Abstract
Paclitaxel, a chemotherapeutic agent, causes neuropathic pain whose supraspinal pathophysiology is not fully understood. Dysregulation of sodium channel expression, studied mainly in the periphery and spinal cord level, contributes to the pathogenesis of neuropathic pain. We examined gene expression of sodium channel (Nav) subunits by real time polymerase chain reaction (PCR) in the anterior cingulate cortex (ACC) at day 7 post first administration of paclitaxel, when mice had developed paclitaxel-induced thermal hyperalgesia. The ACC was chosen because increased activity in the ACC has been observed during neuropathic pain. In the ACC of vehicle-treated animals the threshold cycle (Ct) values for Nav1.4, Nav1.5, Nav1.7, Nav1.8 and Nav1.9 were above 30 and/or not detectable in some samples. Thus, comparison in mRNA expression between untreated control, vehicle-treated and paclitaxel treated animals was done for Nav1.1, Nav1.2, Nav1.3, Nav1.6, Nax as well as Navβ1–Navβ4. There were no differences in the transcript levels of Nav1.1–Nav1.3, Nav1.6, Nax, Navβ1–Navβ3 between untreated and vehicle-treated mice, however, vehicle treatment increased Navβ4 expression. Paclitaxel treatment significantly increased the mRNA expression of Nav1.1, Nav1.2, Nav1.6 and Nax, but not Nav1.3, sodium channel alpha subunits compared to vehicle-treated animals. Treatment with paclitaxel significantly increased the expression of Navβ1 and Navβ3, but not Navβ2 and Navβ4, sodium channel beta subunits compared to vehicle-treated animals. These findings suggest that during paclitaxel-induced neuropathic pain (PINP) there is differential upregulation of sodium channels in the ACC, which might contribute to the increased neuronal activity observed in the area during neuropathic pain.
Keywords: Neuropathic pain, Anterior cingulate cortex, Paclitaxel, Sodium channel, Gene expression
Introduction
Voltage-gated sodium channels (Nav) are responsible for action potential initiation and propagation in neurons and other excitable cells. Sodium channels are composed of a pore-forming α subunit associated with one or more auxiliary β subunits that modulate channel gating, expression and localisation (Catterall, Goldin & Waxman, 2005; Isom, 2001). There are ten sodium channel α subunits Nav1.1–Nav1.9 and Nax encoded by genes SCN1A–SCN11A, and four β subunits Navβ1–Navβ4, encoded by genes SCN1B–SCN4B (Brackenbury & Isom, 2008; Cummins, Sheets & Waxman, 2007; Yu & Catterall, 2003). These sodium channel subunits are expressed in a wide variety of tissues and the level of expression of each channel varies between tissues.
Sodium channels play an important role in the propagation of nociceptive signals. Changes in sodium channel function or expression can result in altered pain sensitivity and perception in various conditions including neuropathic pain (Bagal et al., 2015; Cummins, Sheets & Waxman, 2007). Dysregulated expression of sodium channels in both the periphery and the central nervous system (CNS), which can result in frequent and ectopic firing in neurons, have been associated with the pathogenesis of neuropathic pain (Craner et al., 2002; Lindia et al., 2005; Pertin et al., 2005; Rogers et al., 2006).
In the periphery, the expression all sodium channel α subunits was downregulated, except for Nav1.2, in the dorsal root ganglia (DRG) of rats with spared nerve injury (SNI) (Laedermann et al., 2014). Another study observed downregulation of Nav1.8 and Nav1.9 in the DRG of a chronic constriction injury (CCI) model of neuropathic pain (Dib-Hajj et al., 1999). However, other studies have observed upregulation of sodium channel subunits such as Nav1.3, Nav1.6, Nav1.9, Navβ2 and Navβ3 in the DRG of animal models of neuropathic pain (Craner et al., 2002; Lindia et al., 2005; Pertin et al., 2005; Shah et al., 2001; Shah et al., 2000).
In the spinal cord Nav1.3 was also found to be upregulated in the dorsal horn neurons of CCI and spinal cord injury (SCI) models of neuropathic pain (Hains et al., 2003; Hains et al., 2004). Sciatic nerve injury (axotomy) resulted in upregulation of Nav1.7 in the spinal cord, which had strong correlation with the level of pain behaviour (Persson et al., 2009). In a model of painful diabetic neuropathy there was upregulation of Navβ3 expression in spinal cord (Shah et al., 2001). Navβ1 expression increased whereas Navβ2 decreased in the spinal cord of neuropathic rats (Blackburn-Munro & Fleetwood-Walker, 1999).
In the brain dysregulation of sodium channel expression has been observed in different areas during neuropathic pain. In the prefrontal cortex Nav1.1 expression was upregulated in mice with SNI (Alvarado et al., 2013). The expression of Nav1.3 was upregulated in the ventral posterolateral (VPL) nucleus of the thalamus of rats with CCI or spinal cord contusion injury (Hains, Saab & Waxman, 2005; Zhao, Waxman & Hains, 2006).
