Abstract
Background: Invasive disease is a poor prognostic factor for patients with differentiated thyroid cancer (DTC). Uncontrolled central neck disease is a common cause of distressing death for patients presenting in this manner. Advances in assessment and management of such cases have led to significant improvements in outcome for this patient group. This article reviews the patterns of invasion and a contemporary approach to investigation and treatment of patients with invasive DTC.
Summary: Aerodigestive tract invasion is reported in around 10% of case series of DTC. Assessment should include not only clinical history and physical examination with endoscopy as indicated, but ultrasound and contrast-enhanced cross-sectional imaging. Further studies including positron emission tomography should be considered, particularly in recurrent cases that are radioactive iodine (RAI) resistant. Both the patient and the extent of disease should be carefully assessed prior to embarking on surgery. The aim of surgery is to resect all gross disease. When minimal visceral invasion is encountered early, “shave” procedures are recommended. In the setting of transmural invasion of the airway or esophagus, however, full thickness excision is required. For intermediate cases in which invasion of the viscera has penetrated the superficial layers but is not evident in the submucosa, opinion is divided. Early reports recommended an aggressive approach. More recently authors have tended to recommend less aggressive resections with postoperative adjuvant therapies. The role of external beam radiotherapy continues to evolve in DTC with support for its use in patients considered to have RAI-resistant tumors.
Conclusions: Patients with invasive DTC require a multidisciplinary approach to investigation and treatment. With detailed assessment, appropriate surgery, and adjuvant therapy when indicated, this patient group can expect durable control of central neck disease, despite the aggressive nature of their primary tumors.
Introduction
The incidence of differentiated thyroid cancer (DTC) is increasing worldwide (1). The majority of lesions now detected present with early primary disease (2). Such patients have excellent long-term outcomes (3,4). However, an increase in the number of patients with large-volume primary tumors has also been observed (5), and locally advanced disease constitutes around 5%–15% of case series (4,6,7). Uncontrolled involvement of the viscera of the central neck is associated with high rates of mortality and a most distressing mode of death. However, several reports have shown that complete surgical resection is associated with higher survival rates compared to incomplete resections (8–11). As such, clinicians dealing with DTC must be aware of the most appropriate ways to investigate and treat patients who present with lesions that involve the viscera of the central neck.
Extrathyroidal extension (ETE) has long been recognized as an independent predictor of poor outcome in patients with DTC (8,10,12,13). Not only does such invasive disease result in higher rates of recurrence, but survival is compromised in this patient group. Cases that present with airway invasion are more likely to present with adverse risk factors including advanced age and aggressive histology. As such, radioactive iodine (RAI) avidity is reduced in this patient group, leading to poor or no response to adjuvant RAI. When the airway is involved by ETE, the situation is complicated not only by the negative prognostic implications of the disease itself (14) but also by the morbidity associated with surgical procedures required to adequately resect disease. Airway invasion commonly accompanies invasion of surrounding structures in the central neck, further complicating the approach to management.
The relative rarity of locally advanced disease and the lack of prospective trials in this particular field has led to controversy over investigation and management of patients who present with DTC involving the airway.
The aim of this paper was to review the patterns of invasion seen in DTC and to consider the approach to investigation and management of patients who present with locally advanced DTC.
Clinical Presentation
Patients with locally advanced DTC may present in a relatively asymptomatic manner; however, most patients will present with hoarseness, stridor, hemoptysis, dysphagia, and pain, alone or in combination. Hoarseness and stridor can develop because of recurrent laryngeal nerve (RLN), tracheal, or laryngeal invasion. Hemopytsis is always a result of intraluminal invasion, and pharyngo-esophageal invasion or extrinsic luminal compression can lead to dysphagia (15).
In patients with advanced disease, the clinician should consider the possibility of dedifferentiated thyroid malignancy or lymphoma. In cases of anaplastic thyroid cancer, the outcome is rapidly fatal and few patients, if any, will be candidates for an aggressive surgical approach. It is therefore critical to have representative tissue prior to definitive therapy. For the majority of patients, fine-needle aspiration biopsy will be the first assessment of the tumor's histological subtype. However, heterogeneity within tumors is common. Therefore, the treatment team may recommend further biopsies in the form of core or open biopsy to minimize the chance of underestimating the aggressive nature of the disease. However, the most biologically aggressive disease is often at the point of maximal invasion, and biopsy from this area is impossible. Diagnostic uncertainty will be present until formal histology is available from the resection specimen, and the patient should be counseled appropriately.
Patterns of Invasion
The anatomical relationship between the thyroid gland and the larynx, trachea, esophagus, and RLN place these structures at risk from both direct involvement from the primary lesion and also from metastatic lymph nodes in the central or lateral neck (16).
Although the infra-hyoid strap muscles are the most common site of invasion from ETE (17), in more than half of locally advanced cases, such anterior extension can be excised with relatively minimal morbidity. In contrast, posterior ETE results in visceral invasion and complicates both diagnosis and management.
The trachea is invaded in around one third of locally advanced cases (17). The trachea tends to be involved by direct extension through the tracheal perichondrium toward the submucosa of the airway. Further invasion results in ulceration of the mucosa with intraluminal tumor, which may in turn present with hemoptysis or stridor.
Classification systems have been proposed by both and Czaja and McCaffrey (14) and Shin et al. (18) based on the extent of laryngo-tracheal invasion (Table 1, Fig. 1). Early invasion results in adherence of the gland to the airway without frank invasion. As the disease progresses, first the perichondrium, the cartilage, and eventually the submucosa are invaded prior to involvement of the mucosal surface. Such systems are useful in considering the surgical approach to managing the airway in advanced cases.
Table 1.
Classification Systems for Staging Extent of Airway Involvement
| Stage | Czaja and McCaffrey (14) | Shin et al. (18) |
|---|---|---|
| I | Extra thyroid extension adherent to airway with no gross invasion | Invasion of perichondrium |
| II | Gross invasion which can be excised without mucosal disruption | Invasion of the cartilage |
| III | Gross invasion which requires mucosal disruption to excise but no intraluminal invasion | Involvement of submucosa |
| IV | Gross intraluminal invasion | Intraluminal invasion |
FIG. 1.

Classification of tracheal invasion as proposed by Shin et al. (18) (courtesy of Memorial Sloan Kettering Cancer Center).
Invasion of the larynx is less common (12%) (17) and may occur via direct invasion through the cartilages or the cricothyroid membrane. In addition, disease may grow posteriorly around the dorsal border of the thyroid cartilage, entering the paraglottic space of the larynx via the constrictor muscles and pyriform fossa (Figs. 2–4). Early invasion may be asymptomatic. However, more advanced disease results in hoarseness and aspiration followed by airway compromise and hemorrhage.
FIG. 2.

Schematic of disease invading posteriorly into the paraglottic space (courtesy of Memorial Sloan Kettering Cancer Center).
