Abstract
Streptococcus mutans often survives as a biofilm on the tooth surface and contributes to the development of dental caries. We investigated the efficacy of ClyR, an engineered chimeolysin, against S. mutans biofilms under physiological and cariogenic conditions. Susceptibility tests showed that ClyR was active against all clinical S. mutans isolates tested as well as S. mutans biofilms that displayed resistance to penicillin. The S. mutans biofilms that formed on hydroxyapatite discs under physiological sugar conditions and cariogenic conditions were reduced ∼2 logs and 3 logs after treatment with 100 μg/ml ClyR, respectively. In comparison, only a 1-log reduction was observed in the chlorhexidine gluconate (ChX)-treated group, and no killing effect was observed in the NaF-treated group. A mouse dental colonization model showed that repeated use of ClyR for 3 weeks (5 μg/day) reduced the number of colonized S. mutans cells in the dental plaques significantly (P < 0.05) and had no harmful effects on the mice. Furthermore, toxicity was not noted at concentrations exceeding those used for the in vitro and in vivo studies, and ClyR-specific antibodies could not be detected in mouse saliva after repeated use of ClyR in the oral cavity. Our data collectively demonstrate that ClyR is active against S. mutans biofilms both in vitro and in vivo, thus representing a preventative or therapeutic agent for use against dental caries.
INTRODUCTION
Streptococcus mutans, as an initiator of dental caries, possesses unique virulence factors that play an important role in caries formation (1, 2). The ability of S. mutans to form biofilms, also known as dental plaque, on tooth surfaces allows the subsequent coaggregation of more fastidious organisms (3). The acidogenic and aciduric properties of S. mutans allow it to metabolize sucrose to lactic acid and to grow at low pH values (4). The acid formation leads to the dissolution of calcium and phosphate in tooth enamel, causing tooth decay, and further promotes adhesion of additional bacteria (5, 6). Thus, the biofilm-forming bacterium S. mutans has been reported to be the primary etiological agent of human dental caries (7).
Most current dental therapies include mechanical removal or broad-spectrum antimicrobial treatments that are focused on eradicating the dental plaque (8). Sodium fluoride (NaF) at 0.05% and chlorhexidine gluconate (ChX) at 0.12% are two different types of antimicrobials used clinically in toothpaste and mouthwashes to reduce plaques and prevent caries (9–11). However, the ability to rapidly form biofilms enhances the virulence of S. mutans and protects the bacteria from the activities of the antimicrobial agents (12). Vaccine strategies have been proposed to be a way to protect from S. mutans, but to date, none have been successful and streptococcal antigens may cause other health issues (13). Therefore, improved approaches are needed to prevent and remove the biofilms of S. mutans.
Bacteriophage-encoded lysins are peptidoglycan hydrolases that digest the bacterial cell wall, leading to the rapid lysis of susceptible Gram-positive bacteria when applied externally (14). In the last decade, natural and engineered lysins have been demonstrated extensively to be therapeutic agents with activity against various Gram-positive pathogens both in vitro and in vivo (15–17). Some investigations have also shown the activities of several lysins against staphylococcal biofilms (16, 18) and streptococcal biofilms (19–21), indicating the advantages of lysins over traditional antibiotics in removing biofilms. However, no studies to date have reported on lysins effective against S. mutans biofilms. ClyR is a chimeolysin engineered from two parental streptococcal lysins and is the first lysin reported to be active against planktonic S. mutans cells (22). In the present study, we report the efficacy of ClyR in preventing and removing biofilms formed by S. mutans under both physiological and cariogenic conditions.
MATERIALS AND METHODS
Bacterial strains.
Bacterial strains (see Table S1 in the supplemental material) were grown at 37°C. Planktonic cells of the S. mutans strains were grown in Todd-Hewitt broth supplemented with 2% yeast extract (THY) medium (Becton, Dickinson and Co., USA). For production of biofilms, THY medium was supplemented with 0.1 mM glucose to mimic physiological conditions or 1% glucose (56 mM) or 5% sucrose (146 mM) to mimic cariogenic conditions. S. mutans-containing clinical samples were isolated from the dental plaques of children, each of whom had a decayed-missing-filled tooth (DMFT) index of greater than 4 (23). The human experiments were approved by the Research Ethics Committee of the Hospital of Stomatology, Wuhan University (approval no. 2013-32). All clinical isolates were identified by 16S rRNA gene sequencing analysis combined with analysis with a biochemistry identification kit following the vendor instructions (Tianhe Microbial, Inc., Hangzhou, China). Escherichia coli BL21(DE3) were grown in Luria broth (LB) medium.
MIC determination.
The susceptibilities of planktonic cells of the S. mutans isolates to penicillin were determined by microtiter broth dilution as described by the Clinical and Laboratory Standards Institute (24). The MIC was defined as the lowest concentration of antibiotic inhibiting visible growth.
Quantification of ClyR lytic activity.
ClyR was expressed in E. coli BL21(DE3) and purified by Ni-nitrilotriacetic acid affinity chromatography, and lytic activity on S. mutans ATCC 25175 cells was determined as previously described (22) with minor modifications. Briefly, overnight cultures of various S. mutans strains (see Table S1 in the supplemental material) were centrifuged, and the pellets were resuspended in phosphate-buffered saline (PBS). The cells were then mixed 1:1 with an equal volume of ClyR (25 μg/ml) to a final optical density at 600 nm (OD600) of 0.8 to 1.2, and the OD600 was monitored by use of a microplate spectrophotometer (SpecraMax 190; Molecular Devices, USA) every 15 s for 20 min at 37°C. Bacteriolytic activity (i.e., susceptibility) was quantified as the reduction in turbidity, measured as the difference between the OD600 of ClyR-treated wells and the OD600 of PBS-treated wells at the final time point, and was recorded as the drop in the OD600 (i.e., –OD600). To test the dose-dependent lytic activity of ClyR, S. mutans ATCC 25175 was treated with ClyR (0 to 25 μg/ml) at 37°C, and the turbidity reduction over 20 min was measured as described above. Alternatively, S. mutans MT8148 was treated with ClyR (0 to 100 μg/ml) for 60 min at 37°C, growth was monitored by a Synergy H1 microplate reader (BioTek, USA), and the viable cell number was calculated by serial dilution plating at the 10-min time point to enumerate the remaining CFU.
Crystal violet assay.
