Abstract
Group A rotaviruses (RVAs) are 11-segmented, double-stranded RNA viruses and important causes of gastroenteritis in the young of many animal species. Previous studies have suggested that human Wa-like RVAs share a close evolutionary relationship with porcine RVAs. Specifically, the VP1-VP3 and NSP2-5/6 genes of these viruses are usually classified as genotype 1 with >81% nucleotide sequence identity. Yet, it remains unknown whether the genotype 1 genes and proteins of human Wa-like strains are distinguishable from those of porcine strains. To investigate this, we performed comprehensive bioinformatic analyses using all known genotype 1 gene sequences. The RVAs analyzed represent wildtype strains isolated from humans or pigs at various geographical locations during the years of 2004–2013, including 11 newly-sequenced porcine RVAs from Brazil. We also analyzed archival strains that were isolated during the years of 1977–1992 as well as atypical strains involved in inter-species transmission between humans and pigs. We found that, in general, the genotype 1 genes of typical modern human Wa-like RVAs clustered together in phylogenetic trees and were separate from those of typical modern porcine RVAs. The only exception was for the NSP5/6 gene, which showed no host-specific phylogenetic clustering. Using amino acid sequence alignments, we identified 34 positions that differentiated the VP1-VP3, NSP2, and NSP3 genotype 1 proteins of typical modern human Wa-like RVAs versus typical modern porcine RVAs and documented how these positions vary in the archival/unusual isolates. No host-specific amino acid positions were identified for NSP4, NSP5, or NSP6. Altogether, the results of this study support the notion that human Wa-like RVAs and porcine RVAs are evolutionarily related, but indicate that some of their genotype 1 genes and proteins have diverged over time possibly as a reflection of sequestered replication and protein co-adaptation in their respective hosts.
Keywords: Group A rotavirus, Genomics, Porcine, Human, Genotype 1, Sub-genotypic diversity, Amino acid changes, Host tropism
1. Introduction
Group A rotaviruses (RVAs) are gastrointestinal pathogens of many animal species. In humans, RVAs are a leading cause of childhood diarrheal death, particularly in developing regions of the world (Tate et al., 2012). RVAs are also a major cause of acute viral diarrhea in suckling and weaned piglets, imparting significant financial losses to the pork industry (Chang et al., 2012). The RVA genome consists of 11 segments of double-stranded RNA, which are encapsidated within a non-enveloped, triple-layered virion particle (Estes and Kapikian, 2007). The outermost layer of the RVA virion is made up of VP4 and VP7, while the middle layer is comprised of VP6. The innermost core shell is formed of VP2, and it surrounds the viral genome and RNA processing enzymes (VP1 and VP3). Five or six viral non-structural proteins (NSP1-NSP5/6) are made within infected cells and play various roles during viral replication.
RVAs are classified according to a system that designates a specific genotype for each of the 11 viral genome segments (i.e., genes) based on their nucleotide sequences and established percent identity cut-off values (Matthijnssens et al., 2008). In this system, the genotype constellation of the VP7-VP4-VP6-VP1-VP2-VP3-NSP1-NSP2-NSP3-NSP4-NSP5/6 genes is described as Gx-Px-Ix-Rx-Cx-Mx-Ax-Nx-Tx-Ex-Hx, where x is a number. The vast majority of human RVAs sequenced to date (n > 300) exhibit the genotype constellation of G1/2/3/4/9/12-P[8]-I1-R1-C1-M1-A1-N1-T1-E1-H1 (Matthijnssens and Van Ranst, 2012). Human RVAs with this genotype constellation are referred to as “Wa-like” because they are genetically similar to the archival reference strain Wa (Wyatt et al., 1980). While fewer porcine RVAs have been sequenced to date (n = 33), the available data suggests that these strains typically exhibit the genotype constellation G3/5/9/11-P[6]/[7]/[13]/[19]/[23]-I5-R1-C1-M1-A8-N1-T1/7-E1-H1 (Kim et al., 2012; Martel-Paradis et al., 2013; Matthijnssens et al., 2008; Monini et al., 2014; Nagai et al., 2015; Okitsu et al., 2013; Silva et al., 2015; Theuns et al., 2015). Thus, it seems that while human Wa-like RVAs and porcine RVAs differ in their VP7, VP4, VP6, and NSP1 gene genotypes, they tend to have similar genotype 1 VP1-VP3 and NSP2-NSP5/6 genes. This observation suggested that human Wa-like RVAs and porcine RVAs share an evolutionary relationship and perhaps a common ancestor (Matthijnssens et al., 2008).
In the current study, we sought to more fully characterize the genotype 1 genes and proteins of human Wa-like RVAs and porcine RVAs and to determine whether specific genetic signatures differentiate those from each host. We constructed neighbor-joining phylogenetic trees to reveal the relationships between the genotype 1 genes of typical and atypical human Wa-like and porcine RVAs. We also employed amino acid sequence alignments to identify positions that varied in the genotype 1 viral proteins in a host-specific manner. Furthermore, we documented how several archival/unusual isolates differ at these host-specific variable positions. Altogether, these results enhance our understanding of RVA genetic diversity and elucidate putative evolutionary signatures of genotype 1 viral proteins from human versus porcine strains.
2. Materials and methods
2.1. Nucleotide sequencing of 11 Brazilian porcine RVAs
Near-complete genome nucleotide sequences were determined for 11 porcine RVAs (ROTA02, ROTA03, ROTA04, ROTA05, ROTA13, ROTA16, ROTA18, ROTA25, ROTA27, ROTA30, and ROTA31) using the same approach described in Silva et al., 2015 (Silva et al., 2015). These porcine strains are considered to represent typical modern isolates as they were found in fecal specimens collected from diarrheic nursing and suckling piglets (<60 days of age) on various Brazilian farms during the years of 2012–2013. The 83 new full or partial gene sequences were genotyped using RotaC2.0 (Maes et al., 2009) and were deposited into GenBank (Table S1). Only the sequences for the genotype 1 VP1-VP3 and NSP2-NSP5/6 genes were analyzed in the current study.
2.2. Sub-genotypic neighbor-joining phylogenetic analyses
During the initial stages of the analyses, we downloaded the nucleotide sequences of genotype 1 VP1-VP3 and NSP2-NSP5/6 genes of all known human Wa-like RVAs and porcine RVAs. Alignments were created for each gene using Geneious Pro v5.6.5 (Biomatters) and the ClustalW algorithm and were trimmed so that the sequences were the same length in each alignment. The following nucleotide regions were analyzed for each gene: VP1 (nts 162-1572), VP2 (nts 28-1431), VP3 (nts 52-1034), NSP2 (nts 76-907), NSP3 (nts 59-977), NSP4 (nts 48-577), and NSP5/6 (nts 43-535). Neighbor-joining phylogenetic trees were created for each gene using Geneious Pro v5.6.5 (Biomatters). The trees were out-group rooted to the genotype 2 genes of strain DS-1 and built using three different distance models (Jukes-Cantor, Tamura-Nei, and HKY) and 100 bootstrap replicates. The overall tree topologies and major groupings were identical irrespective of the distance model chosen. Based upon the clustering of sequences in the initial trees, we then selected 102 representative RVAs to include in the final trees, which were built using the Jukes-Cantor distance model (Table S2 and Figs. S1–S7). For selection of the final strains, we also considered the year, G/P-genotype, and geographical location of the strain. To prepare Fig. 1, major groupings were collapsed using FigTree v1.4 and colorized using Adobe Illustrator CS5 (Adobe Systems).
