Abstract
A novel quantitative method for coding epochs of active and quiet sleep in infants using respiration is reported. The approach uses the variance of the instantaneous breathing rate within brief epochs of sleep. Variances are normalized within subject by dividing by the 75th percentile variance across epochs. Then, a normalized variance active sleep threshold of 0.29 was determined to produce the highest concordance with a method based on visual inspection of respiratory variability (100% and 90% for quiet and active sleep, respectively). The method was independently validated by comparing to standard polysomnographic state coding (87% and 80% concordance for quiet and active sleep) as well as with behavioral state coding (92% and 78% for quiet and active sleep). Validity was also demonstrated by showing that sleep states identified by the method resulted in the expected state differences in infant heart rate variability and electrocortical activity.
Keywords: human infant, sleep state, breathing rate, automated state scoring, heart rate variability, EEG power
1 INTRODUCTION
Human fetuses and infants exhibit two distinct behavioral states denoted as quiet sleep (QS) and active sleep (AS) (Nijhuis, Prechtl, Martin, & Bots, 1982; Prechtl, 1974). In newborn infants, QS is characterized by highly rhythmic respiration, limited gross body movements except for transient startles, absence of eye movements, and the trace alternans pattern in the electroencephalogram (EEG) (Korotchikova, Stevenson, Livingstone, Ryan, & Boylan, 2016; Sahni et al., 2005). In contrast, AS is characterized by irregular breathing, higher heart rate and heart rate variability, eye movements, gross body movements, twitches, and lower amplitude, continuously variable EEG patterns (Anders, Emde, & Parmelee, 1971). The phenomenology of the two sleep states is well characterized and numerous studies have shown that assessments of infant autonomic regulation and neurophysiology are sleep-state dependent (Harper, Kinney, Fleming, & Thach, 2000; Horne, 2014; Mrowka, Patzak, & Rosenblum, 2000; Myers et al., 1997; Peirano, Algarin, & Uauy, 2003; Sahni et al., 1999, 2000, 2005; Schechtman et al., 1988).
Several methodologies for determination of sleep state (“state coding”) in infants have been developed, with a trend toward more quantitative and less qualitative approaches over recent decades. Historically, systematic infant sleep research involved only the qualitative visual observation of infant behavior such as the regularity, or lack thereof, of an infant's breathing (Wolff, 1959). Prechtl (1974) later broadened the characterization of sleep state by including descriptive observations of additional behaviors that reflect state-dependent differences in central nervous system functioning. Hence, direct behavioral observation became the preferred method to assess infant sleep. But, behavioral observation to classify sleep states requires skilled technicians to complete real time scoring of states that is labor intensive and is usually limited to a few hours during daytime (Sadeh, 2015). Other studies have looked to alternatives such as actigraphy to assess infant sleep (e.g., Sadeh, Acebo, Seifer, Aytur, & Carskadon, 1995; Sazonov, Sazonova, Schuckers, Neuman, & Chime Study Group, 2004; So, Buckley, Adamson, & Horne, 2005; Sung, Adamson, & Horne, 2009). While cost-effective and non-intrusive, the use of actigraphy also has disadvantages, namely, it only measures activity and does not provide data on sleep states, breathing, or specific behaviors. Meltzer, Montgomery-Downs, Insana, and Walsh (2012) also noted that the lack of a standardized approach to the utilization and reporting of actigraphy undercuts its reliability in estimating sleep-wake patterns. Currently, polysomography is considered to be the standard for sleep assessment. Comprehensive polysomnographic sleep studies can employ audio and video recording of the infant as well as the typical recordings of respiration, electroencephalography (EEG), electrocardiography (ECG), electromyography (EMG), and electrooculography (EOG). However, the instrumentation required in studies of this detail is only found in dedicated sleep laboratories (Grigg-Damberger et al., 2007). Furthermore, polysomnography requires subjects to tolerate multiple electrodes and sensors along with sleeping in a foreign, intrusive unnatural sleeping environment which poses a challenge to normal sleep patterns and is a major limitation (Mouthon & Huber, 2015). Thus, polysomnography is not a practical option for most infant assessment protocols particularly in the newborn nursery or in home monitoring studies, (Montgomery-Downs, Insana, & Bond, 2012).
Recent advances in technology have allowed more physiological data to be collected, processed, and available for clinicians and doctors to assist them in decision-support when monitoring their patients (Liddle et al., 2012; Smith et al., 2014). These large datasets also benefit researchers by providing an opportunity to mine data for new insights in a variety of fields. But analyses of large datasets require automation for practical application. For infant research, various methods have been explored to develop sleep state coding techniques that require only a subset of standard polysomnographic measures (e.g., Haddad, Jeng, Lai, & Mellins, 1987; Harper, Schechtman, & Kluge, 1987; Myers et al., 1997).
