Abstract
Functional neuroimaging has revealed that in healthy adults retrieval of personal trait knowledge is associated with increased activation in the medial prefrontal cortex (mPFC). Separately, neuropsychology has shown that the self-referential nature of memory can be disrupted in individuals with mPFC lesions. However, it remains unclear whether damage to the mPFC impairs retrieval of personal trait knowledge. Therefore, in this neuropsychological case study we investigated the integrity of personal trait knowledge in J.S., an individual who sustained bilateral damage to the mPFC as a result of an anterior communicating artery aneurysm. We measured both accuracy and consistency of J.S.’s personal trait knowledge as well as his trait knowledge of another, frequently seen person, and compared his performance to a group of healthy adults. Findings revealed that J.S. had severely impaired accuracy and consistency of his personal trait knowledge relative to control participants. In contrast, J.S.’s accuracy and consistency of other-person trait knowledge was intact in comparison to control participants. Moreover, J.S. showed a normal positivity bias in his trait ratings. These results, albeit based on a single case, implicate the mPFC as critical for retrieval of personal trait knowledge. Findings also cast doubt on the likelihood that the mPFC, in particular the ventral mPFC, is necessary for storage and retrieval of trait knowledge of other people. Therefore, this case study adds to a growing body of evidence that mPFC damage can disrupt the link between self and memory.
Keywords: Self, Personal Semantics, Self-referential, Prefrontal cortex
I. Introduction
A remarkable feature of memory is that it can be highly self-referential and thus closely connected to one’s self-concept. For example, episodic memories, which are memories of unique events, are retrieved by mentally projecting the self back in time and recalling phenomenological and other experience-specific details (Tulving, 1972; 1983; 1985). However, not all personal memories are episodic; knowledge stored in semantic memory also can be specific to the self (Renoult, Davidson, Palombo, Moscovitch, & Levine, 2012). Indeed, there are at least two types of personal semantic memory: autobiographical facts (e.g. I was born in California) and personal trait and role knowledge (e.g. “I am active” as a personality trait; “I am a parent” as a personal role). Therefore, the self-concept is represented in different types of memory, bolstering its multidimensionality (Klein, 2012).
Findings from several decades of research in cognitive neuroscience and neuropsychology have provided a great deal of insight into the cognitive and neural bases of episodic memory. Neuropsychological studies have established that the medial temporal lobe (MTL) is critical for retrieval of episodic memory (Scoville & Milner, 1957; Tulving, 1983; 1985), including memories of highly meaningful, self-defining events (Grilli & Verfaellie, 2015). However, episodic memory also can be impaired when other brain regions are damaged, including the parietal lobes (Berryhill et al., 2007), diencephalon, and prefrontal cortex (Kopelman, Stanhope, & Kingsley, 1999; Levine, 2004). Indeed, findings from functional neuroimaging research have indicated that these brain regions form an intricate neural network that supports episodic memory (Svoboda, McKinnon, & Levine, 2006). The contributions of these brain regions to episodic memory vary as a function of retrieval and elaboration (Ford, Addis, & Giovanello, 2011; Holland, Addis, & Kensinger, 2011), vividness (Sheldon & Levine, 2013), and personal significance (Addis, Moscovitch, Crawley, & McAndrews, 2004).