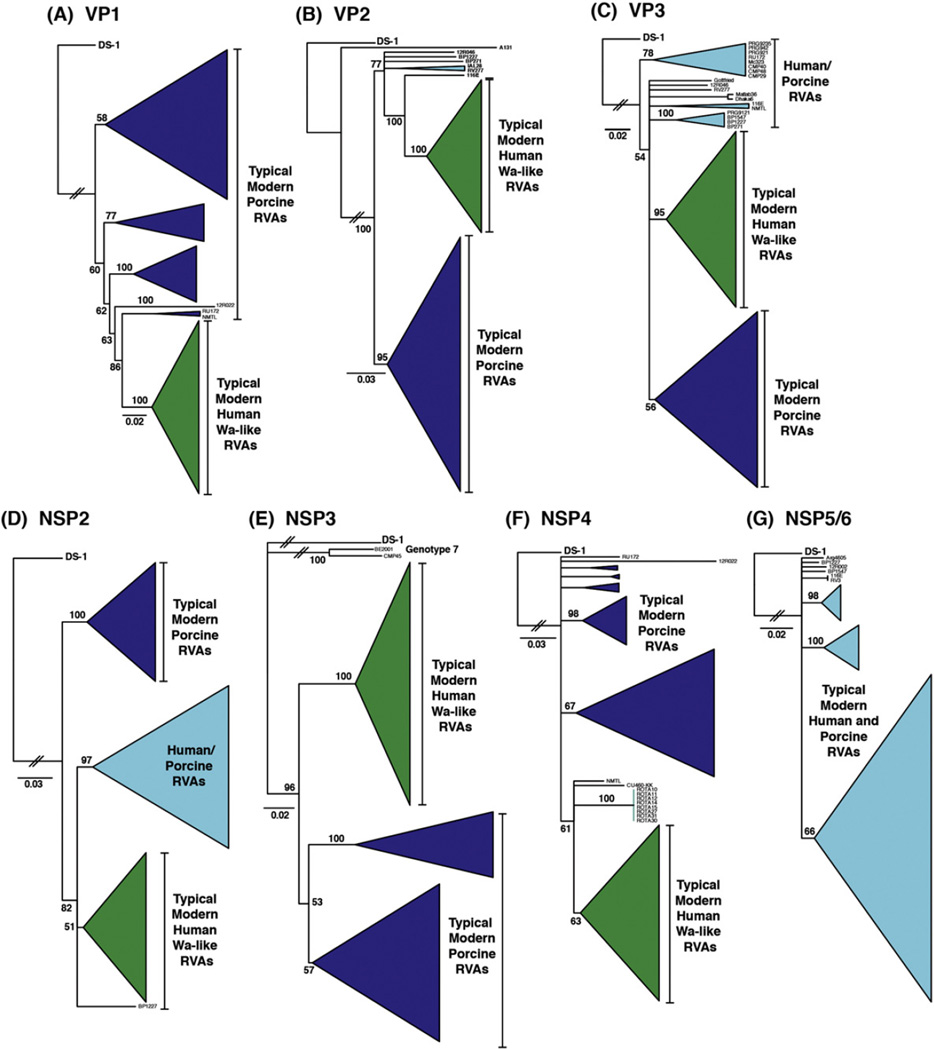
Fig. 1.
Genetic relationships among the genotype 1 genes of human Wa-like RVAs and porcine RVAs. The neighbor-joining phylogenetic trees are outgroup rooted to strain DS-1, and horizontal branch lengths are drawn to scale (nucleotide substitutions per base). Bootstrap values are shown as percentages for key nodes. Monophyletic groupings were collapsed and are shown as cartooned triangles. Groupings comprised mostly of typical modern human RVA genotype 1 genes are shown in green and those of typical modern porcine RVA genes are shown in dark blue. Other groupings that contained genotype 1 genes of archival/unusual isolates or that contained genotype 1 genes from both human and porcine RVAs are shown in cyan.
2.3. Identification of host-specific amino acid changes in genotype 1 proteins
The deduced amino acid sequences of genotype 1 proteins from the 102 representative strains (Table S2) were aligned using Geneious Pro v5.6.5 and the BLOSUM-62 matrix of ClustalW. Amino acids that varied according to host species or viral isolate were identified by visual inspection of the alignments and confirmed by NCBI BLAST analysis. For VP2, which varies in length, the documented position numbers are based on those of strain RVA/Human-wt/PRY/1638SR/2008/G1P[8]. The three dimensional locations of the host-specific variable positions were determined using UCSF Chimera v1.8 and the predicted or known atomic structures of viral proteins: strain UK and SA11 VP1 and VP2 (PDB# 4F5X), strain RRV VP3 (PDB# 2IHP), strain SA11 NSP2 (PDB# 1L9V), and SA11 NSP3 (PDB# 1KNZ and PDB# 1LJ2) (Deo et al., 2002; Estrozi et al., 2013; Pettersen et al., 2004; Groft and Burley, 2002; Jayaram et al., 2002; Ogden et al., 2014).
3. Results
3.1. Genetic relationships between the genotype 1 genes of human Wa-like RVAs and porcine RVAs
To investigate the genetic relationships between human Wa-like RVA and porcine RV genotype 1 genes, we constructed individual phylogenetic trees for VP1-VP3 and NSP2-NSP5/6. Initial trees were constructed using all human Wa-like RVA and porcine RVA genotype 1 nucleotide sequences available in GenBank (data not shown). In this study, we also deduced the full or partial gene sequences for 11 porcine RVAs isolated from diarrheic piglets in Brazil during the years 2012–2013. The final trees included the genotype 1 genes of these 11 new Brazilian porcine RVA sequences as well as those of 91 additional human or porcine RVAs that altogether reflected the genetic diversity seen in the initial trees (Fig. 1). The genotype constellations of the 102 representative strains are shown in Fig. 2.
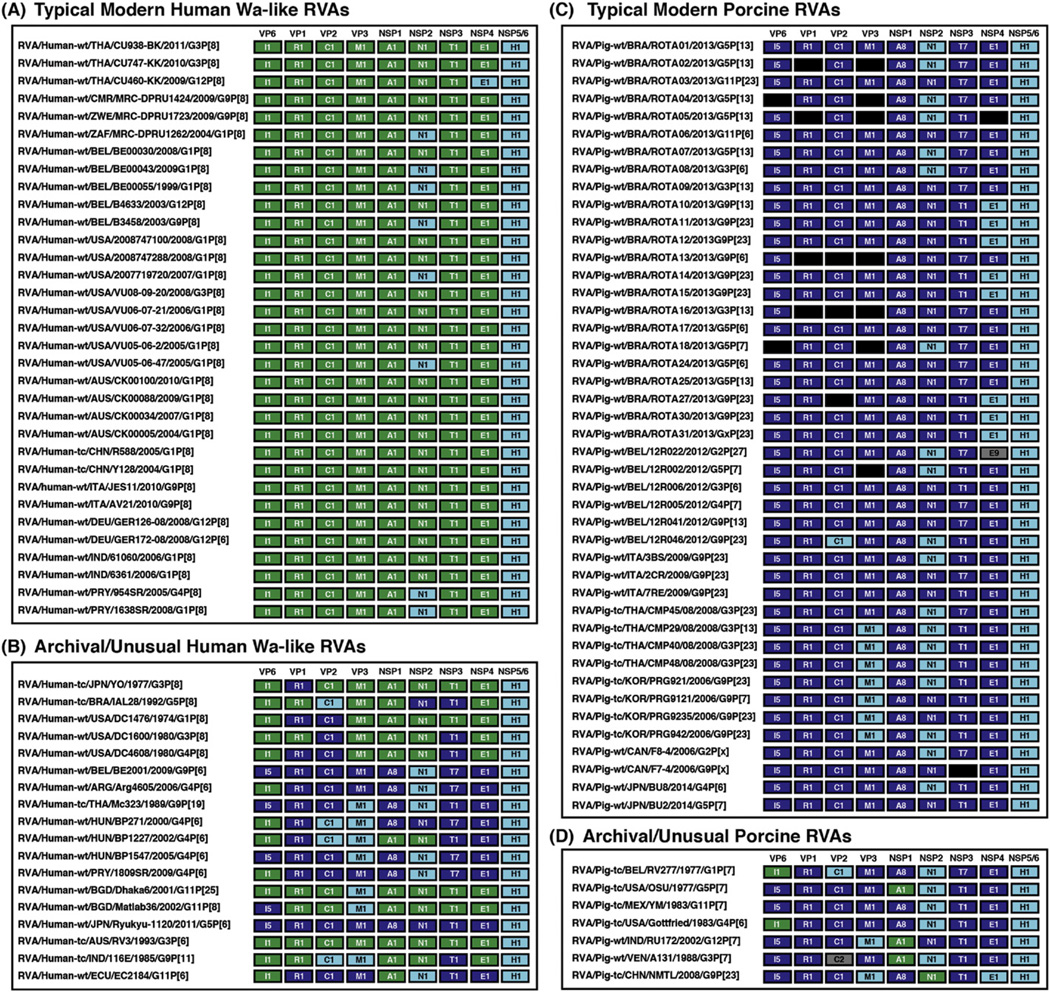
Fig. 2.