Because cardiorespiratory studies on infants can be done in a variety of settings (hospital ward, intensive care unit, or home environment), it is easier to accurately collect good quality cardiorespiratory signals as opposed to signals such as EEG, EOG, or EMG (Southall et al., 1983). Because animal and human models have long established that breathing regulation is modulated in a state-dependent manner, the use of respiratory physiological signals to classify and automate infant sleep provides a promising alternative to polysomnography (Bolton & Herman, 1974; Dawes, Fox, Leduc, Liggins, & Richards, 1972; Hoppenbrouwers, Harper, Hodgman, Sterman, & McGinty, 1978; Stephenson, Liao, Hamrahi, & Horner, 2001; Snyder, Hobson, Morrison, & Goldfrank, 1964). In particular, prior studies have noted that inter-breath intervals are characteristically different between infant active and quiet sleep (Al-Hathlol et al., 2000; Terrill, Wilson, Suresh, & Cooper, 2009; Tobin, Mador, Guenther, Lodato, & Sackner, 1988). More recently, Elder, Campbell, Larsen, and Galletly (2011) confirmed that sleep state is the predominant influence on respiratory variability in healthy term and convalescent preterm infants. Terrill, Wilson, Suresh, Cooper, and Dakin (2012) have shown that using quantification of nonlinear property of respiration alone can achieve sleep state coding that agrees with the polysomnographic standard 80% of the time. This is consonant with an earlier report which found that variation in breathing rate was the single best correlate of infant sleep states (Harper et al., 1987).
In the present study, we developed a quantitative method for automated coding of infant sleep states based on variation in respiratory rate alone. Optimization of the key breathing rate parameter for the method (a threshold based on the normalized variance) was initially conducted by comparing concordance rates between automated and visual state coding in a large number of recordings obtained from a heterogeneous cohort of newborn infants. We then assessed performance of this method by comparing results to a study that used a traditional clinical polysomnographic approach (Crowell et al., 1997). We also applied our method to two extant data sets from newborn sleep studies conducted in our laboratories at Columbia University Medical Center. Finally, as further validation of our method, we computed measures of heart rate variability (HRV) and high frequency EEG power for each coded minute of sleep.
2 METHODS
2.1 Participants
Three groups of infants (a total of 49 subjects) with extant physiological data recordings and which had been sleep state coded employing a range of coding methodologies were used to validate our respiration-based method. Group I used data obtained from the publically available dataset of the Collaborative Home Infant Monitoring Evaluation (Crowell et al., 1997). The CHIME study used a comprehensive clinical state coding method. Each infant underwent an overnight ~8 hr polysomnographic recording with EEG, EMG, EOG, and respiration recorded using the Healthdyne ALICE3 system (versions 1.17 and 1.19) at sampling rates of 100, 100, 40, and 40 Hz, respectively. Data were coded for sleep state in 30 s epochs by experts using the guidelines defined by Anders et al. (1971). For this validation, a subset of 8 CHIME infants recorded at full-term equivalent age, with gestational ages ranging from 26 to 42 weeks (mean [standard deviation] 37.5 [5.4] weeks), were selected based on the requirement that active and quiet sleep states were observed in each subject and that the respiration signal was of usable quality.
Data for Group II were obtained from a set of 20 pre-term infants born between 26 and 34 weeks GA and which were studied near to term age (37–44 weeks post conceptional age) as part of the Family Nurture Intervention study (Welch et al., 2012) conducted at the Columbia University Medical Center (CUMC). Parental consent was obtained with all procedures approved by the local Institutional Review Board. Sixty-minute physiological recordings (EEG, ECG, and respiration) were obtained within 30 min after feeding between 11 am and 4 pm. During the studies, specially trained research assistants assigned one of five state codes (AS, QS, indeterminate, awake, cry) for each minute epoch based on previously established behavioral criteria (Stefanski et al., 1984). ECG and respiration data were collected at 1,000 and 20 Hz, respectively, using custom data acquisition software (Medelex, Inc., New York, NY). R-wave peaks in the ECG were marked automatically (Ledano Solutions, Inc., New York, NY) and visually corrected if needed. R-to-R intervals (RRI) were obtained by measuring times between successive peaks. In this study, EEG was simultaneously recorded with Electrical Geodesic's (EGI, Inc., Eugene, OR) 128-electrode dense array data acquisition system. The EEG was sampled at 1,000 Hz using a vertex reference. During recording, voltage from each lead was band-pass filtered from 0.1 to 400 Hz and digitized with 16 bits per sample. Other details of EEG acquisition are described in prior work (Grieve et al., 2008).
Group III data were obtained from a cohort of 21 full-term infants with recordings made in the Newborn Nursery at CUMC. Parental consent was obtained and all procedures were approved by the local Institutional Review Board. These infants had 10 min of baseline data collection before undergoing a 40 min tilting procedure as part of a large ongoing study of newborn sleep dependent physiology and behavior. ECG, respiration, and EEG were collected using the same systems and analysis procedures as in Group II (except that ECG was sampled at 500 Hz). One of four sleep state codes (AS, QS, indeterminate, and awake) was assigned for each 60 s epoch based on offline visual inspection of the variability in the respiratory signal (similar to that used in the PASS learning set described in the next section) and supplemented with behavioral observations noted during the recordings.
For all three groups, a smoothing process was applied to the manually coded states whereby states were required to persist for a minimum duration of 3 min. If they did not persist, the current state remained unchanged until a new state lasting at least 3 min occurred (Prechtl, 1974).
2.2 Design of automated state coding
The initial derivation of an optimal parameter for the automated state coding method made use of data from a large ongoing study being conducted by the Prenatal Alcohol in SIDS and Stillbirth Network (PASS) which included collection of human infant physiology within the first 4 days of life (Dukes et al., 2014). Respiratory data were obtained from 1,167 newborn infants during recording sessions that lasted ~ 45 min. These infants were a heterogeneous subset comprised of mixed racial, ethnic, and socioeconomic demographics as well as prenatal environmental and exposure conditions. Respiratory data were collected by means of a respiratory inductance belt (Ambulatory Monitoring Inc., Ardsley, NY) and were digitized at 20 samples/sec. The respiratory tracings were displayed and the peak inspiratory value for each breath was marked using specially designed software. After visual verification of these marks, the instantaneous breathing rate (IBR) was computed and displayed. These IBR tracings showed clear contrasts between periods of low and high variability that we and others take to correspond to periods of QS and AS (Figure 1). Each of these records was visually coded with start and stop times for each period of QS and AS.