Recently, we observed increased excitability of the anterior cingulate cortex (ACC) to electrophysiological stimulation in a rat model of paclitaxel-induced neuropathic pain (PINP) (Nashawi et al., 2016). Paclitaxel is a chemotherapeutic drug whose therapeutic use is sometimes limited by the development of dose-dependent painful neuropathy (Scripture, Figg & Sparreboom, 2006; Wolf et al., 2008). The ACC is an area in the brain involved in pain perception and modulation, and has increased activity during neuropathic pain (Hsieh et al., 1995; Vogt, 2005; Xie, Huo & Tang, 2009; Zhuo, 2008). In previous studies, we observed changes in the expression of gamma-aminobutyric acid (GABA)-ergic and glutamatergic molecules in the ACC of a mouse model of PINP (Masocha, 2015a; Masocha, 2015b). However, the expression of sodium channels in the ACC during PINP has not been studied as yet. Studying the expression of sodium channels in the ACC during PINP is important as they might contribute to the increased neuronal excitability, which we observed in the ACC during PINP (Nashawi et al., 2016). Thus, in the current study the gene expression of sodium channel subunits in the ACC was evaluated in mice at a time point when the mice had paclitaxel-induced thermal hyperalgesia (Masocha, 2015a; Nieto et al., 2008; Parvathy & Masocha, 2013). In previous studies, gene expression changes of other molecules were observed in the ACC of mice with paclitaxel-induced thermal hyperalgesia (Masocha, 2015a; Masocha, 2015b).
Materials and Methods
Animals
Female BALB/c mice (8–12 weeks old; 20–30 g; n = 49) supplied by the Animal Resources Centre (ARC) at the Health Sciences Center (HSC), Kuwait University were used. The animals were kept in temperature controlled (24 ± 1 °C) rooms with food and water given ad libitum. Animals were handled in compliance with the Kuwait University, HSC, ARC guidelines and in compliance with Directive 2010/63/EU of the European Parliament and of the Council on the protection of animals used for scientific purposes. All animal experiments were approved by the Ethical Committee for the use of Laboratory Animals in Teaching and in Research, HSC, Kuwait University.
Paclitaxel administration
Paclitaxel (Cat. No. 1097, Tocris, Bristol, UK) was dissolved in a solution made up of 50% Cremophor EL and 50% absolute ethanol to a concentration of 6 mg/ml and then diluted in normal saline (NaCl 0.9%), to a final concentration of 0.2 mg/ml just before administration. Mice were treated intraperitoneally (i.p.) for five consecutive days with paclitaxel 2 mg/kg, the cumulative dose was 10 mg/kg, or its vehicle. This treatment regimen produces painful neuropathy and thermal hyperalgesia in mice on day 7 post first administration (Nieto et al., 2008; Parvathy & Masocha, 2013). A group of control mice was left untreated.
Tissue preparation and real time RT-PCR
Mice were anesthetized with isoflurane, sacrificed by decapitation on day 7 post first administration of paclitaxel. The ACC was dissected and prepared for RNA extraction as described previously (Masocha, 2015b).
Gene transcripts of the 10 sodium channel alpha subunits (Nav1.1, Nav1.2, Nav1.3, Nav1.4, Nav1.5, Nav1.6, Nav1.7, Nav1.8, Nav1.9 and Nax) and four sodium channel beta subunits (Navβ1, Navβ2, Navβ3 and Navβ4) were quantified in the ACC of untreated, vehicle-treated and paclitaxel-treated mice by real time polymerase chain reaction (PCR). Total RNA was xtracted from the fresh frozen ACC using the RNeasy Kit (Qiagen GmbH), reverse-transcribed, and the mRNA levels were quantified on an ABI Prism® 7500 sequence detection system (Applied Biosystems) as previously described (Masocha, 2009; Masocha, 2015a). The primer sequences which were used, listed in Table 1, were ordered from Invitrogen (Life Technologies) and/or synthesized at the Research Core Facility (RCF), HSC, Kuwait University. Threshold cycle (Ct) values for all cDNA samples were obtained and the amount of mRNA of individual animal sample (n = 8–12 per group) was normalized to cyclophilin (housekeeping gene) (ΔCt). The relative amount of target gene transcripts was calculated using the 2−ΔΔCt method as described previously (Livak & Schmittgen, 2001). These values were then used to calculate the mean and standard error of the relative expression of the target gene mRNA in the ACC of paclitaxel- and vehicle-treated mice.
Table 1. PCR primer sequences of cyclophilin, and sodium channel subunits.
| Gene | Polarity | |
|---|---|---|
| Sense Sequence 5′ to 3′ |
Anti-sense Sequence 5′ to 3′ |
|
| Cyclophilin | GCTTTTCGCCGCTTGCT | CTCGTCATCGGCCGTGAT |
| Nav1.1 | AACAAGCTTCATTCACATACAATAAG | AGGAGGGCGGACAAGCTG |
| Nav1.2 | GGGAACGCCCATCAAAGAAG | ACGCTATCGTAGGAAGGTGG |
| Nav1.3 | GGGTGTTGGGTGAGAGTGGAG | AATGTAGTAGTGATGGGCTGATAAGAG |
| Nav1.4 | CGCGCTGTTCAGCATGTT | CTCCACGTCCTTGGACCAAG |
| Nav1.5 | AGACTTCCCTCCATCTCCAGATA | TGTCACCTCCAGAGCTAGGAAG |
| Nav1.6 | AGCAAAGACAAACTGGACGATACC | CACTTGAACCTCTGGACACAACC |
| Nav1.7 | TCCTTTATTCATAATCCCAGCCTCAC | GATCGGTTCCGTCTCTCTTTGC |
| Nav1.8 | ACCGACAATCAGAGCGAGGAG | ACAGACTAGAAATGGACAGAATCACC |
| Nav1.9 | TGAGGCAACACTACTTCACCAATG | AGCCAGAAACCAAGGTACTAATGATG |
| Nax | TGTCTCCTCTAAACTCCCTCAG | TGCGTAAATCCCAAGCAAAGT |
| Navβ1 | GTGTATCTCCTGTAAGCGTCGTAG | ATTCTCATAGCGTAGGATCTTGACAA |
| Navβ2 | GGCCACGGCAAGATTTACCT | CACCAAGATGACCACAGCCA |
| Navβ3 | ACTGAAGAGGCGGGAGAAGAC | GGTGAGGAAGACCAGGAGGATG |
| Navβ4 | CCCTTGGTGTAGAAACTAAGCAGAG | CAGAAGCGAGTCAGTCAGATACG |
Statistical analyses
Statistical analyses were performed using Mann Whitney U test using Graph Pad Prism software (version 5.0). The differences were considered significant at p < 0.05. The results in the text and figures are expressed as the means ± S.E.M.
