Abstract
Thiyl radicals are important intermediates in the redox biology and chemistry of thiols. These radicals can react via hydrogen transfer with various C-H bonds in peptides and proteins, leading to the generation of carbon-centered radicals, and, potentially, to irreversible protein damage. This review summarizes quantitative information on reaction kinetics and product formation, and discusses the significance of these reactions for protein degradation induced by thiyl radical formation.
Keywords: Thiyl radicals, hydrogen atom transfer, carbon-centered radicals, D-amino acids, glutathione
Introduction
An increased level of protein oxidation is an important hallmark of biological oxidative stress [1–7]. Protein oxidation is the result of increased levels of reactive oxygen and nitrogen species (ROS and RNS), generated via various enzymatic and non-enzymatic pathways, and a manifold of different reactions of these ROS and RNS with proteins. Usually, aromatic and sulfur-containing amino acids are more susceptible to attack by ROS and RNS [8]. However, depending on the nature of ROS and RNS, also aliphatic amino acids may be targeted. With few exceptions, the reactions of ROS and RNS with amino acids generate reactive intermediates, which can subsequently react with other, secondary targets [9]. Such secondary reactions can “move” the final reaction products away from the sites of initial attack. In addition, such secondary reactions can trigger processes, which would possibly not have been initiated by the primary reactions of ROS and RNS. The current review focuses specifically on this area, summarizing recent results on hydrogen abstraction reactions of thiyl radicals within proteins and model peptides. These results suggest that secondary, hydrogen abstraction, reactions of thiyl radicals may have the potential for extensive, irreversible protein damage. In the following, we will briefly introduce reactions which can lead to the formation of thiyl radicals in physiologic and pathologic environment, summarize our current knowledge on hydrogen transfer reactions of thiyl radicals, and conclude with a discussion of the relevance of these reactions for protein degradation.
Formation of thiyl radicals
The redox chemistry of thiols has been detailed in many articles [10–19] and shall only be reviewed here with regard to thiyl radical formation. Chemically, protein thiyl radicals are generated via many pathways. While physiologically only a few of them may be significant [11, 19, 20] additional pathways will operate under pathologic conditions, or during the exposure of organisms to exogenous stresses such as, e.g., ionizing radiation. Protein thiyl radicals are generated during the reaction of hydrogen peroxide with heme proteins, including hemoglobin [21–23], and similar pathways are expected for non-heme iron complexes and other protein-associated redox-active transition metals. Significant levels of redox-active transition metals can be present under conditions of iron overload [24], neurodegenerative diseases [25], and as a result of over exposure to other metals, e.g. in the case of manganese [26, 27]. Physiologically, peptide and protein thiyl radicals can form through electron/hydrogen transfer between Cys and tyrosyl radicals [28, 29] or carbon-centered radicals [30]. Chemically, thiyl radicals can be generated by reaction of Cys with tryptophan radicals/radical cations [31], peroxyl radicals [32] (including superoxide [33, 34], where superoxide-induced protein thiyl radical formation has been implicated in S-glutathionylation of mitochondrial complex I [35, 36] and endothelial nitric oxide synthase [37, 38]), carbon-centered radicals [30], nitrogen dioxide (•NO2) [39, 40], carbonate radical (CO3•−) [40] and the hydroxyl radical (HO•). Protein thiyl radicals have been involved in mechanisms leading to S-nitrosation, and specifically in mechanisms of nucleotide exchange of various GTPases [41–47].
Hydrogen transfer reactions of thiyl radicals
Hydrogen abstraction by thiyl radicals from organic substrates had been documented decades ago [48, 49], and thiols had been added to synthetic processes to facilitate hydrogen transfer via “polarity reversal catalysis” [50, 51]. Here, a primary organic radical (R1•) abstracts a hydrogen from a thiol, yielding a thiyl radical, which, in turn, reacts via hydrogen abstraction with a second organic substrate, R2-H. The net reaction is hydrogen transfer between R1• and R2-H, catalyzed by the thiol.
| (1) |
| (2) |
It is this concept of “polarity reversal catalysis”, which, when extended and applied to proteins, suggests that thiyl radicals could be efficient promotors of protein damage, even by radicals or oxidants, which would not react rapidly with most amino acids. A simplified reaction sequence for such protein damage is displayed in reaction sequence 3, 4 and 7. Cys thiyl radicals (Cys-S•) are generated through reaction 3, and a measurable fraction of these thiyl radicals reacts with amino acid (AA) C-H bonds (reaction 4) rather than with one of the biologically available and abundant antioxidants, glutathione (GSH) and ascorbate (Asc−) (reactions 5 and 6, where Asc• represents an ascorbyl radical). In competition to the reverse reaction (−4), the amino acid radical, (AA)C•, must convert into another intermediate or product (reaction 7; see below), which effectively removes (AA)C• from equilibrium 4. For simplicity, the reaction of Cys-S• with molecular oxygen [17] was omitted in this reaction scheme, as this reaction is reversible; however, it should be noted that the radical anion complex [Cys-S-S-G]−• will efficiently react with oxygen.
| (3) |
| (4) |
| (5) |
| (6) |
| (7) |
Reaction sequence 3, 4 and 7 can proceed intra- and intermolecularly: rate constants k4 = 103–105 M−1s−1 have been measured for intermolecular hydrogen transfer reactions [52, 53] while intramolecular reactions (such as reaction 8, measured for several model peptides) proceed with k8 ≈ 105 s−1 (and k−8 ≈ 106 s−1) [54].