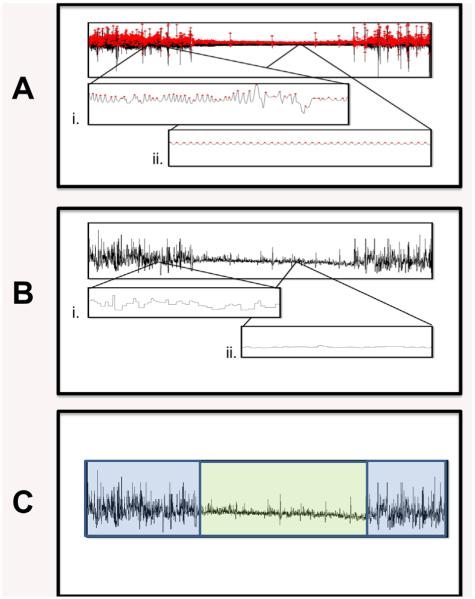
FIGURE 1.
Illustration of manual respiration-based state coding in a typical recording from Group II. Visual inspection of the respiratory waveform (A) reveals distinct contrasts, presumed to be due to active sleep (i) and quiet sleep (ii). Peaks of the waveform are marked by software and inspected visually for errors. Breath-to-breath intervals (B) are derived from the peak times and inverted to obtain the instantaneous breath rate (IBR) which has periods of higher (i) and lower (ii) variability. Recording-wide inspection of IBR (C) is used to assign times to either active (blue) or quiet (green) sleep
The relative ease with which this visual coding of state was accomplished suggested that a quantitative threshold could be derived and used to delineate state based on variance of IBR. Accordingly, we used the data from the PASS infants to find the AS/QS threshold that would provide the best concordance between the automated and visual coding methods. The procedure first involved computing the variance of IBRs within each 60 s epoch for each recording, after removing IBR values greater than five times the interquartile range from the median. The next step was to standardize (normalize) the variances within each study such that the ranges from one subject to another would be similar. Although a standard approach would be to divide each value by the range, variances were positively skewed and ranges can be influenced by high outlier values. We found that the 75th percentile was highly correlated with the range but less affected by the high outliers. Accordingly, the variance of each epoch was divided by the 75th percentile of the variances across all epochs. By iterative variation of AS/QS thresholds, it was determined that a normalized variance threshold of 0.29 dichotomized the epochs into states that produced the best agreement with sleep states originally assigned by visually coded IBRs. This threshold produced a median agreement of 100% with ~75% of records having greater than 90% concordance. This threshold was then applied to three independent groups of infants to validate the method as described below.
2.3 Instrumentation of automated coding
For all three groups, the acquired respiration signal was smoothed with a three-sample moving average. Custom software (Ledano Solutions, Inc.) was used to mark the peaks in the resulting respiratory waveform. Marked peaks were verified by visual inspection and corrected if needed. The breath-to-breath intervals derived from subtracting successive peak times were then inverted to produce the instantaneous breathing rate (IBR). IBR values greater than five times the interquartile range from the median were considered outliers and removed. The normalized variance of each 60 s epoch (30 s epoch for the CHIME data set) of the IBR signal was then calculated as described above. For each subject within each group, sleep state codes were generated epoch by epoch using the optimal normalized variance AS/QS threshold determined from the PASS data. The generated state codes were then aligned with the manually coded states for each group. A typical example of this procedure is shown in Figure 2 for one subject in Group II.
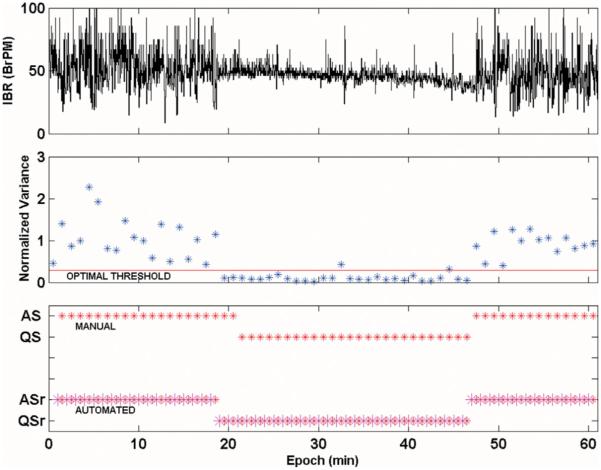
FIGURE 2.
Illustration of automated respiration-based state coding and concordance analysis in the same recording used in Figure 1. The top panel displays the IBR and the middle panel shows the minute by minute normalized variances and the optimal threshold (0.29, red line; see text) for automated sleep state assignment. The bottom panel shows the automatically coded states aligned and compared with the manually coded states, from which concordance is calculated. ASr (QSr) denote respiration-based automated state codes, whereas AS (QS) denote manual, non-automated state codes
2.4 Sleep analysis
To compare automated state coding to manual coding in each subject, we calculated the percent concordance as the percentage of automatically coded epochs of each sleep state that matched the corresponding manually coded state. For each state, the percent agreement was found for each subject and its median value across subjects within each group was calculated.
