Abstract
Background: We examined outcomes in patients treated for radioactive iodine–induced sialadenitis (RAIS) and xerostomia with sialendoscopy.
Methods: Data was prospectively collected for all patients undergoing sialendoscopy for RAIS from a single institution. Interventional details and intraoperative findings were recorded. Qualitative data were obtained through patient examination, telephone interviews, and use of a standard quality of life questionnaire, Xerostomia Questionnaire. Quantitative data were obtained from patients who underwent sialometry.
Results: Twenty-six patients (24 women and 2 men; median age, 43 years; age range, 19–57 years) underwent interventional sialendoscopy after conservative management of symptoms proved unsuccessful. Sialadenitis was present in 25 patients and xerostomia in 22 patients. Mucus plugging in the duct of the gland was the most common finding (22 patients) followed by stenosis (18 patients), inflammation (eight patients), and erythema (eight patients). Median follow-up time was 23.4±12.1 months. Sixteen patients (64%) reported complete resolution; seven (28%), partial resolution; one (4%), no change in symptoms; and one (4%), regression in RAIS-related symptoms. Patients subjectively noted the following regarding their xerostomia symptoms: seven (31.8%) had complete resolution; 10 (45.5%), partial resolution; four (18.2%), no change; and one (4.5%), regression. Statistical analysis of the available sialometry data revealed a statistically significant difference in saliva production at 6 months following sialendoscopy for unstimulated saliva production (p=0.028).
Conclusion: Sialendoscopy is an effective treatment option for the management of RAIS and xerostomia refractory to conservative therapy and medical management. Patients in our cohort report durable improvement in symptoms after intervention.
Introduction
Radioactive iodine (RAI) is frequently used to ablate remnant thyroid tissue in patients who have undergone a thyroidectomy for the treatment of thyroid cancer. The use of RAI has been described as the single most important factor related to favorable disease-free survival (1). However, the use of RAI is associated with significant side effects affecting quality of life. Early and late sialadenitis occurs most frequently (2). Approximately 10%–60% of patients treated with RAI report acute or chronic salivary dysfunction related symptoms (3–7). Symptoms of sialadenitis include pain and swelling of the affected glands, trismus, sour taste in the mouth, and facial weakness. Patients with xerostomia often complain of oral discomfort, pain, increased rate of dental caries and oral infection, dysphagia, dysgeusia, decreased nutritional intake, and consequently reduced body weight (8). Rare serious complications include facial paresis/paralysis and development of salivary gland neoplasms.
The mechanism of salivary injury from RAI occurs via accumulation of 131I in the salivary ductal cells with concentrations of up to 20–100 times higher than plasma levels. The iodine uptake may be mediated by the sodium-iodide symporter, which is expressed in various extrathyroidal tissues, including the ductal cells of the salivary glands (9,10). Serous acini have shown increased uptake of RAI compared to mucinous acini, resulting in injury of the parotid glands more commonly than the submandibular and sublingual glands (2,11).
Conservative management of sialadenitis has traditionally involved adequate hydration, salivary gland massages, sialagogues (i.e., sour candies, juices), warm compresses, systemic steroid doses, and cholinergic medications (12). Sialadenectomy is performed as a last resort when conservative management has failed; nerve weakness or paralysis has been reported in 20%–60% of cases of sialadenectomy for chronic sialadenitis (13,14). Sialendoscopy has been reported to be a potential alternative treatment option (4,12,15,16). This procedure is a minimally invasive technique that avoids the need for external surgery and has been shown to have minimal operative morbidity in sialadenitis cases of varying etiologies (4,17,18).
This study examines the effectiveness of sialendoscopy for salivary gland–related symptoms, specifically sialadenitis and xerostomia, in patients who have been treated with RAI. While previous reports in the literature only focused on the subjective evaluation of patients to determine resolution of symptoms (4,12), our work also quantitates the effect of sialendoscopy on saliva production.
