Abstract
The biosynthesis of coenzyme A (CoA) from pantothenate and the utilization of CoA in essential biochemical pathways represent promising antimalarial drug targets. Pantothenamides, amide derivatives of pantothenate, have potential as antimalarials, but a serum enzyme called pantetheinase degrades pantothenamides, rendering them inactive in vivo. In this study, we characterize a series of 19 compounds that mimic pantothenamides with a stable triazole group instead of the labile amide. Two of these pantothenamides are active against the intraerythrocytic stage parasite with 50% inhibitory concentrations (IC50s) of ∼50 nM, and three others have submicromolar IC50s. We show that the compounds target CoA biosynthesis and/or utilization. We investigated one of the compounds for its ability to interact with the Plasmodium falciparum pantothenate kinase, the first enzyme involved in the conversion of pantothenate to CoA, and show that the compound inhibits the phosphorylation of [14C]pantothenate by the P. falciparum pantothenate kinase, but the inhibition does not correlate with antiplasmodial activity. Furthermore, the compounds are not toxic to human cells and, importantly, are not degraded by pantetheinase.
INTRODUCTION
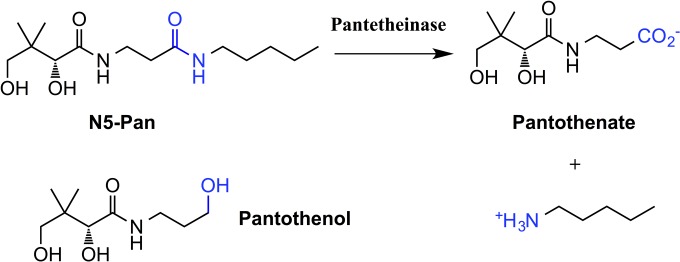
Due to drug resistance by Plasmodium falciparum, the parasite responsible for malaria, it is vital to identify new chemotherapeutics targeting novel parasite pathways. P. falciparum does not survive its asexual red blood cell (RBC) stage without access to exogenous pantothenate (vitamin B5) (1–3). Pantothenate is metabolized by five enzymes into coenzyme A (CoA), a cofactor estimated to be required by 9% of all enzymes (4). The first enzyme in the CoA biosynthetic pathway is pantothenate kinase (PanK), which catalyzes the phosphorylation of pantothenate into phosphopantothenate. Several pantothenate analogues have been shown to inhibit the growth of P. falciparum in vitro, including pantothenol (5), CJ-15,801 (6), and various other analogues (7, 8). Pantothenol (Fig. 1) has also been shown to possess antiplasmodial activity in vivo in a mouse model of malaria (5). Recently, pantothenamides, amides of pantothenate initially investigated for antibacterial activity (9–11), have been shown to possess potent antiplasmodial activity (12). Unfortunately, the effectiveness of pantothenamides as antiplasmodials is attenuated by pantetheinase (12), an enzyme found in human serum (13). The endogenous substrate of pantetheinase is pantetheine, which is broken down by pantetheinase into pantothenate and cysteamine (14). Pantetheinase also breaks down pantothenamides (12), including the prototypical pantothenamide, N-pentylpantothenamide (N5-Pan [Fig. 1]). The breakdown of pantothenamides is an obstacle that needs to be overcome if these compounds are to be of any use as antimicrobial agents.
FIG 1.
Structures of pentylpantothenamide (N5-Pan), its pantetheinase degradation products, and pantothenol. The pantetheinase-sensitive amide bond in N5-Pan and the hydroxy group in pantothenol are shown in blue.
There are two obvious ways by which the breakdown of pantothenamides by pantetheinase can be prevented: (i) development of a pantetheinase inhibitor that can be coadministered with the pantothenamide, a strategy recently demonstrated to be possible in vitro (15), or (ii) modification of the pantothenamide structure so that it is insensitive to pantetheinase-mediated degradation while still maintaining its antiplasmodial activity. The latter strategy has been employed by de Villiers et al. (16) and, more recently, by Macuamule et al. (17) with incomplete success, as either antiplasmodial activity was decreased or pantetheinase resistance was not complete.
Triazoles can act as amide bioisosteres due to their hydrogen bonding abilities and structural properties, including geometry and interatomic distances (18). In this study, we characterize a new series of 19 pantothenamide derivatives in which a triazole group is used as a rigidified, amide isostere to replace the pantetheinase-susceptible amide group.
MATERIALS AND METHODS
Parasite culture.
P. falciparum parasites (strain 3D7) at the red blood cell stage were maintained in continuous culture using an adaptation (19) of a previously described method (20). The parasites were grown within human erythrocytes at ∼4% hematocrit in RPMI 1640 medium supplemented with glucose (11 mM), hypoxanthine (200 μM), gentamicin (24 μg/ml), and Albumax II (0.6%, wt/vol), the source of pantetheinase in the parasite culture medium (12). The cultures were incubated at 37°C with gentle agitation (to prevent settling of erythrocytes) under an atmosphere of 96% nitrogen, 3% carbon dioxide, and 1% oxygen. The parasites were maintained at a parasitemia of ∼5 to 10% by adding fresh uninfected RBCs, as appropriate. To prepare pantetheinase-free parasite culture medium, the fully reconstituted medium described above was incubated at 37°C in the presence of light for 40 h, as described previously (12).
Antiplasmodial activity assay.
