Abstract
Autophagy is a catabolic process during which cellular components including protein aggregates and organelles are degraded via a lysosome-dependent process to sustain metabolic homeostasis during nutrient or energy deprivation. Measuring the rate of proteolysis of long-lived proteins is a classical assay for measurement of autophagic flux. However, traditional methods, such as a radioisotope labeling assay, are technically tedious and have low sensitivity. Here, we report a novel method for quantification of long-lived protein degradation based on L-azidohomoalanine (AHA) labeling in mouse embryonic fibroblasts (MEFs) and in human cancer cells. AHA is a surrogate for L-methionine, containing a bio-orthogonalazide moiety. When added to cultured cells, AHA is incorporated into proteins during active protein synthesis. After a click reaction between an azide and an alkyne, the azide-containing proteins can be detected with an alkyne-tagged fluorescent dye, coupled with flow cytometry. Induction of autophagy by starvation or mechanistic target of rapamycin (MTOR) inhibitors was able to induce a significant reduction of the fluorescence intensity, consistent with other autophagic markers. Coincidently, inhibition of autophagy by pharmacological agents or by Atg gene deletion abolished the reduction of the fluorescence intensity. Compared with the classical radioisotope pulse-labeling method, we think that our method is sensitive, quantitative, nonradioactive, and easy to perform, and can be applied to both human and animal cell culture systems.
Keywords: AHA, autophagy, click-chemistry, flow cytometry, proteolysis
Autophagy is an evolutionarily conserved self-digestive process in response to starvation or other stress conditions to sustain cellular homeostasis.1,2 Autophagy plays crucial roles in development, innate immune defense, tumor suppression, and cell survival.2,3 During the induction of autophagy, portions of cytoplasmic materials, including macromolecules (such as stable, long-lived proteins) are engulfed into specialized double-membrane structures termed phagophores, which mature into autophagosomes; these double-membraned vesicles subsequently fuse with lysosomes to degrade their cargos and regenerate nutrients.4,5 Under normal growth conditions, autophagy is kept at a basal level to maintain turnover of long-lived proteins and damaged organelles. Under starvation conditions, autophagy is induced to enhance the catabolic process and to provide cells with additional internal nutrient supplies. This induction is largely due to inhibition of MTOR, the key negative regulator of autophagy, which otherwise suppresses the ULK1 complex consisting of ULK1, RB1CC1/FIP200, and ATG13.6-8
Among various assays developed to measure the autophagic flux, the classical method is determination of long-lived protein degradation by a radioisotope-labeling assay. In this method, cellular proteins are pulse labeled by the incorporation of radioactive amino acids [14C]-leucine or [14C]-valine, and then followed by a long cold (nonradioactive)-chase to allow for the degradation of short-lived proteins.9-11 Next, the time-dependent release of acid-soluble radioactivity from the degraded proteins in intact cells is measured. Although the radioisotope-based autophagic proteolysis of long-lived proteins assay is considered to be a gold standard in measuring autophagic proteolysis, this method is technically tedious, insensitive, and inconvenient (with a requirement for radioactive containment). Though Doherty et al.12 updated this method by using dynamic stable isotope labeling with amino acids in cell culture (SILAC), coupled with quantitative mass spectrometry, the lengthy and laborious process would limit its widespread application. Thus, a radioactive-free method that can be used to quickly and specifically quantify long-lived protein degradation is in high demand.
Recently, new techniques for labeling a variety of molecules based on the principle of bio-orthogonal metabolic labeling have been developed, such as bio-orthogonal noncanonical amino acid tagging (BONCAT) and fluorescent noncanonical amino acid tagging (FUNCAT) which have been used to tag and identify or visualize newly synthesized proteins.13,14 Such methods are particularly useful when direct labeling or the use of antibodies is not applicable, or efficient. L-azidohomoalanine, an amino acid analog of methionine containing an azide moiety, can be incorporated into de novo protein synthesis and can be readily detected by a chemoselective ligation between an azide and alkyne-bearing tags.14 AHA has been successfully used for the detection of nascent protein synthesis, in combination with proteomic techniques, including gels, blots, and mass spectrometry.14,15
Here we report a novel method, which combines pulse-AHA labeling, click-chemistry tagging and flow cytometry detection to quantitatively measure long-lived protein degradation during autophagy. We tested this method in both normal and cancer cells, under various autophagy induction and inhibition conditions. This assay provides a fast, sensitive, and nonradioactive method for quantification of autophagic proteolysis, applicable to in vitro cell culture systems. This method could also be adapted for high-throughput screening of novel pharmacological agents that enhance or inhibit autophagy, and also of genes that modulate autophagy.
Results
Optimization of AHA labeling
Growth medium, cell density, cell type variations, and other factors may influence the AHA labeling capacity. For initial experiments, we optimized the labeling and reaction conditions in terms of AHA concentration and incubation time. Mouse embryonic fibroblasts were plated at desired density (70~80%) overnight in full culture medium with 10% fetal bovine serum (FBS). After the full medium was removed and washed with phosphate-buffered saline (PBS) twice, cells were then cultured in l-methionine-free DMEM with different dosages of AHA. Cells need to be labeled in methionine-free medium, as methionine is the preferred substrate for methionyl-tRNA transferase.16
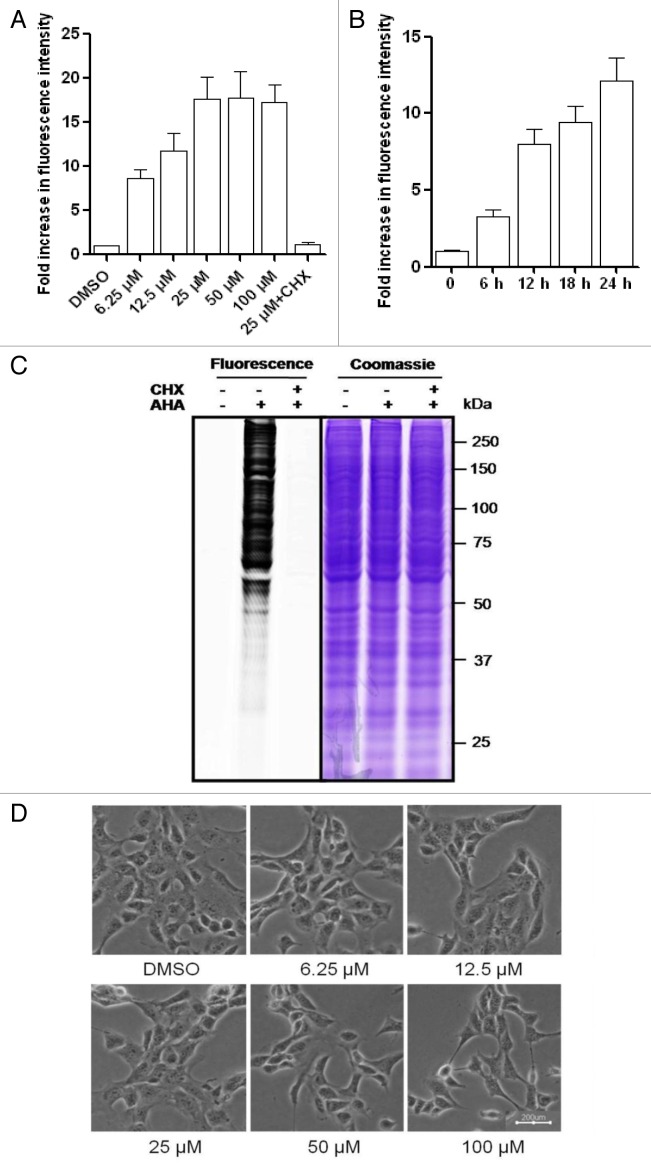
First, we tested a range of AHA concentrations to determine the optimal concentration for our cell type and experimental conditions. As shown in Figure 1A, we observed a dose-dependent increase of the AHA signal intensity up to 100 μM, while the signal reached the plateau by a concentration of 25 μM. Next, we tested the time-course of AHA labeling by incubating MEFs with 25 μM AHA for up to 24 h, resulting in a time-dependent increase of the AHA signal intensity (Fig. 1B). We further confirmed the AHA labeling by examining the fluorescence intensity using SDS-PAGE. As shown in Figure 1C, only the cells labeled with AHA were detected with fluorescence. The Coomassie staining was used to show the equal loading of the protein samples.
