Abstract
High doses of micafungin are advocated in neonates with systemic candidiasis, but limited pharmacokinetic (PK) and safety data are available to support their use. Eighteen preterm neonates and infants with systemic candidiasis, three of whom had meningitis, were treated for at least 14 days with 8 to 15 mg/kg of body weight/day of intravenous micafungin. Plasma micafungin concentrations (four measurements for each patient) were determined after the third dose, and the cerebrospinal fluid (CSF) micafungin concentrations in three patients were also obtained. Population PK analyses were used to identify the optimal model, and the model was further validated using external data (n = 5). The safety of micafungin was assessed by measurement of the levels of liver and kidney function biomarkers. The mean age and weight at the initiation of treatment were 2.33 months (standard deviation [SD], 1.98 months) and 3.24 kg (SD, 1.61 kg), respectively. The optimal PK model was one that scaled plasma clearance to weight and the transaminase concentration ratio. The CSF of three patients was sampled, and the observed concentrations were between 0.80 and 1.80 mg/liter. The model-predicted mean micafungin area under the concentration-time curve over 24 h was 336 mg · h/liter (SD, 165 mg · h/liter) with the 10-mg/kg/day dosage. Eighteen of the 23 subjects (78.2%) had clinical resolution of their infection, but 5 had neurologic impairments. Among the transaminases, alkaline phosphatase measurements were significantly higher posttreatment, with a geometric mean ratio of 1.17 (90% confidence interval, 1.01, 1.37). Furthermore, marked elevations in the gamma-glutamyltransferase (GGT) level were observed in three patients treated with 10- to 15-mg/kg/day doses, and improvement of the GGT level was noted after a dose reduction. Higher weight-based doses of micafungin were generally well tolerated in neonates and infants and achieved pharmacokinetic profiles predictive of an effect.
INTRODUCTION
Invasive candidiasis (IC) is a common infection in newborns and occurs in 7% to 20% of critically ill neonates (1–4). This risk increases progressively in low-birth-weight (LBW) infants (birth weight, <2,500 g), very-low-birth-weight (VLBW) infants (birth weight, <1,500 g), and extremely low-birth-weight (ELBW) infants (birth weight, <1,000 g) (1–4). Moreover, 50% to 64% of ELBW infants are susceptible to central nervous system (CNS) involvement early in the presentation of IC (1). CNS involvement is associated with a high rate of mortality (30 to 60%), and survivors can have significant long-term complications, including neuromotor developmental disorders, chronic lung disease, and severe retinopathy of prematurity (2–4).
To date, most studies of antifungal drugs and clinical experience with such drugs in neonates have centered on older antifungal drugs, such as amphotericin B (AMB) deoxycholate, liposomal amphotericin B (L-AMB) and fluconazole. As a consequence, the current Infectious Diseases Society of America (IDSA) guidance supports the consideration of AMB desoxycholate and fluconazole as first-line therapies (5). However, the renal and bone marrow toxicities associated with AMB deoxycholate and the potential for the presence of Candida species less susceptible to fluconazole require the consideration of alternative agents. L-AMB is an alternative, but less evidence supporting its use is available, especially when candiduria is also present (5). Echinocandins are considered potential salvage therapy for the treatment of CNS invasive candidiasis and candidemia but not for the treatment of CNS infections (5). Among the echinocandins, micafungin is the only agent approved for use in pediatric patients 4 months of age and older, but uncertainty about the dosage remains (6–8). These uncertainties include limited safety and pharmacokinetic (PK) data on the echinocandins in pediatric patients less than 4 months of age and concerns about low CNS concentrations.
No specific dosage recommendations for micafungin for the treatment of neonatal candidiasis are provided in the IDSA guidance. In contrast, the European guidance suggests a higher dosage of 4 to 10 mg/kg of body weight/day of micafungin when the CNS is involved (9). No data for the special population of premature and LBW infants less than 4 months of age are available. It has been proposed that neonates and VLBW neonates may require higher weight-based doses (up to 15 mg/kg/day) of micafungin, in line with allometric scaling of plasma clearance to achieve exposures bioequivalent to those in adults (10). This is sensible because the distribution of the weight of newborn VLBW neonates and 4-month-old infants can be 1 kg to 7 kg, for example, which reflects a 7-fold weight range, whereas the weight range for nonobese adults is 2-fold. This lower weight but wide weight range for neonates raises the questions of whether a standard micafungin dose of 2 mg/kg/day in infants <4 months of age is adequate across this wide expected weight range.
The current study characterizes the plasma pharmacokinetics and safety associated with the use of a high dose (8 to 15 mg/kg/day) of micafungin in neonates and infants with IC. We also measured serial concentrations of micafungin in the cerebrospinal fluid (CSF) where CNS infection was documented and an external shunt was available for sampling.
MATERIALS AND METHODS
Study subjects.
