Abstract
Recent findings have identified Escherichia coli strains that are pan-β-lactam susceptible (PBL-S) but piperacillin-tazobactam resistant (TZP-R) in vitro. We assessed the in vivo significance of this resistance profile in a neutropenic murine pneumonia model using humanized exposures of TZP with 18 clinical E. coli isolates, 8 TZP-S/PBL-S and 10 genotypically confirmed TZP-R/PBL-S. Despite phenotypically and genotypically defined resistance, TZP displayed efficacy against these isolates. Additional studies are required to define the clinical implications of these TZP-R/PBL-S strains.
TEXT
Piperacillin-tazobactam (TZP) is one of the most widely used empirical antimicrobials due to its broad spectrum of activity against Gram-negative bacteria, including Escherichia coli. Consequently, the susceptibility of this agent continues to erode, as a recent surveillance study demonstrated that 9% of E. coli strains are nonsusceptible to TZP; albeit, not all strains demonstrated identical phenotypic resistance patterns to other antibiotics (1). More specifically, we identified a subset of E. coli strains that are susceptible to pan-β-lactam (PBL-S) (i.e., all cephalosporins, monobactams, and carbapenems) but resistant to TZP (TZP-R) (1). Additional molecular studies on these isolates revealed that TZP-R is associated with deleted or dysfunctional porins, as exhibited by significantly lower expression of both ompC and ompF (2). Since the insidious nature of this resistance profile may result in poor clinical outcomes, considering the predominant use of this agent in the hospital setting, we assessed the in vivo significance of this TZP-R profile in an immunocompromised murine model using humanized exposures of TZP (3).
Eighteen clinical isolates of E. coli, 8 TZP susceptible (TZP-S)/PBL-S and 10 genotypically confirmed TZP-R/PBL-S, were tested in an immunocompromised lung infection model. Preinfection TZP MICs were determined in triplicate by broth microdilution according to the 2016 Clinical and Laboratory Standards Institute guidelines, and the modal MIC was reported (4). Specific-pathogen-free female ICR (CD-1) mice weighing 20 to 22 g were obtained from Envigo RMS, Inc. (Indianapolis, IN). The protocol was reviewed and approved by the Institutional Animal Care and Use Committee at Hartford Hospital, Hartford, CT. Bacterial colonies of a fresh subculture of each isolate were suspended in sterile normal saline to produce a suspension of ∼107 CFU/ml. Final inoculum concentrations were confirmed by plating serial dilutions on Trypticase soy agar with 5% sheep blood (BD Biosciences, Sparks, MD) and incubating overnight at ∼37°C in ambient air. Each mouse was intranasally inoculated with 50 μl of the above-described bacterial suspension. The first TZP dose was administered subcutaneously at 2 h postinoculation.
Commercially available TZP (Premier Pro Rx, lot AI9Z/11) was solubilized in normal saline immediately prior to dosing. A pharmacokinetic study was conducted to confirm a TZP dosing regimen that would provide in vivo murine drug exposure similar to 4.5 g every 6 h (q6h) in humans, quantified by the free time above MIC from 0 to 24 h (fT > MIC) (5, 6). A protein binding value of 20% was used for humans and mice (5, 7, 8). Prior to dose administration, one group of mice (n = 6) for each bacterial isolate was euthanized, and the lungs were excised and harvested to assess the initial CFU burden. Lungs from one group each of control (vehicle-dosed) and TZP-treated mice infected with TZP-R/PBL-S or TZP-S/PBL-S E. coli were harvested and processed for quantitative culture after 24 h of treatment. Dilutions of the lung homogenates were plated on Trypticase soy agar with 5% sheep blood and incubated overnight at ∼37°C. Efficacy was calculated as the change in bacterial density (Δlog10 numbers of CFU) obtained in the TZP-treated mice after 24 h relative to the 0-h untreated controls for the E. coli isolates. MICs of the E. coli organisms isolated from the lungs postinfection were assessed to reconfirm the phenotypic profile of the isolates.