The in vitro antiplasmodial activities of selected compounds were investigated using the previously described malaria SYBR Safe-based fluorescence assay (21), with minor modifications. The outer wells of a 96-well plate contained 200 μl of medium to negate the “edge effect” (22). One of the plate columns contained chloroquine (0.25 μM) and was used as a positive control (i.e., 100% inhibition of parasite proliferation). Another column did not contain any drugs and served as a 100% parasite proliferation control. The remaining columns included eight 2-fold dilutions of the test compounds (final volume, 100 μl), with the highest concentration depending on the compounds' potency. Infected RBCs with parasites at the ring stage (parasitemia set to 0.5%) were added to each well (100 μl) such that the final hematocrit was 0.25% and the final total volume was 200 μl. Technical repeats (n = 3) were included for each condition. Chloroquine was dissolved in H2O, and all the other compounds were dissolved in dimethyl sulfoxide (DMSO). The plate was incubated at 37°C in an atmosphere of 96% nitrogen, 3% carbon dioxide, and 1% oxygen for 96 h. Thereafter, the plate was placed at −20°C overnight or processed immediately. For processing, the plate was defrosted (if frozen) and the content of each well was resuspended by pipetting. Next, for each well, 100 μl was transferred to the corresponding well of a new 96-well plate. A 100-μl aliquot of SYBR Safe (0.2 μl/ml) in lysis buffer (KH2PO4-K2HPO4, 10 mM, adjusted to pH 7.4 with KOH) was then added to each well. The amount of fluorescence from each well was measured with a FLUOstar Optima multidetection microplate reader (BMG Labtech) with 490-nm excitation and 520-nm emission wavelengths. The fluorescence from the chloroquine control wells was subtracted from the fluorescence readings in the other wells before further analysis. Sample fluorescence, as a percentage of the fluorescence measured in drug-free control wells, was then plotted against the test compound concentration on a log scale. SigmaPlot for Windows version 11.0 (Systat Software) was then used to fit sigmoidal curves to the data. The concentration of the compounds resulting in a 50% decrease in parasite proliferation (50% inhibitory concentration [IC50]) was determined.
HFF cytotoxicity assay.
Confluent human foreskin fibroblasts (HFF cells) (in a 75-cm2 flask) were harvested by treatment with 1× trypsin–EDTA (0.25% trypsin + 0.2 g/liter EDTA) and diluted in 21 ml of RPMI 1640 with 10% fetal bovine serum (7). Cytotoxicity assays using 96-well plates were set up in a manner similar to that described for the antiplasmodial assay but included cycloheximide (10 μM) as a positive control (instead of chloroquine). The plates were incubated at 37°C in a CO2 incubator for 48 h or until the HFF cells in the drug-free control wells became confluent (up to 62 h). Thereafter, 150 μl of the medium from each well was removed carefully so as to not disturb the cells on the bottom of the wells, and the plates were placed at −20°C overnight. The next day, 150 μl of the SYBR Safe lysis buffer (see above) was added to each well of the plate, and the fluorescence was measured (and data were analyzed) as described above for the antiplasmodial activity assay.
P. falciparum and HFF lysate preparation.
Cultured 3D7 parasites at the trophozoite stage were released from RBCs with saponin (final concentration, 0.05% [wt/vol]) (23), and the isolated parasites were washed three times in 1 ml malaria saline (125 mM NaCl, 5mM KCl, 25 mM HEPES, 20 mM glucose, 1 mM MgCl2, and pH adjusted to 7.1), with separation by centrifugation at 14,000 × g for 40 s before being resuspended in 1 ml of the malaria saline and stored at 37°C. The parasites were then resuspended in a potassium-buffered (KH2PO4-K2HPO4 [10 mM]) hypotonic solution, adjusted to pH 7.4, in order to lyse the cells (23). The cells were then triturated to facilitate rupture of the parasite plasma membrane. The crude lysate was centrifuged at 17,000 × g and 4°C for 30 min, and the clarified lysate (supernatant) was carefully aspirated, transferred to a new tube, and stored at −20°C until required.
HFF cells were detached from their flask by treatment with trypsin (0.25%)–EDTA (0.2 g/liter). Cells were resuspended in 1 ml of potassium lysis buffer (KH2PO4-K2HPO4 [10 mM]) adjusted to pH 7.4 and stored at −20°C until required.
Phosphorylation assays.
Phosphorylation assays were carried out as recently described (24). The assay makes use of the Somogyi reagent [Zn(OH)2 and Ba(OH)2], which precipitates phosphorylated reaction products. We utilized the assay to determine whether the phosphorylation of [14C]pantothenate was inhibited by the pantothenate analogues. The compounds were serially diluted and added to either P. falciparum or HFF lysates. Single time points, within the linear phase of the phosphorylation reaction, were used. For lysates prepared from P. falciparum, this was 40 min. For HFF cell lysates, we determined that the reaction was linear for at least 120 min (data not shown), and this time point was used as it gave a better signal-to-noise ratio than did earlier time points.
Statistical analysis.
Unless otherwise indicated, values are presented as means ± standard errors of the means (SEM). To determine whether the difference between the means of two groups was statistically significant, we carried out confidence interval (CI) analyses using SigmaPlot for Windows version 11.0 (Systat Software, Inc.). The reported CI indicates the 95% confidence interval of the difference between the means. A “power” value between 0.8 and 1 indicates a statistically significant difference. Power values of <0.8 are not statistically significant. Linear regression and nonlinear regression analysis was carried out using SigmaPlot.
Compound synthesis.
Full details regarding compound synthesis are included in the Supplemental text in the supplemental material.