The study was reviewed and approved by the Bambino Gesù Children's Hospital Research Ethics Committee, and parental informed consent was obtained prior to the conduct of any procedures. Preterm neonates and infants with IC, including those with meningitis and hydrocephalus drained by an external shunt, were treated for at least 14 days with micafungin (Mycamine) at an initial dosage ranging from 8 to 15 mg/kg/day. The dosage was formulated as a 2-mg/ml solution and administered intravenously over 1 h once daily. Infants who died within the first 48 h of therapy and those with acute or chronic hepatic diseases and/or with known hypersensitivity to echinocandins were excluded. Dosage modification was performed at the discretion of the physician on the basis of available laboratory information.
Diagnostic criteria.
Candida infections were classified as proven and suspected. In patients with proven infections, Candida species were isolated from blood, CSF, urine, or peritoneal fluid cultures or by the detection of fungal DNA by PCR of blood and/or CSF with the presence of abnormal C-reactive protein levels or white blood cell counts. Urinary tract infections secondary to infection with a Candida species were defined according to standard CDC/NHSN criteria (11). Neonates with a deteriorating clinical presentation on antibiotic therapy, neonates with a mannan antigen concentration in blood of ≥125 pg/ml by enzyme-linked immunosorbent assay (range, 62.5 to 125 pg/ml; Platelia Candida Ag Plus assay; Bio-Rad), and/or neonates from whom a Candida species was isolated from at least two noncontiguous locations (tracheal aspirate, gastric aspirates, stool), all of whom also had a laboratory test suggestive of infection, were classified as having suspected candidiasis.
Pharmacokinetic sampling and assay.
The CSF and plasma levels of micafungin were determined by high-performance liquid chromatography (HPLC). Blood samples were collected at about the time of administration of the third initial dose and after the third modified dose (if changed) of micafungin, which consisted of nominal collection times of a time within 30 min of administration of the third dose and 0.5 to 1, 2, and 8 h after administration of the dose. To optimize timing of blood collection with the timing of blood collection required for clinical care, actual sampling times were overlapped with those required for the routine management of critically ill children. Blood samples were obtained by puncture of the heel, collected in a 0.2-ml capillary tube, and transferred into microtubes; EDTA had been sprayed into both the capillary tube and the microtubes. Plasma was separated by centrifugation at 3,000 × g for 10 min and stored at −80°C until analysis. Anidulafungin (20 μg/ml) served as the internal standard, and both agents were extracted using protein precipitation and chromatographic separation on a reversed-phase column. The effluent was monitored at the two UV wavelengths of 273 nm and 306 nm, which represent the absorption maxima of micafungin and anidulafungin, respectively. In patients with Candida meningitis and hydrocephalus, CSF micafungin concentrations in samples collected from an external ventricular shunt were measured as described above. These CSF samples were collected at times that corresponded with the time of blood sampling.
Population pharmacokinetic analyses.
Pharmacokinetic analyses were conducted using the PMetrics library package, version 1.4.1, implemented through R (12). Initial exploratory analyses were performed using an iterated 2-stage Bayesian (IT2B) method to test 1-, 2-, and 3-compartment models and identify potential covariates of system parameters, followed by use of the nonparametric adaptive grid (NPAG). Observations were weighted to the inverse of the standard deviation (SD) multiplied by the term γ, which captures extra process noise. Covariates of system parameters were individually tested in the model using the NPAG. Given that body weight is a well-recognized covariate of micafungin system parameters, we tested this relationship as linear and nonlinear functions. Model discrimination was based on the Akaike information criterion (AIC) and visual inspection of the goodness of fit of the population predicted and individual predicted plots to the observed plots.
Internal validation of the data was accomplished utilizing the nonparametric distributional error (13). Central tendencies around the final model system parameter statistic were reported as the median absolute weight deviation (MAWD) and the 95% confidence interval (CI) surrounding these estimates (14). The 95% confidence interval limits and MAWD were derived by using an added function in PMetrics that relies on a Monte Carlo approach. The approach simulates 1,000 samples for each support point in the model with replacement from the weighted marginal distribution of each parameter. For each 1,000-sample simulated support point set, the difference between each sample point and the median for this set was used to generate the MAWD and 95% confidence interval for each parameter. External validation of the final model was accomplished through analyses of data for subjects (n = 5) not included in the original population analyses. The final validated model was used for Monte Carlo simulations (n = 5,000) to generate profiles of the micafungin area under the concentration-time curve (AUC) from 48 to 72 h (AUC48–72) for dosages of 2, 3, 4, 8, 10, and 15 mg/kg/day for three doses.
Safety assessment.
Hepatic function was assessed by laboratory assessment of aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), gamma-glutamyltransferase (GGT), total bilirubin (BIL), direct bilirubin (BILd), albumin (ALB), and total protein concentrations. In addition, blood urea nitrogen (BUN) and serum creatinine (SCr) concentrations were assessed as markers of kidney function.