TZP MICs for TZP-S/PBL-S isolates (n = 8) ranged from 2 to 16 μg/ml. TZP MICs for TZP-R/PBL-S isolates (n = 10) ranged from 256 to ≥2,048 μg/ml. MICs of the E. coli isolates against TZP and other commercially available agents are displayed in Table 1. The confirmatory pharmacokinetic study, similar to a previously published humanized TZP 4.5-g q6h regimen, displayed fT > MIC values comparable to those of humans (Table 2) (5, 6).
TABLE 1.
In vitro potency of piperacillin-tazobactam and commercially available antibiotics against each E. coli isolate
| E. coli isolate | MIC (μg/ml) fora: |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TZP | Antibiotic |
||||||||||
| FEP | CRO | CAZ | CIP | CST | ATM | ETP | IPM | MEM | TOB | ||
| C2-9 | 512 | 0.25 | 0.125 | 1 | ≥32 | 0.5 | 0.25 | ≤0.015 | 0.125 | ≤0.06 | 8 |
| C3-23 | ≥2,048 | 1 | ≤0.06 | 2 | 1 | 1 | 0.5 | 0.03 | 0.125 | ≤0.06 | 1 |
| C6-25 | 2,048 | 0.5 | 0.125 | 1 | ≥32 | 0.5 | 0.25 | 0.03 | 0.25 | ≤0.06 | 2 |
| C7-1 | 256 | 0.5 | ≤0.06 | 2 | ≤0.015 | 1 | 1 | 0.125 | 0.25 | ≤0.06 | 2 |
| C10-11 | ≥2,048 | 0.25 | ≤0.06 | 0.5 | ≤0.015 | 1 | 0.125 | ≤0.015 | 0.25 | ≤0.06 | 1 |
| C11-14 | ≥2,048 | 0.5 | 0.125 | 1 | ≤0.015 | 1 | 0.25 | 0.06 | 0.5 | ≤0.06 | 1 |
| C12-1 | 512 | 0.25 | 0.06 | 0.5 | ≤0.015 | 0.5 | 0.125 | ≤0.015 | 0.125 | ≤0.06 | 1 |
| C14-26 | ≥2,048 | 8 | 0.5 | 4 | 0.5 | 0.5 | 1 | 2 | 0.5 | 0.25 | 2 |
| C18-6 | ≥2,048 | 0.125 | ≤0.06 | 0.25 | 0.03 | 2 | ≤0.06 | ≤0.015 | 0.125 | ≤0.06 | 2 |
| C30-5 | 256 | 0.125 | ≤0.06 | 0.25 | ≤0.015 | 0.5 | 0.125 | ≤0.015 | 0.5 | ≤0.06 | 8 |
| C1-6 | 16 | ≤0.06 | 2 | 2 | 0.015 | 0.5 | 2 | 0.03 | 1 | 0.06 | 4 |
| C1-7 | 4 | 0.125 | ≤0.06 | 0.5 | 8 | 0.5 | ≤0.06 | ≤0.015 | 0.25 | ≤0.06 | 4 |
| C1-23 | 2 | ≤0.06 | ≤0.06 | 0.25 | ≤0.015 | 1 | ≤0.06 | ≤0.015 | 0.125 | ≤0.06 | 4 |
| C2-5 | 4 | 0.125 | 0.25 | 0.5 | 16 | 0.5 | 0.5 | ≤0.015 | 0.125 | ≤0.06 | 1 |
| C2-14 | 2 | ≤0.06 | 0.125 | 0.25 | 0.03 | 8 | 0.25 | ≤0.015 | 0.125 | ≤0.06 | 2 |
| C2-19 | 4 | ≤0.06 | ≤0.06 | 2 | ≤0.015 | 0.5 | 2 | ≤0.015 | 0.125 | ≤0.06 | 0.5 |
| C2-27 | 2 | ≤0.06 | ≤0.06 | 0.125 | ≤0.015 | 0.5 | ≤0.06 | ≤0.015 | 0.25 | ≤0.06 | 2 |
| C3-2 | 4 | 0.125 | ≤0.06 | 0.5 | 0.06 | 0.5 | 0.125 | ≤0.015 | 0.125 | ≤0.06 | 2 |
TZP, piperacillin-tazobactam; FEP, cefepime; CRO, ceftriaxone; CAZ, ceftazidime; CIP, ciprofloxacin; CST, colistin; ATM, aztreonam; ETP, ertapenem; IPM, imipenem; MEM, meropenem; TOB, tobramycin.
