Abstract
The prenatal hormonal milieu is widely believed to shape health later in life, however there are considerable methodological challenges associated with measuring the in utero hormonal environment. Two potential biomarkers of prenatal androgen exposure that can be measured postnatally have been proposed: anogenital distance (AGD) and the ratio of the second to fourth digits of the hand (2D:4D). Although both measures are widely used research tools, their use in adult women may be complicated by the dramatic fluctuations in reproductive hormones across the menstrual cycle. To determine whether there is cyclical variation in these biomarkers, we conducted a longitudinal study of 12 naturally cycling, nulliparous adult women. Trained examiners assessed two measures of AGD (anus to clitoris [AGD-AC] and anus to fourchette [AGD-AF]) and 2D:4D in both hands for the duration of three menstrual cycles, taking measurements during the follicular, peri-ovulatory, and luteal phases of each cycle. Despite the small sample size, longer (more masculine) AGD was associated with lower (more masculine) digit ratios, as predicted by the literature. Using multi-level linear regression models, we found that AGD and 2D:4D measurements did not differ significantly across cycle phases. AGD-AF and digit ratios in both hands were associated with age at menarche, suggesting a possible common developmental trajectory. These results demonstrate that AGD and 2D:4D are stable across the menstrual cycle. Additional research is needed to determine how reliably these measures reflect the in utero hormonal milieu.
Keywords: anogenital distance, digit ratio, 2D:4D, menstrual cycle, prenatal androgens
INTRODUCTION
The in utero hormonal milieu is believed to play an important role in fetal programming of later health and disease. Prenatal androgen exposure is of particular interest, given the important role that sex hormones play in the development of many mammalian body systems including the reproductive system and brain. In animal models, manipulation of sex-typical androgen concentrations during critical periods of early development can elicit changes in reproductive function 1-3 as well as behavior (both reproductive and social) 4, 5. In humans, evidence from clinical conditions such as congenital adrenal hyperplasia (CAH), in which typical fetal endocrine activity is altered, reinforce the important role that these hormones play during fetal development 6-9. Much less is known about how the typical range of variation in fetal androgen exposure contributes to postnatal outcomes.
Unfortunately, gaining insight into the fetal hormonal milieu presents a considerable challenge to human research. Indexing fetal hormone concentrations through sampling of fetal blood or amniotic fluid is impossible to do on a population level and is no longer a viable research tool. Assessment of exposure through alternative media (including maternal serum saliva, and hair) is feasible, but is still problematic because it: (1) may not be informative about fetus’ level of exposure 10; and (2) requires recruitment during pregnancy, which is impractical for many types of studies, particularly those examining health outcomes presenting much later in life. For these reasons, there has been great interest in establishing biomarkers of prenatal exposure to sex steroids, particularly androgens, that can be reliably measured later in life. Two putative biomarkers of prenatal androgen exposure, anogenital distance (AGD) and 2:4 digit length ratio, have emerged based on animal models and the human literature. Both biomarkers are gaining popularity as research tools and are appealing because their measurement is non-invasive, inexpensive, and replicable11, 12.
AGD, the distance from the anus to the genitals, is 50-100% longer in males than in females in humans and most other species. Androgen-insensitive male rodents show shortened AGD 13, and prenatal exposure to anti-androgens is associated with shorter AGD in males in both animal models and humans infants 14-17. AGD may serve as a marker of reproductive health in humans. Infant boys with hypospadias and cryptorchidism tend to have shorter AGD than controls 14, 18 and in adult men, short AGD is associated with poorer semen quality 19, 20 and lower testosterone levels 21. By contrast, prenatally androgenized female rodents show longer, more masculine AGD22 and the same is true of female infants with CAH, who experienced elevated androgen levels in utero23. Little research has examined the extent to which AGD is an indicator of reproductive health in women, however one study found that women with longer AGD are more likely to have multi-follicular ovaries than women with shorter AGD 24.
A second proposed measure of prenatal androgen exposure, the ratio of the lengths of the 2nd and 4th digits (or 2D:4D), is also sexually dimorphic and has been widely, and controversially, studied in relation to psychosocial and physiological endpoints in both sexes12, 25. Typically, in males, the 4th digit is longer than the 2nd, while the opposite is frequently true in women, resulting in females having a higher 2D:4D ratio, on average, than males 26. In rodent models, experimental manipulation of prenatal androgen and estrogen activity alters these sex-typical digit ratios27, 28. Evidence from clinical populations provides additional support for a possible relationship between prenatal hormones and digit ratios. Some, but not all, studies have found that women with CAH tend to have a lower, more masculine 2D:4D than female controls29-33, whereas males with complete androgen insensitivity (AIS) have a higher, more feminine 2D:4D than controls34. In cross-sectional studies, 2D:4D ratio has been linked to adult circulating sex steroid hormone concentrations, (most notably, testosterone and estradiol) in both sexes35, 36. Like AGD, variation in 2D:4D ratio has been associated with semen quality and fertility in males35, 37, 38. In females, associations between 2D:4D and reproductive function are less well-characterized, however digit ratio has been linked to sexual orientation and breast cancer, among other endpoints 29, 39, 40.
One potential complication for the use of these measures as biomarkers of prenatal androgen exposure in reproductive-age women is the possibility that they also covary with the dramatic cyclical changes in reproductive hormone concentrations across the menstrual cycle. For instance, a rodent study found that although AGD measurements were consistent within estrous cycle stages, they varied across stages, and after adjusting for body weight, AGD was longer in dioestrus than metoestrus 41. In a study of adult women, use of hormonal contraception predicted shorter AGD, further suggesting that adult circulating hormone levels could affect the measurement 24. We know of no direct assessment of changes in anogenital distance in women across the menstrual cycle, however other aspects of genital anatomy and physiology in cycling women, such as clitoral volume and vascularization, do show cyclic variation42. A study of 2D:4D ratio in adult women also found within-cycle variation in finger lengths and digit ratios in naturally cycling women (n=13), whereby the ratio tended to be higher during the pre-ovulatory cycle phases and decline after ovulation; in hormonal contraception users (n=6), patterns differed slightly 43. However, the validity of the digit measurement technique used in that study, which was based on photocopies of the hand, has been questioned37.
