Abstract
Current therapies against toxoplasmosis are limited, and drugs have significant side effects and low efficacies. We evaluated the potential anti-Toxoplasma activity of propranolol at a dose of 2 or 3 mg/kg of body weight/day in vivo in the acute and chronic phases. Propranolol as a cell membrane-stabilizing agent is a suitable drug for inhibiting the entrance of Toxoplasma gondii tachyzoites into cells. The acute-phase assay was performed using propranolol, pyrimethamine, and propranolol plus pyrimethamine before (pretreatment) and after (posttreatment) intraperitoneal challenge with 1 × 103 tachyzoites of the virulent T. gondii strain RH in BALB/c mice. Also, in the chronic phase, treatment was performed 12 h before intraperitoneal challenge with 1 × 106 tachyzoites of the virulent strain RH of T. gondii in rats. One week (in the acute phase) and 2 months (in the chronic phase) after postinfection, tissues were isolated and DNA was extracted. Subsequently, parasite load was calculated using quantitative PCR (qPCR). In the acute phase, in both groups, significant anti-Toxoplasma activity was observed using propranolol (P < 0.001). Propranolol in the pretreatment group showed higher anti-Toxoplasma activity than propranolol in posttreatment in brain tissues, displaying therapeutic efficiency on toxoplasmosis. Also, propranolol combined with pyrimethamine reduced the parasite load as well as significantly increased survival of mice in the pretreatment group. In the chronic phase, anti-Toxoplasma activity and decreased parasite load in tissues were observed with propranolol. In conclusion, the presented results demonstrate that propranolol, as an orally available drug, is effective at low doses against acute and latent murine toxoplasmosis, and the efficiency of the drug is increased when it is used in combination therapy with pyrimethamine.
INTRODUCTION
Toxoplasmosis is a widespread infection caused by the obligate intracellular protozoan parasite Toxoplasma gondii (1). Toxoplasma is classified by the National Institutes of Health and Centers for Disease Control (CDC) as a category B human-infectious pathogen. Over 1 billion people are estimated to be infected with T. gondii globally (2). Toxoplasmosis in immunocompetent people is usually asymptomatic or appears as flulike symptoms, swollen lymph glands, muscle aches, and pain that may last from a few days to several weeks (3). In HIV-positive individuals and patients with other immunosuppressive disorders, toxoplasmosis may cause severe symptoms, such as necrotizing encephalitis, pneumonitis, chorioretinitis, and myocarditis, and death (4, 5).
Furthermore, acute infection during pregnancy is a major cause of spontaneous abortion, gastrointestinal complications, intrauterine fetal death, preterm delivery, or serious fetal malformations, including encephalitis and ocular and cerebral syndromes. Recently, infections with T. gondii have also been linked to possible increased risk for autism and early onset of schizophrenia (2, 5). In addition, toxoplasmosis is considered the third most common nosocomially acquired foodborne disease and can lead to death if it is not treated (2).
The recommended drugs for treatment or prophylaxis of toxoplasmosis are pyrimethamine and sulfadiazine. These drugs have side effects such as neutropenia, severe drop of platelet count, thrombocytopenia, leucopenia, elevation in serum creatinine and serum liver enzymes, hematological abnormalities, and hypersensitivity reactions (6). In addition, other drugs, such as azithromycin, clarithromycin, spiramycin, atovaquone, dapsone, and co-trimoxazole (trimethoprim-sulfamethoxazole), have been used for clinical toxoplasmosis (7–10). Spiramycin monotherapy can be effective during the early stage of pregnancy to prevent prenatal transmission. More than 50% of patients treated with spiramycin retained T. gondii DNA in blood and remained infected (2). So, for prevention/eradication of the chronic infection or protection against congenital toxoplasmosis, current chemotherapy is not safe or effective. Therefore, it is necessary to investigate new and more-effective therapeutic agents with low toxicity to treat this disease (2, 7, 11).
T. gondii during the host cell invasion attaches to the host cell with its apical end. This mechanism often occurs as a direct result of active gliding motility. Moreover, surface antigens such as perforinlike protein aid in the interaction between the tachyzoite and the host cell. The process is accompanied by three sequentially discharged sets of morphologically and functionally distinct secretory organelles: micronemes, rhoptries, and dense granules. Invasion occurs faster than phagocytosis. Disruption of any of these essential functions would be expected to kill or inhibit the parasite (12, 13).
It is known that the cell membrane-stabilizing drugs change the resistance of the cell membrane by blocking the actin gel and interfering with microfilament functions (14, 15). Previously, we have shown that ketotifen at 2 mg/kg of body weight (16), chromolyn sodium at 10 mg/kg (17), and propranolol at 2 or 3 mg/kg (18) used as cell membrane-stabilizing drugs inhibited T. gondii penetration into nucleated cells. The aim of the present study was to determine propranolol anti-Toxoplasma activity during the acute and chronic phases of experimental toxoplasmosis in a murine model.
MATERIALS AND METHODS
Parasites used in acute and chronic phases.