Methods
Patients who underwent sialendoscopy between March 2010 and June 2013 and had salivary gland-related symptoms following RAI therapy that were refractory to conservative management were included in this study. Data collected for each patient from the electronic medical record, following Institutional Review Board approval, included age, sex, race/ethnicity, social history, cancer diagnosis, type of cancer treatment rendered, total dosage of RAI administered, number and location of symptomatic salivary glands, number of sialendoscopy procedures, findings at sialendoscopy, complications during sialendoscopy procedure, symptom resolution, patient satisfaction, and actual quantity of saliva produced before and after sialendoscopy. Data were collected from the medical record when patients presented for routine follow-up visits to the Head and Neck Surgery clinic. Data were also collected via telephone interview for patients who had not been recently seen at our institution for follow-up.
All sialendoscopy procedures were performed on an outpatient basis in the operating room with the patient under general anesthesia. The sialendoscopic technique involved serial dilation and introduction of the Karl Storz multi-channel sialendoscope (Karl Storz Endoscopy, El Segundo, CA). The ductal lumen was inspected and continuously irrigated with saline solution. Debris, mucous plugs, and/or stones were removed as indicated with irrigation, grasping forceps, or wire basket when necessary. At the end of procedure, the ductal system was irrigated with a steroid (Kenalog-40) in 5 mL of sterile saline solution as previously described (4). In some cases, sialodochoplasty or stent placement was performed. Findings from the procedure, as well as the intervention details, were recorded.
Quantitative data on the amount of saliva production before and after sialendoscopy were obtained from the electronic medical record of patients who had undergone sialometry. Unstimulated and stimulated saliva data were collected from patients who had the study performed. For the collection of unstimulated saliva, patients did not receive anything by mouth for a minimum of 30 minutes before collection. The patients were instructed to first clear the mouth by swallowing. With the head held slightly forward, the patients were instructed not to swallow during the 5-minute collection, but instead allow saliva to collect in floor of mouth. Patients were instructed to repeat this procedure four more times for a total collection time of 5 minutes. At the end of the 5 minutes, the collection vials were promptly sealed and weighed. For stimulated saliva collection, a patient held 20 mL of citric acid solution in his or her mouth for 1 minute, and then expectorated into a waste area or container. The stimulated saliva was also collected for a total of 5 minutes.
Qualitative data of patient reported salivary symptoms were obtained during follow-up appointments at the Head and Neck Surgery clinic and by telephone interviews. Additionally, a standard quality of life Xerostomia Questionnaire (XQ) was administered during patient follow-up visits at the head and neck clinic and via telephone interviews (Supplementary Materials; Supplementary Data are available online at www.liebertpub.com/thy).
This study contains descriptive analysis of aggregate data from the patients identified whose disease and recorded information met the criteria described above. Frequencies of study patients within the categories for each of the parameters of interest were enumerated and descriptive statistics on scaled data (i.e., sialometry results, follow-up time, etc.) were calculated. Possible differences between groups for scaled parameters were assessed by one-way ANOVA. The cut-off value for statistical significance was taken to be p<0.05. The statistical calculations were performed with the assistance of the Statistica (version 12, StatSoft, Inc., Tulsa, OK; www.statsoft.com) data analysis software system.
Results
Twenty-six patients underwent sialendoscopy out of 56 patients who were evaluated for concerns and complaints following RAI treatment, including 24 female and two male patients. The median age of patients was 43 years (range, 19–57 years). All patients had undergone RAI treatment. Papillary thyroid carcinoma was the most common malignancy, seen in 20 patients (77%). Four patients (15.4%) presented with the follicular variant of papillary thyroid carcinoma, while poorly differentiated thyroid carcinoma was seen in two patients (7.8%). All patients had a total thyroidectomy. The median RAI dose for patients who had undergone RAI once was 107±34.5 mCi (range, 98–809 mCi). Twenty-three patients had undergone RAI treatment only once, while three patients had undergone RAI treatment four, three, and two times, receiving a total dose of 809, 550, and 400 mCi, respectively.
Patient's symptoms were recorded during their initial visit to the Head and Neck Surgery clinic. Predominant symptoms observed were sialadenitis, described as pain and swelling, in 25 patients (96.2%), 21 patients (80.8%) had pain, and 22 patients (84.6%) experienced xerostomia. Dysgeusia, dysphagia, and trismus were the other symptoms reported (Table 1). Prior to the sialendoscopy procedure, conservative treatment measures, including gland massage, sialagogues, warm compresses, hydration, analgesics, artificial saliva, antibiotics, and steroids, were tried in all cases but did not result in resolution of symptoms. The median time from the date of last RAI dose to the sialendoscopy procedure was 13.8±6.75 months (range, 4.5–33 months). Four patients had more than one sialendoscopy procedure for either worsening of symptoms following the initial procedure or development of symptoms in other major salivary glands.
