals can be generated via a manifold of pathways but physiologically only a few of them may be significant. Protein thiyl radical generation has been documented for the reaction of hydrogen peroxide with heme proteins, including hemoglobin[18, 19]. Analogous pathways may operate with non-heme iron and other protein-associated redox-active transition metals present under conditions of iron overload[20] or neurodegenerative diseases[21]. Protein thiyl radicals form through electron transfer between Cys and protein tyrosyl radicals[22], where tyrosyl radicals are generated through one-electron oxidation of Tyr. The latter can be induced through peroxynitrite-decomposition in in the presence of CO2, generating •CO3− and •NO2. For example, peroxynitrite-induced tyrosyl radicals were detected in human blood plasma[23]. However, the extent to which tyrosyl radicals will involve in electron-transfer with protein Cys residues will depend on the rate-constant for intramolecular electron transfer, which has to compete with the reduction of the tyrosyl radical by GSH and ascorbate. Protein thiyl radicals have been involved in mechanisms leading to S-nitrosation, and specifically in mechanisms of nucleotide exchange of various GTPases[11–17].
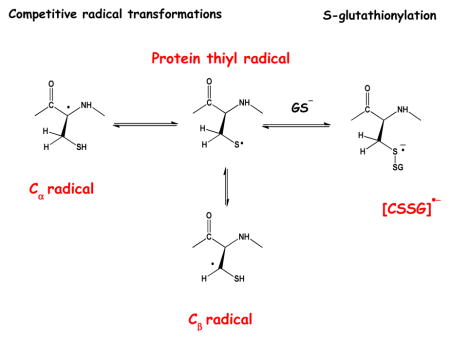
Theoretical calculations predicted that thiyl radicals should react with αC-H bonds of most amino acids in proteins when located in flexible or β-sheet structures but not in α-helices[24–27]. Our kinetic NMR experiments confirmed that intermolecular hydrogen atom transfer (HAT) reactions of the general type displayed in Scheme 1, equilibrium 2, occur between thiyl radicals and several amino acids[28]. However, these HAT reactions occurred not only with the αC-H bonds but also with the C-H bonds of the amino acid side chains[29]. Radiation chemistry, ESR, NMR and mass spectrometry experiments then provided evidence for intramolecular HAT reactions in model peptides[30–32] as well as in glutathione (GSH)[33–38], where HAT was observed between the Cys thiyl radical and the αC-H bonds of γ-Glu, Cys and Gly. More recently, pulse radiolysis experiments with Cys and several model compounds provided rate constants for intramolecular HAT reactions with the αC-H and the βC-H bond (Scheme 1, equilibria 2 and 1)[39]. The consequence of these inter- and intramolecular HAT equilibria is the intermediary generation of significant fractions of carbon-centered radicals, which are at the origin of various products. When oxygen is available, these carbon-centered radicals can react with oxygen to yield peroxyl radicals, where peroxyl radical formation may lead to irreversible protein damage, including fragmentation[40]. However, under physiological conditions the availability of oxygen may be limited for various reasons. First, oxygen concentrations can range from ca. 3–70 μM[41–43], depending on the nature of the tissue. Second, oxygen diffusion into the interior of proteins may be limited[44] and peroxyl radical formation may be slow when carbon-centered radicals are generated within buried domains of proteins, for example through HAT reactions induced by thiyl radicals. Under these conditions, carbon-centered radicals may undergo additional reactions such as the addition to aromatic amino acids[45].
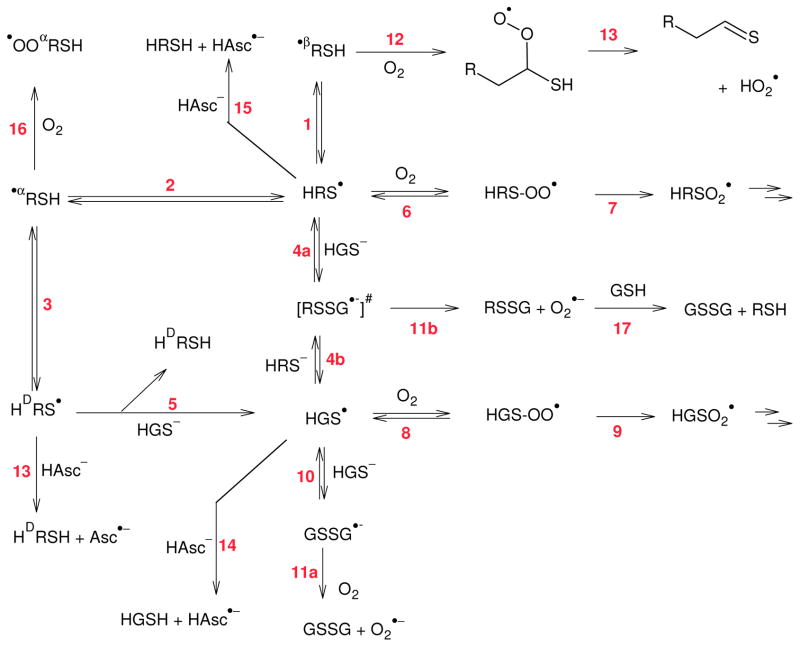
Scheme 1. Reactions of potein thiyl radicals considered in the kinetic simulations.
