Abstract
Background and purpose — Short-term experimental studies have confirmed that there is superior fixation of cementless implants inserted with compaction compared to broaching of the cancellous bone.
Patients and methods — 1-stage, bilateral primary THA was performed in 28 patients between May 2001 and September 2007. The patients were randomized to femoral bone preparation with broaching on 1 side and compaction on the other side. 8 patients declined to attend the postoperative follow-up, leaving 20 patients (13 male) with a mean age of 58 (36–70) years for evaluation. The patients were followed with radiostereometric analysis (RSA) at baseline, at 6 and 12 weeks, and at 1, 2, and 5 years, and measurements of periprosthetic bone mineral density (BMD) at baseline and at 1, 2, and 5 years. The subjective part of the Harris hip score (HHS) and details of complications throughout the observation period were obtained at a mean interval of 6.3 (3.0–9.5) years after surgery.
Results — Femoral stems in the compaction group had a higher degree of medio-lateral migration (0.21 mm, 95% CI: 0.03–0.40) than femoral stems in the broaching group at 5 years (p = 0.02). No other significant differences in translations or rotations were found between the 2 surgical techniques at 2 years (p > 0.4) and 5 years (p > 0.7) postoperatively. There were no individual stems with continuous migration. Periprosthetic BMD in the 7 Gruen zones was similar at 2 years and at 5 years. Intraoperative femoral fractures occurred in 2 of 20 compacted hips, but there were none in the 20 broached hips. The HHS and dislocations were similar in the 2 groups at 6.3 (3.0–9.5) years after surgery.
Interpretation — Bone compaction as a surgical technique with the Bi-Metric stem did not show the superior outcomes expected compared to conventional broaching. Furthermore, 2 periprosthetic fractures occurred using the compaction technique, so we cannot recommend compaction for insertion of the cementless Bi-Metric stem.
Initial stability is crucial for osseointegration between a cementless implant and the surrounding bone (Soballe et al. 1992, Jasty et al. 1997). In cemented and uncemented femoral stems, early and ongoing migration (subsidence and retroversion) has been considered to be a pattern of implant failure (Karrholm 1989, Hauptfleisch et al. 2006). Since the introduction of cementless femoral stems more than 3 decades ago (Hansen and Rechnagel 1977, Brown and Ring 1985), significant advances have been made in implant design, materials, coating, and geometry (Learmonth et al. 2007), whereas less focus has been placed on optimizing the quality and quantity of the host bone that comes in contact with the implant. Bone preparation by conventional broaching technique partly removes cancellous bone by the use of toothed broaches. In contrast, the compaction technique sequentially compresses the existing cancellous bone using increasing sizes of smooth tamps (Chareancholvanich et al. 2002). Short-term experimental in vitro and in vivo studies have shown some advantages of bone compaction such as increased initial implant stability (Channer et al. 1996, Green et al. 1999, Chareancholvanich et al. 2002, Kold et al. 2003a, 2005b, c, d) and preserved periprosthetic bone (Green et al. 1999).
On the other hand, possible disadvantages include concern that compression of cancellous bone might lead to micro-fracturing and brakeage of trabeculae, which could result in non-vital periprosthetic bone and therefore to a loss of implant fixation (Kold et al. 2005c, Windolf et al. 2009). Furthermore, human cadaver studies have found a greater risk of femoral fracture using smooth tamps than using toothed broaches (Breusch et al. 2001, Kold et al. 2003b, 2005a).
Ideally, a phased introduction including small-scale randomized radiostereometric studies of all new implant designs, cements, or surgical procedures should be performed (Malchau et al. 1995, Malchau 2000, Karrholm et al. 2006, Nelissen et al. 2011).
In this small-scale, randomized clinical study, we investigated the possible advantages and disadvantages of compaction technique compared to broaching technique for bone preparation prior to cementless femoral stem insertion. To eliminate the effect of individual differences, operations were performed using 1-stage, bilateral THA. We evaluated 3 hypotheses: (1) that compaction of the cancellous femoral bone provides superior stem fixation compared to broaching of the femoral cancellous bone, (2) that compaction of the cancellous femoral bone increases the periprosthetic BMD in comparison to broaching, and (3) that compaction of the cancellous femoral bone does not increase the risk of intraoperative femoral fractures compared to broaching.
Patients and methods
1-stage, bilateral primary THA was performed in 28 patients between May 2001 and September 2007. The inclusion criteria were having bilateral, symptomatic, and radiographically verified osteoarthritis of the hips, being aged between 18 and 70 years, and having sufficient bone quality to allow insertion of a cementless femoral stem (as assessed by preoperative radiographs and by intraoperative evaluation). The exclusion criteria were having severe bone deformities unsuitable for the use of the Bi-Metric stem, having metabolic or inflammatory bone disorders (including rheumatoid arthritis), having neuromuscular or vascular diseases of the legs, undergoing regular systemic glucocorticoid treatment, having active cancer or chemotherapy treatment, planning pregnancy (women), having a chronic infectious disease, and having a diagnosis of osteoporosis. 8 patients declined to attend the postoperative follow-up, leaving 20 patients (13 males) with a mean age of 58 (36–70) years for evaluation.
Randomization was performed with computer software, and consisted of block randomization using sealed envelopes in blocks of 10 patients. The patients were positioned at the operating table before the envelope was drawn. Half of the patients were initially positioned on the right side, and the other half on the left side. All patients were kept from knowing the operation technique that was used on each hip. The research worker who performed the RSA analyses and the DXA analyses (XY) was also kept blind. All operations took place at Farsoe Hospital or Aalborg University Hospital, and follow-up occasions with RSA and DXA took place at either Aarhus University Hospital (n = 26 hips, 8 men) or Farsoe Hospital (n = 14 hips, 5 men). Stem sizes used for broaching and compaction were similar (Table 1).
Table 1.