RESULTS
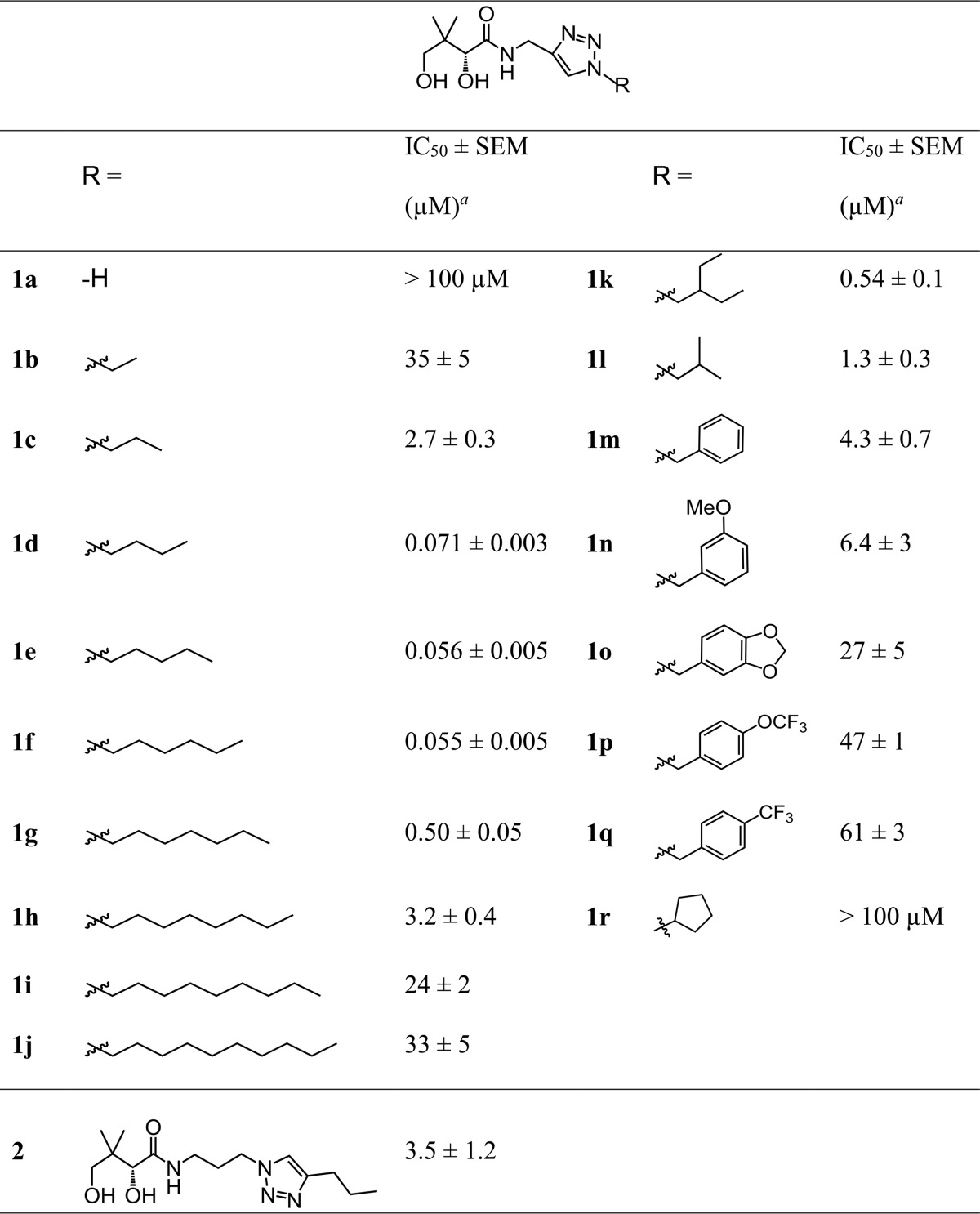
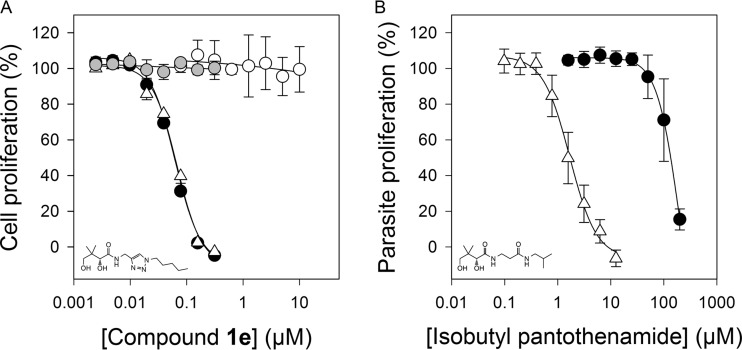
Triazole-containing compound 1e (Fig. 2A, Table 1), previously reported as part of an investigation into the structural requirements for substrate binding to the Escherichia coli PanK (25), was identified as a more-stable derivative of N5-Pan. Based on the description of how the triazole may mimic amide bonds by Genazzani and coworkers, a closer mimic of N5-Pan would have had its triazole moiety one carbon further away from the pantoyl group than does compound 1e; however, docking studies with selected CoA-binding enzymes suggest that compound 1e might be a better mimic of N5-Pan (note that three-dimensional [3D] structures have not been reported for any of the P. falciparum CoA biosynthesis enzymes). Following synthesis of compound 1e, its in vitro antiplasmodial activity was tested in the presence or absence of pantetheinase activity (Fig. 2A). Compound 1e was found to be an effective antiplasmodial against the asexual RBC stage of the parasite's life cycle, in both the presence and absence of pantetheinase activity, with IC50s of 56 ± 5 nM (n = 9) and 59 ± 6 nM (n = 2), respectively. These data are consistent with compound 1e being completely stable in the presence of pantetheinase during the 96-h assay. Isobutyl pantothenamide, a compound shown previously to be degraded by pantetheinase (12), was included as a positive control (Fig. 2B). As expected and unlike compound 1e, the antiplasmodial activity of isobutyl pantothenamide was reduced (70-fold) in the presence of pantetheinase activity.