An overnight culture of S. mutans ATCC 25175 (1 ml) was mixed with 500 μl of fresh THY medium supplemented with 0.1 mM glucose in 24-well CellBIND plates (Corning, USA). After an additional 24 h of incubation at 37°C in 5% CO2, the liquid was aspirated and samples were washed with PBS to remove unattached cells. The biofilms were treated for 1 h with penicillin (100 μg/ml), ClyR (25 to 400 μg/ml), ChX (0.12%), or NaF (0.05%). After incubation, the liquid was again aspirated and the biofilms were washed with PBS and allowed to dry overnight. On the following morning, the biofilms were stained with 0.1% crystal violet for 15 min at room temperature and washed three times with PBS. The crystal violet was extracted from the biofilm matrix with 1% SDS and quantified in a spectrophotometer at OD590.
Treatment of biofilms on hydroxyapatite discs.
Sterile hydroxyapatite discs (5 mm in diameter by 2 mm thick; Clarkson Chromatography Products, Inc., USA) were individually placed in 24-well plates containing 1 ml of S. mutans ATCC 25175 and mixed with 500 μl of fresh THY medium with 0.1 mM glucose. After 24 h of incubation at 37°C in 5% CO2 to allow bacterial adhesion, the liquid was aspirated and the discs were washed three times with PBS, separated into three groups, and placed in new 24-well plates for treatment. In group 1, 1 ml of ClyR (25 to 400 μg/ml), ChX (0.12%), or NaF (0.05%) was added to each well for 30 min. The discs were then washed with PBS three times before being transferred into 500 μl of PBS in sterile centrifuge tubes. The tubes were sonicated twice by sonication procedures consisting of three 10-s pulses with a 5-s interval between each pulse. An aliquot (100 μl) of the suspension was serially diluted to 104 and plated on THY medium plates. The plates were incubated at 37°C in 5% CO2 overnight, and the residual CFU was enumerated. For the other two treatment groups, the discs were exposed 3 times per day for 30 min each time to 1 ml of either 1% glucose (55.6 mM) (group 2) or 5% sucrose (146 mM) (group 3). One milliliter of ClyR (25 to 400 μg/ml), ChX (0.12%), or NaF (0.05%) was added after the first and the last exposure of the day for 30 min in each of the two groups. These procedures were repeated for the next 2 days, and after each sugar exposure and treatment, the liquid was aspirated and the discs were washed three times with PBS before being placed in fresh THY medium. At the end of the second day of cariogenic exposure and treatment, bacterial viability was enumerated as described above for the first treatment group.
Electron microscopy.
Planktonic S. mutans MT8148 cells were exposed to 50 μg/ml ClyR or PBS, cross-linked with 2.5% glutaraldehyde after 5 min of incubation, and viewed by thin-section transmission electron microscopy (TEM) using a Tecnai G2 20 Twin TEM (FEI, Hillsboro, OR). Alternatively, S. mutans MT8148 biofilms were grown on a round cover glass (tissue culture treated; Nest, USA) in 6-well plates (Nest, USA) for 24 h, washed twice with PBS, and then treated with 1 ml of 50 μg/ml ClyR or PBS for 5 min before they were washed twice with PBS and fixed with 2.5% glutaraldehyde. The sample was dehydrated using graded ethanol and subjected to scanning electron microscopy (SEM) on an SU8010 SEM (Hitachi, Japan).
Confocal laser scanning microscopy (CLSM).
S. mutans MT8148 biofilms were grown in THY medium on confocal dishes (Nest, USA) for 72 h, washed twice with PBS, and treated with 1 ml of ClyR (50 μg/ml) for 5 min. The biofilms were then labeled with the Live/Dead Bac-Light bacterial viability stain (Invitrogen, USA) for 15 min, washed twice with PBS, and imaged by a confocal fluorescence microscope (UltraVIEW VoX; PerkinElmer, USA). The Volocity (version 6.3.0) software supplied with the instrument was used for three-dimensional image reconstruction and subsequent calculations.
Mouse dental assay.
All animal experiments were conducted with the approval of the Animal Experiments Committee of the Wuhan Institute of Virology, Chinese Academy of Sciences (approval no. WIVA17201401). Female BALB/c mice (6 weeks old) were fed a diet supplemented with 0.1% ampicillin, 0.1% chloramphenicol, 0.1% carbenicillin, and water containing 4,000 U/ml penicillin for 3 days. Starting on the 4th day, mice were infected orally with S. mutans MT8148 cells twice a day for 6 days and provided a cariogenic Keyes 2000 diet (25) (containing 56% powdered sucrose to facilitate implantation of S. mutans and initiation of plaque formation) and sterile water. For each infection, a total bacterial suspension volume of 200 μl (2 × 109 CFU/ml) was divided into 10 parts and inoculated by pipette. Water was withheld for 30 min after each inoculation. When the 6-day inoculation was completed, mice were randomly divided into two groups. Mice were treated orally with 100 μl ClyR (50 μg/ml), divided into 10 equal parts for administration (group 1, n = 7), or 100 μl PBS (group 2, n = 7) once a day for 3 weeks. Beginning at the onset of treatment and continuing for 5 weeks, the viable cell number within the dental plaques of each mouse, collected by the use of oral swabs, was determined. The swabs were placed in 400 μl PBS, sonicated for 1 min, and plated on mitis salivarius-bacitracin agar plates containing 15% sucrose, 0.2 μg/ml bacitracin, 0.001% potassium tellurate for enumeration of the plaque CFU. After the 3-week treatment, the serum and saliva were monitored for the presence of ClyR-specific antibodies for another 2 weeks by enzyme-linked immunosorbent assay (ELISA), as described previously (22). The body weight of each mouse was also measured weekly.
Cytotoxicity testing.
The cytotoxicity of ClyR against Caco-2 and CHO cells was determined by a Cell Counting Kit-8 (CCK-8) assay (Dojindo Molecular Technologies, Kumamoto, Japan) according to the manufacturer's protocol. Caco-2 and CHO cells were grown in Dulbecco's modified Eagle medium (DMEM; Sigma-Aldrich, Shanghai, China) supplemented with 10 to 15% fetal bovine serum, 1% penicillin, and 1% streptomycin in a humidified atmosphere of 5% CO2 at 37°C. To perform the CCK-8 assay, Caco-2 and CHO cells were seeded in 96-well plates at a density of 5,000 cells per well and incubated under conditions of 5% CO2 at 37°C for 24 h. The cells were then exposed to a series of concentrations of ClyR (0, 125, 250, 500, 1,000, 2,000 μg/ml) for another 24 h. Afterwards, the contents of the plates were replaced with fresh medium containing 10% CCK-8 solution and incubated at 37°C for 1.5 h. The final optical density at OD450 was noted by use of a microplate reader (SynergyH1; BioTek, USA). The results were expressed as relative cell viability, expressed as a percentage of the growth of cells in control wells treated with PBS only.
