Abstract
Prader–Willi syndrome (PWS), a neurodevelopmental disorder caused by loss of paternal gene expression from 15q11–q13, is characterised by growth retardation, hyperphagia and obesity. However, as single gene mutation mouse models for this condition display an incomplete spectrum of the PWS phenotype, we have characterised the metabolic impairment in a mouse model for ‘full’ PWS, in which deletion of the imprinting centre (IC) abolishes paternal gene expression from the entire PWS cluster. We show that PWS-ICdel mice displayed postnatal growth retardation, with reduced body weight, hyperghrelinaemia and marked abdominal leanness; proportionate retroperitoneal, epididymal/omental and inguinal white adipose tissue (WAT) weights being reduced by 82%, 84% and 67%, respectively. PWS-ICdel mice also displayed a 48% reduction in proportionate interscapular brown adipose tissue (isBAT) weight with significant ‘beiging’ of abdominal WAT, and a 2°C increase in interscapular surface body temperature. Maintenance of PWS-ICdel mice under thermoneutral conditions (30°C) suppressed the thermogenic activity in PWS-ICdel males, but failed to elevate the abdominal WAT weight, possibly due to a normalisation of caloric intake. Interestingly, PWS-ICdel mice also showed exaggerated food hoarding behaviour with standard and high-fat diets, but despite becoming hyperphagic when switched to a high-fat diet, PWS-ICdel mice failed to gain weight. This evidence indicates that, unlike humans with PWS, loss of paternal gene expression from the PWS cluster in mice results in abdominal leanness. Although reduced subcutaneous insulation may lead to exaggerated heat loss and thermogenesis, abdominal leanness is likely to arise from a reduced lipid storage capacity rather than increased energy utilisation in BAT.
Keywords: Prader–Willi syndrome, fat mass, thermogenesis, food hoarding
Introduction
Prader–Willi syndrome (PWS) is caused by a lack of paternal gene expression from the 15q11–q13 imprinting cluster and results from large chromosomal deletions, chromosome 15 maternal uniparental disomy or imprinting-centre (IC) mutations. This disorder is associated with significant metabolic impairment. After severe neonatal hypotonia and a failure to thrive in infancy, subsequent development of hyperphagia and reduced satiety responses result in obesity, unless managed carefully. Indeed, as PWS is the most common syndromal cause of morbid obesity (Butler 1990), elucidating the aetiology of this metabolic disturbance will not only benefit individuals with PWS but also may have wider implications for ameliorating obesity.
Insight into the relationship between the imprinted genes within the PWS cluster and impaired function has been gained by comparing the phenotype of mouse models bearing different PWS-associated mutations. For example, neonatal failure to thrive and early life metabolic impairment are seen in many models (Yang et al. 1998, Ge et al. 2002, Stefan et al. 2005), including a number of single gene knock-out mice (Ding et al. 2008, Schaller et al. 2010). In contrast, evidence for feeding and metabolic changes in adult mice are inconsistent. For instance, despite being hypophagic (Kozlov et al. 2007), mice null for Magel2 become obese (Bischof et al. 2007), whereas mice carrying a deletion of the small nucleolar (sno)RNA Snord116 show mild hyperphagia, and impaired meal termination, coupled with leanness, even on a high-fat diet (Ding et al. 2008).
Given that single gene mutations constitute a rare subset of PWS individuals (Sahoo et al. 2008) and display an incomplete phenotypic spectrum (Peters 2008), we have characterised the metabolic impairment in a mouse model for ‘full’ PWS, the PWS-ICdel mouse. IC deletion in this model results in the complete loss of paternal gene expression from the PWS interval (Chamberlain et al. 2004), including its expression in the central nervous system (Relkovic et al. 2010). Although PWS-ICdel mice suffer considerable postnatal lethality, sufficient survivors have enabled us to demonstrate many of the behavioural aspects of PWS in this model, including reduced locomotor activity and deficits in cognitive function, such as attention and impulse control (Doe et al. 2009, Relkovic et al. 2010). However, although we have shown that hyperphagia in PWS-ICdel mice is driven primarily by calorie seeking rather than hedonic processes (Davies et al. 2015), there has been no systematic characterisation of metabolism in this or any other mouse model for ‘full’ PWS (Bervini & Herzog 2013).
We have therefore characterised the metabolic phenotype of the PWS-ICdel model in this study, quantifying appetitive and consummatory feeding behaviour, lipid profiling and storage, short-term responses to high-fat diet exposure and assessment of thermogenic function.
Materials and methods
Animals
The PWS-IC+/− (referred throughout as PWS-ICdel) and wild-type (WT) mice used in this study were bred under the Authority of the Animals (scientific procedures) Act 1986 (UK), with subsequent procedures conforming with the institutional and national guidelines, including those for genetically modified animals, and specifically approved by local ethical review.
PWS-ICdel mice and WT littermates were generated by crossing ICdel-positive males with WT females. Given the nature of the epigenetic regulation of imprinted genes, paternally inherited IC deletion results in a lack of gene expression from the PWS interval. As PWS-ICdel animals on a pure C57BL/6J background suffer severe postnatal lethality, we crossed ICdel-positive males with CD1 females and selectively culled WT littermates (identified on the basis of their increased size 48 h after birth) leaving only 1 or 2 WT pups per litter, as previously described (Chamberlain et al. 2004, Davies et al. 2015). Animals were weaned at approximately 4 weeks of age and were single-sexed group housed with WT littermates (2–5 animals per cage). This procedure generated the six cohorts of adult mice used below.
All animals were maintained on a 12-h light/darkness cycle (lights on 07:00 h), with ad libitum access to water and standard laboratory chow (Rat and Mouse No. 3 Breeding Diet, Special Diet Services Ltd., Witham, Essex, UK, containing 4.2% crude fat; 22.4% crude protein; 4.2% crude fibre; 7.6% crude ash), unless stated otherwise.
Study 1. Adiposity profile in PWS-ICdel mice
After an overnight fast (with water available ad libitum), 18-month-old male WT and PWS-ICdel littermate mice were weighed and killed by cervical dislocation. Inguinal, retroperitoneal and epididymal white adipose tissue (WAT) and interscapular brown adipose tissue (isBAT) fat depots and liver were excised, weighed, snap frozen and stored at −70°C for subsequent histological analysis (see below). Left tibiae were excised, and the length was determined with a hand-held micrometre.
Study 2. Plasma lipid profiles in PWS-ICdel mice
Two groups of 16- to 17-month-old female WT and PWS-IC littermate mice were weighed and killed by cervical dislocation after 24 h of total food removal or ad libitum feeding (with water available to both groups ad libitum). After thoracotomy, blood samples were obtained by cardiac puncture using BD Microtainers (SST Amber Tubes, BD Biosciences, UK), and aliquots of separated plasma were stored at −20°C for the quantification of circulating ghrelin and lipid profiling (see below).
Study 3. Thermogenesis in PWS-ICdel mice
After analysis of BAT histology from the mice in study 1, surface body temperature was measured in 6- to 10-month-old male WT and PWS-IC mice as previously described (Wells et al. 2012, Schultz et al. 2013). Thermal images obtained using an FLIR A40 camera (FLIR Systems, Boston MA, USA) were analysed by ImageJ to determine surface body temperatures for the head, interscapular, dorso-lumbar and tail root regions and for a region of cage immediately adjacent to the right shoulder.
Study 4. The effect of suppressing thermogenesis in PWS-ICdel mice
To determine the impact of thermogenesis on intra-abdominal adiposity, 6- to 15-month-old male and female PWS-ICdel mice, and WT littermates, were group housed (2–3 mice/cage) at either standard room temperature (20–22°C) or at a thermoneutral ambient temperature (30°C) for 9 weeks. 30°C represents the lower critical temperature in mice, below which non-shivering thermogenesis is induced (Overton 2010). Mice maintained at thermoneutrality were housed in standard cages inside a warming chamber (Scanbur Type 48-VS-IV, Karslunde, Denmark), beginning at room temperature and increasing the temperature by no more than 2°C per day until a stable chamber temperature of 30°C was attained on day 8. Body weight was monitored 3 times/week. In weeks 7–8, mice were individually housed in metabolic cages under standard or thermoneutral ambient temperatures. After 3 days acclimatisation, ad libitum food intake was measured on two consecutive days and mean daily food intake calculated. The mice were then returned to their original group cages for the remainder of the 9 weeks, after which mice were weighed and killed by decapitation under isoflurane anaesthesia. Interscapular BAT, inguinal, epididymal/omental and retroperitoneal WAT and liver were excised, weighed, snap frozen and stored at −80°C for subsequent analysis.