Abbreviations used:
HAsc−, ascorbate; HAsc•−, ascorbyl radical; HRSH, intact protein; HDRSH, protein with incorporated D-Cys; HRS−, protein thiolate; HRS•, protein thiyl radical; •βRSH, alpha-mercaptoalkyl radical of protein; •αRSH, backbone radical of protein at Cys; •OOαRSH, backbone peroxyl radical; HRS-OO•, oxygen adduct to a protein thiyl radical; HRSO2•, protein sulfenyl radical; HGSH, Glutathione; HGS•, Glutathione thiyl radical; #, This intermediate is invoked by analogy to known chemistry
In vitro photochemical studies with several model peptides[31–35, 46, 47] and proteins[48–50], including IgG1 and IgG2, have demonstrated that thiyl radicals can generate intermediary carbon-centered radicals on proteins in a sequence-specific manner. While thiyl radicals were not generated through physiological processes in these studies, they are relevant to physiology as they demonstrate what can happen when thiyl radicals are generated on proteins. In the absence of oxygen, the reversible generation of carbon-centered radicals has led to the conversion of L-Ala to D-Ala in a model peptide[32], and to the sequence-specific generation of D-amino acids on IgG1[51]. Hence, thiyl radical generation on proteins is a viable source for D-amino acid generation, and such reactions have to be taken into account when simulating thiyl radical reaction pathways in proteins (see below).
Kinetic simulations
The chemistry according to scheme 1 has been simulated and the results are summarized in Table 1. We evaluated the yield of several products and reaction channels starting from a hypothetical protein thiyl radical. The published rate constants were used where available. We assumed, that reaction rates for intramolecular hydrogen transfer (reactions 1–3) are the same for free Cys[39] and for Cys located within the protein and that the repair of the protein thiyl radicals by ascorbate (reactions 12 and 13) proceeds with the same rate constant as the repair of glutathione thiyl radicals by ascorbate (reaction 14)[52]. In our simulation we further assume that there is a 50% probability to form D-Cys upon repair of the αC•-radical (k2 = k3), i.e. that there are no geometrical restraints on the orientation of the Cys. Reaction 10 and 11 are well known[53, 54], and represent the main detoxification route of “radical repair processes” by thiols, e.g. reactions involving thiyl radicals [55]. The equilibrium between the thiyl radical the disulfide radical anion is documented not only for GSH, but also for other thiols, including Cys residues in proteins[56]. We, therefore, conclude that such a reaction is also to be expected between protein thiyl radicals and GSH (reaction 4a) as well as for glutathione thiyl radicals and the protein thiolate (reaction 4b). Therefore, there is a chain reaction possible for the epimerization of protein cysteine residues via reactions −2, 3, 5, 4b and 4a. In order to simplify the simulation, the protonation equilibria of GSH and protein thiol have not been explicitly simulated. Instead, we calculated the amount of thiolate in equilibrium with pK(GSH)=9.2 and pK(protein thiol)=8.4, respectively, and used the resulting concentrations to evaluate the equilibration reactions 4a, 4b and 10. As a further simplification, HAT reactions with amino acids other than Cys are not considered. The protein concentration was arbitrarily chosen to be 1 mM. This number does not refer to one particular protein but constitutes an assumed total concentration of all proteins carrying at least one solvent exposed thiol. The reaction of thiyl radicals with oxygen has been discussed in a number of publications; we base our simulation on the rate constants of Zhang et al. [9]. The following rate constants were used for the kinetic simulations: k1 = 1×105 s−1 [39], k−1 = 1.5×10−5 s−1[39], k2 = 8×104 s−1[39], k−2 = 7×105 s−1[39], k3 = 7×105 s−1[39], and k−3 = 8×104 s−1[39], k4a = k4b = k5 = 1.2×109 M−1s−1 (by analogy[57]), k-4a = k-4b = k-5 = 8×106 s−1 (by analogy[57]), k6 = k8 = 2.2×109 M−1s−1 (by analogy[9]), k−6 = k−8 = 6.2×105 s−1 (by analogy[9]), k7 = k9 = 2×103 s−1 (by analogy[9]), k10 = 1×108 M−1s−1[53], k−10 = 2.5×105 s−1[53], k11a [55]= k11b (by analogy[55]) = 2×108 M−1s−1, k12 = k16 = 1×109 M−1s−1 (estimate), and k13 = k14 = k15 = 5×108 M−1s−1 (by analogy[52]).