TABLE 2.
Comparison of %fT > MIC values achieved with piperacillin-tazobactam at each MIC in humans and mice receiving the humanized regimen
| Drug | Species | %fT > MIC for MIC (μg/ml) of: |
||||||
|---|---|---|---|---|---|---|---|---|
| 4 | 8 | 16 | 32 | 64 | 128 | 256 | ||
| TZPa | Mouseb | 85.00 | 75.00 | 61.67 | 45.00 | 28.33 | 13.33 | 6.67 |
| Humanc | 83.33 | 70.00 | 56.67 | 43.33 | 28.33 | 13.33 | 0.00 | |
Piperacillin-tazobactam (8:1).
Dosing regimen: 500 mg/kg (0 h), 100 mg/kg (0.25 h), 200 mg/kg (2.5 h), 75 mg/kg (5 h) q6h.
Dosing regimen: 4.5 g q6h, 30-min infusion.
At 0 h, TZP-R/PBL-S and TZP-S/PBL-S initial bacterial densities were 6.97 ± 0.16 and 6.99 ± 0.29 (mean ± standard deviation) log CFU, respectively, in the lungs of untreated controls. At 24 h, the TZP-R/PBL-S and TZP-S/PBL-S isolates reached 9.25 ± 0.20 and 9.05 ± 0.68 log CFU growth, respectively. The humanized TZP regimen achieved a ≥2-log kill against 5 TZP-S/PBL-S isolates and a ≥1-log kill against the remaining 3 isolates (Fig. 1). Despite the TZP-R phenotype, humanized dosing of TZP resulted in a ≥2-log kill against 2 TZP-R/PBL-S isolates (MIC ≥2,048 μg/ml), a ≥1-log kill against 6 isolates, and stasis against the remaining 2 isolates (Fig. 1). Repeat MICs of the recovered TZP-R isolates posttreatment revealed similar preexposure values.
FIG 1.
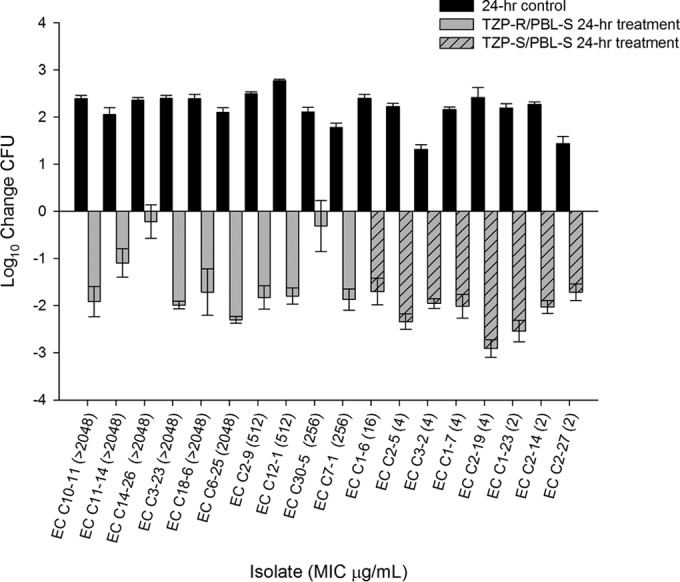
Reduction in bacterial density of TZP-R/PBL-S and TZP-S/PBL-S E. coli (EC) isolates over 24 h after administration of TZP.