| (8) |
The actual extent to which reaction sequence 3, 4 and 7 may lead to protein damage will be defined by the respective rate constants and the availability of glutathione and ascorbate. Physiologic concentrations of glutathione and ascorbate will favor reactions 5 and 6 if these antioxidants have access to Cys-S•. However, this may not always be the case with proteins where Cys residues are frequently buried in the interior [55] (see also below). A role for thiols [56, 57] in the protection of cells against ROS (for example during exposure to ionizing radiation) has been established. However, it has been realized that secondary radicals from thiol or ascorbate oxidation may induce damage as well, for example during γ-irradiation of solutions containing deoxyguanosine [58]. Radiation chemistry, ESR, NMR and mass spectrometry experiments have provided complimentary evidence for intramolecular hydrogen atom transfer reactions between thiyl radicals and C-H bonds in model peptides [54, 59, 60] and glutathione (GSH) [30, 61–65]. The equilibria between thiyl and carbon-centered radicals were directly monitored by ESR spectrometry, while NMR and mass spectrometry monitored the loss of proton signal or covalent incorporation of deuterium into reaction products, respectively, when reactions were carried out in D2O. In these experiments, covalent deuterium incorporation is the result of reactions (9)-(12).
| (9) |
| (10) |
| (11) |
| (12) |
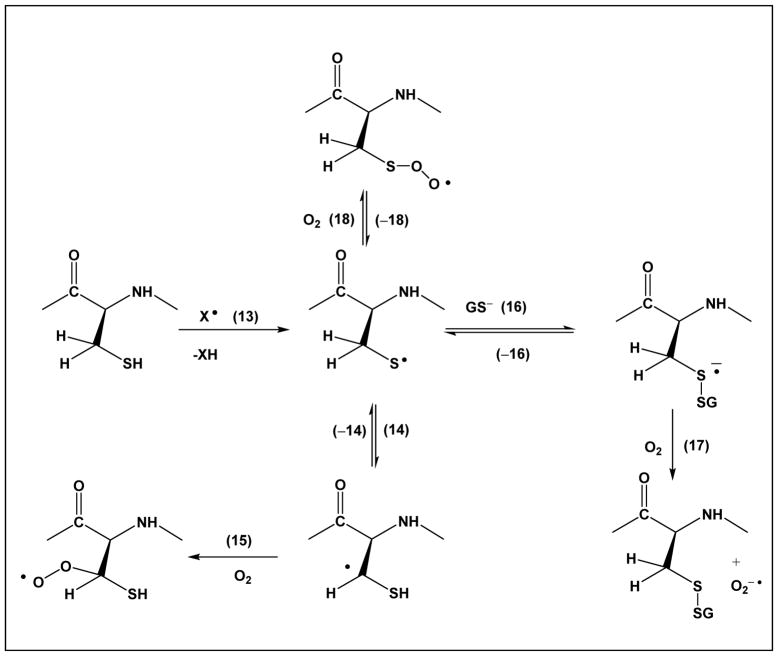
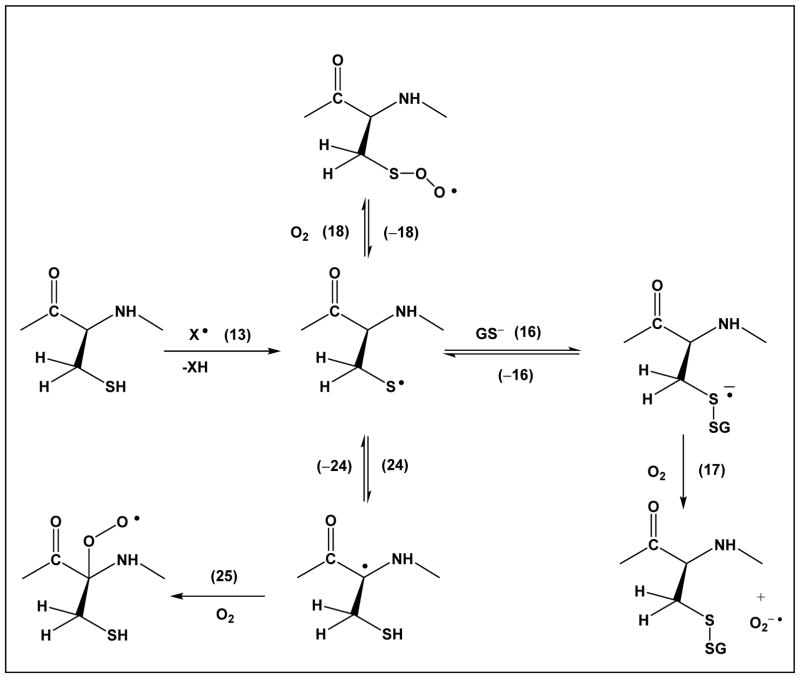
Importantly, thiyl radicals of glutathione react with the C-H bonds of all three amino acids present in glutathione: γ-Glu, Cys and Gly [62, 63]. Here, the intramolecular reactions of thiyl radicals with C-H bonds of the Cys residue itself suggests 1,2 – and 1,3-hydrogen transfer processes (Scheme 1, equilibrium 14, and Scheme 3, equilibrium 24, respectively), reactions, which were confirmed recently by pulse radiolysis studies of a series of model compounds.
Scheme 1.
1,2-Hydrogen transfer of Cys thiyl radicals and competitive reactions
Scheme 3.