Genotype constellations of representative human Wa-like RVAs and porcine RVAs. Strain names are listed to the left of the corresponding genotype constellation for the strains analyzed in this study. Each gene is shown as a box and is color-coded according to genotype or according to the results of Fig. 1. Specifically, genes characteristic of human Wa-like RVAs are shown in green, and those characteristic of porcine RVAs are shown in dark blue. Genes that formed distinct clusters or those that could not be resolved in Fig. 1 are shown in cyan. Black boxes represent genes for which no sequence information is available, and grey boxes represent genes with genotypes not generally found in human Wa-like or porcine strains. (A) Typical modern human Wa-like RVAs. These strains are considered to be wildtype, as they were isolated from human clinical fecal specimens during the years of 2004–2011 and have G/P-genotypes normally associated with human RVAs. (B) Archival/unusual human Wa-like RVAs. These human strains are considered atypical as they were isolated >20 years ago, are putative reassortants, and/or were involved in inter-species transmission events. (C) Typical modern porcine RVAs. These strains are considered to be wildtype, as they were isolated from porcine clinical fecal specimens during the years of 2006–2014 and have G/P-genotypes normally associated with porcine RVAs. (D) Archival/unusual porcine RVAs. These porcine strains are considered atypical as they were isolated from porcine fecal specimens >30 years ago or are putative reassortants.
The human Wa-like RVAs chosen as representatives of typical modern strains (Fig. 2A) included those that were isolated during the years of 2004–2011 from diarrheic children at various geographical locations: Thailand (strains CU938-BK, CU747-KK, and CU460-KK), Africa (strains MRC-DPRU1424, MRC-DPRU1723, and MRC-DPRU1262), Belgium (strains BE00030, BE00043, BE00055, B4633, and B3458), the United States (strains 2008747100, 2008747288, 2007719720, VU08-09-20, VU06-07-21, VU06-07-32, VU05-06-2, and VU05-06-47), Australia (strains CK00100, CK00088, CK00034, and CK00005), China (strains R588 and Y128), Italy (strains JES11 and AV21), Germany (strains GER126-08 and GER172-08), India (strains 61060 and 6361), and Paraguay (strains 954SR and 1638SR) (Arora and Chitambar, 2011; Ianiro et al., 2013; Matthijnssens et al., 2008; McDonald et al., 2012; Nyaga et al., 2013; Pietsch and Liebert, 2009; Rahman et al., 2007; Shintani et al., 2012; Theamboonlers et al., 2014; Zeller et al., 2015).
We also included in our phylogenetic analyses the genotype 1 gene sequences of archival/unusual human strains (Fig. 2B) that were either isolated prior to 1992 (strains RV3, 116E, YO, IAL28, DC1476, DC1600, and DC4608) or that were likely the result of pig-to-human interspecies transmission events (strains BE2001, Arg4605, Mc323, BP271, BP1227, BP1547, 1809SR, Dhaka6, Matlab36, Ryukyu-1120, and EC2184) (Banyai et al., 2009; Degiuseppe et al., 2013; Ghosh et al., 2012; Heiman et al., 2008; Komoto et al., 2013; Martinez et al., 2014; Matthijnssens et al., 2008; Matthijnssens et al., 2010; McDonald et al., 2011; McDonald et al., 2009; Papp et al., 2013; Rippinger et al., 2010; Zeller et al., 2012; Zhang et al., 2014).
For the porcine RVAs, we included in the final trees typical modern strains for which complete or near-complete genome sequence information is available (Fig. 2C). These strains were considered to be wildtype porcine RVAs because they had G/P-genotypes normally associated with porcine RVAs and they were found in the feces of diarrheic or non-diarrheic piglets during the years of 2006–2014. The porcine RVAs were also from various geographical locations: Brazil (strains ROTA01-10, ROTA24-25, ROTA27, and ROTA30-31), Belgium (strains 12R022, 12R002, 12R006, 12R005, 12R041, and 12R046), Italy (strains 3BS, 2CR, and 7RE), Thailand (strains CMP45, CMP29, CMP40, and CMP48), Korea (strains PRG921, PRG9121, PRG9235, and PRG942), Canada (strains F8-4 and F7-4), and Japan (strains BU8 and BU2) (Kim et al., 2012; Martel-Paradis et al., 2013; Monini et al., 2014; Nagai et al., 2015; Okitsu et al., 2013; Silva et al., 2015; Theuns et al., 2015). We also included the gene sequences of several atypical strains (Fig. 2D) that were either archival and isolated prior to 1983 (strains RV277, OSU, YM, and Gottfried) or that were predicted to represent human-porcine RVA reassortants (strains RU172, A131, and NMTL) (Ghosh et al., 2010; Matthijnssens et al., 2008; Shi et al., 2012; Theuns et al., 2015).
For VP1, we found that the genotype 1 genes of typical modern human Wa-like RVAs clustered together in the phylogenetic tree (Fig. 1A and Fig. 2A; green) and were generally separate from those of both porcine RVAs and unusual/archival strains. The genotype 1 genes of typical modern porcine RVAs, on the other hand, were found in several different phylogenetic clusters interspersed with those of archival/unusual strains (Fig. 1A and Fig. 2B–D; dark blue). Consistent with previous reports, the genotype VP1 genes of archival human strains YO, DC1476, and DC4608 clustered with those of porcine RVAs rather than with typical human strains, suggesting that they might have been acquired via gene reassortment (Fig. 1A and Fig. 2B; dark blue) (McDonald et al., 2011; Zhang et al., 2014). Moreover, as reported previously, the genotype 1 VP1 genes of several unusual human isolates (i.e., BE2001, Arg4605, Mc323, BP271, BP1227, BP1547, 1809SR, Ryukyu-1120, and EC2184) clustered with those of typical porcine RVAs, indicative of these viruses taking part in inter-species transmission events (Fig. 1A and Fig. 2B; dark blue) (Degiuseppe et al., 2013; Ghosh et al., 2012; Komoto et al., 2013; Martinez et al., 2014; Papp et al., 2013; Zeller et al., 2012).
For VP2 and VP3, two major lineages were seen in each respective phylogenetic tree, which were comprised mostly of the genotype 1 genes of: (i) typical modern human Wa-like RVAs (Fig. 1B–C and Fig. 2A; green) or (ii) typical modern porcine RVAs (Fig. 1B–C and Fig. 2C; dark blue). The VP2 tree also showed several additional branches, which represented the genotype 1 genes of one modern Belgium porcine RVA (strain 12R046) and several archival/unusual RVAs found in pigs or humans (strains RV277, IAL28, BP271, BP1227, and 116E) (Fig. 1B andFig. 2B–D; cyan). Likewise, for VP3, additional branches and clusters were identified and found to be comprised of genotype 1 genes of modern porcine RVAs from Thailand (strains CMP29, CMP40, and CMP48) or Korea (strains PRG921, PRG9121, PRG9235, and PRG942), as well as genes of archival/unusual porcine or human isolates (strains RU172, NMTL, Mc323, BP271, BP1227, BP1547, Dhaka6, Matlab38, and 116E) (Fig. 1C and Fig. 2B–D; cyan). Consistent with previous reports, the VP2 and/or VP3 genotype 1 genes of several archival/unusual human RVAs (e.g., DC1476, BE2001, 1809SR, and Ryukyu-1120) clustered with those of typical porcine RVAs (Fig. 1B–C and Fig. 2B–D; dark blue), reflecting either gene reassortment events or pig-to-human transmission of the strains (Komoto et al., 2013; Martinez et al., 2014; McDonald et al., 2011; Zeller et al., 2012; Zhang et al., 2014).