Table 1.
Patient-Reported Symptoms Following Radioactive Iodine Treatment
| Symptom | n | % |
|---|---|---|
| Swelling | 25 | 96.2 |
| Pain | 21 | 80.8 |
| Xerostomia | 22 | 84.6 |
| Dysgeusia | 5 | 19.2 |
| Dysphagia | 4 | 15.4 |
| Trismus | 1 | 3.8 |
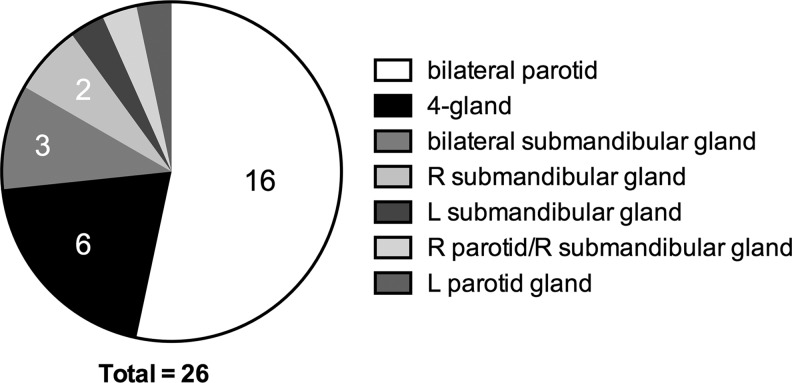
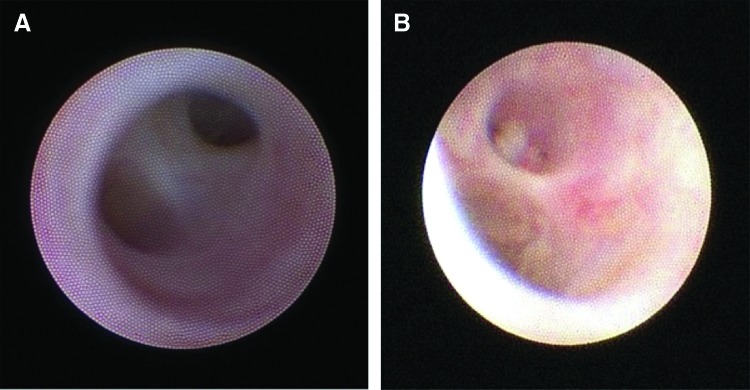
A total of 68 glands were treated with the sialendoscopy procedure. Sixteen patients had bilateral parotid gland endoscopy, six patients underwent endoscopy of all four glands (Fig. 1). Findings at the time of the procedure included the presence of inflammation, paleness of the duct, stenosis, mucous plugs, and erythema (Table 2). No ductal stones were identified. Endoscopic images of normal and RAI-induced sialadenitis (RAIS) ducts are seen in Figure 2. Steroid irrigation was performed to address generalized inflammation of the duct, while stent placement and sialodochoplasty were done to address to localized stenosis.
FIG. 1.
Distribution of glands requiring sialendoscopy. The number of procedures performed is indicated if more than one procedure was done.
Table 2.
Intraoperative Findings During Sialendoscopy
| Finding | n | % |
|---|---|---|
| Mucus plug | 22 | 84.6 |
| Stenosis | 18 | 69.2 |
| Inflammation | 8 | 30.8 |
| Ductal pallor | 11 | 42.3 |
| Papilla effacement | 5 | 19.2 |
FIG. 2.
Intraoperative appearance of a normal (A) and an inflamed (B) parotid duct seen during sialendoscopy. Note the pale mucosa and the duct with retained debris as a nidus for obstruction and infection.
The average follow-up time was calculated from the date of sialendoscopy to the last follow-up appointment with the Head and Neck Surgery team or telephone interview. The median follow-up time was 23.4±12.1 months, with a maximum follow-up time of 40 months (Supplementary Fig. S1). Four patients could not be contacted for the telephone interview and were considered lost to follow-up.