Table 1.
Simulated yields of products as a function of experimental parameters
| Conditions | pH | 7.4 | 7.4 | 7.4 | 7.4 | 7.4 | 7.4 |
| [GSH]/mM | 10 | 10 | 10 | 0 | 10 | 10 | |
| [Hasc-]/mM | 1 | 0.1 | 0 | 0.1 | 0.1 | 0 | |
| [O2]/μM | 30 | 30 | 30 | 30 | 200 | 200 | |
|
| |||||||
|
|
|||||||
| Calculated yield/initial protein thiyl radical (in %) | |||||||
| Product yield | GSSGa | 0.2 | 12 | 30 | 0 | 12 | 32 |
| (Thio-)aldehydeb | 3.6 | 34 | 62 | 18 | 32 | 52 | |
| D-Cys in Protein | 7.5 | 16 | 133 | 39 | 20 | 33 | |
| Protein peroxyl radical | 0.5 | 2 | 7.9 | 23 | 8 | 13 | |
| Ascorbyl radicalc | 96 | 78 | 0 | 79 | 47 | 0 | |
| O2•− d | 3.7 | 20 | 44 | 18 | 44 | 84 | |
| GSSG(total)e | 51 | 64 | 64 | 0 | 70 | 94 | |
| Protein damage (sum) | 12 | 52 | 203 | 80 | 60 | 98 | |
via oxidation of GSSG•−
via oxidation of alpha-mercaptoalkyl radicals of Cys by O2
via reaction with GS• and RS•, reacts with O2 to form O2•−
via a) and b), dismutates to form H2O2
via a), and oxdation by protein hydroperoxide, H2O2, and reduction of protein peroxyl radicals either by ascorbate or GSH
Thermodynamic considerations
The energetics of Scheme 1 are evaluated with known electrode potentials at pH 7: E°′(asc•−, H+/Hasc) = +0.28 V[58], E°′(HRS•, H+/HRSH) = E°′(GS•, H+/GSH) = +0.92 V[59]; E°′(O2, O2•−,) = −0.35 V[60], E°′(RSSG/RSSG•−) = −1.3 V[61], E°′(•αRSH, H+/HRSH) = +0.98 (based on K = 0.1 for the equilibrium HRS• ⇌ •αRSH[33]) and E°′(•βRSH, H+/HRSH) = +0.92 (based on K = 1 for the equilibrium HRS• ⇌ •βRSH[39]). As more •βRSH radicals are formed than •αRSH from HRS•, we assume that E°′(•βRSH, H+/HRSH) < E°′(•αRSH, H+/HRSH).
The cycle consisting of reactions 2, 3, 5, 4b and 4a is, of course, thermoneutral. The reaction of a thiyl radical with dioxygen, reaction 8, is an equilibrium[62], and compared with the fast and favorable reaction of thiyl radicals with Hasc−, not very important. The reaction of a carbon-centered radical with O2 is considered to be irreversible, due to follow-up reactions (reactions 12 and 16). As follows from the Table 1, Hasc−, even at a concentration of only 0.10 mM, limits protein damage more effectively than 10 mM GSH. The energetics for both superoxide and ascorbyl radical formation are quite favourable, but the former reaction requires the presence of a substantial concentration of GSH to form RSSG•−, which then reacts with O2, while the latter, repair by Hasc−, is a fast straightforward bimolecular reaction.
Discussion
The formation of GSSG has frequently been used as proof for repair of protein radicals by GSH. With our simulations we differentiate between the following reaction channels: (a) direct repair by GSH via reaction 11, (b) repair by ascorbate via reactions 13,14 and 15, (c) formation of thioaldehyde via reaction 12 (significant yields of thioaldehyde or its hydrolysis product, the aldehyde, were detected when thiyl radicals were generated photochemically[31]), (d) epimerization via reaction (3), (e) peroxidation via reaction (16) and (f) oxidation of the protein thiol via reaction 7. In all cases studied protein thiol oxidation, reaction channel (f), was negligible and, therefore, is not referenced to in Table 1. Because of reactions 18 – 22 (in vivo, H2O2 is predominantly generated via catalysis by SOD, reaction 21), the experimentally determined GSSG formation will be much higher than indicated by the simulation results on channel (a) only.