Previously conducted in vivo murine studies demonstrated that 40% to 50% fT > MIC is required to demonstrate TZP efficacy (9). However, here, we demonstrate that humanized exposures of TZP result in substantive in vivo killing against highly in vitro-resistant organisms where the fT > MIC corresponds to 0%. Thus, this study reveals overt discordance between the in vitro resistance profile and in vivo efficacy of human TZP exposures against this novel TZP-R/PBL-S phenotypic profile. These observations combined with the recovery of TZP-R isolates with MICs similar to those of pretreatment values suggest reduced in vivo expression of this phenotype.
This paradox has been reported among β-lactams against Gram-negative organisms (10–13). It has been proposed that the rapid hydrolysis of antimicrobials within the in vitro setting may be due to the unnatural accumulation of enzymes, resulting in resistant phenotypes (13, 14). Since genotypic profiling of these isolates revealed that TZP resistance was associated with deleted or dysfunctional porins secondary to frameshifts, insertions, and deletions, albeit in the background of TEM-1 and testing negative for TEM or SHV extended-spectrum β-lactamases, KPC, NDM, IMP, VIM, OXA-48, and CTX-M, it appears that this previously noted enzymatic explanation does not fully elucidate the mechanism(s) for the discordance observed in the current study (2). Interestingly, other possibilities for this in vitro-in vivo discordance may result from alterations in resistance expression, as observed in pandemic ST131-H30 E. coli or NDM-1-producing E. coli, where the acquisition of resistant mechanisms potentially reduces the fitness/virulence of these organisms (15, 16). Given these collective findings regarding the TZP-R strain, i.e., the potential for multiple mechanisms to explain our observed in vitro-in vivo discordance, the prevalence of E. coli as an infecting pathogen, and extensive use of TZP in the clinical setting, additional investigations are warranted to evaluate the clinical implications of this TZP-R/PBL-S phenotype.
ACKNOWLEDGMENTS
We thank Jennifer Tabor-Rennie, Sara Robinson, Debora Santini, Elizabeth Cyr, Christina Sutherland, Kimelyn Greenwood, Islam Ghazi, Abrar Thabit, Kamilia Abdelraouf, and Mordechai Grupper from the Center for Anti-Infective Research and Development, Hartford, CT, for assistance with the conduct of the study.
This study was internally funded by the Center for Anti-Infective Research and Development, Hartford Hospital, Hartford, CT.
Funding Statement
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
REFERENCES
- 1.Sutherland CA, Nicolau DP. 2015. Susceptibility profile of ceftolozane/tazobactam and other parenteral antimicrobials against Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa from US hospitals. Clin Ther 37:1564–1571. doi: 10.1016/j.clinthera.2015.05.501. [DOI] [PubMed] [Google Scholar]
- 2.Mediavilla JR, Schneider Z, Nwaigwe C, Chavda K, Chen L, Satlin M, Jenkins SG, Nicolau DP, Kreiswirth BN. 2015. Molecular characterization of cephalosporin/carbapenem/monobactam susceptible but piperacillin-tazobactam (TZP) resistant E. coli, abstr 1181 Abstr ID Week 2015, San Diego, CA. [Google Scholar]