TABLE 1.
Antiplasmodial activity of modified pantothenamides that contain a triazole with different substituents attached at the R position
a In medium containing pantetheinase. IC50s are averaged from three or more independent experiments, each carried out in triplicate.
FIG 2.
Comparison of in vitro antiplasmodial activity, serum stability, and cytotoxicity of compound 1e (A) and the pantetheinase-susceptible isobutyl pantothenamide (B) under various conditions. (A) Dose-response relationship of compound 1e in the presence (black circles) and absence (white triangles) of pantetheinase. The effect of increasing the concentration of pantothenate (from 1 μM to 100 μM) in the presence of pantetheinase on the antiplasmodial activity of compound 1e was also tested (gray circles). The white circles show the lack of an effect of compound 1e on the proliferation of HFF cells. (B) Isobutyl pantothenamide activity against the parasite was tested in the presence (black circles) and absence (white triangles) of pantetheinase. This assay served as a positive control and was carried out in paired experiments with compound 1e. The data are averaged from 2 to 9 independent experiments, each carried out in triplicate. Error bars represent the range divided by 2 (if n = 2) or SEM (if n ≥ 3).
To determine whether compound 1e was acting on the CoA biosynthesis and/or utilization pathway, its antiplasmodial activity was tested in the presence of 100 μM pantothenate, which is 100 times more than the pantothenate concentration in standard medium. As can be seen in Fig. 2A, the higher pantothenate concentration completely attenuated the effect of compound 1e, consistent with the compound targeting the CoA biosynthesis and/or utilization pathway. Whether compound 1e was toxic to human cells was next investigated by exposing HFF cells to compound 1e at concentrations of up to 10 μM, 200-fold the IC50 against the parasite. No toxicity was observed (Fig. 2A).
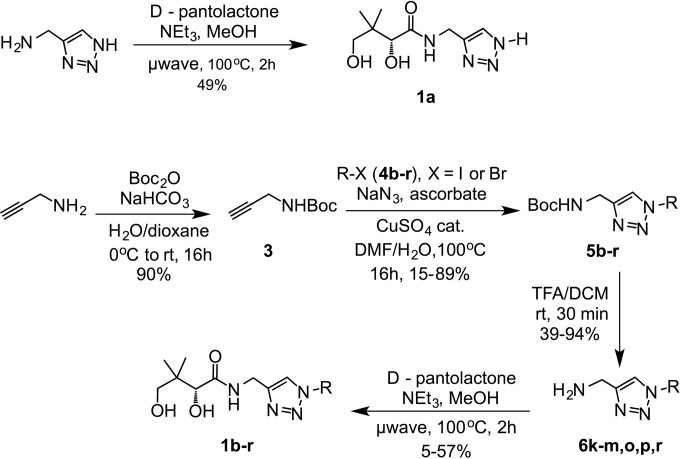
On the basis of the data obtained with compound 1e, a series of analogues was created (Fig. 3 and Table 1), to determine structure-activity relationships. Based on previous pantothenamide studies (4), it was envisaged that changing the length of the carbon chain of compound 1e might have a large effect on activity. Compounds 1a to j were designed to verify this hypothesis. The target scope also included a variety of N-substituents to verify the effect of branching, with (compound 1r) or without (compounds 1k and 1l) cyclization, aromatic rings of various electron densities (compounds 1m to q), including a 1,3-benzodioxole group (as in compound 1o) inspired from a previously reported pantothenamide (16). In all cases, the triazole group was maintained to ensure resistance to pantetheinase. Compound 1a was synthesized directly from 1H-1,2,3-triazole-5-methanamine (Fig. 3). The synthetic path used to access the triazole-containing pantothenamides 1b to r (Fig. 3) begins with the Boc (tert-butyloxycarbonyl) protection of propargylamine to yield compound 3. Next, a two-step one-pot process, involving first the formation of the desired azide from the corresponding bromide, followed by a 1,3-dipolar cycloaddition with compound 3, generated the 1,4-substituted regioisomers 5b to 5r selectively. The copper-catalyzed cycloaddition was optimized by performing the reaction in a sealed vessel (to counteract the volatility of compounds 4b to r), which increased yields up to 80%, from 40% in an open vessel. Next, Boc deprotection of compounds 5b to r was followed by a reaction with d-pantolactone, either with purification of the intermediate (compounds 6k, l, m, o, p, r) or not (for compounds 5b to j, n, q). The latter proved to be less efficient.
FIG 3.
Synthetic scheme for the preparation of compounds 1a through 1r. Specific yields can be found in Table S1 in the supplemental material.
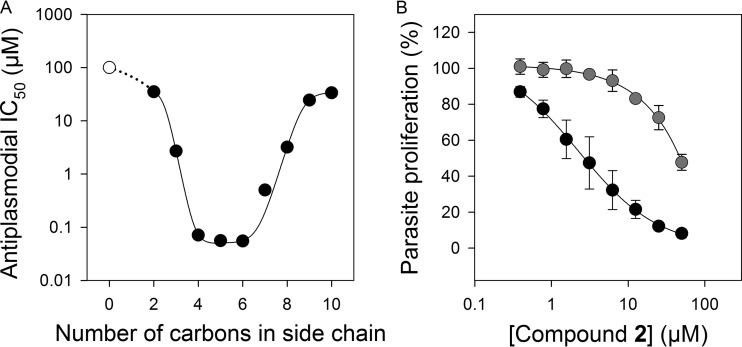
The in vitro antiplasmodial activity of compounds 1a to r, measured in the presence of pantetheinase, is shown in Table 1. Compounds 1a to j served to investigate the relationship between the length of the hydrocarbon chain at the R position and the antiplasmodial activity of the compounds. A direct relationship was found between the length of the hydrocarbon tail and the compounds' IC50s (Fig. 4A), with the IC50 decreasing as the number of carbons is increased up to 4 and then increasing as the length is increased beyond 6 carbons. There were a total of four compounds (1d to g) in this series that had submicromolar IC50s, with the most potent compound, 1f, having an IC50 of 55 ± 5 nM (n = 7), very similar to that of compound 1e (56 ± 5 nM, n = 9).