Tachyzoites of the virulent strain RH of T. gondii, routinely obtained by serial intraperitoneal passages in BALB/c female mice, were used for experiments. To prepare fresh tachyzoites, briefly, 0.5 ml of parasite suspension in sterile phosphate-buffered saline (PBS; pH 7.4) containing 100 IU/ml penicillin and 100 μg/ml streptomycin was injected in the mouse peritoneum, and tachyzoites were harvested after 3 to 4 days from peritoneal exudates. The number of tachyzoites was determined by counting in a hemocytometer under a light microscope. For challenge, suspensions adjusted to 1 × 103 tachyzoites/ml of PBS were inoculated intraperitoneally to female BALB/c mice in the acute phase. In the chronic phase, 1 × 106 tachyzoites/ml were inoculated intraperitoneally into female rats (19, 20).
Mice in the acute phase and rats in the chronic phase.
Six-week-old inbred female BALB/c mice weighing 18 to 20 g were used for this experiment in the acute phase; 8- to 10-week-old female rats weighing 100 to 120 g were used for this experiment in the chronic phase. The study underwent ethical review and was given approval by the Ethics Committee of Mazandaran University of Medical Sciences. Care and use of experimental animals complied with local animal welfare laws, guidelines, and policies. All experimental animals were housed in cages (n = 4) under standard laboratory conditions (with an average temperature of 20 to 25°C) and were given drinking water and a regular mouse or rat diet (21).
Experimental design and groups in acute and chronic phases.
Acute-phase experiments were performed in two separate pretreatment and posttreatment groups. In each group, 24 female BALB/c mice in six subgroups (n = 4) were used for the assessment of anti-Toxoplasma effects of drugs, including propranolol at 2 mg/kg/day, propranolol at 3 mg/kg/day, pyrimethamine (50 mg/kg/day) plus propranolol (2 mg/kg/day), pyrimethamine (50 mg/kg/day) plus propranolol (3 mg/kg/day), pyrimethamine (50 mg/kg/day) (as the positive control), and PBS (as the negative control). Drugs were dissolved and diluted in PBS and used freshly. Initially, for control of drug side effects, a preliminary experiment was conducted on mice receiving the same dose of drugs for 3 days, and no mortality or clinically significant toxicity was observed.
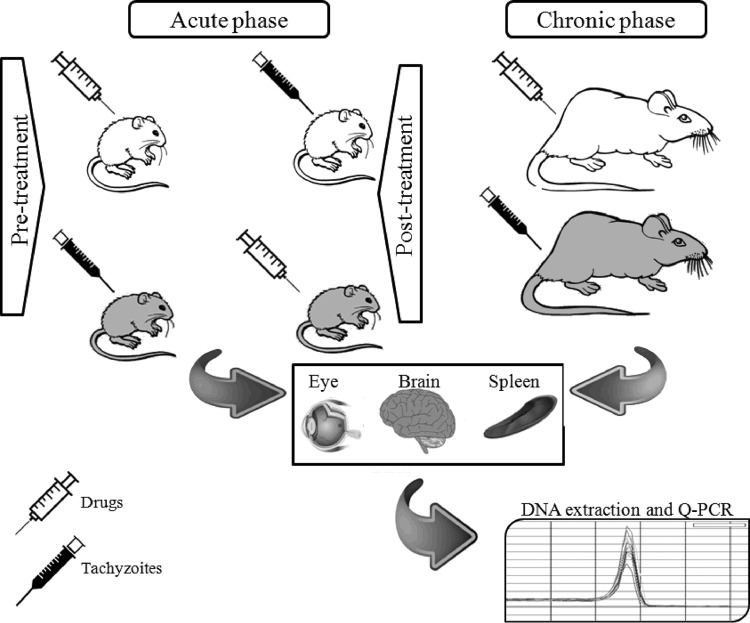
In the acute-phase assays, the study was performed in two sections: pretreatment and posttreatment. In the pretreatment group, drug injections were performed 48, 24, and 4 h before challenge with 1 × 103 T. gondii tachyzoites. In another set of experiments, in the posttreatment group, drugs were injected 4, 24, and 48 h after challenge with the same dose. Infected mice were kept for 7 days, and the animals were observed daily for mortality. Afterwards, brain and eye tissues of BALB/c mice were isolated. Parasite load was calculated using quantitative PCR (qPCR) targeting the RE gene (38). In the chronic phase, 20 female rats in four subgroups (n = 5) were used for assessment of anti-Toxoplasma effects of drugs, including propranolol at 2 mg/kg/day, propranolol at 3 mg/kg/day, pyrimethamine (50 mg/kg/day) (as the positive control), and PBS (as the negative control). Drugs were dissolved and diluted in PBS and used fresh. Initially, for control of drug side effects, a preliminary experiment was conducted on mice receiving the same dose of drugs for 1 week, and no mortality or clinically significant toxicity was observed. Treatment was performed 12 h before intraperitoneal challenge with 1 × 106 tachyzoites of the virulent strain RH of T. gondii in rats. Two months after infection, brain, eye, and spleen tissues were isolated and the parasite load was calculated using qPCR targeting the RE gene (Fig. 1).
FIG 1.
Schematic diagram of the study design.
DNA extraction.