Thus, before AGD and 2D:4D can be more widely implemented as a research tools in adult women, it is methodologically important to establish whether there is cyclical variation in AGD and 2D:4D measurements. It is also of additional interest to determine the extent to which these two proposed biomarkers of the prenatal hormonal milieu are correlated. Although two observational studies in rodents have not found correlations between AGD and 2D:4D 44, 45, in experimental models, male rodents with feminized digit ratios also developed hypospadias28, suggesting that digit ratios and genital development may share common developmental pathways. This is further supported by human evidence that certain HOX gene mutations are associated with altered limb and reproductive development in both sexes46. However to our knowledge, to date, no published work has examined the association between digit ratio and AGD in a human population. To this end, in the current study, we employed a longitudinal study design, measuring AGD and 2D:4D digit ratio in twelve naturally cycling women for the duration of three menstrual cycles, taking measurements during the follicular, peri-ovulatory, and luteal phases of each cycle.
METHODS
Study population and overview of study activities
Subjects were recruited through flyers posted at the University of Rochester Medical Center from 2013 to 2014. Eligibility criteria included age 18-40, nulliparous with no pregnancy lasting more than ten weeks, not currently using any form of hormonal contraception, regularly menstruating, body mass index of 20-35 kg/m2, no evidence of any hormonal disorder (including polycystic ovary syndrome), and no history of injury to or surgery on the genital region. All study activities were approved by the Research Subjects Review Board (RSRB) at the University of Rochester, and written informed consent was obtained from all subjects. At consent, information was collected regarding subjects’ demographics, lifestyle, and gynecological history. Participants were instructed to contact the study team by day three of their next menstrual cycle (where day 1 is the first day of bleeding) to schedule physical exams for the early follicular phase (days 5-9), mid-cycle (days 13-15), and the luteal phase (days 19-22). At each visit, the subjects underwent: 1) AGD measurements; and 2) digit measurements. This process was repeated for two additional months, for a total of three months of follow-up with each subject (for a total of nine study visits per subject). Two trained examiners conducted all study activities, including AGD and digit measurements.
Anogenital distance measurements
Following procedures described elsewhere24, AGD measurements were taken using Vernier (dial) calipers. All measurements were made with the subject lying on an examination table in the lithotomy position, with the thighs positioned at a 45° angle (as measured with a protractor) in relation to the table. First, AGD-AC was measured as the distance from the center of the anus to the superior aspect of the clitoris (Figure 1). Second, AGD-AF was measured as the distance from the anus to the base of the fourchette (the bottom opening of the vagina). Each measurement was repeated three times with the calipers closed in between, to ensure independence of measurements.
Figure 1.
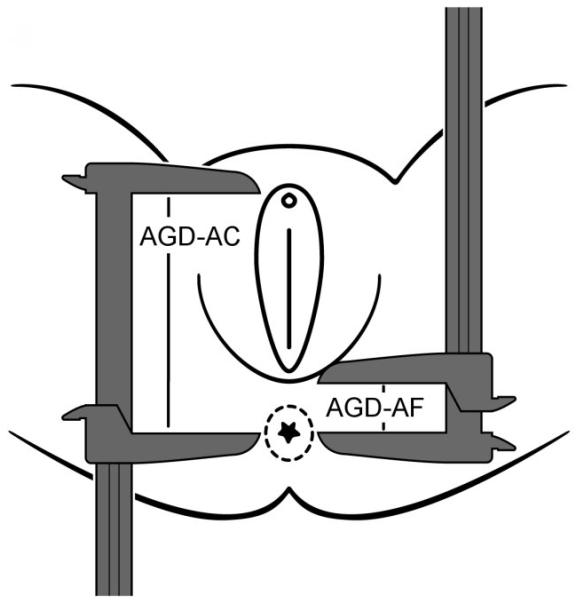
Measurement of anogenital distance in women as adapted with permission from Sathayanarayana et al. (2010)11.
Digit measurements
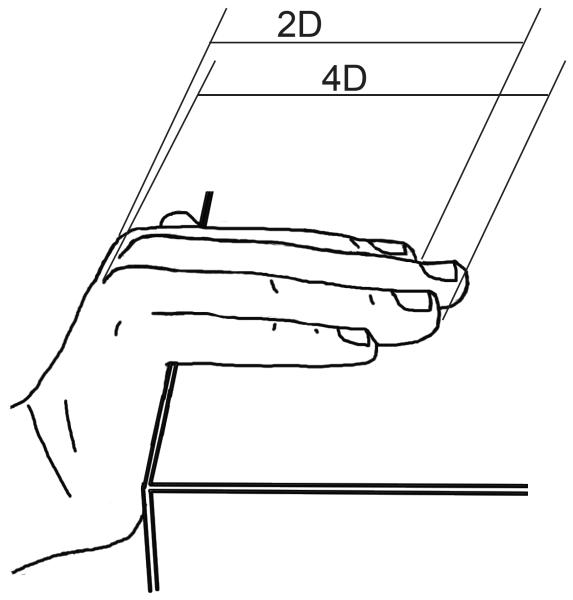
Digit measurements were made based on fixed anatomical landmarks using methods developed and described by Augur and Eustache (2011). Of several measurement methods tested in their study, this one showed by far the highest correlation with digit measurements made by the gold standard, radiographs. Per their method, the subject’s hand was positioned such that the digits were flat on the edge of the surface of the table with the palm wrapped downward at an angle of 100-120° relative to the fingers (Figure 2). Using a clean set of Vernier calipers set flat on their side, the second and fourth digits of each hand were measured from the base of the proximal phalanx to the end of the distal phalanx. Each digit was measured three times, for a total of 12 measurements per subject per visit (2 fingers × 2 hands × 3 measurements/finger). At each visit, 2D:4D ratio was calculated as the mean of the three measurements of the second digit divided by the mean of the three measurements of the 4th digit. Thus each subject had a total of 18 digit ratio values (one per hand per visit), in contrast to 27 sets of AGD measurements (three per visit each of AGD-AC and AGD-AF).
Figure 2.
Direct measurement of digit lengths using calipers as described by Auger and Eustache (2011)37.
Statistical analyses
We first calculated summary statistics on the study population for our variables of interest. These included our four outcome variables AGD-AF, AGD-AC, 2D:4D right hand, 2D:4D left hand) as well as several potential covariates (BMI, height, weight, age at menarche, and examiner). We used t-tests and correlations to examine relationships between these variables, and investigate possible multi-collinearity. In particular, we examined the correlation between AGD measures and digit ratios, using the woman, rather than the visit or cycle, as the unit of analysis. To do so, we averaged all measurements taken at all 9 visits for each subject and calculated Spearman’s correlations based on that average. We first examined correlations across all subjects, and secondarily, recalculated correlations excluding subjects who reported having ever suffered an injury to the 2nd or 4th digits.