Descriptive data regarding arthroplasty components
| Broaching | Compaction | |
|---|---|---|
| Variable | (n = 20) | (n = 20) |
| Size of femoral stemb | 12 (11–14) | 12 (10–14) |
| Size of acetabular cupb | 60 (54–68) | 60 (54–68) |
| Implant side, right/left | 9/11 | 11/9 |
Mean (range)
Continuous subsidence has been considered to be a pattern of failure of femoral stems (Karrholm 1989), and the predefined primary endpoint was therefore subsidence at 5 years. The patients were followed with RSA at baseline, at 6 and 12 weeks, and at 1, 2, and 5 years after surgery to examine migration of the femoral stem. The RSA at baseline was obtained before weight bearing. Secondary outcomes were measurements of periprosthetic BMD, outcome values of the Harris hip score (HHS), and risk of femoral bone fracture. DXA scans were performed postoperatively and at 1, 2, and 5 years after surgery, and the subjective part of the HHS and complications throughout the observation period were obtained cross-sectionally at mean 6.3 (3–9.5) years after surgery.
Surgery and prosthesis
1 experienced orthopedic hip surgeon (PHC) undertook the operations using a posterolateral approach. The decision to perform a cementless procedure was based on the inclusion criteria and an intraoperative clinical assessment by the experienced hip surgeon that the bone quality was satisfactory for a cementless procedure. All patients received cementless Bi-Metric stems without HA coating (Biomet Inc., Warsaw, IN) and 28-mm chrome-cobalt femoral heads.
On the acetabular side, 6 patients received cementless Trilogy fiber-mesh shells and Trilogy ultra-high-molecular-weight polyethylene (UHMWPE) 10° elevated rim liners (Zimmer Inc., Warsaw, IN), 6 patients received cementless HA-coated Trilogy fiber-mesh shells and Trilogy UHMWPE 10° elevated rim liners (Zimmer), and 8 patients received Longevity highly crosslinked 10° elevated rim liners (Zimmer). All patients were instructed to walk with 40 kg of weight bearing (aided by crutches) for the first 6 weeks after surgery, and full weight bearing was allowed thereafter.
Instruments
Instruments for a cementless primary hip were used (Bi-Metric Hip; Biomet Inc, Warsaw, IN). The upper half of the toothed broaches had a diamond-shaped surface and the remaining distal part had a smooth surface. The tamps had only a smooth surface. For each broach size, the corresponding tamp size had the same base volume as the broaches, but without the teeth (Figure 1).
Figure 1.

The 2 different instrument configurations used in this study: smooth tamp used for the compaction technique (left) and sharped rasp used for the broaching technique (right).
Technique for preparation of femoral bone
The compaction procedure included distal reaming with cylindrical reamers and proximal bone preparation with smooth tamps of increasing sizes. Conventional broaching was prepared as suggested by the manufacturer of the instruments/stems. Thus, the broaching procedure included distal reaming with cone-shaped reamers and preparation of proximal bone with toothed broaches of increasing sizes.
Radiostereometric analysis
For RSA measurements, 8–10 tantalum markers (1 mm caliber) were inserted into the greater and lesser trochanter during surgery. Furthermore, all stems had been modified with 3 small marker towers (tantalum beads; Wennbergs Finmek, Gunnilse, Sweden) distributed with 1 marker tower distally on the tip of the stem, 1 marker tower proximal-medial (calcar region), and 1 marker tower proximal-lateral (shoulder of the stem). The stereo radiographs were obtained at Aarhus University Hospital and Farsoe Hospital using a standard RSA setup with 2 synchronized ceiling-fixed roentgen tubes angled towards each other at 40° (Arco-Ceil/Medira; Santax Medico, Bromma, Sweden). The uniplanar carbon calibration box at Aarhus University Hospital was Box 24 (Medis Specials, Leiden, the Netherlands) and the uniplanar carbon calibration box at Farsoe Hospital was uniplanar no. 43 (RSA Biomedical, Umeå, Sweden). All stereo radiographs were obtained with the patients in standard position: supine, body parallel to the examination table, and the big toes pointing straight up, with the calibration box placed under the examination table. Implant migration was assessed on all follow-up stereo radiographs using the first postoperative exposure as the reference. Model-based (MB) RSA version 3.2 (RSAcore, Leiden, the Netherlands) was used to calculate implant migration. Stereo radiographs were analyzed using the combined large-marker hip model (LMHM). However, stereo radiographic series of 4 patients (3 broaching and 1 compaction) had to be analyzed using an EGS hip-stem model (Kaptein et al. 2006) due to technical problems with missing markers on the implant. Translations (implant movement along the axes) were expressed as x-translations (medial-lateral direction), y-translations (proximal-distal direction), and z-translations (anterior-posterior direction). Rotations were expressed as rotations about the x-axis (anterior/posterior tilt), rotations about the y-axis (retroversion/anteversion), and rotations about the z-axis (valgus/varus tilt). The total translation (TT) and the total rotation (TR) were calculated using the Pythagorean theorem (TT = √(x2 + y2 + z2) and TR = √(x2 + y2 + z2)). The distribution of the implant and femoral bone markers was assessed using the condition number (CN), and an upper limit of ≤150 has been suggested (Valstar et al. 2005). The mean CN of the markers on the stem and in the femur was 8.5 (SD 5.1) and 17.2 (SD 10.3), respectively. The rigid body error (RBE) represents the stability of the markers. The mean RBE in the analysis of the markers of the stem and femur was 0.1 (SD 0.1) and 0.2 (SD 0.1). The rigid body match threshold was set at 0.5 mm.
Sample size and precision of the radiostereometric analysis
The study was designed to include 20 hips in each group, since small-scale randomized studies have been suggested for preclinical testing of new implants and procedures (Malchau et al. 1995, Malchau 2000, Nelissen et al. 2011). The study design was further strengthened by the bilateral design, with each patient being his/her own control. No pre-study power analysis was performed.