Sensitivity and specificity were also calculated for each state. Sensitivity was calculated by taking the total number of true positives over the sum of true positive and false negative epochs. Specificity was calculated by taking the total number of true negatives divided by the sum of true negatives and false positives.
2.5 Heart rate variability analysis
For both Groups II and III, each R-wave in the recorded ECG of each subject was marked using custom software (Ledano Solutions, Inc.) and visually corrected if needed. As a measure of HRV, the standard deviations of the RR-intervals (RRISD) were computed for every 30 or 60 s epoch. Mean differences in RRISD between the two states (AS vs. QS) within each method (automated and manual) were compared using Student's t-tests. Similarly, mean differences in RRISD between the two methods for each state were also compared using Student's t-tests.
2.6 EEG analysis
For each electrode, the power spectrum of the EEG was computed using methods specified by Grieve et al. (2008) for successive epochs. In this instance, mean power of 60 FFTs applied to 1 s non-overlapping segments after half-cosine windowing. To control for variability of raw EEG power values across subjects, we took the ratio of mean EEG power in the beta band (16 to 30 Hz) to that in the delta band (1 to 4 Hz). We used this ratio because comprehensive adult sleep studies have shown that this measure of relative high frequency EEG power is the single best EEG metric to discriminate between sleep states (Krakovska & Mezeiova, 2011; Susmakova & Krakovska, 2008). Median EEG power at each frequency over all non-excluded electrodes was used to compute the beta to delta power ratio for each epoch. For each subject, the median beta/delta power across epochs was found for each sleep state. For Groups II and III, Student's t-tests were used to test for significant differences between combinations of coding methods and sleep states.
3 RESULTS
3.1 Concordance with non-automated methods
The median concordance for AS and QS in each Group is shown in Table 1. The median percent concordance within Group I was 80% an 87% for AS and QS, respectively. For the behaviorally coded Group II, the respective concordances were 78% and 92%, while for the visually respiratory coded group (Group III), they were 90% and 100%. In all three groups, QS had higher concordance than AS. The interquartile ranges for AS and QS in all groups were also reported. The interquartile ranges for AS in Groups I, II, and III were 70–90%, 60–95%, and 76–100% respectively.
TABLE 1.
Sleep state concordance for active and quiet sleep (AS/QS)
| Group I N = 8 | Group II N = 20 | Group III N = 21 | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| AS | QS | AS | QS | AS | QS | |
|
| ||||||
| Median concordance (%) | 80 | 87 | 78 | 92 | 90 | 100 |
|
| ||||||
| Interquartile range | 70–90 | 69–93 | 60–95 | 75–100 | 76–100 | 100 |
Automated sleep states were compared to non-automated states coded bypolysomnography (Group I), behavioral observation (Group II), or visual inspection of the respiratory recording (Group III). The median percent concordances in all three groups were above 75% for both active and quiet sleep. Interquartile ranges (IQR) are shown in the bottom row. The 25th percentile for quiet sleep in Group III was 100%.
To test whether the method would be valid for various study lengths, we checked whether there was any correlation between study length and concordance within Group I (Groups II and III had similar study lengths of ~ 1 hr, while the studies in Group I were more variable). We found that for neither state was concordance in that group correlated with study length (p = 0.38 and 0.25 for AS and QS, respectively).
3.2 Sensitivity and specificity
The sensitivity and specificity percentages for AS and QS in each Group is shown in Table 2. The sensitivity for all groups was higher than the specificity for AS (83%, 86%, and 99% vs. 78%, 68%, and 80% for Groups I, II, and III, respectively). For QS the reverse was true, with the sensitivity for all groups lower than the specificity (78%, 68%, and 80% vs. 83%, 86%, and 99% for Groups I, II, and III, respectively).
TABLE 2.
Sensitivity and secificity for active and quiet sleep (AS/QS)
| Group I N = 8 |
Group II N = 20 |
Group III N = 21 |
||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| AS | QS | AS | QS | AS | QS | |
|
| ||||||
| Sensitivity (%) | 83 | 78 | 86 | 68 | 99 | 80 |
|
| ||||||
| Specificity (%) | 78 | 83 | 68 | 86 | 80 | 99 |
Specificity and sensitivity percentages were calculated for all groups. Sensitivity values in AS and specificity values in QS were higher in all groups while sensitivity values in QS and specificity values in QS were lower in all groups.
3.3 HRV validation
As predicted, group mean variability of heart rate as quantified by RRISD was higher in AS compared to QS in both Groups II and III (see Figure 3). Automated coding based on respiratory variability in both Groups II and III for AS and QS was denoted as ASr and QSr to differentiate it against the original manual state coding. Mean variability of heart rate for Group II for manual and automated are presented in the upper panel while mean variability of heart rate for Group III for both methods are displayed on the bottom panel. Sleep state differences were significant in Group II for both methods of state coding (p = .0002 and .001 for manual and automated coding, respectively). In Group III, there was a significant difference for automated coding, but not for manual coding (p = .006 and .06, respectively). For each state, there were no significant RRISD differences between manual and automated state coded minutes.
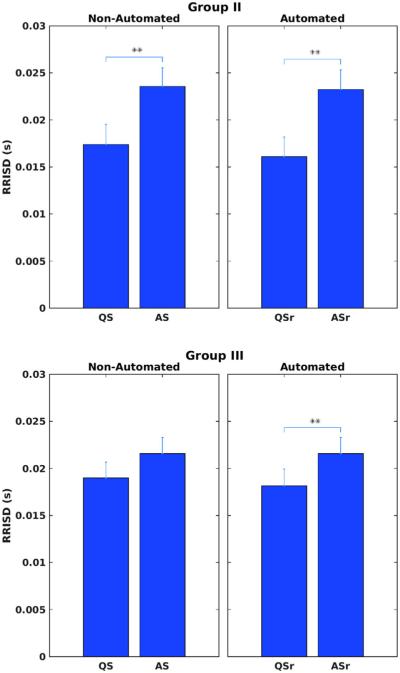
FIGURE 3.