For NSP2, the phylogenetic tree showed the same two major lineages that were identified in the VP2 and VP3 trees, which were comprised of genotype 1 genes of: (i) typical modern human Wa-like RVAs (Fig. 1D and Fig. 2A; green) and (ii) typical modern porcine RVAs (Fig. 1D and Fig. 2C; dark blue). However, for NSP2, a third larger lineage was also identified, and it was made up of genotype 1 genes of both typical and atypical human and porcine strains (Fig. 1D and Fig. 2; cyan). Specifically, the NSP2 genes of 19 of 44 modern porcine RVAs, 5 archival/unusual porcine RVAs (strains RV277, OSU, YM, Gottfried, and A131), and 6 pig-to-human transmitted RVAs (strains BE2001 Arg4605, Mc323, BP1547, 1809SR, and EC2184) clustered in this unique grouping (Fig. 2B and 2D; cyan). Fewer modern human Wa-like RVA NSP2 genes were found in this grouping; yet, those that were are considered to be typical wildtype isolates from South Africa (strain MRC-DPRU1262), Belgium (strains BE00043, BE00055, and B3458), United States (strains 2007719720 and VU05-06-47), and Paraguay (strains 954SR and 1638SR) (Fig. 2A; cyan).
The NSP3 genes of human Wa-like RVAs are almost exclusively designated as genotype 1; however, the NSP3 genes of porcine RVAs can either be genotype 1 or genotype 7 (i.e., T1 or T7, respectively). We found that 23 of the typical modern porcine RVAs and 19 of the archival/unusual porcine or human RVAs have genotype 1 NSP3 genes (Fig. 2B–D). The phylogenetic tree showed that the genotype 1 NSP3 genes of modern human Wa-like strains clustered together (Fig. 1E and Fig. 2A; green) and distinctly from those of porcine strains, which formed two separate sub-clusters (Fig. 1E and Fig. 2C; dark blue). The genotype 1 NSP3 genes of several archival/unusual human strains (i.e., IAL28, DC1600, DC4608, Mc323, BP1227, Ryukyu-1120, 116E, and EC2184) were found to be porcine RVA-like (Fig. 2B; dark blue), consistent with the reports of these strains being reassortants or the result of inter-species transmission events (Banyai et al., 2009; Ghosh et al., 2012; Komoto et al., 2013; McDonald et al., 2011; Papp et al., 2013; Rippinger et al., 2010).
For NSP4, the genotype 1 genes of typical modern human Wa-like RVAs clustered together in the phylogenetic tree (Fig. 1F and Fig. 2A; green) and were largely separate from those of porcine RVAs and those of archival/unusual strains. The genotype 1 NSP4 genes of typical modern porcine RVAs, on the other hand, were found in several different phylogenetic clusters interspersed with those of archival/unusual strains (Fig. 1F and Fig. 2C; dark blue). A small grouping of genes from 8 Brazilian porcine RVAs formed a sub-cluster distinct from the genes of typical human RVAs and other porcine RVAs (Fig. 1F and Fig. 2C–D; cyan). Related to this sub-cluster were the NSP4 genes of the unusual porcine RVA from China (strain NMTL) and a single modern human Wa-like RVA from Thailand (strain CU460-KK) (Shi et al., 2012; Theamboonlers et al., 2014).
For NSP5/6, we did not find any specific clustering pattern that corresponded with host or virus type (Fig. 1G and Fig. 2; cyan). Despite a few clusters in the tree, the genotype 1 genes of modern human Wa-like RVAs and porcine RVAs could not be delineated. Likewise, the genotype 1 NSP5/6/genes of modern strains were not separate from those of archival/unusual strains.
3.2. Amino acid residues distinguishing typical modern human Wa-like vs. typical modern porcine RVAs
Having found that the genotype 1 VP1-VP3 and NSP2-NSP4 genes of typical modern human Wa-like RVAs generally clustered together in phylogenetic trees and were distinct from those of typical modern porcine RVAs, we next wondered whether their encoded proteins exhibited specific amino acid changes. To investigate this, we created amino acid sequence alignments and identified positions that were conserved among all typical modern human Wa-like RVAs but that varied compared to typical modern porcine RVAs and vice versa. Altogether, we found 34 host-specific variant positions for VP1-VP3, NSP2, and NSP3; no such positions were identified for NSP4, a nonstructural protein involved in virion morphogenesis and virulence.
For VP1, the viral RNA-dependent RNA polymerase, we identified 2 positions (284 and 1049) that varied in a host-specific manner (Fig. 3A). Residue 284 is a buried residue located in the N-terminal domain of the polymerase, whereas residue 1049 is a surface-exposed residue in the C-terminal bracelet domain (Lu et al., 2008) (Fig. 3A and data not shown). Neither of these residues are within the catalytic motifs of VP1.
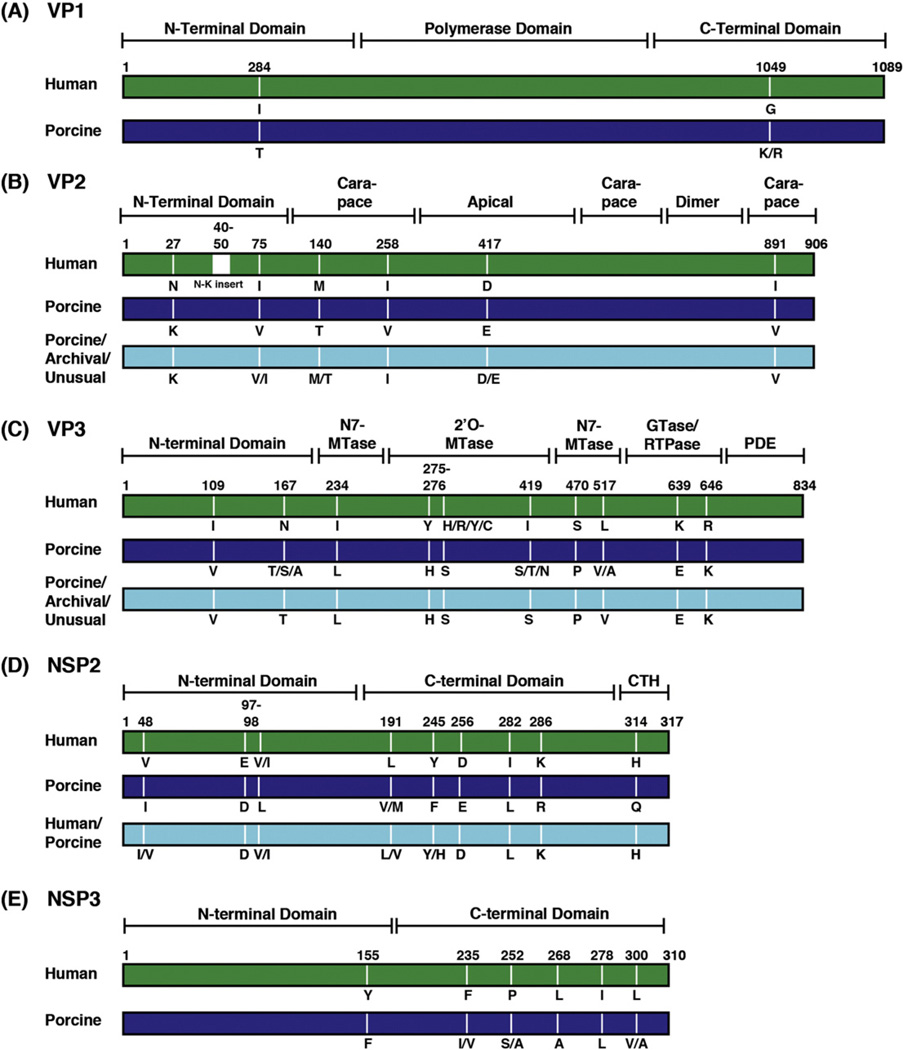
Fig. 3.
Host-specific variable positions in genotype 1 viral proteins. The rectangles represent linear schematics of genotype 1 viral proteins (not drawn to scale) and are color-coded based on the results of Fig. 1. Typical modern human RVA genotype 1 proteins shown in green, and typical modern porcine RVA genotype 1 proteins shown in dark blue. Genotype 1 proteins of archival/unusual isolates or those from additional phylogenetic groupings containing both human and porcine RVAs are shown in cyan. Known or predicted domains/subdomains of each protein are bracketed and labeled. Positions in the amino acid sequence alignments that varied in a host-specific manner are labeled and the corresponding residues are listed below each protein schematic.