| (18) |
| (19) |
| (20) |
| (21) |
| (22) |
We, therefore, added a line in our Table, that indicates the experimentally expected yield. This number probably is underestimated. Reactions 23 and 24 (where reaction 24 is catalyzed by glutathione peroxidase) probably also occur nearly quantitatively.
| (23) |
| (24) |
In the absence of ascorbate, the protein peroxyl radicals will probably oxidize GSH via
| (25) |
A kinetic simulation with as many reactions as ours is inherently prone to substantial errors: rate constants of very rapid reactions, as used in our simulations here, typically have errors larger ±10%, even if these rate constants are reproducible. Additionally, some rate constants were taken from analogous reactions. There are several competition situations which influence yields, for example k1 + k2 vs. k−4a, which determines the minimum damage via formation of carbon-centered radicals, or k11 vs. k12, which strongly influences the maximum production of thioaldehyde. Also, the assumed total concentration of protein (i.e. 1 mM) influences the chain reaction, i.e. the yield of reaction −4b. We calculated several sets of probable combinations, based on the spread of rate constants published in the literature. For most starting concentrations, uncertainties are below ± 20%.1 Higher uncertainties, up to ± 60%, were found in simulations for complete absence of ascorbate. Overall, we believe our results to be semiquantitatively correct, the simulations give important insight into the relevant reaction pathways.
The simulations let us expect a substantial yield of GSSG for typical in vitro experiments in the absence of ascorbate (200 μM O2, 10 mM GSH), in line with the commonly used reaction scheme based solely on reactions 2, 4a, 4b, 10, and 11a[63]. However, this does not imply quantitative repair. Our model suggests the formation of additional products, mainly protein aldehydes from the hydrolysis of thioaldehydes and protein-bound D-cysteine (or other D-amino acids[51]). Such species would not be found in standard assays. In vivo, the concentration of oxygen is typically around 3–70 μM[41–43], much lower than in air saturated solutions. Under such conditions the reaction chain gets much longer and we expect a distinct increase of the formation of D-Cys. In the presence of ascorbate, damage to the protein strongly decreases and the uncertainties of the simulation sharply drop because the determining influence of ascorbate on the half-life of thiyl radicals. In the presence of 30 μM oxygen, the yield of damage per initial protein thiyl radical decreases from 2.5 irreversibly damaged protein molecules per initiating protein radical in the absence of ascorbate, i.e. a chain length of over two, to 0.1 damaged protein molecules in the presence of 1 mM ascorbate. Ascorbate suppresses the propagation step of the chain reaction, repair by 100 μM ascorbate is about one order of magnitude more efficient than repair by 10 mM GSH.
Conclusion
An important result of our simulations is the fact, that thiyl radical formation may lead to protein damage even in the presence of 10 mM GSH and 1mM ascorbate. Under these conditions, a simulated yield of ca. 0.5 equivalents of GSSG per initial protein thiyl radical would suggest that protein damage might be negligible; nevertheless, our simulated yields for thioaldehyde, D-Cys and protein peroxyl radical account for ca. 12% protein damage under such conditions. These yields increase as the concentrations of GSH and ascorbate are lowered, where specifically the ascorbate concentration has a significant effect.
Highlights.
Recent experimental evidence suggests the possibility of intramolecular hydrogen atom transfer reactions of thiyl radicals in proteins
Kinetic simulations including experimental rate constants for hydrogen atom transfer reactions suggest that thiyl radical reactions in proteins may lead to additional products different from disulfides
The extent to which such hydrogen atom transfer reactions may occur under physiological conditions will depend on the presence of oxygen and antioxidants
The potential for intramolecular hydrogen atom transfer reactions will increase upon a decrease of antioxidant levels, such as observed under conditions of oxidative stress
Acknowledgments
Support of this work by the ETH Zürich and the NIH (P01AG12993) is gratefully acknowledged.