1,3-Hydrogen transfer of Cys thiyl radicals and competitive reactions
In equilibrium 14, a thiyl radical exists in equilibrium with an α-mercaptoalkyl radical through a formal 1,2-hydrogen transfer. Rate constants for this equilibrium are on the order of k14 ≈ 105 s−1 and k−14 ≈ 1.5×105 s−1 at acidic pH [66]. When thiyl radicals were generated from glutathione in D2O, we observed covalent H/D-exchange for a total of two C-H bonds within Cys, consistent with at least one deuterium incorporated into the original βC-H bond (and the other deuterium either into the second βC-H bond or the αC-H bond) [62]. Complimentary evidence for such hydrogen transfer processes comes from recent ESR spectroscopy studies on E. coli class III ribonucleotide reductase, where covalent deuterium incorporation into the βC-H bond of Cys-175 was observed during experiments carried out in D2O [67]. Theoretical calculations suggest that equilibrium 19 should be located predominantly on the left hand side [68], and analogous calculations were performed for the equilibrium between HO-CH2CH2S• and HO-CH2-•CH-SH [69]. However, more recent data by Morris et al. indicate that deprotonation of the mercapto group (equilibrium 20) lowers the C-H bond energy (of CH3S•) by ca. 49.4 kJ/mol compared to that of CH3SH [70]. By analogy to carbon-centered radicals from aliphatic alcohols [71, 72], α-mercaptoalkyl radicals may have significantly lower pKa values of the mercapto group compared to alkyl mercaptanes, i.e. the deprotonation reaction 21 is expected to shift equilibrium 19 towards the right hand side.
| (19) |
| (20) |
| (21) |
In fact, the covalent H/D-exchange at the βC-H bond of Cys-175 of ribonucleotide reductase was rationalized by the intermediary formation of a deprotonated α-mercaptoalkyl radical [67].
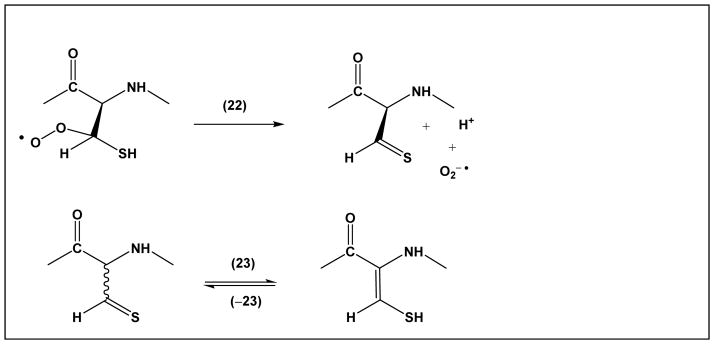
For the covalent modifications of proteins, the potential formation of α-mercaptoalkyl radicals is significant. Addition of oxygen (reaction 15) leads to a peroxyl radical, which may react via hydrogen abstraction or electron transfer with other amino acids, or via elimination of superoxide (Scheme 2, reaction 22). Hydrogen abstraction and electron transfer reactions will lead to additional protein radicals, while reaction 22 yields thioaldehyde, a tautomeric form of dehydrocysteine (equilibrium 23).
Scheme 2.
Formation and tautomers of Cys thioaldehyde
Such products were, in fact, observed when thiyl radicals were generated in several model peptides [59, 60, 73, 74] and proteins [75], but, interestingly, also in iron regulatory protein 2 (IRP2), potentially as a result of iron-dependent degradation [76, 77]. Importantly, both the reactions of thiyl radicals of model peptides and Cys oxidation in IRP2 also reveal the conversion of Cys to Ala. The mechanism for Ala formation likely involves β-elimination, which may proceed via another radical intermediate, αC• radicals (see below).
Noteworthy, the reaction of α-mercaptoalkyl radicals with oxygen proceeds in competition with other pathways. The reaction of thiyl radicals with molecular oxygen [17] proceeds with k18 = 2.2×109 M−1s−1 (rate constant measured for the addition of oxygen to the thiyl radical from β-mercaptoethanol) but the efficient reverse reaction, k−18 = 6.2×105 s−1, likely precludes significant product formation via this pathway. Reaction 16, with the deprotonated form of glutathione, GS−, proceeds with k16 ca. 4.5×108 M−1s−1 [65], and generates a disulfide radical anion. Analogous reactions will occur with other protein Cys residues, i.e. can proceed intramolecularly when the protein structure permits. While the reverse reaction proceeds with k−16 ca. 2×105 s−1 [65], the efficient reaction of the disulfide radical anion with molecular oxygen (reaction 17) will generate significant yields of disulfide. Schemes 1 and 3 do not display any reaction of thiyl radicals with ascorbate. While this reaction is efficient, it will only occur with thiyl radicals of protein Cys residues which can be accessed by ascorbate. As frequently Cys residues are buried [55], accessibility by ascorbate cannot be necessarily taken for granted.
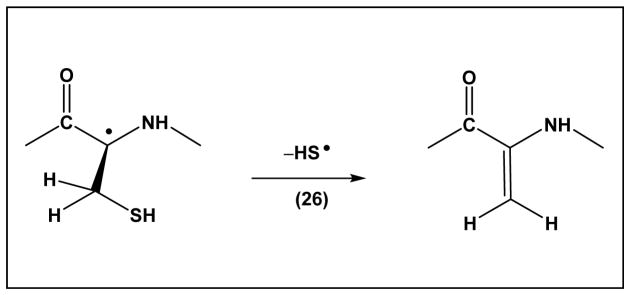
Scheme 3, equilibrium 24, displays a 1,3-hydrogen transfer reaction of the Cys thiyl radical, where by analogy to reactions of penicillamine thiyl radicals, k24 ≈ 8×104 s−1 and k−24 ≈ 1.4×106 s−1 [66]. This reaction leads to αC• radicals, which can react with molecular oxygen (reaction 25). The generation of peroxyl radicals at the αC position will lead to additional hydrogen and electron transfer processes, as well as fragmentation reactions [78]. Moreover, the αC• radicals can eliminate •SH (Scheme 4, reaction 26), which proceeds with k26 ≈ 5×103 s−1 [66]. The latter reaction generates dehydroalanine, an electrophile which can cross-link with nucleophilic amino acids such as Cys and Lys.
Scheme 4.