FIG. 3.

Computed tomography (CT) scan showing recurrent papillary thyroid cancer invading in to pyriform fossa at level of cricoid cartilage.
FIG. 4.

Endoscopic image of same patient under general anesthetic with disease evidence in the hypopharynx.
In contrast to the airway structures, the mucosa of the esophagus is relatively resistant to invasion. Direct extension into the muscular layers of the pharynx or esophagus is more common, occurring in around 20% of locally advanced cases (Fig. 5) (17).
FIG. 5.
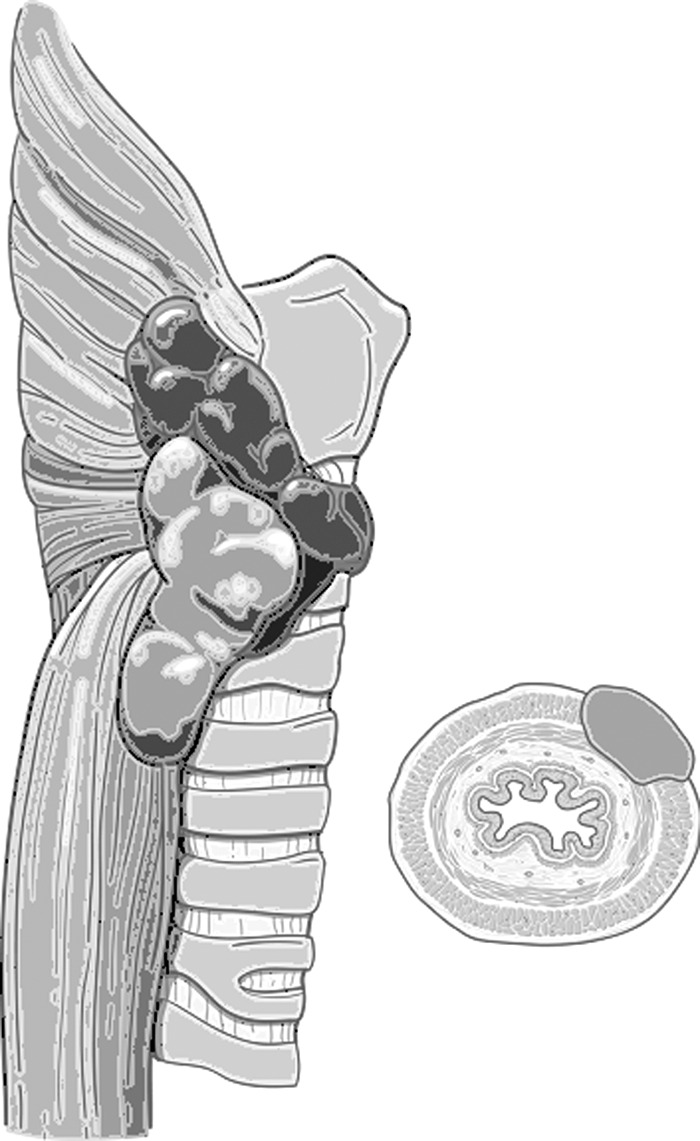
Schematic of esophageal invasion (courtesy of Memorial Sloan Kettering Cancer Center).
Around half of locally advanced cases will also have involvement of the RLN (17). The nerve may be invaded directly from primary thyroid disease (Fig. 6) or encased in metastatic lymph nodes (Fig. 7) in the trachesophageal groove. Disease first abuts and then encases the nerve, and function will be lost at some point during this process (Fig. 8). Work by experts in intraoperative nerve monitoring (IONM) suggests that around half of patients with a clinically invaded RLN will have normal vocal cord function, and that in 60% of cases with RLN invasion, electrophysiological function can be detected (19). Some patients with vocal cord dysfunction may develop compensation from the contralateral vocal cord, and voice changes may be very subtle.
FIG. 6.
Primary disease encases the left recurrent laryngeal nerve at the level of the tubercle of Zuckerkandl.
FIG. 7.
Metastatic lymph nodes encasing the recurrent laryngeal nerve.
FIG. 8.

Schematic of invasion of the recurrent laryngeal nerve by primary disease (courtesy of Memorial Sloan Kettering Cancer Center).
Rarely, the great vessels in the neck are directly invaded by DTC because extracapsular involvement is very infrequent even in the presence of large-volume lateral neck nodes. Such invasion can be due to primary or nodal disease directly invading the lumen of the internal jugular vein, mainly due to intravascular growth in follicular carcinomas. As disease progresses it may extend to encase the great vessels. Direct involvement of the carotid artery usually represents transformation of DTC to poorly differentiated or anaplastic histology.
Assessment of Invasion
Clinical history may suggest that a thyroid lesion involves the viscera of the central neck. Gaissert et al. (20) report hemoptysis, dyspnea, neck mass, and voice change in 28%, 26%, 37%, and 36% of cases, respectively. Only 12% of locally advanced cases were asymptomatic in this series.
Examination is also critical. The neck should be palpated with focus on fixation of the mass to deep structures. The presence of palpable central neck disease in the setting of recurrence should alert the clinician to the possibility of invasive disease. Assessment of the mucosa of the laryngopharynx and trachea as well as the function of the vocal cords is also critical. This assessment should be performed in the office setting to allow a dynamic evaluation of laryngeal function (21). Additional assessment using tracheoscopy and esophagoscopy may also be required under general anesthesia to complete the clinical examination of the viscera of the central neck. Office examination allows assessment of laryngo-pharyngeal function. However, accurate assessment of the mucosa of the subglottis, trachea, and esophagus is more accurately performed under general anesthesia. Echoendoscopy may also be used to further assess the degree of disease involvement in the trachea or esophagus.
Although ultrasound can be used to identify ETE (22), cross-sectional imaging is critical in determining the extent of invasion and for accurate presurgical planning. Such additional imaging is not required in all cases of DTC. However, if evidence of nodal disease or suspicion of extra thyroid extension exists, consideration should be given to imaging that should include the mediastinal lymph nodes. Contrast-enhanced computed tomography (CT) scanning allows accurate assessment of the degree of extension while also allowing the mediastinal lymph nodes and great vessels of the neck to be assessed without the movement artifact encountered with magnetic resonance imaging (MRI). In addition, synchronous examination of the chest can be used to assess for distant disease and respiratory comorbidities. MRI may provide additionally useful information about the presence or extent of prevertebral compartment invasion in select cases.
In terms of the relationship between the disease and major blood vessels, the presence of a fat plane on imaging suggests that surgical dissection will be possible. Disease that surrounds >180° of the vessel circumference is suggestive of encasement, which prevents successful dissection. In exceptional cases, resection with bypass of major vessels can be considered. However, not only does this approach represent a significant clinical risk and a great technical challenge, but it also suggests particularly aggressive disease, making the decision to offer such complex surgery questionable.