Automated sleep state coding for Groups II (upper panel) and III (lower panel) were validated by how well they distinguished heart rate variability (RRISD) between sleep states by Student's t-tests, compared with the same tests using non-automated state coding. Single (double) asterisks denote p values <.05 (.01). ASr(QSr) denote respiration-based automated state codes, whereas AS (QS) denoted manual, non-automated state codes. RRISD was significantly higher in active versus quiet sleep in both groups except for non-automated codes in Group III
3.4 EEG validation
As hypothesized, group mean high frequency EEG power as quantified by the beta/delta ratio was higher in AS compared to QS in both Groups II and III for both automated and manual state coding (see Figure 4). Again, automated coding based on respiratory variability for AS and QS was denoted as ASr and QSr in both groups. The state difference in both groups was significant for both manual and automated coding (Group II: p = .00006 and .000024, respectively; Group III: p = .0094 and .036, respectively). For each state, there were no significant beta/delta power differences between manual and automated state coded epochs.
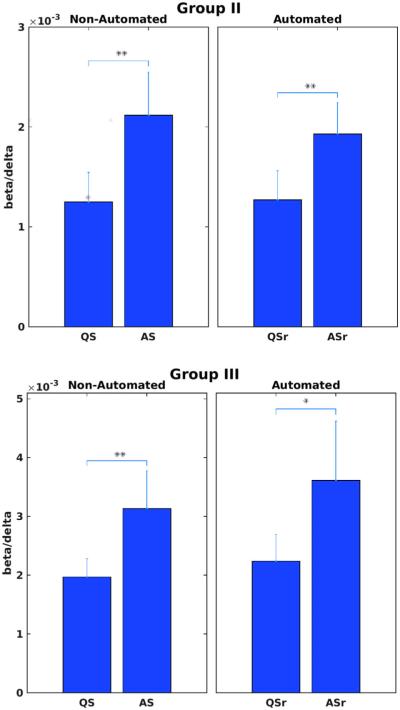
FIGURE 4.
Automated sleep state coding for Groups II (upper panel) and III (lower panel) were validated by how well they distinguish high frequency content in the EEG (beta/delta power ratio) between sleep states by Student's t-tests, compared with the same tests using non-automated state coding. Single (double) asterisks denote p values <.05 (.01). ASr (QSr) denote respiration-based automated state codes, whereas AS (QS) denote manual, non-automated state codes. The beta/delta power ratio was significantly higher in active versus quiet sleep in both groups
4 DISCUSSION
We developed a quantitative method to automatically code for quiet versus active sleep using only respiratory variability, designed for use in relatively brief (~ 1 hr) infant studies. Concordance with other state coding methods was approximately 80% or better. Previous studies have also developed automated sleep state coding strategies for use in infancy based on fewer signals than the full suite of measures used in the standard polysomnographic studies. Based on a prior visual method (Sahni, Schulze, Stefanski, Myers, & Fifer, 1995), Myers et al. (1997) developed an algorithm to automatically detect trace alternans episodes in the EEG and assign 1 min epochs to QS or AS based on the presence or absence of that EEG pattern. They found concordance with behaviorally coded state of 82% and 79% for QS and AS.
Several infant sleep studies developed state coding methods based solely on respiratory activity, as we do here. Haddad et al. (1987) used a statistical decision rule in a suite of cardiorespiratory measures (mean and coefficient of variation of RR intervals, inter-breath intervals (IBI), and tidal volume) in nine 1-month-old infants. Using their algorithm, they found that the coefficient of variability of the IBI was sufficient to achieve 93% (QS) and 99% (AS) concordance with standard polysomnographic manual coding. In 25 one-week-old infants, Harper et al. (1987) performed discriminant analysis on seven cardiorespiratory measures (median and interquartile range of IBR and heart rate (HR) and three RR measures) with 12 infants as a training set and 13 as a validation set. They found concordances of 84% (QS) and 88% (AS) using all seven variables. Using just the two respiratory measures the corresponding concordances were 83% and 85%, suggesting respiratory measures alone are sufficient to code state. Another study that used a machine learning approach was Sazonova, Sazonov, Tan, and Schuckers (2006). They used movement and respiratory measurements (maximum absolute value and standard deviation of accelerometer signal and tidal volume and mean and standard deviation of IBI) to classify state in a two-stage process in 26 infants (13 made up the training set and 13 the validation set). First, they derived a model to distinguish wake from sleep, and then used a second model to classify AS and QS within sleep. The best single classifier of AS/QS was the coefficient of variation of IBI, with a validation rate of 70%. Most recently, Terrill et al. (2012) used a nonlinear dynamical measure of IBI data to classify 30 s epochs as AS, QS or awake. Their method achieved a concordance with standard polysomnographic manual coding of 80% across the three states in 25 two-week-old infants.
In comparison to the above referenced efforts to automate state coding in infants, our results with concordances of 87% (QS) and 80% (AS) are within the range found in other studies. One reason that most of these methods, including ours, often achieve concordance of no better than 80–90% with the standard may be because regular polysomnographic studies rely on state coders to make subjective judgments about the presence or absence of certain state markers (e.g., specific patterns in the EEG). As a result, inter-rater reliability is also in the range of 80–90% (Grigg-Damberger et al., 2007).