Statistical analysis.
Experimental data are presented as means ± standard errors of means. Student's t tests were performed for the statistical analysis. P values of <0.05 and <0.01 were considered statistically significant and very significant, respectively.
RESULTS
Lytic activity of ClyR against planktonic S. mutans cells.
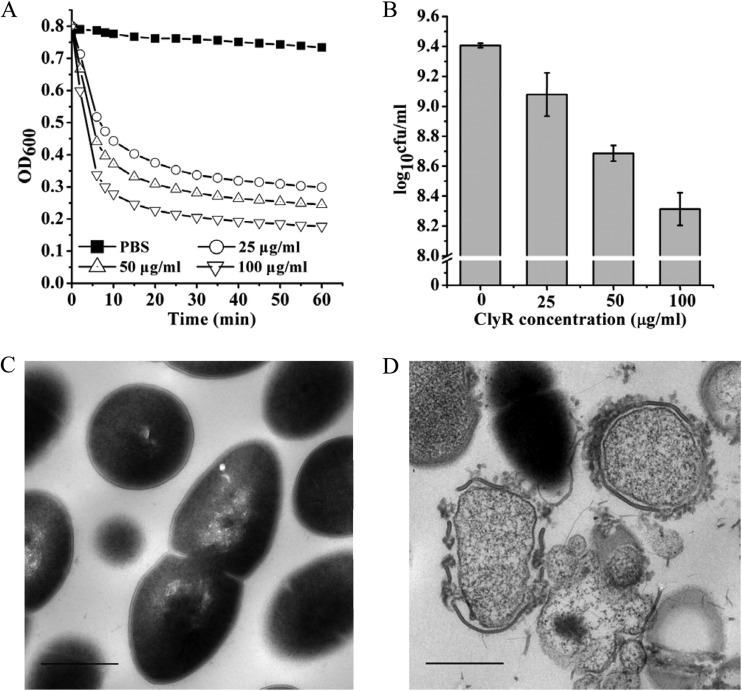
Our previous study demonstrated that ClyR was active against numerous streptococcal species, including S. mutans (22). In the present study, the time-killing curves for ClyR and the dose-dependent bactericidal activity of ClyR against an extended range of laboratory and clinical S. mutans isolates were further determined. As shown in Fig. 1A, a rapid decrease in cell turbidity was observed when S. mutans MT8148 cells were treated with 25 to 100 μg/ml ClyR. A corresponding 90% decrease in cell viability (∼1.1 logs) was achieved after treatment with 100 μg/ml ClyR for only 10 min (Fig. 1B). Likewise, a dose-dependent lytic activity of ClyR against S. mutans ATCC 25175 was also observed at low concentrations (0 to 25 μg/ml) (see Fig. S1 in the supplemental material). TEM analysis revealed that untreated S. mutans MT8148 cells had an intact cellular morphology and peptidoglycan integrity (Fig. 1C); however, after exposure to ClyR for 5 min, an obvious breach of the peptidoglycan was seen at multiple sites, resulting in disruption of the bacterial membrane and ejection of cellular contents (Fig. 1D). These observations are consistent with those of previously published reports of lysin-mediated osmotic lysis of Gram-positive bacteria (26).
FIG 1.
Lytic activity of ClyR against planktonic S. mutans cells. (A) Time-killing curves of ClyR. The turbidity of S. mutans MT8148 cells treated with different concentrations of ClyR at 37°C for 60 min was monitored by a microplate reader. (B) Dose-dependent lytic activity of ClyR. S. mutans MT8148 cells were treated with different concentrations of ClyR at 37°C for 10 min, and the residual cell numbers were calculated by plating. (C) TEM image of untreated S. mutans MT8148 cells. (D) TEM image of S. mutans MT8148 cells exposed to 50 μg/ml ClyR for 5 min. Bars = 0.5 μm.
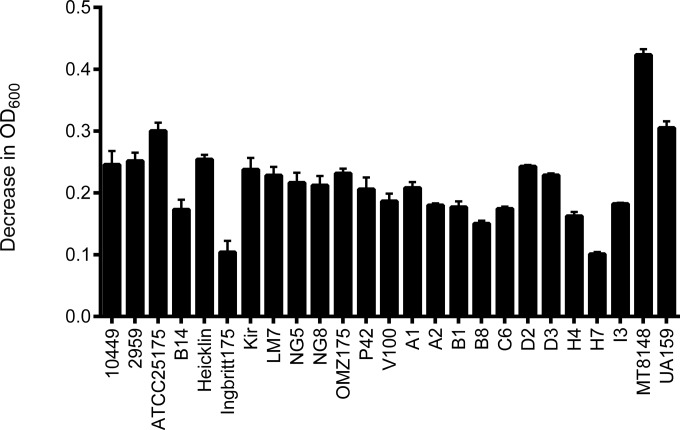
We also tested the susceptibility of a wide range of S. mutans strains to ClyR since our initial study included only a few strains (22). We tested 15 well-characterized strains available from commercial collections, including representatives of the c, e, f, and d/g serovars, as well as 10 clinical isolates from children with a DMFT index of greater than 4 (see Table S1 in the supplemental material). The turbidity reduction analysis showed that ClyR was active against all S. mutans strains tested (Fig. 2), demonstrating the robust activity of ClyR against S. mutans.
FIG 2.
Susceptibility of S. mutans isolates to ClyR. Reference as well as clinical S. mutans isolates representing several serotypes were washed once with PBS and resuspended to a final OD600 of 0.8 to 1.2. After the cells were treated with 25 μg/ml of ClyR at 37°C for 20 min, lytic activity was quantified by determination of the net change in the OD600, obtained by subtraction of the OD600 for treated cells from the OD600 for PBS-treated control cells. Experiments were done in triplicate, and the error bars show the standard deviations.
In vitro antibiofilm activity of ClyR.