Tissues were harvested from each mouse. Afterward, tissue and tachyzoite DNAs were extracted using the Viogen Tissue DNA extraction kit (catalog number GG2001-50) according to the manufacturer's instructions.
Detection of T. gondii RE gene by qPCR.
DNA samples were dissolved in distilled water and used to amplify and quantify DNA from the T. gondii highly conserved RE gene, yielding 200 to 300 copies. Amplification was performed using qPCR, and each amplification included positive (a DNA extract from 5 × 106 tachyzoites of strain RH per ml) and negative (water and DNA extracts from a blood sample of a healthy mouse) controls. qPCR was performed using forward primer F 5′-AGG GAC AGA AGT CGA AGG GG-3′ and reverse primer R 5′-GCA GCC AAG CCG GAA ACA TC-3′, amplifying a 164-bp fragment of gene RE with SYBR green master mix (10 μl; Thermo Scientific catalog number K0221) mixed with 1.4 μl template DNA to reach a final volume of 20 ml containing 7 μl distilled water and 0.8 μl of each primer at a concentration of 1 pmol/μl. The following amplification protocol was applied: 10 min at 95°C, 40 cycles at 94°C for 30 s (denaturation), 55°C for 30 s (annealing), and 72°C for 30 s (amplification). Melting curve analysis was performed to verify the correct product size and ensure the absence of side products or primer dimers. Subsequently, qPCR assays were performed in triplicate with three independent tissue samples for each experimental group. The number of parasites in the samples was calculated from the qPCR threshold cycle (CT) value according to a standard curve (y = −3.32x + 40.639; R2 = 0.9829) obtained with DNA samples from a range of serial dilutions (5 × 106 to 5 × 101/ml) of strain RH tachyzoites. The results were expressed as T. gondii tachyzoite equivalents per milligram of tissue.
Survival study in the acute phase.
As previously mentioned for pretreatment groups, the survival rate among different groups was studied in acute-phase toxoplasmosis. Overall, 36 female BALB/c mice in six subgroups (n = 6) were used. The survival periods were recorded daily until all mice died.
Statistical analysis.
Statistical analysis was performed using SPSS software version 15. Differences between test and control groups (positive and negative) were analyzed by analysis of variance (ANOVA) and the Newman-Keuls multiple-comparison test. A P value of less than 0.05 was considered statistically significant. The Kaplan-Meier curve was used to show the survival time, and the survival rates among different groups were compared using the log rank test. Differences were considered statistically significant when P was <0.05.
RESULTS
Brain and eye tissue samples were collected 1 week after postinfection, and qPCR analysis was applied for detection and quantification of Toxoplasma DNA in all groups. The qPCR protocol was performed using RE gene primers and successfully amplified a 164-bp fragment of T. gondii DNA. All samples, standards, and negative controls were analyzed in triplicate. There was no amplification observed in negative-control reactions. Over the study period, parasite burden outbursts occurred in all the Toxoplasma-inoculated animals, and all samples obtained from both case and control groups were positive for T. gondii in acute and chronic phases.
Anti-Toxoplasma effects of drugs in pretreatment groups in the acute phase.
The findings showed that in the pretreatment group, the highest parasite load (copy number) of T. gondii was seen in negative-control groups in different tissues (brain, 1,018,000 parasites/ml; eye, 961,367 parasites/ml). In contrast, in the treatment group, in the brain, the lowest parasite load (14,013 parasites/ml) was observed with propranolol at 3 mg/kg/day combined with pyrimethamine, whereas the lowest parasite load in the eye (4,007 parasites/ml) was seen with propranolol at 2 mg/kg/day combined with pyrimethamine. Also, the brain tissue obtained from mice treated with propranolol (2 mg/kg/day) displayed a lower parasite load (43,172 parasites/ml) than did eye tissue. There was a significant difference between each drug group and the untreated group (negative control) (P < 0.001) in different tissues in pretreatment groups (Table 1).
TABLE 1.
Anti-Toxoplasma activity of different doses of propranolol drug in brain and eye tissues in the acute phase (pretreatment group)
| Drug | Dose (mg/kg) | Brain |
Eye |
||
|---|---|---|---|---|---|
| CTa (mean parasite load) | P value | CT (mean parasite load) | P value | ||
| Propranolol | 2 | 24.50 ± 1.34 (43,172) | <0.001 | 24.08 ± 1.64 (192,931) | <0.001 |
| 23.13 ± 1.26 (119,020) | <0.001 | 23.47 ± 1.53 (275,649) | <0.001 | ||
| Propranolol + pyrimethamine | 2 + 50 | 22.75 ± 4.50 (70,928) | <0.001 | 26.19 ± 1.27 (4,007) | <0.001 |
| 3 + 50 | 23.83 ± 1.29 (14,013) | <0.001 | 28.19 ± 0.87 (33,230) | <0.001 | |
| Pyrimethamine (positive control) | 50 mg | 25.74 ± 2.23 (40,439) | <0.001 | 27.04 ± 2.09 (38,643) | <0.001 |
| PBS (negative control) | 19.64 ± 1.88 (1,018,000) | 20.07 ± 1.22 (961,367) | |||
CT values are means ± SD.