Of the other studies mentioned, the most similar to ours was Haddad et al. (1987). That study used a threshold on the coefficient of variation of the IBI for AS/QS determination, very similar to our use of a threshold on normalized IBR variance for the same purpose. Both approaches have the virtue of using rapidly computed quantities on easily recorded data to determine sleep state. A further strength of the approach taken here is that our threshold was determined using an optimization procedure on a very large group (>1,000) of sleeping newborn infants.
Of the three Groups used for this paper, median concordance was highest (100% for QS, 90% for AS) in Group III. This is not surprising, as we developed our method explicitly to automate a manual procedure based on visual inspection of IBR variability that was initially used for that group.
Sensitivity values calculated for all three groups underscore a higher sensitivity for AS as opposed to QS while specificity was higher for QS. Thus, if the goal when applying this method is to determine group differences in a state dependent variable (e.g., heart rate variability), results for QS will be less affected by uncertainty of state coding.
For independent validation of our state coding method, we examined two physiological markers of infant sleep state: heart rate variability and high frequency EEG power. Using RRI-SD as a measure of the former and the beta/delta power ratio as a measure of the latter, we expected that both would be higher in AS than in QS. Indeed, in all cases for both groups, that is what we found. Interestingly, p values suggested that using our automated state coding resulted in more robust discrimination between states in the majority of these measures than using manually coded state.
In conclusion, a quantitative method was developed for coding AS and QS in infants using only respiratory variability. It was designed to automate a method based on visual inspection of respiratory variability, and it achieved that goal with 100% and 90% reliability for QS and AS, respectively. It was validated in two ways: by its high concordance with standard state scoring and by the ability to detect predicted sleep state differences in neonatal physiology, namely higher variability of heart rate and greater high frequency EEG power in AS versus QS. Because the approach does not require the full suite of physiological measures necessary for polysomnographic studies, it offers an automated method of state coding for both short and long duration studies at a low cost by relying on an easy-to-compute measure (variance). Thus, this reliable estimate of sleep state from respiration alone may be particularly efficient given recent advances in home monitoring technologies used to study sleep state-dependent processes.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the PASS Research Network for providing access to data used in the initial derivation of parameters for the automated coding method. We also thank Dr. Martha Welch, Director of the Nurture Science Program at Columbia University Medical Center, and the Einhorn Family Charitable Trust for providing access to and funding for the Group II data that were used for validating this method. In addition, we would like to acknowledge the following individuals for their input and support with respect to many aspects of this project: Raymond Stark, Philip Grieve, Joel Yang, J. David Nugent, Carmen Condon, Albany Perez, Julia Chafkin, Maria Ordonez Retamar, Margaret Shair, Daianna Rodriguez, and Cynthia Rodriguez. The writing of this manuscript was supported by the Sackler Institute of Developmental Psychobiology at Columbia University and by National Institute of Health Grant R37 HD032774 (to W. P. F).
Funding information
National Institute of Health, Grant number: R37 HD032774; Einhorn Family Charitable Trust; Director of the Nurture Science Program
REFERENCES
- Al-Hathlol K, Idiong N, Hussain A, Kwiatkowski K, Alvaro RE, Weintraub Z, Rigatto H. A study of breathing pattern and ventilation in newborn infants and adult subjects. Acta Paediatrica. 2000;89:1420–1425. doi: 10.1080/080352500456570. [DOI] [PubMed] [Google Scholar]
- Anders TF, Emde RN, Parmelee AH. A manual of standardized terminology, techniques and criteria for scoring of states of sleep and wakefulness in newborn infants. UCLA Brain Information Service/BRI Publications Office, NINDS Neurological Information Network; Los Angeles, CA: 1971. [Google Scholar]
- Bolton DP, Herman S. Ventilation and sleep state in the new-born. The Journal of Physiology. 1974;240:67–77. doi: 10.1113/jphysiol.1974.sp010599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell DH, Brooks LJ, Colton T, Corwin MJ, Hoppenbrouwers TT, Hunt CE, Ward SL. Infant polysomnography: Reliability. Collaborative Home Infant Monitoring Evaluation (CHIME) Steering Committee. Sleep. 1997;20:553–560. [PubMed] [Google Scholar]