Elimination of HS• from Cys αC• radical
Beyond 1,2- and 1,3-hydrogen transfer reactions, thiyl radicals will be able to react with C-H bonds of other amino acids when the protein structure permits, i.e. via “long range hydrogen transfer” (based on the position of amino acids within the protein sequence) (reaction 8). Theoretical calculations by Rauk and co-workers show that thiyl radicals should react with αC-H bonds of protein amino acids when located in flexible or β-sheet structures but not within α-helices [79–82].
Our data on thiyl radical reactions within insulin are consistent with this prediction [83]. Importantly, thiyl radicals can react with both αC-H and side chain C-H bonds [52, 53]. As a consequence, these inter- and intramolecular hydrogen transfer reactions equilibria generate significant fractions of intermediary carbon-centered radicals. As shown for αC• and βC• radicals in Schemes 3 and 1, respectively, but generally applicable to any carbon-centered radical, these are precursors for peroxyl radicals (reaction 27) and the various routes of peroxyl radical chemistry[78].
| (27) |
The extent to which peroxyl radical formation will compete against alternative pathways will depend on the oxygen concentration, which, in tissue is in the range of ca. 3–70 μM [84–86], and oxygen diffusion across the three-dimensional structure of the proteins [87].
The reversibility of the 1,3-hydrogen transfer (reaction 24) and of any long-range hydrogen transfer between thiyl radicals and αC-H bonds bear the potential for epimerization. In fact, D-alanine formation was detected in model peptides [60], and during light-induced thiyl radical generation in IgG1[88]. These observations are consistent with synthetic applications, where thiyl radicals where used for the racemization of amines [89].
Significance for protein degradation
Based on the reactions summarized above, any reactive species capable of forming a protein thiyl radical is theoretically able to induce protein degradation via the general reaction sequence 3, 4 and 7 (where reaction 27 represents one potential pathway for the irreversible conversion of an amino acid radical (AA)C• according to the general reaction 7). An interesting case can be made for superoxide: superoxide would not efficiently react with any of the essential amino acids except Cys (for which rate constants have been measured on the order of 102–103 M−1s−1 [33, 34]). Part of the reaction of superoxide with Cys generates thiyl radicals, and, therefore, via the mechanisms summarized above, superoxide is theoretically able to induce damage of aliphatic amino acids, promoted by thiyl radicals. To what extent superoxide would practically react with protein thiols certainly depends on the environment and especially the availability of superoxide dismutases.
However, in this context it is important to note that a role of superoxide was discussed with respect to thiyl radical formation in endothelial NOS [37, 38] and mitochondrial NADH dehydrogenase [90], where protein thiyl radicals appear to play a role in self-inactivation [90]. In the latter case the authors utilized immunospin-trapping for the localization of radicals on Cys and Tyr, suggesting a hydrogen transfer equilibrium between radicals from Cys and Tyr. Additional examples for thiyl radical-dependent protein degradation are forthcoming for 3-glyceraldehyde phosphate dehydrogenase (GAPDH) and the sarco/endoplasmic reticulum Ca-ATPase (SERCA) (Mozziconacci and Schöneich, unpublished results). Importantly, hydrogen transfer reactions to protein thiyl radicals are not restricted to proteins but may proceed between Cys-S• and lipids, carbohydrates and DNA, provided close contact of Cys-S• with the respective C-H bonds such as potentially present in protein complexes with these molecules.
Acknowledgments
I would like to acknowledge the contributions of various co-workers and collaborators to our research on thiyl radicals, including Drs. D. Pogocki, V. Sharov, O. Mozziconacci, S. Zhou, B.A. Kerwin, Y. J. Wang, T. Nauser and W.H. Koppenol. Financial support by the NIH (P01AG12993), Amgen and Genentech is gratefully acknowledged.
References
- 1.Davies KJ. Protein damage and degradation by oxygen radicals. I. General aspects. J Biol Chem. 1987;262:9895–9901. [PubMed] [Google Scholar]
- 2.Davies KJ, Delsignore ME, Lin SW. Protein damage and degradation by oxygen radicals. II. Modification of amino acids. J Biol Chem. 1987;262:9902–9907. [PubMed] [Google Scholar]
- 3.Davies KJ, Delsignore ME. Protein damage and degradation by oxygen radicals. III. Modification of secondary and tertiary structure. J Biol Chem. 1987;262:9908–9913. [PubMed] [Google Scholar]
- 4.Davies KJ, Goldberg AL. Proteins damaged by oxygen radicals are rapidly degraded in extracts of red blood cells. J Biol Chem. 1987;262:8227–8234. [PubMed] [Google Scholar]
- 5.Stadtman ER. Protein oxidation and aging. Science. 1992;257:1220–1224. doi: 10.1126/science.1355616. [DOI] [PubMed] [Google Scholar]
- 6.Stadtman ER, Oliver CN, Levine RL, Fucci L, Rivett AJ. Implication of protein oxidation in protein turnover, aging, and oxygen toxicity. Basic Life Sci. 1988;49:331–339. doi: 10.1007/978-1-4684-5568-7_50. [DOI] [PubMed] [Google Scholar]