Statistical analyses.
All statistical analyses were performed by the use of the Stata SE, version 13.1, program (StataCorp LP, College Station, TX, USA) and through R. Descriptive statistics were used to characterize the demographic and baseline laboratory parameters. Graph matrix plots were used for visual inspection of scatter plots and aid with consideration of the linear and nonlinear models for covariate relationships. The sign test of matched pairs was used to compare laboratory values at the end of therapy to those at the baseline. A two-sided P value of <0.05 was considered statistically significant.
RESULTS
Study subjects.
The study enrolled 18 subjects (n = 13 male subjects) for the initial pharmacokinetic analyses. A summary of the subject demographics is included in Table 1. The mean gestational age of 10 babies who were born preterm and weighed <2.5 kg was 34.9 weeks (SD, 3.92 weeks); of these, 5 had weights that met the definition of VLBW. The weight distribution of the infants at the time of initiation of micafungin treatment was 1.20 kg to 6.76 kg, and the median weight was approximately 3 kg. The baseline laboratory parameters are also reported in Table 1. Although at the baseline 50.0% and 22.2% of patients had AST and ALT concentrations above the normal concentrations, respectively, only one patient had a value 2 times above the upper limit of normal (ULN). At the baseline, the ALP concentration was above the ULN in 2 patients, while the GGT concentration was above the ULN and 3 times the ULN in 83.3% of patients and 50.0% of patients, respectively. Other laboratory markers were within the normal limits for the majority of patients at the baseline.
TABLE 1.
Demographic parameters of neonates and infants at birth and at initiation of treatment and baseline laboratory parameters at initiation of treatmenta
| Parameter | Mean (SD) | Median (range) |
|---|---|---|
| Gestational age (wk) | 34.9 (3.92) | 36.7 (26.9–39.0) |
| Birth wt (kg) | 2.29 (0.926) | 2.38 (1.10–3.80) |
| Age (mo) | 2.33 (1.98) | 1.34 (0.107–6.07) |
| Wt (kg) | 3.24 (1.61) | 2.98 (1.2–6.76) |
| AST concn (IU/liter) | 46.6 (35.2) | 39.5 (16–172) |
| ALT concn (IU/liter) | 37.6 (44.2) | 24 (10–199) |
| GGT concn (IU/liter) | 204 (207) | 146 (26–829) |
| ALP concn (IU/liter) | 679 (274) | 622 (317–1216) |
| BIL concn (mg/dl) | 1.42 (1.67) | 0.585 (0.100–4.65) |
| BILd concn (mg/dl) | 0.791 (1.21) | 0.240 (0.010–4.02) |
| ALB concn (g/dl) | 3.48 (0.811) | 3.50 (1.80–5.00) |
| Total protein concn (g/dl) | 4.68 (0.893) | 4.80 (3.30–6.20) |
| BUN concn (mg/dl) | 14.7 (12.0) | 10.0 (2.00–52.0) |
| SCr concn (mg/dl) | 0.642 (0.687) | 0.400 (0.150–2.61) |
Data are for 18 neonates and infants.
Infectious etiology and treatment.
Infection with Candida species was proven by culture in 17 of the 18 subjects, with positivity for antigenemia supporting this diagnosis in 1 case (Table 2). Candida albicans was the most common species (50%) detected in blood and urine and from the CNS in 9 cases. Susceptibility testing was performed, and the micafungin MIC ranged from ≤0.0075 mg/liter to 0.015 mg/liter. Candida parapsilosis (38.9%) was the second most frequent species isolated, and micafungin MIC values against this pathogen were within expected epidemiologic values of 1 mg/liter or 2 mg/liter. The requirement for total parental nutrition (TPN) and/or enteral nutrition was present in over three-quarters of the study population. The majority of subjects were treated for 14 to 21 days with an initial dose of 8 or 10 mg/kg, and treatment was initiated with a dose of 15 mg/kg in only two subjects (both of whom were infected with C. albicans). The dosage was reduced in three subjects receiving 10 mg/kg (n = 2) or 15 mg/kg (n = 1) due to an elevation in GGT concentrations.
TABLE 2.