Anti-Toxoplasma effects of drugs in posttreatment groups in the acute phase.
In the treatment group, in the brain, the lowest parasite load (2,267 parasites/ml) was observed in the subgroup treated with propranolol (2 mg/kg/day) combined with pyrimethamine, whereas the lowest parasite load in the eye (112.5 parasites/ml) was seen in the subgroup treated with propranolol (3 mg/kg/day) combined with pyrimethamine. There was a significant difference between each drug group and the untreated group (negative control; P < 0.001) in the eye tissues in posttreatment groups. Also, results indicated a significant difference in brain tissues between the effects of propranolol (2 or 3 mg/kg/day) combined with pyrimethamine and what was seen in the negative control (P < 0.001) (Table 2).
TABLE 2.
Anti-Toxoplasma activity of different doses of propranolol drug in brain and eye tissues in the acute phase (posttreatment group)
| Drug | Dose (mg/kg) | Brain |
Eye |
||
|---|---|---|---|---|---|
| CTa (mean parasite load) | P value | CT (mean parasite load) | P value | ||
| Propranolol | 2 | 21.14 ± 0.66 (612,623) | >0.05 | 24.70 ± 3.18 (227,979) | <0.001 |
| 3 | 21.17 ± 0.44 (870,668) | >0.05 | 22.86 ± 1.52 (428,559) | <0.001 | |
| Propranolol + pyrimethamine | 2 + 50 | 29.58 ± 0.5 (2,267) | <0.001 | 30.82 ± 3.17 (491) | <0.001 |
| 3 + 50 | 31.68 ± 3.76 (2,548) | <0.001 | 31.08 ± 2.58 (112.5) | <0.001 | |
| Pyrimethamine (positive control) | 50 | 30.62 ± 2.19 (1,741) | <0.001 | 32.03 ± 3.14 (3,577) | <0.001 |
| PBS (negative control) | 19.64 ± 1.88 (1,018,000) | 20.07 ± 1.22 (961,367) | |||
CT values are means ± SD.
Survival rates of acutely infected mice in the pretreatment group.
Clinically, the number of mice in untreated infected groups (negative control) started to show reduction on the eighth day of the study, and all mice died by the ninth day. Mice in the treatment groups (propranolol alone and in combination with pyrimethamine) showed statistically higher survival rates than untreated infected control mice (P < 0.05). There was no significant difference between each drug and the positive control (P > 0.05) in the different subgroups (Fig. 2).
FIG 2.
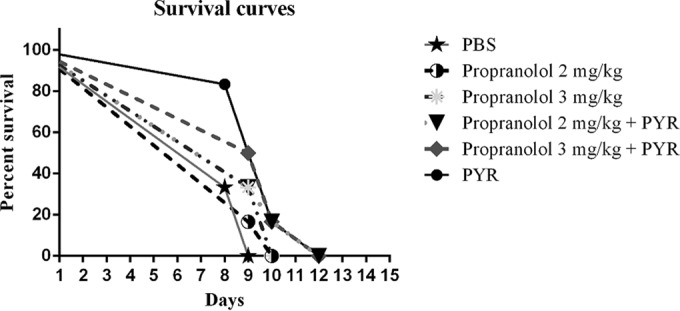
Survival curves of mice following acute toxoplasmosis in the pretreatment group. BALB/c mice groups (n = 6) were treated with propranolol at 2 or 3 mg/kg/day, pyrimethamine (PYR; 50 mg/kg/day) plus propranolol at 2 or 3 mg/kg/day, pyrimethamine (50 mg/kg/day) (as the positive control), or PBS (as the negative control) 48, 24, and 4 h before challenge with 1 × 103 T. gondii tachyzoites.
Comparison of different doses of drugs in pretreatment and posttreatment groups in the acute phase.
All infected mice in different groups, in comparison with the negative-control group, displayed a drastic decrease of parasite load or circulating parasite DNA levels in their brain and eye tissues, as shown in Tables 1 and 2.
In brain tissue, animals receiving propranolol dosed at 2 mg/kg/day (98,740 parasites/ml) and propranolol at 3 mg/kg/day (244,700 parasites/ml) in the pretreatment group showed lower parasite loads than those receiving propranolol at 2 mg/kg/day (796,400 parasites/ml) and propranolol at 3 mg/kg/day (754,000 parasites/ml) in posttreatment groups (P < 0.001).
There was no significant difference between different doses of drugs in eye tissue in both groups (P > 0.05). All drugs, compared to the negative-control group, significantly reduced the parasite load (P < 0.001).
Anti-Toxoplasma effect of drugs in the chronic phase.
In the chronic phase, the findings showed that the highest parasite load was seen in negative-control groups in different tissues (brain, 28,900 parasites/ml; eye, 4,340 parasites/ml; spleen, 15,800 parasites/ml) as shown in Table 3. In contrast, propranolol was highly effective in the reduction of parasite loads in the treatment groups. The results indicated that treatment with propranolol (2 mg/kg/day) decreased the parasite load in the brain (1,630 parasites/ml) and spleen (2,170 parasites/ml) tissues. In the eye tissue, propranolol at 3 mg/kg/day was more effective (3,320 parasites/ml) than propranolol at 2 mg/kg/day (4,180 parasites/ml). Despite the anti-Toxoplasma effect of propranolol in the chronic phase, there was no significant difference between each drug and the untreated group (negative control) (P > 0.05) in the different tissues (Table 3).