For VP2, the inner core shell protein, we identified 6 positions (27, 75, 140, 258, 417, and 891) that varied in a host-specific manner (Fig. 3B). We also found that the VP2 proteins of typical modern human Wa-like strains, but not porcine strains, have an asparagine-lysine (N-K)-rich insertion at positions ~40–50 in the alignment. This insertion differs in length among human Wa-like strains and is not seen in human DS-1-like or AU-like strains with genotype 2 or 3 VP2 proteins, respectively (McDonald and Patton, 2008). Residues 140, 258, and 891 of VP2 reside in the central carapace subdomain of the core shell, whereas reside 417 is in the apical subdomain near the fivefold axis and near predicted VP1 contact sites (Fig. 3B and data not shown) (Estrozi et al., 2013). The extreme N-terminal domain of VP2 (residues ~1–100) is not resolved in any published rotavirus structure. However, this region of the core shell protein is predicted to reside within the particle interior, suggesting that host-specific variable positions 27, 40–50, and 75 may be proximal to the location of RNA, VP1, and VP3 (McClain et al., 2010). VP2 residues 258 and 891 are on the outer surface of the core shell at the VP6 interface (data not shown).
For VP3, an RNA capping enzyme that has innate immune antagonist activities via a C-terminal 3′5′-phosphodiesterase domain (PDE), we identified 10 positions that varied (109, 167, 234, 275, 276, 419, 470, 517, 639, and 646) between typical modern human Wa-like RVAs and typical modern porcine RVAs (Fig. 3C) (Morelli et al., 2015). According a VP3 homology model, residues 109 and 167 are located in the N-terminal adapter domain, residues 234, 470, and 517 are in the guanine-N7-methyltransferase (N7-MTase) domain, residues 275, 276 and 419 are in the ribose 2′ O-methyltransferase (2′ O-MTase) domain, and residues 639 and 646 are located in the guanylyltransferase/RNA 5′ triphosphatase (GTase/RTPase) domain (Ogden et al., 2014) (Fig. 3C and data not shown). All of these residues are distant from the predicted catalytic motifs of VP3, with the exception of residue 470, which is located immediately adjacent to the major S-adenosyl-l-homocysteine (SAH)-binding surface in the N7-MTase domain (Ogden et al., 2014). These residues are predicted to be fully or partially surface-exposed in the context of the homology model (data not shown). No host-specific variable positions were identified in the C-terminal PDE domain of VP3.
For NSP2, which is an octameric nonstructural protein that plays roles during viral genome replication and early particle assembly, we identified 9 positions (48, 97, 98, 191, 245, 256, 282, 286, and 314) that varied between typical modern human Wa-like RVAs and typical modern porcine RVAs (Fig. 3D). Based on the structure of simian rotavirus strain SA11 NSP2, residues 48, 97, and 98 are located in the N-terminal domain, residues 191, 245, 256, 282, and 286 are located in the C-terminal domain, and residue 314 is located in the extreme C-terminal alpha-helix (Jayaram et al., 2002) (Fig. 3D and data not shown). These residues are predicted to be surface-exposed in the NSP2 octamer (data not shown).
For NSP3, a nonstructural protein involved in protein synthesis, we identified 6 positions (155, 235, 252, 268, 278, and 300) that varied in a host-specific manner (Fig. 3E). Residue 155 is surface-exposed and located in the N-terminal RNA-binding domain of NSP3. Residues 235, 252, 268, 278, and 300 are located within the C-terminal eIF4G-binding domain (Groft and Burley, 2002). (Fig. 3E and data not shown). Residues 235, 252, 278, and 300 are buried and contribute to NSP3 dimerization, while residue 258 is surface-exposed (Fig. 3E and data not shown) (Deo et al., 2002).
3.3. Amino acid changes associated with unusual/archival RVAs
When creating the amino acid sequence alignments, we noticed that many of the 34 host-specific variable positions differed in the sequence of archival/unusual isolates (Fig. 4). Because the changes in these atypical RVAs might be influenced by the genetic context of the entire virus, we sought to document and interpret them in the context of the viral genotype constellations. Based on this analysis, we divided the archival/ unusual isolates into 3 groups:
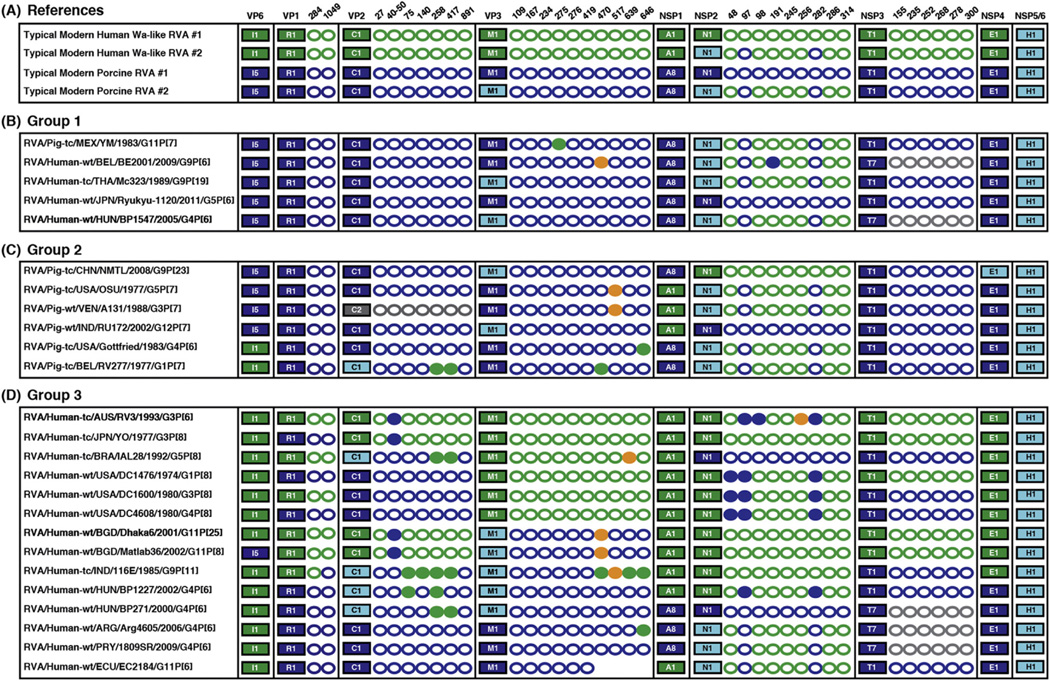
Fig. 4.
Amino acid differences in archival/unusual RVA strains at host-specific variable positions. Genotype constellations of RVAs are shown as in Fig. 2. For proteins VP1-VP3, NSP2, and NSP3, the host-specific variable positions identified in Fig. 3 are listed to the right of the corresponding protein. Green circles represent amino acid residues identical to those of typical modern human Wa-like RVAs. Dark blue circles represent amino acid residues identical to those of typical modern porcine RVAs. Orange circle represent amino acid residues that are unique, differing from those of human/porcine RVAs. Grey circles are shown for non-genotype 1 proteins (i.e. T7). Filled circles indicate that the residue found at that position is not what would be expected based on the evolutionary origin of the protein, indicating amino acid changes in archival/unusual isolates at host-specific amino acid positions. (A) Typical modern human Wa-like RVA and typical modern porcine RVA constellations/positions are shown as references. (B) Group 1 is comprised of porcine RVAs isolated from pigs or humans. (C) Group 2 is comprised of putative reassortant RVAs isolated from pigs but containing one or more human RVA-like genes. (D) Group 3 is comprised of putative reassortants isolated from humans but containing one or more porcine RVA-like genes.