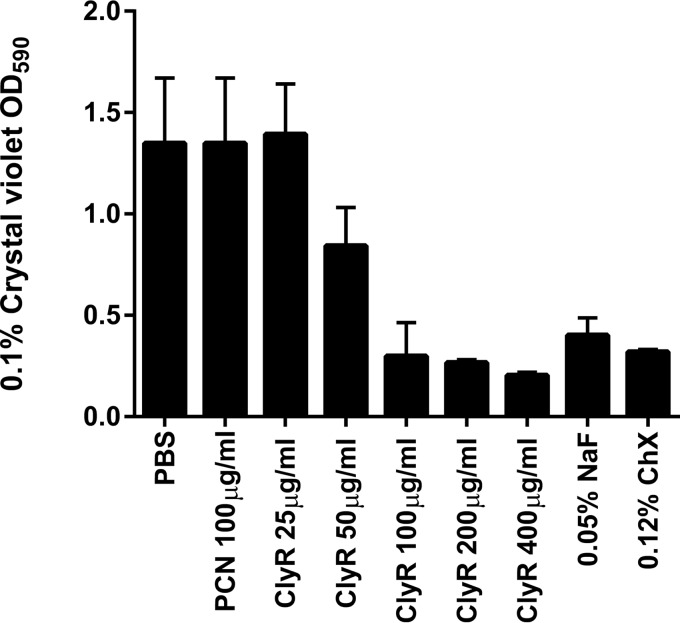
We tested the activity of ClyR against biofilms formed by S. mutans in comparison with the activities of antibiotics (i.e., penicillin) and chemotherapeutic agents (i.e., NaF [27] and ChX [28], which are commonly used in toothpaste or mouth rinses as a precaution against dental caries). Table S1 in the supplemental material shows that planktonic cells of most of the S. mutans strains tested were susceptible to penicillin (22/23), with MICs being below 0.06 μg/ml for all strains except S. mutans B8. We initially examined the antibiofilm activity of penicillin against S. mutans ATCC 25175. The crystal violet assay demonstrated that 100 μg/ml penicillin had no appreciable effect on the biofilm biomass, suggesting that S. mutans ATCC 25175 cells within biofilms were at least 3,300-fold more resistant to penicillin than their planktonic counterparts (Fig. 3). In contrast, these biofilms were susceptible to ClyR at concentrations of 25 μg/ml or greater, with a biomass reduction of over 80% being observed after treatment with 100 μg/ml ClyR, similar to that achieved with 0.05% NaF or 0.12% ChX.
FIG 3.
ClyR displays dose-dependent antimicrobial activity against biofilms of S. mutans ATCC 25175. The amount of biofilm in each well is represented by the quantity of crystal violet staining of biomass at the OD590 for each concentration of ClyR as well as penicillin (PCN), NaF, and ChX. Experiments were done in triplicate, and the error bars represent the standard deviations.
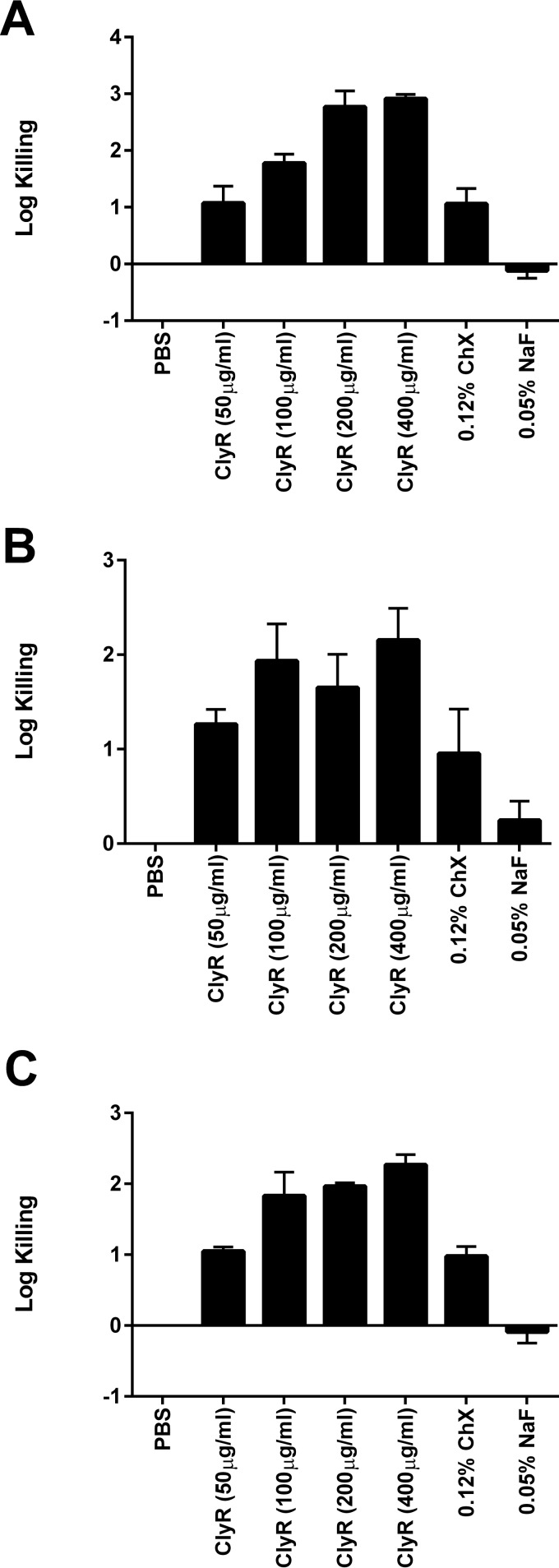
Next, we tested the activity of ClyR against S. mutans biofilms formed on hydroxyapatite discs, representing the tooth enamel, under normal physiological sugar conditions and cariogenic conditions in comparison with that of 0.05% NaF and 0.12% ChX. Under physiological conditions (i.e., 0.1 mM glucose), the activity of ClyR against S. mutans biofilms showed a dose-response relationship, with reported killing of ∼1 log at 50 μg/ml, ∼2 logs at 100 μg/ml, and ∼3 logs at 200 μg/ml (Fig. 4A). In comparison, ChX caused a reduction of only ∼1 log and NaF did not demonstrate any killing compared to that achieved with the PBS-treated controls. Under cariogenic condition with 1% glucose (Fig. 4B) or 5% sucrose (Fig. 4C), ClyR treatment again achieved ∼1-log killing at 50 μg/ml and ∼2-log killing at 100 μg/ml, although higher doses did not appreciably increase the log fold killing. Likewise, ChX produced ∼1-log killing under both conditions and NaF performed poorly (∼0.25-log killing under conditions with 1% glucose and no killing under conditions with 5% sucrose). These results collectively show that ClyR has robust antibiofilm activity against S. mutans under both physiological and cariogenic conditions.