TABLE 3.
Anti-Toxoplasma activity of different doses of propranolol drug in different tissues in the chronic phase
| Drug | Dose (mg/kg) | Brain |
Eye |
Spleen |
|||
|---|---|---|---|---|---|---|---|
| CTa (mean parasite load) | P value | CT (mean parasite load) | P value | CT (mean parasite load) | P value | ||
| Propranolol | 2 | 30.29 ± 1.28 (1,630) | >0.05 | 28.97 ± 0.14 (4,180) | >0.05 | 30.05 ± 1.71 (2,170) | >0.05 |
| 3 | 29.45 ± 0.75 (2,540) | >0.05 | 30.37 ± 3.35 (3,320) | >0.05 | 29.82 ± 2.91 (4,100) | >0.05 | |
| Pyrimethamine (positive control) | 50 | 29.75 ± 0.79 (2,110) | >0.05 | 29.99 ± 2.20 (2,950) | >0.05 | 29.52 ± 1.33 (2,960) | >0.05 |
| PBS (negative control) | 26.52 ± 2.01 (28,900) | 28.40 ± 0.53 (4,340) | 27.96 ± 3.15 (15,800) | ||||
CT values are means ± SD.
DISCUSSION
The concept of host targeting for the prevention and treatment of parasitic infectious diseases has recently emerged (22). The success of current therapy against toxoplasmosis is limited, and the drugs can often cause many side effects. Thus, finding less toxic new drugs against Toxoplasma is critically needed (23, 24). Since T. gondii must invade host nuclear cells to proliferate, this interaction between the parasite and host cells could be an appropriate potential site for intervention in the control of Toxoplasma infections (22, 25). In the present study, we examined the anti-Toxoplasma effect of propranolol (2 or 3 mg/kg/day), pyrimethamine (50 mg/kg/day) (positive control), propranolol (2 or 3 mg/kg/day) plus pyrimethamine, and PBS (negative control). Treatment was performed 4, 24, and 48 h before and after intraperitoneal challenge using 1 × 103 tachyzoites of the virulent strain RH of Toxoplasma gondii in pretreatment and posttreatment groups in BALB/c mice in the acute phase and using 1 × 106 tachyzoites in rats in pretreatment in the chronic phase and then was evaluated by determination of the parasite load using qPCR. T. gondii RE gene as a target was amplified in different tissues, including brain and eye in the acute phase and brain, eye, and spleen in the chronic phase. Also, the survival rate was evaluated in infected mice in acute toxoplasmosis as mentioned above for pretreatment groups.
Previously, we have shown the effects of propranolol (2 or 3 mg/kg/day) against T. gondii infection in vivo in pre- and posttreatment groups. Propranolol, as a beta blocker, stabilizes the cell membrane and inhibits parasite entry into the host cells (18). It is known that the cell membrane-stabilizing drugs, with interference in microfilament functions and blocking of the actin gel, change the resistance of the cell membrane. Rezaei et al. have shown that the cell membrane-stabilizing drugs such as cromolyn sodium and ketotifen, similar to propranolol, are able to inhibit T. gondii penetration into nucleated cells at appropriate concentrations in vitro and in vivo (17). Similarly, our data demonstrated that membrane-stabilizing action of beta-blocking agents such as propranolol (2 or 3 mg/kg/day) (26, 27) alone and combined with pyrimethamine in tissues of BALB/c mice in both pre- and posttreatment groups reduced the burden of parasites. Previous studies have shown that treatment with cytochalasins impairs entry of T. gondii and Plasmodium falciparum into their respective host cells. Cytochalasins, which disrupt actin filaments, block parasite gliding and host phagocytic responses, thus confusing the interpretation of the observed inhibition of invasion. So, T. gondii actin filaments are essential for host cell invasion (12, 15). A few studies have assessed the efficacy of these drugs on infectious diseases, especially parasitic diseases. Ohnishi et al. investigated the effects of several membrane-acting drugs on malaria and sickle cell anemia and found that propranolol inhibited the growth of P. falciparum (in vitro) and Plasmodium vinckei (in vivo) (28).
The present results clearly demonstrated that propranolol (2 or 3 mg/kg/day), with its membrane-stabilizing effects, significantly inhibited the intracellular proliferations of the tachyzoites of the highly virulent strain RH of T. gondii in brain tissues in pretreatment groups (P < 0.001). However, propranolol at 2 mg/kg/day showed higher anti-Toxoplasma activity than did propranolol at 3 mg/kg/day, and thus the former dose may have greater treatment efficiency on toxoplasmosis and fewer side effects than the latter.
Propranolol is a dose-dependent drug, as Benedetto et al. have shown that propranolol at high concentrations causes resistance to T. gondii in rats (29).
Our data demonstrated that the anti-Toxoplasma activity of propranolol combined with pyrimethamine in murine infection with the parasite strain RH in pre- and posttreatment groups significantly decreased parasite burden in the brain and eye tissues (P < 0.001). Moreover, combination therapy improved survival rates in the pretreatment group.