Results
The mRNA expression of sodium channel subunits were analysed in the ACC at day 7, a time when the mice treated with paclitaxel had developed thermal hyperalgesia as we described previously (Masocha, 2014; Parvathy & Masocha, 2013) i.e. reduction in reaction latency compared to the baseline latency and vehicle-treated mice (5.7 ± 0.3 s compared to 9.6 ± 0.3 and 9.3 ± 0.3 s, respectively; n = 8 vehicle-treated mice and 10 paclitaxel treated-mice; p < 0.01 for both comparisons).
Expression of sodium channel alpha subunits transcripts in the ACC at seven days after paclitaxel administration
In vehicle-treated animals the Ct values for Nav1.4, Nav1.5, Nav1.7, Nav1.8 and Nav1.9 were above 30 and not detectable in some samples, whereas the Ct values for Nav1.1, Nav1.2, Nav1.3, Nav1.6 and Nax were below 30. Thus, comparison in mRNA expression between control and paclitaxel treated animals was done for Nav1.1, Nav1.2, Nav1.3, Nav1.6 and Nax.
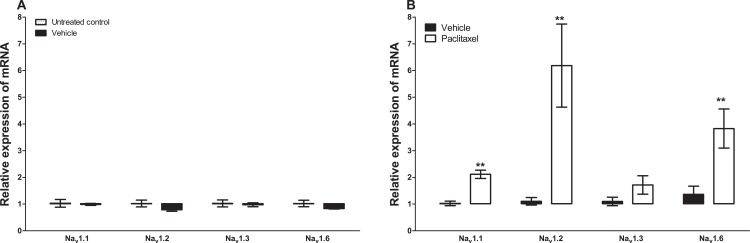
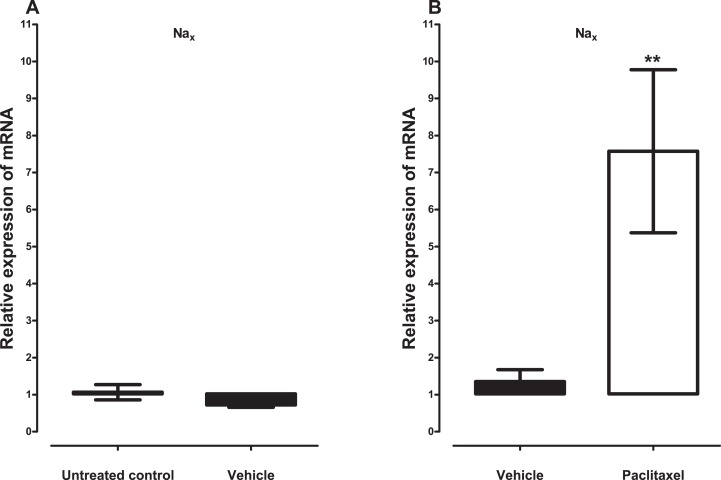
Treatment with vehicle did not alter the expression of the five sodium channel alpha subunits evaluated, Nav1.1 (p = 1.000), Nav1.2 (p = 0.1143), Nav1.3 (p = 0.6857), Nav1.6 (p = 0.3429) and Nax (p = 0.3429), compared to untreated control (Figs. 1A and 2A). Amongst the five sodium channel alpha subunits (Nav1.1, Nav1.2, Nav1.3, Nav1.6 and Nax) treatment with paclitaxel did not significantly alter the mRNA expression of the Nav1.3 (p = 0.1379), but significantly increased the expression of Nav1.1 by 2.1 ± 0.2 fold (p = 0.0002), Nav1.2 by 6.2 ± 1.6 fold (p = 0.0003), Nav1.6 by 3.8 ± 0.7 fold (p = 0.0051), compared to vehicle-treated controls (Fig. 1B). Nax was significantly upregulated by 7.6 ± 2.2 fold (p = 0.0012) in the ACC by treatment with paclitaxel compared to treatment with vehicle (Fig. 2B). The most upregulated sodium channel alpha subunits were Nav1.2 and Nax, which were increased by more than sixfold after treatment with paclitaxel.
Figure 1. Effects of paclitaxel on sodium channel alpha subunits transcript levels in the anterior cingulate cortex (ACC).
Relative mRNA expression of sodium channel alpha subunits Nav1.1, Nav1.2, Nav1.3 and Nav1.6 in the ACC of BALB/c mice (A) vehicle-treated mice versus untreated mice. Each bar represents the mean ± S.E.M of the values obtained from four untreated mice and four vehicle-treated mice. (B) Relative mRNA expression of sodium channel alpha subunits on day 7 after first administration of the drug or its vehicle. Each bar represents the mean ± S.E.M of the values obtained from 9 to 11 vehicle-treated mice and 12 paclitaxel-treated mice. ** p < 0.01 compared to vehicle-treated mice.
Figure 2. Effects of paclitaxel on the sodium channel alpha subunit Nax transcript levels in the anterior cingulate cortex (ACC).