FIG 4.
ClyR displays bactericidal activity against S. mutans biofilms grown on hydroxyapatite discs under normal and cariogenic conditions. (A) Normal oral sugar conditions with 0.1 mM glucose; (B) cariogenic oral sugar conditions with 1% glucose (55.6 mM); (C) cariogenic oral sugar conditions with 5% sucrose (146 mM). Experiments were done in triplicate, and the error bars represent the standard deviations.
Microscopy analysis of S. mutans biofilms treated with ClyR.
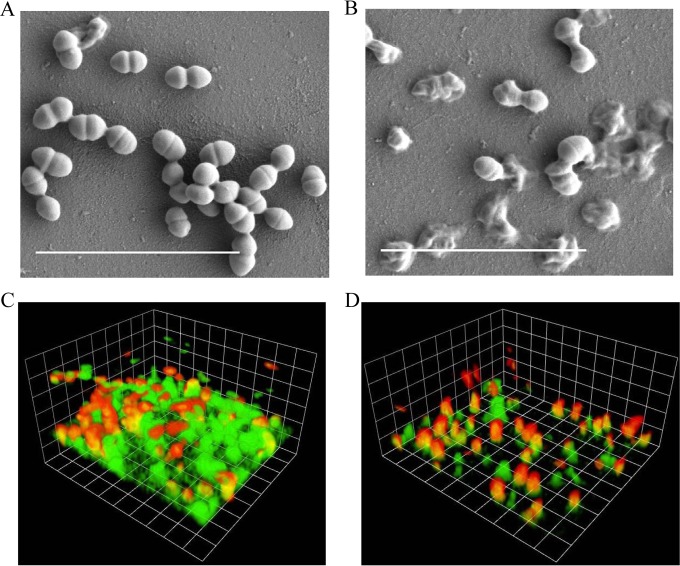
SEM and CLSM were used to study the changes in biofilm morphology and composition after treatment with ClyR. As shown in Fig. 5A, S. mutans MT8148 cells cultured for 24 h formed smooth biofilms on a cover glass. After treatment with 50 μg/ml ClyR for 5 min, the cells began to lyse, resulting in the formation of ghost cells (Fig. 5B), suggesting that ClyR was able to disrupt S. mutans biofilms by direct cell killing. This phenomenon is consistent with what is observed when Streptococcus pyogenes biofilms are treated with the PlyC lysin (19).
FIG 5.
Microscope analysis of S. mutans biofilms. (A and B) SEM images of intact S. mutans MT8148 biofilms and those treated with ClyR. The S. mutans biofilms (24 h old) were treated with PBS (A) or 50 μg/ml ClyR (B) for 5 min and washed with PBS, and the biofilms were imaged by SEM. Bars = 5 μm. (C and D) CLSM images of intact S. mutans MT8148 biofilms and those treated with ClyR under an imaging box size of 17.5 μm by 14.8 μm. The S. mutans biofilms (72 h old) were treated with PBS (C) or 50 μg/ml ClyR (D) for 5 min, stained with the Live/Dead dyes, and imaged by CLSM.
CLSM analysis of S. mutans MT8148 biofilms cultured for 72 h without ClyR treatment showed heavy green fluorescence (indicating viable cells), which was the result of staining by the Syto9 component of the Live/Dead stain. Dead cells, as indicated by staining by the red propidium iodide component of the Live/Dead stain, naturally appeared in the biofilm at a low frequency (Fig. 5C). Further analysis illustrated that the height of the intact S. mutans biofilm was 12.67 ± 1.97 μm, and the ratio of dead cells to live cells was 0.39 ± 0.015 (Fig. 5C). After treatment with 50 μg/ml ClyR for 5 min, the percentage of viable cells decreased significantly compared with that for the untreated controls (P < 0.05), with the ratio of dead cells to live cells increasing to 1.09 ± 0.088 (Fig. 5D). As a result of the robust activity of ClyR, the height of the S. mutans biofilm decreased to 7.88 ± 0.80 μm (Fig. 5D), which was a significant (P < 0.01) decrease compared with the height of the untreated controls (Fig. 5C).
Mouse dental colonization model.
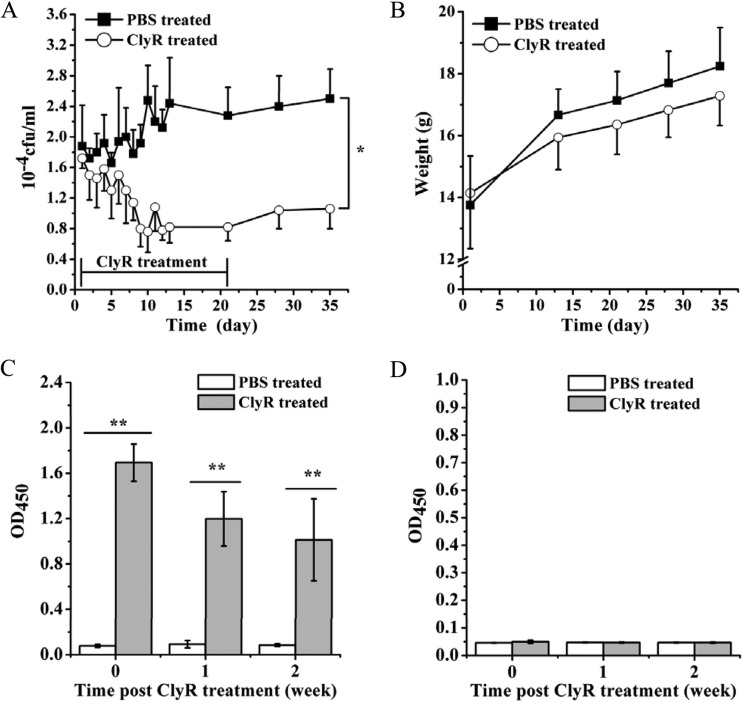
The in vivo activity of ClyR against S. mutans MT8148 was evaluated in a mouse dental colonization model. As shown in Fig. 6A, the average number of S. mutans cells in ClyR-treated mice was significantly decreased compared to the average number in PBS-treated control mice over the entire course of the 5-week experiment (P < 0.05), and a decrease in viable cell numbers of ∼1.5 logs was still observed 2 weeks after the cessation of ClyR treatment. No significant difference (P = 0.48) in body weight was observed between the ClyR- and PBS-treated groups (Fig. 6B). As a protein therapeutic, ClyR evoked a detectable immune response in mouse serum after repeated oral administration (Fig. 6C). However, no ClyR-specific antibody was detected in mouse saliva after repeated oral administration (Fig. 6D), suggesting that potential neutralizing antibodies are avoided when ClyR is used for oral applications.