The precision of the RSA analyses was assessed by double examination of 14 patients (with 2 pairs of stereo radiographs being recorded from the same patient within 10–15 min) (Ranstam et al. 2000, Valstar et al. 2005). The postoperative stereo radiograph was used as the reference in migration analysis of the double examinations; the expected difference in displacement between the 2 calculations reflects the systematic error of the RSA system (bias) and should (optimally) be equal to zero. The standard deviation of the mean difference (SDdiff) between the double examinations represents the precision of the system, and the coefficient of repeatability (CR) (± 1.96 × SDdiff) is the lower limit within which it is possible to detect implant migration based on an individual patient (Altman 2009) (Table 2).
Table 2.
Measurement of error for the RSA double-examination stereo radiographs (n = 14), for translations and rotations
| Translation, mm |
Rotation, degrees |
|||||||
|---|---|---|---|---|---|---|---|---|
| Axis | x | y | z | TTa | x | y | z | TRb |
| Mean diff.c | −0.06 | −0.24 | 0.16 | 0.11 | −0.09 | 0.32 | −0.12 | −0.16 |
| SDdiff. d | 0.47 | 0.71 | 0.59 | 0.83 | 0.51 | 1.26 | 0.39 | 0.84 |
| CRe | 0.92 | 1.39 | 1.16 | 1.63 | 0.99 | 2.47 | 0.76 | 1.65 |
The total translation was calculated using the 3-D Pythagorean theorem (TT = √(x2 + y2 + z2)).
The total rotation was calculated using the 3-D Pythagorean theorem (TR = √(x2 + y2 + z2)).
Mean difference represents the systematic error of the system.
SDdiff. is the random variation in the method comparing the double examinations.
Coefficient of repeatability (1.96 × SDdiff.) reflects the precision of the system on the individual basis.
Dual-energy X-ray absorptiometry scans
Postoperatively (within 1 week of surgery) and at 1, 2, and 5 years after surgery, quantitative measurements of the periprosthetic BMD (g/cm2) was assessed with DXA scans. At Aarhus University Hospital, scans were performed with a Hologic QDR 4500 (Holic Inc., Waltham, MA), and Hologic Apex software version 13 was used for analysis. At Farsoe Hospital, scans were performed with a pencil-beam bone densitometer Norland XR 36 scanner (Norland Corporation, Fort Atkinson, WI), and Illuminatus DXA software version 4.2 (Norland Corporation) was used for analysis. Automatic metal artifact removal was used on both scanners. Changes in the periprosthetic BMD were measured in all 7 Gruen zones according to the model of Gruen, which was placed identically on all scans. The first DXA scan served as baseline for the subsequent scans, as recommended (Kroger et al. 1996). Patients were placed in a standardized position: supine, body parallel to the examination table, and the feet 15 degrees internally rotated and fixed on a triangle device with foot straps. On both DXA scanners, calibration was performed on a daily basis using a phantom, according to the manufacturer’s guidelines, to verify the reliability of the systems. This was within the acceptable range throughout the follow-up.
Clinical outcome measures and complications
At a mean interval of 6.3 (3.0–9.5) years after surgery, all patients filled out a questionnaire regarding patient satisfaction, any revision surgery, and the subjective part of the HHS. At the same time, perioperative and later complications were obtained through a systemic cross-check reading of all patient files. The following details were recorded: intraoperative femoral fractures, infections, dislocations, and revision surgery.
Statistics
Migrations were assessed using a linear mixed-model analysis to take into account the longitudinal nature of the data and the repeated measurements in individual patients. All available examinations were included in the mixed-model analysis. Statistical analyses of differences in migration from 0 to 2 years and from 0 to 5 years, and of differences in BMD from 0 to 2 years and from 0 to 5 years were pre-specified in the study protocol. Post hoc test estimates were used to assess the difference between broaching and compaction. Measured values of migration and BMD at all follow-up intervals are reported as the mean with 95% confidence interval (CI) (Tables 3 and 4). Clinical outcome scores were analyzed with a paired t-test. The significance level was kept at 5%. For assessments of individual migrations, however, migrations should exceed the precision limit (as calculated on the basis of double stereoradiographic examinations) to be regarded as measurable and relevant. All analyses were performed using STATA software version 13.
Table 3.