We then selected variables for inclusion in multivariable models. We chose height as our preferred measurement of body size given that digit lengths are more plausibly related to skeletal size than body mass47. Our primary predictor, cycle phase, was modelled as two categorical variables (follicular and luteal phases, with mid-cycle as the referent). Thus, our final models included cycle phase, height, and age at menarche as independent variables. In analyses predicting digit ratios, we included self-reported history of injury to the relevant digits as an additional covariate. Due to the nested structure of the data (including multiple measurements, multiple visits, and multiple cycles for each subject), to optimize power we fit multi-level linear regression models with a variance components covariance matrix because we had no assumptions as to the relationship between the measurements across cycle phases. Four models were fit, one for each of the four outcomes (AGD-AC, AGD-AF, 2D:4D right, 2D:4D left). We considered the addition of an examiner term in multivariable models, however there were no significant differences in AGD or digit measurements across examiners, and thus examiner was not retained in final models. In sensitivity analyses, we considered weight and BMI as possible covariates, rather than height. Intra-observer variation was calculated as the coefficient of variation (CV). Across all analyses, standard model assumptions were checked. All analyses were conducted in SAS Version 9.3 (SAS Institute, Cary, NC, USA) and all p-values reported are two-tailed with an alpha-level of 0.05.
RESULTS
Twelve women participated in this longitudinal study of variation across the menstrual cycle, and complete measurements were obtained for all subjects at all study visits. The mean age was 25.6 years (min-max: 19-30) and all women reported cycling regularly (11-13 menstrual periods per year). The average age at menarche was 11.7 (min-max: 9-16) and the mean BMI was 23.1 kg/m2 (min-max: 20-26). Eight subjects were Caucasian and four were African-American. All subjects reported being right handed. Because the primary outcome measure of the study was AGD, potential subjects were not pre-screened for digit injuries and indeed, three subjects reported a history of injury to the 2nd or 4th digits of the right hand, and three subjects reported injuries to the digits of the left hand. These included digit dislocations, fractures, breaks, all occurring at least several years prior to participation in the study.
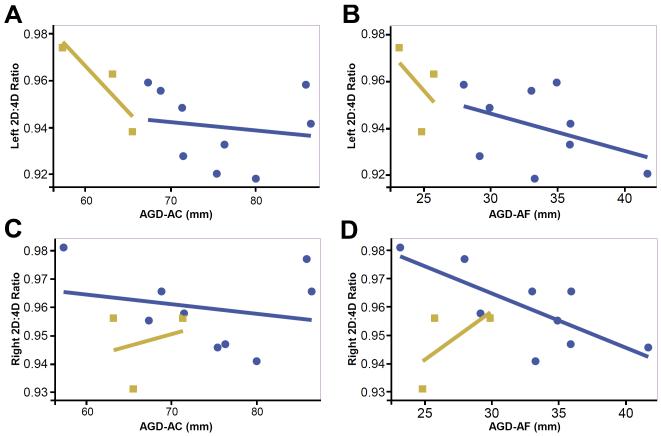
Digit ratios in the right and left hand were highly correlated (r=0.70) and the two AGD measurements (AGD-AC and AGD-AF) were moderately correlated with one another (r=0.53). In bivariate analyses, AGD and digit ratios were generally inversely related (Table 2; Figure 3). When all subjects were included in the analyses, the left hand digit ratio was significantly, inversely associated with AGD-AF (r=−0.57, p=0.05) and results were similar, albeit weaker, for AGD-AF (r=−0.52, p=0.08). No statistically significant associations were observed with right hand digit ratio and AGD measurement. When subjects with a history of digit injury were excluded from analyses, associations between left hand digit ratio and AGD were attenuated (AGD-AC: r=−0.37, p=0.33; AGD-AF: r=−0.33, p=0.38), however a moderate correlation was observed between right hand digit ratio and AGD-AF (r=−0.61, p=0.08).
Table 2.
Spearman correlations between anogenital distance and digit length measures within the entire cohort and the subset of women with no history of digit injuries [r (p-value)]1.
| All subjects (n=12) |
Subjects with no history of digit injuries (left hand: n=9; right hand: n=9) |
|||
|---|---|---|---|---|
|
| ||||
| 2D:4D Left | 2D:4D Right | 2D:4D Left | 2D:4D Right | |
| AGD-AC | −0.57 (0.05) | −0.002 (0.99) | −0.37 (0.33) | −0.13 (0.73) |
| AGD-AF | −0.52 (0.08) | −0.29 (0.35) | −0.33 (0.38) | −0.62 (0.08) |
For each individual, AGD and digit ratio values used in the correlations represent the average of all values over all visits (i.e. average of 9 sets of digit ratios and 27 sets of AGD measurements per woman).
Figure 3.
Bivariate relationships between AGD measures (AGD-AC, AGD-AF) and digit measurements (right and left hands). Blue circles indicate subjects with no history of digit injuries. Tan squares indicate subjects with a history of digit injuries.
In multivariable models, AGD-AF and AGD-AC measurements taken during the follicular and luteal phases did not differ from measurements taken mid-cycle (Table 3). Similarly, there were no significant differences in digit ratio in either hand across the menstrual cycle. Age at menarche was inversely associated with AGD-AC (β=−2.26, 95% CI: −3.38, −1.14), but not AGD-AF. By contrast, age at menarche was positively associated with digit ratio in both the left (β=0.005, 95% CI: 0.002, 0.007) and right (β=0.003, 95% CI: 0.001, 0.004) hands. Height was similarly positively associated with right hand digit ratio (β=0.003, 95% CI: 0.0001, 0.004), but not left hand digit ratio. Finally, self-report of history of injury to the right digits showed a weak association with right hand digit ratio (β=−0.009, 95% CI: −0.017, 0.001), however self-report of history of injury to the left digits was not associated with left hand digit ratio (β=0.010, 95% CI: −0.002, −0.022). In sensitivity analyses, the inclusion of BMI or weight (rather than height) did not change the estimates for the effects of cycle phase on AGD or digit ratios (not shown). Associations with age at menarche at AGD-AC were attenuated when either BMI (β=−1.20, 95% CI: −2.51, 0.11) or weight (β=−1.20, 95% CI: −2.46, 0.06) was included rather than height. Associations between age at menarche and digit ratios on both hands were slightly attenuated, but still statistically significant when BMI or weight was considered instead of height (not shown).