Concerns about the impact of iodine-based contrast on the ability to effectively deliver adjuvant RAI therapy are likely to have been overestimated (23). Clinicians should remember that such locally aggressive disease is likely to have lost RAI avidity, and the benefit from accurate preoperative imaging to define the extent and location of invasion to facilitate complete surgical removal far outweighs any theoretical limitations about RAI administration (24). Preoperative contrast-enhanced CT does not preclude the use of therapeutic RAI postoperatively. If concern remains about iodine avidity of residual thyroid tissue due to prior contrast uptake, a washout period of between 4 and 8 weeks is generally felt to be sufficient (23), which is not a clinically significant delay.
[18F]-Fluorodeoxyglucose (FDG) positron emission tomography (PET ±CT) is a useful imaging modality in patients with advanced disease because it can provide information about the biology of the tumor and thereby aid treatment decisions. Poorly differentiated and dedifferentiating tumors become FDG avid as they lose RAI avidity because of the loss of expression of the sodium iodide symporter (25). Therefore, PET positivity is associated with more aggressive biology and a tumor that is unlikely to respond to RAI therapy. Such an assessment of disease biology may affect therapy. For example, those patients who present with significant distant disease demonstrating high levels of FDG avidity are likely to have a more limited life expectancy. The association between this finding and dedifferentiated aggressive disease may impact on the decision to offer aggressive surgery. In contrast, for non–FDG-avid distant disease, there is a greater chance of response to RAI, which may justify a more aggressive surgical approach to the primary tumor. If PET-CT is not available, additional investigations including MRI of the spine may be considered to screen for bone metastases in advanced disease.
Further assessment should be tailored to the individual patient. Assessment of the eventual comorbidities and fitness for radical surgery is critical. If life-threatening disease is encountered on preoperative assessment, be it primary disease, distant metastases, or comorbid conditions, an attempt to compare the trajectory of primary versus systemic disease should be made. This assessment of disease biology will allow clinicians to select those patients most likely to benefit from major surgery.
Surgical Management
The aim of surgery for locally advanced DTC is to achieve an optimal oncological outcome, minimize surgical morbidity, and maximize quality of life. It is widely accepted that incomplete resection of gross disease compromises oncological outcome (11,14,26,27). However, the impact of microscopic residual disease is controversial.
Preoperative assessment of the extent of primary, regional, and distant disease as well as comorbidities is critical. Details of specific surgical procedures are beyond the scope of this article and are well reported elsewhere (28,29). However, in order to manage such cases appropriately, the treating team should have experience with advanced techniques of resection and reconstruction (30). As such, consideration should be given to referring such cases to tertiary centers that have the multidisciplinary experience to manage such complex cases (31).
Invasion of the airway
When disease abuts the perichondrium or invades the perichondrium but does not involve the cartilage of the trachea or larynx, the consensus is that procedures that shave a margin of cartilage but maintain the integrity of the airway lumen are sufficient (31). For most patients managed in this way, the role of adjuvant external beam radiotherapy (EBRT) following resection of all gross disease remains controversial (32) unless there is strong suggestion that the disease is RAI resistant.
In more advanced cases, when disease extends into the cartilage without definite evidence of disease in the submucosa, the situation is more complex. In this situation, determining the exact extent of invasion requires histopathological assessment that can only be achieved postoperatively. Therefore, some authors recommend a full-thickness excision including the mucosa of the airway (9,20,33–37). This approach ensures a histologically negative margin but results in increased surgical complexity and significant functional impairment. Other groups have experienced similar oncological outcomes with an approach that reserves full-thickness excision only for disease that grossly involves the submucosa or mucosa itself (6,14,27,38–44). This approach accepts a higher risk of leaving microscopically positive margins but results in lower immediate surgical morbidity.
The groups advocating an aggressive approach report low mortality and morbidity from extensive procedures with favorable long-term results in those patients treated in this manner (20). Similarly, groups employing a less aggressive approach also report excellent outcomes with local control rates of up to 95% and 5-year survival of 93% (41). In addition, such groups report that significant numbers of patients (over 50% in one study) are unwilling to consent to more aggressive surgery because of the risk of functional impairment (27).
All studies in the literature are retrospective, and it is highly unlikely that prospective data will become available to confirm the most appropriate surgical approach to cartilage invasion. In their absence, it is interesting to consider the reasons that may explain the differences in approach between centers. The specifics of the presenting populations may explain some of the variation. Some centers include high rates of previously operated disease in their reported series (over 50% of those treated in the Massachusetts General Hospital experience (20)), whereas other groups report only primary surgeries. Clearly, for patients who have had prior treatment, postoperative fibrosis will make conservative surgery more challenging. In addition, tertiary referral centers are likely to attract more aggressive pathology and patients who may require a more aggressive surgical approach. Some centers have included dedifferentiated histologies (including anaplastic thyroid cancer) in their series (20), whereas others excluded less differentiated disease (6,14,16). In particular, anaplastic cases may bias results because almost all patients with such aggressive disease will not be candidates for an aggressive surgical approach. Patient age may also be a factor; for example, Kowalski et al. reported a mean age of 41 years in their cohort (11,14,26,27) compared with 64 years reported by Gaissert et al. (20). It is also likely that individual surgical experience contributes to varied international institutional approaches. Institutions with higher rates of recurrent disease in older patients are more likely to encounter RAI-resistant disease and favor a more aggressive surgical approach.
In the most advanced cases, when disease extends through cartilage to involve the submucosa or the mucosa of the airway, a full-thickness resection of airway structures is required. For small tracheal segments, window or wedge resection with reconstruction may suffice (45). For more extensive tracheal invasion, sleeve resection may be required with primary anastomosis. When the larynx is invaded, disease is often well lateralized and can be excised with a partial laryngectomy. However, extensive laryngeal invasion, particularly when comorbid respiratory disease is present, will require total laryngectomy (28).
Airway sacrifice is associated with a significant psycho-social impact on the patient. Many aspects of lifestyle from appearance, feeding, communication, and in some cases even independent living will be affected. For this reason, the decision to perform a major airway resection must be individualized. In a younger patient with a good life expectancy, who is considered able to manage a laryngeal stoma independently, laryngectomy in order to achieve good surgical margins and optimize oncological outcome is well justified. In contrast, for elderly frail patients who may require prolonged hospitalizations after such a procedure, all options must be considered. If such patients have symptoms related to central neck disease and are expected to have a reasonable quality of life following surgery, palliative laryngectomy should be considered. The alternative in this situation would be to either accept death from asphyxiation, or to place a temporary tracheostomy in order to bypass the nonfunctioning larynx (which may later obstruct secondary to tumor). In contrast, patients with minimal life expectancy, irrespective of central neck disease, and in whom quality of life would be negatively impacted by radical surgery, should be managed with a palliative approach.