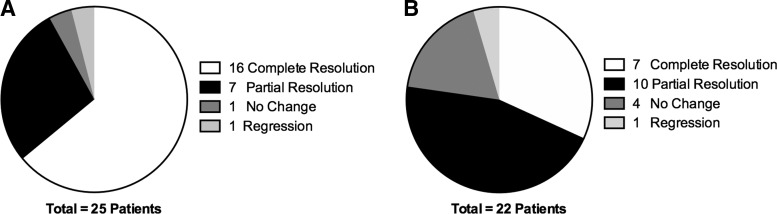
Subjective response to treatment in the 25 patients initially reporting pain and swelling of their salivary glands demonstrated 16 patients (64%) with a complete response, seven patients (28%) with a partial response, and one patient (4%) with no change in symptoms; one patient (4%) had a regression in sialadenitis symptoms (Fig. 3A). Median duration of response to one sialendoscopy treatment was 19.9±12.1 months, with a maximum follow-up of 40.3 months to assess symptom relief.
FIG. 3.
Subjective response to sialendoscopy. Resolution of patient symptoms as related to (A) sialadenitis and (B) xerostomia.
Patient-reported change in xerostomia after sialendoscopy included seven patients (31.8%) with a complete response, 10 patients (45.5%) with a partial response, four patients (18.2%) with no change in their symptoms, and one patient (4.5%) with regression (Fig. 3B).
Nine patients were available to answer a baseline and follow-up XQ. The average follow-up time for the XQs of these nine patients was 19.5 months with a maximum follow-up time of 27.7 months. The mean percentage improvement noted from analysis of data obtained from the XQ was 13.5%. Six out of nine patients had an improved XQ scores, while the remaining three patients experienced regression in their scores.
Seven patients had baseline and 6-month follow-up sialometry data. For unstimulated salivary flow, we found a statistically significant improvement in salivary flow after sialendoscopy (p=0.028) (Supplementary Fig. S2). An evaluation of stimulated salivary flow did not demonstrate a significant improvement in saliva production (p=0.50).
Discussion
This study represents the largest cohort of patients with RAIS. We demonstrated that sialendoscopy has a positive effect on the disease with a median duration of response of 19.9 months; thus, sialendoscopy is an attractive addition to the otherwise limited treatment options in patients with RAIS.
RAI treatment can have deleterious effects on the salivary glands, especially the parotid gland. This effect is likely due to the parotid gland consisting mostly of serous cells, which are more prone to injury from RAI treatment (19). Traditional methods of treating nonneoplastic disorders of the salivary glands are conservative treatment or salivary gland excision (20). Conservative therapy, consisting of salivary massage, sialagogues, and hydration, has been found to control symptoms in 71% of patients in one study (15). For those who do not respond to conservative therapy, surgical excision of the salivary gland carries a risk of significant complications. The application of sialendoscopy in chronic inflammatory salivary disease has expanded beyond obstructive salivary disease related to sialolithiasis.
Symptoms of RAIS include obstructive symptoms, consisting of pain and swelling, and xerostomia, which can be related to direct injury of the RAI to acinar cells (2). Our patient cohort demonstrated significant improvement for obstructive symptoms but not xerostomia; therefore, we report these two symptoms separately in contrast to previous reports in which these symptoms were combined. Based on our results, we believe the separation of obstructive symptoms and xerostomia to be critical in counseling patients.
We found that sialadenitis symptoms are greatly reduced by the sialendoscopy procedure, with 23 patients (92%) having either a partial or complete resolution of obstructive symptoms after only one procedure. Xerostomia symptoms, on the other hand, had partial or complete reduction in 17 patients (77.3%). These findings are consistent with our experience that salivary obstruction and xerostomia are distinct symptom complexes with different mechanisms of onset. The benefits of sialendoscopy in patients with xerostomia are likely due to physical dilation of the salivary papilla and ducts, saline irrigation of the ductal lumen and removal of debris and mucus plugs, which removes any mechanical obstruction to salivary flow without restoration of direct acinar damage following RAI treatment, although this hypothesis requires further investigation with prospectively collected data. Moreover, the addition of cholinergic agents (e.g., pilocarpine or cevimeline) as an adjunct to sialendoscopy may increase salivary flow after the mechanical obstruction has been relieved, which may improve the outcomes of patients who previously did not benefit from sialendoscopy alone.