Group 1 consisted of 5 RVAs that are quite similar to typical modern porcine RVAs (Fig. 4A–B). Only one member of this group was isolated from a pig (strain YM), whereas the other 4 members (strains BE2001, Mc323, Ryukyu-1120, and BP1547) were isolated from humans following inter-species transmission events (Ghosh et al., 2012; Komoto et al., 2013; Matthijnssens et al., 2008; Papp et al., 2013; Zeller et al., 2012). In general, few changes were detected at the host-specific amino acid positions for this group. Strain YM showed a single change at position 275 in VP3, while strain BE2001 showed changes at position 470 in VP3 and position 191 in NSP2. Strains Mc323, Ryukyu-1120, and BP1547 did not show any changes at the host-specific positions, suggesting that little if any adaptation occurred during their replication in the human host.
Group 2 consisted of 6 putative reassortant RVAs (strains NMTL, OSU, A131, RU172, and Gottfried, RV277) that were isolated from pigs but that have one or more genes more similar to those of human Wa-like RVAs (Fig. 4A and 4C) (Ghosh et al., 2010; Matthijnssens et al., 2008; Shi et al., 2012; Theuns et al., 2015). Four of the viruses in this group showed changes at host-specific variable positions in VP3. Additionally, the VP2 gene of the archival Belgium strain RV277, which clustered distinctly in the phylogenetic tree from the those of typical modern porcine RVAs, was found to match the cognate human Wa-like RVA residues at positions 258 and 417. Similar changes were found for the VP2 proteins of human strains IAL28, 116E, BP1227, and BP271 (see below).
Group 3 consisted of 14 putative reassortant RVAs (strains RV3, YO, IAL28, DC1476, DC1600, DC4608, Dhaka6, Matlab36, 116E, BP1227, BP271, Arg4605, 1809SR, and EC2184) that were isolated from humans but that have one or more porcine RVA-like genes (Fig. 4A and 4D) (Banyai et al., 2009; Degiuseppe et al., 2013; Heiman et al., 2008; Martinez et al., 2014; Matthijnssens et al., 2008; Papp et al., 2013; Rippinger et al., 2010). We found several changes at host-specific amino acid positions in VP2, VP3, and NSP2 for many members of this group. For example, archival human strains DC1476 and DC4608 have porcine RVA-like VP1 and VP2 genes/proteins. While the NSP2 proteins of these two isolates are most similar to those of typical modern human Wa-like RVAs (with cognate amino acids at 6 of the 9 host-specific sites), they differed at positions 48, 97, and 282, showing amino acid residues typical of porcine RVAs. It is interesting to speculate that perhaps the amino acid changes seen in these human-porcine reassortant RVAs reflects ongoing adaptive evolution of the virus as a result of the new (i.e., reassortant) gene constellation.
4. Discussion
Previous comparative genomic studies have reported a likely evolutionary relationship between human Wa-like RVAs and porcine RVAs (Matthijnssens et al., 2008; Matthijnssens and Van Ranst, 2012; Theuns et al., 2015). However, until now, there had not been a systematic analysis of the genes and proteins from these seemingly related RVAs strains mainly because we lacked sufficient porcine RVA genome sequences. In fact, prior to 2015, <20 complete/near-complete genome sequences had been reported for wildtype porcine RVAs (i.e., those found in the feces of symptomatic piglets), whereas >300 wildtype human Wa-like RVA genome sequences existed. Recently, we described the near-complete genome sequencing and analysis of 12 Brazilian porcine RVAs isolated in 2012–2013 (Silva et al., 2015). Here, we report an additional 11 near-complete genome sequences of porcine RVAs from this same sample collection, thereby adding significantly to the number of wildtype porcine RVA sequences available in GenBank. Importantly, the genotype and sub-genotype constellations of our Brazilian strains mirror those of RVAs isolated from pigs in Belgium, Italy, Thailand, Korea, Canada, and Japan suggesting that they are representative of porcine RVA genetic diversity (Martel-Paradis et al., 2013; Kim et al., 2012; Monini et al., 2014; Nagai et al., 2015; Okitsu et al., 2013; Theuns et al., 2015). As such, in the current study, we were well-positioned to ask the following questions: Do the genes and proteins of human Wa-like RVAs differ from those of porcine RVAs? If so, are there specific genetic signatures that distinguish human versus porcine strains?
By comparing the genotype constellations of typical modern human Wa-like RVAs to those of typical modern porcine RVAs (Fig. 2), we confirmed previous reports that the most divergent genes are those encoding outer capsid proteins VP7 and VP4, the intermediate capsid protein VP6, and the nonstructural innate immune antagonist protein NSP1 (Kim et al., 2012; Martel-Paradis et al., 2013; Monini et al., 2014; Nagai et al., 2015; Okitsu et al., 2013; Silva et al., 2015; Theuns et al., 2015). Indeed, human Wa-like RVAs and porcine RVAs usually exhibit different genotypes for these four genes, which by definition share <85% nucleotide sequence identity (Matthijnssens et al., 2008). Consistent with previous reports, we also observed that the NSP3 gene can sometimes, but not always, differ in genotype between human Wa-like RVAs and porcine RVAs (Kim et al., 2012; Martel-Paradis et al., 2013; Monini et al., 2014; Nagai et al., 2015; Okitsu et al., 2013; Silva et al., 2015; Theuns et al., 2015). Thus, it is likely that the VP7, VP4, VP6, NSP1, and NSP3 genes/proteins dictate whether an RVA productively infects a human versus a pig. However, we hypothesized that host-specific changes might be found in the six other viral genes, which share the genotype 1 designation (i.e., VP1-VP3 and NSP2-5/6) and >81% nucleotide sequence identity. We posited that such host-specific changes could contribute to host tropism or could reflect evolutionary changes resulting from sequestered replication of the virus. In support of this notion, our phylogenetic analyses showed that the genotype 1 genes from typical modern human Wa-like strains generally clustered together and were separate from those of typical modern porcine RVAs (Figs. 1 and 2). We did not observe any host-specific clustering for the NSP5/6 gene, suggesting that either this gene is genetically identical in human and porcine strains, or that our current data is insufficient to resolve host-specific differences.
To investigate whether the proteins encoded by the genotype 1 genes differed depending upon the host species (i.e., human vs. pig), we performed amino acid sequence alignments (Fig. 3). For this analysis, it was critical that we stratify typical modern strains away from archival/ unusual isolates because we noticed that the latter showed amino acid variations at positions in the alignment that were otherwise invariable within a host (Fig. 4). Altogether, we identified a total of 34 amino acid positions that are conserved among the typical modern human Wa-like RVAs, but that differed for typical modern porcine RVAs or vice versa. Of these 34 host-specific variable positions, 29 of them are located at surface-exposed regions of viral replication proteins VP1, VP2, VP3, NSP2, or NSP3. Given the three-dimensional locations of these host-specific residues, we hypothesize that protein co-adaptation may have been a selection pressure that influenced their emergence. Protein co-adaptation occurs when a fitness-diminishing mutation in one viral protein is compensated for by a fitness-restoring change in a second viral protein. As a consequence of protein co-adaptation, gene segments become genetically-linked and stable constellations are observed (Heiman et al. 2008; Iturriza-Gòmara et al., 2003; Miño et al., 2016; Zhang et al., 2015). This hypothesis may explain why several of the putative reassortant viruses (i.e., archival/unusual isolates) show variations at the identified host-specific positions; the viral proteins may be trying to re-adapt to a new genetic context. Indeed, strains with gene segments derived from animal rotaviruses do not appear to be major causes of infections in humans, suggesting that their overall gene/protein constellations confer decreased fitness (compared to wildtype human Wa-like strains) in the absence of compensatory changes. However, several of the archival/unusual isolates, including reassortants were grown in cell culture for many passages before sequencing, which could account for the observed changes. Moreover, the role of co-adaptive RNA-RNA or RNA-protein interactions cannot be excluded as additional possible selection pressure leading to the emergence of host-specific changes or variations following reassortment. For instance, although we observed some host-specific segregation of genotype 1 NSP4 genes, we did not find any amino acid positions that differentiated these proteins in a host-specific manner, suggesting a role for the viral RNA molecule itself. Of course, it is also possible that the host-specific amino acid (or nucleotide) residues emerged as a result of evolutionary pressure(s) from cellular factors, which would differ between humans and pigs. A fuller understanding of RVA evolution will require additional genome sequences of isolates found in humans, pigs, and other animal hosts. Nevertheless, the results of the current study provide a baseline to compare future sequences to as we continue to resolve RVA genetic diversity and identify determinants of host tropism.