Martins-Duarte et al. performed a similar study in vivo that proved that fluconazole combined with sulfadiazine and pyrimethamine against T. gondii was highly effective (25). Combination therapy is known as the most effective treatment for toxoplasmic encephalitis (30, 31). Pyrimethamine monotherapy for treatment of toxoplasmosis is not recommended, seeing that patients receiving maintenance treatment with pyrimethamine alone were susceptible to relapses of toxoplasmosis (32).
In addition, the combined administration of pyrimethamine and propranolol might encourage a decrease in drug dosages and consequently decreased drug side effects and is an emerging therapy against toxoplasmosis. Murphy et al. showed that erythrocyte G proteins are functional and that propranolol, as an antagonist of G protein-coupled b-adrenergic receptors, inhibits G protein activity in erythrocytes. They also discovered that like other b2 antagonists, propranolol inhibited blood-stage parasite growth, such that propranolol used in combination with existing antimalarial agents in cell culture reduced the 50% and 90% inhibitory concentrations for existing drugs against P. falciparum and was also effective in reducing drug doses in animal models of infection (33).
Interestingly, propranolol (2 or 3 mg/kg/day) was more effective in brain in the pretreatment group (P < 0.001) than different doses of drugs in posttreatment groups. The findings of this research thus show that propranolol as a cell membrane-stabilizing compound is a suitable drug for inhibiting the entrance of T. gondii tachyzoites into nucleated cells in pretreatment groups and has potential as a therapeutic agent for the prevention of toxoplasmosis in humans.
In the present study, the effect of propranolol (2 or 3 mg/kg/day) was analyzed in vivo during the chronic stages of Toxoplasma infection in the rats. The observations in the infected animals implied that propranolol diminished the number of parasites in the brain, eye, and spleen tissues after 2 months. We found that propranolol (2 or 3 mg/kg/day) diminished the number of parasites of strain RH in the brains of infected rats. However, propranolol at low doses (2 mg/kg/day) was more effective than at a higher dose (3 mg/kg/day) in controlling infection. Similar effects were reported for endochin-like quinolone: ELQ-271 and ELQ-316 at low doses were highly active against the cyst form of T. gondii in mice, reducing cyst burden by 76 to 88% (34). Moreover, spiramycin coadministered with metronidazole was highly efficient against chronic toxoplasmosis, since metronidazole increased spiramycin brain penetration, causing a significant reduction of T. gondii brain cysts, with potential clinical translatability for chronic toxoplasmosis treatment (35).
However, propranolol (2 mg/kg/day) was more effective in reduction of the brain tissue parasites in rat. Our results show that all rats survived until the end of the experiment (2 months); also, no mortality or clinically significant toxicity was observed. However, Benedetto et al. reported impairment of natural resistance to T. gondii infection in rats pretreated with a high concentration of beta blockers within 4 weeks (36). In addition, decreasing parasite loads in the brain, eye, and spleen tissues indicate the proper dose (2 mg/kg/day) of medication in the prevention and treatment of toxoplasmosis. Propranolol stabilizes cell membrane and inhibits parasite entry into the host cells via interference in microfilament functions and blocking of the actin gel, changing the resistance of the cell membrane (15). The present results clearly demonstrate that propranolol (2 mg/kg/day) inhibited the intracellular proliferations of the tachyzoites of the highly virulent strain RH of T. gondii in brain tissues in latent infection after 2 months (1,630 parasites/ml), which suggests that propranolol at low doses can prevent the formation of tissue cysts. Thus, propranolol has the potential to reduce latent infection at low doses.
The beta blocker propranolol has been approved for human use and is typically prescribed at 0.8 to 4.5 mg/kg/day. It is also recommended at low doses for use in pregnant women to reduce the risk of fetal placental transmission (37).
In conclusion, the presented results demonstrate that propranolol, as an orally available drug, is effective at low doses against acute and latent murine toxoplasmosis and that the efficiency of drug is increased when used in combination therapy with pyrimethamine (propranolol-pyrimethamine).
ACKNOWLEDGMENTS
This study was prepared from M. Montazeri's M.S. thesis and supported by a grant (number 92-471) from the Deputy of Research, Mazandaran University of Medical Sciences, Sari, Iran.
The sponsor or funding organization had no role in the design or conduct of this research.