Figure 1. Dose- and time-dependent metabolic labeling of AHA in MEFs. (A) MEFs were treated in l-methionine-free medium with different dosages of AHA for 18 h. Cell were then cultured in regular DMEM with 10× l-methionine (2 mM) for 2 h. Finally, the cells were harvested, fixed, and permeabilized for the click reaction, as described in Materials and Methods. (B) MEFs were labeled with 25 μM AHA at different time points (6, 12, 18, and 24 h) and cellular fluorescence intensity was analyzed as described. (C) Visualization of AHA-labeled proteins. MEFs were labeled with AHA (25 μM × 18 h) and then the cells were harvested for click tagging and in-gel fluorescence scanning. Coomassie staining was used to show the equal protein loading. Cycloheximide (CHX, 10 μg/ml) was added to inhibit de novo protein synthesis and the cells cultured in regular DMEM were used as the negative control. (D) Morphological changes of MEFs with different dosages of AHA labeling were examined and photographed with an inverted microscope (scale bar: 200 μm).
To determine the specificity of AHA incorporation into newly synthesized proteins, we added the protein synthesis inhibitor cycloheximide (CHX) into the labeling medium along with AHA. As shown in Figure 1C, the presence of CHX completely abolished the fluorescence signal in cells with AHA labeling, confirming that this procedure labels newly synthesized proteins with high specificity.
It has been reported that the metabolic labeling of mammalian cell with AHA did not alter global protein synthesis rates or protein degradation.14 As shown in Figure 1D, labeling with different concentrations of AHA for 18 h did not change the cell morphology and did not cause any obvious reduction of cell viability measured by a propidium iodide (PI)-live cell exclusion test (Fig. S1A and S1B), indicating that AHA is not toxic to MEFs.
Autophagy-mediated protein degradation was detected by reduced AHA fluorescence intensity
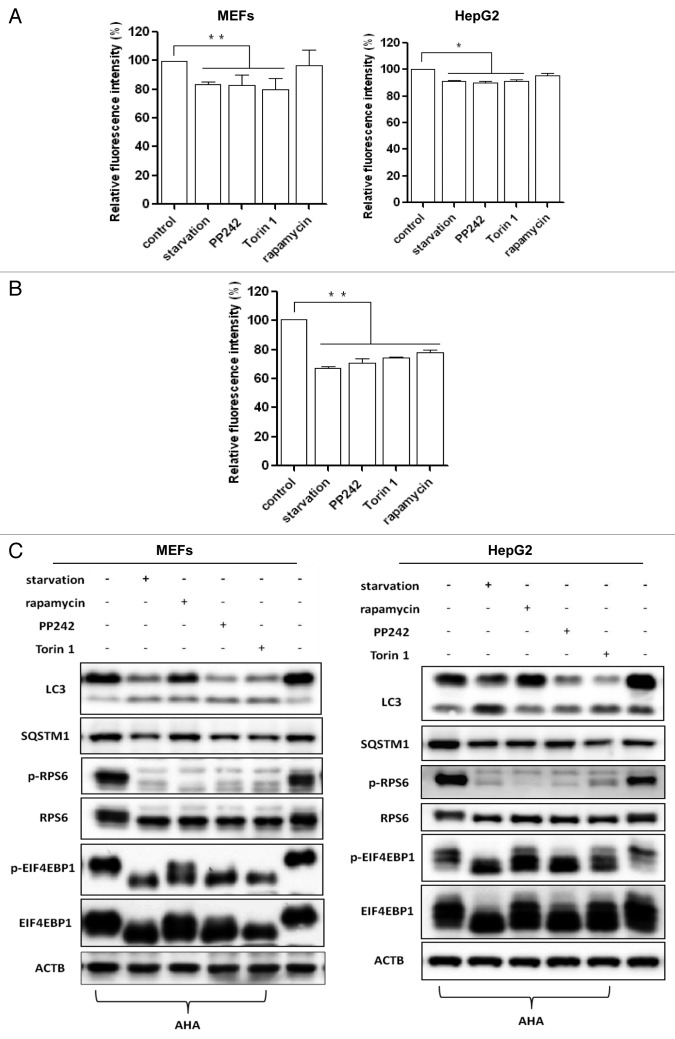
We next tested the changes of AHA fluorescence intensity in MEFs undergoing autophagy induced by a well-established autophagy inducer, starvation (culturing cells in amino acid-free medium).17 As shown in the left panel of Figure 2A, starvation for 3 h caused a 20% reduction in the AHA signal intensity, and the magnitude of reduction was agreeable with a previous report that detected the degradation of mitochondria.18 Starvation for a longer period of time (6 h) induced a further reduction in cell fluorescence intensity, with about 70% of the original signal intensity remaining (Fig. 2B).
Figure 2. Autophagy induction increased long-lived protein degradation. (A) Cells were first labeled with AHA as described, and then the cells were cultured in starvation medium or treated with MTOR inhibitors (rapamycin, 1 μM; PP242, 1 μM; or Torin 1, 1 μM) for 3 h. Left panel for MEFs; right panel for HepG2 cells. Data for the relative signal intensity were expressed as the ratio of treated cells to control cells, as mean ± SD from 3 independent experiments, *P < 0.05, **P < 0.01, the Student t test. (B) MEFs were subjected to starvation or treated with MTOR inhibitors for 6 h and cellular fluorescence intensity was measured. (C) MEFs and HepG2 cells were treated as in (A) and cell lysates were prepared for western blot. ACTB/β-actin was used as the loading control.