- Dawes G, Fox HE, Leduc BM, Liggins GC, Richards RT. Respiratory movements and rapid eye movement sleep in the foetal lamb. The Journal of Physiology. 1972;220:119–143. doi: 10.1113/jphysiol.1972.sp009698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukes KA, Burd L, Elliott AJ, Fifer WP, Folkerth RD, Hankins GD, Kinney HC. The safe passage study: Design, methods, recruitment, and follow-up approach. Paediatric and Perinatal Epidemiology. 2014;28:455–465. doi: 10.1111/ppe.12136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder DE, Campbell AJ, Larsen PD, Galletly D. Respiratory variability in preterm and term infants: Effect of sleep state, position and age. Respiratory Physiology & Neurobiology. 2011;175:234–238. doi: 10.1016/j.resp.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Grieve PG, Isler JR, Izraelit A, Peterson BS, Fifer WP, Myers MM, Stark RI. EEG functional connectivity in term age extremely low birth weight infants. Clinical Neurophysiology. 2008;119:2712–2720. doi: 10.1016/j.clinph.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigg-Damberger M, Gozal D, Marcus CL, Quan SF, Rosen CL, Chervin RD, Iber C. The visual scoring of sleep and arousal in infants and children. Journal of Clinical Sleep Medicine. 2007;3:201–240. [PubMed] [Google Scholar]
- Haddad GG, Jeng HJ, Lai TL, Mellins RB. Determination of sleep state in infants using respiratory variability. Pediatric Research. 1987;21:556–562. doi: 10.1203/00006450-198706000-00010. [DOI] [PubMed] [Google Scholar]
- Harper RM, Schechtman VL, Kluge KA. Machine classification of infant sleep state using cardiorespiratory measures. Electroencephalography and Clinical Neurophysiology. 1987;67:379–387. doi: 10.1016/0013-4694(87)90126-x. [DOI] [PubMed] [Google Scholar]
- Harper RM, Kinney HC, Fleming PJ, Thach BT. Sleep influences on homeostatic functions: Implications for sudden infant death syndrome. Respiration Physiology. 2000;119:123–132. doi: 10.1016/s0034-5687(99)00107-3. [DOI] [PubMed] [Google Scholar]
- Hoppenbrouwers T, Harper RM, Hodgman JE, Sterman MB, McGinty DJ. Polygraphic studies on normal infants during the first six months of life. II. Respiratory rate and variability as a function of state. Pediatric Research. 1978;12:120–125. doi: 10.1203/00006450-197802000-00011. [DOI] [PubMed] [Google Scholar]
- Horne RS. Cardio-respiratory control during sleep in infancy. Paediatric Respiratory Reviews. 2014;15:163–169. doi: 10.1016/j.prrv.2013.02.012. [DOI] [PubMed] [Google Scholar]
- Korotchikova I, Stevenson NJ, Livingstone V, Ryan CA, Boylan GB. Sleep-wake cycle of the healthy term newborn infant in the immediate postnatal period. Clinical Neurophysiology. 2016;127:2095–2101. doi: 10.1016/j.clinph.2015.12.015. [DOI] [PubMed] [Google Scholar]
- Krakovska A, Mezeiova K. Automatic sleep scoring: A search for an optimal combination of measures. Artificial Intelligence in Medicine. 2011;53:25–33. doi: 10.1016/j.artmed.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Liddle S, Grover L, Zhang R, Khitrov M, Brown JC, Cobb JP, Reisner AT. Safety evaluation of a medical device data system. Engineering in Medicine and Biology Society (EMBC); 2012 Annual International Conference of the IEEE; IEEE; 2012. pp. 5899–5902. [DOI] [PubMed] [Google Scholar]
- Meltzer LJ, Montgomery-Downs HE, Insana SP, Walsh CM. Use of actigraphy for assessment in pediatric sleep research. Sleep Medicine Reviews. 2012;16:463–475. doi: 10.1016/j.smrv.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouthon AL, Huber R. Methods in pediatric sleep research and sleep medicine. Sleep. 2015;14:7. doi: 10.1055/s-0035-1550232. [DOI] [PubMed] [Google Scholar]
- Montgomery-Downs HE, Insana SP, Bond JA. Movement toward a novel activity monitoring device. Sleep and Breathing. 2012;16:913–917. doi: 10.1007/s11325-011-0585-y. [DOI] [PubMed] [Google Scholar]
- Mrowka R, Patzak A, Rosenblum M. Quantitative analysis of cardiorespiratory synchronization in infants. International Journal of Bifurcation and Chaos. 2000;10:2479–2488. [Google Scholar]
- Myers MM, Fifer WP, Grose-Fifer J, Sahni R, Stark RI, Schulze KF. A novelquantitative measure of Tracé-alternant EEG activity and its association with sleep states of preterm infants. Developmental Psychobiology. 1997;31:167–174. doi: 10.1002/(sici)1098-2302(199711)31:3<167::aid-dev1>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Nijhuis JG, Prechtl HF, Martin CJ, Bots RSGM. Are there behavioural states in the human fetus? Early Human Development. 1982;6:177–195. doi: 10.1016/0378-3782(82)90106-2. [DOI] [PubMed] [Google Scholar]
- Peirano P, Algarın C, Uauy R. Sleep-wake states and their regulatory mechanisms throughout early human development. The Journal of Pediatrics. 2003;143:70–79. doi: 10.1067/s0022-3476(03)00404-9. [DOI] [PubMed] [Google Scholar]
- Prechtl HF. The behavioural states of the newborn infant (a review) Brain Research. 1974;76:185–212. doi: 10.1016/0006-8993(74)90454-5. [DOI] [PubMed] [Google Scholar]
- Sadeh A. sleep assessment methods. Monographs of the Society for Research in Child Development. 2015;80:33–48. doi: 10.1111/mono.12143. Iii. [DOI] [PubMed] [Google Scholar]
- Sadeh A, Acebo C, Seifer R, Aytur S, Carskadon MA. Activity-based assessment of sleep-wake patterns during the 1st year of life. Infant Behavior and Development. 1995;18:329–337. [Google Scholar]
- Sahni R, Schulze KF, Stefanski M, Myers MM, Fifer WP. Methodological issues in coding sleep states in immature infants. Developmental Psychobiology. 1995;28:85–101. doi: 10.1002/dev.420280203. [DOI] [PubMed] [Google Scholar]