Study 5. Feeding behaviour in PWS-ICdel mice
Study 5a. Ad libitum feeding
Groups of 5- to 9-month-old male and female WT and PWS-ICdel mice were housed individually in metabolic cages. After 5 days acclimatisation, mice were subjected to ad libitum feeding with a standard diet (SDS 10% Atwater fuel energy (AFE) Fat, in which energy sources (3.68 kcal/g) were Crude Fat 10%; Crude Protein 20%; Carbohydrate 70% (Special Diet Services, Witham, Essex, UK)) for 1 week, followed by a further 1-week period with a high-fat diet (SDS 45% AFE Fat, in which energy sources (4.54 kcal/g) were: Crude Fat 45% Crude Protein 20%; Carbohydrate 35%). Body weight and food intake were monitored daily, food consumption being normalised to body weight. After the first day of exposure to each diet, the proportion of ‘hoarded’ food was determined by expressing the mass of food in the inner (‘spill’) compartment of the food hopper (Supplementary Fig. 1A and B, see section on supplementary data given at the end of this article) as a percentage of the total remaining diet. The proportion of diet consumed during the darkness and light phases was determined on the fifth day of exposure to each diet. At the end of the dietary manipulations, mice were killed by decapitation under isoflurane anaesthesia and trunk blood was collected. Separated plasma was stored at −20°C prior to subsequent analysis of plasma ghrelin (total) concentration (see below).
Study 5b. Post-fast feeding
Group housed 4- to 6-month-old male and female WT and PWS-ICdel mice were subjected to a 16-h overnight fast (17:00–09:00) (with water provided ad libitum). Mice were placed individually in normal shoe-box cages under low lighting levels and presented with a pre-weighed pot of wet mash (1 part standard diet (SDS RM3):1 part water). Wet mash was used to overcome the propensity of PWS-ICdel mice to hoard diet pellets and spill powdered diet (Supplementary Fig. 1). Mice were allowed to feed freely for 60 min, the pots being weighed after 30 and 60 min to determine food consumption.
Tissue analysis
Adipocyte size and hepatic lipid content were quantified as previously described (Davies et al. 2009). Samples of retroperitoneal WAT were paraffin-embedded and 5 µm sections were stained with Masson’s Trichrome. Cryostat sections (15 µm) of liver were stained with oil red-O and counterstained with Meyer’s haematoxylin. Digital images (one image per section, three images per mouse) were obtained under light microscopy. Adipocyte size was quantified in cells with transversely sectioned peripheral nuclei (minimum 9 cells per section/3 sections per sample) using ImageJ and hepatic lipid droplets measured using Leica Q-Win. The degree of ‘beiging’ was determined in three random full screen digital images of retroperitoneal WAT. Areas of ‘beiging’ were identified (see Fig. 2a and b), delineated, filled and quantified using ImageJ, the data being expressed as %-‘beiging’ of total field.
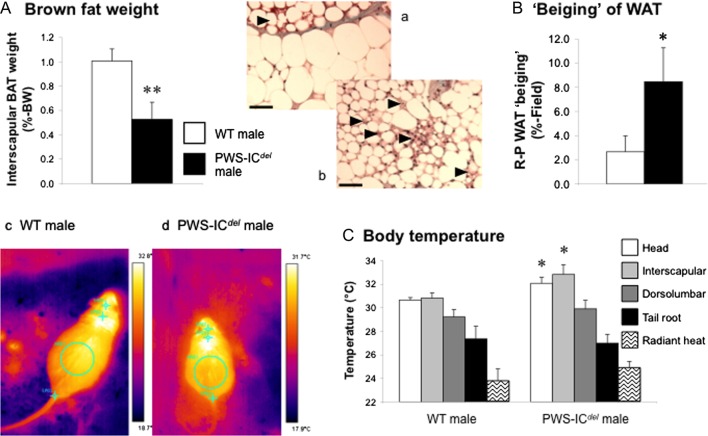
Figure 2.
PWS-ICdel mice show enhanced thermogenesis. Quantification of BAT mass (A) and ‘beiging’ in retroperitoneal (R-P) WAT (inset pictures a (WT) and b (PWS-ICdel) (arrowheads: areas of ‘beige’ or ‘brite’ adipose tissue; scale bars: 50 µm)) (B) in 18-month-old male WT and PWS-ICdel littermate mice and determination of surface body temperatures (head, interscapular, dorsolumbar and tail root regions and an estimation of heat loss) from thermal images (inset pictures c and d) taken of 5- to 9-month-old male WT and PWS-ICdel mice (C). Data shown are mean ± s.e.m. (n = 6 for all groups), with statistical comparisons made with either Student’s t-test (A and B; *P < 0.05, **P < 0.01) or 1-way ANOVA and Bonferroni’s selected pairs post hoc test (C; *P < 0.05). A full colour version of this figure is available at http://dx.doi.org/10.1530/JOE-16-0367.
Uncoupling protein-1 (Ucp-1) mRNA expression in isBAT and inguinal WAT was quantified as previously described (Wells et al. 2012). In brief, total RNA from lysed tissue was purified using the Qiagen RNeasy Mini extraction kit before quantification and assessment of RNA purity. RNA was subsequently reverse transcribed using the Precision nanoScript Reverse Transcription kit (Primerdesign, Southampton, UK), 1 µg RNA being reverse transcribed in 20 µL reactions containing 1 µL oligoDT and 1 µL random decamers (Primerdesign) prior to freeze storing at −20°C. cDNA was diluted 1:10 in dd.H2O prior to quantification of Ucp-1 and β-actin cDNA by quantitative real-time PCR amplification using a Bio-Rad IQ5 thermal cycler and Precision 2× qPCR Mastermix reagents (Primerdesign). Oligonucleotide primers for mouse Ucp-1 were purchased from Primerdesign (Ucp-1 sense primer, GGTAAAAACAAGATTCATCAACTCTC: Ucp-1 anti-sense primer, TCGCAGAAAAGAAGCCACAA). PCR thermal cycling conditions included an initial enzyme activation for 10 min at 95°C (1 cycle), followed by 51 cycles of denaturation at 95°C for 15 s and real-time Fluorogenic Data Collection (FDC) at 60°C for 1 min. The final FDCs were acquired at 0.5°C temperature increments between 55°C and 95°C at 10-s intervals (81 cycles). The PCR products were quantified by incorporation of SYBR green into double stranded DNA. Single amplicon identity was verified by melt curve analysis. Individual samples were normalised to the expression of β-actin (ACTB Primer mix, Primer Design # ge-SY-6; Batch # 12474). The quantity of cDNA in each reaction was calculated by reference to a standard curve constructed from serial dilutions of cDNA from WT BAT (Wells et al. 2012).
Plasma analysis
Plasma ghrelin (total) concentrations were determined by RIA (Millipore/Linco (IAV: 3.28–8.05%)). Plasma lipids were extracted by the Folch method (Kates 1986). Classes of individual non-polar lipids (triacyglycerols, free fatty acids and sterol esters), and total polar lipids were separated by thin-layer chromatography and quantified by their fatty acid contents. Fatty acid methyl esters were separated on a Clarus 500 gas chromatograph (PerkinElmer) identified by comparing retention times with fatty acid standards (Nu-Chek Prep Inc., Elysian, MN, USA) and quantified against an internal pentadecanoate standard.
Statistical analysis
Results are expressed as mean ± s.e.m., and differences were established by 1-way and 2-way repeated measures ANOVA and Bonferroni post hoc test or unpaired Student’s t-test (using GraphPad Prism, GraphPad Software), as indicated in the figure and table legends, with P < 0.05 considered significantly different.
Results
Growth and endocrine status in PWS-ICdel mice
Although neither foetal growth (E18.5 PWS-ICdel foetuses weighed 96% of their WT counterparts (WT: 1.18 ± 0.044 g (n = 7); PWS-ICdel: 1.13 ± 0.044 g (n = 7); P = 0.45)) nor placental weight (WT: 100 ± 6.5 mg (n = 7); PWC-ICdel: 92 ± 4.1 mg (n = 7); P = 0.32) were significantly affected in PWS-ICdel pregnancies, PWS-ICdel mice failed to thrive after 24 h post-partum, leading to increased neonatal mortality. PWS-ICdel mice that survived to adulthood were significantly growth retarded, adult PWS-ICdel males showing a 40% reduction in body weight (Fig. 1A; P < 0.001), accompanied by a 7% reduction in tibial length (data not shown; P < 0.001). Comparable reductions in body weight were observed in similarly-aged PWS-ICdel females (Table 1).