Supplementary Material
Acknowledgments
We are grateful to members of the McDonald laboratory for scientific and editorial suggestions. FDFS was supported by funding from the Brazilian government/Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Project 1079750). SMM was supported by grants from the NIH/NIAID (R01-AI116815-01, R21-AI113402-01, R21-AI119588-01). FG was supported by Foundation of Support to Research of the São Paulo State (Project 2011/00870-0).
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.meegid.2016.05.014.
References
- Arora R, Chitambar SD. Full genomic analysis of Indian G1P[8] rotavirus strains. Infect. Genet. Evol. 2011;11:504–511. doi: 10.1016/j.meegid.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Banyai K, Esona MD, Kerin TK, Hull JJ, Mijatovic S, Vasconez N, Torres C, de Filippis AM, Foytich KR, Gentsch JR. Molecular characterization of a rare, human-porcine reassortant rotavirus strain, G11P[6], from Ecuador. Arch. Virol. 2009;154:1823–1829. doi: 10.1007/s00705-009-0499-1. [DOI] [PubMed] [Google Scholar]
- Chang KS, Kim Y, Saif LJ. Rotavirus and reovirus. In: Zimmerman JJ, Karriker LA, Ramirez A, Schwartz KJ, Stevenson GW, editors. Diseases of Swine. United Kingdom: Wiley-Blackwell; 2012. pp. 621–634. [Google Scholar]
- Degiuseppe JI, Beltramino J, Millan A, Stupka JA, Parra GI. Complete genome analyses of G4P[6] rotavirus detected in Argentinean children with diarrhoea provides evidence of interspecies transmission from swine. Clin. Microbiol. Infect. 2013;19:367–371. doi: 10.1111/1469-0691.12216. [DOI] [PubMed] [Google Scholar]
- Deo RC, Groft CM, Rajashankar KR, Burley SK. Recognition of the rotavirus mRNA 3′ consensus by an asymmetric NSP3 homodimer. Cell. 2002;108:71–81. doi: 10.1016/s0092-8674(01)00632-8. [DOI] [PubMed] [Google Scholar]
- Estes MK, Kapikian AZ. Rotaviruses and their replication. In: Knipe DM, Howley PM, editors. Fields Virology. fifth. Philadelphia: Lippincott Williams and Wilkens; 2007. pp. 1917–1974. [Google Scholar]
- Estrozi LF, Settembre EC, Goret G, McClain B, Zhang X, Chen JZ, Grigorieff N, Harrison SC. Location of the dsRNA-dependent polymerase, VP1, in rotavirus particles. J. Mol. Biol. 2013;425:124–132. doi: 10.1016/j.jmb.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Kobayashi N, Nagashima S, Chawla-Sarkar M, Krishnan T, Ganesh B, Naik TN. Full genomic analysis and possible origin of a porcine G12 rotavirus strain RU172. Virus Genes. 2010;40:382–388. doi: 10.1007/s11262-010-0454-y. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Urushibara N, Taniguchi K, Kobayashi N. Whole genomic analysis reveals the porcine origin of human G9P[19] rotavirus strains Mc323 and Mc345. Infect. Genet. Evol. 2012;12:471–477. doi: 10.1016/j.meegid.2011.12.012. [DOI] [PubMed] [Google Scholar]
- Groft CM, Burley SK. Recognition of eIF4G by rotavirus NSP3 reveals a basis for mRNA circularization. Mol. Cell. 2002;9:1273–1283. doi: 10.1016/s1097-2765(02)00555-5. [DOI] [PubMed] [Google Scholar]
- Heiman EM, McDonald SM, Barro M, Taraporewala ZF, Bar-Magen T, Patton JT. Group A human rotavirus genomics: evidence that gene constellations are influenced by viral protein interactions. J. Virol. 2008;82:11106–11116. doi: 10.1128/JVI.01402-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ianiro G, Heylen E, Delogu R, Zeller M, Matthijnssens J, Ruggeri FM, Van Ranst M, Fiore L. Genetic diversity of G9P[8] rotavirus strains circulating in Italy in 2007 and 2010 as determined by whole genome sequencing. Infect. Genet. Evol. 2013;16:426–432. doi: 10.1016/j.meegid.2013.03.031. [DOI] [PubMed] [Google Scholar]
- Iturriza-Gòmara M, Anderton E, Kang G, Gallimore C, Phillips W, Desselberger U, Gray J. Evidence for genetic linkage between the gene segments encoding NSP4 and VP6 proteins in common and reassortant human rotavirus strains. J. Clin. Microbiol. 2003;41:3566–3573. doi: 10.1128/JCM.41.8.3566-3573.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaram H, Taraporewala Z, Patton JT, Prasad BV. Rotavirus protein involved in genome replication and packaging exhibits a HIT-like fold. Nature. 2002;417:311–315. doi: 10.1038/417311a. [DOI] [PubMed] [Google Scholar]
- Kim HH, Matthijnssens J, Kim HJ, Kwon HJ, Park JG, Son KY, Ryu EH, Kim DS, Lee WS, Kang MI, Yang DK, Hyun BH, Park SI, Park SJ, Cho KO. Full-length genomic analysis of porcine G9P[23] and G9P[7] rotavirus strains isolated from pigs with diarrhea in South Korea. Infect. Genet. Evol. 2012;12:1427–1435. doi: 10.1016/j.meegid.2012.04.028. [DOI] [PubMed] [Google Scholar]
- Komoto S, Maeno Y, Tomita M, Matsuoka T, Ohfu M, Yodoshi T, Akeda H, Taniguchi K. Whole genomic analysis of a porcine-like human G5P[6] rotavirus strain isolated from a child with diarrhoea and encephalopathy in Japan. J. Gen. Virol. 2013;94:1568–1575. doi: 10.1099/vir.0.051011-0. [DOI] [PubMed] [Google Scholar]
- Lu X, McDonald SM, Tortorici MA, Tao YJ, Vasquez-Del Carpio R, Nibert ML, Patton JT, Harrison SC. Mechanism for coordinated RNA packaging and genome replication by rotavirus polymerase VP1. Structure. 2008;16:1678–1688. doi: 10.1016/j.str.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes P, Matthijnssens J, Rahman M, Van Ranst M. RotaC: a web-based tool for the complete genome classification of group A rotaviruses. BMC Microbiol. 2009;9:238. doi: 10.1186/1471-2180-9-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel-Paradis O, Laurin MA, Martella V, Sohal JS, L'Homme Y. Full-length genome analysis of G2, G9 and G11 porcine group A rotaviruses. Vet. Microbiol. 2013;162:94–102. doi: 10.1016/j.vetmic.2012.08.028. [DOI] [PubMed] [Google Scholar]
- Martinez M, Galeano ME, Akopov A, Palacios R, Russomando G, Kirkness EF, Parra GI. Whole-genome analyses reveals the animal origin of a rotavirus G4P[6] detected in a child with severe diarrhea. Infect. Genet. Evol. 2014;27:156–162. doi: 10.1016/j.meegid.2014.07.020. [DOI] [PubMed] [Google Scholar]
- Matthijnssens J, Van Ranst M. Genotype constellation and evolution of group A rotaviruses infecting humans. Curr. Opin. Virol. 2012;2:426–433. doi: 10.1016/j.coviro.2012.04.007. [DOI] [PubMed] [Google Scholar]