Footnotes
Every rate constant used has an uncertainty and contributes to an error propagation in the calculations. The total error is dependent on all the errors of the rate constants used and to the weight of each induvidual reaction in the reaction network. We chose a pragmatic approach to estimate errors: we first analysed the robustness of the simulations to changes of single rate constants. Then, we concentrated our analysis on reactions with a high influence on the overall error. With the spread of reported rate constants of such reactions, we calculated the cumulative maximum impact on our result, the yields. The result represents a range where the true result is expected. The true error, however, may be even higher: many rate constants have been determined only once, and we suspect that in such cases the error may be larger than the statistical reproducibility usually given. Because of this, and because we neglected “minor influence reactions” in our determination, we estimate our simulation to have a total error of double the range derived above.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Winterbourn CC, Hampton MB. Thiol chemistry and specificity in redox signaling. Free Radic Biol Med. 2008;45:549–561. doi: 10.1016/j.freeradbiomed.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Winterbourn CC. Are Free Radicals Involved in Thiol-Based Redox Signaling? Free Radic Biol Med. 2014 doi: 10.1016/j.freeradbiomed.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Santamarina S, Boronat S, Hidalgo E. Reversible cysteine oxidation in hydrogen peroxide sensing and signal transduction. Biochemistry. 2014;53:2560–2580. doi: 10.1021/bi401700f. [DOI] [PubMed] [Google Scholar]
- 4.Jones DP. Radical-free biology of oxidative stress. Am J Physiol Cell Physiol. 2008;295:C849–868. doi: 10.1152/ajpcell.00283.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schöneich C. Mechanisms of protein damage induced by cysteine thiyl radical formation. Chem Res Toxicol. 2008;21:1175–1179. doi: 10.1021/tx800005u. [DOI] [PubMed] [Google Scholar]
- 6.Akhlaq MS, Schuchmann HP, von Sonntag C. The reverse of the ‘repair’ reaction of thiols: H-abstraction at carbon by thiyl radicals. Int J Radiat Biol Relat Stud Phys Chem Med. 1987;51:91–102. doi: 10.1080/09553008714550531. [DOI] [PubMed] [Google Scholar]
- 7.Karoui H, Hogg N, Frejaville C, Tordo P, Kalyanaraman B. Characterization of sulfur-centered radical intermediates formed during the oxidation of thiols and sulfite by peroxynitrite. ESR-spin trapping and oxygen uptake studies. J Biol Chem. 1996;271:6000–6009. doi: 10.1074/jbc.271.11.6000. [DOI] [PubMed] [Google Scholar]
- 8.Sevilla MD, Becker D, Yan M. The formation and structure of the sulfoxyl radicals RSO(.), RSOO(.), RSO2(.), and RSO2OO(.) from the reaction of cysteine, glutathione and penicillamine thiyl radicals with molecular oxygen. Int J Radiat Biol. 1990;57:65–81. doi: 10.1080/09553009014550351. [DOI] [PubMed] [Google Scholar]
- 9.Zhang X, Zhang N, Schuchmann H-P, von Sonntag C. Pulse radiolysis of 2-mercaptoethanol in oxygenated aqueous solution. Generation and reactions of the thiylperoxyl radical. J Phys Chem-Us. 1994;98:6541–6547. [Google Scholar]
- 10.Bonifacic M, Asmus KD. Adduct Formation and Absolute Rate Constants in the Displacement Reaction of Thiyl Radicals with Disulfides. J Phys Chem-Us. 1984;88:6286–6290. [Google Scholar]
- 11.Jourd’heuil D, Jourd’heuil FL, Feelisch M. Oxidation and nitrosation of thiols at low micromolar exposure to nitric oxide. Evidence for a free radical mechanism. J Biol Chem. 2003;278:15720–15726. doi: 10.1074/jbc.M300203200. [DOI] [PubMed] [Google Scholar]