FIG 4.
(A) Relationship between the length of the hydrocarbon side chain (at the R position) of compounds 1a to j and their antiplasmodial IC50s. The IC50 for compound 1a (white symbol) could not be determined because it is higher than the highest concentration tested (100 μM), which had <10% inhibitory effect. The assay was carried out in the presence of pantetheinase activity and 1 μM pantothenate. (B) In vitro antiplasmodial activity of compound 2. Dose-response relationship of compound 2 in the presence (black circles) of pantetheinase. The effect of increasing the concentration of pantothenate (from 1 μM to 100 μM) in the presence of pantetheinase on the antiplasmodial activity of compound 2 was also tested (gray circles). Values are means from three or more independent experiments, each carried out in triplicate. Where not shown, the error bars (SEM) are smaller than the symbols.
An additional series of compounds (1k to r) included more chemical diversity, but most of these were not as effective as compounds 1e or 1f. Interestingly, however, branched compound 1k, which has the same number of carbon atoms as compound 1f, had a submicromolar IC50 (Table 1). Moreover, compounds 1d and 1l, which also have the same number of carbon atoms, have similar activities, with the branched compound 1l being less active. In order to confirm the optimal positioning of the amide-mimicking triazole group, a compound in which the triazole ring of compound 1e is shifted two carbon atoms down the chain was designed (Table 1, compound 2). It was synthesized as previously reported (25). As expected, the antiplasmodial activity of compound 2 against asexual, blood-stage P. falciparum was significantly less than that of compound 1e, with an IC50 of 3.5 ± 1.2 μM (n = 3; CI = 2.0 to 4.9 μM, power = 0.998).
Although less potent than compound 1e, compound 2 also inhibited parasite growth by targeting the CoA biosynthesis/utilization pathway as demonstrated by the fact that 100 times the normal pantothenate concentration in the culture medium antagonized, at least partially, its antiplasmodial activity (Fig. 4B). Taken together, these data support our hypothesis that the triazole moiety of compounds 1a to r mimics the labile amide group of pantothenamides yet is resistant to hydrolysis.
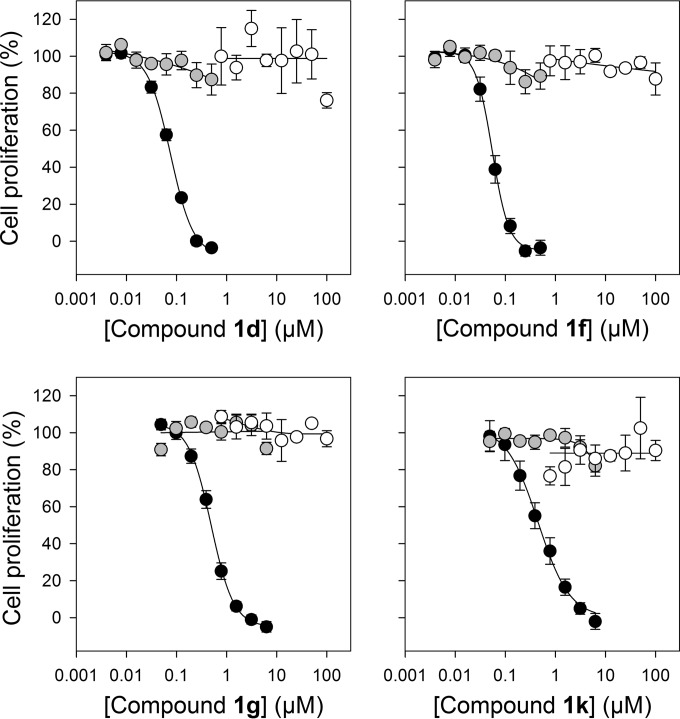
The activities of the compounds that, in addition to compound 1e, exhibited submicromolar IC50s against the parasite (i.e., compounds 1d, 1f, 1g, and 1k) were next investigated in more detail. In the presence of 100 μM pantothenate, the antiplasmodial activity of all four compounds was antagonized, consistent with the compounds acting on the target pathway (Fig. 5). The compounds were not toxic to HFF cells at concentrations of up to 100 μM (Fig. 5).
FIG 5.
In vitro antiplasmodial activity and cytotoxicity screening of a selection of the compounds tested. The antiplasmodial activity of the compounds was tested in the presence of pantetheinase in medium containing 1 μM (black circles) or 100 μM pantothenate (gray circles). The white circles show cytotoxicity screens performed against HFF cells using concentrations (up to 100 μM) much higher than those required to inhibit parasite growth (IC50 = 55 nM to 0.54 μM). The data are averaged from three independent experiments, each carried out in triplicate, and the error bars represent SEM.