Clinical and treatment characteristics of the patients by Candida speciesa
| Characteristic | Antigen | C. krusei | C. parapsilosis | C. albicans |
|---|---|---|---|---|
| No. of patients | 1 | 1 | 7 | 9 |
| MIC value (mg/liter)b | ND | ND | 1.0–2.0 (5) | ≤0.015 (5) |
| Clinical sourceb | Blood (1) | Urine (1) | Blood (5), urine (2) | Blood (4), urine (3), CNS (2) |
| No. of patients with: | ||||
| Sepsis or SIRS | 1 | 0 | 3 | 3 |
| Renal dysfunction | 0 | 0 | 1 | 2 |
| Hepatic dysfunction | 0 | 0 | 1 | 1 |
| Requirement for TPN or enteral nutrition | 1 | 0 | 6 | 7 |
| Wt (kg) | 2 | 3.2 | 3.41 (1.95) | 3.25 (1.55) |
| Dose (mg/kg) | 8 | 8 | 8.71 (1.89) | 10.2 (2.86) |
| Dose (mg) | 16 | 25.6 | 30.0 (20.4) | 35.2 (26.3) |
| Duration of treatment (days) | 14 | 21 | 18.3 (3.40) | 19.0 (8.98) |
| Plasma Ctrough (mg/liter) | 3.50 | 14.1 | 8.15 (4.78) | 6.40 (3.50) |
| Plasma Cpeak (mg/liter) | 12.5 | 30.7 | 30.5 (7.92) | 23.0 (9.72) |
| No. of patients whose CSF was sampled | 0 | 0 | 2 | 1 |
| CSF Crange (mg/liter)c | 1.23 (10), 1.3–1.80 (8) | 0.8–1.1 (10) | ||
| AUC48–72 (mg · h/liter) | 181 | 437 | 320 (139) | 278 (101) |
Abbreviations: ND, not determined; SIRS, systemic inflammatory response syndrome; Ctrough, trough concentration; Cpeak, peak concentration; Crange, concentration range. Values are reported as mean (SD) unless specified otherwise.
Values in parentheses in this row are the numbers of patients.
Values in parentheses in this row are the dose (in milligrams per kilogram).
Population pharmacokinetic analyses.
The plasma concentrations of micafungin were measured in four samples from each of 18 subjects, for a total of 72 plasma concentrations of micafungin. Summaries of the trough concentrations measured before the third dose and the peak concentrations measured after the third dose are provided in Table 2. The peak concentrations were a median of 3.83-fold (range, 1.46 to 8.20-fold) higher than the trough concentrations. CSF micafungin concentrations were measured in three CSF samples from each of three subjects, for a total of nine CSF concentrations. Unlike the range of measurements in plasma, the range of measurements in the CSF was narrow, and the central tendency was a concentration of 1.0 to 1.5 mg/liter.
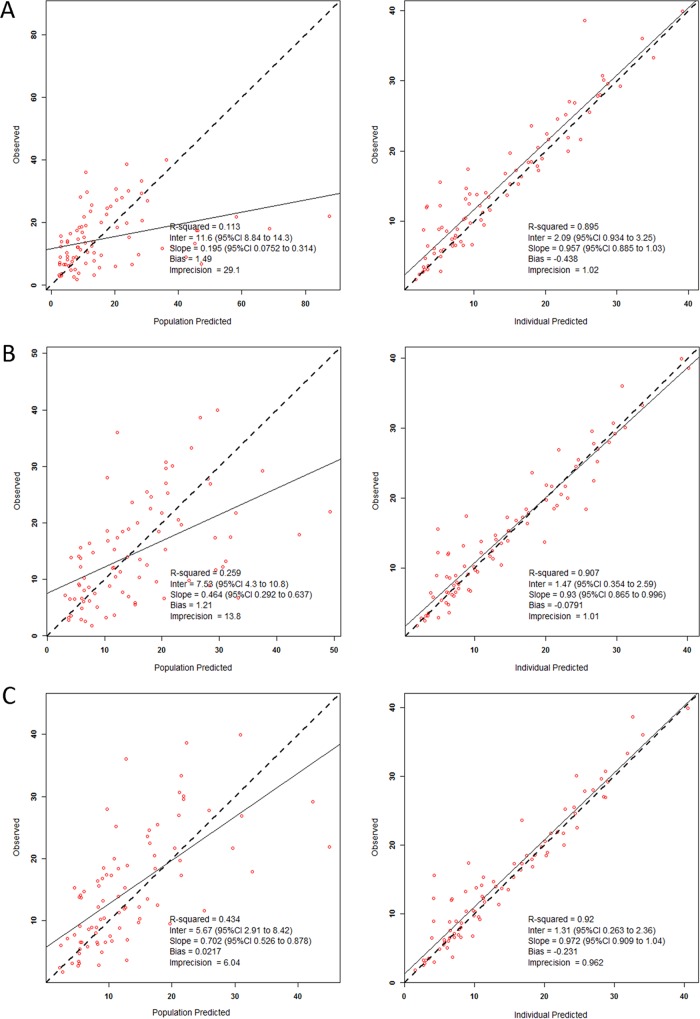
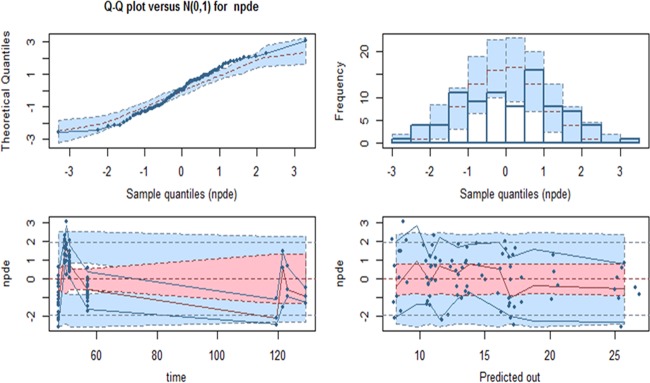
The initial exploratory approach using the IT2B method identified a 2-compartment model to be optimal for characterization of the plasma concentrations. The sparse number and range of CSF measurements limited construction of a plasma-CSF comodel. Approximately 30 alternate models were tested using the NPAG to explore the relationships of body size and other covariates to system parameters. The base (no covariates), secondary (allometrically scaled), and final structural models are provided in Table 3. The population predicted concentrations versus the observed concentrations for the stated models demonstrated clear improvement with the inclusion of covariates (Fig. 1). The final model for clearance (CL) included a fixed slope term with a supralinear exponent (>1.0) term over weight and a slope term function of the AST/ALT ratio. Weight was also an important covariate of both the volume of distribution of the central compartment (Vc) and distribution clearance (CLd) but not the volume of distribution of the peripheral compartment (Vp). Given that the lowest observed weight was approximately 1 kg, weight was not benchmarked to a 70-kg individual as in previous analyses. The internal validity of each model was tested and compared as well with the final model, and the results are detailed in Fig. 2. The median (5th and 95th percentile) AUC48–72 values observed in the patients (n = 18), the AUC48–72 