Table 3.
Multi-level, multivariable linear regression models predicting four proposed biomarkers of prenatal androgen exposure AGD-AS, AGD-AF, 2D:4D (right); 2D:4D (left) (n=12 women, measured at three points in the menstrual cycle, for three cycles).
| Outcome; β (95%CI) | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| AGD-AF | p-value | AGD-AC | p-value | 2D:4D (right) | p-value | 2D:4D (left) | p-value | |
| Cycle phase1 | ||||||||
| Follicular (day 5-9) | 0.367 (−2.885, 3.619) | 0.82 | −0.961 (−5.777, 3.855) | 0.70 | 0.004(−0.003, 0.012) | 0.24 | −0.001 (−0.010, 0.009) | 0.92 |
| Luteal (day 19-22) | 0.021 (−3.231, 3.273) | 0.99 | −0.471 (−5.287, 4.344) | 0.85 | 0.001 (−0.006, 0.009) | 0.72 | 0.0001 (−0.010, 0.010) | 0.98 |
| Height (inches) | 0.297 (−0.364, 0.957) | 0.40 | 0.913 (−0.065, 1.891) | 0.10 | 0.003 (0.001, 0.004) | 0.01 | 0.001 (−0.001, 0.003) | 0.37 |
| Age at menarche (yrs) | −0.687 (−1.445, 0.071) | 0.11 | −2.259 (−3.381, −1.137) | 0.003 | 0.003 (0.001, 0.004) | 0.02 | 0.005 (0.002, 0.007) | 0.008 |
| History of finger injury2 | −0.009 (−0.017, 0.001) | 0.06 | 0.010 (−0.002, 0.022) | 0.15 | ||||
Referent: mid-cycle (day 13-15).
For right ratio, this is right finger injuries and for the left ratio, this is left finger injuries.
Inter-examiner variation (as calculated by CV) was low and consistent across all cycle phases for both AGD and digit lengths (Table 4). The CVs were higher for AGD-AF (ranging from 5.0% to 5.6% across phases) than for AGD-AC (ranging from 2.1% to 3.1% across phases). CVs for digit lengths were under 1.0% for all cycle phases.
Table 4.
Mean (SD) for the coefficient of variation (CV)1 within examiner by cycle phase.
| AGD | Digit lengths | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| AGD-AC | AGD-AF | 2D-right | 4D-right | 2D-left | 4D-left | |
| Follicular | 2.7% (2.1%) | 5.0% (2.6%) | 0.5% (0.3%) | 0.5% (0.3%) | 0.8% (1.1%) | 0.7% (0.8%) |
| Mid-Cycle | 3.1% (2.4%) | 5.4% (2.7%) | 0.6% (0.3%) | 0.5% (0.4%) | 0.7% (0.5%) | 0.7% (0.7%) |
| Luteal | 2.1% (1.5%) | 5.6% (3.8%) | 0.5% (0.3%) | 0.5% (0.4%) | 0.6% (0.4%) | 0.5% (0.2%) |
CV=(standard deviation/mean)*100
DISCUSSION
In the current longitudinal study of cycling women, we investigated whether two proposed biomarkers of prenatal androgen exposure, anogenital distance and digit ratio, vary according to menstrual cycle phase. Our results demonstrate that both measures are stable across the menstrual cycle, which is important if they are to be used as research tools in adult women. In addition, despite the small sample size, we found inverse associations between AGD and 2D:4D, as would be predicted based on the literature, and in particular, left hand digit ratios showed stronger associations with AGD measures. We found further evidence of the developmental origins of these biomarkers in that both AGD and digit ratios were associated with age at menarche.
Previous work has suggested that AGD may vary in relation to circulating hormone levels in adult women. A rodent study found that AGD was significantly different during dioestrus as compared to metoestrus41, and in a population of young Spanish women, those using hormonal contraception had significantly shorter AGD than naturally cycling women, indicating that perhaps AGD is responsive to exogenous hormones during adulthood. Unfortunately, in that cross-sectional study, all AGD measurements were taken during the early follicular phase, so cyclical variation could not be examined24. However, our results suggest that within-woman variation in endogenous ovarian hormones across the menstrual cycle is unlikely to alter AGD. A longitudinal study is needed to determine whether this is true of women using hormonal contraception as well.
One study reported cyclical fluctuation in digit ratios in naturally cycling women, but not hormonal contraception users, across the menstrual cycle43. One possible explanation for the discrepancy between the current results and that study is differences in measurement techniques. The technique used in the current study was specifically chosen because, of all methods examined, it most closely correlated with digit lengths measured from the gold standard, radiographs, which reflect only bone length and not the fat pad at the distal phalanx37. Other popular measurement techniques based on photocopying the hand or drawing an outline show typically lower correlation with radiograph-based measurements and capture greater variation in fat deposition 37, 48. Fat tissue has estrogen and androgen receptors49, 50, and indeed, there appears to be cyclical variation in other soft-tissue traits in women, including those that are bilaterally symmetrical, such as breast and ear size 51. This may explain the within-cycle variation in digit ratios observed in previous work using photocopy-based measurements43. Given that variation in bone length, rather than fat mass, is typically of greatest interest with respect to prenatal exposures, the current measurement technique, which is stable across the cycle, may be preferable as a research tool in the future.
If digit ratios and AGD both convey information about prenatal androgen exposure, then it follows that the two anatomical measures may be associated. Even within this small sample, the two biomarkers showed relationships in the expected direction, such that longer (more “masculine”) AGD was associated with lower (more “masculine”) digit ratio, and vice versa. These correlations were significant only for the left hand ratio and only when we considered the full sample (including women with a history of digit injuries). Unfortunately, for each hand, three (of twelve) subjects reported having sustained an injury to either the 2nd or 4th digit at some point in their lifetimes and it is unknown whether those injuries may have altered their digit ratio. Further research in a larger sample of women is needed to confirm this preliminary finding of within-individual associations between AGD and digit ratios, as is analogous work in men and children.