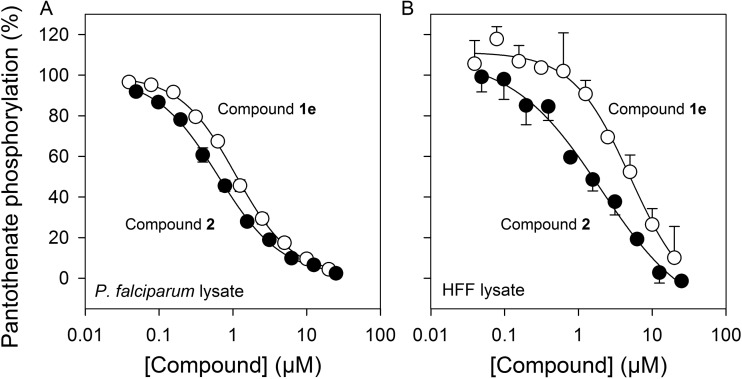
Additional experiments were carried out with compound 1e (and compound 2 as a control) to investigate further its mechanism of action. Specifically, we looked for a correlation between the antiplasmodial activity of the compounds and their activity against a possible target. Given the similarity of compound 1e to pantothenate, compound 1e was expected to interact with PanK, the first enzyme involved in CoA biosynthesis. The phosphorylation of [14C]pantothenate by PanK in P. falciparum lysates (23) following a 40-min incubation was determined in the presence of increasing concentrations of compounds 1e or 2 (Fig. 6). As expected, it was found that as the concentration of compounds 1e or 2 was increased, the amount of [14C]pantothenate phosphorylated by the P. falciparum lysate was reduced. Surprisingly, however, compound 1e was significantly less effective at inhibiting pantothenate phosphorylation (IC50, 1.1 ± 0.1 μM; n = 3) compared to its antiplasmodial activity (IC50 = 56 ± 5 nM; CI = 0.96 to 1.21 μM; power = 1), while compound 2 was equally effective at inhibiting pantothenate phosphorylation (IC50 = 0.6 ± 0.1 μM; n = 3) and parasite growth (IC50 = 3.5 ± 1.2 μM; CI = −6.4 to 0.6 μM; power = 0.348). Although the potency of compound 1e and that of compound 2 at inhibiting pantothenate phosphorylation by P. falciparum PanK were similar (<2-fold difference), they were statistically significantly different (CI = 0.36 to 0.65 μM; power = 1.0).
FIG 6.
Effect of compounds 1e and 2 on [14C]pantothenate phosphorylation by P. falciparum (A) and HFF cell (B) lysates. PanK activity was detected using [14C]pantothenate and Somogyi reagent. The assay was carried out for 40 min (P. falciparum) or 120 min (HFF cells) in the presence or absence of increasing concentrations of compound 1e (white circles) and compound 2 (black circles). Data are expressed as a percentage of the total amount of [14C]pantothenate phosphorylated in the absence of inhibitors and are averaged from three independent experiments (±SEM), each carried out in duplicate.
The fact that the difference observed in the antiplasmodial IC50s of compounds 1e and 2 is not carried through in the pantothenate phosphorylation assay points to the target of compound 1e being something other than PanK. This is further supported by our observation that N5-Pan, the benchmark pantothenamide to which compound 1e is the closest mimic (10), inhibits phosphorylation of [14C]pantothenate by P. falciparum lysates with an IC50 of 0.13 ± 0.03 μM (n = 3) (see Fig. S1 in the supplemental material), making it 8-fold more active than compound 1e in this assay (CI = 0.7 to 1.3 μM; power = 1.0) despite being 35-fold less active in the in vitro antiplasmodial assay (IC50 = 2 ± 0.2 μM in the absence of pantetheinase) (12).
To examine whether the lack of activity of compound 1e (and other analogues) against HFF cell viability was due to a poor interaction between compound 1e and the HFF cell PanK, the ability of compounds 1e and 2 to inhibit [14C]pantothenate phosphorylation by PanK in lysates prepared from HFF cells was also tested. The results show that both compounds inhibited the phosphorylation of [14C]pantothenate by HFF cell lysate, with IC50s of 5.2 ± 0.4 μM (n = 3) for compound 1e and 1.6 ± 0.5 μM (n = 3) for compound 2. These values are similar to those obtained for compounds 1e and 2 when tested against the P. falciparum PanK (1.1 ± 0.1 μM and 0.6 ± 0.1 μM, respectively), consistent with the lack of cytotoxicity by the analogues against HFF cells not being due to a lack of an interaction with the HFF PanK.
DISCUSSION
Pantothenamides may be considered promising leads for the development of new antimalarial agents due to their excellent potency against P. falciparum (some with IC50s comparable to that of chloroquine against the RBC stage of the parasite), the fact that they act on a pathway not targeted by current drugs (12, 17), and their low toxicity against mammalian cells (7, 17). Their susceptibility to degradation by serum pantetheinase (12), however, has prevented their further development.
In this study, we show that replacement of the susceptible amide moiety of pantothenamides with a triazole isostere produces pantothenamide-mimicking compounds, which, for the first time, both show nanomolar antiplasmodial activity and are fully resistant to pantetheinase activity. In particular, one of the most active triazoles, compound 1e (IC50 = 56 nM), is also the closest mimic to the benchmark compound N5-Pan but is much more potent (N5-Pan; 2 μM in the absence of pantetheinase) (12). A further six triazole-bearing analogues (1f, 1l, 1m, 1n, 1p, 1q) resemble pantothenamides tested previously for antiplasmodial activity in pantetheinase-free medium (16). The triazole-bearing compounds were equivalent to (for compound 1l), or between 2 times (compound 1q) and >45 times (compound 1m) more potent than the pantothenamides that they mimic. The triazole moiety, therefore, not only prevents pantetheinase-mediated degradation of these compounds but also enhances their antiplasmodial potency. One possible explanation for the enhanced activity is the fact that the triazole group is more rigid than an amide bond, which may reduce the entropic cost of binding to the target. It is well established that by reducing this entropic term, the overall free energy of binding can be improved dramatically (26).