- 12.Bosworth CA, Toledo JC, Jr, Zmijewski JW, Li Q, Lancaster JR., Jr Dinitrosyliron complexes and the mechanism(s) of cellular protein nitrosothiol formation from nitric oxide. Proc Natl Acad Sci U S A. 2009;106:4671–4676. doi: 10.1073/pnas.0710416106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aaron Hobbs G, Mitchell LE, Arrington ME, Gunawardena HP, DeCristo MJ, Loeser RF, Chen X, Cox AD, Campbell SL. Redox Regulation of Rac1 by Thiol Oxidation. Free Radic Biol Med. 2014 doi: 10.1016/j.freeradbiomed.2014.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis MF, Vigil D, Campbell SL. Regulation of Ras proteins by reactive nitrogen species. Free Radic Biol Med. 2011;51:565–575. doi: 10.1016/j.freeradbiomed.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis MF, Zhou L, Ehrenshaft M, Ranguelova K, Gunawardena HP, Chen X, Bonini MG, Mason RP, Campbell SL. Detection of Ras GTPase protein radicals through immuno-spin trapping. Free Radic Biol Med. 2012;53:1339–1345. doi: 10.1016/j.freeradbiomed.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitchell L, Hobbs GA, Aghajanian A, Campbell SL. Redox regulation of Ras and Rho GTPases: mechanism and function. Antioxid Redox Signal. 2013;18:250–258. doi: 10.1089/ars.2012.4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raines KW, Bonini MG, Campbell SL. Nitric oxide cell signaling: S-nitrosation of Ras superfamily GTPases. Cardiovasc Res. 2007;75:229–239. doi: 10.1016/j.cardiores.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 18.Maples KR, Kennedy CH, Jordan SJ, Mason RP. In vivo thiyl free radical formation from hemoglobin following administration of hydroperoxides. Arch Biochem Biophys. 1990;277:402–409. doi: 10.1016/0003-9861(90)90596-q. [DOI] [PubMed] [Google Scholar]
- 19.Venters HD, Jr, Bonilla LE, Jensen T, Garner HP, Bordayo EZ, Najarian MM, Ala TA, Mason RP, Frey WH., 2nd Heme from Alzheimer’s brain inhibits muscarinic receptor binding via thiyl radical generation. Brain Res. 1997;764:93–100. doi: 10.1016/s0006-8993(97)00425-3. [DOI] [PubMed] [Google Scholar]
- 20.Meneghini R. Iron homeostasis, oxidative stress, and DNA damage. Free Radic Biol Med. 1997;23:783–792. doi: 10.1016/s0891-5849(97)00016-6. [DOI] [PubMed] [Google Scholar]
- 21.Schrag M, Mueller C, Zabel M, Crofton A, Kirsch WM, Ghribi O, Squitti R, Perry G. Oxidative stress in blood in Alzheimer’s disease and mild cognitive impairment: a meta-analysis. Neurobiol Dis. 2013;59:100–110. doi: 10.1016/j.nbd.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 22.Petruk AA, Bartesaghi S, Trujillo M, Estrin DA, Murgida D, Kalyanaraman B, Marti MA, Radi R. Molecular basis of intramolecular electron transfer in proteins during radical-mediated oxidations: computer simulation studies in model tyrosine-cysteine peptides in solution. Arch Biochem Biophys. 2012;525:82–91. doi: 10.1016/j.abb.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pietraforte D, Minetti M. Direct ESR detection or peroxynitrite-induced tyrosine-centred protein radicals in human blood plasma. Biochem J. 1997;325(Pt 3):675–684. doi: 10.1042/bj3250675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Block DA, Yu D, Armstrong DA, Rauk A. On the influence of secondary structure on the alpha-C-H bond dissociation energy of proline residues in proteins: a theoretical study. Can J Chem. 1998;76:1042–1049. [Google Scholar]
- 25.Rauk A, Yu D, Armstrong DA. Oxidative damage to and by cysteine in proteins: An ab initio study of the radical structures, C-H, S-H, and C-C bond dissociation energies, and transition structures for H abstraction by thiyl radicals. Journal of the American Chemical Society. 1998;120:8848–8855. [Google Scholar]
- 26.Rauk A, Yu D, Armstrong DA. Toward site specificity of oxidative damage in proteins: C-H and C-C bond dissociation energies and reduction potentials of the radicals of alanine, serine, and threonine residues - An ab initio study. Journal of the American Chemical Society. 1997;119:208–217. [Google Scholar]
- 27.Reid DL, Armstrong DA, Rauk A, von Sonntag C. H-atom abstraction by thiyl radicals from peptides and cyclic dipeptides. A theoretical study of reaction rates. Physical Chemistry Chemical Physics. 2003;5:3994–3999. [Google Scholar]
- 28.Nauser T, Schöneich C. Thiyl radicals abstract hydrogen atoms from the (alpha)C-H bonds in model peptides: absolute rate constants and effect of amino acid structure. J Am Chem Soc. 2003;125:2042–2043. doi: 10.1021/ja0293599. [DOI] [PubMed] [Google Scholar]
- 29.Nauser T, Pelling J, Schöneich C. Thiyl radical reaction with amino acid side chains: rate constants for hydrogen transfer and relevance for posttranslational protein modification. Chem Res Toxicol. 2004;17:1323–1328. doi: 10.1021/tx049856y. [DOI] [PubMed] [Google Scholar]