- 7.Amici A, Levine RL, Tsai L, Stadtman ER. Conversion of amino acid residues in proteins and amino acid homopolymers to carbonyl derivatives by metal-catalyzed oxidation reactions. J Biol Chem. 1989;264:3341–3346. [PubMed] [Google Scholar]
- 8.Davies MJ. The oxidative environment and protein damage. Biochim Biophys Acta. 2005;1703:93–109. doi: 10.1016/j.bbapap.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 9.Neuzil J, Gebicki JM, Stocker R. Radical-induced chain oxidation of proteins and its inhibition by chain-breaking antioxidants. The Biochemical journal. 1993;293(Pt 3):601–606. doi: 10.1042/bj2930601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia-Santamarina S, Boronat S, Hidalgo E. Reversible cysteine oxidation in hydrogen peroxide sensing and signal transduction. Biochemistry. 2014;53:2560–2580. doi: 10.1021/bi401700f. [DOI] [PubMed] [Google Scholar]
- 11.Winterbourn CC, Hampton MB. Thiol chemistry and specificity in redox signaling. Free radical biology & medicine. 2008;45:549–561. doi: 10.1016/j.freeradbiomed.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Jones DP. Radical-free biology of oxidative stress. American journal of physiology Cell physiology. 2008;295:C849–C868. doi: 10.1152/ajpcell.00283.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schöneich C. Mechanisms of protein damage induced by cysteine thiyl radical formation. Chem Res Toxicol. 2008;21:1175–1179. doi: 10.1021/tx800005u. [DOI] [PubMed] [Google Scholar]
- 14.Akhlaq MS, Schuchmann HP, von Sonntag C. The reverse of the ‘repair’ reaction of thiols: H-abstraction at carbon by thiyl radicals. Int J Radiat Biol Relat Stud Phys Chem Med. 1987;51:91–102. doi: 10.1080/09553008714550531. [DOI] [PubMed] [Google Scholar]
- 15.Karoui H, Hogg N, Frejaville C, Tordo P, Kalyanaraman B. Characterization of sulfur-centered radical intermediates formed during the oxidation of thiols and sulfite by peroxynitrite. ESR-spin trapping and oxygen uptake studies. J Biol Chem. 1996;271:6000–6009. doi: 10.1074/jbc.271.11.6000. [DOI] [PubMed] [Google Scholar]
- 16.Sevilla MD, Becker D, Yan M. The formation and structure of the sulfoxyl radicals RSO(.), RSOO(.), RSO2(.), and RSO2OO(. ) from the reaction of cysteine, glutathione and penicillamine thiyl radicals with molecular oxygen. Int J Radiat Biol. 1990;57:65–81. doi: 10.1080/09553009014550351. [DOI] [PubMed] [Google Scholar]
- 17.Zhang X, Zhang N, Schuchmann H-P, von Sonntag C. Pulse radiolysis of 2-mercaptoethanol in oxygenated aqueous solution. Generation and reactions of the thiylperoxyl radical. Journal of Physical Chemistry. 1994;98:6541–6547. [Google Scholar]
- 18.Bonifacic M, Asmus KD. Adduct Formation and Absolute Rate Constants in the Displacement Reaction of Thiyl Radicals with Disulfides. Journal of Physical Chemistry. 1984;88:6286–6290. [Google Scholar]
- 19.Winterbourn CC. Are free radicals involved in thiol-based redox signaling? Free radical biology & medicine. 2015;80:164–170. doi: 10.1016/j.freeradbiomed.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 20.Nauser T, Koppenol WH, Schöneich C. Protein thiyl radical reactions and product formation: a kinetic simulation. Free radical biology & medicine. 2015;80:158–163. doi: 10.1016/j.freeradbiomed.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maples KR, Kennedy CH, Jordan SJ, Mason RP. In vivo thiyl free radical formation from hemoglobin following administration of hydroperoxides. Archives of biochemistry and biophysics. 1990;277:402–409. doi: 10.1016/0003-9861(90)90596-q. [DOI] [PubMed] [Google Scholar]
- 22.Venters HD, Jr, Bonilla LE, Jensen T, Garner HP, Bordayo EZ, Najarian MM, et al. Heme from Alzheimer’s brain inhibits muscarinic receptor binding via thiyl radical generation. Brain research. 1997;764:93–100. doi: 10.1016/s0006-8993(97)00425-3. [DOI] [PubMed] [Google Scholar]
- 23.Svistunenko DA. Reaction of haem containing proteins and enzymes with hydroperoxides: the radical view. Biochim Biophys Acta. 2005;1707:127–155. doi: 10.1016/j.bbabio.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 24.Meneghini R. Iron homeostasis, oxidative stress, and DNA damage. Free Radic Biol Med. 1997;23:783–92. doi: 10.1016/s0891-5849(97)00016-6. [DOI] [PubMed] [Google Scholar]
- 25.Schrag M, Mueller C, Zabel M, Crofton A, Kirsch WM, Ghribi O, et al. Oxidative stress in blood in Alzheimer’s disease and mild cognitive impairment: a meta-analysis. Neurobiology of disease. 2013;59:100–110. doi: 10.1016/j.nbd.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 26.Chen P, Chakraborty S, Mukhopadhyay S, Lee E, Paoliello MM, Bowman AB, et al. Manganese Homeostasis in the Nervous System. J Neurochem. 2015 May 16; doi: 10.1111/jnc.13170. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen P, Parmalee N, Aschner M. Genetic factors and manganese-induced neurotoxicity. Front Genet. 2014;5:265. doi: 10.3389/fgene.2014.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petruk AA, Bartesaghi S, Trujillo M, Estrin DA, Murgida D, Kalyanaraman B, et al. Molecular basis of intramolecular electron transfer in proteins during radical-mediated oxidations: computer simulation studies in model tyrosine-cysteine peptides in solution. Arch Biochem Biophys. 2012;525:82–91. doi: 10.1016/j.abb.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pietraforte D, Minetti M. Direct ESR detection or peroxynitrite-induced tyrosine-centred protein radicals in human blood plasma. Biochem J. 1997;325( Pt 3):675–684. doi: 10.1042/bj3250675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grierson L, Hildenbrand K, Bothe E. Intramolecular transformation reaction of the glutathione thiyl radical into a non-sulphur-centred radical: a pulse-radiolysis and EPR study. Int J Radiat Biol. 1992;62:265–277. doi: 10.1080/09553009214552111. [DOI] [PubMed] [Google Scholar]