Previously published reports of sialendoscopy in patients with RAIS have included 15, 6, 12, and 11 patients (4,12,15,21). Success rates reported ranged from 50% (15) to 100% (21). Nahlieli and Nazarian (21) published the first report, which included 15 patients who presented with vague obstructive symptoms, and noted resolution of symptoms after one procedure between 1 and 4 years of follow-up, but follow-up times and exact symptom resolution were not stated. Kim et al. (15) found symptom improvement in three of six patients with sialendoscopy over a duration of 10 months; however, their work did not define the symptoms the patients were experiencing. Bomeli et al. (4) demonstrated 75% improvement in their patient series with limited duration of remission lasting a median of 6 months, with a maximum of 33 months symptom free. Interestingly, one patient in their series underwent four separate interventions without relief. The most recent report from Prendes et al. (12) demonstrated a more complete picture of symptom resolution with 6 of 11 patients (54%) having complete resolution over a median follow-up of 16.5 months and partial improvement in four patients (36%). One patient in their series underwent parotidectomy after two failed endoscopies.
In the previously published series, symptom changes were reported subjectively. Our series demonstrates some objective improvement with sialometry, albeit in a small subset of our patients. Moreover, we found that xerostomia and obstructive symptoms have distinct responses to sialendoscopy. Both sialadenitis and xerostomia are primarily patient-reported issues, and objective measurement of these symptoms has not been reported previously. In addition to subjective evaluation of the patient during clinical examinations and telephone conversations, we performed quantitative evaluation with XQ and sialometry. While the XQ was useful for assessing changes in perceived comfort level following the sialendoscopy procedure, it was influenced by patient disposition and recall at the time of the telephone interview. Sialometry provided an accurate representation of the true quantity of saliva produced by the patient, but this may also vary due to various factors such as changes in medications, time of day, and hydration. Future studies should be directed at addressing these limitations and correlating objective findings with subjective patient responses.
Although our initial plan was to perform XQ and sialometry on every patient, scheduling and follow-up issues prevented consistent testing. One patient in this study, who reported a regression in symptoms of sialadenitis and xerostomia 13 days after the procedure, was unavailable for contact by telephone for follow-up. The quantitative data set was limited because only patients with both a baseline and postoperative assessment at 6 months could be analyzed. Thus, specific comparisons and correlation with qualitative assessment are limited. However, the findings from this study suggest that prospective quantitative and imaging (pre- and post- endoscopy) data could be employed in future studies to determine the effectiveness of sialendoscopy. Further investigation is needed for the application of sialendoscopy to relieve obstructive symptoms and concomitant treatment with cholinergic medications for xerostomia as stated above, which may more fully address the side effects of RAI treatment.
Despite the limitations related to collection of quantitative data, this study contains the largest series of patients with RAI-induced sialadenitis and xerostomia undergoing sialendoscopy compared to previously published reports. It also has extended follow-up time. The experience demonstrates that the benefits of sialendoscopy for RAIS appear to persist over the long term.
Conclusion
Sialendoscopy appears to be a viable therapeutic option in the management of RAIS when conservative measures have proven unsuccessful. The beneficial effects are most often seen in patients with obstructive sialadenitis and to a lesser extent, in patients with RAI-induced xerostomia.