Signed migrations of the Bi-metric femoral stem as mean (95% CI) along and about the 3 orthogonal axes, measured with RSA at 6 weeks, 12 weeks, 1 year, 2 years, and 5 years after surgery
| Rotations, degrees |
Rotations, degrees |
||||
|---|---|---|---|---|---|
| Broaching | Compaction | Broaching | Compaction | ||
| Medial-lateral (x-axis) | Anterior/posterior tilt (x-axis) | ||||
| 6 weeks | 0.07 (0.006 to 0.13) | 0.19 (0.08 to 0.30) | 6 weeks | −0.09 (−0.45 to 0.28) | 0.01 (−0.20 to 0.23) |
| 12 weeks | 0.06 (−0.07 to 0.20) | 0.17 (0.03 to 0.30) | 12 weeks | −0.04 (−0.31 to 0.22) | 0.08 (−1.70 to 0.03) |
| 1 year | 0.07 (−0.05 to 0.18) | 0.19 (0.05 to 0.33) | 1 year | −0.13 (−0.49 to 0.23) | −0.01 (−0.35 to 0.33) |
| 2 years | −0.02 (−0.20 to 0.15) | 0.07 (−0.22 to 0.35) | 2 years | −0.13 (−0.63 to 0.36) | 0.01 (−0.49 to 0.53) |
| 5 years | −0.01 (−0.15 to 0.13) | 0.16 (−0.003 to 0.32) | 5 years | −0.40 (−0.82 to −0.05) | −0.21 (−0.62 to 0.26) |
| Proximal-distal (y-axis) | Anteversion/retroversion (y-axis) | ||||
| 6 weeks | −1.06 (−1.85 to −0.27) | −0.90 (−1.62 to −0.16) | 6 weeks | 1.99 (0.53 to 3.47) | 1.89 (0.69 to 3.10) |
| 12 weeks | −1.05 (−1.91 to −0.19) | −0.96 (−1.72 to −0.19) | 12 weeks | 1.71 (0.24 to 3.18) | 1.78 (0.50 to 3.08) |
| 1 year | −0.99 (−1.81 to −0.18) | −0.82 (−1.61 to −0.04) | 1 year | 1.99 (0.44 to 3.56) | 1.93 (0.71 to 3.15) |
| 2 years | −0.54 (−1.10 to 0.20) | −0.32 (−0.67 to 0.03) | 2 years | 0.91 (0.28 to 1.54) | 1.08 (0.18 to 2.09) |
| 5 years | −0.88 (−1.77 to 0.003) | −0.67 (−1.45 to 0.12) | 5 years | 1.67 (0.13 to 3.50) | 1.61 (0.37 to 2.86) |
| Anterior-posterior (z-axis) | Valgus/varus tilt (z-axis) | ||||
| 6 weeks | −0.46 (−0.74 to −0.17) | −0.37 (−0.66 to 0.07) | 6 weeks | −0.18 (−0.29 to −0.06) | −0.24 (−0.36 to −0.13) |
| 12 weeks | −0.32 (−0.65 to 0.007) | −0.41 (−0.60 to −0.21) | 12 weeks | −0.16 (−0.27 to −0.04) | −0.24 (−0.40 to −0.09) |
| 1 year | −0.41 (−0.78 to −0.03) | −0.37 (−0.61 to −0.14) | 1 year | −0.24 (−0.45 to −0.04) | −0.25 (−0.43 to −0.05) |
| 2 years | −0.11 (−0.14 to −0.02) | −0.20 (−0.53 to −0.12) | 2 years | −0.15 (−0.32 to −0.02) | −0.04 (−0.32 to 0.25) |
| 5 years | −0.18 (−0.61 to −0.03) | −0.11 (−0.37 to −0.14) | 5 years | −0.39 (−0.68 to −0.11) | −0.30 (−0.51 to −0.09) |
Table 4.
Bone mineral density in the femoral Gruen zones, as mean (95% CI) percentage of baseline values at 1, 2, and 5 years
| Femoral zone | Broaching | Compaction |
|---|---|---|
| Gruen 1 | ||
| 1 year | 103 (94–113) | 93 (86–99) |
| 2 years | 102 (94–110) | 93 (86–100) |
| 5 years | 100 (91–109) | 93 (84–101) |
| Gruen 2 | ||
| 1 year | 95 (86–105) | 94 (86–101) |
| 2 years | 92 (87–98) | 91 (85–98) |
| 5 years | 93 (84–102) | 95 (87–102) |
| Gruen 3 | ||
| 1 year | 103 (96–110) | 95 (99–101) |
| 2 years | 101 (94–108) | 93 (88–97) |
| 5 years | 103 (94–112) | 98 (93–104) |
| Gruen 4 | ||
| 1 year | 101 (98–106) | 97 (93–101) |
| 2 years | 102 (96–107) | 97 (94–101) |
| 5 years | 102 (95–109) | 100 (95–105) |
| Gruen 5 | ||
| 1 year | 108 (95–111) | 102 (94–111) |
| 2 years | 102 (96–109) | 102 (96–107) |
| 5 years | 103 (96–110) | 104 (97–113) |
| Gruen 6 | ||
| 1 year | 92 (83–102) | 93 (87–99) |
| 2 years | 90 (81–99) | 92 (85–98) |
| 5 years | 87 (76–97) | 92 (83–101) |
| Gruen 7 | ||
| 1 year | 76 (66–86) | 72 (66–77) |
| 2 years | 71 (63–78) | 69 (63–75) |
| 5 years | 67 (59–76) | 68 (60–76) |
| Mean for all zones | ||
| 1 year | 96 (90–103) | 92 (88–97) |
| 2 years | 94 (90–98) | 91 (87–95) |
| 5 years | 93 (88–99) | 93 (87–98) |
Ethics and registration
All examinations were designed and carried out in accordance with the Helsinki Declaration (II). All patients gave informed consent before entering the study. The study was approved by the Central Denmark Regional Committee on Biomedical Research (entry no. 2000065; issue date January 4, 2000) and by the Danish Data Protection Agency (protocol no. 1-16-02-62-09). The project was registered with www.clinicaltrials.gov (bilateral sub-study NCT00317889). The reporting of data from this trial complies with the CONSORT statement.
Results
Radiostereometric analysis
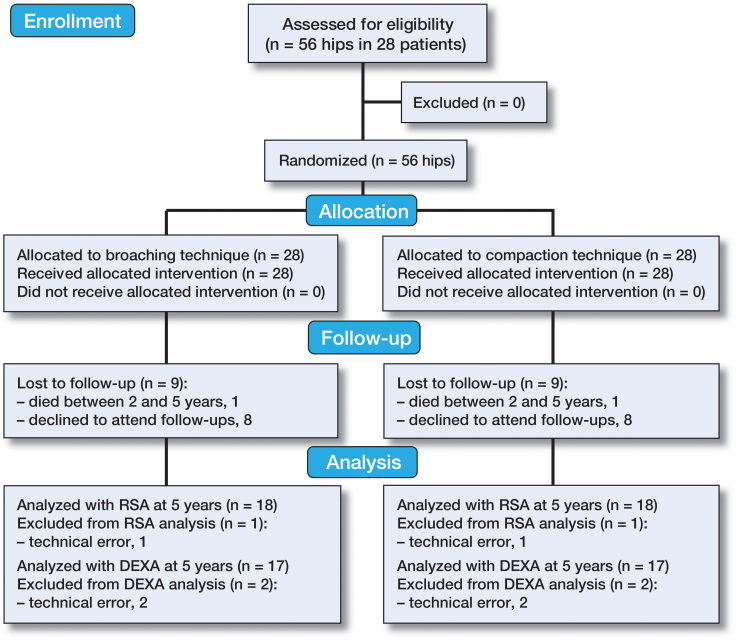
1 male patient did not show up for the 6-week RSA examination and 1 female patient did not show up for the 12-week RSA examination. Patients operated at Farsoe Hospital (n = 7) were not examined with RSA at 2 years, and 1 patient had died at 5 years (Figure 2).