Since our method is designed to detect long-lived protein degradation, we also tested the effect of different nonradioactive chase durations to minimize the interference from short-lived proteins. As shown in Figure S2A, a longer chase time (2–12 h) markedly reduced the basal fluorescence level in the control group without starvation. Interestingly, as shown in Figure S2B, a better signal-noise ratio was achieved when the chase duration increased from 0.5 h to 2 h, whereas no further improvement was found with longer chase time (more than 2 h). For the classical protein degradation assay using radioisotope labeling, a 2 h chase time has been commonly used.11 Therefore, we used 2 h chase time for all the subsequent experiments.
In addition to starvation, we also measured autophagic protein degradation induced by different MTOR inhibitors, including rapamycin (an allosteric MTOR inhibitor), and PP242 and Torin 1 (2 catalytic MTOR inhibitors).19 As shown in the left panel of Figure 2A, both PP242 and Torin 1 were as effective as starvation in causing protein degradation, and also in a similar time-dependent manner. Interestingly, rapamycin failed to induce a significant decrease of AHA fluorescence intensity with a shorter treatment duration (3 h) and the degree of autophagic proteolysis induced by rapamycin was weaker compared with other treatments, which is consistent with previous studies showing that rapamycin is a relatively weaker autophagy inducer.18,20 Moreover, we also examined our data using histograms. As shown in Figure S3, we observed a left shift of the histogram, indicating a reduced fluorescence intensity in cells treated with various autophagy inducers.
Similar results were also obtained in HepG2 cells (Fig. 2A, right panel), although the average AHA fluorescence intensity reduction was about 10% in cells under starvation conditions and in the presence of MTOR inhibitors (PP242 and Torin 1) for 3 h, which was relatively weaker when compared with MEFs, suggesting that cell type variations may influence the autophagic proteolysis rate measured by this assay.
To further evaluate the efficiency of autophagy induction, we also performed western blots to determine changes in MTOR activity and to monitor autophagy markers. As shown in Figure 2C, starvation and MTOR inhibitors, such as rapamycin, PP242 and Torin 1, increased the LC3-II level and decreased the SQSTM1 level, indicating higher autophagy activity. We also performed the same experiments in MEF cells with stable expression of green fluorescent protein GFP-LC3 and observed increased GFP-LC3 puncta representing autophagic vacuoles formed in the cytoplasm (Fig. S4A and S4B). Meanwhile, the effectiveness of starvation and MTOR inhibitors was verified with the phosphorylation blockage of ribosomal protein S6 (RPS6) (Ser235/236) seen in MEFs and HepG2 cells (Fig. 2C). Different from other treatments, rapamycin was largely ineffective on phospho-EIF4EBP1 (Thr37/46). Such observations are consistent with the current understanding that rapamycin is an allosteric inhibitor of MTOR and only suppresses part of MTOR function, while both PP242 and Torin 1 are catalytic inhibitors that are able to fully suppress MTOR.19
Autophagy inhibitors reversed the reduction of AHA fluorescence
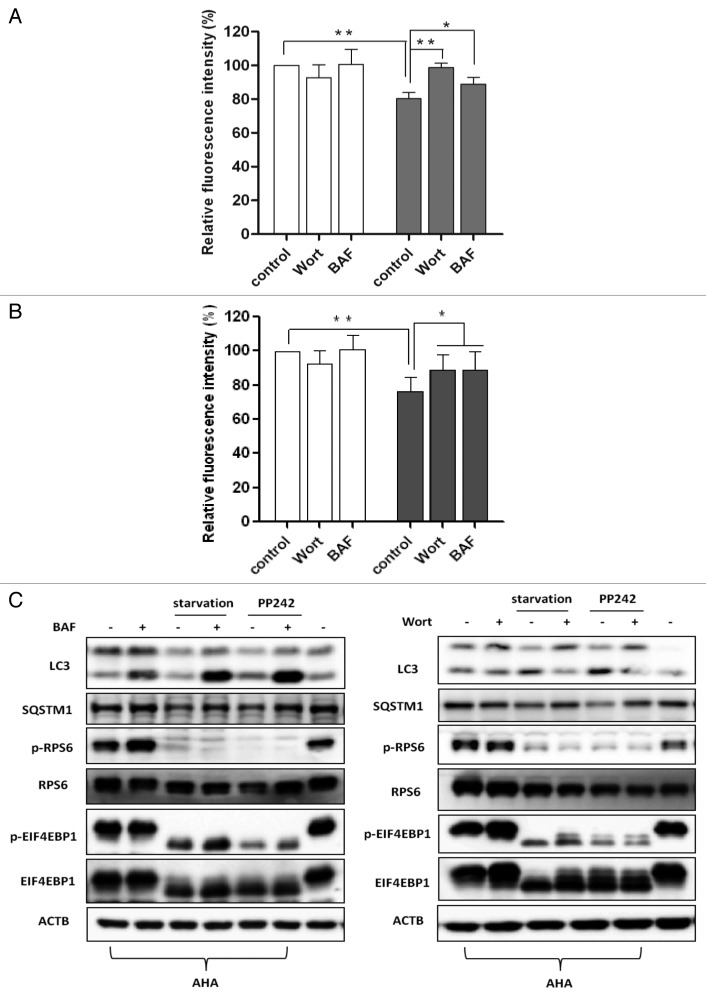
To verify this method of autophagic proteolysis, we utilized pharmacological inhibitors of autophagy to observe the changes of AHA fluorescence. Wortmannin, a phosphatidylinositol-3-kinase (PtdIns3-kinase) inhibitor, can inhibit an early step of macroautophagy.21,22 Bafilomycin A1, a selective inhibitor of the lysosomal V-ATPase, is able to block the fusion between autophagosomes and lysosomes.23 In MEFs (Fig. 3A) and HepG2 cells (Fig. S5A) under starvation for 3 h), both inhibitors were able to significantly reverse the reduction of AHA fluorescence intensity, although wortmannin appeared to be more effective than bafilomycin A1.
Figure 3. Autophagy inhibition blocked long-lived protein degradation. (A) MEFs were labeled with AHA as described in Figure 2A and then cultured in regular DMEM with 10× l-methionine (2 mM) for 2 h in the presence or absence of autophagy inhibitors (wortmannin [Wort], 100 nM; or bafilomycin A1 [BAF], 50 nM), followed by starvation for 3 h. (B) MEFs were treated as described in (A), followed by PP242 (×1 μM) for 3 h. Data for the relative signal intensity were expressed as the ratio of treated cells to control cells, as mean ± SD from 3 independent experiments, *P < 0 0.05, **P < 0.01, the Student t test. (C) MEFs were treated as described in (A and B). Cells were then harvested and protein from cell lysates was analyzed with western blot. ACTB/β-actin was used as a loading control.
We also tested the effects of these 2 autophagy inhibitors on autophagy induction by PP242. As shown in Figure 3B (for MEFs) and Figure S5B (for HepG2 cells), both inhibitors were also able to abolish the reduction of AHA fluorescence.