Clearly these decisions are difficult, and all recommendations need to be individualized. Early involvement of the patient, family, and members of the wider disease management team are critical in ensuring appropriate decision making at a stressful time for all involved.
Invasion of the RLN
Management of the involved RLN demands a thorough clinical examination preoperatively. Only when the surgeon has recorded the function of both vocal cords can appropriate decisions be made.
Invasion of the RLN is common in cases of locally advanced DTC but has not convincingly been shown to be independently predictive of poor outcome (17). In addition, resection of involved nerves has not been shown to be associated with survival benefit (46). This situation has led to controversy about the optimal management of the nerve during surgery for advanced disease.
When vocal cord function is normal preoperatively, all attempts should be made to preserve the integrity of the RLN. If disease is minimally adherent to the nerve, shaving the tumor from the nerve is recommended. If disease encases the nerve, the situation is more complex. If dissecting the nerve from the tumor without leaving gross residual disease is possible, it should be attempted. If such dissection results in gross residual disease, the nerve function remains at high risk if disease progresses. In this circumstance resection of the nerve to achieve macroscopic clearance should be performed.
When the ipsilateral vocal cord is dysfunctional and disease encases the RLN at surgery, resection is indicated. If the nerve appears to be minimally adherent to tumor but is dysfunctional, shaving the disease from the nerve is recommended. In the rare setting of contralateral cord dysfunction and ipsilateral invasion (with a normally functioning ipsilateral cord), the surgeon should consider sparing the ipsilateral RLN in an attempt to avoid tracheostomy (31). The clinical team should consider that such patients are at high risk of requiring tracheostomy, either temporarily following surgery or because of subsequent disease progression resulting in RLN invasion and bilateral cord palsy (Table 2).
Table 2.
Management of the Recurrent Laryngeal Nerve
| Vocal cord function normal | Ipsilateral vocal cord dysfunction | Contralateral vocal cord dysfunction | |
|---|---|---|---|
| Disease minimally adherent to the nerve | Shave | Shave | Shave |
| Disease encasing the nerve | Dissect nerve out unless results in gross residual disease | Resect nerve en bloc | Consider preserving the nerve to avoid tracheostomy |
The role of IONM is controversial in thyroid surgery. Support now exists for the use of IONM for complex cases, particularly in the revision setting or when the RLN is suspected to be invaded by disease (47). IONM may be used as a diagnostic adjunct, with some authors recommending sparing even grossly involved RLNs which retain a positive electromyographic positive signal (48).
Invasion of the esophagus
When disease invades the muscular layer of the esophagus, it can often be dissected from the underlying mucosa without the need for formal reconstruction (Fig. 9). Limited luminal invasion can be addressed by resection with primary closure. However, when extensive invasion is encountered, resection with appropriate reconstruction is required.
FIG. 9.
Disease involving the muscular layer of the esophagus. The muscularis layer of the esophagus is usually very resistant to invasion.
Management of the regional lymph nodes
During preoperative investigation, lymph nodes should be assessed from the base of the skull to the mediastinum. More than 20% of advanced DTC cases will have clinical evidence of nodal metastases (49). Indeed, some series suggest that over 95% of patients with ETE will have evidence of nodal metastases on histological examination of elective neck dissection specimens (50). In the setting of clinically evident nodal metastases, a compartment-oriented neck dissection should be performed with the aim of resecting all gross disease. Those patients considered free of nodal disease (cN0) are at high risk of harboring occult nodal metastases, particularly in the central compartment. However the clinical impact of such occult metastases is uncertain. An individualized approach seems most appropriate. If the patient is encountered in the salvage setting following prior central neck dissection, further nodal surgery may carry significant risk. However, in the primary setting most groups now recommend considering elective nodal clearance in the presence of gross ETE (16,51,52).
Patients with distant metastases at presentation
Patients with distant metastases account for up to one third of reported series of invasive thyroid cancers (27). In a series of 1038 previously untreated patients with differentiated carcinoma of the thyroid, most of them papillary carcinomas, the risk of distant metastasis for papillary, follicular, and Hürthle cell carcinomas was 10%, 22%, and 33%, respectively (53). Distant metastasis are frequent in childhood, especially in the lungs. La Quaglia et al. (54) showed that the survival rate of children with distant metastases is excellent, but very few such patients present with locally advanced primary tumors.
Patients with invasive thyroid cancer and distant metastasis will require RAI therapy, which should be considered when planning the surgical approach because resection of all gross disease facilitates 131I imaging and treatment. Analysis of data of 1516 patients of all ages with papillary carcinoma depicted 102 patients with lung metastases only or with other organ involvement (55). The 10- and 20-year survival rates in patients with papillary thyroid carcinoma without distant metastasis and in the lung metastasis groups were 98.5%, 98.0%, and 75.0%, 51.2%, respectively. The prognosis of patients with papillary thyroid carcinoma and lung metastasis at time of diagnosis is the same as for those whose lung metastases are discovered later. Survival analysis suggests that patients with multi-organ metastases have poorer survival than those with metastases limited to the lung (56). Furthermore, Shoup et al. (57) identified 336 patients with distant metastasis from differentiated thyroid carcinomas, in whom distant disease was either detected at the time of diagnosis or represented the first site of recurrence. Ten-year disease-specific survival from identification of distant metastasis was 26%, and multivariate analysis identified age 45 years or more, symptoms, site other than lung only or bone only, and no RAI treatment for the metastasis as predictors of poor outcomes.
Although cure cannot be achieved for many patients with distant metastases, the aim of therapy is to palliate symptoms and alter the mode of death. Controlling invasive disease in the central neck prevents the distress of death from asphyxiation or upper airway hemorrhage. However, as stated above, decision making in such complex cases must be individualized. For patients with rapidly progressing distant disease and well-controlled central neck disease, surgical resection would not be appropriate. In contrast, patients who present with resectable central neck disease and small-volume distant metastases remain candidates for aggressive airway resection as supported by the promising oncological outcomes reported above.
Adjuvant Therapies
The majority of patients with high-risk DTC will be considered for adjuvant RAI as part of initial therapy (51,52). However, patients with invasive DTC are at high risk of harboring RAI-resistant disease, which is often associated with aggressive histological subtypes (areas of poorly differentiated disease, tall cell or insular carcinoma) and advanced age. For that reason, RAI is likely to be less effective in this subgroup of patients (58). Nonetheless, the relatively low side effect profile of treatment and some limited evidence of effectiveness in this high-risk group (59) means that most patients will be considered for adjuvant RAI.
More controversial is the role of EBRT for invasive DTC. No prospective trials have been performed, and a recent German study designed to address this issue was closed because only 45 of 311 patients recruited agreed to randomization (60).
In the absence of prospective evidence, the role of EBRT remains controversial. Treatment decisions should be made following multidisciplinary discussion and should be based upon patient, tumor, and surgical factors.