Figure 2.
CONSORT flow diagram showing the inclusion/exclusion process and follow-up until 5 years.
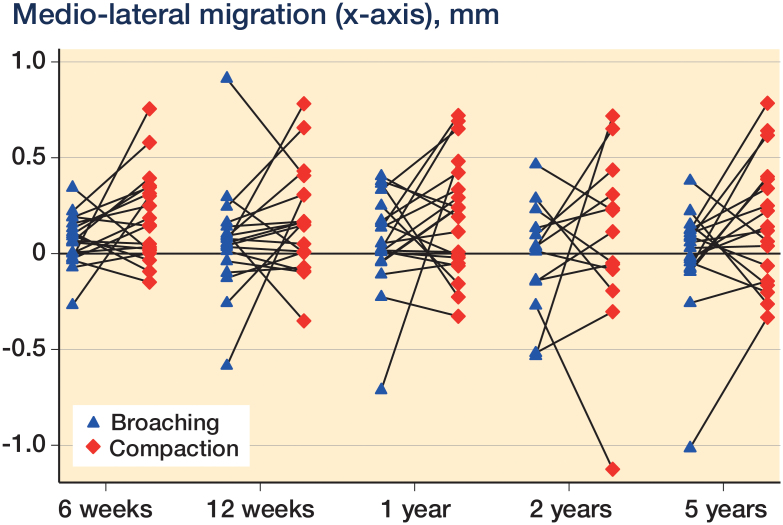
Linear mixed-model analysis showed that femoral stems in the compaction group had an increased (p = 0.02) medio-lateral migration of 0.21 (95% CI: 0.03–0.40) mm compared to femoral stems in the broaching group at 5 years (Figure 3 and Table 3). No other significant differences in translations or rotations were found between the 2 surgical techniques at 2 years and 5 years postoperatively (p > 0.4 and p > 0.7). Age, sex, mean BMD, and stem size had no statistically significant influence on migration of the stems (all p > 0.2). There were no individual stems with continuous migration (Figure 4).
Figure 3.
Medio-lateral migration (in mm) illustrated as a pairwise comparison of broaching and compaction at 6 weeks, 12 weeks, 1 year, 2 years, and 5 years after surgery.
Figure 4.
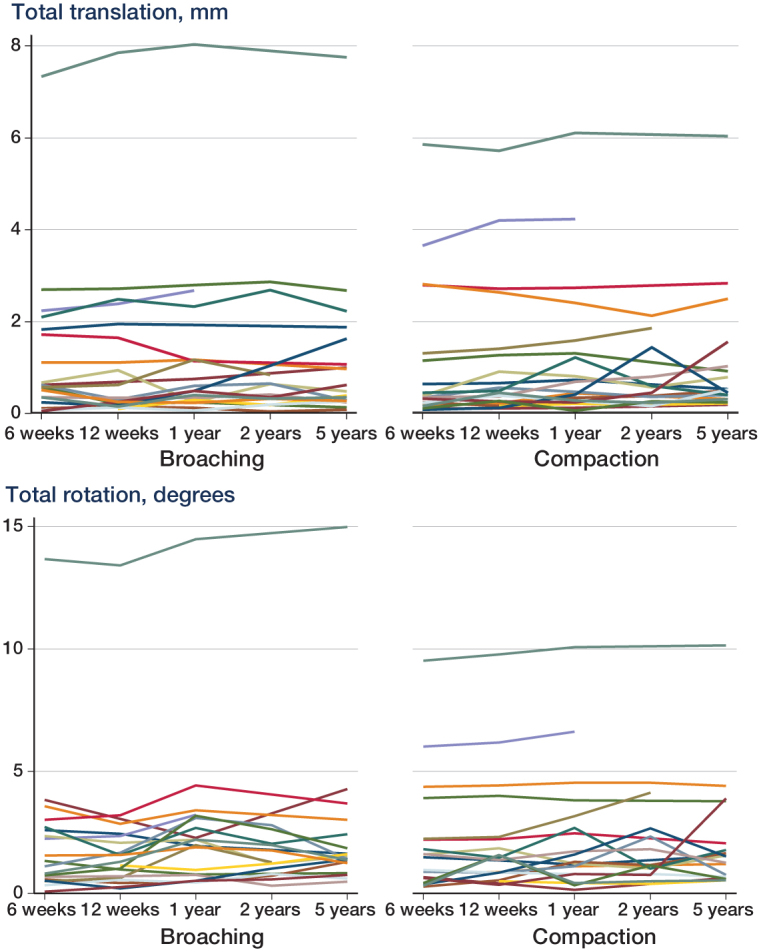
Total rotations and total translations measured at 6 weeks, 12 weeks, 1 year, 2 years, and 5 years.
At 2 years, 1 stem inserted with bone compaction had a measurable (above the precision limit of the x-axis of 0.92 mm) medio-lateral translation of 1.12 mm, and at 5 years, 1 stem inserted with bone broaching had a measurable medio-lateral translation of 1.01 mm.