We also show that compound 1e is about 60 times more effective than compound 2 at inhibiting the RBC stage of the parasite's life cycle, yet both were much less potent in the kinase assay than the antiplasmodial activity of compound 1e. This, together with our observation that N5-Pan is a much weaker antiplasmodial yet more potent inhibitor of pantothenate phosphorylation than compound 1e, is consistent with PanK not being the target of compound 1e. Moreover, the transport of pantothenate into the parasite is an unlikely target in light of the fact that it takes place via a low-affinity (Km, ∼23 mM) transport mechanism (27), whereas the antiplasmodial activity of compound 1e is in the nanomolar range. Nevertheless, the observation that the antiplasmodial effect of compound 1e (and the other analogues tested) can be antagonized by increasing the extracellular concentration of pantothenate (Fig. 2 and 5) is consistent with the compound acting on the CoA biosynthesis and/or utilization pathway. An alternative interpretation whereby compound 1e has a mechanism of action that is unrelated to CoA biosynthesis/utilization but its uptake into the parasite is reduced when the extracellular pantothenate concentration is increased to 100 μM (via competition at the pantothenate transporter) can be excluded because the transporter would not be saturated under these conditions (i.e., at substrate concentrations that are 230-fold lower than the Km of the transporter). The target of compound 1e is therefore likely to be downstream of PanK (4). Alternatively, compound 1e (and not compound 2) may accumulate within the intact parasite to the micromolar level required to inhibit pantothenate phosphorylation by PanK.
Our data are consistent with the compounds being nontoxic. Given that compound 1e is equally as effective at inhibiting pantothenate phosphorylation by PanK from both P. falciparum and HFF cells, a possible explanation for the lack of activity against HFF cells is that the compounds may not permeate the latter cell type. The high specificity of the human sodium-dependent multivitamin transporter (SMVT) and the previous demonstration that several triazole-conjugated molecules are not substrates of the human SMVT are consistent with this explanation (28).
We have characterized a series of novel rigidified pantothenate-mimicking analogues that show potent antiplasmodial activity by targeting the CoA biosynthesis/utilization pathway. The incorporation of a strategically positioned triazole group renders the compounds resistant to degradation by pantetheinase in human serum. Furthermore, as they appear to be nontoxic to human cells, we believe that compounds 1d to g and k are potential lead compounds for the development of a novel antimalarial. Whether they have additional, hitherto-unknown liabilities that prevent them from being active in vivo remains to be determined and will be investigated in future work.
Supplementary Material
ACKNOWLEDGMENTS
We thank the Canberra branch of the Australian Red Cross for the RBCs, Giel van Dooren (Research School of Biology, Australian National University) for HFF cells, and Erick Strauss (Department of Biochemistry, Stellenbosch University) for the isobutyl pantothenamide.
Funding Statement
This work was partly funded by grants from Gouvernement du Canada | Natural Sciences and Engineering Research Council of Canada (NSERC) and Gouvernement du Canada | Canadian Institutes of Health Research (CIHR) to Karine Auclair. The efforts of Vanessa M. Howieson were funded by an Australian Government scholarship. The efforts of Annabelle Hoegl were funded by a Canadian Institutes of Health Research-McGill Drug Development Training Program (DDTP) scholarship. The efforts of Elisa Tran were funded by a Gouvernement du Canada | Canadian Institutes of Health Research (CIHR) scholarship.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.01436-16.
REFERENCES
- 1.Divo AA, Geary TG, Davis NL, Jensen JB. 1985. Nutritional requirements of Plasmodium falciparum in culture. I. Exogenously supplied dialyzable components necessary for continuous growth. J Protozool 32:59–64. doi: 10.1111/j.1550-7408.1985.tb03013.x. [DOI] [PubMed] [Google Scholar]
- 2.Spry C, Saliba KJ. 2009. The human malaria parasite Plasmodium falciparum is not dependent on host coenzyme A biosynthesis. J Biol Chem 284:24904–24913. doi: 10.1074/jbc.M109.025312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spry C, van Schalkwyk DA, Strauss E, Saliba KJ. 2010. Pantothenate utilization by Plasmodium as a target for antimalarial chemotherapy. Infect Disord Drug Targets 10:200–216. doi: 10.2174/187152610791163390. [DOI] [PubMed] [Google Scholar]
- 4.Spry C, Kirk K, Saliba KJ. 2008. Coenzyme A biosynthesis: an antimicrobial drug target. FEMS Microbiol Rev 32:56–106. doi: 10.1111/j.1574-6976.2007.00093.x. [DOI] [PubMed] [Google Scholar]