values for patients evaluated by use of a Monte Carlo simulation (n = 5,000 patients per dose), and the AUC48–72 values observed in the external validation data set (n = 5) are included in Fig. S1 in the supplemental material. As shown in Fig. S1, the AUC48–72 values for the five subjects whose data were used for the external validation data set were well within the expectations generated by the model and observed in the data set used to develop the model. These five ELBW subjects had a median weight of 1.90 kg (range, 1.00 to 3.45 kg) and a median age of 2.83 months (range, 1.26 to 7.87 months) at the initiation of treatment because of suspected IC by positive antigenemia findings.
TABLE 3.
Pharmacokinetic system parameter estimates of the micafungin base and final modelsa
| Parameter | Base model | Allometrically scaled model | Final model |
|---|---|---|---|
| CL (liters/h) | 0.143 (0.013–0.267) | 0.0481 (0.0131–0.0541) | 0.011 (fixed) |
| Wθ1 | θ1 = 0.75 (fixed) | θ1 = 1.57 (1.18–2.28) | |
| (AST/ALT) · θ2 | θ2 = 0.0364 (0.005–0.0593) | ||
| Vc (liter) | 0.435 (0.008–1.05) | 0.142 (0.0368–0.238) | 0.216 (0.174–0.680) |
| Wθ3 | θ3 = 1.00 (fixed) | θ3 = 1.30 (0.010–1.31) | |
| CLd (liters/h) | 0.019 (0.006–0.049) | 0.111 (0.0352–0.240) | 0.156 (0.0198–0.324) |
| Wθ4 | θ4 = 0.131 (0.011–3.04) | ||
| Vp (liters) | 1.19 (0.0566–2.51) | 2.25 (0.482–5.93) | 1.81 (1.21–3.18) |
| AIC | 490 | 485 | 481 |
| γ | 2.12 | 2.10 | 2.09 |
The data are reported as the median (95% confidence interval around the median absolute weighted deviation). W, weight.
FIG 1.
Scatter and linear fit plots of the population and individual predicted versus observed concentrations with an 2-compartment base model (A), an allometrically scaled 2-compartment model (B), and a 2-comparment covariate structured final model (C).
FIG 2.
Quantile-quantile (Q-Q) plot (top left) of the nonparametric distributional error (npde) versus the corresponding quantiles of a normal distribution [N(0,1)], and histogram (top right) of the npde showing symmetric light tailed distributions centered at zero. The nonparametric distributional error versus time (in hours) of the sample and predicted out (micafungin concentrations in mg/liter) is shown, with horizontal lines representing the −2, 0, and +2 standard deviations for the ideal normal distribution and the 95% error, shown by the bottom blue, pink, and top blue regions, respectively.
Clinical outcomes and safety.
The infection was resolved in 18 of the 23 subjects (78.2%) studied, and 13 were discharged home without major deficits, while 5 had neurologic impairments at the time of discharge. Five (21.7%) subjects died during hospitalization, but death was not attributed to micafungin administration. Three subjects died from septic shock and were documented to have concomitant bacterial infections (a Serratia sp. in two cases and Klebsiella pneumoniae in one case). No statistically significant differences between the posttreatment and baseline laboratory test values for liver and kidney function, with the exception of the values for ALP, were observed. The median ALP concentration posttherapy was 835 IU/liter (5th and 95th percentile concentrations, 286 and 1,714 IU/liter, respectively), and the geometric mean ratio was 1.17 (90% confidence interval, 1.01, 1.37). As noted above, the GGT level was elevated at the baseline in the majority of the patients. Nonetheless, the micafungin dose was reduced in three cases due to a marked elevation (>7 times the ULN) of the GGT level that returned to normal limits at treatment completion. No clear relationship between changes in laboratory parameters and micafungin exposure or treatment duration could be discerned (see Fig. S2 in the supplemental material).