FIG 6.
Mouse dental colonization model. (A) Mice were provided with a cariogenic diet, infected orally with S. mutans MT8148 for 6 days (8 × 108 CFU/mouse/day), and treated with ClyR (5 μg/mouse/day) once a day for 3 weeks. Starting on the first day that ClyR was administered, the viable cell count within dental plaques collected from each mouse by use of oral swabs was calculated, and the plaques were plated for 5 weeks. PBS-treated groups were used as controls. (B) Body weight of mice during the experiment. (C and D) Test of immunity induced by ClyR-specific antibodies. After ClyR treatment, mouse serum (C) or saliva (D) was collected weekly, and the titers of the ClyR-specific antibodies in each sample were detected by ELISA. * and **, P < 0.05 and P < 0.01, respectively.
Cytotoxicity assay.
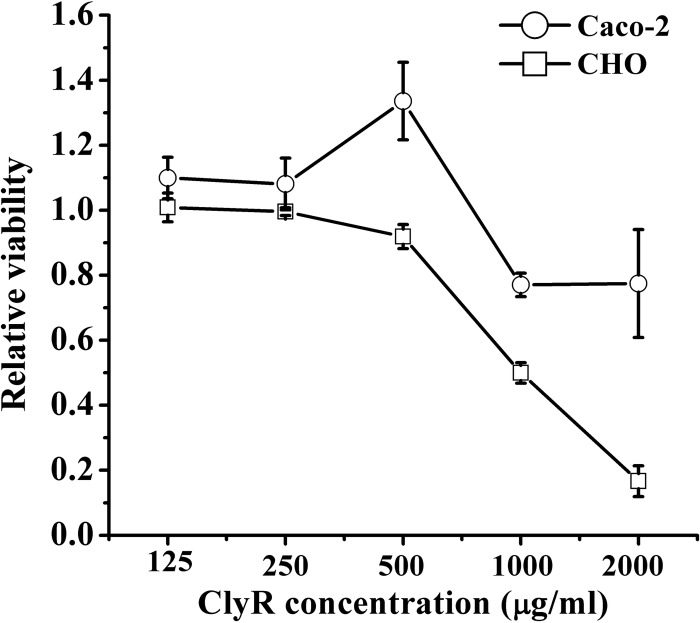
The CCK-8 assay showed that CHO cells were more susceptible to ClyR than Caco-2 cells, demonstrating a dose-dependent profile. No obvious cytotoxicity was observed in either cell line at ClyR concentrations of less than 500 μg/ml (Fig. 7). A relative cell viability of 50% was observed for CHO cells after exposure to 1,000 μg/ml ClyR for 24 h, whereas Caco-2 cells displayed 77% viability when exposed to 2,000 μg/ml ClyR, the highest concentration tested, for the same amount of time.
FIG 7.
Relative viability of Caco-2 and CHO cells exposed to different concentrations of ClyR. Cells were cultured in DMEM supplemented with 10 to 15% fetal bovine serum, 1% penicillin, and 1% streptomycin for 24 h. After the cells were exposed to a series of concentrations of ClyR (0, 125, 250, 500, 1,000, 2,000 μg/ml) for another 24 h, the relative viability of the cells after each treatment was determined by the CCK-8 assay.
DISCUSSION
Considerable interest in the use of bacteriophage lysins for species-specific antimicrobial chemotherapy has occurred in the past 15 years as the incidence of antibiotic resistance continues to rise (16). Lysins have been isolated from every major Gram-positive pathogen and have been characterized, and corresponding in vivo efficacy models have been described for many of these enzymes. For example, PlyGBS has been tested in a vaginal model of Streptococcus agalactiae infection (29); PlyCD has been tested in a gastrointestinal model of Clostridium difficile infection (30); CF-301 has been tested in a model of Staphylococcus aureus septicemia; and Cpl-1 has been tested in models of blood pneumococcal infection (31), endocarditis caused by pneumococci (32), meningitis caused by pneumococci (33), and lung pneumococcal infection (34). Despite demonstrated success in all of these animal models, potential issues surrounding delivery, pharmacokinetics, and immunological consequences remain to be worked out for the vaginal, gastrointestinal, blood, cerebrospinal fluid, and lung application of lysins. Therefore, a monospecies infection whereby a lysin can be applied topically/orally is desired for the initial translational development of this technology. Toward this end, S. mutans has long been viewed as an ideal target for lysin therapy because of its causal relationship to dental caries. However, despite the isolation of multiple S. mutans phages (35), their reported lytic activity on S. mutans biofilms (36), and sequencing of their genomes (37), no lysin active against S. mutans had been reported until our group created ClyR, a chimeric lysin, in 2015 (22).
The present study expanded the testing of ClyR from a few S. mutans strains to 25 strains, including clinical isolates and isolates of serovars c, e, f, and d/g, all of which have uniform sensitivity to ClyR (Fig. 2). A viability reduction of >90% could be achieved when 2.5 × 109 CFU/ml S. mutans was treated with 100 μg/ml ClyR for only 10 min (Fig. 1B). The rapid decrease in the number of viable cells was due to the disruption and deformation of the S. mutans cell wall on exposure to ClyR (Fig. 1D). These observations indicate that ClyR lyses S. mutans through degradation of its peptidoglycan, similar to its actions against Streptococcus dysgalactiae reported previously (22) and consistent with the actions of other lysins, including PlyC (38), PlySs2 (39), PlyGBS (29), and PAL (40). However, unlike these enzymes, which have a host range defined by one or a few closely related species, ClyR is active against virtually all streptococcal species (S. pyogenes, S. agalactiae, S. dysgalactiae, Streptococcus equi, S. mutans, Streptococcus pneumoniae, Streptococcus salivarius, Streptococcus gordonii, Streptococcus parasanguis) as well as some staphylococcal (i.e., S. aureus) and enterococcal (i.e., Enterococcus faecalis) species (22). Thus, while further research is required, it is speculated that ClyR may recognize a common moiety shared by the peptidoglycan of many Gram-positive pathogens rather than a species-specific teichoic acid, as demonstrated for some lysins.