- 31.Chen Y, Barkley MD. Toward understanding tryptophan fluorescence in proteins. Biochemistry. 1998;37:9976–9982. doi: 10.1021/bi980274n. [DOI] [PubMed] [Google Scholar]
- 32.Hildenbrand K, Schulte-Frohlinde D. Time-resolved EPR studies on the reaction rates of peroxyl radicals of poly(acrylic acid) and of calf thymus DNA with glutathione. Re-examination of a rate constant for DNA. Int J Radiat Biol. 1997;71:377–385. doi: 10.1080/095530097143996. [DOI] [PubMed] [Google Scholar]
- 33.Jones CM, Lawrence A, Wardman P, Burkitt MJ. Kinetics of superoxide scavenging by glutathione: an evaluation of its role in the removal of mitochondrial superoxide. Biochem Soc Trans. 2003;31:1337–1339. doi: 10.1042/bst0311337. [DOI] [PubMed] [Google Scholar]
- 34.Jones CM, Lawrence A, Wardman P, Burkitt MJ. Electron paramagnetic resonance spin trapping investigation into the kinetics of glutathione oxidation by the superoxide radical: re-evaluation of the rate constant. Free Radic Biol Med. 2002;32:982–990. doi: 10.1016/s0891-5849(02)00791-8. [DOI] [PubMed] [Google Scholar]
- 35.Kang PT, Chen CL, Chen YR. Increased mitochondrial prooxidant activity mediates up-regulation of Complex I S-glutathionylation via protein thiyl radical in the murine heart of eNOS(−/−) Free Radic Biol Med. 2015;79:56–68. doi: 10.1016/j.freeradbiomed.2014.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang PT, Zhang L, Chen CL, Chen J, Green KB, Chen YR. Protein thiyl radical mediates S-glutathionylation of complex I. Free Radic Biol Med. 2012;53:962–973. doi: 10.1016/j.freeradbiomed.2012.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen CA, Lin CH, Druhan LJ, Wang TY, Chen YR, Zweier JL. Superoxide induces endothelial nitric-oxide synthase protein thiyl radical formation, a novel mechanism regulating eNOS function and coupling. J Biol Chem. 2011;286:29098–29107. doi: 10.1074/jbc.M111.240127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zweier JL, Chen CA, Druhan LJ. S-glutathionylation reshapes our understanding of endothelial nitric oxide synthase uncoupling and nitric oxide/reactive oxygen species-mediated signaling. Antioxidants & redox signaling. 2011;14:1769–1775. doi: 10.1089/ars.2011.3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ford E, Hughes MN, Wardman P. Kinetics of the reactions of nitrogen dioxide with glutathione, cysteine, and uric acid at physiological pH. Free Radic Biol Med. 2002;32:1314–1323. doi: 10.1016/s0891-5849(02)00850-x. [DOI] [PubMed] [Google Scholar]
- 40.Augusto O, Bonini MG, Amanso AM, Linares E, Santos CC, De Menezes SL. Nitrogen dioxide and carbonate radical anion: two emerging radicals in biology. Free Radic Biol Med. 2002;32:841–859. doi: 10.1016/s0891-5849(02)00786-4. [DOI] [PubMed] [Google Scholar]
- 41.Davis MF, Vigil D, Campbell SL. Regulation of Ras proteins by reactive nitrogen species. Free Radic Biol Med. 2011;51:565–575. doi: 10.1016/j.freeradbiomed.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davis MF, Zhou L, Ehrenshaft M, Ranguelova K, Gunawardena HP, Chen X, et al. Detection of Ras GTPase protein radicals through immuno-spin trapping. Free Radic Biol Med. 2012;53:1339–1345. doi: 10.1016/j.freeradbiomed.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mitchell L, Hobbs GA, Aghajanian A, Campbell SL. Redox regulation of Ras and Rho GTPases: mechanism and function. Antioxidants & Redox Signaling. 2013;18:250–258. doi: 10.1089/ars.2012.4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raines KW, Bonini MG, Campbell SL. Nitric oxide cell signaling: S-nitrosation of Ras superfamily GTPases. Cardiovascular Research. 2007;75:229–239. doi: 10.1016/j.cardiores.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 45.Jourd’heuil D, Jourd’heuil FL, Feelisch M. Oxidation and nitrosation of thiols at low micromolar exposure to nitric oxide. Evidence for a free radical mechanism. J Biol Chem. 2003;278:15720–15726. doi: 10.1074/jbc.M300203200. [DOI] [PubMed] [Google Scholar]
- 46.Bosworth CA, Toledo JC, Jr, Zmijewski JW, Li Q, Lancaster JR., Jr Dinitrosyliron complexes and the mechanism(s) of cellular protein nitrosothiol formation from nitric oxide. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:4671–4676. doi: 10.1073/pnas.0710416106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hobbs GA, Mitchell LE, Arrington ME, Gunawardena HP, DeCristo MJ, Loeser RF, et al. Redox regulation of Rac1 by thiol oxidation. Free Radic Biol Med. 2015;79:237–250. doi: 10.1016/j.freeradbiomed.2014.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pryor WA, Gojon G, Church DF. Relative Rate Constants for Hydrogen Atom Abstraction by the Cyclohexanethiyl and Benzenethiyl Radicals. J Org Chem. 1978;43:793–800. [Google Scholar]