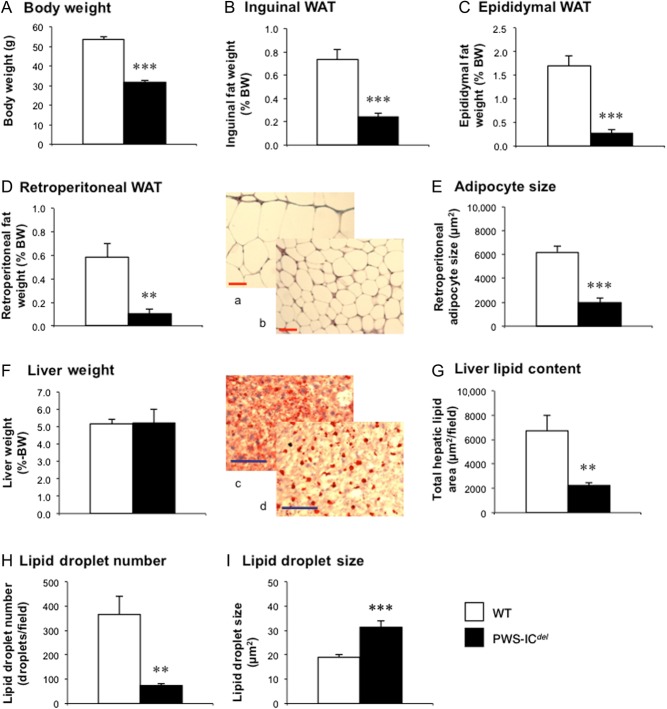
Figure 1.
PWS-ICdel mice show profound abdominal leanness and reduced hepatic lipid storage. Quantification of body weight (A), proportionate inguinal (B), epididymal (C) and retroperitoneal (D) WAT, and liver (F) weights in 18-month-old male WT (n = 6) and PWS-ICdel (n = 6) littermate mice. Retroperitoneal adipocyte size (E) was quantified on digital images from WT (a) and PWS-ICdel (b) mice (scale bar 50 µm). Hepatic lipid content (G), lipid droplet number (H) and size (I) were determined by digital analysis of oil red-O-stained sections in WT (c) and PWS-ICdel (d) mice (scale bar 100 µm). Values shown are mean ± s.e.m., with statistical comparisons performed by Student’s unpaired t-test (**P < 0.01; ***P < 0.001 vs WT). A full colour version of this figure is available at http://dx.doi.org/10.1530/JOE-16-0367.
Table 1.
The effect of fasting on the plasma lipid profile and circulating ghrelin in female W-T and PWS-ICdel littermate mice.
| W-T female | PWC-ICdel female | |||
|---|---|---|---|---|
| Fed (n = 9) | Fasted (n = 9) | Fed (n = 8) | Fasted (n = 4) | |
| Body weight (g) | 43.1 ± 3.0 | 42.2 ± 2.6 | 26.2 ± 0.7aaa | 22.9 ± 1.1ccc |
| Total polar lipids (µg/µL) | 0.313 ± 0.021 | 0.366 ± 0.043 | 0.247 ± 0.014 | 0.269 ± 0.040 |
| Free fatty acids (µg/µL) | 0.045 ± 0.004 | 0.072 ± 0.013 | 0.049 ± 0.017 | 0.069 ± 0.021 |
| Triacylglycerol (µg/µL) | 0.191 ± 0.026 | 0.105 ± 0.023 | 0.128 ± 0.031 | 0.085 ± 0.042 |
| S1 sterol esters (µg/µL) | 0.036 ± 0.005 | 0.057 ± 0.011 | 0.029 ± 0.005 | 0.025 ± 0.009 |
| S2 sterol esters (µg/µL) | 0.058 ± 0.004 | 0.058 ± 0.010 | 0.045 ± 0.007 | 0.030 ± 0.009 |
| Plasma [ghrelin (total)] (ng/mL) | 9.82 ± 2.24 | 54.94 ± 10.03aa | 13.12 ± 1.95 | 62.86 ± 15.43bb |
S1, arachidonic acid-enriched sterol esters;
S2, sterol esters with shorter chain fatty acids.
P < 0.01
P < 0.001 vs fed W–T female
P < 0.01 vs fed PWC-ICdel female
P < 0.001 vs fasted W-T female.
Study 1. Adiposity profile in PWS-ICdel mice
However, in contrast to humans with PWS, PWS-ICdel mice were surprisingly lean, proportionate inguinal (Fig. 1B), epididymal (Fig. 1C) and retroperitoneal (Fig. 1D) WAT weights being reduced by 67% (P < 0.01), 84% (P < 0.001) and 82% (P < 0.01), respectively, and reflected in a 69% reduction in adipocyte size (Fig. 1E and inset pictures a and b; P < 0.001). Although proportionate liver weight was unaffected (Fig. 1F), hepatic lipid storage in PWS-ICdel mice was disrupted. In contrast to the diffuse lipid staining in WT livers (Fig. 1c inset), PWS-ICdel livers showed punctate staining (Fig. 1d inset), with fewer (80% of WT (Fig. 1H; P < 0.01)), larger (increased by 66% (Fig. 1I; P < 0.001)) lipid droplets, with total lipid content reduced by 67% (Fig. 1G; P < 0.01).
Study 2. Plasma lipid profiles in PWS-ICdel mice
We therefore investigated whether the reduction in WAT mass was due to impaired lipid handling. Although circulating total polar lipid in fed PWS-ICdel mice was only 79% of that in WT littermates (P > 0.05; Table 1), none of the markers of dietary lipid intake (S1 and S2 sterol esters), hepatic lipid output (triacylglycerol: TAG) or WAT lipid output (free fatty acids: FFAs) were significantly different. Similarly, the effect of fasting on circulating lipids was comparable in WT and PWS-ICdel mice (Table 1). As shown previously (Davies et al. 2015), circulating ghrelin (total) in PWS-ICdel females was 134% of that in female WT littermates (Table 1), but was regulated appropriately after the 24-h fast.
Study 3. Thermogenesis in PWS-ICdel mice
We subsequently investigated the thermogenic activity in PWS-ICdel mice. Proportionate isBAT weight was reduced by 48% in PWS-ICdel mice (Fig. 2A; P < 0.01), consistent with elevated thermogenesis. Similarly, retroperitoneal WAT (Fig. 2 pictures a and b) showed a four-fold elevation in the degree of differentiation to ‘beige’ or ‘brite’ adipose tissue (Fig. 2B; P < 0.05). Thermal imaging revealed that surface temperatures of the head and interscapular regions were increased in PWS-ICdel mice by 1.4°C and 2.0°C, respectively (Fig. 2C and pictures c and d; P < 0.05), whereas dorsolumbar tail root and radiant temperatures were not significantly affected (Fig. 2C).