A key feature of S. mutans is its propensity to colonize the tooth enamel as a biofilm. S. mutans cells grown in a biofilm are several thousand times more resistant to antibiotics than their planktonic counterparts. For example, there is no observed effect of 100 μg/ml penicillin against S. mutans ATCC 25175 biofilms compared to the effect against the PBS controls (Fig. 3A), whereas planktonic cells of the same strain have a penicillin MIC of <0.03 μg/ml (see Table S1 in the supplemental material), which may be a part of the reason for the current difficulties in the prevention and cure of dental caries. We therefore attempted to ascertain the activity of ClyR against S. mutans biofilms under physiological and cariogenic conditions. The antibiofilm activity of ClyR was found to be comparable to or better than that of ChX or NaF, as revealed by crystal violet staining (Fig. 3). Significantly, ChX and NaF are commonly used as the active ingredients in toothpastes and mouth rinses at the concentrations tested in the present study to prevent dental caries. When similar biofilm tests were extended to hydroxyapatite discs to replicate growth on the tooth enamel, ClyR outperformed ChX by ∼2 logs under normal sugar conditions and by ∼1 log under cariogenic conditions (Fig. 4). Surprisingly, NaF had little to no effect on S. mutans biofilms when they were grown on hydroxyapatite discs under all conditions.
The results collectively demonstrate that ClyR has robust lytic activity against both planktonic and biofilm S. mutans cells and under both physiological and cariogenic conditions on dental surfaces in vitro and in vivo. Furthermore, despite the formation of anti-ClyR antibodies in the serum, no neutralizing antibodies could be detected in the saliva after repeated exposure. Finally, toxicity was not observed at concentrations exceeding those used for the in vitro and in vivo experiments. When these findings are taken together, ClyR represents a potential topical prevention and/or therapeutic agent for dental caries that avoids the delivery, pharmacokinetic, and immunological issues associated with the development of other lysins for their infection niches.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to the Core Facility and Technical Support of Wuhan Institute of Virology for assistance in the microscopy studies.
H. Wei and H. Yang declare that a patent application of ClyR has been transferred to Wuhan Phagelux Biotechnology Inc., China.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.01872-16.
REFERENCES
- 1.Hamada S, Slade HD. 1980. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev 44:331–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loesche WJ. 1986. Role of Streptococcus mutans in human dental decay. Microbiol Rev 50:353–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bos R, van der Mei HC, Busscher HJ. 1999. Physico-chemistry of initial microbial adhesive interactions—its mechanisms and methods for study. FEMS Microbiol Rev 23:179–230. doi: 10.1111/j.1574-6976.1999.tb00396.x. [DOI] [PubMed] [Google Scholar]
- 4.Wen ZT, Burne RA. 2002. Functional genomics approach to identifying genes required for biofilm development by Streptococcus mutans. Appl Environ Microbiol 68:1196–1203. doi: 10.1128/AEM.68.3.1196-1203.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Narisawa N, Kawarai T, Suzuki N, Sato Y, Ochiai K, Ohnishi M, Watanabe H, Senpuku H. 2011. Competence-dependent endogenous DNA rearrangement and uptake of extracellular DNA give a natural variant of Streptococcus mutans without biofilm formation. J Bacteriol 193:5147–5154. doi: 10.1128/JB.05240-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paes Leme AF, Koo H, Bellato CM, Bedi G, Cury JA. 2006. The role of sucrose in cariogenic dental biofilm formation—new insight. J Dent Res 85:878–887. doi: 10.1177/154405910608501002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burne RA. 1998. Oral streptococci products of their environment. J Dent Res 77:445–452. doi: 10.1177/00220345980770030301. [DOI] [PubMed] [Google Scholar]
- 8.Li LN, Guo LH, Lux R, Eckert R, Yarbrough D, He J, Anderson M, Shi WY. 2010. Targeted antimicrobial therapy against Streptococcus mutans establishes protective non-cariogenic oral biofilms and reduces subsequent infection. Int J Oral Sci 2:66–73. doi: 10.4248/IJOS10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bradshaw DJ, Marsh PD, Hodgson RJ, Visser JM. 2002. Effects of glucose and fluoride on competition and metabolism within in vitro dental bacterial communities and biofilms. Caries Res 36:81–86. doi: 10.1159/000057864. [DOI] [PubMed] [Google Scholar]
- 10.Hope CK, Wilson M. 2004. Analysis of the effects of chlorhexidine on oral biofilm vitality and structure based on viability profiling and an indicator of membrane integrity. Antimicrob Agents Chemother 48:1461–1468. doi: 10.1128/AAC.48.5.1461-1468.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen Y, Stojicic S, Haapasalo M. 2011. Antimicrobial efficacy of chlorhexidine against bacteria in biofilms at different stages of development. J Endod 37:657–661. doi: 10.1016/j.joen.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Limsong J, Benjavongkulchai E, Kuvatanasuchati J. 2004. Inhibitory effect of some herbal extracts on adherence of Streptococcus mutans. J Ethnopharmacol 92:281–289. doi: 10.1016/j.jep.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Simon-Soro A, Mira A. 2015. Solving the etiology of dental caries. Trends Microbiol 23:76–82. doi: 10.1016/j.tim.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 14.Fischetti VA. 2010. Bacteriophage endolysins: a novel anti-infective to control Gram-positive pathogens. Int J Med Microbiol 300:357–362. doi: 10.1016/j.ijmm.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischetti VA. 5 January 2016. Lysin therapy for Staphylococcus aureus and other bacterial pathogens. Curr Top Microbiol Immunol doi: 10.1007/82_2015_5005. [DOI] [PubMed] [Google Scholar]
- 16.Yang H, Yu J, Wei H. 2014. Engineered bacteriophage lysins as novel anti-infectives. Front Microbiol 5:542. doi: 10.3389/fmicb.2014.00542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodriguez-Rubio L, Gutierrez D, Donovan DM, Martinez B, Rodriguez A, Garcia P. 2015. Phage lytic proteins: biotechnological applications beyond clinical antimicrobials. Crit Rev Biotechnol 36:542–552. doi: 10.3109/07388551.2014.993587. [DOI] [PubMed] [Google Scholar]