At 5 years, mean subsidence for stems operated with broaching was −0.88 (95% CI: −1.77 to 0.003) mm and for stems operated with compaction it was −0.67 (CI: −1.45 to 0.12). The individual patient migration patterns revealed 4 stems in the broaching group and 3 stems in the compaction group with measurable subsidence (precision limit: 1.39 mm) of mean −3.44 (−6.88 to −1.78) mm and mean −3.51 (−5.96 to −2.1) mm, respectively (p = 0.9). Stem retroversion was similar between groups at any time point, and the individual migration patterns showed 6 stems with measurable rotation into retroversion at 5 years: 2 stems in the broaching group had a mean rotation of 8.95 (2.95–14.7) degrees and 4 stems in the compaction group had a mean rotation of 5.17 (2.72–10.0) degrees (p = 0.5).
Dual-energy X-ray absorptiometry scans
The differences in periprosthetic BMD as percentage change in baseline values were similar between groups at 2 years (p > 0.06) and at 5 years (p > 0.17) (Table).
In the entire study group, the periprosthetic BMD in Gruen zone 7 was reduced the most, by mean 32% (95% CI: 27–38) at 5 years after surgery.
Clinical outcome measures and complications
At a mean interval of 6.3 (3.0–9.5) years after surgery, the patient-reported clinical outcome measure of HHS was similar in hips operated with broaching technique (mean 91 (33–100)) and in hips operated with compaction technique (mean 94 (59–100)).
Intraoperative trochanter fractures occurred in 2 of the 20 femurs operated with compaction and in none of the 20 femurs operated with broaching. The 2 fractures were fixed using cable systems, and they were stable between 6 weeks and 5 years after surgery. At 5 years, the HHS (subjective outcome) in both hips was 100 points. 2 hips (1 broaching and 1 compaction) dislocated several times, and they were finally treated with revision of the femoral heads and liners at 2 and 3 years (respectively) after surgery. During surgery, the orthopedic surgeon judged both stems to be stable. Another hip (broaching) dislocated once during the study period, but it was successfully treated with closed reduction. There were no deep or superficial infections, and no stems or cups were revised.
Discussion
We hypothesized that compaction as the surgical preparation of cancellous metaphyseal and proximal diaphyseal femoral bone would provide a better outcome in cementless femoral stems than would broaching. However, we could not confirm that bone canal preparation with compaction was superior to broaching technique in cementless femoral stems, regarding migration rates and changes in BMD. Furthermore, intraoperative femoral fractures only occurred in the compaction group.
Radiostereometric analysis
Our findings were not in agreement with our expectations, and they are not in line with results of previously published preclinical research. In vitro studies on tibias and femurs from fresh-frozen human cadavers have demonstrated higher initial stability with compaction than with conventional press-fit, by mechanical testing (Channer et al. 1996, Chareancholvanich et al. 2002). Additionally, 2 dog studies of loaded hydroxyapatite-coated implants and unloaded porous-coated implants found an improvement in initial implant stability with bone compaction compared to drilling at 0 and 2 weeks (Green et al. 1999), and at 0 and 3 weeks (Kold et al. 2006).
Another RSA study (Moritz et al. 2011) on 61 women operated with anatomically shaped femoral stems reported that the importance of surgical preservation of intertrochanteric cancellous bone has been exaggerated for osseointegration of cementless stems. This might also be the case for the Bi-Metric stem, and thus we did not find major differences in migration between compaction and broaching.
Continuous subsidence, retroversion, and medial migration has been considered to be a pattern of failure in femoral stems (Karrholm 1989, Karrholm et al. 1994, Hauptfleisch et al. 2006). In the entire study group, 7 stems (4 broaching and 3 compaction) had measurable subsidence (above the precision limit of 1.39 mm) of mean −0.77 (95% CI: −1.33 to −0.21) mm, and 6 stems had measurable rotations into retroversion (above the precision limit of 2.47 degrees) of mean 7.06 (2.72–14.7) degrees at 5 years after surgery. However, when analyzing these individual measurable migrations between 6 weeks and 5 years, we found migration patterns to be non-progressive, and we therefore consider these femoral stems to be stable.
Dual-energy X-ray absorptiometry scans
A previous dog study that investigated the histological bone surrounding titanium implants found that compaction of cancellous bone was significantly associated with increased peri-implant BMD compared to drilling at 0 and 2 weeks, but not at 4 weeks (Kold et al. 2005b). In agreement with this, another dog study reported that porous-coated implants inserted with compaction increased the peri-implant BMD compared to drilling at 0 and 3 weeks, but not at 9 weeks (Green et al. 1999). We were unable to demonstrate this possibly favorable effect of compaction on the periprosthetic BMD. A reasonable explanation for these different findings between the 2 dog studies and our study would be that those studies compared compaction of cancellous bone with drilling of the cancellous bone, and drilling may remove more bone than broaching during bone preparation—and may therefore not resemble the broaching technique.
In the entire study group, the greatest percentage reduction in periprosthetic BMD of 27–38% was observed in the calcar region (Gruen zone 7). Previous reports of the cementless proximally HA-coated Bi-Metric stems have found a similar pattern of bone reduction in the proximal region of Gruen zone 7 (Boden et al. 2004, 2006, Skoldenberg et al. 2006). But even though the proximal periprosthetic BMD is reduced to a large extent in patients with Bi-Metric stems, it has been reported that stable Bi-Metric stems commonly have a proximal bone reduction whereas unstable Bi-Metric stems usually have a bone reduction along the entire stem (Boden et al. 2004).
Clinical outcome measures and complications
At a mean interval of 6.3 (3–9.5) years after surgery, no stems were revised and the mean HHS score was excellent (above 90). In accordance with our clinical results, a former study on the cementless Bi-Metric stem described no stem revisions and mean HHS scores of 95 points at a minimum of 6 years of follow-up (Goosen et al. 2005). Additionally, studies by Boden et al. (2006), Meding et al. (2004), and Takatori et al. (2002) also found no stem revisions and mean HHS scores of 92 points at 10 years after surgery.