Relative mRNA expression of Nax in the ACC of BALB/c mice (A) vehicle-treated mice versus untreated mice. Each bar represents the mean ± S.E.M of the values obtained from four untreated mice and four vehicle-treated mice. (B) Relative mRNA expression of sodium channel alpha subunits on day 7 after first administration of the drug or its vehicle. Each bar represents the mean ± S.E.M of the values obtained from 11 vehicle-treated mice and 12 paclitaxel-treated mice. ** p < 0.01 compared to vehicle-treated control mice.
Expression of sodium channel beta subunits transcripts in the ACC at seven days after paclitaxel administration
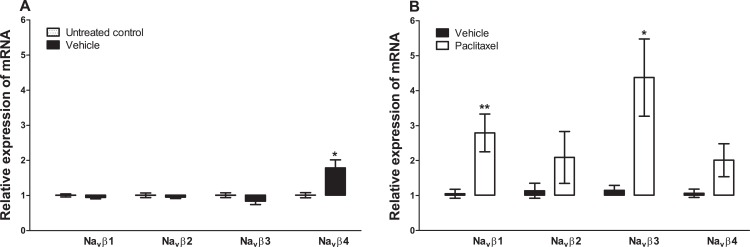
Treatment with vehicle did not alter the expression of three sodium channel beta subunits, Navβ1 (p = 0.2000), Navβ2 (p = 0.4857), Navβ3 (p = 0.6857), but significantly increased the expression of Navβ4 (p = 0.0286), compared to untreated control (Fig. 3A). Amongst the four sodium channel beta subunits analysed treatment with paclitaxel significantly increased the expression of Navβ1 by 2.8 ± 0.5 fold (p = 0.0047) and Navβ3 by 4.4 ± 1.1 fold (p = 0.0127), but not Navβ2 (p = 0.2301) and Navβ4 (p = 0.0525), compared to vehicle-treated controls (Fig. 3). The most upregulated sodium channel beta subunit was Navβ3, which was increased by more than fourfold after treatment with paclitaxel.
Figure 3. Effects of paclitaxel on sodium channel beta subunits transcript levels in the anterior cingulate cortex (ACC).
Relative mRNA expression of sodium channel beta subunits Navβ1 to four in the ACC of BALB/c mice (A) vehicle-treated mice versus untreated mice. Each bar represents the mean ± S.E.M of the values obtained from four untreated mice and four vehicle-treated mice. * p < 0.05 compared to untreated mice. (B) Relative mRNA expression of sodium channel beta subunits on day 7 after first administration of the drug or its vehicle. Each bar represents the mean ± S.E.M of the values obtained from 8 to 11 vehicle-treated control mice and 8–12 paclitaxel-treated mice. * p < 0.05 and ** p < 0.01 compared to vehicle-treated mice.
Discussion
This study presents the first comprehensive analysis of the expression of transcripts of sodium channel subunits in the ACC during neuropathic pain, specifically PINP. The ACC is an area of the brain associated with pain perception and modulation (Vogt, 2005; Xie, Huo & Tang, 2009; Zhuo, 2008).
No reports about the expression of sodium channels in the ACC specifically were found. However, Nav1.1, Nav1.2, Nav1.3, Nav1.6 and also Nax have been reported to be expressed predominantly (but not exclusively) in the brain with differential expression in different brain areas such as hippocampus, thalamus, cerebellum etc. (Beckh et al., 1989; Catterall, 2000; Gautron et al., 1992; Levy-Mozziconacci et al., 1998; Schaller & Caldwell, 2003; Westenbroek, Merrick & Catterall, 1989; Whitaker et al., 2000; Whitaker et al., 2001). On the other hand, Nav1.4 is expressed principally in the skeletal muscle, Nav1.5 is mainly expressed in cardiac muscle, while Nav1.7, Nav1.8 and Nav1.9 are expressed preferentially in peripheral neurons (Cummins, Sheets & Waxman, 2007; Dib-Hajj, Black & Waxman, 2015). In the current study using real time PCR all the 10 α subunits and four β subunits were detected in the ACC with different degrees of expression. Nav1.1, Nav1.2, Nav1.3, Nav1.6 and Nax as well as Navβ1–Navβ4 were highly expressed in the ACC. On the other hand, although Nav1.4, Nav1.5, Nav1.7, Nav1.8 and Nav1.9 were detected in the ACC they were lowly expressed and/or were not detectable in some samples. Thus, the findings of this study are in agreement with studies described above. This suggests that the different sodium channel subunits have different roles in the ACC and the brain in general. Nav1.1, Nav1.2, Nav1.3, Nav1.6 and Nax as well as Navβ1–Navβ4 most likely have more important roles in neuronal activity in the ACC than Nav1.4, Nav1.5, Nav1.7, Nav1.8 and Nav1.9. This could be important for drug development of specific sodium channel blockers; for example a specific blocker of Nav1.1 or Nav1.2 would more likely have more effect in the ACC compared to a specific inhibitor of Nav1.7 or Nav1.8 based on their expression patterns. Further studies are necessary to understand the specific properties and activities of specific sodium channel subunits in the ACC under normal conditions and during neuropathic pain.