- 49.Pryor WA, Gojon G, Stanley JP. Hydrogen abstraction by thiyl radicals. J Am Chem Soc. 1973;95:945–946. [Google Scholar]
- 50.Dang HS, Roberts BP, Sekhon J, Smits TM. Deoxygenation of carbohydrates by thiol-catalysed radical-chain redox rearrangement of the derived benzylidene acetals. Org Biomol Chem. 2003;1:1330–1341. doi: 10.1039/b212303g. [DOI] [PubMed] [Google Scholar]
- 51.Dang HS, Roberts BP, Tocher DA. Thiol-catalysed radical-chain redox rearrangement reactions of benzylidene acetals derived from terpenoid diols. Org Biomol Chem. 2003;1:4073–4084. doi: 10.1039/b309060b. [DOI] [PubMed] [Google Scholar]
- 52.Nauser T, Pelling J, Schöneich C. Thiyl radical reaction with amino acid side chains: rate constants for hydrogen transfer and relevance for posttranslational protein modification. Chem Res Toxicol. 2004;17:1323–1328. doi: 10.1021/tx049856y. [DOI] [PubMed] [Google Scholar]
- 53.Nauser T, Schöneich C. Thiyl radicals abstract hydrogen atoms from the (alpha)C-H bonds in model peptides: absolute rate constants and effect of amino acid structure. J Am Chem Soc. 2003;125:2042–2043. doi: 10.1021/ja0293599. [DOI] [PubMed] [Google Scholar]
- 54.Nauser T, Casi G, Koppenol WH, Schöneich C. Reversible intramolecular hydrogen transfer between cysteine thiyl radicals and glycine and alanine in model peptides: absolute rate constants derived from pulse radiolysis and laser flash photolysis. J Phys Chem B. 2008;112:15034–15044. doi: 10.1021/jp805133u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marino SM, Gladyshev VN. Cysteine function governs its conservation and degeneration and restricts its utilization on protein surfaces. J Mol Biol. 2010;404:902–916. doi: 10.1016/j.jmb.2010.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Biaglow JE, Ayene IS, Tuttle SW, Koch CJ, Donahue J, Mieyal JJ. Role of vicinal protein thiols in radiation and cytotoxic responses. Radiat Res. 2006;165:307–317. doi: 10.1667/rr3505.1. [DOI] [PubMed] [Google Scholar]
- 57.Chatterjee A. Reduced glutathione: a radioprotector or a modulator of DNA-repair activity? Nutrients. 2013;5:525–42. doi: 10.3390/nu5020525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Svoboda P, Harms-Ringdahl M. Protection or sensitization by thiols or ascorbate in irradiated solutions of DNA or deoxyguanosine. Radiat Res. 1999;151:605–616. [PubMed] [Google Scholar]
- 59.Mozziconacci O, Sharov V, Williams TD, Kerwin BA, Schöneich C. Peptide cysteine thiyl radicals abstract hydrogen atoms from surrounding amino acids: the photolysis of a cystine containing model peptide. J Phys Chem B. 2008;112:9250–9257. doi: 10.1021/jp801753d. [DOI] [PubMed] [Google Scholar]
- 60.Mozziconacci O, Kerwin BA, Schöneich C. Reversible hydrogen transfer between cysteine thiyl radical and glycine and alanine in model peptides: covalent H/D exchange, radical-radical reactions, and L- to D-Ala conversion. J Phys Chem B. 2010;114:6751–6762. doi: 10.1021/jp101508b. [DOI] [PubMed] [Google Scholar]
- 61.Hofstetter D, Nauser T, Koppenol WH. Hydrogen exchange equilibria in glutathione radicals: rate constants. Chem Res Toxicol. 2010;23:1596–1600. doi: 10.1021/tx100185k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mozziconacci O, Williams TD, Schöneich C. Intramolecular hydrogen transfer reactions of thiyl radicals from glutathione: formation of carbon-centered radical at Glu, Cys, and Gly. Chem Res Toxicol. 2012;25:1842–1861. doi: 10.1021/tx3000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hofstetter D, Thalmann B, Nauser T, Koppenol WH. Hydrogen exchange equilibria in thiols. Chem Res Toxicol. 2012;25:1862–1867. doi: 10.1021/tx300045f. [DOI] [PubMed] [Google Scholar]
- 64.Zhao R, Lind J, Merenyi G, Eriksen TE. Significance of the intramolecular transformation of glutathione thiyl radicals to alpha-aminoalkyl radicals. Thermochemical and biological implications. J Chem Soc Perk T. 1997;2:569–574. [Google Scholar]
- 65.Zhao R, Lind J, Merenyi G, Eriksen TE. Kinetics of One-Electron Oxidation of Thiols and Hydrogen Abstraction by Thiyl Radicals from Alpha-Amino C-H Bonds. J Am Chem Soc. 1994;116:12010–12015. [Google Scholar]
- 66.Nauser T, Koppenol WH, Schöneich C. Reversible hydrogen transfer reactions in thiyl radicals from cysteine and related molecules: absolute kinetics and equilibrium constants determined by pulse radiolysis. J Phys Chem B. 2012;116:5329–5341. doi: 10.1021/jp210954v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wei Y, Mathies G, Yokoyama K, Chen J, Griffin RG, Stubbe J. A chemically competent thiosulfuranyl radical on the Escherichia coli class III ribonucleotide reductase. J Am Chem Soc. 2014;136:9001–13. doi: 10.1021/ja5030194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fossay J, Sorba J. J Mol Struct. 1989;186:305–319. [Google Scholar]
- 69.Naumov S, Von Sonntag C. UV/Vis absorption spectra of alkyl-, vinyl-, aryl- and thioperoxyl radicals and some related radicals in aqueous solution. J Phys Org Chem. 2005;18:586–594. [Google Scholar]