2 periprosthetic fractures occurred in 20 patients using the compaction technique, and no periprosthetic fractures occurred using the broaching technique. This outcome is of some concern, as periprosthetic fractures can substantially affect the outcome of THA (Brun and Maansson 2013). The main weakness of our study is that patients had been followed with RSA and DXA on 2 sites, but the setups were standardized with the same guidelines, and patients were followed with the same equipment throughout follow-up. Since mainly changes from baseline until 5 years follow-up were assessed between groups, we expected only minor noise in the data. With the limited sample size, there remains a risk of residual confounding. We did not investigate the degree of osteoarthritis preoperatively, which may be a residual confounder—as the degree of osteoarthritis before surgery may be related to early stem migration. Notable strengths of our study were the randomized study design and the same patients being the treatment and control groups due to the bilateral THA treatment. To reduce the influence of surgeon-related differences in surgical technique, all the operations were performed by one experienced orthopedic surgeon. However, the external validity of the study may have been compromised by the single-surgeon design.
In summary, we could not verify the superior outcomes of compaction compared to broaching that have been shown in previous short-term in vitro and in vivo studies. Although the stems inserted with the compaction technique migrated more at 5 years, the differences were small and were clinically irrelevant. However, we find it clinically relevant that 2 periprosthetic fractures occurred using the compaction technique, so we cannot recommend the compaction technique for insertion of the cementless Bi-Metric stem.
SK, MS, PHC, PTN, and KS were involved in formulating the study hypothesis and follow-up examinations. MHH performed the data analysis and wrote the first draft of the manuscript. MS, SK, KS, PTN, and PHC helped revise it.
We thank Lone Løvgren Andersen and Gitte Broholm for help with project coordination, and Rikke Mørup for help in analyzing the stereo radiographs. We also thank Professor Bente Lomholt Langdahl and biomedical laboratory technician Lisbeth Flyvbjerg. Biomet Inc. and the Danish Rheumatism Association kindly supported the study financially.
No competing interests declared.
Altman D G. Practical Statistics for Medical Research. Chapman & Hall 2009.
References
- Boden H, Adolphson P, Oberg M.. Unstable versus stable uncemented femoral stems: a radiological study of periprosthetic bone changes in two types of uncemented stems with different concepts of fixation. Arch Orthop Trauma Surg 2004; 124(6): 382–92. [DOI] [PubMed] [Google Scholar]
- Boden H, Salemyr M, Skoldenberg O, Ahl T, Adolphson P.. Total hip arthroplasty with an uncemented hydroxyapatite-coated tapered titanium stem: results at a minimum of 10 years’ follow-up in 104 hips. J Orthop Sci 2006; 11(2): 175–9. [DOI] [PubMed] [Google Scholar]
- Breusch S J, Norman T L, Revie I C, Lehner B, Caillouette J T, Schneider U, Blaha J D, Lukoschek M.. Cement penetration in the proximal femur does not depend on broach surface finish. Acta Orthop Scand 2001; 72(1): 29–35. [DOI] [PubMed] [Google Scholar]
- Brown I W, Ring P A.. Osteolytic changes in the upper femoral shaft following porous-coated hip replacement. J Bone Joint Surg Br 1985; 67(2): 218–21. [DOI] [PubMed] [Google Scholar]
- Brun O C, Maansson L.. Fractures of the greater trochanter following total hip replacement. Hip Int 2013; 23(2): 143–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channer M A, Glisson R R, Seaber A V, Vail T P.. Use of bone compaction in total knee arthroplasty. J Arthroplasty 1996; 11(6): 743–9. [DOI] [PubMed] [Google Scholar]
- Chareancholvanich K, Bourgeault C A, Schmidt A H, Gustilo R B, Lew W D.. In vitro stability of cemented and cementless femoral stems with compaction. Clin Orthop Relat Res 2002; (394): 290–302. [DOI] [PubMed] [Google Scholar]
- Goosen J H, Swieringa A J, Keet J G, Verheyen C C.. Excellent results from proximally HA-coated femoral stems with a minimum of 6 years follow-up: a prospective evaluation of 100 patients. Acta Orthop 2005; 76(2): 190–7. [DOI] [PubMed] [Google Scholar]
- Green J R, Nemzek J A, Arnoczky S P, Johnson L L, Balas M S.. The effect of bone compaction on early fixation of porous-coated implants. J Arthroplasty 1999; 14(1): 91–7. [DOI] [PubMed] [Google Scholar]
- Hansen F W, Rechnagel K.. The Monk hip arthroplasty. Preliminary report on the uncemented standard Monk prosthesis. Acta Orthop Scand 1977; 48(4): 394–9. [DOI] [PubMed] [Google Scholar]
- Hauptfleisch J, Glyn-Jones S, Beard D J, Gill H S, Murray D W.. The premature failure of the Charnley Elite-Plus stem: a confirmation of RSA predictions. J Bone Joint Surg Br 2006; 88(2): 179–83. [DOI] [PubMed] [Google Scholar]
- Jasty M, Bragdon C, Burke D, O’Connor D, Lowenstein J, Harris W H.. In vivo skeletal responses to porous-surfaced implants subjected to small induced motions. J Bone Joint Surg Am 1997; 79(5): 707–14. [DOI] [PubMed] [Google Scholar]