Meanwhile, we performed western blots to confirm the effectiveness of wortmannin and bafilomycin A1 to block autophagy induced by starvation and PP242. In MEFs (Fig. 3C) and HepG2 cells (Fig. S5C), starvation and PP242 increased the autophagic flux evidenced by increased LC3-II and reduced SQSTM1 protein levels. As expected, bafilomycin A1 blocked autophagic flux by suppressing lysosomal degradation, as evidenced by a further increase of the LC3-II and decrease of the SQSTM1 levels in cells subjected to starvation or treated with PP242 (Fig. 3C; Fig. S5C). Meanwhile, wortmannin prevented the inecrease in LC3-II levels and decrease of SQSTM1 levels induced by starvation or PP242 (Fig. 3C; Fig. S5C). In addition, the effectiveness of starvation and PP242 was verified with the phosphorylation blockage of RPS6 (Ser235/236) and EIF4EBP1 (Thr37/46). Thus, data from this part of our study further support the notion that the degradation of proteins by autophagy can be effectively measured by our proposed AHA-labeling system.
Autophagy deficiency prevented protein degradation measured by AHA labeling
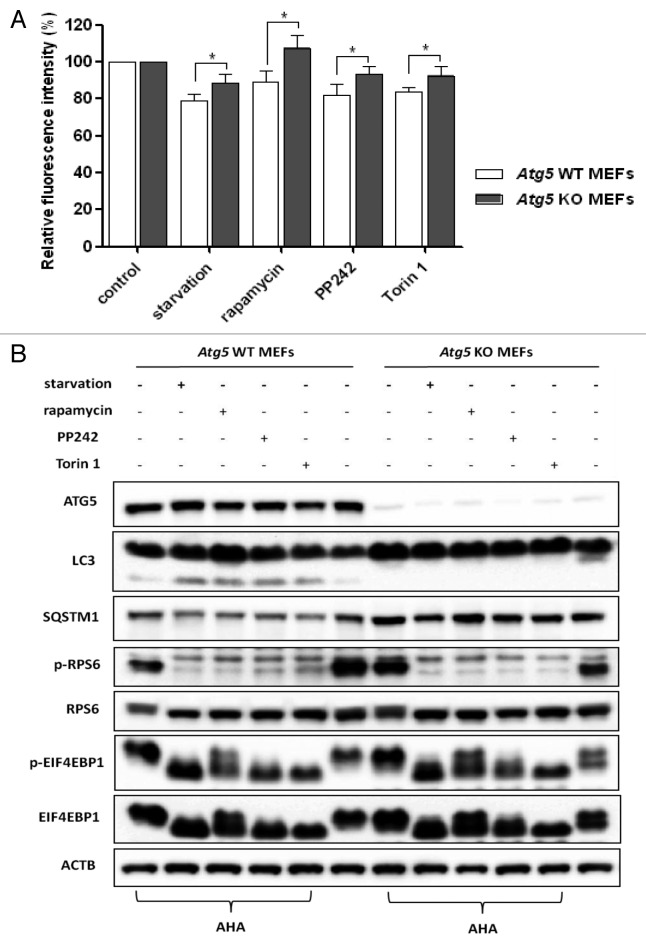
To further examine the usefulness of this assay in measuring autophagic proteolysis, we measured the changes of AHA fluorescence in cells deficient in key autophagy-related genes including Atg5 and Atg7. We observed only a minor degree of protein degradation in both Atg5 KO MEFs and Atg7 KO MEFs in the presence of various autophagy inducers, in comparison to their respective wild-type counterparts (Fig. 4A and Fig. S6A, respectively). Interestingly, we also observed some reduction of AHA fluorescence intensity in the Atg5 or Atg7 KO MEFs, especially in Atg7 KO MEFs under starvation conditions, suggesting the possibility of autophagy-independent protein degradation in the treated cells, such as proteasome-mediated protein degradation.24 Meanwhile, western blot analysis was performed to determine the identity of the cellular and autophagy-specific markers induced by starvation or MTOR inhibitors (Fig. 4B; Fig. S6B). Autophagy inducers increased LC3-II levels and decreased SQSTM1 levels in wild-type MEFs, but not in autophagy-deficient cells, although their suppressive effects on MTORC1 activity were similar.
Figure 4. Defective autophagy impaired long-lived protein degradation. (A) Atg5 WT and KO MEFs were labeled with AHA and then treated with starvation or MTOR inhibitors for 3 h, as described in Figure 2A. Data for the relative signal intensity were expressed as the ratio of treated cells to control cells, as mean ± SD from 3 independent experiments, *P < 0.05, the Student t test. (B) Atg5 WT and KO MEFs were treated as described in (A), harvested and proteins from cell lysates were analyzed by western blot. ACTB served as the loading control.
Discussion
At present, measurement of “autophagic flux” in the autophagy study is still technically challenging.25 Several assays have been described and commonly used, including (i) turnover of autophagy markers such as LC3-II and GFP-LC3 puncta in the presence of lysosomal inhibitors such as chloroquine; (ii) changes of SQSTM1/p62 protein levels, (iii) use of the GFP and RFP tandemly tagged LC3 (tfLC3), and (iv) quantification of autophagic proteolysis by radioisotope labeling. In the present study, we described a novel assay to quantify long-lived protein degradation in autophagy using AHA labeling coupled with flow cytometry. Compared with the classical radioisotope labeling method, this assay is nonradioactive, sensitive, specific, quantitative, relatively easy to perform, and versatile in the mammalian cell culture system, and thus offers a good tool for many laboratories in the field of autophagy research.
Recently, the BONCAT technique has been used to identify newly synthesized proteins in mammalian cells through the cotranslational introduction of azide groups into proteins and the chemoselective tagging of azide-labeled proteins with an alkyne affinity tag.14,15 Based upon this technique, the metabolic labeling of AHA into newly synthesized proteins has been widely used in cultured mammalian cell lines, primary neuronal cells and organotypic brain slice cultures, and larval zebrafish.14,26,27 Labeling with AHA is similar to the traditional metabolic labeling with radioactive amino acids (35S-labeled methionine or cysteine). Unlike other labels, the azide is small enough to be tagged with biomolecules (such as sugars and amino acids) that are acceptable as substrates for the enzymes to incorporate them into proteins. However, the application of AHA labeling in measuring protein degradation has not yet been reported at present. In this study, we established a novel technique for the study of autophagic proteolysis. In cells induced by starvation or MTOR inhibitors to activate autophagy, there was a significant reduction of the AHA signal intensity (Fig. 2A and B). Consistently, suppression of autophagy by pharmacological inhibitors effectively prevented the reduction of AHA signal intensity (Fig. 3; Fig. S5). More importantly, no obvious reduction of AHA signal was found in MEFs with deletion of Atg5 or Atg7 (Fig. 4A; Fig. S6A). Therefore, it is thus thought that the reduction of the AHA signal intensity specifically measures the protein degradation due to autophagy.