Administration of tetrodotoxin (TTX), a voltage-gated sodium channel blocker, was reported to prevent and treat signs of PINP such as thermal hyperalgesia, cold and mechanical allodynia in mice, suggesting that TTX-sensitive voltage-gated sodium channels play a role in the pathophysiology of PINP (Nieto et al., 2008). Mexiletine, a non-selective voltage-gated sodium channel blocker was also found to have antinociceptive effects in rats with paclitaxel-induced mechanical allodynia and hyperalgesia (Xiao, Naso & Bennett, 2008). However, we found no studies that investigated the expression of sodium channels in the periphery or CNS during PINP. In the current study, Nav1.1, Nav1.2, Nav1.6 and Nax as well as NavB1 and NavB3 were upregulated in the ACC of mice with paclitaxel-induced thermal hyperalgesia. Upregulation of sodium channel expression has been observed in other areas of the brain during neuropathic pain. In the prefrontal cortex Nav1.1 expression was upregulated in mice with SNI (Alvarado et al., 2013). Thus, our data are in agreement with the findings of Alvarado et al. (2013) and the suggestion that over-expression of Nav1.1 is involved in increased cortical excitability associated with chronic pain. It is also possible that the increased expression of Nav1.2, Nav1.6, Nax, NavB1 and NavB3 in the ACC are involved in the increased excitability of this area observed during PINP (Nashawi et al., 2016). Although Nav1.3 was not significantly altered in the ACC during PINP it was reported to be upregulated in the VPL nucleus of the thalamus of rats with CCI and spinal cord contusion injury (Hains, Saab & Waxman, 2005; Zhao, Waxman & Hains, 2006). The findings of the current study suggest that upregulation of specific sodium channel subunits might contribute to hyperexcitability in the ACC. Hyperexcitability has been associated with dysregulation in sodium channels (Devor, 2006). A link between upregulation of Nav1.3 and hyperexcitability of neurons in the spinal cord was found in neuropathic pain after SCI (Hains et al., 2003). Recently, we observed increased excitability of the ACC to electrophysiological stimulation in a rat model PINP (Nashawi et al., 2016), which could be in part be due upregulation of sodium channels amongst other mechanisms such as decreased GABA availability at the synapse because of increased GABA transporter 1 (GAT-1) expression (Masocha, 2015b). Changes in the expression of other molecules such as those of the GABAergic, glutamatergic, muscarinic dopaminergic systems have also been observed in the ACC during experimental neuropathic pain (Masocha, 2015a; Masocha, 2015b; Ortega-Legaspi et al., 2011; Ortega-Legaspi et al., 2010). These findings suggest that the ACC plays an important role in the pathophysiology of PINP in addition to other brain areas, the spinal cord and peripheral nerve damage. Paclitaxel has limited ability to cross the blood-brain barrier (Glantz et al., 1995; Kemper et al., 2003), thus a direct effect of paclitaxel in the ACC is unlikely. In a rat model paclitaxel induced microglial activation in the spinal cord (Peters et al., 2007). They proposed (Peters et al., 2007) that paclitaxel-induced nerve injury possibly induced neurochemical reorganization within the spinal cord leading to central sensitization (Cata et al., 2006) and that the microglial reaction they observed occurred as a result of degeneration of central terminals of injured primary afferent fibers or possibly due to the spinal release of factors from injured neurons rather than direct injury of spinal cord neurons by paclitaxel. In the periphery, paclitaxel causes nerve damage by direct effects on the neurons (Cavaletti et al., 2000; Scuteri et al., 2006; Theiss & Meller, 2000) or via inflammation and the increased infiltration of macrophages into the DRG (Peters et al., 2007; Zhang et al., 2016), which cause further nerve damage. Thus, the changes observed in the ACC could be due to an increased nociceptive input from the peripheral nerves damaged by paclitaxel resulting in central sensitization. However, information on protein expression is critical to subsequently define the meaning of expression changes in the mRNA level observed in the ACC.
Conclusions
In conclusion, the findings of this study show that sodium channel subunit transcripts are differentially expressed in the ACC; with those known to be preferentially expressed in the CNS being highly expressed in the ACC, whereas those known to be preferentially expressed in the periphery being lowly expressed in the ACC. More importantly, the results show that during experimental PINP there is increased expression of various sodium channel subunit transcripts in the ACC, which could contribute to the increased excitability and activity observed in this brain region during neuropathic pain.
Supplemental Information
Acknowledgments
I am grateful to Dr. Subramanian S. Parvathy, Ms. Salini Soman, Ms. Amal Thomas from the Department of Pharmacology and Therapeutics, Faculty of Pharmacy, for their technical assistance and to the staff from the Animal Resources Centre, HSC, Kuwait University for their support.
Funding Statement
Funding was provided by Kuwait University Research Sector: PT01/09, SRUL02/13. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The author declares that he has no competing interests.
Author Contributions
Willias Masocha conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Animal Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
All animal experiments were approved by the Ethical Committee for the use of Laboratory Animals in Teaching and in Research, HSC, Kuwait University.
Data Deposition
The following information was supplied regarding data availability:
The raw data has been supplied as Supplemental Dataset Files.