- Kaptein B L, Valstar E R, Spoor C W, Stoel B C, Rozing P M.. Model-based RSA of a femoral hip stem using surface and geometrical shape models. Clin Orthop Relat Res 2006; 448: 92–7. [DOI] [PubMed] [Google Scholar]
- Karrholm J. Roentgen stereophotogrammetry. Review of orthopedic applications. Acta Orthop Scand 1989; 60(4): 491–503. [DOI] [PubMed] [Google Scholar]
- Karrholm J, Borssen B, Lowenhielm G, Snorrason F.. Does early micromotion of femoral stem prostheses matter? 4-7-year stereoradiographic follow-up of 84 cemented prostheses. J Bone Joint Surg Br 1994; 76(6): 912–7. [PubMed] [Google Scholar]
- Karrholm J, Gill R H, Valstar E R.. The history and future of radiostereometric analysis. Clin Orthop Relat Res 2006; 448: 10–21. [DOI] [PubMed] [Google Scholar]
- Kold S, Bechtold J E, Ding M, Chareancholvanich K, Rahbek O, Soballe K.. Compacted cancellous bone has a spring-back effect. Acta Orthop Scand 2003a; 74(5): 591–595. [DOI] [PubMed] [Google Scholar]
- Kold S, Mouzin O, Bourgeault C, Soballe K, Bechtold J E.. Femoral fracture risk in hip arthroplasty: smooth versus toothed instruments. Clin Orthop Relat Res 2003b;(408): 180–188. [DOI] [PubMed] [Google Scholar]
- Kold S, Bechtold J E, Mouzin O, Bourgeault C, Soballe K.. Importance of pre-clinical testing exemplified by femoral fractures in vitro with new bone preparation technique. Clin Biomech (Bristol, Avon) 2005a; 20(1): 77–82. [DOI] [PubMed] [Google Scholar]
- Kold S, Rahbek O, Toft M, Ding M, Overgaard S, Soballe K.. Bone compaction enhances implant fixation in a canine gap model. J Orthop Res 2005b; 23(4): 824–30. [DOI] [PubMed] [Google Scholar]
- Kold S, Rahbek O, Vestermark M, Overgaard S, Soballe K.. Bone compaction enhances fixation of weightbearing titanium implants. Clin Orthop Relat Res 2005c;(431): 138–44. [DOI] [PubMed] [Google Scholar]
- Kold S, Rahbek O, Zippor B, Bechtold J E, Soballe K.. Bone compaction enhances fixation of hydroxyapatite-coated implants in a canine gap model. J Biomed Mater Res B Appl Biomater 2005d; 75(1): 49–55. [DOI] [PubMed] [Google Scholar]
- Kold S, Rahbek O, Vestermark M, Overgaard S, Soballe K.. Bone compaction enhances fixation of weight-bearing hydroxyapatite-coated implants. J Arthroplasty 2006; 21(2): 263–70. [DOI] [PubMed] [Google Scholar]
- Kroger H, Miettinen H, Arnala I, Koski E, Rushton N, Suomalainen O.. Evaluation of periprosthetic bone using dual-energy x-ray absorptiometry: precision of the method and effect of operation on bone mineral density. J Bone Miner Res 1996; 11(10): 1526–30. [DOI] [PubMed] [Google Scholar]
- Learmonth I D, Young C, Rorabeck C.. The operation of the century: total hip replacement. Lancet 2007; 370(9597): 1508–19. [DOI] [PubMed] [Google Scholar]
- Malchau H. Introducing new technology: a stepwise algorithm. Spine (Phila Pa 1976) 2000; 25(3): 285. [DOI] [PubMed] [Google Scholar]
- Malchau H, Karrholm J, Wang Y X, Herberts P.. Accuracy of migration analysis in hip arthroplasty. Digitized and conventional radiography, compared to radiostereometry in 51 patients. Acta Orthop Scand 1995; 66(5): 418–424. [DOI] [PubMed] [Google Scholar]
- Meding J B, Keating E M, Ritter M A, Faris P M, Berend M E.. Minimum ten-year follow-up of a straight-stemmed, plasma-sprayed, titanium-alloy, uncemented femoral component in primary total hip arthroplasty. J Bone Joint Surg Am 2004; 86-A(1): 92–7. [DOI] [PubMed] [Google Scholar]
- Moritz N, Alm J J, Lankinen P, Makinen T J, Mattila K, Aro H T.. Quality of intertrochanteric cancellous bone as predictor of femoral stem RSA migration in cementless total hip arthroplasty. J Biomech 2011; 44(2): 221–7. [DOI] [PubMed] [Google Scholar]
- Nelissen R G, Pijls B G, Karrholm J, Malchau H, Nieuwenhuijse M J, Valstar E R.. RSA and registries: the quest for phased introduction of new implants. J Bone Joint Surg Am 2011; 93Suppl3: 62–65. [DOI] [PubMed] [Google Scholar]
- Ranstam J, Ryd L, Onsten I.. Accurate accuracy assessment: review of basic principles. Acta Orthop Scand 2000; 71(1): 106–8. [DOI] [PubMed] [Google Scholar]
- Skoldenberg O G, Boden H S, Salemyr M O, Ahl T E, Adolphson P Y.. Periprosthetic proximal bone loss after uncemented hip arthroplasty is related to stem size: DXA measurements in 138 patients followed for 2-7 years. Acta Orthop 2006; 77(3): 386–92. [DOI] [PubMed] [Google Scholar]
- Soballe K, Hansen E S, Rasmussen H, Jorgensen P H, Bunger C.. Tissue ingrowth into titanium and hydroxyapatite-coated implants during stable and unstable mechanical conditions. J Orthop Res 1992; 10(2): 285–99. [DOI] [PubMed] [Google Scholar]
- Takatori Y, Nagai I, Moro T, Kuruta Y, Karita T, Mabuchi A, Ninomiya S.. Ten-year follow-up of a proximal circumferential porous-coated femoral prosthesis: radiographic evaluation and stability. J Orthop Sci 2002; 7(1): 68–73. [DOI] [PubMed] [Google Scholar]
- Valstar E R, Gill R, Ryd L, Flivik G, Borlin N, Karrholm J.. Guidelines for standardization of radiostereometry (RSA) of implants. Acta Orthop 2005; 76(4): 563–572. [DOI] [PubMed] [Google Scholar]
- Windolf M, Muths R, Braunstein V, Gueorguiev B, Hanni M, Schwieger K.. Quantification of cancellous bone-compaction due to DHS Blade insertion and influence upon cut-out resistance. Clin Biomech (Bristol, Avon) 2009; 24(1): 53–8. [DOI] [PubMed] [Google Scholar]