Supplementary Material
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Samaan NA, Schultz PN, Hickey RC, Goepfert H, Haynie TP, Johnston DA, Ordonez NG. 1992. The results of various modalities of treatment of well differentiated thyroid carcinomas: a retrospective review of 1599 patients. J Clin Endocrinol Metab 75:714–720 [DOI] [PubMed] [Google Scholar]
- 2.Mandel SJ, Mandel L. 2003. Radioactive iodine and the salivary glands. Thyroid 13:265–271 [DOI] [PubMed] [Google Scholar]
- 3.Allweiss P, Braunstein GD, Katz A, Waxman A. 1984. Sialadenitis following I-131 therapy for thyroid carcinoma: concise communication. J Nucl Med 25:755–758 [PubMed] [Google Scholar]
- 4.Bomeli SR, Schaitkin B, Carrau RL, Walvekar RR. 2009. Interventional sialendoscopy for treatment of radioiodine-induced sialadenitis. Laryngoscope 119:864–867 [DOI] [PubMed] [Google Scholar]
- 5.DiRusso G, Kern KA. 1994. Comparative analysis of complications from I-131 radioablation for well-differentiated thyroid cancer. Surgery 116:1024. [PubMed] [Google Scholar]
- 6.Edmonds C, Smith T. 1986. The long-term hazards of the treatment of thyroid cancer with radioiodine. Br J Radiol 59:45–51 [DOI] [PubMed] [Google Scholar]
- 7.Jeong SY, Lee J. 2010. Radiation sialadenitis induced by high-dose radioactive iodine therapy. Nucl Med Mol Imaging 44:102–109 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Chambers MS, Garden AS, Kies MS, Martin JW. 2004. Radiation-induced xerostomia in patients with head and neck cancer: pathogenesis, impact on quality of life, and management. Head Neck 26:796–807 [DOI] [PubMed] [Google Scholar]
- 9.Van Nostrand D. 2011. Sialoadenitis secondary to 131I therapy for well-differentiated thyroid cancer. Oral Dis 17:154–161 [DOI] [PubMed] [Google Scholar]
- 10.La Perle KM, Kim DC, Hall NC, Bobbey A, Shen DH, Nagy RS, Wakely PE, Jr, Lehman A, Jarjoura D, Jhiang SM. 2013. Modulation of sodium/iodide symporter expression in the salivary gland. Thyroid 23:1029–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Helman J, Turner RJ, Fox PC, Baum BJ. 1987. 99mTc-pertechnetate uptake in parotid acinar cells by the Na+/K+/Cl− co-transport system. J Clin Invest 79:1310–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prendes BL, Orloff LA, Eisele DW. 2012. Therapeutic sialendoscopy for the management of radioiodine sialadenitis. Arch Otolaryngol Head Neck Surg 138:15–19 [DOI] [PubMed] [Google Scholar]
- 13.Bron LP, O'Brien CJ. 1997. Facial nerve function after parotidectomy. Arch Otolaryngol Head Neck Surg 123:1091–1096 [DOI] [PubMed] [Google Scholar]
- 14.Amin MA, Bailey BM, Patel SR. 2001. Clinical and radiological evidence to support superficial parotidectomy as the treatment of choice for chronic parotid sialadenitis: a retrospective study. Br J Oral Maxillofac Surg 39:348–352 [DOI] [PubMed] [Google Scholar]
- 15.Kim JW, Han GS, Lee SH, Lee DY, Kim YM. 2007. Sialoendoscopic treatment for radioiodine induced sialadenitis. Laryngoscope 117:133–136 [DOI] [PubMed] [Google Scholar]
- 16.Nahlieli O, Nazarian Y. 2006. Sialadenitis following radioiodine therapy–a new diagnostic and treatment modality. Oral Dis 12:476–479 [DOI] [PubMed] [Google Scholar]
- 17.Strychowsky JE, Sommer DD, Gupta MK, Cohen N, Nahlieli O. 2012. Sialendoscopy for the management of obstructive salivary gland disease: a systematic review and meta-analysis. Arch Otolaryngol Head Neck Surg 138:541–547 [DOI] [PubMed] [Google Scholar]
- 18.Zenk J, Koch M, Klintworth N, Konig B, Konz K, Gillespie MB, Iro H. 2012. Sialendoscopy in the diagnosis and treatment of sialolithiasis: a study on more than 1000 patients. Otolaryngol Head Neck Surg 147:858–863 [DOI] [PubMed] [Google Scholar]
- 19.Bowen MA, Tauzin M, Kluka EA, Nuss DW, DiLeo M, McWhorter AJ, Schaitkin B, Walvekar RR. 2011. Diagnostic and interventional sialendoscopy: a preliminary experience. Laryngoscope 121:299–303 [DOI] [PubMed] [Google Scholar]
- 20.Marchal F, Kurt A-M, Dulguerov P, Becker M, Oedman M, Lehmann W. 2001. Histopathology of submandibular glands removed for sialolithiasis. Ann Otol Rhinol Laryngol 110:464–469 [DOI] [PubMed] [Google Scholar]
- 21.Nahlieli O, Nazarian Y. 2006. Sialadenitis following radioiodine therapy—a new diagnostic and treatment modality. Oral Dis 12:476–479 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.