References
- Alvarado et al. (2013).Alvarado S, Tajerian M, Millecamps M, Suderman M, Stone LS, Szyf M. Peripheral nerve injury is accompanied by chronic transcriptome-wide changes in the mouse prefrontal cortex. Molecular Pain. 2013;9(1):21. doi: 10.1186/1744-8069-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagal et al. (2015).Bagal SK, Marron BE, Owen RM, Storer RI, Swain NA. Voltage gated sodium channels as drug discovery targets. Channels. 2015;9(6):360–366. doi: 10.1080/19336950.2015.1079674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckh et al. (1989).Beckh S, Noda M, Lübbert H, Numa S. Differential regulation of three sodium channel messenger RNAs in the rat central nervous system during development. EMBO Journal. 1989;8(12):3611–3616. doi: 10.1002/j.1460-2075.1989.tb08534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn-Munro & Fleetwood-Walker (1999).Blackburn-Munro G, Fleetwood-Walker SM. The sodium channel auxiliary subunits β1 and β2 are differentially expressed in the spinal cord of neuropathic rats. Neuroscience. 1999;90(1):153–164. doi: 10.1016/S0306-4522(98)00415-1. [DOI] [PubMed] [Google Scholar]
- Brackenbury & Isom (2008).Brackenbury WJ, Isom LL. Voltage-gated Na+ channels: potential for β subunits as therapeutic targets. Expert Opinion on Therapeutic Targets. 2008;12(9):1191–1203. doi: 10.1517/14728222.12.9.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cata et al. (2006).Cata JP, Weng H-R, Lee BN, Reuben JM, Dougherty PM. Clinical and experimental findings in humans and animals with chemotherapy-induced peripheral neuropathy. Minerva Anestesiologica. 2006;72(3):151–169. [PubMed] [Google Scholar]
- Catterall (2000).Catterall WA. From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron. 2000;26(1):13–25. doi: 10.1016/S0896-6273(00)81133-2. [DOI] [PubMed] [Google Scholar]
- Catterall, Goldin & Waxman (2005).Catterall WA, Goldin AL, Waxman SG. International Union of Pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacological Reviews. 2005;57(4):397–409. doi: 10.1124/pr.57.4.4. [DOI] [PubMed] [Google Scholar]
- Cavaletti et al. (2000).Cavaletti G, Cavalletti E, Oggioni N, Sottani C, Minoia C, D’Incalci M, Zucchetti M, Marmiroli P, Tredici G. Distribution of paclitaxel within the nervous system of the rat after repeated intravenous administration. Neurotoxicology. 2000;21(3):389–393. [PubMed] [Google Scholar]
- Craner et al. (2002).Craner MJ, Klein JP, Renganathan M, Black JA, Waxman SG. Changes of sodium channel expression in experimental painful diabetic neuropathy. Annals of Neurology. 2002;52(6):786–792. doi: 10.1002/ana.10364. [DOI] [PubMed] [Google Scholar]
- Cummins, Sheets & Waxman (2007).Cummins TR, Sheets PL, Waxman SG. The roles of sodium channels in nociception: implications for mechanisms of pain. Pain. 2007;131(3):243–257. doi: 10.1016/j.pain.2007.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devor (2006).Devor M. Sodium channels and mechanisms of neuropathic pain. Journal of Pain. 2006;7(1):S3–S12. doi: 10.1016/j.jpain.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Dib-Hajj, Black & Waxman (2015).Dib-Hajj SD, Black JA, Waxman SG. NaV1.9: a sodium channel linked to human pain. Nature Reviews Neuroscience. 2015;16(9):511–519. doi: 10.1038/nrn3977. [DOI] [PubMed] [Google Scholar]
- Dib-Hajj et al. (1999).Dib-Hajj SD, Fjell J, Cummins TR, Zheng Z, Fried K, LaMotte R, Black JA, Waxman SG. Plasticity of sodium channel expression in DRG neurons in the chronic constriction injury model of neuropathic pain. Pain. 1999;83(3):591–600. doi: 10.1016/S0304-3959(99)00169-4. [DOI] [PubMed] [Google Scholar]
- Gautron et al. (1992).Gautron S, Dos Santos G, Pinto-Henrique D, Koulakoff A, Gros F, Berwald-Netter Y. The glial voltage-gated sodium channel: cell- and tissue-specific mRNA expression. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(15):7272–7276. doi: 10.1073/pnas.89.15.7272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glantz et al. (1995).Glantz MJ, Choy H, Kearns CM, Mills PC, Wahlberg LU, Zuhowski EG, Calabresi P, Egorin MJ. Paclitaxel disposition in plasma and central nervous systems of humans and rats with brain tumors. Journal of the National Cancer Institute. 1995;87(14):1077–1081. doi: 10.1093/jnci/87.14.1077. [DOI] [PubMed] [Google Scholar]
- Hains et al. (2003).Hains BC, Klein JP, Saab CY, Craner MJ, Black JA, Waxman SG. Upregulation of sodium channel Nav1.3 and functional involvement in neuronal hyperexcitability associated with central neuropathic pain after spinal cord injury. Journal of Neuroscience. 2003;23(26):8881–8892. doi: 10.1523/JNEUROSCI.23-26-08881.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hains et al. (2004).Hains BC, Saab CY, Klein JP, Craner MJ, Waxman SG. Altered sodium channel expression in second-order spinal sensory neurons contributes to pain after peripheral nerve injury. Journal of Neuroscience. 2004;24(20):4832–4839. doi: 10.1523/JNEUROSCI.0300-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hains, Saab & Waxman (2005).Hains BC, Saab CY, Waxman SG. Changes in electrophysiological properties and sodium channel Nav1.3 expression in thalamic neurons after spinal cord injury. Brain. 2005;128(10):2359–2371. doi: 10.1093/brain/awh623. [DOI] [PubMed] [Google Scholar]