- 30.Nauser T, Casi G, Koppenol WH, Schöneich C. Reversible intramolecular hydrogen transfer between cysteine thiyl radicals and glycine and alanine in model peptides: absolute rate constants derived from pulse radiolysis and laser flash photolysis. J Phys Chem B. 2008;112:15034–15044. doi: 10.1021/jp805133u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mozziconacci O, Sharov V, Williams TD, Kerwin BA, Schöneich C. Peptide cysteine thiyl radicals abstract hydrogen atoms from surrounding amino acids: the photolysis of a cystine containing model peptide. J Phys Chem B. 2008;112:9250–9257. doi: 10.1021/jp801753d. [DOI] [PubMed] [Google Scholar]
- 32.Mozziconacci O, Kerwin BA, Schöneich C. Reversible hydrogen transfer between cysteine thiyl radical and glycine and alanine in model peptides: covalent H/D exchange, radical-radical reactions, and L- to D-Ala conversion. J Phys Chem B. 2010;114:6751–6762. doi: 10.1021/jp101508b. [DOI] [PubMed] [Google Scholar]
- 33.Hofstetter D, Nauser T, Koppenol WH. Hydrogen exchange equilibria in glutathione radicals: rate constants. Chem Res Toxicol. 2010;23:1596–1600. doi: 10.1021/tx100185k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mozziconacci O, Williams TD, Schöneich C. Intramolecular hydrogen transfer reactions of thiyl radicals from glutathione: formation of carbon-centered radical at Glu, Cys, and Gly. Chem Res Toxicol. 2012;25:1842–1861. doi: 10.1021/tx3000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hofstetter D, Thalmann B, Nauser T, Koppenol WH. Hydrogen exchange equilibria in thiols. Chem Res Toxicol. 2012;25:1862–1867. doi: 10.1021/tx300045f. [DOI] [PubMed] [Google Scholar]
- 36.Grierson L, Hildenbrand K, Bothe E. Intramolecular transformation reaction of the glutathione thiyl radical into a non-sulphur-centred radical: a pulse-radiolysis and EPR study. Int J Radiat Biol. 1992;62:265–277. doi: 10.1080/09553009214552111. [DOI] [PubMed] [Google Scholar]
- 37.Zhao R, Lind J, Merenyi G, Eriksen TE. Significance of the intramolecular transformation of glutathione thiyl radicals to alpha-aminoalkyl radicals. Thermochemical and biological implications. J Chem Soc Perk T. 1997;2:569–574. [Google Scholar]
- 38.Zhao R, Lind J, Merenyi G, Eriksen TE. Kinetics of One-Electron Oxidation of Thiols and Hydrogen Abstraction by Thiyl Radicals from Alpha-Amino C-H Bonds. Journal of the American Chemical Society. 1994;116:12010–12015. [Google Scholar]
- 39.Nauser T, Koppenol WH, Schöneich C. Reversible hydrogen transfer reactions in thiyl radicals from cysteine and related molecules: absolute kinetics and equilibrium constants determined by pulse radiolysis. J Phys Chem B. 2012;116:5329–5341. doi: 10.1021/jp210954v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garrison WM. Reaction-Mechanisms in the Radiolysis of Peptides, Polypeptides, and Proteins. Chemical Reviews. 1987;87:381–398. [Google Scholar]
- 41.Hogan MC. Phosphorescence quenching method for measurement of intracellular PO2 in isolated skeletal muscle fibers. J Appl Physiol. 1999;86:720–724. doi: 10.1152/jappl.1999.86.2.720. [DOI] [PubMed] [Google Scholar]
- 42.Richardson RS, Newcomer SC, Noyszewski EA. Skeletal muscle intracellular PO(2) assessed by myoglobin desaturation: response to graded exercise. J Appl Physiol (1985) 2001;91:2679–2685. doi: 10.1152/jappl.2001.91.6.2679. [DOI] [PubMed] [Google Scholar]
- 43.Carreau A, El Hafny-Rahbi B, Matejuk A, Grillon C, Kieda C. Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J Cell Mol Med. 2011;15:1239–1253. doi: 10.1111/j.1582-4934.2011.01258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strambini G, Cioni P. The effect of protein structure on oxygen quenching of phosphorescence. J Am Chem Soc. 1999;121:8337–8344. [Google Scholar]
- 45.Nauser T, Carreras A. Carbon-centered radicals add reversibly to histidine - implications. Chem Commun (Camb) 2014 doi: 10.1039/c4cc05316h. [DOI] [PubMed] [Google Scholar]