To our knowledge, no prior studies have simultaneously examined AGD and digit ratios in humans, but there are several reports in animal models. In a study of female rhesus macaques exposed to exogenous androgens during early to mid-gestation, AGD was masculinized (lengthened) compared to controls, whereas 2D:4D was increased, due to elongation of the 2nd digit52. Because absolute digit lengths are longer in male macaques than females, the elongation of the 2nd digit was interpreted as masculinization by the authors, however the overall effect was unexpectedly, a feminization of the 2D:4D ratio. Notably, the elongation was limited to the right hand and was only evident in photocopy-based measurement, not in radiographs, suggesting that soft tissue, but not bone growth, was affected in androgenized animals 52. Interestingly, these results contrast with work in rodents, in which manipulation of the androgen to estrogen signaling preferentially altered the growth of the 4th digit28. In CD-1 mice, the 4th digit shows increased expression of androgen receptors (AR) and estrogen receptor α (ER-α) as compared to the 2nd digit. Inactivation of the AR or the administration of estrogen from gestational day 12.5 to 15.5 resulted in decreased growth of the 4th digit in male mice, whereas inactivation of ER-α or the administration of androgens during the same time period increased growth of the 4th digit in females, leading the authors to conclude that 4th digit bone growth drives sex differences in 2D:4D ratio. Although that study did not specifically report on the correlation between digit ratios and AGD in their models, the authors noted that males with experimentally feminized digit ratios also had hypospadias28, a hallmark of the testicular dysgenesis syndrome. That mutations in certain HOX genes are linked to alterations in both limb and reproductive development in humans (males and females) is additional evidence of shared ontogeny46. It is worth noting that two observational studies in rodents have not found correlations between AGD and digit ratios, however in those studies, no sexual dimorphism in digit lengths was observed 44, 45. Further observational work examining the association between digit ratios and genital development in both control and clinical populations (e.g. CAH, AIS, and males with genital anomalies) is needed to inform this discussion.
We also found that age at menarche was a predictor of both digit ratio and AGD. Earlier age at menarche was associated with longer (more masculine) AGD and a lower, more masculine digit ratio. The direction of the relationship is somewhat unexpected given that a later age at menarche (here associated with more feminine digit ratio and AGD) has been associated with subfecundity and lower ovarian hormone concentrations in some, but not all, studies 53-55. In the only study of AGD in adult women, no associations with age at menarche were reported24. However our results are in line with recent work on age at menarche and digit ratios. A prospective study which measured digit lengths in pre-menarcheal girls and followed them until the occurrence of menarche found a positive relationship between the two56. Similarly, in two large, independent samples (The Avon Longitudinal Study of Parents and Children and the Brisbane Adolescent Twin Study), a single variant in the LIN28B gene, a regulator of developmental timing, was linked to both high digit ratios and delayed menarche 57. However two other studies have found inverse associations between menarcheal timing and 2D:4D 58, 59 and another has found no association 60. Notably, the studies finding inverse associations relied on self-reported age at menarche, and in one study, self-measured digit lengths, raising the possibility of considerable measurement error58. It is plausible that pubertal timing might be associated with AGD and digit ratios through the prenatal hormonal milieu61. It is also possible that there are genetic polymorphisms that influence genital and digit development, as well as postnatal developmental trajectories 57, 62. For example, polymorphisms of ESR1, encoding estrogen receptor α, are associated with both AGD in boys as well as growth and timing of puberty 62, 63 and it is plausible that similar genetic mechanisms may operate in females. LIN28B presents one possibility that merits further research.
As in other studies, relationships across AGD measures and digit ratios across hands were not always consistent. Here, age at menarche was strongly associated with AGD-AC, the longer of the two AGD measures, but more weakly with AGD-AF. Notably, in this and other work 24, AGD-AF shows greater inter-observer variation, suggesting that the fourchette landmark may be more difficult to identify. Similarly, the associations between AGD measures and left hand digit ratio were far stronger than the associations with right hand digit ratio. However, it is also possible that digit injuries altered the digit ratio, particularly on the right hand, thus obscuring our ability to detect relationships, if any. Differences in digit ratios in the right and left hands are frequently observed in studies, and there is conflicting evidence as to which hand may be a better index of prenatal androgen exposure, if any 56, 64-67. Handedness may be important to consider in this respect. In this study, all women were right handed, however future research including both right and left handed individuals may help to further explain the differences observed when right or left hand 2D:4D is considered.
A notable strength of our study was the longitudinal design, which provided increased power even with a small sample. Each subject had three study visits at pre-defined points in the cycle, for three cycles. At each study visit, three sets of AGD and digit measurements were made. Despite this intensive protocol, all subjects completed all study visits, so our data set is complete. We used multi-level modelling to take advantage of the numerous measurements per subject. Nevertheless, it is possible that the study was insufficiently powered to detect associations and a larger sample size is preferable for future work. This is particularly true for the “woman-level” questions emerging from our data, namely further exploring the relationship between AGD and 2D:4D as well as better understanding how these biomarkers relate to other developmental endpoints, such as age at menarche.
Ultimately, additional research is needed to determine whether either of these anatomic measures is a valid index of prenatal androgen exposure, and the extent to which they predict reproductive outcomes in women. Our results simplify this future work, by demonstrating that using the measurement techniques that we have followed herein, there is little, if any, cyclical variation in the measures in naturally cycling, nulliparous women. Whether these findings can be extrapolated to women using hormonal contraception or parous women (whose AGD may be altered after vaginal childbirth) is unknown. Nevertheless, the results speak to the feasibility of using both measures as research tools that may aid in understanding the downstream sequelae of the prenatal hormonal milieu in women.
Table 1.
Characteristics of the study population (n=12).
| Characteristic | Mean (SD) |
|---|---|
| Age (years) | 25.6 (2.9) |
| Height (inches) | 65.4 (2.2) |
| Weight (pounds) | 140.8 (17.9) |
| BMI (kg/m2) | 23.1 (2.2) |
| Age at menarche (years) | 11.7 (1.9) |
| Number of periods per year | 11.9 (0.5) |
| Typical number of days of bleeding | 5.2 (0.6) |
| Anogenital distance (mm)1 | |
| Anus to fourchette (AGD-AF) | 31.3 (5.5) |
| Anus to clitoris (AGD-AC) | 72.4 (8.9) |
| Digit lengths and ratios1 | |
| 2D (right) | 97.8 (5.0) |
| 4D (right) | 102.2 (5.5) |
| 2D (left) | 96.8 (4.4) |
| 4D (left) | 102.6 (6.2) |
| 2D:4D (right) | 0.96 (0.01) |
| 2D:4D (left) | 0.94 (0.02) |
|
| |
| % (n) | |
|
| |
| Race | |
| Caucasian | 67 (8) |
| African-American | 33 (4) |
| Smoker | 8 (1) |
| Alcohol use (any) | 83 (10) |
To obtain this value, we took the within-woman mean for each measurement, then took the mean across women.