DISCUSSION
Safe and effective treatment options for neonates and infants with IC are limited (5). Compared to polyenes and triazoles, echinocandins are generally well tolerated, have a favorable pharmacokinetic profile, and have a low potential for drug-drug interactions (15). At present, micafungin at a dosage of 2 mg/kg every 24 h is the only echinocandin approved for use by the U.S. FDA for the treatment of candidemia, acute disseminated candidiasis, and Candida peritonitis and abscess in infants 4 months of age or older (16). Similar dosing recommendations are provided in the EMEA-approved label, but the recommendations include a black box warning related to hepatotoxicity and specifically refer to the development of hepatocellular tumors in rats treated with micafungin for a period of 3 months or longer (17). These potential dose-limiting concerns are important, given that micafungin doses above 2 mg/kg every 24 h are likely necessary on the basis of preclinical models and bridging studies of CNS-related candidiasis (9, 10). Current well-characterized PK/pharmacodynamic models have shown that doses of 10 mg/kg/day are necessary.
Stronger evidence to support higher micafungin doses for the treatment of neonatal candidiasis has been sought. A phase 3 randomized, double-blind, multinational clinical trial comparing micafungin at 10 mg/kg/day to AMB deoxycholate at 1 mg/kg/day was recently terminated due to insufficient enrollment over an approximately 7-year period (ClinicalTrials.gov registration no. NCT00815516) (18). This trial enrolled 30 neonates and infants who were randomized in a 2:1 ratio to receive micafungin or AMB deoxycholate. The PK profile of micafungin was measured in 12 subjects, who had mean concentration of 25.1 mg/liter within 15 min postinfusion and 14.1 mg/liter at 15 to 24 h postinfusion. The period of fungus-free survival was a mean of 60.0% (95% CI, 36.1% to 80.9%) of patients in the micafungin arm and 70.0% (95% CI, 34.8% to 93.3%) of patients in the AMB deoxycholate arm. Although major conclusions cannot be drawn from this well-designed study (ClinicalTrials.gov registration no. NCT00815516), the findings support the narrative that few data that support the clinical use of high-dose micafungin in neonates and infants are available at present. The current study therefore provides independent insights into micafungin plasma and CNS pharmacokinetic and safety data in an underrepresented population. Our results substantiate recommendations for the use of higher micafungin doses in this pediatric population (9, 10).
The current study has several strengths that include the evaluation of doses that are 4- to 7.5-fold higher than those approved by regulatory agencies. Multiple plasma samples for determination of micafungin concentrations were collected from neonates, and concentrations in CSF were observed to be at or above 0.8 mg/liter in three infants. The majority of infants (there was one exception) had documented IC, and susceptibility testing was performed on more than half of the collected samples. Nonparametric population pharmacokinetic analyses were used, and these permitted the identification of the AST/ALT ratio to be a covariate of micafungin clearance from plasma. The population PK model was further validated with an external data set. Importantly, the safety of micafungin was documented through assessment of the levels of biomarkers of liver and renal function. The elevations in GGT levels observed in three patients improved when the dose was reduced. No clear relationships between the micafungin AUC or the duration of therapy and the elevation in the levels of biomarkers of liver function were observed.
The results of the current study support the conclusions made by Hope and colleagues, who provided the most comprehensive population PK model of micafungin and its metabolites in children and adolescents ranging from 4 months to 16 years of age (19). Their comprehensive population PK analysis included two studies that were the first to suggest that higher micafungin doses (10 to 15 mg/kg/day) may be needed in neonates (20, 21). They demonstrated micafungin clearance to be allometrically scaled to weight and to be a fixed exponent of the AST and total bilirubin concentrations (19). They benchmarked their pediatric doses to values of the micafungin area under the concentration-time curve over 24 h (AUC24) in adults receiving 100 mg/day, with the 10th and 90th percentile expected AUC24 values being 75.0 mg · h/liter and 139 mg · h/liter, respectively. A 2-mg/kg/day dose in patients ranging from 4 months to less than 2 years of age was expected to achieve a similar AUC24 distribution in 82.8% of their simulated population (19). Our work suggests that a 3- to 4-mg/kg/day dose in patients weighing 1 kg to 7 kg will achieve AUC24 values of 75.0 to 139 mg · h/liter in 40.5% to 43.3% of the simulated population. Also, the mean AUC24 predicted by the model was 336 mg · h/liter (SD, 165 mg · h/liter), with the 10th and 90th percentile AUC24 values being 186 mg · h/liter and 572 mg · h/liter, respectively, with the 10-mg/kg/day dosage. This less than 5-fold increase in the AUC, despite a dose based on a 5-fold higher weight, supports our observation that micafungin CL may not scale by the standard allometric assumption with body weight in this low weight range (1 to 7 kg). Wang et al. have shown a body weight-dependent allometric exponent for the scaling of clearance with a sigmoidal relationship when the weight is <10 kg (22). Our observations support consideration of the sigmoidal model proposed by Wang and colleagues when scaling micafungin clearance across a wider weight distribution (22). However, the limited weight distribution and sample size in this study restricted our evaluation of this alternate model and the definition of a simple dosage regimen to mimic micafungin AUC24 expectations in adults.