REFERENCES
- 1.Dubey JP. 2009. Toxoplasmosis of animals and humans. CRC Press, Boca Raton, FL. [Google Scholar]
- 2.Oz HS. 2014. Novel synergistic protective efficacy of atovaquone and diclazuril on fetal-maternal toxoplasmosis. Int J Clin Med 5:921–932. doi: 10.4236/ijcm.2014.515124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiss LM, Dubey JP. 2009. Toxoplasmosis: a history of clinical observations. Int J Parasitol 39:895–901. doi: 10.1016/j.ijpara.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmadpour E, Daryani A, Sharif M, Sarvi S, Aarabi M, Mizani A, Rahimi MT, Shokri A. 2014. Toxoplasmosis in immunocompromised patients in Iran: a systematic review and meta-analysis. J Infect Dev Countries 8:1503–1510. [DOI] [PubMed] [Google Scholar]
- 5.Krivogorsky B, Pernat JA, Douglas KA, Czerniecki NJ, Grundt P. 2012. Structure-activity studies of some berberine analogs as inhibitors of Toxoplasma gondii. Bioorg Med Chem Lett 22:2980–2982. doi: 10.1016/j.bmcl.2012.02.038. [DOI] [PubMed] [Google Scholar]
- 6.Daryani A, Ebrahimzadeh MA, Sharif M, Ahmadpour E, Edalatian S, Esboei BR, Sarvi S. 2015. Anti-Toxoplasma activities of methanolic extract of Sambucus nigra (Caprifoliaceae) fruits and leaves. Rev Biol Trop 63:7–12. [PubMed] [Google Scholar]
- 7.Al-Zanbagi NA. 2007. Effectiveness of myrrh and spiramycin as inhibitors for Toxoplasma gondii tachyzoites in vivo. Mansoura J Forensic Med Clin Toxicol XV:117–128. [Google Scholar]
- 8.Araujo FG, Remington JS. 1992. Recent advances in the search for new drugs for treatment of toxoplasmosis. Int J Antimicrob Agents 1:153–164. doi: 10.1016/0924-8579(92)90002-9. [DOI] [PubMed] [Google Scholar]
- 9.Petersen E, Schmidt DR. 2003. Sulfadiazine and pyrimethamine in the postnatal treatment of congenital toxoplasmosis: what are the options? Expert Rev Anti Infect Ther 1:175–182. doi: 10.1586/14787210.1.1.175. [DOI] [PubMed] [Google Scholar]
- 10.Serranti D, Buonsenso D, Valentini P. 2011. Congenital toxoplasmosis treatment. Eur Rev Med Pharmacol Sci 15:193–198. [PubMed] [Google Scholar]
- 11.Wei S, Daniel BJ, Brumlik MJ, Burow ME, Zou W, Khan IA, Wadsworth S, Siekierka J, Curiel TJ. 2007. Drugs designed to inhibit human p38 mitogen-activated protein kinase activation treat Toxoplasma gondii and Encephalitozoon cuniculi infection. Antimicrob Agents Chemother 51:4324–4328. doi: 10.1128/AAC.00680-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sibley DL, Charron A, Hakansson S, Mordue D. 2013. Invasion and intracellular survival by Toxoplasma. In Madame Curie Bioscience Database. Landes Bioscience, Austin, TX: http://www.ncbi.nlm.nih.gov/books/NBK6450/. [Google Scholar]
- 13.Walker DM, Oghumu S, Gupta G, McGwire BS, Drew ME, Satoskar AR. 2014. Mechanisms of cellular invasion by intracellular parasites. Cell Mol Life Sci 71:1245–1263. doi: 10.1007/s00018-013-1491-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D'Angelo JG, Bordón C, Posner GH, Yolken R, Jones-Brando L. 2009. Artemisinin derivatives inhibit Toxoplasma gondii in vitro at multiple steps in the lytic cycle. J Antimicrob Chemother 63:146–150. doi: 10.1093/jac/dkn451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryning FW, Remington JS. 1978. Effect of cytochalasin D on Toxoplasma gondii cell entry. Infect Immun 20:739–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daryani A, Ebrahimzadeh M-A, Sharif M, Rezaei F, Ahmadpour E, Sarvi S, Ajami A, Ziaei H, Khalilian A. 2014. The inhibitory effect of ketotifen on entrance of Toxoplasma gondii tachyzoites into macrophages of mouse. J Mazandaran Univ Med Sci (JMUMS) 23:75–80. [Google Scholar]
- 17.Rezaei F, Ebrahimzadeh MA, Daryani A, Sharif M, Ahmadpour E, Sarvi S. 2016. The inhibitory effect of cromolyn sodium and ketotifen on Toxoplasma gondii entrance into host cells in vitro and in vivo. J Parasit Dis 40:1001–1005. doi: 10.1007/s12639-014-0623-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montazeri M, Daryani A, Ebrahimzadeh M, Ahmadpour E, Sharif M, Sarvi S. 2015. Effect of propranolol alone and in combination with pyrimethamine on acute murine toxoplasmosis. Jundishapur J Microbiol 8:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daryani A, Hosseini AZ, Dalimi A. 2003. Immune responses against excreted/secreted antigens of Toxoplasma gondii tachyzoites in the murine model. Vet Parasitol 113:123–134. doi: 10.1016/S0304-4017(03)00044-X. [DOI] [PubMed] [Google Scholar]
- 20.Saadatnia G, Haj Ghani H, Khoo B, Maimunah A, Noordin R. 2010. Optimization of toxoplasma gondii cultivation in VERO cell line. Trop Biomed 27:125–130. [PubMed] [Google Scholar]