Acknowledgments
We thank Lynda Kochman, RN and Shaya Greathouse for their work and Dr. Kathy Hoeger for her assistance with study design. In addition, we thank Dr. Sarah Thurston for her biostatistical expertise and Rebecca Rowley for her illustration.
Financial support. Funding for the current study was provided by the Mae Stone Goode foundation with additional support from the National Institutes of Health (K12 ES019851-01; P30 ES001247).
Footnotes
Conflicts of interest. None.
Ethical Standards. The authors assert that all procedures contributing to this work comply with the ethical standards of the Helsinki Declaration of 1975, as revised in 2008, and it has been approved by the institutional committee at the University of Rochester School of Medicine and Dentistry.
References
- 1.Birch RA, Padmanabhan V, Foster DL, Unsworth WP, Robinson JE. Prenatal programming of reproductive neuroendocrine function: fetal androgen exposure produces progressive disruption of reproductive cycles in sheep. Endocrinology. 2003;144(4):1426–1434. doi: 10.1210/en.2002-220965. [DOI] [PubMed] [Google Scholar]
- 2.Sullivan SD, Moenter SM. Prenatal androgens alter GABAergic drive to gonadotropin-releasing hormone neurons: implications for a common fertility disorder. Proc Natl Acad Sci U S A. 2004;101(18):7129–7134. doi: 10.1073/pnas.0308058101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Witham EA, Meadows JD, Shojaei S, Kauffman AS, Mellon PL. Prenatal exposure to low levels of androgen accelerates female puberty onset and reproductive senescence in mice. Endocrinology. 2012;153(9):4522–4532. doi: 10.1210/en.2012-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goy RW, McEwen BS. Sexual differentiation of the brain. MIT Press; Cambridge, NA: 1980. [Google Scholar]
- 5.Hines M. Brain gender. Oxford University Press; New York: 2004. [Google Scholar]
- 6.Constantinescu M, Hines M. Relating Prenatal Testosterone Exposure to Postnatal Behavior in Typically Developing Children: Methods and Findings. Child Development Perspectives. 2012;6(4):407–413. [Google Scholar]
- 7.Giusti RM, Iwamoto K, Hatch EE. Diethylstilbestrol revisited: a review of the long-term health effects. Ann Intern Med. 1995;122(10):778–788. doi: 10.7326/0003-4819-122-10-199505150-00008. [DOI] [PubMed] [Google Scholar]
- 8.Hines M. Psychosexual development in individuals who have female pseudohermaphroditism. Child Adolesc Psychiatr Clin N Am. 2004;13(3):641–656. ix. doi: 10.1016/j.chc.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 9.Mueller SC, Grissom EM, Dohanich GP. Assessing gonadal hormone contributions to affective psychopathologies across humans and animal models. Psychoneuroendocrinology. 2014;46C:114–128. doi: 10.1016/j.psyneuen.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 10.Kuijper EA, Ket JC, Caanen MR, Lambalk CB. Reproductive hormone concentrations in pregnancy and neonates: a systematic review. Reproductive biomedicine online. 2013;27(1):33–63. doi: 10.1016/j.rbmo.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 11.Sathyanarayana S, Beard L, Zhou C, Grady R. Measurement and correlates of ano-genital distance in healthy, newborn infants. Int J Androl. 2010;33(2):317–323. doi: 10.1111/j.1365-2605.2009.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McIntyre MH. The use of digit ratios as markers for perinatal androgen action. Reproductive biology and endocrinology : RB&E. 2006;4:10. doi: 10.1186/1477-7827-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yeh S, Tsai MY, Xu Q, et al. Generation and characterization of androgen receptor knockout (ARKO) mice: an in vivo model for the study of androgen functions in selective tissues. Proc Natl Acad Sci U S A. 2002;99(21):13498–13503. doi: 10.1073/pnas.212474399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swan SH, Main KM, Liu F, et al. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ Health Perspect. 2005;113(8):1056–1061. doi: 10.1289/ehp.8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suzuki Y, Yoshinaga J, Mizumoto Y, Serizawa S, Shiraishi H. Foetal exposure to phthalate esters and anogenital distance in male newborns. Int J Androl. 2012;35(3):236–244. doi: 10.1111/j.1365-2605.2011.01190.x. [DOI] [PubMed] [Google Scholar]
- 16.Bustamante-Montes LP, Hernandez-Valero MA, Flores-Pimentel D, et al. Prenatal exposure to phthalates is associated with decreased anogenital distance and penile size in male newborns. Journal of developmental origins of health and disease. 2013;4(4) doi: 10.1017/S2040174413000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Welsh M, Saunders PT, Fisken M, et al. Identification in rats of a programming window for reproductive tract masculinization, disruption of which leads to hypospadias and cryptorchidism. J Clin Invest. 2008;118(4):1479–1490. doi: 10.1172/JCI34241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsieh MH, Eisenberg ML, Hittelman AB, Wilson JM, Tasian GE, Baskin LS. Caucasian male infants and boys with hypospadias exhibit reduced anogenital distance. Hum Reprod. 2012;27(6):1577–1580. doi: 10.1093/humrep/des087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mendiola J, Stahlhut RW, Jorgensen N, Liu F, Swan SH. Shorter anogenital distance predicts poorer semen quality in young men in Rochester, New York. Environ Health Perspect. 2011;119(7):958–963. doi: 10.1289/ehp.1103421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eisenberg ML, Shy M, Walters RC, Lipshultz LI. The relationship between anogenital distance and azoospermia in adult men. Int J Androl. 2012;35(5):726–730. doi: 10.1111/j.1365-2605.2012.01275.x. [DOI] [PubMed] [Google Scholar]
- 21.Eisenberg ML, Jensen TK, Walters RC, Skakkebaek NE, Lipshultz LI. The relationship between anogenital distance and reproductive hormone levels in adult men. J Urol. 2012;187(2):594–598. doi: 10.1016/j.juro.2011.10.041. [DOI] [PubMed] [Google Scholar]