As with any study with the sample size used in this study, the generalizability of our findings is limited. The open-label nature of the study limits the direct attribution of clinical outcomes and safety to the dosage of micafungin. The majority of subjects had four sample collections at specified times, and this number of samples limits consideration of more complex structural models. Measurement of the micafungin concentration in CSF samples could be performed only for patients with external shunts, and the values observed over the sampling period were within a narrow range. The micafungin concentrations observed in CSF were severalfold higher than the MIC values against C. albicans but 0.5- to 1.0-fold the MIC values against C. parapsilosis. Translation of these concentrations measured in CSF to brain tissue concentrations is not simple because neonatal candidiasis that affects the CNS is typically a meningoencephalitis (10). Preclinical studies of experimental hematogenous Candida meningoencephalitis have shown that the concentrations of micafungin are measurable in the meninges and choroid and are severalfold higher than those in the CSF (10). Although other CNS tissues were not sampled, our ability to measure the micafungin concentration in CSF suggests the possibility that the concentrations are higher in brain tissue, but this could not be determined with certainty.
In summary, we evaluated the safety and pharmacokinetics of micafungin at the high doses proposed to be necessary but not approved by regulatory agencies to manage neonatal candidiasis. We demonstrate that micafungin was well tolerated by this critically ill population at doses up to 15 mg/kg. The pharmacokinetic model defined by this data set supports the continued evaluation of the clinical outcomes achieved with a 10-mg/kg/day dose of micafungin for the treatment of candidiasis in neonates and infants with body weights of <7 kg, especially when CNS involvement is suspected.
Supplementary Material
Funding Statement
No extramural financial support was received to conduct this work.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.01172-16.
REFERENCES
- 1.Benjamin DK Jr, Stoll BJ, Fanaroff AA, McDonald SA, Oh W, Higgins RD, Duara S, Poole K, Laptook A, Goldberg R, National Institute of Child Health and Human Development Neonatal Research Network. 2006. Neonatal candidiasis among extremely low birth weight infants: risk factors, mortality rates, and neurodevelopmental outcomes at 18 to 22 months. Pediatrics 117:84–92. doi: 10.1542/peds.2004-2292. [DOI] [PubMed] [Google Scholar]
- 2.Friedman S, Richardson SE, Jacobs SE, O'Brien K. 2000. Systemic Candida infection in extremely low birth weight infants: short term morbidity and long term neurodevelopmental outcome. Pediatr Infect Dis J 19:499–504. doi: 10.1097/00006454-200006000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Saiman L, Ludington E, Pfaller M, Rangel-Frausto S, Wiblin RT, Dawson J, Blumberg HM, Patterson JE, Rinaldi M, Edwards JE, Wenzel RP, Jarvis W. 2000. Risk factors for candidemia in neonatal intensive care unit patients. The National Epidemiology of Mycosis Survey Study Group. Pediatr Infect Dis J 19:319–324. [DOI] [PubMed] [Google Scholar]
- 4.Lee BE, Cheung PY, Robinson JL, Evanochko C, Robertson CM. 1998. Comparative study of mortality and morbidity in premature infants (birth weight, <1250 g) with candidemia or candidal meningitis. Clin Infect Dis 27:559–565. doi: 10.1086/514712. [DOI] [PubMed] [Google Scholar]
- 5.Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, Reboli AC, Schuster MG, Vazquez JA, Walsh TJ, Zaoutis TE, Sobel JD. 2016. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 62:e1–e50. doi: 10.1093/cid/civ933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ascher S, Smith PB, Benjamin DK Jr. 2011. Safety of micafungin in infants: insight to optimal dosing. Expert Opin Drug Saf 10:281–286. doi: 10.1517/14740338.2011.545345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yanni SB, Smith PB, Benjamin DK Jr, Augustijns PF, Thakker DR, Annaert PP. 2011. Higher clearance of micafungin in neonates compared to adults: role of age dependent micafungin serum binding. Biopharm Drug Dispos 32:222–232. doi: 10.1002/bdd.