When compared with radioisotope labeling in the measurement of autophagic proteolysis, there are a few advantages of AHA labeling. First, AHA labeling is much simpler and easier to perform, nontoxic, and does not affect global rates of protein synthesis or degradation.14 In contrast, radioisotope labeling is more laborious and requires a high level of technical expertise to operate the instruments and conduct experiments. Moreover, radioisotope labeling diminishes half-lives of proteins and alters metabolic stability.28 For example, commercially available radioisotope-labeled amino acids from bacteria contain bacterial components, which may influence protein stabilities through triggering cellular innate immune receptors.29
Second, in AHA-labeled cells, the cellular fluorescence intensity reflects the amount of remaining proteins, including both cytosolic and membrane proteins, and thus exclude factors related to free AHA release, indicating that this is a more accurate and reliable method.14 In contrast, in radioisotope-labeled cells, only the acid-soluble radioactivity released into the medium from the labeled proteins in intact cells is measured.9,10,28 Therefore, the latter method seems less accurate and sensitive compared with our methodology.
There are several important technical issues in establishing this assay for further discussion. First, AHA labeling has to be performed in methionine-free medium with dialyzed FBS (to eliminate l-methionine from other sources), because the incorporation efficiency of AHA is much lower than natural methionine.16 Therefore, theoretically the presence of endogenous methionine inside the cells may adversely affect AHA labeling. Second, AHA labeling is unable to pick up proteins without methionine. Based on the estimation that such proteins only constitute 1.02% of all entries in a human protein database and that 5.08% of the human proteome possess only a single, N-terminal methionine that may be removed by posttranslational modification,14 AHA labeling is applicable to about 94% of the mammalian proteomes. Third, we acknowledge that this assay is unable to measure the basal proteolysis rate in control cells, as it only determines the extent of protein degradation in treated cells in comparison to the control cells. Fourth, we acknowledge that the percentage of fluorescence reduction induced by autophagy is rather moderate (maximum about 30%). For instance, by using a CHO-K1 cell line stably expressing a GFP in the mitochondrial matrix (mtGFP-CHO), it was found that starvation for 12 h led to 20% reduction of the mtGFP fluorescence intensity measured by flow cytometry.18 In another study by Shvets et al. (2008),30 in CHO cells with stable expression of GFP-LC3, treatment with rapamycin and starvation for 3 h resulted in 20% and 40% reduction of the GFP fluorescence intensity, respectively. Based on the well-known fact that GFP-LC3 is the specific substrate for autophagy and the GFP fluorescence is subject to a quenching effect in the acidic autolysosomes,31 a higher percentage of reduction of GFP fluorescent intensity is thus reasonably understood. Therefore, we think that our data may reflect the real degree of global protein degradation caused by autophagy. Last, similar to other protein degradation assays, this technique cannot be used to measure the percentage of autophagic cells.
One interesting finding in our study is that rapamycin at shorter treatment duration (3 h) had no clear effect on protein degradation (Fig. 2A). Coincidently, rapamycin was only partially effective in blocking phospho-EIF4EBP1 (Thr37/46) (Fig. 2C), being consistent with the report that rapamycin, an allosteric inhibitor, is unable to fully suppress MTOR.19 The lower autophagic proteolysis induced by rapamycin is also consistent with a recent study from our laboratory showing that rapamycin treatment is largely ineffective in promoting lysosomal function.20
In summary, we have successfully developed a novel method by using AHA labeling as a simple, sensitive, specific, and quantitative tool to measure autophagic protein degradation. This assay is mainly applicable to cell culture systems in vitro and it could be easily adapted for high-throughput screening of autophagy modulators.
Materials and Methods
Reagents and antibodies
The chemicals used in our experiments were: bafilomycin A1 (Sigma, B1793), PP242 (Sigma, P0037), rapamycin (Sigma, R8781), Torin 1 (Tocris Bioscience, 4247), dimethyl sulfoxide (Sigma, D2650), Click-iT® AHA (L-azidohomoalanine) reagent (Invitrogen, C10289), Tris[(1-benzyl-1H-1,2,3-triazol-4-yl)methyl]amine (TBTA, Sigma, 678937), Tris(2-carboxyethyl) phosphine (TCEP, Sigma, C4706), CuSO4 (Sigma, 451657), Rhodamine B alkyne was obtained from a commercial source (Jinlan Co, Guangzhou, PRC), Dulbecco’s Modified Eagle's Medium (DMEM containing 4500 mg/L D-glucose, without l-glutamine, sodium pyruvate, l-methionine, and L-cystine) (Invitrogen, 21013), dialyzed fetal bovine serum (Invitrogen, 26400044), methanol (Sigma, 34860), 4% formaldehyde in PBS (1.54 mM potassium phosphate monobasic, 155.2 mM NaCl, 2.7 mM sodium phosphate dibasic, pH 7.2), 0.5% TritonTM X-100 in PBS, 3% bovine serum albumin (BSA) in PBS (3% BSA in PBS, pH 7.4), 1% SDS in 50 mM TRIS-HCl (pH 8.0), and amino acid-free medium.
The antibodies used in our experiments included: microtubule-associated protein 1 light chain 3 (LC3, Sigma, L7543), SQSTM1 (Sigma, P0067), ATG7 (ProScience, 3617), ATG5 (Nanotools, 0262), ACTB/β-actin (Sigma, A5441), phospho-RPS6/S6 (Ser235/236) (Cell Signaling Technology, 2211), RPS6 (Cell Signaling Technology, 2217), phospho-EIF4EBP1 (Thr37/46) (Cell Signaling Technology, 2855) and EIF4EBP1 (Cell Signaling Technology, 9452).
Cell culture
Atg5 wild-type (Atg5 WT), and Atg5 knockout (Atg5 KO) MEFs and MEFs with stably expressing GFP-LC3 were kindly provided by Dr N Mizushima.32,33Atg7 WT and Atg7 KO MEFs were kindly provided Dr M Komatsu.34 All cell lines were maintained in DMEM (Sigma, D1152) containing 10% fetal bovine serum (HyClone, SV30160.03) in a 5% CO2 atmosphere at 37 °C.