- Hsieh et al. (1995).Hsieh J-C, Belfrage M, Stone-Elander S, Hansson P, Ingvar M. Central representation of chronic ongoing neuropathic pain studied by positron emission tomography. Pain. 1995;63(2):225–236. doi: 10.1016/0304-3959(95)00048-W. [DOI] [PubMed] [Google Scholar]
- Isom (2001).Isom LL. Sodium channel β subunits: anything but auxiliary. Neuroscientist. 2001;7(1):42–54. doi: 10.1177/107385840100700108. [DOI] [PubMed] [Google Scholar]
- Kemper et al. (2003).Kemper EM, van Zandbergen AE, Cleypool C, Mos HA, Boogerd W, Beijnen JH, van Tellingen O. Increased penetration of paclitaxel into the brain by inhibition of P-Glycoprotein. Clinical Cancer Research. 2003;9(7):2849–2855. [PubMed] [Google Scholar]
- Laedermann et al. (2014).Laedermann CJ, Pertin M, Suter MR, Decosterd I. Voltage-gated sodium channel expression in mouse DRG after SNI leads to re-evaluation of projections of injured fibers. Molecular Pain. 2014;10(1):19. doi: 10.1186/1744-8069-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy-Mozziconacci et al. (1998).Levy-Mozziconacci A, Alcaraz G, Giraud P, Boudier J-A, Caillol G, Couraud F, Autillo-Touati A. Expression of the mRNA for the β2 subunit of the voltage-dependent sodium channel in rat CNS. European Journal of Neuroscience. 1998;10(9):2757–2767. doi: 10.1046/j.1460-9568.1998.00283.x. [DOI] [PubMed] [Google Scholar]
- Lindia et al. (2005).Lindia JA, Köhler MG, Martin WJ, Abbadie C. Relationship between sodium channel NaV1.3 expression and neuropathic pain behavior in rats. Pain. 2005;117(1):145–153. doi: 10.1016/j.pain.2005.05.027. [DOI] [PubMed] [Google Scholar]
- Livak & Schmittgen (2001).Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Masocha (2009).Masocha W. Systemic lipopolysaccharide (LPS)-induced microglial activation results in different temporal reduction of CD200 and CD200 receptor gene expression in the brain. Journal of Neuroimmunology. 2009;214(1–2):78–82. doi: 10.1016/j.jneuroim.2009.06.022. [DOI] [PubMed] [Google Scholar]
- Masocha (2014).Masocha W. Paclitaxel-induced hyposensitivity to nociceptive chemical stimulation in mice can be prevented by treatment with minocycline. Scientific Reports. 2014;4:6719. doi: 10.1038/srep06719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masocha (2015a).Masocha W. Astrocyte activation in the anterior cingulate cortex and altered glutamatergic gene expression during paclitaxel-induced neuropathic pain in mice. PeerJ. 2015a;3:e1350. doi: 10.7717/peerj.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masocha (2015b).Masocha W. Comprehensive analysis of the GABAergic system gene expression profile in the anterior cingulate cortex of mice with Paclitaxel-induced neuropathic pain. Gene Expression. 2015b;16(3):145–153. doi: 10.3727/105221615X14181438356337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashawi et al. (2016).Nashawi H, Masocha W, Edafiogho IO, Kombian SB. Paclitaxel causes electrophysiological changes in the anterior cingulate cortex via modulation of the γ-aminobutyric acid-ergic system. Medical Principles and Practice. 2016;25(5):423–428. doi: 10.1159/000447775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto et al. (2008).Nieto FR, Entrena JM, Cendán CM, Del Pozo E, Vela JM, Baeyens JM. Tetrodotoxin inhibits the development and expression of neuropathic pain induced by paclitaxel in mice. Pain. 2008;137(3):520–531. doi: 10.1016/j.pain.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Ortega-Legaspi et al. (2011).Ortega-Legaspi JM, de Gortari P, Garduño-Gutiérrez R, Amaya M, León-Olea M, Coffeen U, Pellicer F. Expression of the dopaminergic D1 and D2 receptors in the anterior cingulate cortex in a model of neuropathic pain. Molecular Pain. 2011;7(1):97. doi: 10.1186/1744-8069-7-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-Legaspi et al. (2010).Ortega-Legaspi JM, León-Olea M, de Gortari P, Amaya MI, Coffeen U, Simón-Arceo K, Pellicer F. Expression of muscarinic M1 and M2 receptors in the anterior cingulate cortex associated with neuropathic pain. European Journal of Pain. 2010;14(9):901–910. doi: 10.1016/j.ejpain.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Parvathy & Masocha (2013).Parvathy SS, Masocha W. Matrix metalloproteinase inhibitor COL-3 prevents the development of paclitaxel-induced hyperalgesia in mice. Medical Principles and Practice. 2013;22(1):35–41. doi: 10.1159/000341710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson et al. (2009).Persson A-K, Thun J, Xu X-J, Wiesenfeld-Hallin Z, Ström M, Devor M, Lidman O, Fried K. Autotomy behavior correlates with the DRG and spinal expression of sodium channels in inbred mouse strains. Brain Research. 2009;1285:1–13. doi: 10.1016/j.brainres.2009.06.012. [DOI] [PubMed] [Google Scholar]