- 5.Saliba KJ, Ferru I, Kirk K. 2005. Provitamin B5 (pantothenol) inhibits growth of the intraerythrocytic malaria parasite. Antimicrob Agents Chemother 49:632–637. doi: 10.1128/AAC.49.2.632-637.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saliba KJ, Kirk K. 2005. CJ-15,801, a fungal natural product, inhibits the intraerythrocytic stage of Plasmodium falciparum in vitro via an effect on pantothenic acid utilisation. Mol Biochem Parasitol 141:129–131. doi: 10.1016/j.molbiopara.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Hoegl A, Darabi H, Tran E, Awuah E, Kerdo ESC, Habib E, Saliba KJ, Auclair K. 2014. Stereochemical modification of geminal dialkyl substituents on pantothenamides alters antimicrobial activity. Bioorg Med Chem Lett 24:3274–3277. doi: 10.1016/j.bmcl.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spry C, Chai CLL, Kirk K, Saliba KJ. 2005. A class of pantothenic acid analogs inhibits Plasmodium falciparum pantothenate kinase and represses the proliferation of malaria parasites. Antimicrob Agents Chemother 49:4649–4657. doi: 10.1128/AAC.49.11.4649-4657.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akinnusi TO, Vong K, Auclair K. 2011. Geminal dialkyl derivatives of N-substituted pantothenamides: synthesis and antibacterial activity. Bioorg Med Chem 19:2696–2706. doi: 10.1016/j.bmc.2011.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clifton G, Bryant SR, Skinner CG. 1970. N1-(substituted) pantothenamides, antimetabolites of pantothenic acid. Arch Biochem Biophys 137:523–528. doi: 10.1016/0003-9861(70)90470-4. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y-M, Frank MW, Virga KG, Lee RE, Rock CO, Jackowski S. 2004. Acyl carrier protein is a cellular target for the antibacterial action of the pantothenamide class of pantothenate antimetabolites. J Biol Chem 279:50969–50975. doi: 10.1074/jbc.M409607200. [DOI] [PubMed] [Google Scholar]
- 12.Spry C, Macuamule C, Lin Z, Virga KG, Lee RE, Strauss E, Saliba KJ. 2013. Pantothenamides are potent, on-target inhibitors of Plasmodium falciparum growth when serum pantetheinase is inactivated. PLoS One 8(2):e54974. doi: 10.1371/journal.pone.0054974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin F, Malergue F, Pitari G, Philippe J, Philips S, Chabret C, Granjeaud S, Mattei M, Mungall A, Naquet P, Galland F. 2001. Vanin genes are clustered (human 6q22–24 and mouse 10A2B1) and encode isoforms of pantetheinase ectoenzymes. Immunogenetics 53:296–306. doi: 10.1007/s002510100327. [DOI] [PubMed] [Google Scholar]
- 14.Dupre S, Rosei MA, Bellussi L, Del Grosso E, Cavallini D. 1973. The substrate specificity of pantethinase. Eur J Biochem 40:103–107. doi: 10.1111/j.1432-1033.1973.tb03173.x. [DOI] [PubMed] [Google Scholar]
- 15.Pett HE, Jansen PA, Hermkens PH, Botman PN, Beuckens-Schortinghuis CA, Blaauw RH, Graumans W, Van De Vegte-Bolmer M, Koolen KM, Rutjes FP, Dechering KJ, Sauerwein RW, Schalkwijk J. 2015. Novel pantothenate derivatives for anti-malarial chemotherapy. Malar J 14:169. doi: 10.1186/s12936-015-0673-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Villiers M, Macuamule C, Spry C, Hyun YM, Strauss E, Saliba KJ. 2013. Structural modification of pantothenamides counteracts degradation by pantetheinase and improves antiplasmodial activity. ACS Med Chem Lett 4:784–789. doi: 10.1021/ml400180d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Macuamule CJ, Tjhin ET, Jana CE, Barnard L, Koekemoer L, de Villiers M, Saliba KJ, Strauss E. 2015. A pantetheinase-resistant pantothenamide with potent, on-target, and selective antiplasmodial activity. Antimicrob Agents Chemother 59:3666–3668. doi: 10.1128/AAC.04970-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tron GC, Pirali T, Billington RA, Canonico PL, Sorba G, Genazzani AA. 2008. Click chemistry reactions in medicinal chemistry: applications of the 1,3-dipolar cycloaddition between azides and alkynes. Med Res Rev 28:278–308. doi: 10.1002/med.20107. [DOI] [PubMed] [Google Scholar]
- 19.Allen RJW, Kirk K. 2010. Plasmodium falciparum culture: the benefits of shaking. Mol Biochem Parasitol 169:63–65. doi: 10.1016/j.molbiopara.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Trager W, Jensen JB. 1976. Human malaria parasites in continuous culture. Science 193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 21.Smilkstein M, Sriwilaijaroen N, Kelly JX, Wilairat P, Riscoe M. 2004. Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrob Agents Chemother 48:1803–1806. doi: 10.1128/AAC.48.5.1803-1806.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson JD, Dennull RA, Gerena L, Lopez-Sanchez M, Roncal NE, Waters NC. 2007. Assessment and continued validation of the malaria SYBR Green I-based fluorescence assay for use in malaria drug screening. Antimicrob Agents Chemother 51:1926–1933. doi: 10.1128/AAC.01607-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saliba KJ, Horner HA, Kirk K. 1998. Transport and metabolism of the essential vitamin pantothenic acid in human erythrocytes infected with the malaria parasite Plasmodium falciparum. J Biol Chem 273:10190–10195. doi: 10.1074/jbc.273.17.10190. [DOI] [PubMed] [Google Scholar]
- 24.Spry C, Saliba KJ, Strauss E. 2014. A miniaturized assay for measuring small molecule phosphorylation in the presence of complex matrices. Anal Biochem 451:76–78. doi: 10.1016/j.ab.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 25.Awuah E, Ma E, Hoegl A, Vong K, Habib E, Auclair K. 2014. Exploring structural motifs necessary for substrate binding in the active site of Escherichia coli pantothenate kinase. Bioorg Med Chem 22:3083–3090. doi: 10.1016/j.bmc.2014.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Broughton HB, Watson IA. 2004. Selection of heterocycles for drug design. J Mol Graph Model 23:51–58. doi: 10.1016/j.jmgm.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 27.Saliba KJ, Kirk K. 2001. H+-coupled pantothenate transport in the intracellular malaria parasite. J Biol Chem 276:18115–18121. doi: 10.1074/jbc.M010942200. [DOI] [PubMed] [Google Scholar]
- 28.Chirapu SR, Rotter CJ, Miller EL, Varma MV, Dow RL, Finn MG. 2013. High specificity in response of the sodium-dependent multivitamin transporter to derivatives of pantothenic acid. Curr Top Med Chem 13:837–842. doi: 10.2174/1568026611313070006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.