Metabolic labeling of newly synthesized proteins with AHA
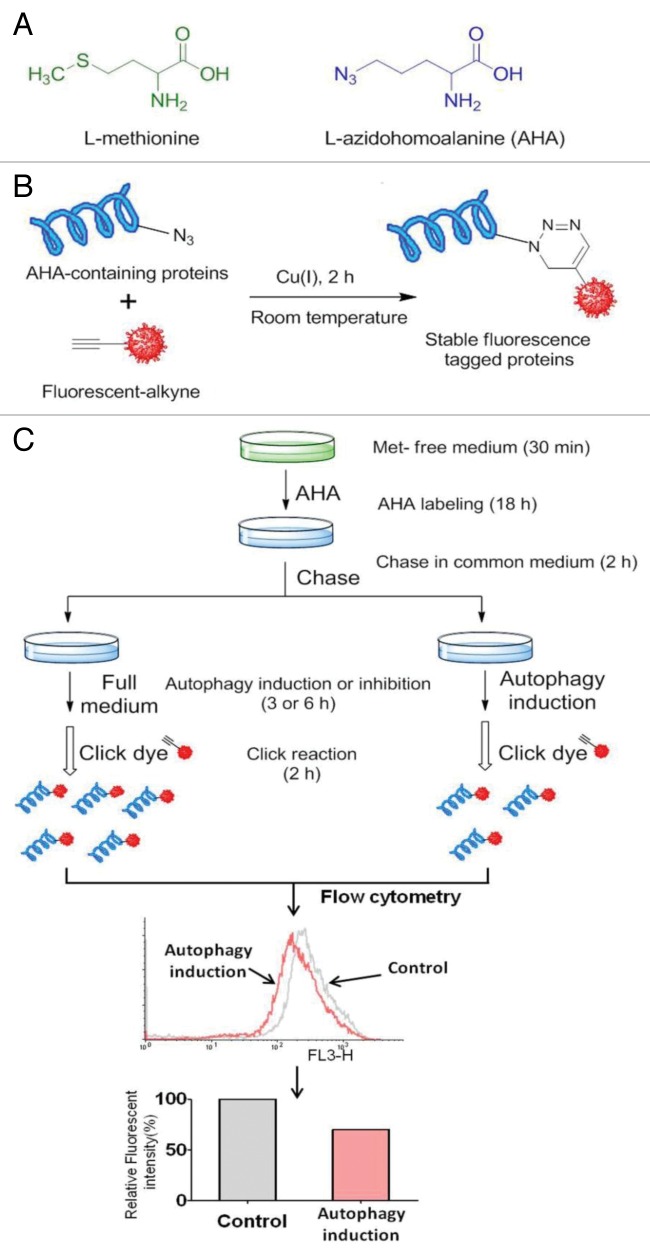
As shown in Figure 5A, AHA is an effective surrogate for methionine, an essential amino acid, that does not require any further manipulations to be incorporated as a substrate by the methionyl-tRNA synthetase into proteins during de novo protein synthesis.16,35
Figure 5. AHA labeling of newly synthesized proteins. (A) Structure of L-azidohomoalanine (AHA), an analog of methionine containing an azide moiety. (B) Click azide/alkyne reaction. The azide and alkyne moieties are interchangeable, where the molecule can be labeled with an alkyne and react with a fluorophore- or hapten-azide. (C) Workflow for AHA labeling-based quantitative analysis of protein degradation. The bio-orthogonal noncanonical amino acid tagging (BONCAT) strategy for labeling, detection and identification of newly synthesized proteins. Cells are incubated with AHA to allow protein synthesis. After incubation, cells are harvested, fixed, and permeabilized. Nascent protein synthesis is detected following a click reaction with Rhodamine B alkyne and analysis is performed using flow cytometry.
Cells with ~70–80% confluency in a 6-well plate were washed with warm PBS and cultured in l-methionine-free DMEM for 30 min to deplete the intracellular methionine reserves. Following methionine depletion, the cells were labeled with AHA in 10% FBS DMEM (methionine-free) for 18 h. In this assay, dialyzed FBS was used to eliminate l-methionine from this other source. After labeling, the cells were washed with PBS and cultured in regular DMEM containing 10× l-methionine (2 mM) for 2 h to chase out short-lived proteins. The cells then underwent various treatments as indicated.
Cell fixation and permeabilization
After incubation, the medium containing AHA was removed and the cells were washed once with PBS. Following the removal of PBS, cells were treated as indicated in the different experiments. The cells were then harvested and fixed in 4% formaldehyde in PBS for 15 min at room temperature. After fixation, the cells were washed twice with 3% BSA in PBS and permeabilized with 0.5% TritonTM X-100 in PBS for 20 min at room temperature. Finally, the cells were resuspended in PBS and stored at 4 °C for the detection of the corresponding alkyne-tagged detection molecule.
Click AHA detection
The amount of AHA incorporated into the protein can be detected using the “click” reaction between an azide and an alkyne, and a broad range of functionally and biochemically diverse proteins that incorporated AHA can be specifically tagged with a corresponding alkyne-containing dye or hapten, and subject to subsequent analysis by flow cytometry or standard biochemistry techniques such as gel electrophoresis (Fig. 5B).
The click reaction was performed based on a reported protocol with modification.15 Basically, the cells were washed by PBS and 3% BSA in PBS. For each reaction, Rhodamine B alkyne (10 μM), Tris(2-carboxyethyl) phosphine (TCEP) (1 mM, 100× fresh stock in water), Tris[(1-benzyl-1H-1,2,3-triazol-4-yl)methyl] amine (TBTA ligand) (100 μM, 100× stock in dimethyl sulfoxide), and CuSO4 (1 mM, 100× stock in water) were added into the suspended cells. The samples were incubated at room temperature for 2 h, and then the reaction cocktail was removed and the cells were washed once with 3% BSA in PBS.
In-gel fluorescence detection
After AHA labeling, the cells were lysed in 0.2% SDS in PBS. Equal amounts (100 μg) of proteins were tagged with the fluorescent dye and clicked proteins were precipitated by acetone and air-dried. One × SDS loading buffer (100 μL) was added to dissolve the sample, and the sample (50 μL) was separated by 10% SDS-PAGE. Finally, gels were visualized using a Typhoon 9410 laser scanner (GE Health Care) and images were analyzed by Image Quant software (GE Health Care).
Analysis of AHA signal intensity using flow cytometry
After fluorescence tagging, nascent protein synthesis was assessed by flow cytometry, and AHA signal intensity was determined in the FL3 channel. We quantified the cell’s fluorescence intensity and calculated the ratio of the treated cell’s fluorescence intensity to the control cell’s fluorescence intensity. This represented the rate of degradation of long-lived proteins at given time points. The average and standard deviation were calculated as follows:
Relative fluorescence intensity (%) = (treated group − negative control)/(control group − negative control) × 100
Based on the AHA labeling and click reaction as described above, we here developed a protocol for quantification of long-lived protein degradation in autophagy, as shown in Figure 5C.
Autophagy induction and inhibition
The cells were labeled with AHA as described above and cultured in regular DMEM with 10× l-methionine (2 mM) for 2 h to chase out short-lived proteins in the presence or absence of autophagy inhibitors (wortmannin and bafilomycin A1). The medium was removed by aspiration and the cells were then incubated in amino acid-free medium (plus 0.1% BSA and 2 mM l-methionine) or treated with MTOR inhibitors (rapamycin, PP242 or Torin 1) to induce autophagy in the presence or absence of autophagy inhibitors at the indicated time points.