- Sahni R, Schulze KF, Kashyap S, Ohira-Kist K, Myers MM, Fifer WP. Body position, sleep states, and cardiorespiratory activity in developing low birth weight infants. Early Human Development. 1999;54:197–206. doi: 10.1016/s0378-3782(98)00104-2. [DOI] [PubMed] [Google Scholar]
- Sahni R, Schulze KF, Kashyap S, Ohira-Kist K, Fifer WP, Myers MM. Maturational changes in heart rate and heart rate variability in low birth weight infants. Developmental Psychobiology. 2000;37:73–81. doi: 10.1002/1098-2302(200009)37:2<73::aid-dev2>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Sahni R, Schulze KF, Kashyap S, Ohira-Kist K, Fifer WP, Myers MM. Sleeping position and electrocortical activity in low birthweight infants. Archives of Disease in Childhood-Fetal and Neonatal Edition. 2005;90:F311–F315. doi: 10.1136/adc.2004.055327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sazonov E, Sazonova N, Schuckers S, Neuman M, CHIME Study Group Activity-based sleep? Wake identification in infants. Physiological Measurement. 2004;25:1291. doi: 10.1088/0967-3334/25/5/018. [DOI] [PubMed] [Google Scholar]
- Sazonova NA, Sazonov EE, Tan B, Schuckers SA. Sleep state scoring in infants from respiratory and activity measurements. Engineering in Medicine and Biology Society; EMBS'06. 28th Annual International Conference of the IEEE; IEEE; 2006. 2006. pp. 2462–2465. [DOI] [PubMed] [Google Scholar]
- Schechtman VL, Harper RM, Kluge KA, Wilson AJ, Hoffman HJ, Southall DP. Cardiac and respiratory patterns in normal infants and victims of the sudden infant death syndrome. Sleep. 1988;11:413–424. doi: 10.1093/sleep/11.5.413. [DOI] [PubMed] [Google Scholar]
- SO K, Buckley P, Adamson TM, Horne RS. Actigraphy correctly predicts sleep behavior in infants who are younger than six months, when compared with polysomnography. Pediatric Research. 2005;58:761–765. doi: 10.1203/01.PDR.0000180568.97221.56. [DOI] [PubMed] [Google Scholar]
- Southall DP, Richards JM, Shinebourne EA, Franks CI, Wilson AJ, Alexander JR. Prospective population-based studies into heart rate and breathing patterns in newborn infants: Prediction of infants at risk of SIDS. In: Tildon JY, Roeder LM, Steinschneider A, editors. Sudden infant death syndrome. Academic Press; New York, NY: 1983. pp. 621–651. [Google Scholar]
- Stefanski M, Schulze K, Bateman D, Kairam R, Pedley TA, Masterson J, James LS. A scoring system for states of sleep and wakefulness in term and preterm infants. Pediatric Research. 1984;18:58–62. [PubMed] [Google Scholar]
- Stephenson R, Liao KS, Hamrahi H, Horner RL. Circadian rhythms and sleep have additive effects on respiration in the rat. The Journal of Physiology. 2001;536:225–235. doi: 10.1111/j.1469-7793.2001.00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susmakova K, Krakovska A. Discrimination ability of individual measures used in sleep stages classification. Artificial Intelligence in Medicine. 2008;44:261–277. doi: 10.1016/j.artmed.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Smith JB, Reisner AT, Edla S, Liu J, Liddle S, Reifman J. A platform for real-time acquisition and analysis of physiological data in hospital emergency departments. Engineering in Medicine and Biology Society (EMBC); 36th Annual International Conference of the IEEE; IEEE; 2014. 2014. pp. 2678–2681. [DOI] [PubMed] [Google Scholar]
- Snyder F, Hobson JA, Morrison DF, Goldfrank F. Changes in respiration, heart rate, and systolic blood pressure in human sleep. Journal of Applied Physiology. 1964;19:417–422. doi: 10.1152/jappl.1964.19.3.417. [DOI] [PubMed] [Google Scholar]
- Sung M, Adamson TM, Horne RS. Validation of actigraphy for determining sleep and wake in preterm infants Acta Paediatrica. 2009;98:52–57. doi: 10.1111/j.1651-2227.2008.01002.x. [DOI] [PubMed] [Google Scholar]
- Terrill PI, Wilson SJ, Suresh S, Cooper DM, Dakin C. Application of recurrence quantification analysis to automatically estimate infant sleep states using a single channel of respiratory data. Medical & Biological Engineering & Computing. 2012;50:851–865. doi: 10.1007/s11517-012-0918-4. [DOI] [PubMed] [Google Scholar]
- Terrill PI, Wilson SJ, Suresh S, Cooper DM. Characterising infant inter-breath interval patterns during active and quiet sleep using recurrence plot analysis. Engineering in Medicine and Biology Society; EMBC 2009. Annual International Conference of the IEEE; IEEE; 2009. 2009. pp. 6284–6287. [DOI] [PubMed] [Google Scholar]
- Tobin MJ, Mador MJ, Guenther SM, Lodato RF, Sackner MA. Variability of resting respiratory drive and timing in healthy subjects. Journal of Applied Physiology. 1988;65:309–317. doi: 10.1152/jappl.1988.65.1.309. [DOI] [PubMed] [Google Scholar]
- Welch MG, Hofer MA, Brunelli SA, Stark RI, Andrews HF, Austin J, Myers MM. Family nurture intervention (FNI): Mmethods and treatment protocol of a randomized controlled trial in the NICU. BMC Pediatrics. 2012;12:14. doi: 10.1186/1471-2431-12-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff PH. Observations on newborn infants. Psychosomatic Medicine. 1959;21:110–118. doi: 10.1097/00006842-195903000-00004. [DOI] [PubMed] [Google Scholar]