- 21.Akins CK, Panicker SE, Cunningham CL. 2005. Laboratory animals in research and teaching: ethics, care, and methods. American Psychological Association, Washington, DC. [Google Scholar]
- 22.Weiss LM, Kim K. 2011. Toxoplasma gondii: the model apicomplexan. Perspectives and methods. Academic Press, New York, NY. [Google Scholar]
- 23.Maubon D, Bougdour A, Wong Y-S, Brenier-Pinchart M-P, Curt A, Hakimi M-A, Pelloux H. 2010. Activity of the histone deacetylase inhibitor FR235222 on Toxoplasma gondii: inhibition of stage conversion of the parasite cyst form and study of new derivative compounds. Antimicrob Agents Chemother 54:4843–4850. doi: 10.1128/AAC.00462-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang X, Huang B, Chen J, Huang S, Zheng H, Lun Z-R, Shen J, Wang Y, Lu F. 2012. In vitro effects of aqueous extracts of Astragalus membranaceus and Scutellaria baicalensis GEORGI on Toxoplasma gondii. Parasitol Res 110:2221–2227. doi: 10.1007/s00436-011-2752-2. [DOI] [PubMed] [Google Scholar]
- 25.Martins-Duarte ES, de Souza W, Vommaro RC. 2013. Toxoplasma gondii: the effect of fluconazole combined with sulfadiazine and pyrimethamine against acute toxoplasmosis in murine model. Exp Parasitol 133:294–299. doi: 10.1016/j.exppara.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 26.Hong C, Yang W, Chiang B. 1983. Importance of membrane stabilizing effect in massive overdose of propranolol: plasma level study in a fatal case. Hum Toxicol 2:511–517. doi: 10.1177/096032718300200307. [DOI] [PubMed] [Google Scholar]
- 27.Takeo S, Yamada H, Tanonaka K, Hayashi M, Sunagawa N. 1990. Possible involvement of membrane-stabilizing action in beneficial effect of beta adrenoceptor blocking agents on hypoxic and posthypoxic myocardium. J Pharmacol Exp Ther 254:847–856. [PubMed] [Google Scholar]
- 28.Ohnishi ST, Sadanaga KK, Katsuoka M, Weidanz WP. 1989. Effects of membrane acting-drugs on plasmodium species and sickle cell erythrocytes. Mol Cell Biochem 91:159–165. doi: 10.1007/BF00228091. [DOI] [PubMed] [Google Scholar]
- 29.Schelowski M, Tewes U. 1999. Psychoneuroimmunology: an interdisciplinary introduction. Kluwer Academic/Plenum Publishers, New York, NY. [Google Scholar]
- 30.Fung HB, Kirschenbaum HL. 1996. Treatment regimens for patients with toxoplasmic encephalitis. Clin Ther 18:1037–1056. doi: 10.1016/S0149-2918(96)80059-2. [DOI] [PubMed] [Google Scholar]
- 31.Petersen E. 2007. Prevention and treatment of congenital toxoplasmosis. Expert Rev Anti Infect Ther 5:285–293. doi: 10.1586/14787210.5.2.285. [DOI] [PubMed] [Google Scholar]
- 32.Foppa CU, Bini T, Gregis G, Lazzarin A, Esposito R, Moroni M. 1991. A retrospective study of primary and maintenance therapy of toxoplasmic encephalitis with oral clindamycin and pyrimethamine. Eur J Clin Microbiol Infect Dis 10:187–189. doi: 10.1007/BF01964458. [DOI] [PubMed] [Google Scholar]
- 33.Murphy SC, Harrison T, Hamm HE, Lomasney JW, Mohandas N, Haldar K. 2006. Erythrocyte G protein as a novel target for malarial chemotherapy. PLoS Med 3:e528. doi: 10.1371/journal.pmed.0030528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doggett JS, Nilsen A, Forquer I, Wegmann KW, Jones-Brando L, Yolken RH, Bordón C, Charman SA, Katneni K, Schultz T. 2012. Endochin-like quinolones are highly efficacious against acute and latent experimental toxoplasmosis. Proc Natl Acad Sci U S A 109:15936–15941. doi: 10.1073/pnas.1208069109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chew WK, Segarra I, Ambu S, Mak JW. 2012. Significant reduction of brain cysts caused by Toxoplasma gondii after treatment with spiramycin coadministered with metronidazole in a mouse model of chronic toxoplasmosis. Antimicrob Agents Chemother 56:1762–1768. doi: 10.1128/AAC.05183-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benedetto N, Folgore A, Galdiero M. 1993. Impairment of natural resistance to Toxoplasma gondii infection in rats treated with beta adrenergics, beta blockers, corticosteroids or total body irradiation. Pathol Biol 41:404–409. [PubMed] [Google Scholar]
- 37.Beers M, Berkow R. 1999. The Merck manual of diagnosis and therapy. Merck and Co., Inc., Whitehouse Station, NJ. [Google Scholar]
- 38.Daryani A, Sharif M, Dadimoghaddam Y, Souteh MBH, Ahmadpour E, Khalilian A, Sarvi S, Farazmand T, Kalani H, Rasouli M. 2014. Determination of parasitic load in different tissues of murine toxoplasmosis after immunization by excretory-secretory antigens using Real time QPCR. Exp Parasitol 143:55–59. doi: 10.1016/j.exppara.2014.05.008. [DOI] [PubMed] [Google Scholar]