Western blot
At the end of the designated treatments, cells were lysed in Laemmli SDS buffer (62.5 mM Tris(hydroxymethyl)aminomethane, pH 6.8 (Sigma, 252859), 25% glycerol (Sigma, G5516), 2% sodium dodecyl sulfate (Sigma, L3771), phosphatase inhibitor and proteinase inhibitor (Thermo Scientific, 88668). An equal amount of protein was resolved by SDS-PAGE and transferred onto PVDF membrane. After blocking with 5% nonfat milk, the membrane was probed with the indicated primary and secondary antibodies, developed with the enhanced chemiluminescence method and visualized with the Kodak Image Station 4000R (Kodak).
Immunofluorescence staining and confocal microscopy
MEFs with stable expression of GFP-LC3 were seeded onto a cover glass slide chamber (Lab-Tek, NUNC, 155411), and after the designated treatments, cells were examined and recorded using a confocal microscope (Olympus Fluoview FV1000); representative cells were selected and photographed.
Quantification of cell viability
A propidium iodide exclusion test coupled with flow cytometry was used to detect cell viability/cell death quantitatively, as described previously.36,37 Briefly, after the indicated treatments, cells were harvested with trypsin. Then, cell pellets were obtained and resuspended in 1× PBS containing PI at a final concentration of 5 µg/ml and incubated for 10 min at 37 °C. Ten thousand cells from each sample were analyzed with FACSCalibur flow cytometry (BD Bioscience) using CellQuest software.
Statistical analysis
All western blot and image data presented are representatives of at least 3 independent experiments. The numerical data are presented as means ± SD from 3 independent experiments and analyzed using the Student t test.
Supplementary Material
Glossary
Abbreviations:
- AHA
L-azidohomoalanine
- BAF
bafilomycin A1
- CHX
cycloheximide
- MEFs
mouse embryonic fibroblasts
- MTOR
mechanistic target of rapamycin
- PtdIns3-kinase
phosphatidylinositol 3-kinase
- PI
propidium iodide
- RPS6
ribosomal protein S6
- Wort
wortmannin
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors thank Dr N Mizushima (University of Tokyo) and Dr M Komatsu (Tokyo Metropolitan Institute of Medical Science) for providing the indicated reagents. This work was supported by research grants to HMS (NMRC-1260/2010 and NMRC-CIRG/1346/2012). JZ, JW, and SN are supported by NUS Research Scholarships.
References
- 1.Mizushima N, Komatsu M. . Autophagy: renovation of cells and tissues. Cell 2011; 147:728 - 41; http://dx.doi.org/ 10.1016/j.cell.2011.10.026; PMID: 22078875 [DOI] [PubMed] [Google Scholar]
- 2.Levine B, Kroemer G. . Autophagy in the pathogenesis of disease. Cell 2008; 132:27 - 42; http://dx.doi.org/ 10.1016/j.cell.2007.12.018; PMID: 18191218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehrpour M, Esclatine A, Beau I, Codogno P. . Autophagy in health and disease. 1. Regulation and significance of autophagy: an overview. Am J Physiol Cell Physiol 2010; 298:C776 - 85; http://dx.doi.org/ 10.1152/ajpcell.00507.2009; PMID: 20089931 [DOI] [PubMed] [Google Scholar]
- 4.Mizushima N. . Autophagy: process and function. Genes Dev 2007; 21:2861 - 73; http://dx.doi.org/ 10.1101/gad.1599207; PMID: 18006683 [DOI] [PubMed] [Google Scholar]
- 5.Nakatogawa H, Suzuki K, Kamada Y, Ohsumi Y. . Dynamics and diversity in autophagy mechanisms: lessons from yeast. Nat Rev Mol Cell Biol 2009; 10:458 - 67; http://dx.doi.org/ 10.1038/nrm2708; PMID: 19491929 [DOI] [PubMed] [Google Scholar]
- 6.Chan EY, Tooze SA. . Evolution of Atg1 function and regulation. Autophagy 2009; 5:758 - 65; PMID: 19411825 [DOI] [PubMed] [Google Scholar]
- 7.Laplante M, Sabatini DM. . mTOR signaling in growth control and disease. Cell 2012; 149:274 - 93; http://dx.doi.org/ 10.1016/j.cell.2012.03.017; PMID: 22500797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jung CH, Ro SH, Cao J, Otto NM, Kim DH. . mTOR regulation of autophagy. FEBS Lett 2010; 584:1287 - 95; http://dx.doi.org/ 10.1016/j.febslet.2010.01.017; PMID: 20083114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogier-Denis E, Houri JJ, Bauvy C, Codogno P. . Guanine nucleotide exchange on heterotrimeric Gi3 protein controls autophagic sequestration in HT-29 cells. J Biol Chem 1996; 271:28593 - 600; http://dx.doi.org/ 10.1074/jbc.271.45.28593; PMID: 8910489 [DOI] [PubMed] [Google Scholar]
- 10.Roberts EA, Deretic V. . Autophagic proteolysis of long-lived proteins in nonliver cells. Methods Mol Biol 2008; 445:111 - 7; http://dx.doi.org/ 10.1007/978-1-59745-157-4_6; PMID: 18425445 [DOI] [PubMed] [Google Scholar]
- 11.Bauvy C, Meijer AJ, Codogno P. . Assaying of autophagic protein degradation. Methods Enzymol 2009; 452:47 - 61; http://dx.doi.org/ 10.1016/S0076-6879(08)03604-5; PMID: 19200875 [DOI] [PubMed] [Google Scholar]
- 12.Doherty MK, Hammond DE, Clague MJ, Gaskell SJ, Beynon RJ. . Turnover of the human proteome: determination of protein intracellular stability by dynamic SILAC. J Proteome Res 2009; 8:104 - 12; http://dx.doi.org/ 10.1021/pr800641v; PMID: 18954100 [DOI] [PubMed] [Google Scholar]
- 13.Best MD. . Click chemistry and bioorthogonal reactions: unprecedented selectivity in the labeling of biological molecules. Biochemistry 2009; 48:6571 - 84; http://dx.doi.org/ 10.1021/bi9007726; PMID: 19485420 [DOI] [PubMed] [Google Scholar]
- 14.Dieterich DC, Link AJ, Graumann J, Tirrell DA, Schuman EM. . Selective identification of newly synthesized proteins in mammalian cells using bioorthogonal noncanonical amino acid tagging (BONCAT). Proc Natl Acad Sci U S A 2006; 103:9482 - 7; http://dx.doi.org/ 10.1073/pnas.0601637103; PMID: 16769897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dieterich DC, Lee JJ, Link AJ, Graumann J, Tirrell DA, Schuman EM. . Labeling, detection and identification of newly synthesized proteomes with bioorthogonal non-canonical amino-acid tagging. Nat Protoc 2007; 2:532 - 40; http://dx.doi.org/ 10.1038/nprot.2007.52; PMID: 17406607 [DOI] [PubMed] [Google Scholar]