- 46.Mozziconacci O, Kerwin BA, Schöneich C. Photolysis of an intrachain peptide disulfide bond: primary and secondary processes, formation of H2S, and hydrogen transfer reactions. J Phys Chem B. 2010;114:3668–3688. doi: 10.1021/jp910789x. [DOI] [PubMed] [Google Scholar]
- 47.Mozziconacci O, Kerwin BA, Schöneich C. Reversible hydrogen transfer reactions of cysteine thiyl radicals in peptides: the conversion of cysteine into dehydroalanine and alanine, and of alanine into dehydroalanine. J Phys Chem B. 2011;115:12287–12305. doi: 10.1021/jp2070453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mozziconacci O, Williams TD, Kerwin BA, Schöneich C. Reversible intramolecular hydrogen transfer between protein cysteine thiyl radicals and alpha C-H bonds in insulin: control of selectivity by secondary structure. J Phys Chem B. 2008;112:15921–15932. doi: 10.1021/jp8066519. [DOI] [PubMed] [Google Scholar]
- 49.Mozziconacci O, Haywood J, Gorman EM, Munson E, Schöneich C. Photolysis of recombinant human insulin in the solid state: formation of a dithiohemiacetal product at the C-terminal disulfide bond. Pharm Res. 2012;29:121–133. doi: 10.1007/s11095-011-0519-1. [DOI] [PubMed] [Google Scholar]
- 50.Zhou S, Mozziconacci O, Kerwin BA, Schöneich C. The photolysis of disulfide bonds in IgG1 and IgG2 leads to selective intramolecular hydrogen transfer reactions of cysteine Thiyl radicals, probed by covalent H/D exchange and RPLC-MS/MS analysis. Pharm Res. 2013;30:1291–1299. doi: 10.1007/s11095-012-0968-1. [DOI] [PubMed] [Google Scholar]
- 51.Mozziconacci O, Schöneich C. Sequence-specific formation of D-amino acids in a monoclonal antibody during light exposure. Mol Pharm. 2014 doi: 10.1021/mp500508w. [DOI] [PubMed] [Google Scholar]
- 52.Wolfenden BS, Willson RL. Radical-Cations as Reference Chromogens in Kinetic-Studies of One-Electron Transfer-Reactions - Pulse-Radiolysis Studies of 2,2′-Azinobis-(3-Ethylbenzthiazoline-6-Sulphonate) J Chem Soc Perk T. 1982;2:805–812. [Google Scholar]
- 53.Mezyk SP. Rate constant determination for the reaction of hydroxyl and glutathione thiyl radicals with glutathione in aqueous solution. J Phys Chem-Us. 1996;100:8861–8866. [Google Scholar]
- 54.Barton JP, Packer JE. The radiolysis of oxygenated cysteine solutions at neutral pH. the role of RSSR− and O2−. Int J Radiat Phys Chem. 1970;2:159–166. [Google Scholar]
- 55.Winterbourn CC. Superoxide as an intracellular radical sink. Free Radic Biol Med. 1993;14:85–90. doi: 10.1016/0891-5849(93)90512-s. [DOI] [PubMed] [Google Scholar]
- 56.Favaudon V, Tourbez H, Houée-Levin C, Lhoste JM. CO2.- radical induced cleavage of disulfide bonds in proteins. A gamma-ray and pulse radiolysis mechanistic investigation. Biochemistry. 1990;29:10978–10989. doi: 10.1021/bi00501a016. [DOI] [PubMed] [Google Scholar]
- 57.Mezyk SP. LINAC/LASER determination of the absolute rate constant for thiyl and hydroxyl radical reaction with sulfhydryls in aqueous solution: Mercaptoethanol, cysteamine, and N-acetyl-L-cysteine. J Phys Chem-Us. 1996;100:8295–8301. [Google Scholar]
- 58.Williams NH, Yandell JK. Outer-Sphere Electron-Transfer Reactions of Ascorbate Anions. Aust J Chem. 1982;35:1133–1144. [Google Scholar]
- 59.Madej E, Wardman P. The oxidizing power of the glutathione thiyl radical as measured by its electrode potential at physiological pH. Arch Biochem Biophys. 2007;462:94–102. doi: 10.1016/j.abb.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 60.Koppenol WH, Stanbury DM, Bounds PL. Electrode potentials of partially reduced oxygen species, from dioxygen to water. Free Radic Biol Med. 2010;49:317–322. doi: 10.1016/j.freeradbiomed.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 61.Nauser T, Steinmann D, Grassi G, Koppenol WH. Why selenocysteine replaces cysteine in thioredoxin reductase: a radical hypothesis. Biochemistry. 2014;53:5017–5022. doi: 10.1021/bi5003376. [DOI] [PubMed] [Google Scholar]
- 62.Turnipseed AA, Barone SB, Ravishankara AR. Observation of Ch3s Addition to O-2 in the Gas-Phase. J Phys Chem-Us. 1992;96:7502–7505. [Google Scholar]
- 63.Winterbourn CC. In: Cellular Implications of Redox Signaling. Gitler C, Danon A, editors. Imperial College Press; 2003. pp. 175–190. [Google Scholar]