Study 4. The effect of suppressing thermogenesis in PWS-ICdel mice
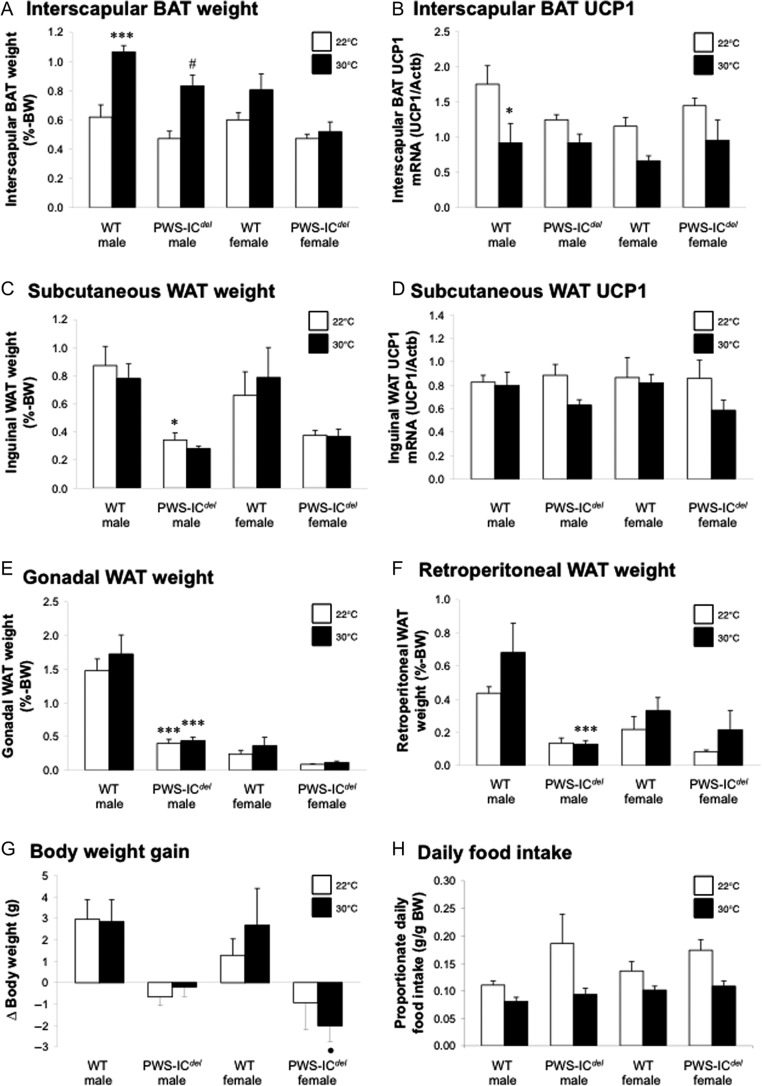
To establish whether the increased energy demands of elevated thermogenesis account for the lean phenotype, PWS-ICdel mice and WT littermates, were maintained under thermoneutral conditions (30°C). After 9 weeks, proportionate isBAT weight in WT and PWS-ICdel males was elevated by 72% and 76%, respectively (Fig. 3A), indicating reduced lipid utilisation; these changes being less pronounced in WT and PWS-ICdel females. Thermoneutrality halved Ucp-1 mRNA expression, the classic marker of thermogenic activity, in WT males (Fig. 3B), but this effect was not significant in PWS-ICdel animals (P = 0.053 (males); P = 0.183 (females)). Despite suppressed thermogenesis, proportionate WAT mass (total: data not shown, or individual depots: Fig. 3C, E and F) was not increased. For example, although proportionate retroperitoneal WAT mass in WT males at thermoneutrality was 156% of that in corresponding mice at room temperature, these means were not significantly different (Fig. 3E; P = 0.09), whereas for male PWS-ICdel mice at thermoneutrality, retroperitoneal WAT mass was 97% of that in PWS-ICdel mice at room temperature. In addition, thermoneutrality had no significant effect on WAT Ucp-1 mRNA expression (Fig. 3D). However, thermoneutrality reduced liver weight in WT males by 12% (data not shown) suggesting either reduced hepatic liver storage or increased lipid export. Interestingly, suppression of thermogenesis failed to increase body weight gain in any of the groups (Fig. 3G), although WT females at thermoneutrality showed a transient increase in weight gain between days 11 and 15 (P < 0.05; data not shown). This lack of weight gain may arise from the suppressive influence of thermoneutrality on daily food intake (Fig. 3H), mean proportionate daily food intake in WT males, PWS-ICdel males, WT females and PWS-ICdel females at thermoneutrality being 73%, 50%, 74% and 62% of that in mice housed at room temperature. Proportionate hyperphagia in PWS-ICdel mice was completely abolished at thermoneutrality (Fig. 3H).
Figure 3.
Thermoneutrality suppresses thermogenesis and hyperphagia in PWS-ICdel mice. Quantification of interscapular BAT mass (A) and Ucp-1 mRNA expression (B), inguinal WAT mass (C) and Ucp-1 mRNA expression (D), epididymal/omental (E) and retroperitoneal (R-P: F) WAT mass, body weight gain (G) and daily food intake (H) in 6- to 14-month-old male and female WT and PWS-ICdel littermate mice maintained at either standard room temperature (20–22°C; white bars) or thermoneutrality (30°C; black bars) for 9 weeks. Data shown are mean ± s.e.m. (n = 5 (WT and PWS-ICdel males at 30°C) and 6 (all other groups)), with statistical comparisons made with 1-way ANOVA and Bonferroni’s selected pairs post hoc test (*P < 0.05, ***P < 0.001 vs WT male (room temperature); #P < 0.05 vs PWS-ICdel male (at room temperature); •P < 0.05 vs WT female (at thermoneutrality)).
Study 5. Feeding behaviour in PWS-ICdel mice
Study 5a. Ad libitum feeding
We subsequently examined feeding behaviour in PWS-ICdel mice at normal ambient temperature. Food intake was measured in male and female WT and PWC-ICdel mice, maintained for 1 week on standard and high-fat diets. Male and female WT mice ate roughly similar amounts of both diets (Fig. 4C). Although PWS-ICdel females consumed 32% less of the high-fat diet (compared to WT females; P < 0.05; Fig. 4C), when corrected for body weight, PWS-ICdel males consumed 76% more of the high-fat diet (compared to WT males; P < 0.01; Fig. 4D). Interestingly, although WT males maintained a constant proportionate caloric intake irrespective of which diet they were eating, PWS-ICdel males were susceptible to overconsumption when eating the high-fat diet (Fig. 4E). Similar patterns of caloric intake were observed in WT and PWS-ICdel females (Fig. 4E). Adiposity is influenced by the timing of food consumption (Glad et al. 2011), but day:night food intake ratios were not significantly different between WT and PWS-ICdel mice (Fig. 4G).
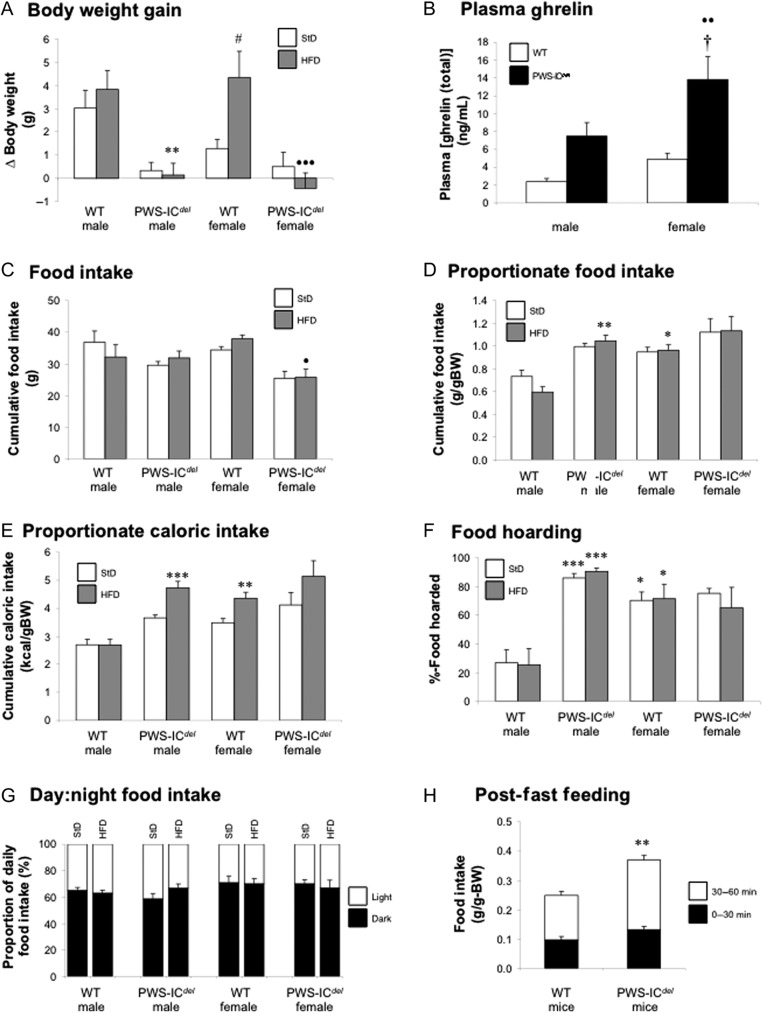
Figure 4.
PWS-ICdel mice show hyperghrelinaemia and disproportionately high food intake with exaggerated food hoarding behaviour and impaired meal termination. Analysis of body weight gain (A), terminal plasma ghrelin (total) concentration (B), food intake (C), weight-corrected food intake (D), weight-corrected caloric intake (E), food hoarding behaviour (F) and day:night feeding (G) in 5- to 9-month-old male and female WT and PWS-ICdel littermate mice when fed for 1 week with standard (StD) and high-fat (HFD) diets. Post-fast feeding (H) was determined in 4- to 6-month-old WT and PWS-ICdel littermate mice for 0–30 and 30–60 min after a 16-h overnight fast. Data shown are mean ± s.e.m. (n = 6 for all groups), with statistical comparisons made with 1-way ANOVA (A–G) or repeated measures 2-way ANOVA (H) and Bonferroni’s selected pairs post hoc test (*P < 0.05, **P < 0.01, ***P < 0.001 vs WT male (same diet); #P < 0.05 vs WT female (standard diet); •P < 0.05; ••P < 0.01; •••P < 0.001 vs WT female (same diet); †P < 0.05 vs PWS-ICdel male (same diet)).