- 16.Kiick KL, Saxon E, Tirrell DA, Bertozzi CR. . Incorporation of azides into recombinant proteins for chemoselective modification by the Staudinger ligation. Proc Natl Acad Sci U S A 2002; 99:19 - 24; http://dx.doi.org/ 10.1073/pnas.012583299; PMID: 11752401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu YT, Tan HL, Huang Q, Kim YS, Pan N, Ong WY, Liu ZG, Ong CN, Shen HM. . Autophagy plays a protective role during zVAD-induced necrotic cell death. Autophagy 2008; 4:457 - 66; PMID: 18253089 [DOI] [PubMed] [Google Scholar]
- 18.Kawai A, Takano S, Nakamura N, Ohkuma S. . Quantitative monitoring of autophagic degradation. Biochem Biophys Res Commun 2006; 351:71 - 7; http://dx.doi.org/ 10.1016/j.bbrc.2006.09.168; PMID: 17054905 [DOI] [PubMed] [Google Scholar]
- 19.Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y, Reichling LJ, Sim T, Sabatini DM, Gray NS. . An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J Biol Chem 2009; 284:8023 - 32; http://dx.doi.org/ 10.1074/jbc.M900301200; PMID: 19150980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou J, Tan SH, Nicolas V, Bauvy C, Yang ND, Zhang J, Xue Y, Codogno P, Shen HM. . Activation of lysosomal function in the course of autophagy via mTORC1 suppression and autophagosome-lysosome fusion. Cell Res 2013; 23:508 - 23; http://dx.doi.org/ 10.1038/cr.2013.11; PMID: 23337583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu YT, Tan HL, Shui G, Bauvy C, Huang Q, Wenk MR, Ong CN, Codogno P, Shen HM. . Dual role of 3-methyladenine in modulation of autophagy via different temporal patterns of inhibition on class I and III phosphoinositide 3-kinase. J Biol Chem 2010; 285:10850 - 61; http://dx.doi.org/ 10.1074/jbc.M109.080796; PMID: 20123989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petiot A, Ogier-Denis E, Blommaart EF, Meijer AJ, Codogno P. . Distinct classes of phosphatidylinositol 3′-kinases are involved in signaling pathways that control macroautophagy in HT-29 cells. J Biol Chem 2000; 275:992 - 8; http://dx.doi.org/ 10.1074/jbc.275.2.992; PMID: 10625637 [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto A, Tagawa Y, Yoshimori T, Moriyama Y, Masaki R, Tashiro Y. . Bafilomycin A1 prevents maturation of autophagic vacuoles by inhibiting fusion between autophagosomes and lysosomes in rat hepatoma cell line, H-4-II-E cells. Cell Struct Funct 1998; 23:33 - 42; http://dx.doi.org/ 10.1247/csf.23.33; PMID: 9639028 [DOI] [PubMed] [Google Scholar]
- 24.Vabulas RM, Hartl FU. . Protein synthesis upon acute nutrient restriction relies on proteasome function. Science 2005; 310:1960 - 3; http://dx.doi.org/ 10.1126/science.1121925; PMID: 16373576 [DOI] [PubMed] [Google Scholar]
- 25.Klionsky DJ, Abdalla FC, Abeliovich H, Abraham RT, Acevedo-Arozena A, Adeli K, Agholme L, Agnello M, Agostinis P, Aguirre-Ghiso JA, et al. . Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 2012; 8:445 - 544; http://dx.doi.org/ 10.4161/auto.19496; PMID: 22966490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dieterich DC, Hodas JJ, Gouzer G, Shadrin IY, Ngo JT, Triller A, Tirrell DA, Schuman EM. . In situ visualization and dynamics of newly synthesized proteins in rat hippocampal neurons. Nat Neurosci 2010; 13:897 - 905; http://dx.doi.org/ 10.1038/nn.2580; PMID: 20543841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hinz FI, Dieterich DC, Tirrell DA, Schuman EM. . Non-canonical amino acid labeling in vivo to visualize and affinity purify newly synthesized proteins in larval zebrafish. ACS Chem Neurosci 2012; 3:40 - 9; http://dx.doi.org/ 10.1021/cn2000876; PMID: 22347535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yewdell JW, Lacsina JR, Rechsteiner MC, Nicchitta CV. . Out with the old, in with the new? Comparing methods for measuring protein degradation. Cell Biol Int 2011; 35:457 - 62; http://dx.doi.org/ 10.1042/CBI20110055; PMID: 21476986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lelouard H, Gatti E, Cappello F, Gresser O, Camosseto V, Pierre P. . Transient aggregation of ubiquitinated proteins during dendritic cell maturation. Nature 2002; 417:177 - 82; http://dx.doi.org/ 10.1038/417177a; PMID: 12000969 [DOI] [PubMed] [Google Scholar]
- 30.Shvets E, Fass E, Elazar Z. . Utilizing flow cytometry to monitor autophagy in living mammalian cells. Autophagy 2008; 4:621 - 8; PMID: 18376137 [DOI] [PubMed] [Google Scholar]
- 31.Kimura S, Noda T, Yoshimori T. . Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3. Autophagy 2007; 3:452 - 60; PMID: 17534139 [DOI] [PubMed] [Google Scholar]
- 32.Hosokawa N, Hara Y, Mizushima N. . Generation of cell lines with tetracycline-regulated autophagy and a role for autophagy in controlling cell size. FEBS Lett 2006; 580:2623 - 9; http://dx.doi.org/ 10.1016/j.febslet.2006.04.008; PMID: 16647067 [DOI] [PubMed] [Google Scholar]
- 33.Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T, Ohsumi Y, Tokuhisa T, Mizushima N. . The role of autophagy during the early neonatal starvation period. Nature 2004; 432:1032 - 6; http://dx.doi.org/ 10.1038/nature03029; PMID: 15525940 [DOI] [PubMed] [Google Scholar]
- 34.Komatsu M, Waguri S, Ueno T, Iwata J, Murata S, Tanida I, Ezaki J, Mizushima N, Ohsumi Y, Uchiyama Y, et al. . Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J Cell Biol 2005; 169:425 - 34; http://dx.doi.org/ 10.1083/jcb.200412022; PMID: 15866887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Link AJ, Tirrell DA. . Cell surface labeling of Escherichia coli via copper(I)-catalyzed [3+2] cycloaddition. J Am Chem Soc 2003; 125:11164 - 5; http://dx.doi.org/ 10.1021/ja036765z; PMID: 16220915 [DOI] [PubMed] [Google Scholar]
- 36.Ng S, Wu YT, Chen B, Zhou J, Shen HM. . Impaired autophagy due to constitutive mTOR activation sensitizes TSC2-null cells to cell death under stress. Autophagy 2011; 7:1173 - 86; http://dx.doi.org/ 10.4161/auto.7.10.16681; PMID: 21808151 [DOI] [PubMed] [Google Scholar]
- 37.Wu YT, Tan HL, Huang Q, Sun XJ, Zhu X, Shen HM. . zVAD-induced necroptosis in L929 cells depends on autocrine production of TNFα mediated by the PKC-MAPKs-AP-1 pathway. Cell Death Differ 2011; 18:26 - 37; http://dx.doi.org/ 10.1038/cdd.2010.72; PMID: 20539307 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.