In patients whose disease is considered unresectable or in those who have gross residual disease following surgery, EBRT may be considered. The term “very advanced disease” is now preferred to the term unresectable, but either way some patients will present with disease that the surgical team feels in not amenable to a surgical approach. This definition will depend not only on the tumor, but also on the patient. Classically, disease encircling the carotid artery or invading the prevertebral fascia was considered unresectable. However, some patients will tolerate sacrifice of the carotid artery without major cerebrovascular effects. In contrast, if disease extends into the mediastinum and surgical resection would require sternotomy, there are patients who are considered insufficiently physiologically fit to justify such an approach to resect disease even if major arteries are not grossly involved. In such patients, although treatment intent is palliative, disease control can be achieved in a high percentage of patients (61–64).
Patients who undergo successful surgical excision of macroscopic disease remain at high risk of loco-regional recurrence. In this situation, careful discussion of risk factors should be undertaken within the multidisciplinary team, and EBRT can be considered for maximizing local control and organ preservation. RAI should not be discounted, although aggressive histological subtypes predict a poorer treatment response. Nonetheless, it may still be a useful adjunctive therapy. The optimal sequencing of RAI and EBRT in this situation is unknown. Some favor RAI first because EBRT may lead to reduced iodine avidity. However, concern remains about airway safety, EBRT may be administered first with RAI given subsequently.
When the approach to EBRT is considered, doses will range from 70 Gy for areas of gross disease, 60–66 Gy for postoperative high-risk sites including positive surgical margins, and 50–54 Gy for areas of lower risk including elective nodal sites (32). Treatment volumes can be restricted to areas at high risk of relapse based on preoperative radiology, intraoperative findings, and postoperative review of the pathology in order to minimize treatment toxicity. Intensity-modulated radiation techniques must be employed to achieve prescribed dose to the target volumes while remaining within critical structure tolerances (52).
If disease progresses and is refractory to RAI, palliative systemic therapies with targeted agents such as tyrosine kinase inhibitors may then be considered (65,66). The definition of RAI-refractory disease includes at least one of the following: (a) disease does not take up iodine at documented sites of metastatic disease, (b) disease progresses despite RAI treatment and confirmed uptake, (c) distant disease grows over a 1-year period after RAI, or (d) total cumulative dose of RAI exceeds 600 mCi (67).
Although the use of tyrosine kinase inhibitors has been reported in a neo-adjuvant setting (68), experience is extremely limited and their use is not currently considered to be indicated.
Prognosis
When outcomes for patients with invasive thyroid cancer are considered, limitations of the reported studies must be kept in mind. No prospective trials exist and all experience represents retrospective surgical cohorts. Any patient deemed unfit for surgery based on tumor or patient grounds will therefore have been excluded, and the cases that are included are likely to represent the fittest patients who present with relatively limited disease.
However, despite these limitations, there are selected patients in whom prognosis may be favorable. Long-term rates of local control exceed 90% in some studies (41). Long-term survival can also be achieved in a significant group of patients, with 5-year survival approaching 90% in these selected cases (17,41).
Factors predictive of outcome have been poorly reported to date. Some investigators have demonstrated an association between the extent of airway invasion and outcome. Shin et al. (18) found in a study of 22 patients that 5-year survival dropped from 100% versus 29% for disease that spared or involved the luminal airway, respectively. Czaja and McCaffrey (14) also demonstrated a significant association between extent of airway invasion and survival in 286 patients with invasive disease. Univariate analysis of outcome showed survival of 60% versus 80% at 10 years for patients with and without airway invasion respectively (p < 0.05).
Conclusion
Visceral invasion occurs in at least 5% of patients with DTC. In patients with resectable disease, long-term outcomes are good, with up to 95% local control rates reported. Initial series reported central neck disease as the most common cause of death (69,70). However, advances in our understanding of the biology of this disease and an aggressive surgical approach to management have resulted in improved outcomes for this patient group, with death from uncontrolled central neck disease now uncommon (71–74). Even for patients who present with distant metastatic disease, not only can meaningful prolongation of life be achieved, but control of central neck disease can avoid a distressing mode of death.
Although the presence and degree of airway invasion is likely to be the strongest predictor of outcome for this patient group, controversy about the surgical approach to these cases exists. The consensus is that excision of all gross disease should be the aim of surgery. Some groups recommend full-thickness airway resections for all but the most limited of disease and report excellent outcomes with this approach. However, with careful patient selection, appropriate preoperative investigation and with a selective approach to adjuvant therapy, most groups now prefer a more conservative approach with minimal airway resection in the absence of disease extending deep to the cartilage of the trachea or larynx.
Patients with airway invasion from DTC may present in the primary or recurrent setting, with localized or systemic disease and in the presence of complex comorbidities. As such, these patients require multidisciplinary evaluation and planning to achieve optimal outcomes.
Author Disclosure Statement
Dr. Kate Newbold has consultancy and advisory roles with Eisai, Astra-Zeneca, and Genzyme. The other authors have nothing to disclose.