During this experiment, a marked difference in appetitive behaviour became apparent. When presented with refilled diet hoppers PWS-ICdel mice immediately began moving the diet from the outer ‘fill’ compartment to the inner ‘spill’ compartment (Supplementary Fig. 1A and B). When quantifying the amount of diet in the ‘spill’ compartment as a percentage of the remaining diet, PWS-ICdel males were found to ‘hoard’ more than 3-times as much diet as WT males (P < 0.001; Fig. 4F and Supplementary Fig. 1A). This behaviour was prominent with both standard and high-fat diets, PWS-ICdel males hoarding 86–91% of the remaining diet. Interestingly, this pattern of hoarding in PWS-ICdel males was broadly similar to that in both WT and PWS-ICdel females (Fig. 4F).
Although constrained by the short period of monitoring, it is particularly striking that although PWS-ICdel mice consumed most calories when maintained on the high-fat diet, they not only failed to gain weight but also gained markedly less weight than their respective WT littermates (P < 0.01; Fig. 4A). Analysis of terminal blood samples revealed that mean circulating ghrelin (total) was tripled in PWS-ICdel mice in comparison to their sex-matched littermates (Fig. 4B), although this was not statistically significant in PWS-ICdel males (P = 0.116).
Study 5b. Post-fast feeding
As PWS-ICdel mice show proportionate hyperphagia, we investigated individual meal consumption patterns after an overnight fast. When adjusted for body weight, PWS-ICdel mice consumed 48% more diet than WT littermates (Fig. 4H; repeated measures ANOVA, main effect of GENOTYPE F1,11 = 6.01, P = 0.032). This was due mainly to continued consumption after 30 min as evidenced by a significant interaction with the time of measurement (F1,11 = 13.43, P = 0.004).
Discussion
Loss of paternal gene expression from the imprinted gene cluster on human chromosome 15q11–q13 impairs neuroendocrine and metabolic function in PWS. However, investigating these impairments in mouse models for PWS has been hampered by high postnatal lethality. We have utilised a mixed genetic background to increase survival, enabling us to characterise metabolic status in a mouse model in which deletion of the homologous PWS-IC results in complete loss of paternal gene expression from the entire PWS locus (Chamberlain et al. 2004, Relkovic et al. 2010). We now report that, with one notable exception, the metabolic phenotype of PWS is replicated in adult PWS-ICdel mice.
The growth abnormalities normally associated with PWS are recapitulated in PWS-ICdel mice, with reductions in body weight and limb bone length combined with a 40–50% reduction in circulating IGF-1 (unpublished data). As similar growth results have been reported in another ‘full’ genetic mouse model for PWS (Stefan et al. 2005, Ding et al. 2008), whereas mice with specific deletion of Magel2 exhibit normal skeletal growth (Bischof et al. 2007); this aspect of the PWS phenotype appears to be dependent upon lack of expression of one or more of the other genes within the PWS locus. Indeed, the reduced circulating IGF-1 and elevated GH-releasing hormone mRNA reported in Snord116del mice (Qi et al. 2016) indicate that loss of Snord116 expression causes a pituitary-specific deficiency in the GH axis.
Despite growth retardation, PWS-ICdel mice displayed a remarkable reduction in adiposity, affecting intra-abdominal, subcutaneous and intramedullary (data not presented) depots. Although reduced visceral adiposity has been reported in human females with PWS (Goldstone et al. 2001, 2004), the profound leanness in the PWS-ICdel mice is unlike the obesity usually associated with this condition in humans. Indeed, the degree of leanness seen in the PWS-ICdel model is considerably greater than the transient reduction in triglycerides in TgPWS mice (Stefan et al. 2005), and the mild reduction in fat mass in Snord116del mice (Ding et al. 2008, Qi et al. 2016). Given this surprising result, which corroborates previous reports in younger mice with IC deletions (Yang et al. 1998, Chamberlain et al. 2004), we examined the aetiology of this leanness, quantifying indices of energy intake, storage and utilisation.
Leanness in PWS-ICdel mice is clearly not due to impaired energy input, with males in particular showing proportionate hyperphagia, especially with a high-fat diet. Similarly, the normal day:night consumption ratios indicate that the altered lipid storage efficiency associated with daytime feeding in nocturnal rodents (Glad et al. 2011) does not contribute to the lean phenotype. The hyperphagia seen in PWS-ICdel mice, which is driven by calorie seeking rather than hedonic processes (Davies et al. 2015), was even more exaggerated after overnight fasting, where prolonged consumption indicates a failure in meal termination characteristic of PWS. These indices of increased consummatory behaviour are likely to reflect the sustained hyperghrelinaemia observed in both the current and previous studies (Davies et al. 2015), but as this is not accompanied by an increase in NPY mRNA expression (unpublished data), the hypothalamic mechanisms underlying proportionate hyperphagia remain to be elucidated. That said, as Magel2-null mice show hypophagia (Bischof et al. 2007), whereas specific deletion of Snord116 results in a similar combination of impaired meal termination and leanness (Ding et al. 2008), these aspects of the PWS-ICdelmouse phenotype probably result from loss of Snord116 expression.
When monitoring food intake, it became rapidly apparent that PWS males also displayed a remarkable increase in appetitive behaviour; moving crushed diet from the fill compartment to the spill compartment (Supplementary Fig. 1A and B). When supplied with pelleted diet, individual PWS-ICdel mice from our Cardiff colony displayed increased diet distribution in both home and metabolic cages (Supplementary Fig. 1C), whereas PWS-ICdel mice in a Florida colony packed bedding around high-fat diet pellets in the food hopper (Supplementary Fig. 1D). This hoarding behaviour, previously described in birds, rodents and, in certain circumstances, humans (Deacon 2006, Keen-Rinehart et al. 2010) is most prominent in species not readily storing energy in the form of accumulated WAT (Keen-Rinehart et al. 2010) and is exaggerated after surgical removal of epididymal WAT (Dailey & Bartness 2008). This implies that the lean phenotype of these mice may be a significant causal factor in this behaviour. Interestingly, our data also indicate that in WT mice, hoarding appears to be a predominantly female behaviour. Therefore, the hyperphagia and hoarding behaviour observed in PWS-ICdel males suggest that loss of paternal gene expression in the PWS locus results in a feminisation of feeding behaviour. Given that the central circuits regulating both consummatory and appetitive feeding behaviours are activated by ghrelin (Nakazato et al. 2001, Lawrence et al. 2002, Keen-Rhinehart & Bartness 2005), the elevation in food intake and diet hoarding may result from the sustained hyperghrelinaemia seen in this model (Davies et al. 2015) and in humans with PWS (Cummings et al. 2002, DelParigi et al. 2002, Haqq et al. 2003, Erdie-Lalena et al. 2006, Bizzari et al. 2010). However, it is interesting to note that PWS-ICdel mice show a normal ghrelin response to fasting (Table 1) and re-feeding (unpublished data), as reported recently in humans with this condition (Kuppens et al. 2016).
It is also possible that proportionate hyperphagia may arise from impairment of the anorexic oxytocin circuitry. Although we have yet to quantify oxytocin expression in PWS-ICdel mice, a reduction in the population of parvocellular oxytocin neurons has previously been described in humans with PWS (Swaab et al. 1995) and in mice with a specific deletion of Magel2 (Schaller et al. 2010). Indeed, postnatal injections of oxytocin have been reported to restore suckling in Magel2-null mice, preventing neonatal mortality (Schaller et al. 2010) and ameliorating deficits in social and learning behaviour in adults (Meziane et al. 2015). As Magel2 expression is absent in PWS-ICdel mice (Chamberlain et al. 2004, Relkovic et al. 2010), it is likely that correcting any imbalance in the oxytocin system in these animals may have similar beneficial effects, but whether this will prevent hyperphagia in adults and restore fat deposition remains to be determined.