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manzoni P, Wu C, Tweddle L, Roilides E. 2014. Micafungin in premature and non-premature infants. A systematic review of 9 clinical trials. Pediatr Infect Dis J 33:e291–e298. doi: 10.1097/INF.0000000000000434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hope WW, Castagnola E, Groll AH, Roilides E, Akova M, Arendrup MC, Arikan-Akdagli S, Bassetti M, Bille J, Cornely OA, Cuenca-Estrella M, Donnelly JP, Garbino J, Herbrecht R, Jensen HE, Kullberg BJ, Lass-Flörl C, Lortholary O, Meersseman W, Petrikkos G, Richardson MD, Verweij PE, Viscoli C, Ullmann AJ, ESCMID Fungal Infection Study Group. 2012. ESCMID guideline for the diagnosis and management of Candida diseases 2012: prevention and management of invasive infections in neonates and children caused by Candida spp. Clin Microbiol Infect 18(Suppl 7):S38–S52. doi: 10.1111/1469-0691.12040. [DOI] [PubMed] [Google Scholar]
- 10.Hope WW, Mickiene D, Petraitis V, Petraitiene R, Kelaher AM, Hughes JE, Cotton MP, Bacher J, Keirns JJ, Buell D, Heresi G, Benjamin DK Jr, Groll AH, Drusano GL, Walsh TJ. 2008. The pharmacokinetics and pharmacodynamics of micafungin in experimental hematogenous Candida meningoencephalitis: implications for echinocandin therapy in neonates. J Infect Dis 197:163–171. doi: 10.1086/524063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horan TC, Andrus M, Dudeck MA. 2008. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 36:309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Neely MN, van Guilder MG, Yamada WM, Schumitzky A, Jelliffe RW. 2012. Accurate detection of outliers and subpopulations with Pmetrics, a nonparametric and parametric pharmacometric modeling and simulation package for R. Ther Drug Monit 34:467–476. doi: 10.1097/FTD.0b013e31825c4ba6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Comets E, Brendel K, Mentré F. 2008. Computing normalised prediction distribution errors to evaluate nonlinear mixed-effect models: the npde add-on package for R. Comput Methods Programs Biomed 90:154–166. doi: 10.1016/j.cmpb.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Neely M, Margol A, Fu X, van Guilder M, Bayard D, Schumitzky A, Orbach R, Liu S, Louie S, Hope W. 2015. Achieving target voriconazole concentrations more accurately in children and adolescents. Antimicrob Agents Chemother 59:3090–3097. doi: 10.1128/AAC.00032-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arrieta AC, Maddison P, Groll AH. 2011. Safety of micafungin in pediatric clinical trials. Pediatr Infect Dis J 30:e97–e102. doi: 10.1097/INF.0b013e3182127eaf. [DOI] [PubMed] [Google Scholar]
- 16.U.S. Food and Drug Administration. 2016. Mycamine (micafungin sodium) for injection. Product label U.S. Food and Drug Administration, Rockville, MD: http://www.accessdata.fda.gov/drugsatfda_docs/label/2007/021506s009lbl.pdf Accessed 15 April 2016. [Google Scholar]
- 17.European Medicines Agency. 2016. Mycamine (micafungin). Product label European Medicines Agency, London, United Kingdom: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000734/WC500031075.pdf Accessed 15 April 2016. [Google Scholar]
- 18.National Institutes of Health. Study to compare the efficacy and safety of micafungin versus conventional amphotericin B for the treatment of neonatal candidiasis (MAGIC-2). National Institutes of Health, Bethesda, MD: https://clinicaltrials.gov/ct2/show/NCT00815516 Accessed 15 April 2016. [Google Scholar]
- 19.Hope WW, Kaibara A, Roy M, Arrieta A, Azie N, Kovanda LL, Benjamin DK Jr. 2015. Population pharmacokinetics of micafungin and its metabolites M1 and M5 in children and adolescents. Antimicrob Agents Chemother 59:905–913. doi: 10.1128/AAC.03736-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith PB, Walsh TJ, Hope WW, Arrieta A, Takada A, Kovanda LL, Kearns GL, Kaufman D, Sawamoto T, Buell DN, Benjamin DK Jr. 2009. Pharmacokinetics of an elevated dosage of micafungin in premature neonates. Pediatr Infect Dis J 28:412–415. doi: 10.1097/INF.0b013e3181910e2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hope WW, Smith PB, Arrieta A, Buell DN, Roy M, Kaibara A, Walsh TJ, Cohen-Wolkowiez M, Benjamin DK Jr. 2010. Population pharmacokinetics of micafungin in neonates and young infants. Antimicrob Agents Chemother 54:2633–2637. doi: 10.1128/AAC.01679-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang C, Peeters MY, Allegaert K, Blussé van Oud-Alblas HJ, Krekels EH, Tibboel D, Danhof M, Knibbe CA. 2012. A bodyweight-dependent allometric exponent for scaling clearance across the human life-span. Pharm Res 29:1570–1581. doi: 10.1007/s11095-012-0668-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.