- 22.Dean A, Smith LB, Macpherson S, Sharpe RM. The effect of dihydrotestosterone exposure during or prior to the masculinization programming window on reproductive development in male and female rats. Int J Androl. 2012;35(3):330–339. doi: 10.1111/j.1365-2605.2011.01236.x. [DOI] [PubMed] [Google Scholar]
- 23.Callegari C, Everett S, Ross M, Brasel JA. Anogenital ratio: measure of fetal virilization in premature and full-term newborn infants. J Pediatr. 1987;111(2):240–243. doi: 10.1016/s0022-3476(87)80075-6. [DOI] [PubMed] [Google Scholar]
- 24.Mendiola J, Roca M, Minguez-Alarcon L, et al. Anogenital distance is related to ovarian follicular number in young Spanish women: a cross-sectional study. Environmental Health. 2012;11:90. doi: 10.1186/1476-069X-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dean A, Sharpe RM. Clinical review: Anogenital distance or digit length ratio as measures of fetal androgen exposure: relationship to male reproductive development and its disorders. J Clin Endocrinol Metab. 2013;98(6):2230–2238. doi: 10.1210/jc.2012-4057. [DOI] [PubMed] [Google Scholar]
- 26.Peters M, Mackenzie K, Bryden P. Finger length and distal finger extent patterns in humans. Am J Phys Anthropol. 2002;117(3):209–217. doi: 10.1002/ajpa.10029. [DOI] [PubMed] [Google Scholar]
- 27.Auger J, Le Denmat D, Berges R, et al. Environmental levels of oestrogenic and antiandrogenic compounds feminize digit ratios in male rats and their unexposed male progeny. Proceedings Biological sciences / The Royal Society. 2013;280(1768):20131532. doi: 10.1098/rspb.2013.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng Z, Cohn MJ. Developmental basis of sexually dimorphic digit ratios. Proc Natl Acad Sci U S A. 2011;108(39):16289–16294. doi: 10.1073/pnas.1108312108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown WM, Finn CJ, Cooke BM, Breedlove SM. Differences in finger length ratios between self-identified "butch" and "femme" lesbians. Arch Sex Behav. 2002;31(1):123–127. doi: 10.1023/a:1014091420590. [DOI] [PubMed] [Google Scholar]
- 30.Buck JJ, Williams RM, Hughes IA, Acerini CL. In-utero androgen exposure and 2nd to 4th digit length ratio-comparisons between healthy controls and females with classical congenital adrenal hyperplasia. Hum Reprod. 2003;18(5):976–979. doi: 10.1093/humrep/deg198. [DOI] [PubMed] [Google Scholar]
- 31.Rivas MP, Moreira LM, Santo LD, Marques AC, El-Hani CN, Toralles MB. New studies of second and fourth digit ratio as a morphogenetic trait in subjects with congenital adrenal hyperplasia. American journal of human biology : the official journal of the Human Biology Council. 2014;26(4):559–561. doi: 10.1002/ajhb.22545. [DOI] [PubMed] [Google Scholar]
- 32.Ciumas C, Linden Hirschberg A, Savic I. High fetal testosterone and sexually dimorphic cerebral networks in females. Cereb Cortex. 2009;19(5):1167–1174. doi: 10.1093/cercor/bhn160. [DOI] [PubMed] [Google Scholar]
- 33.Okten A, Kalyoncu M, Yaris N. The ratio of second- and fourth-digit lengths and congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Early Hum Dev. 2002;70(1-2):47–54. doi: 10.1016/s0378-3782(02)00073-7. [DOI] [PubMed] [Google Scholar]
- 34.Berenbaum SA, Bryk KK, Nowak N, Quigley CA, Moffat S. Fingers as a marker of prenatal androgen exposure. Endocrinology. 2009;150(11):5119–5124. doi: 10.1210/en.2009-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manning JT, Scutt D, Wilson J, Lewis-Jones DI. The ratio of 2nd to 4th digit length: a predictor of sperm numbers and concentrations of testosterone, luteinizing hormone and oestrogen. Hum Reprod. 1998;13(11):3000–3004. doi: 10.1093/humrep/13.11.3000. [DOI] [PubMed] [Google Scholar]
- 36.McIntyre MH, Chapman JF, Lipson SF, Ellison PT. Index-to-ring finger length ratio (2D:4D) predicts levels of salivary estradiol, but not progesterone, over the menstrual cycle. American journal of human biology : the official journal of the Human Biology Council. 2007;19(3):434–436. doi: 10.1002/ajhb.20623. [DOI] [PubMed] [Google Scholar]
- 37.Auger J, Eustache F. Second to fourth digit ratios, male genital development and reproductive health: a clinical study among fertile men and testis cancer patients. Int J Androl. 2011;34(4 Pt 2):e49–58. doi: 10.1111/j.1365-2605.2010.01124.x. [DOI] [PubMed] [Google Scholar]
- 38.Lu H, Huo ZH, Liu YJ, Shi ZY, Zhao JL. Correlations between digit ratio and infertility in Chinese men. Early Hum Dev. 2012;88(11):865–869. doi: 10.1016/j.earlhumdev.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 39.Hong L, Zhan-Bing M, Zhi-Yun S, Xiao-Xia S, Jun-Li Z, Zheng-Hao H. Digit ratio (2D:4D) in Chinese women with breast cancer. American journal of human biology : the official journal of the Human Biology Council. 2014;26(4):562–564. doi: 10.1002/ajhb.22546. [DOI] [PubMed] [Google Scholar]
- 40.Muller DC, Baglietto L, Manning JT, et al. Second to fourth digit ratio (2D:4D), breast cancer risk factors, and breast cancer risk: a prospective cohort study. Br J Cancer. 2012;107(9):1631–1636. doi: 10.1038/bjc.2012.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dusek A, Bartos L. Variation in ano-genital distance in spontaneously cycling female mice. Reproduction in domestic animals = Zuchthygiene. 2012;47(6):984–987. doi: 10.1111/j.1439-0531.2012.02003.x. [DOI] [PubMed] [Google Scholar]
- 42.Morotti E, Battaglia B, Persico N, et al. Clitoral changes, sexuality, and body image during the menstrual cycle: a pilot study. The journal of sexual medicine. 2013;10(5):1320–1327. doi: 10.1111/jsm.12103. [DOI] [PubMed] [Google Scholar]