In the context of elevated proportionate caloric intake (which was seen despite correcting for hoarding behaviour), it is possible that leanness occurs in PWS-ICdel mice as a result of impaired nutrient absorption. For the dietary lipids at least this does not appear to be the case, as circulating sterol esters, which reflect dietary lipid absorption (Jones & Glomset 1985), are unaffected (Table 1). In addition, although lipid processing in the liver may be disturbed (Fig. 1), the absence of any significant reduction in circulating TAG (Table 1) indicates that hepatic lipid output was not significantly curtailed.
The normal circulating lipid profile in the context of proportionate hyperphagia implies an increase in substrate utilisation. However, we have previously reported that locomotor activity in PWS-ICdel mice is significantly reduced (Doe et al. 2009, Relkovic et al. 2010). We therefore examined whether the energy utilisation associated with BAT-mediated thermogenesis was elevated in this model. This appears to be the case. The halving of isBAT weight, which reflects reduced TAG storage, together with the increased transdifferentiation of white adipocytes to ‘beige’ or ‘brite’ adipocytes, is consistent with an increased demand for heat production (Barbatelli et al. 2010, Cinti 2011, Schultz et al. 2013). Similarly, the increased surface temperature in the head and interscapular regions (directly above the isBAT) in PWS-ICdel mice indicates an elevation in non-shivering thermogenesis. In this context, it should be noted that although reduced locomotor activity in PWS-ICdel mice may increase the infra-red signal measured, this does not result in an increase in surface temperature in those regions not associated with BAT activity, or in radiant temperature, which would be particularly susceptible to a reduction in movement. Thus, despite an absence of increased UCP1 expression, the classic marker of thermogenic activity, in PWS-ICdel mice (Fig. 3B), our data indicate that lipid utilisation in BAT is elevated in this model.
Evidence of elevated thermogenesis in PWS is sketchy, but our data extend previous reports that Snord116del mice show elevated daytime subcutaneous temperature (Lassi et al. 2016) and an improved ability to maintain core body temperature on cold exposure (Ding et al. 2008). In humans, a small but statistically significant proportion of PWS children are reported to experience persistent (Ince et al. 2005) or episodic hypothermia (surface temperature <94°F) (Williams et al. 1994), whereas fingertip temperature is unaltered (DiMario & Burleson 2002). As BAT is now emerging as a potentially important tissue in adult humans, particularly in the context of obesity (Cannon & Nedergaard 2010, Cinti 2011), a comprehensive analysis of BAT function in individuals with PWS is now required.
Elevated thermogenesis in PWS-ICdel mice is potentially significant because elevating BAT activity is highly effective in sequestering circulating lipid (Bartelt et al. 2011), thereby ‘starving’ WAT of energy substrate. When compounded by the increased energy utilisation of the more abundant ‘beige’/‘brite’ adipocytes within the WAT stores, this could result in the intra-abdominal leanness observed. To test this hypothesis, we examined the adiposity in PWS-ICdel mice after suppressing thermogenic activity in a thermoneutral environment. Although the thermoneutral set point may be higher in smaller PWS-ICdel mice (due to their increased surface area to volume ratio (Speakman & Keijer 2012)), maintaining mice at 30°C was clearly effective in suppressing thermogenesis, BAT mass increasing in WT and in PWS-ICdel males (Fig. 3A). However, this failed to induce a corresponding elevation in WAT mass, body weight gain only being elevated transiently in WT females. This surprising outcome is most likely due to the influence of ambient temperature on food intake, proportionate hyperphagia in PWS-ICdel males being abolished at thermoneutrality. Although this may suggest that simple elevations in ambient temperature may reduce orexigenic drive in human PWS, it demonstrates that elevated thermogenesis is not the primary cause of abdominal leanness in PWS-ICdel mice.
In the absence of reduced energy intake/absorption or a causal increase in energy expenditure, leanness may result from a primary failure of lipid storage in WAT. Storage capacity is determined by adipogenesis (the terminal differentiation of pre-adipocytes), whereas lipid accumulates in response to increased substrate supply/uptake, de novo lipogenesis and/or decreased lipolysis or lipid export, resulting in hypertrophy (Wells 2009).
We did not quantify intra-abdominal adipogenesis directly, but the reduced adiposity in the tibial marrow of PWS-ICdel mice was partly due to a 50% reduction in adipocyte number (data not shown). Evidence of reduced adipogenesis in subcutaneous WAT has recently been reported in human PWS, adipocyte progenitor number being halved (Cadoudal et al. 2014). These findings are consistent with a reduction in circulating GH, as GH promotes pre-adipocyte differentiation (Guller et al. 1989), and GH replacement in children with PWS restores pre-adipocyte numbers (Cadoudal et al. 2014).
Despite a reduction in lipid storage capacity, humans with PWS still develop obesity, whereas in PWS-ICdel mice, intra-abdominal adipocyte size is reduced by 69% (Fig. 1E). With lipolysis and lipid export apparently unaffected (as indicated by normal circulating FFAs and normal lipolytic responses in cultured human adipocytes (Cadoudal et al. 2014)), the only option remaining is a failure of lipid uptake and/or synthesis. To test this, we monitored weight gain in mice maintained on standard and high-fat diets. Although we acknowledge the limitation of short time periods used, it is remarkable that despite increased caloric intake on the high-fat diet, PWS-ICdel males, unlike WT littermates, failed to gain weight. When taken together with the reduced adipocyte size and the absence of elevated WAT mass at thermoneutrality, this implies that PWS-ICdel adipocytes fail to import energy substrate.
In summary, when considered alongside our previous studies, the current data indicate that PWS-ICdel mice display many of the endocrine and metabolic characteristics of human PWS, including marked hyperghrelinaemia and proportionate hyperphagia (Fig. 5). However, despite these obesogenic influences (Thompson et al. 2004, Wells 2009, Overton 2010), PWS-ICdel mice show profound intra-abdominal leanness. Although this leanness may arise in part from a reduction in lipid storage capacity, the primary cause appears to be a failure of lipid accumulation in white adipocytes. Intra-abdominal leanness may result in elevated food hoarding and a compensatory increase in thermogenesis. Given our demonstration that the obesogenic environment of PWS may be overcome, determination of the functionality of parallel mechanisms in mice and humans may help to alleviate the morbid obesity usually present in children with this condition.
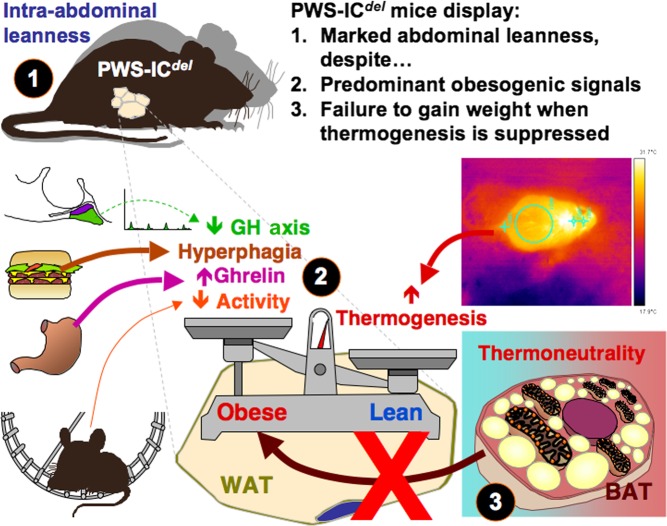
Figure 5.
Mechanisms of leanness in PWS-ICdel mice. PWS-ICdel mice display marked abdominal leanness (1; current study) despite a preponderance of obesogenic signals (underactive GH axis (current study), proportionate hyperphagia (with pronounced food hoarding) (current study; Davies et al. 2015), hyperghrelinaemia (current study; Davies et al. 2015) and reduced physical activity (Doe et al. 2009, Relkovic et al. 2010)) (2). Suppression of elevated thermogenesis (current study) in brown adipose tissue (BAT) by maintenance in a thermoneutral environment (3) fails to elevate white adipose tissue (WAT) mass (current study). A full colour version of this figure is available at http://dx.doi.org/10.1530/JOE-16-0367.
Supplementary data
This is linked to the online version of the paper at http://dx.doi.org/10.1530/JOE-16-0367.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This works was supported a Research Council UK Dorothy Hodgkin Postgraduate Award in collaboration with GlaxoSmithKline (to D R); the Prader–Willi Syndrome Association UK (to A R I and T W), Biotechnology and Biological Sciences Research Council (to A R I; BB/J016756/1) and Foundation for Prader–Willi Research (to T W); and Seed Corn Funding (Schools of Biosciences and the MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University) (to T W and A R I).