- 18.Son JS, Lee SJ, Jun SY, Yoon SJ, Kang SH, Paik HR, Kang JO, Choi YJ. 2010. Antibacterial and biofilm removal activity of a Podoviridae Staphylococcus aureus bacteriophage SAP-2 and a derived recombinant cell-wall-degrading enzyme. Appl Microbiol Biotechnol 86:1439–1449. doi: 10.1007/s00253-009-2386-9. [DOI] [PubMed] [Google Scholar]
- 19.Shen Y, Koller T, Kreikemeyer B, Nelson DC. 2013. Rapid degradation of Streptococcus pyogenes biofilms by PlyC, a bacteriophage-encoded endolysin. J Antimicrob Chemother 68:1818–1824. doi: 10.1093/jac/dkt104. [DOI] [PubMed] [Google Scholar]
- 20.Meng X, Shi Y, Ji W, Meng X, Zhang J, Wang H, Lu C, Sun J, Yan Y. 2011. Application of a bacteriophage lysin to disrupt biofilms formed by the animal pathogen Streptococcus suis. Appl Environ Microbiol 77:8272–8279. doi: 10.1128/AEM.05151-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Domenech M, Garcia E, Moscoso M. 2011. In vitro destruction of Streptococcus pneumoniae biofilms with bacterial and phage peptidoglycan hydrolases. Antimicrob Agents Chemother 55:4144–4148. doi: 10.1128/AAC.00492-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang H, Linden SB, Wang J, Yu J, Nelson DC, Wei H. 2015. A chimeolysin with extended-spectrum streptococcal host range found by an induced lysis-based rapid screening method. Sci Rep 5:17257. doi: 10.1038/srep17257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naidu BV, Reginald BA. 2016. Quantification and correlation of oral Candida with caries index among different age groups of school children: a case-control study. Ann Med Health Sci Res 6:80–84. doi: 10.4103/2141-9248.181843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clinical and Laboratory Standards Institute. 2014. Performance standards for antimicrobial susceptibility testing, 23rd informational supplement. CLSI M100-S23. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 25.Keyes PH, Jordan HV. 1964. Periodontal lesions in the Syrian hamster. III. Findings related to an infectious and transmissible component. Arch Oral Biol 9:377–400. [DOI] [PubMed] [Google Scholar]
- 26.Daniel A, Euler C, Collin M, Chahales P, Gorelick KJ, Fischetti VA. 2010. Synergism between a novel chimeric lysin and oxacillin protects against infection by methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 54:1603–1612. doi: 10.1128/AAC.01625-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Comar LP, Cardoso CDAB, Charone S, Grizzo LT, Buzalaf MA, Magalhaes AC. 2015. TiF4 and NaF varnishes as anti-erosive agents on enamel and dentin erosion progression in vitro. J Appl Oral Sci 23:14–18. doi: 10.1590/1678-775720140124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderson MH. 2003. A review of the efficacy of chlorhexidine on dental caries and the caries infection. J Calif Dent Assoc 31:211–214. [PubMed] [Google Scholar]
- 29.Cheng Q, Nelson D, Zhu S, Fischetti VA. 2005. Removal of group B streptococci colonizing the vagina and oropharynx of mice with a bacteriophage lytic enzyme. Antimicrob Agents Chemother 49:111–117. doi: 10.1128/AAC.49.1.111-117.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Q, Euler CW, Delaune A, Fischetti VA. 2015. Using a novel lysin to help control Clostridium difficile infections. Antimicrob Agents Chemother 59:7447–7457. doi: 10.1128/AAC.01357-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loeffler JM, Fischetti VA. 2003. Synergistic lethal effect of a combination of phage lytic enzymes with different activities on penicillin-sensitive and -resistant Streptococcus pneumoniae strains. Antimicrob Agents Chemother 47:375–377. doi: 10.1128/AAC.47.1.375-377.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Entenza JM, Loeffler JM, Grandgirard D, Fischetti VA, Moreillon P. 2005. Therapeutic effects of bacteriophage Cpl-1 lysin against Streptococcus pneumoniae endocarditis in rats. Antimicrob Agents Chemother 49:4789–4792. doi: 10.1128/AAC.49.11.4789-4792.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grandgirard D, Loeffler JM, Fischetti VA, Leib SL. 2008. Phage lytic enzyme Cpl-1 for antibacterial therapy in experimental pneumococcal meningitis. J Infect Dis 197:1519–1522. doi: 10.1086/587942. [DOI] [PubMed] [Google Scholar]
- 34.Witzenrath M, Schmeck B, Doehn JM, Tschernig T, Zahlten J, Loeffler JM, Zemlin M, Muller H, Gutbier B, Schutte H, Hippenstiel S, Fischetti VA, Suttorp N, Rosseau S. 2009. Systemic use of the endolysin Cpl-1 rescues mice with fatal pneumococcal pneumonia. Crit Care Med 37:642–649. doi: 10.1097/CCM.0b013e31819586a6. [DOI] [PubMed] [Google Scholar]
- 35.Delisle AL, Rostkowski CA. 1993. Lytic bacteriophages of Streptococcus mutans. Curr Microbiol 27:163–167. doi: 10.1007/BF01576015. [DOI] [PubMed] [Google Scholar]
- 36.Dalmasso M, de Haas E, Neve H, Strain R, Cousin FJ, Stockdale SR, Ross RP, Hill C. 2015. Isolation of a novel phage with activity against Streptococcus mutans biofilms. PLoS One 10:e0138651. doi: 10.1371/journal.pone.0138651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Delisle AL, Guo M, Chalmers NI, Barcak GJ, Rousseau GM, Moineau S. 2012. Biology and genome sequence of Streptococcus mutans phage M102AD. Appl Environ Microbiol 78:2264–2271. doi: 10.1128/AEM.07726-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nelson D, Loomis L, Fischetti VA. 2001. Prevention and elimination of upper respiratory colonization of mice by group A streptococci by using a bacteriophage lytic enzyme. Proc Natl Acad Sci U S A 98:4107–4112. doi: 10.1073/pnas.061038398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gilmer DB, Schmitz JE, Euler CW, Fischetti VA. 2013. Novel bacteriophage lysin with broad lytic activity protects against mixed infection by Streptococcus pyogenes and methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 57:2743–2750. doi: 10.1128/AAC.02526-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loeffler JM, Nelson D, Fischetti VA. 2001. Rapid killing of Streptococcus pneumoniae with a bacteriophage cell wall hydrolase. Science 294:2170–2172. doi: 10.1126/science.1066869. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.