- 70.Morris M, Chan B, Radom L. Heteroatomic deprotonation of substituted methanes and methyl radicals: theoretical insights into structure, stability, and thermochemistry. J Phys Chem A. 2012;116:12381–12387. doi: 10.1021/jp3101927. [DOI] [PubMed] [Google Scholar]
- 71.Asmus KD, Henglein A, Wigger A, Beck G. Pulsradiolytische Versuche zur elektrolytischen Dissoziation von aliphatischen Alkoholradikalen. Berichte der Bunsengesellschaft für Physikalische Chemie. 1966;70:756–758. [Google Scholar]
- 72.Simic M, Neta P, Hayon E. Pulse radiolysis study of alcohols in aqueous solution. J Phys Chem. 1969;73:3794–3800. [Google Scholar]
- 73.Mozziconacci O, Kerwin BA, Schöneich C. Photolysis of an intrachain peptide disulfide bond: primary and secondary processes, formation of H2S, and hydrogen transfer reactions. J Phys Chem B. 2010;114:3668–3688. doi: 10.1021/jp910789x. [DOI] [PubMed] [Google Scholar]
- 74.Mozziconacci O, Kerwin BA, Schöneich C. Reversible hydrogen transfer reactions of cysteine thiyl radicals in peptides: the of cysteine into dehydroalanine and alanine, and of alanine into dehydroalanine. J Phys Chem B. 2011;115:12287–12305. doi: 10.1021/jp2070453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mozziconacci O, Haywood J, Gorman EM, Munson E, Schöneich C. Photolysis of recombinant human insulin in the solid state: formation of a dithiohemiacetal product at the C-terminal disulfide bond. Pharm Res. 2012;29:121–133. doi: 10.1007/s11095-011-0519-1. [DOI] [PubMed] [Google Scholar]
- 76.Dae-Kyung K, Jeong J, Drake SK, Wehr NB, Rouault TA, Levine RL. Iron regulatory protein 2 as iron sensor. Jounal of Biological Chemistry. 2003;278:14857–14864. doi: 10.1074/jbc.M300616200. [DOI] [PubMed] [Google Scholar]
- 77.Jeong J, Rouault TA, Levine RL. Identification of a heme-sensing domain in iron regulatory protein 2. Journal of Biological Chemistry. 2004;279:45450–45454. doi: 10.1074/jbc.M407562200. [DOI] [PubMed] [Google Scholar]
- 78.Garrison WM. Reaction mechanisms in the radiolysis of peptides, polypeptides, and proteins. Chem Rev. 1987;87:381–398. [Google Scholar]
- 79.Block DA, Yu D, Armstrong DA, Rauk A. On the influence of secondary structure on the alpha-C-H bond dissociation energy of proline residues in proteins: a theoretical study. Can J Chem. 1998;76:1042–1049. [Google Scholar]
- 80.Rauk A, Yu D, Armstrong DA. Oxidative damage to and by cysteine in proteins: An ab initio study of the radical structures, C-H, S-H, and C-C bond dissociation energies, and transition structures for H abstraction by thiyl radicals. J Am Chem Soc. 1998;120:8848–8855. [Google Scholar]
- 81.Rauk A, Yu D, Armstrong DA. Toward site specificity of oxidative damage in proteins: C-H and C-C bond dissociation energies and reduction potentials of the radicals of alanine, serine, and threonine residues - An ab initio study. J Am Chem Soc. 1997;119:208–217. [Google Scholar]
- 82.Reid DL, Armstrong DA, Rauk A, von Sonntag C. H-atom abstraction by thiyl radicals from peptides and cyclic dipeptides. A theoretical study of reaction rates. Phys Chem Chem Phys. 2003;5:3994–3999. [Google Scholar]
- 83.Mozziconacci O, Williams TD, Kerwin BA, Schöneich C. Reversible intramolecular hydrogen transfer between protein cysteine thiyl radicals and alpha C-H bonds in insulin: control of selectivity by secondary structure. J Phys Chem B. 2008;112:15921–15932. doi: 10.1021/jp8066519. [DOI] [PubMed] [Google Scholar]
- 84.Hogan MC. Phosphorescence quenching method for measurement of intracellular PO2 in isolated skeletal muscle fibers. Journal of applied physiology. 1999;86:720–724. doi: 10.1152/jappl.1999.86.2.720. [DOI] [PubMed] [Google Scholar]
- 85.Richardson RS, Newcomer SC, Noyszewski EA. Skeletal muscle intracellular PO(2) assessed by myoglobin desaturation: response to graded exercise. J Appl Physiol (1985) 2001;91:2679–2685. doi: 10.1152/jappl.2001.91.6.2679. [DOI] [PubMed] [Google Scholar]
- 86.Carreau A, El Hafny-Rahbi B, Matejuk A, Grillon C, Kieda C. Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia Journal of cellular and molecular medicine. 2011;15:1239–1253. doi: 10.1111/j.1582-4934.2011.01258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Strambini G, Cioni P. The effect of protein structure on oxygen quenching of phosphorescence. J Am Chem Soc. 1999;121:8337–8344. [Google Scholar]
- 88.Mozziconacci O, Schöneich C. Sequence-specific formation of D-amino acids in a monoclonal antibody during light exposure. Molecular pharmaceutics. 2014;11:4291–4297. doi: 10.1021/mp500508w. [DOI] [PubMed] [Google Scholar]
- 89.Routaboul L, Vanthuyne N, Gastaldi S, Gil G, Bertrand M. Highly efficient photochemically induced thiyl radical-mediated racemization of aliphatic amines at 30 degrees C. J Org Chem. 2008;73:364–368. doi: 10.1021/jo702241y. [DOI] [PubMed] [Google Scholar]
- 90.Chen Y, Chen C, Zhang L, Green-Church KB, Zweier JL. Superoxide generation from mitochondrial NADH dehydrogenase induces self-inactivation with specific protein radical formation. Jounal of Biological Chemistry. 2005;280:37339–37348. doi: 10.1074/jbc.M503936200. [DOI] [PubMed] [Google Scholar]