Acknowledgements
The authors thank JBIOS staff and Derek Scarborough (Cardiff University) for excellent technical support and Sachiko Nishimura (School of Optometry and Vision Sciences, Cardiff University) and Prof. Sam Evans (School of Engineering, Cardiff University) for thermal image acquisition and quantification.
References
- Barbatelli G, Murano I, Madsen L, Hao Q, Jimenez M, Kristiansen K, Giacobino JP, De Matteis R, Cinti S. 2010. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by brown adipocyte transdifferentiation. American Journal of Physiology: Endocrinology and Metabolism 298 E1244–E1253. ( 10.1152/ajpendo.00600.2009) [DOI] [PubMed] [Google Scholar]
- Bartelt A, Bruns OT, Reimer R, Hohenberg H, Ittrich H, Peldschus K, Kaul MG, Tromsdorf UI, Weller H, Waurisch C, et al. 2011. Brown adipose tissue activity controls triglyceride clearance. Nature Medicine 17 200–205. ( 10.1038/nm.2297) [DOI] [PubMed] [Google Scholar]
- Bervini S, Herzog H. 2013. Mouse models of Prader-Willi syndrome: a systematic review. Frontiers in Neuroendocrinology 34 107–119. ( 10.1016/j.yfrne.2013.01.002) [DOI] [PubMed] [Google Scholar]
- Bischof JM, Stewart CL, Wevrick R. 2007. Inactivation of the mouse Magel2 gene results in growth abnormalities similar to Prader-Willi syndrome. Human Molecular Genetics 16 2713–2719. ( 10.1093/hmg/ddm225) [DOI] [PubMed] [Google Scholar]
- Bizzari C, Rigamonti AE, Luce A, Cappa M, Cella SG, Berini J, Sartorio A, Müller EE, Salvatoni A. 2010. Children with Prader-Willi syndrome exhibit more evident meal-induced responses in plasma ghrelin and peptide YY levels than obese and lean children. European Journal of Endocrinology 162 499–505. ( 10.1530/EJE-09-1033) [DOI] [PubMed] [Google Scholar]
- Butler M. 1990. Prader-Willi syndrome: current understanding of cause and diagnosis. American Journal of Medical Genetics 35 319–332. ( 10.1002/ajmg.1320350306) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadoudal T, Buléon M, Segenès C, Diene G, Desneulin F, Molinas C, Eddiry S, Conte-Auriol F, Daviaud D, Martin PG, et al. 2014. Impairment of adipose tissue in Prader-Willi syndrome rescued by growth hormone treatment. International Journal of Obesity 38 1234–1240. ( 10.1038/ijo.2014.3) [DOI] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. 2010. Metabolic consequences of the presence or absence of the thermogenic capacity of brown adipose tissue in mice (and probably humans). International Journal of Obesity 34 S7–S16. ( 10.1038/ijo.2010.177) [DOI] [PubMed] [Google Scholar]
- Chamberlain SJ, Johnstone KA, DuBose AJ, Bartolomei MS, Resnick JL, Brannan CI. 2004. Evidence for genetic modifiers of postnatal lethality in PWS-IC deletion mice. Human Molecular Genetics 13 2971–2977. ( 10.1093/hmg/ddh314) [DOI] [PubMed] [Google Scholar]
- Cinti S. 2011. Between brown and white: novel aspects of adipocyte differentiation. Annals of Medicine 43 104–115. ( 10.3109/07853890.2010.535557) [DOI] [PubMed] [Google Scholar]
- Cummings DE, Clement K, Purnell JQ, Vaisse C, Foster KE, Frayo RS, Schwartz MW, Basdevant A, Weigle DS. 2002. Elevated plasma ghrelin levels in Prader-Willi syndrome. Nature Medicine 8 643–644. ( 10.1038/nm0702-643) [DOI] [PubMed] [Google Scholar]
- Dailey ME, Bartness TJ. 2008. Fat pad-specific effects of lipectomy on foraging, food hoarding, and food intake. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology 294 R321–R328. ( 10.1152/ajpregu.00230.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies JR, Humby T, Dwyer DM, Garfield AS, Furby H, Wilkinson AL, Wells T, Isles AR. 2015. Calorie seeking, but no hedonic response, contributes to hyperphagia in a mouse model for Prader-WIlli Syndrome. European Journal of Neuroscience 42 2105–2113. ( 10.1111/ejn.12972) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies JS, Kotokorpi P, Eccles SR, Barnes SK, Tokarczuk PF, Allen SK, Whitworth HS, Guschina IA, Evans BA, Mode A, et al. 2009. Ghrelin induces abdominal obesity via GHS-R-dependent lipid retention. Molecular Endocrinology 23 914–924. ( 10.1210/me.2008-0432) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon RMJ. 2006. Assessing hoarding in mice. Nature Protocols 1 2828–2830. ( 10.1038/nprot.2006.171) [DOI] [PubMed] [Google Scholar]
- DelParigi A, Tschöp M, Heiman ML, Salbe AD, Vozarova B, Sell SM, Bunt JC, Tataranni PA. 2002. High circulating ghrelin: a potential cause for hyperphagia and obesity in Prader-Willi syndrome. Journal of Clinical Endocrinology and Metabolism 87 5461–5464. ( 10.1210/jc.2002-020871) [DOI] [PubMed] [Google Scholar]
- DiMario FJ, Jr, Burleson JA. 2002. Cutaneous blood flow and thermoregulation in Prader-Willi syndrome patients. Pediatric Neurology 26 130–133. ( 10.1016/S0887-8994(01)00386-1) [DOI] [PubMed] [Google Scholar]
- Ding F, Li HH, Zhang S, Solomon NM, Camper SA, Cohen P, Francke U. 2008. SnoRNA Snord116 (Pwcr1/MBII-85) deletion causes growth deficiency and hyperphagia in mice. PLoS ONE 3 e1709 ( 10.1371/journal.pone.0001709) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doe CM, Relkovic D, Garfield AS, Dalley JW, Theobald DE, Humby T, Wilkinson LS, Isles AR. 2009. Loss of the imprinted snoRNA mbii-52 leads to increased 5htr2c pre-RNA editing and altered 5HT2CR-mediated behaviour. Human Molecular Genetics 18 2140–2148. ( 10.1093/hmg/ddp137) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdie-Lalena CR, Holm VA, Kelly PC, Frayo RS, Cummings DE. 2006. Ghrelin levels in young children with Prader-Willi syndrome. Journal of Pediatrics 149 199–204. ( 10.1016/j.jpeds.2006.04.011) [DOI] [PubMed] [Google Scholar]
- Ge Y, Ohta T, Driscoll DJ, Nicholls RD, Kalra SP. 2002. Anorexigenic melanocort signalling in the hypothalamus is augmented in association with failure-to-thrive in a transgenic mouse model for Prader-Willi syndrome. Brain Research 957 42–45. ( 10.1016/S0006-8993(02)03583-7) [DOI] [PubMed] [Google Scholar]
- Glad CA, Kitchen EE, Russ GC, Harris SM, Davies JS, Gevers EF, Gabrielsson BG, Wells T. 2011. Reverse feeding suppresses the activity of the GH axis and induces a preobesogenic state. Endocrinology 152 689–882. ( 10.1210/en.2010-0768) [DOI] [PubMed] [Google Scholar]
- Goldstone AP, Thomas EL, Brynes AE, Bell JD, Frost G, Saeed N, Hajnal JV, Holland A, Bloom SR. 2001. Visceral adipose tissue and metabolic complications of obesity are reduced in Prader-Willi syndrome female adults: evidence for novel influences on body fat distribution. Journal of Clinical Endocrinology and Metabolism 86 4330–4338. ( 10.1210/jcem.86.9.7814) [DOI] [PubMed] [Google Scholar]
- Goldstone AP, Thomas EL, Brynes AE, Castroman G, Edwards R, Ghatei MA, Frost G, Holland AJ, Grossman AB, Korbonits M, et al. 2004. Elevated fasting plasma ghrelin in Prader-Willi syndrome is not solely explained by their reduced visceral adiposity and insulin resistance. Journal of Clinical Endocrinology and Metabolism 89 1718–1726. ( 10.1210/jc.2003-031118) [DOI] [PubMed] [Google Scholar]
- Guller S, Sonenberg M, Wu KY, Szabo P, Corin RE. 1989. Growth hormone-dependent events in adipose differentiation of 3T3-F442A fibroblasts: modulation of macromolecular synthesis. Endocrinology 125 2360–2367. ( 10.1210/endo-125-5-2360) [DOI] [PubMed] [Google Scholar]