- Pertin et al. (2005).Pertin M, Ji R-R, Berta T, Powell AJ, Karchewski L, Tate SN, Isom LL, Woolf CJ, Gilliard N, Spahn DR, Decosterd I. Upregulation of the voltage-gated sodium channel beta2 subunit in neuropathic pain models: characterization of expression in injured and non-injured primary sensory neurons. Journal of Neuroscience. 2005;25(47):10970–10980. doi: 10.1523/JNEUROSCI.3066-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters et al. (2007).Peters CM, Jimenez-Andrade JM, Jonas BM, Sevcik MA, Koewler NJ, Ghilardi JR, Wong GY, Mantyh PW. Intravenous paclitaxel administration in the rat induces a peripheral sensory neuropathy characterized by macrophage infiltration and injury to sensory neurons and their supporting cells. Experimental Neurology. 2007;203(1):42–54. doi: 10.1016/j.expneurol.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Rogers et al. (2006).Rogers M, Tang L, Madge DJ, Stevens EB. The role of sodium channels in neuropathic pain. Seminars in Cell & Developmental Biology. 2006;17(5):571–581. doi: 10.1016/j.semcdb.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Schaller & Caldwell (2003).Schaller KL, Caldwell JH. Expression and distribution of voltage-gated sodium channels in the cerebellum. Cerebellum. 2003;2(1):2–9. doi: 10.1080/14734220309424. [DOI] [PubMed] [Google Scholar]
- Scripture, Figg & Sparreboom (2006).Scripture C, Figg W, Sparreboom A. Peripheral neuropathy induced by paclitaxel: recent insights and future perspectives. Current Neuropharmacology. 2006;4(2):165–172. doi: 10.2174/157015906776359568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scuteri et al. (2006).Scuteri A, Nicolini G, Miloso M, Bossi M, Cavaletti G, Windebank AJ, Tredici G. Paclitaxel toxicity in post-mitotic dorsal root ganglion (DRG) cells. Anticancer Research. 2006;26(2A):1065–1070. [PubMed] [Google Scholar]
- Shah et al. (2001).Shah BS, Gonzalez MI, Bramwell S, Pinnock RD, Lee K, Dixon AK. β3, a novel auxiliary subunit for the voltage gated sodium channel is upregulated in sensory neurones following streptozocin induced diabetic neuropathy in rat. Neuroscience Letters. 2001;309(1):1–4. doi: 10.1016/S0304-3940(01)01976-0. [DOI] [PubMed] [Google Scholar]
- Shah et al. (2000).Shah BS, Stevens EB, Gonzalez MI, Bramwell S, Pinnock RD, Lee K, Dixon AK. β3, a novel auxiliary subunit for the voltage-gated sodium channel, is expressed preferentially in sensory neurons and is upregulated in the chronic constriction injury model of neuropathic pain. European Journal of Neuroscience. 2000;12(11):3985–3990. doi: 10.1046/j.1460-9568.2000.00294.x. [DOI] [PubMed] [Google Scholar]
- Theiss & Meller (2000).Theiss C, Meller K. Taxol impairs anterograde axonal transport of microinjected horseradish peroxidase in dorsal root ganglia neurons in vitro. Cell and Tissue Research. 2000;299(2):213–224. doi: 10.1007/s004419900120. [DOI] [PubMed] [Google Scholar]
- Vogt (2005).Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nature Reviews Neuroscience. 2005;6(7):533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westenbroek, Merrick & Catterall (1989).Westenbroek RE, Merrick DK, Catterall WA. Differential subcellular localization of the RI and RII Na+ channel subtypes in central neurons. Neuron. 1989;3(6):695–704. doi: 10.1016/0896-6273(89)90238-9. [DOI] [PubMed] [Google Scholar]
- Whitaker et al. (2000).Whitaker WRJ, Clare JJ, Powell AJ, Chen YH, Faull RLM, Emson PC. Distribution of voltage-gated sodium channel α-subunit and β-subunit mRNAs in human hippocampal formation, cortex, and cerebellum. Journal of Comparative Neurology. 2000;422(1):123–139. doi: 10.1002/(SICI)1096-9861(20000619)422:1<123::AID-CNE8>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Whitaker et al. (2001).Whitaker WRJ, Faull RLM, Waldvogel HJ, Plumpton CJ, Emson PC, Clare JJ. Comparative distribution of voltage-gated sodium channel proteins in human brain. Molecular Brain Research. 2001;88(1–2):37–53. doi: 10.1016/S0169-328X(00)00289-8. [DOI] [PubMed] [Google Scholar]
- Wolf et al. (2008).Wolf S, Barton D, Kottschade L, Grothey A, Loprinzi C. Chemotherapy-induced peripheral neuropathy: prevention and treatment strategies. European Journal of Cancer. 2008;44(11):1507–1515. doi: 10.1016/j.ejca.2008.04.018. [DOI] [PubMed] [Google Scholar]
- Xiao, Naso & Bennett (2008).Xiao W, Naso L, Bennett GJ. Experimental studies of potential analgesics for the treatment of chemotherapy-evoked painful peripheral neuropathies. Pain Medicine. 2008;9(5):505–517. doi: 10.1111/j.1526-4637.2007.00301.x. [DOI] [PubMed] [Google Scholar]
- Xie, Huo & Tang (2009).Xie Y-F, Huo F-Q, Tang J-S. Cerebral cortex modulation of pain. Acta Pharmacologica Sinica. 2009;30(1):31–41. doi: 10.1038/aps.2008.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu & Catterall (2003).Yu FH, Catterall WA. Overview of the voltage-gated sodium channel family. Genome Biology. 2003;4(3):207. doi: 10.1186/gb-2003-4-3-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. (2016).Zhang H, Li Y, de Carvalho-Barbosa M, Kavelaars A, Heijnen CJ, Albrecht PJ, Dougherty PM. Dorsal root ganglion infiltration by macrophages contributes to paclitaxel chemotherapy-induced peripheral neuropathy. Journal of Pain. 2016;17(7):775–786. doi: 10.1016/j.jpain.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Waxman & Hains (2006).Zhao P, Waxman SG, Hains BC. Sodium channel expression in the ventral posterolateral nucleus of the thalamus after peripheral nerve injury. Molecular Pain. 2006;2(1):27. doi: 10.1186/1744-8069-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo (2008).Zhuo M. Cortical excitation and chronic pain. Trends in Neurosciences. 2008;31(4):199–207. doi: 10.1016/j.tins.2008.01.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.