References
- 1.Davies L, Welch HG. 2006. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA 295:2164–2167 [DOI] [PubMed] [Google Scholar]
- 2.Hughes DT, Haymart MR, Miller BS, Gauger PG, Doherty GM. 2011. The most commonly occurring papillary thyroid cancer in the United States is now a microcarcinoma in a patient older than 45 years. Thyroid 21:231–236 [DOI] [PubMed] [Google Scholar]
- 3.Nixon IJ, Ganly I, Patel SG, Palmer FL, Whitcher MM, Ghossein R, Tuttle MR, Shaha AR, Shah JP. 2012. Changing trends in well differentiated thyroid carcinoma over eight decades. Int J Surg 10:618–623 [DOI] [PubMed] [Google Scholar]
- 4.Hay ID, Thompson GB, Grant CS, Bergstralh EJ, Dvorak CE, Gorman CA, Maurer MS, McIver B, Mullan BP, Oberg AL, Powell CC, van Heerden JA, Goellner JR. 2002. Papillary thyroid carcinoma managed at the Mayo Clinic during six decades (1940–1999): temporal trends in initial therapy and long-term outcome in 2444 consecutively treated patients. World J Surg 26:879–885 [DOI] [PubMed] [Google Scholar]
- 5.Morris LG, Myssiorek D. 2010. Improved detection does not fully explain the rising incidence of well-differentiated thyroid cancer: a population-based analysis. Am J Surg 200:454–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Segal K, Shpitzer T, Hazan A, Bachar G, Marshak G, Popovtzer A. 2006. Invasive well-differentiated thyroid carcinoma: effect of treatment modalities on outcome. Otolaryngol Head Neck Surg 134:819–822 [DOI] [PubMed] [Google Scholar]
- 7.Falk SA, McCaffrey TV. 1995. Management of the recurrent laryngeal nerve in suspected and proven thyroid cancer. Otolaryngol Head Neck Surg 113:42–48 [DOI] [PubMed] [Google Scholar]
- 8.Hay ID, Bergstralh EJ, Goellner JR, Ebersold JR, Grant CS. 1993. Predicting outcome in papillary thyroid carcinoma: development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery 114:1050–1057; discussion 1057–1058 [PubMed] [Google Scholar]
- 9.Grillo HC, Zannini P. 1986. Resectional management of airway invasion by thyroid carcinoma. Ann Thorac Surg 42:287–298 [DOI] [PubMed] [Google Scholar]
- 10.Andersen PE, Kinsella J, Loree TR, Shaha AR, Shah JP. 1995. Differentiated carcinoma of the thyroid with extrathyroidal extension. Am J Surg 170:467–470 [DOI] [PubMed] [Google Scholar]
- 11.Kowalski LP, Filho JG. 2002. Results of the treatment of locally invasive thyroid carcinoma. Head Neck 24:340–344 [DOI] [PubMed] [Google Scholar]
- 12.Robbins RJ, Wan Q, Grewal RK, Reibke R, Gonen M, Strauss HW, Tuttle RM, Drucker W, Larson SM. 2006. Real-time prognosis for metastatic thyroid carcinoma based on 2-[18F]fluoro-2-deoxy-D-glucose-positron emission tomography scanning. J Clin Endocrinol Metab 91:498–505 [DOI] [PubMed] [Google Scholar]
- 13.Lang BH, Lo CY, Chan WF, Lam KY, Wan KY. 2007. Prognostic factors in papillary and follicular thyroid carcinoma: their implications for cancer staging. Ann Surg Oncol 14:730–738 [DOI] [PubMed] [Google Scholar]
- 14.Czaja JM, McCaffrey TV. 1997. The surgical management of laryngotracheal invasion by well-differentiated papillary thyroid carcinoma. Arch Otolaryngol Head Neck Surg 123:484–490 [DOI] [PubMed] [Google Scholar]
- 15.McCaffrey TV, Lipton RJ. 1990. Thyroid carcinoma invading the upper aerodigestive system. Laryngoscope 100:824–830 [DOI] [PubMed] [Google Scholar]
- 16.Machens A, Hinze R, Lautenschlager C, Thomusch O, Dralle H. 2001. Thyroid carcinoma invading the cervicovisceral axis: routes of invasion and clinical implications. Surgery 129:23–28 [DOI] [PubMed] [Google Scholar]
- 17.McCaffrey TV, Bergstralh EJ, Hay ID. 1994. Locally invasive papillary thyroid carcinoma: 1940–1990. Head Neck 16:165–172 [DOI] [PubMed] [Google Scholar]
- 18.Shin DH, Mark EJ, Suen HC, Grillo HC. 1993. Pathologic staging of papillary carcinoma of the thyroid with airway invasion based on the anatomic manner of extension to the trachea: a clinicopathologic study based on 22 patients who underwent thyroidectomy and airway resection. Hum Pathol 24:866–870 [DOI] [PubMed] [Google Scholar]
- 19.Kamani D, Darr EA, Randolph GW. 2013. Electrophysiologic monitoring characteristics of the recurrent laryngeal nerve preoperatively paralyzed or invaded with malignancy. Otolaryngol Head Neck Surg 149:682–688 [DOI] [PubMed] [Google Scholar]
- 20.Gaissert HA, Honings J, Grillo HC, Donahue DM, Wain JC, Wright CD, Mathisen DJ. 2007. Segmental laryngotracheal and tracheal resection for invasive thyroid carcinoma. Ann Thorac Surg 83:1952–1959 [DOI] [PubMed] [Google Scholar]
- 21.Jeannon JP, Orabi AA, Bruch GA, Abdalsalam HA, Simo R. 2009. Diagnosis of recurrent laryngeal nerve palsy after thyroidectomy: a systematic review. Int J Clin Pract 63:624–629 [DOI] [PubMed] [Google Scholar]
- 22.Kamaya A, Tahvildari AM, Patel BN, Willmann JK, Jeffrey RB, Desser TS. 2015. Sonographic detection of extracapsular extension in papillary thyroid cancer. J Ultrasound Med 34:2225–2230 [DOI] [PubMed] [Google Scholar]
- 23.Padovani RP, Kasamatsu TS, Nakabashi CC, Camacho CP, Andreoni DM, Malouf EZ, Marone MM, Maciel RM, Biscolla RP. 2012. One month is sufficient for urinary iodine to return to its baseline value after the use of water-soluble iodinated contrast agents in post-thyroidectomy patients requiring radioiodine therapy. Thyroid 22:926–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yeh MW, Bauer AJ, Bernet VA, Ferris RL, Loevner LA, Mandel SJ, Orloff LA, Randolph GW, Steward DL, American Thyroid Association Surgical Affairs Committee Writing Task Force 2015. American Thyroid Association statement on preoperative imaging for thyroid cancer surgery. Thyroid 25:3–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moon SH, Oh YL, Choi JY, Baek CH, Son YI, Jeong HS, Choe YS, Lee KH, Kim BT. 2013. Comparison of 18F-fluorodeoxyglucose uptake with the expressions of glucose transporter type 1 and Na+/I- symporter in patients with untreated papillary thyroid carcinoma. Endocr Res 38:77–84 [DOI] [PubMed] [Google Scholar]
- 26.Shaha AR. 2004. Implications of prognostic factors and risk groups in the management of differentiated thyroid cancer. Laryngoscope 114:393–402 [DOI] [PubMed] [Google Scholar]