- 3.Díaz-Martín A, Martínez-González ML, Ferrer R, Ortiz-Leyba C, Piacentini E, Lopez-Pueyo MJ, Martín-Loeches I, Levy MM, Artigas A, Garnacho-Montero J. 2012. Antibiotic prescription patterns in the empiric therapy of severe sepsis: combination of antimicrobials with different mechanisms of action reduces mortality. Crit Care 16:R223. doi: 10.1186/cc11869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clinical and Laboratory Standards Institute. 2016. Performance standards for antimicrobial susceptibility testing; 24th informational supplement. CLSI document M100-S25. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 5.Bulik CC, Tessier PR, Keel RA, Sutherland CA, Nicolau DP. 2012. In vivo comparison of CXA-101 (FR264205) with and without tazobactam versus piperacillin-tazobactam using human simulated exposures against phenotypically diverse gram-negative organisms. Antimicrob Agents Chemother 56:544–549. doi: 10.1128/AAC.01752-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mattoes HM, Capitano B, Kim MK, Xuan D, Quintiliani R, Nightingale CH, Nicolau DP. 2002. Comparative pharmacokinetic and pharmacodynamic profile of piperacillin/tazobactam 3.375g q4hr and 4.5g q6hr. Chemotherapy 48:59–63. doi: 10.1159/000057663. [DOI] [PubMed] [Google Scholar]
- 7.Beam TR. 1983. Recent developments in antimicrobial therapy with beta-lactam antibiotics. J Med 14:307–336. [PubMed] [Google Scholar]
- 8.Komuro M, Kakuo H, Matsushita H, Shimada J. 1994. Inhibition of the renal excretion of tazobactam by piperacillin. J Antimicrob Chemother 34:555–564. doi: 10.1093/jac/34.4.555. [DOI] [PubMed] [Google Scholar]
- 9.Craig WA. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis 26:1–12. doi: 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- 10.Bulik CC, Christensen H, Li P, Sutherland CA, Nicolau DP, Kuti JL. 2010. Comparison of the activity of a human simulated, high-dose, prolonged infusion of meropenem against Klebsiella pneumoniae producing the KPC carbapenemase versus that against Pseudomonas aeruginosa in an in vitro pharmacodynamics model. Antimicrob Agents Chemother 54:804–810. doi: 10.1128/AAC.01190-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bulik CC, Nicolau DP. 2010. In vivo efficacy of simulated human dosing regimens of prolonged-infusion doripenem against carbapenemase-producing Klebsiella pneumoniae. Antimicrob Agents Chemother 54:4112–4115. doi: 10.1128/AAC.00026-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bulik CC, Nicolau DP. 2011. Double-carbapenem therapy for carbapenemase-producing Klebsiella pneumoniae. Antimicrob Agents Chemother 55:3002–3004. doi: 10.1128/AAC.01420-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crandon JL, Schuck VJ, Banevicius MA, Beaudoin ME, Nichols WW, Tanudra MA, Nicolau DP. 2012. Comparative in vitro and in vivo efficacies of human simulated doses of ceftazidime and ceftazidime-avibactam against Pseudomonas aeruginosa. Antimicrob Agents Chemother 56:6137–6146. doi: 10.1128/AAC.00851-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coleman K, Levasseur P, Girard AM, Borgonovi M, Miossec C, Merdjan H, Drusano G, Shlaes D, Nichols WW. 2014. Activities of ceftazidime and avibactam against β-lactamase-producing Enterobacteriaceae in a hollow-fiber pharmacodynamic model. Antimicrob Agents Chemother 58:3366–3372. doi: 10.1128/AAC.00080-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson JR, Thuras P, Johnston BD, Weissman SJ, Limaye AP, Riddell K, Scholes D, Tchesnokova V, Sokurenko E. 2016. The pandemic H30 subclone of Escherichia coli sequence type 131 is associated with persistent infections and adverse outcomes independent from its multidrug resistance and associations with compromised hosts. Clin Infect Dis 15:1529–1536. doi: 10.1093/cid/ciw193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Göttig S, Riedel-Christ S, Saleh A, Kempf VA, Hamprecht A. 2016. Impact of blaNDM-1 on fitness and pathogenicity of Escherichia coli and Klebsiella pneumoniae. Int J Antimicrob Agents 47:430–435. doi: 10.1016/j.ijantimicag.2016.02.019. [DOI] [PubMed] [Google Scholar]