- Haqq AM, Farooqi IS, O’Rahilly S, Stadler DD, Rosenfeld RG, Pratt KL, LaFranchi SH, Purnell JQ. 2003. Serum ghrelin levels are inversely correlated with body mass index, age, ad insulin concentrations in normal children and are markedly increased in Prader-Willi syndrome. Journal of Clinical Endocrinology and Metabolism 88 174–178. ( 10.1210/jc.2002-021052) [DOI] [PubMed] [Google Scholar]
- Ince E, Çiftçi E, Tekin M, Kendirli T, Tutar E, Dalgiç S, Öncel S, Dogru Ü. 2005. Characteristics of hyperthermia and its complications in patients with Prader Willi syndrome. Pediatrics International 47 550–553. ( 10.1111/j.1442-200x.2005.02124.x) [DOI] [PubMed] [Google Scholar]
- Jones A, Glomset J. 1985. Chapter 4 Biosynthesis, function and metabolism of sterol esters. New Comprehensive Biochemistry 12 95–119. ( 10.1016/s0167-7306(08)60680-8) [DOI] [Google Scholar]
- Kates M. 1986. Techniques in Lipidology, 2nd ed. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- Keen-Rhinehart E, Bartness TJ. 2005. Peripheral ghrelin injections stimulate food intake, foraging and food hoarding in Siberian hamsters. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology 288 R716–R722. ( 10.1152/ajpregu.00705.2004) [DOI] [PubMed] [Google Scholar]
- Keen-Rinehart E, Dailey MJ, Bartness T. 2010. Physiological mechanisms for food-hoarding motivation in animals. Philosophical Transactions of the Royal Society B 365 961–975. ( 10.1098/rstb.2009.0225) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlov SV, Bogenpohl JW, Howell MP, Wevrick R, Panda S, Hogenesch JB, Muglia LJ, Van Gelder RN, Herzog ED, Stewart CL. 2007. The imprinted gene Magel2 regulates normal circadian output. Nature Genetics 39 1266–1272. ( 10.1038/ng2114) [DOI] [PubMed] [Google Scholar]
- Kuppens RJ, Delhanty PJ, Huisman TM, van der Lely AJ, Hokken-Koelega AC. 2016. Acylated and unacylated ghrelin during OGTT in Prader-Willi syndrome: support for normal response to food intake. Clinical Endocrinology 85 488–494. ( 10.1111/cen.13036) [DOI] [PubMed] [Google Scholar]
- Lassi G, Priano L, Maggi S, Garcia-Garcia C, Balzani E, El-Assawy N, Pagani M, Tinarelli F, Giardino D, Mauro A, et al. 2016. Deletion of the Snord116/SNORD116 alters sleep in mice and patients with Prader-Willi syndrome. Sleep 39 637–644. ( 10.5665/sleep.5542) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence CB, Snape AC, Baudoin FM, Luckman SM. 2002. Acute central ghrelin and GH secretagogues induce feeding and activate appetite centers. Endocrinology 143 155–162. ( 10.1210/endo.143.1.8561) [DOI] [PubMed] [Google Scholar]
- Meziane H, Schaller F, Bauer S, Villard C, Matarazzo V, Riet F, Guillon G, Lafitte D, Desarmenien MG, Tauber M, et al. 2015. An early postnatal oxytocin treatment prevents social and learning deficits in adult mice deficient in Magel2, a gene involved in Parder-Willi sundrome. Biological Psychiatry 78 85–94. ( 10.1016/j.biopsych.2014.11.010) [DOI] [PubMed] [Google Scholar]
- Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangaw K, Matsukura S. 2001. A role for ghrelin in the central regulation of feeding. Nature 409 194–198. ( 10.1038/35051587) [DOI] [PubMed] [Google Scholar]
- Overton JM. 2010. Phenotyping small animals as models for the human metabolic syndrome: thermoneutrality matters. International Journal of Obesity 34 S53–S58. ( 10.1038/ijo.2010.240) [DOI] [PubMed] [Google Scholar]
- Peters J. 2008. Prader-Willi and snoRNAs. Nature Genetics 40 688–689. ( 10.1038/ng0608-688) [DOI] [PubMed] [Google Scholar]
- Qi Y, Purtell L, Fu M, Lee MJ, Aepler J, Zhang L, Loh K, Enriquez RF, Baldock PA, Zolotukhin S, et al. 2016. Snord116 is critical in the regulation of food intake and body weight. Scientific Reports 6 18614 ( 10.1038/srep18614) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relkovic D, Doe CM, Humby T, Johnstone KA, Resnick JL, Holland AJ, Hagan JJ, Wilkinson LS, Isles AR. 2010. Behavioural and cognitive abnormalities in an imprinting centre deletion mouse model for Prader-Willi syndrome. European Journal of Neuroscience 31 156–164. ( 10.1111/j.1460-9568.2009.07048.x) [DOI] [PubMed] [Google Scholar]
- Sahoo T, del Gaudio D, German JR, Shinawi M, Peters SU, Person RE, Garnica A, Cheung SW, Beaudet AL. 2008. Prader-Willi phenotype caused by deficiency for the HBII-85 C/D box small nucleolar RNA cluster. Nature Genetics 40 719–721. ( 10.1038/ng.158) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller F, Watrin F, Sturny R, Massacrier A, Szepetowski P, Muscatelli F. 2010. A single postnatal injection of oxytocin rescues the lethal feeding behaviour in mouse newborns deficient for the imprinted Magel2 gene. Human Molecular Genetics 19 4895–4905. ( 10.1093/hmg/ddq424) [DOI] [PubMed] [Google Scholar]
- Schultz TJ, Huang P, Huang TL, Xue R, McDougall LE, Townsend KL, Cypess AM, Mishina Y, Gussoni E, Tseng Y. 2013. Brown-fat paucity due to impaired BMP signalling induces compensatory browning of white fat. Nature 495 379–383. ( 10.1038/nature11943) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speakman JR, Keijer J. 2012. Not so hot: optimal housing temperatures for mice to mimic the thermal environment of humans. Molecular Metabolism 21 5–9. ( 10.1016/j.molmet.2012.10.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan M, Ji H, Simmons RA, Cummings DE, Ahima RS, Friedman MI, Nicholls RD. 2005. Hormonal and metabolic defects in a prader-willi syndrome mouse model with neonatal failure to thrive. Endocrinology 146 4377–4385. ( 10.1210/en.2005-0371) [DOI] [PubMed] [Google Scholar]
- Swaab DF, Purba JS, Hofman MA. 1995. Alterations in the hypothalamic parventricular nucleus and its oxytocin neurons (putative satiety cells) in Prader-Willi syndrome: a study of five cases. Journal of Clinical Endocrinology and Metabolism 80 573–579. ( 10.1210/jcem.80.2.7852523) [DOI] [PubMed] [Google Scholar]
- Thompson NM, Gill DA, Davies R, Loveridge N, Houston PA, Robinson ICAF, Wells T. 2004. Ghrelin and des-octanoyl ghrelin promote adipogenesis directly in-vivo by a mechanism independent of the type 1a growth hormone secretagogue receptor. Endocrinology 145 234–242. ( 10.1210/en.2003-0899) [DOI] [PubMed] [Google Scholar]
- Wells T. 2009. Ghrelin – defender of fat. Progress in Lipid Research 48 257–274. ( 10.1016/j.plipres.2009.04.002) [DOI] [PubMed] [Google Scholar]
- Wells T, Davies JR, Guschina IA, Ball DJ, Davies JS, Davies VJ, Evans BAJ, Votruba M. 2012. Opa3, a novel regulator of mitochondrial function, controls thermogenesis and abdominal fat mass in a mouse model for Costeff syndrome. Human Molecular Genetics 21 4836–4844. ( 10.1093/hmg/dds315) [DOI] [PubMed] [Google Scholar]
- Williams MS, Rooney BL, Williams J, Josephson K, Pauli R. 1994. Investigation of thermoregulatory characteristics in patients with Prader-Willi syndrome. American Journal of Medical Genetics 49 302–307. ( 10.1002/ajmg.1320490312) [DOI] [PubMed] [Google Scholar]
- Yang T, Adamson TE, Resnick JL, Leff S, Wevrick R, Francke U, Jenkins NA, Copeland NG, Brannan CI. 1998. A mouse model for Prader-Willi syndrome imprinting-centre mutations. Nature Genetics 19 25–31. ( 10.1038/ng0598-25) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a