- 27.Wada N, Nakayama H, Masudo Y, Suganuma N, Rino Y. 2006. Clinical outcome of different modes of resection in papillary thyroid carcinomas with laryngotracheal invasion. Langenbecks Arch Surg 391:545–549 [DOI] [PubMed] [Google Scholar]
- 28.Price DL, Wong RJ, Randolph GW. 2008. Invasive thyroid cancer: management of the trachea and esophagus. Otolaryngol Clin North Am 41:1155–1168, ix-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah JP, Patel SG, Singh B. 2012. Jatin Shah's Head and Neck Surgery and Oncology. 4th ed. Elsevier/Mosby, Philadelphia, PA [Google Scholar]
- 30.Youngwirth LM, Adam MA, Scheri RP, Roman SA, Sosa JA. 2016. Patients treated at low-volume centers have higher rates of incomplete resection and compromised outcomes: analysis of 31,129 patients with papillary thyroid cancer. Ann Surg Oncol 23:403–409 [DOI] [PubMed] [Google Scholar]
- 31.Shindo ML, Caruana SM, Kandil E, McCaffrey JC, Orloff LA, Porterfield JR, Shaha A, Shin J, Terris D, Randolph G. 2014. Management of invasive well-differentiated thyroid cancer: an American Head and Neck Society consensus statement. AHNS consensus statement. Head Neck 36:1379–1390 [DOI] [PubMed] [Google Scholar]
- 32.Kiess AP, Agrawal N, Brierley JD, Duvvuri U, Ferris RL, Genden E, Wong RJ, Tuttle RM, Lee NY, Randolph GW. 2016. External-beam radiotherapy for differentiated thyroid cancer locoregional control: a statement of the American Head and Neck Society. Head Neck 38:493–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grillo HC, Suen HC, Mathisen DJ, Wain JC. 1992. Resectional management of thyroid carcinoma invading the airway. Ann Thorac Surg 54:3–9; discussion 9–10 [DOI] [PubMed] [Google Scholar]
- 34.Musholt TJ, Musholt PB, Behrend M, Raab R, Scheumann GF, Klempnauer J. 1999. Invasive differentiated thyroid carcinoma: tracheal resection and reconstruction procedures in the hands of the endocrine surgeon. Surgery 126:1078–1087; discussion 1087–1078 [DOI] [PubMed] [Google Scholar]
- 35.Bayles SW, Kingdom TT, Carlson GW. 1998. Management of thyroid carcinoma invading the aerodigestive tract. Laryngoscope 108:1402–1407 [DOI] [PubMed] [Google Scholar]
- 36.Honings J, Stephen AE, Marres HA, Gaissert HA. 2010. The management of thyroid carcinoma invading the larynx or trachea. Laryngoscope 120:682–689 [DOI] [PubMed] [Google Scholar]
- 37.Fujimoto Y, Obara T, Ito Y, Kodama T, Yashiro T, Yamashita T, Nozaki M, Suzuki K. 1986. Aggressive surgical approach for locally invasive papillary carcinoma of the thyroid in patients over forty-five years of age. Surgery 100:1098–1107 [PubMed] [Google Scholar]
- 38.McCaffrey JC. 2000. Evaluation and treatment of aerodigestive tract invasion by well-differentiated thyroid carcinoma. Cancer Control 7:246–252 [DOI] [PubMed] [Google Scholar]
- 39.Nishida T, Nakao K, Hamaji M. 1997. Differentiated thyroid carcinoma with airway invasion: indication for tracheal resection based on the extent of cancer invasion. J Thorac Cardiovasc Surg 114:84–92 [DOI] [PubMed] [Google Scholar]
- 40.Xu XF, Li ZJ, Wang X, Tang PZ. 2004. [The management and prognosis of laryngotracheal invasion by well-differentiated thyroid carcinoma]. Zhonghua yi xue za zhi 84:1888–1891. (In Chinese.) [PubMed] [Google Scholar]
- 41.Tsukahara K, Sugitani I, Kawabata K. 2009. Surgical management of tracheal shaving for papillary thyroid carcinoma with tracheal invasion. Acta Otolaryngol 129:1498–1502 [DOI] [PubMed] [Google Scholar]
- 42.Breaux GP, Jr, Guillamondegui OM. 1980. Treatment of locally invasive carcinoma of the thyroid: how radical? Am J Surg 140:514–517 [DOI] [PubMed] [Google Scholar]
- 43.Kim H, Jung HJ, Lee SY, Kwon TK, Kim KH, Sung MW, Hun Hah J. 2016. Prognostic factors of locally invasive well-differentiated thyroid carcinoma involving the trachea. Eur Arch Otorhinolaryngol 273:1919–1926 [DOI] [PubMed] [Google Scholar]
- 44.Li S, Li Y, Tang Q, He X, Liu J, Liu B, Yang M, Yang X. 2014. [Management and prognosis of differentiated thyroid carcinoma with tracheal invasion]. Zhonghua er bi yan hou tou jing wai ke za zhi 49:802–806. (In Chinese.) [PubMed] [Google Scholar]
- 45.Varvares MA, Walker RJ. 2016. Letter to the editor regarding: Management of invasive well-differentiated thyroid cancer: an American Head and Neck Society Consensus Statement. Head Neck 38:328–329 [DOI] [PubMed] [Google Scholar]
- 46.Nishida T, Nakao K, Hamaji M, Kamiike W, Kurozumi K, Matsuda H. 1997. Preservation of recurrent laryngeal nerve invaded by differentiated thyroid cancer. Ann Surg 226:85–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chandrasekhar SS, Randolph GW, Seidman MD, Rosenfeld RM, Angelos P, Barkmeier-Kraemer J, Benninger MS, Blumin JH, Dennis G, Hanks J, Haymart MR, Kloos RT, Seals B, Schreibstein JM, Thomas MA, Waddington C, Warren B, Robertson PJ; American Academy of Otolaryngology-Head and Neck Surgery 2013. Clinical practice guideline: improving voice outcomes after thyroid surgery. Otolaryngol Head Neck Surg 148:S1–S37 [DOI] [PubMed] [Google Scholar]
- 48.Randolph GW, Kamani D. 2014. Intraoperative neural monitoring in thyroid cancer surgery. Langenbecks Arch Surg 399:199–207 [DOI] [PubMed] [Google Scholar]
- 49.Nixon IJ, Wang LY, Ganly I, Patel SG, Morris LG, Migliacci JC, Tuttle RM, Shah JP, Shaha AR. 2016. Outcomes for patients with papillary thyroid cancer who do not undergo prophylactic central neck dissection. Br J Surg 103:218–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shi L, Song H, Zhu H, Li D, Zhang N. 2013. Pattern, predictors, and recurrence of cervical lymph node metastases in papillary thyroid cancer. Contemp Oncol (Pozn) 17:504–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haugen BRM, Alexander EK, Bible KC, Doherty G, Mandel SJ, Nikiforov YE, Pacini F, Randolph G, Sawka A, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward D, Tuttle RMM, Wartofsky L. 2016. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer. Thyroid 26:1–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perros P, Boelaert K, Colley S, Evans C, Evans RM, Gerrard Ba G, Gilbert J, Harrison B, Johnson SJ, Giles TE, Moss L, Lewington V, Newbold K, Taylor J, Thakker RV, Watkinson J, Williams GR, British Thyroid A. 2014. Guidelines for the management of thyroid cancer. Clin Endocrinol (Oxf) 81(Suppl 1):1–122 [DOI] [PubMed] [Google Scholar]