- 43.Mayhew TM, Gillam L, McDonald R, Ebling FJ. Human 2D (index) and 4D (ring) digit lengths: their variation and relationships during the menstrual cycle. J Anat. 2007;211(5):630–638. doi: 10.1111/j.1469-7580.2007.00801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manno FA., 3rd Measurement of the digit lengths and the anogenital distance in mice. Physiol Behav. 2008;93(1-2):364–368. doi: 10.1016/j.physbeh.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 45.Hurd PL, Bailey AA, Gongal PA, Yan RH, Greer JJ, Pagliardini S. Intrauterine position effects on anogenital distance and digit ratio in male and female mice. Arch Sex Behav. 2008;37(1):9–18. doi: 10.1007/s10508-007-9259-z. [DOI] [PubMed] [Google Scholar]
- 46.Goodman FR, Scambler PJ. Human HOX gene mutations. Clin Genet. 2001;59(1):1–11. doi: 10.1034/j.1399-0004.2001.590101.x. [DOI] [PubMed] [Google Scholar]
- 47.Barut C, Tan U, Dogan A. Association of height and weight with second to fourth digit ratio (2D:4D) and sex differences. Percept Mot Skills. 2008;106(2):627–632. doi: 10.2466/pms.106.2.627-632. [DOI] [PubMed] [Google Scholar]
- 48.Wallen K. Does finger fat produce sex differences in second to fourth digit ratios? Endocrinology. 2009;150(11):4819–4822. doi: 10.1210/en.2009-0986. [DOI] [PubMed] [Google Scholar]
- 49.Dieudonne MN, Leneveu MC, Giudicelli Y, Pecquery R. Evidence for functional estrogen receptors alpha and beta in human adipose cells: regional specificities and regulation by estrogens. American journal of physiology Cell physiology. 2004;286(3):C655–661. doi: 10.1152/ajpcell.00321.2003. [DOI] [PubMed] [Google Scholar]
- 50.Laughlin GA, Barrett-Connor E, May S. Sex-specific association of the androgen to oestrogen ratio with adipocytokine levels in older adults: the Rancho Bernardo Study. Clin Endocrinol (Oxf) 2006;65(4):506–513. doi: 10.1111/j.1365-2265.2006.02624.x. [DOI] [PubMed] [Google Scholar]
- 51.Scutt D, Manning JT. Symmetry and ovulation in women. Hum Reprod. 1996;11(11):2477–2480. doi: 10.1093/oxfordjournals.humrep.a019142. [DOI] [PubMed] [Google Scholar]
- 52.Abbott AD, Colman RJ, Tiefenthaler R, Dumesic DA, Abbott DH. Early-to-mid gestation fetal testosterone increases right hand 2D:4D finger length ratio in polycystic ovary syndrome-like monkeys. PloS one. 2012;7(8):e42372. doi: 10.1371/journal.pone.0042372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Apter D, Reinila M, Vihko R. Some endocrine characteristics of early menarche, a risk factor for breast cancer, are preserved into adulthood. Int J Cancer. 1989;44(5):783–787. doi: 10.1002/ijc.2910440506. [DOI] [PubMed] [Google Scholar]
- 54.Wise LA, Mikkelsen EM, Rothman KJ, et al. A prospective cohort study of menstrual characteristics and time to pregnancy. Am J Epidemiol. 2011;174(6):701–709. doi: 10.1093/aje/kwr130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guldbrandsen K, Hakonsen LB, Ernst A, et al. Age of menarche and time to pregnancy. Hum Reprod. 2014;29(9):2058–2064. doi: 10.1093/humrep/deu153. [DOI] [PubMed] [Google Scholar]
- 56.Oberg AS, Villamor E. Low digit ratio predicts early age at menarche in Colombian schoolgirls. Paediatr Perinat Epidemiol. 2012;26(5):448–455. doi: 10.1111/j.1365-3016.2012.01310.x. [DOI] [PubMed] [Google Scholar]
- 57.Medland SE, Zayats T, Glaser B, et al. A variant in LIN28B is associated with 2D:4D finger-length ratio, a putative retrospective biomarker of prenatal testosterone exposure. Am J Hum Genet. 2010;86(4):519–525. doi: 10.1016/j.ajhg.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Manning JT, Fink B. Is low digit ratio linked with late menarche? Evidence from the BBC internet study. American journal of human biology : the official journal of the Human Biology Council. 2011;23(4):527–533. doi: 10.1002/ajhb.21186. [DOI] [PubMed] [Google Scholar]
- 59.Matchock RL. Low digit ratio (2D:4D) is associated with delayed menarche. American journal of human biology : the official journal of the Human Biology Council. 2008;20(4):487–489. doi: 10.1002/ajhb.20763. [DOI] [PubMed] [Google Scholar]
- 60.Helle S. Does second-to-fourth digit length ratio (2D:4D) predict age at menarche in women? American journal of human biology : the official journal of the Human Biology Council. 2010;22(3):418–420. doi: 10.1002/ajhb.21000. [DOI] [PubMed] [Google Scholar]
- 61.Yermachenko A, Dvornyk V. Nongenetic determinants of age at menarche: a systematic review. BioMed research international. 2014;2014:371583. doi: 10.1155/2014/371583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sathyanarayana S, Swan SH, Farin FM, et al. A pilot study of the association between genetic polymorphisms involved in estrogen signaling and infant male genital phenotypes. Asian journal of andrology. 2012;14(5):766–772. doi: 10.1038/aja.2012.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kang BH, Kim SY, Park MS, Yoon KL, Shim KS. Estrogen receptor alpha polymorphism in boys with constitutional delay of growth and puberty. Annals of pediatric endocrinology & metabolism. 2013;18(2):71–75. doi: 10.6065/apem.2013.18.2.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ventura T, Gomes MC, Pita A, Neto MT, Taylor A. Digit ratio (2D:4D) in newborns: influences of prenatal testosterone and maternal environment. Early Hum Dev. 2013;89(2):107–112. doi: 10.1016/j.earlhumdev.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 65.Cattrall FR, Vollenhoven BJ, Weston GC. Anatomical evidence for in utero androgen exposure in women with polycystic ovary syndrome. Fertil Steril. 2005;84(6):1689–1692. doi: 10.1016/j.fertnstert.2005.05.061. [DOI] [PubMed] [Google Scholar]
- 66.van Anders SM, Vernon PA, Wilbur CJ. Finger-length ratios show evidence of prenatal hormone-transfer between opposite-sex twins. Horm Behav. 2006;49(3):315–319. doi: 10.1016/j.yhbeh.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 67.Putz DA, Gaulin SJC, Sporter RJ, McBurney DH. Sex hormones and finger length: what does 2D:4D indicate? Evolution and Human Behavior. 2004;25:182–199. [Google Scholar]