- Matthijnssens J, Ciarlet M, Heiman E, Arijs I, Delbeke T, McDonald SM, Palombo EA, Iturriza-Gomara M, Maes P, Patton JT, Rahman M, Van Ranst M. Full genome-based classification of rotaviruses reveals a common origin between human Wa-like and porcine rotavirus strains and human DS-1-like and bovine rotavirus strains. J. Virol. 2008;82:3204–3219. doi: 10.1128/JVI.02257-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthijnssens J, Rahman M, Ciarlet M, Zeller M, Heylen E, Nakagomi T, Uchida R, Hassan Z, Azim T, Nakagomi O, Van Ranst M. Reassortment of human rotavirus gene segments into G11 rotavirus strains. Emerg. Infect. Dis. 2010;16:625–630. doi: 10.3201/eid1604.091591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain B, Settembre E, Temple BR, Bellamy AR, Harrison SC. X-ray crystal structure of the rotavirus inner capsid particle at 3.8 A resolution. J. Mol. Biol. 2010;397:587–599. doi: 10.1016/j.jmb.2010.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald SM, Patton JT. Molecular characterization of a subgroup specificity associated with the rotavirus inner capsid protein VP2. J. Virol. 2008;82:2752–2764. doi: 10.1128/JVI.02492-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald SM, Matthijnssens J, McAllen JK, Hine E, Overton L, Wang S, Lemey P, Zeller M, Van Ranst M, Spiro DJ, Patton JT. Evolutionary dynamics of human rotaviruses: balancing reassortment with preferred genome constellations. PLoS Pathog. 2009;5:e1000634. doi: 10.1371/journal.ppat.1000634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald SM, Davis K, McAllen JK, Spiro DJ, Patton JT. Intra-genotypic diversity of archival G4P[8] human rotaviruses from Washington, DC. Infect. Genet. Evol. 2011;11:1586–1594. doi: 10.1016/j.meegid.2011.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald SM, McKell AO, Rippinger CM, McAllen JK, Akopov A, Kirkness EF, Payne DC, Edwards KM, Chappell JD, Patton JT. Diversity and relationships of cocirculating modern human rotaviruses revealed using large-scale comparative genomics. J. Virol. 2012;86:9148–9162. doi: 10.1128/JVI.01105-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miño S, Barrandeguy M, Parreño V, Parra GI. Genetic linkage of capsid protein-encoding RNA segments in group A equine rotaviruses. J. Gen. Virol. 2016;97:912–921. doi: 10.1099/jgv.0.000397. [DOI] [PubMed] [Google Scholar]
- Monini M, Zaccaria G, Ianiro G, Lavazza A, Vaccari G, Ruggeri FM. Full-length genomic analysis of porcine rotavirus strains isolated from pigs with diarrhea in northern Italy. Infect. Genet. Evol. 2014;25:4–13. doi: 10.1016/j.meegid.2014.03.024. [DOI] [PubMed] [Google Scholar]
- Morelli M, Ogden KM, Patton JT. Silencing the alarms: innate immune antagonism by rotavirus NSP1 and VP3. Virology. 2015;479–480:75–84. doi: 10.1016/j.virol.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai M, Shimada S, Fujii Y, Moriyama H, Oba M, Katayama Y, Tsuchiaka S, Okazaki S, Omatsu T, Furuya T, Koyama S, Shirai J, Katayama K, Mizutani T. H2 genotypes of G4P[6], G5P[7], and G9[23] porcine rotaviruses show super-short RNA electropherotypes. Vet. Microbiol. 2015;176:250–256. doi: 10.1016/j.vetmic.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Nyaga MM, Jere KC, Peenze I, Mlera L, van Dijk AA, Seheri ML, Mphahlele MJ. Sequence analysis of the whole genomes of five African human G9 rotavirus strains. Infect. Genet. Evol. 2013;16:62–77. doi: 10.1016/j.meegid.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Ogden KM, Snyder MJ, Dennis AF, Patton JT. Predicted structure and domain organization of rotavirus capping enzyme and innate immune antagonist VP3. J. Virol. 2014;88:9072–9085. doi: 10.1128/JVI.00923-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okitsu S, Khamrin P, Thongprachum A, Kongkaew A, Maneekarn N, Mizuguchi M, Hayakawa S, Ushijima H. Whole-genomic analysis of G3P[23], G9P[23] and G3P[13] rotavirus strains isolated from piglets with diarrhea in Thailand, 2006–2008. Infect. Genet. Evol. 2013;18:74–86. doi: 10.1016/j.meegid.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Papp H, Borzak R, Farkas S, Kisfali P, Lengyel G, Molnar P, Melegh B, Matthijnssens J, Jakab F, Martella V, Banyai K. Zoonotic transmission of reassortant porcine G4P[6] rotaviruses in Hungarian pediatric patients identified sporadically over a 15 year period. Infect. Genet. Evol. 2013;19:71–80. doi: 10.1016/j.meegid.2013.06.013. [DOI] [PubMed] [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Pietsch C, Liebert UG. Human infection with G12 rotaviruses, Germany. Emerg. Infect. Dis. 2009;15:1512–1515. doi: 10.3201/eid1509.090497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M, Matthijnssens J, Yang X, Delbeke T, Arijs I, Taniguchi K, Iturriza-Gomara M, Iftekharuddin N, Azim T, Van Ranst M. Evolutionary history and global spread of the emerging G12 human rotaviruses. J. Virol. 2007;81:2382–2390. doi: 10.1128/JVI.01622-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippinger CM, Patton JT, McDonald SM. Complete genome sequence analysis of candidate human rotavirus vaccine strains RV3 and 116E. Virology. 2010;405:201–213. doi: 10.1016/j.virol.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Chen J, Li H, Sun D, Wang C, Feng L. Molecular characterization of a rare G9P[23] porcine rotavirus isolate from China. Arch. Virol. 2012;157:1897–1903. doi: 10.1007/s00705-012-1363-2. [DOI] [PubMed] [Google Scholar]
- Shintani T, Ghosh S, Wang YH, Zhou X, Zhou DJ, Kobayashi N. Whole genomic analysis of human G1P[8] rotavirus strains from different age groups in China. Viruses. 2012;4:1289–1304. doi: 10.3390/v4081289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva FD, Espinoza LR, Tonietti PO, Barbosa BR, Gregori F. Whole-genomic analysis of 12 porcine group a rotaviruses isolated from symptomatic piglets in Brazil during the years of 2012–2013. Infect. Genet. Evol. 2015;32:239–254. doi: 10.1016/j.meegid.2015.03.016. [DOI] [PubMed] [Google Scholar]
- Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect. Dis. 2012;12:136–141. doi: 10.1016/S1473-3099(11)70253-5. [DOI] [PubMed] [Google Scholar]
- Theamboonlers A, Maiklang O, Thongmee T, Chieochansin T, Vuthitanachot V, Poovorawan Y. Complete genotype constellation of human rotavirus group a circulating in Thailand, 2008–2011. Infect. Genet. Evol. 2014;21:295–302. doi: 10.1016/j.meegid.2013.11.020. [DOI] [PubMed] [Google Scholar]
- Theuns S, Heylen E, Zeller M, Roukaerts ID, Desmarets LM, Van Ranst M, Nauwynck HJ, Matthijnssens J. Complete genome characterization of recent and ancient Belgian pig group A rotaviruses and assessment of their evolutionary relationship with human rotaviruses. J. Virol. 2015;89:1043–1057. doi: 10.1128/JVI.02513-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt RG, James WD, Bohl EH, Theil KW, Saif LJ, Kalica AR, Greenberg HB, Kapikian AZ, Chanock RM. Human rotavirus type 2: cultivation in vitro. Science. 1980;207:189–191. doi: 10.1126/science.6243190. [DOI] [PubMed] [Google Scholar]
- Zeller M, Heylen E, De Coster S, Van Ranst M, Matthijnssens J. Full genome characterization of a porcine-like human G9P[6] rotavirus strain isolated from an infant in Belgium. Infect. Genet. Evol. 2012;12:1492–1500. doi: 10.1016/j.meegid.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Zeller M, Donato C, Trovao NS, Cowley D, Heylen E, Donker NC, McAllen JK, Akopov A, Kirkness EF, Lemey P, Van Ranst M, Matthijnssens J, Kirkwood CD. Genome-wide evolutionary analyses of G1P[8] strains isolated before and after rotavirus vaccine introduction. Genome Biol. Evol. 2015;7:2473–2483. doi: 10.1093/gbe/evv157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, McDonald PW, Thompson TA, Dennis AF, Akopov A, Kirkness EF, Patton JT, McDonald SM. Analysis of human rotaviruses from a single location over an 18-year time span suggests that protein coadaption influences gene constellations. J. Virol. 2014;88:9842–9863. doi: 10.1128/JVI.01562-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.