- 53.Shaha AR, Shah JP, Loree TR. 1996. Patterns of nodal and distant metastasis based on histologic varieties in differentiated carcinoma of the thyroid. Am J Surg 172:692–694 [DOI] [PubMed] [Google Scholar]
- 54.La Quaglia MP, Black T, Holcomb GW, 3rd, Sklar C, Azizkhan RG, Haase GM, Newman KD. 2000. Differentiated thyroid cancer: clinical characteristics, treatment, and outcome in patients under 21 years of age who present with distant metastases. A report from the Surgical Discipline Committee of the Children's Cancer Group. J Pediatr Surg 35:955–959; discussion 960 [DOI] [PubMed] [Google Scholar]
- 55.Lin JD, Chao TC, Chou SC, Hsueh C. 2004. Papillary thyroid carcinomas with lung metastases. Thyroid 14:1091–1096 [DOI] [PubMed] [Google Scholar]
- 56.Wang LY, Palmer FL, Nixon IJ, Thomas D, Patel SG, Shaha AR, Shah JP, Tuttle RM, Ganly I. 2014. Multi-organ distant metastases confer worse disease-specific survival in differentiated thyroid cancer. Thyroid 24:1594–1599 [DOI] [PubMed] [Google Scholar]
- 57.Shoup M, Stojadinovic A, Nissan A, Ghossein RA, Freedman S, Brennan MF, Shah JP, Shaha AR. 2003. Prognostic indicators of outcomes in patients with distant metastases from differentiated thyroid carcinoma. J Am Coll Surg 197:191–197 [DOI] [PubMed] [Google Scholar]
- 58.Fatourechi V, Hay ID, Javedan H, Wiseman GA, Mullan BP, Gorman CA. 2002. Lack of impact of radioiodine therapy in tg-positive, diagnostic whole-body scan-negative patients with follicular cell-derived thyroid cancer. J Clin Endocrinol Metab 87:1521–1526 [DOI] [PubMed] [Google Scholar]
- 59.Jung TS, Kim TY, Kim KW, Oh YL, Park DJ, Cho BY, Shong YK, Kim WB, Park YJ, Jung JH, Chung JH. 2007. Clinical features and prognostic factors for survival in patients with poorly differentiated thyroid carcinoma and comparison to the patients with the aggressive variants of papillary thyroid carcinoma. Endocr J 54:265–274 [DOI] [PubMed] [Google Scholar]
- 60.Biermann M, Pixberg M, Riemann B, Schuck A, Heinecke A, Schmid KW, Willich N, Dralle H, Schober O, group Ms. 2009. Clinical outcomes of adjuvant external-beam radiotherapy for differentiated thyroid cancer—results after 874 patient-years of follow-up in the MSDS-trial. Nuklearmedizin 48:89–98; quiz N15 [DOI] [PubMed] [Google Scholar]
- 61.Chow SM, Yau S, Kwan CK, Poon PC, Law SC. 2006. Local and regional control in patients with papillary thyroid carcinoma: specific indications of external radiotherapy and radioactive iodine according to T and N categories in AJCC 6th edition. Endocr Relat Cancer 13:1159–1172 [DOI] [PubMed] [Google Scholar]
- 62.Terezakis SA, Lee KS, Ghossein RA, Rivera M, Tuttle RM, Wolden SL, Zelefsky MJ, Wong RJ, Patel SG, Pfister DG, Shaha AR, Lee NY. 2009. Role of external beam radiotherapy in patients with advanced or recurrent nonanaplastic thyroid cancer: Memorial Sloan-Kettering Cancer Center experience. Int J Radiat Oncol Biol Phys 73:795–801 [DOI] [PubMed] [Google Scholar]
- 63.Romesser PB, Sherman EJ, Shaha AR, Lian M, Wong RJ, Sabra M, Rao SS, Fagin JA, Tuttle RM, Lee NY. 2014. External beam radiotherapy with or without concurrent chemotherapy in advanced or recurrent non-anaplastic non-medullary thyroid cancer. J Surg Oncol 110:375–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schwartz DL, Lobo MJ, Ang KK, Morrison WH, Rosenthal DI, Ahamad A, Evans DB, Clayman G, Sherman SI, Garden AS. 2009. Postoperative external beam radiotherapy for differentiated thyroid cancer: outcomes and morbidity with conformal treatment. Int J Radiat Oncol Biol Phys 74:1083–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brose MS, Nutting CM, Sherman SI, Shong YK, Smit JW, Reike G, Chung J, Kalmus J, Kappeler C, Schlumberger M. 2011. Rationale and design of decision: a double-blind, randomized, placebo-controlled phase III trial evaluating the efficacy and safety of sorafenib in patients with locally advanced or metastatic radioactive iodine (RAI)-refractory, differentiated thyroid cancer. BMC Cancer 11:349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, Habra MA, Newbold K, Shah MH, Hoff AO, Gianoukakis AG, Kiyota N, Taylor MH, Kim SB, Krzyzanowska MK, Dutcus CE, de las Heras B, Zhu J, Sherman SI. 2015. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med 372:621–630 [DOI] [PubMed] [Google Scholar]
- 67.Dadu R, Cabanillas ME. 2012. Optimizing therapy for radioactive iodine-refractory differentiated thyroid cancer: current state of the art and future directions. Minerva Endocrinol 37:335–356 [PMC free article] [PubMed] [Google Scholar]
- 68.Danilovic D, Castro G, Kulsar M, Maia MC, Bonani F, Marui S, Hoff A. 2015. Potential role of sorafenib as neoadjuvant therapy in unresectable papillary thyroid cancer. Endocrine Society's 97th Annual Meeting and Expo, San Diego, CA [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tollefsen HR, Decosse JJ, Hutter RV. 1964. Papillary carcinoma of the thyroid. A clinical and pathological study of 70 fatal cases. Cancer 17:1035–1044 [DOI] [PubMed] [Google Scholar]
- 70.Smith SA, Hay ID, Goellner JR, Ryan JJ, McConahey WM. 1988. Mortality from papillary thyroid carcinoma. A case-control study of 56 lethal cases. Cancer 62:1381–1388 [DOI] [PubMed] [Google Scholar]
- 71.Kobayashi T, Asakawa H, Tamaki Y, Umeshita K, Monden M. 1996. Fatal differentiated thyroid cancer. J Surg Oncol 62:123–127 [DOI] [PubMed] [Google Scholar]
- 72.Kitamura Y, Shimizu K, Nagahama M, Sugino K, Ozaki O, Mimura T, Ito K, Tanaka S. 1999. Immediate causes of death in thyroid carcinoma: clinicopathological analysis of 161 fatal cases. J Clin Endocrinol Metab 84:4043–4049 [DOI] [PubMed] [Google Scholar]
- 73.Ronga G, Filesi M, Montesano T, Melacrinis FF, Di Nicola A, Ventroni G, Antonaci A, Vestri AR. 2002. Death from differentiated thyroid carcinoma: retrospective study of a 40-year investigation. Cancer Biother Radiopharm 17:507–514 [DOI] [PubMed] [Google Scholar]
- 74.Nixon IJ, Ganly I, Palmer FL, Whitcher MM, Patel SG, Tuttle RM, Shaha AR, Shah JP. 2011. Disease-related death in patients who were considered free of macroscopic disease after initial treatment of well-differentiated thyroid carcinoma. Thyroid 21:501–504 [DOI] [PubMed] [Google Scholar]