In recent years, there has been increased interest in uncovering the cognitive and neural bases of personal semantic memory, with particular attention directed towards autobiographical fact knowledge. Early functional neuroimaging studies noted that retrieval of autobiographical facts recruited brain regions shared with episodic memory, including the MTL (Maguire & Mummery, 1999; Maguire & Frith, 2003). A recent meta-analysis revealed that autobiographical fact knowledge is associated with a distributed neural network, including the parahippocampus, anterior and posterior cingulate cortices, mPFC, lateral PFC, left superior and middle temporal gyrus, left thalamus, and left fusiform gyrus (Martinelli et al., 2013). Consistent with the functional neuroimaging results, a review of evidence from adults with amnesia secondary to MTL damage revealed that autobiographical fact knowledge depends on the medial temporal lobe for retrieval, albeit less so than episodic memory (Grilli & Verfaellie, 2014). Philippi and colleagues (Philippi, Tranel, Duff, & Rudrauf, 2015) also demonstrated that impaired autobiographical fact knowledge retrieval was associated with lesions to left MTL, whereas impaired episodic memory retrieval was related to lesions to right MTL. Grilli and Verfaellie (2016) further found that autobiographical fact retrieval was impaired in a group of adults with amnesia secondary to isolated MTL lesions, only if such knowledge had not been fully extracted from the spatiotemporal context from which it was derived. Lesion studies have further implicated the lateral temporal lobe (Grilli & Verfaellie, 2014; 2016) and prefrontal cortex (Philippi et al., 2015) in autobiographical fact retrieval, consistent with recent functional neuroimaging research showing that the neural signature of autobiographical fact retrieval has commonalities with general semantic memory retrieval (Coronel & Federmeier, 2016; Renoult et al., 2016). Therefore, while much remains unanswered, cognitive neuroscience and neuropsychology are rapidly shedding light on the neural bases of autobiographical fact knowledge.
In regard to personal trait knowledge, extensive evidence from functional neuroimaging research has converged on the notion that the medial prefrontal cortex (mPFC) plays a critical role in the ability to retrieve this type of personal semantic memory. Recent meta-analyses of fMRI studies contrasting personal trait knowledge to trait knowledge of others have revealed that activation in the mPFC is increased when individuals reflect on the self-descriptiveness of traits relative to personality traits of others (Martinelli et al., 2013; van der Meer et al., 2010). This finding is in line with studies comparing self-referential processing more broadly (i.e., evaluation of knowledge, beliefs, and agency) to cognitive processes involved in representing others (for recent meta-analyses, see Denny, Kober, Wager, & Ochsner, 2012; Murray, Schaer, & Debbane, 2012; Murray et al., 2015; Qin & Northoff, 2011). However, while these functional neuroimaging findings strongly implicate the mPFC in personal trait knowledge retrieval, studies also have associated other brain regions with retrieval of this type of personal semantic memory, including anterior and posterior cingulate, medial parietal cortex, precuneus, and the temporal poles (Craik et al., 1999; D’Argembeau et al., 2007; Kelley et al., 2002). Therefore, converging evidence from neuropsychology would complement the functional neuroimaging studies and shed light on whether the mPFC is necessary for retrieval of personal trait and role knowledge.
There is evidence that mPFC lesions can disrupt the self-referential nature of memory. For example, recent studies have found that individuals with mPFC lesions generate fewer event-specific details (Bertossi, Tesini, Cappelli, & Ciaramelli, in press), and self-referential pronouns during episodic memory retrieval (Kurczek et al., 2015; c.f. Bertossi et al., in press). Moreover, Philippi and colleagues (2015) found that impairment in autobiographical fact knowledge was associated with lesions in the mPFC in addition to the MTL. There also is evidence that individuals with mPFC lesions do not show a self-reference effect – the well-established boost in episodic memory associated with processing new information in relation to the self (Philippi, Duff, Denburg, Tranel, & Rudrauf, 2011). Thus, given that personal trait knowledge is another type of self-related memory, it too might be impaired by mPFC lesions.
Several case studies have investigated whether the integrity of personality trait knowledge is compromised in neuropsychological populations. Most of these studies have focused on understanding whether personal trait knowledge can be spared in the face of episodic memory impairment. In a series of case studies, Klein and colleagues showed that the ability to reliably judge one’s own personality traits was intact in adults with amnesia secondary to cardiac arrest and anoxia (Klein, Loftus, & Kihlstrom, 2002), posttraumatic amnesia from head injury (Klein, Loftus, & Kihlstrom, 1996), Alzheimer’s disease (Klein, Cosmides, & Costabile, 2003), and autism (Klein, Chan, & Loftus, 1999). Tulving (1993) also showed that K.C., who acquired amnesia secondary to traumatic brain injury, had stable knowledge of his personality traits. Although these studies indicate that stability of personal trait knowledge can be spared in adults with episodic memory impairment, mPFC damage was not reported in any of these cases.
In contrast, Philippi and colleagues (Philippi et al., 2012) investigated various aspects of the self-concept, including personal trait knowledge, in patient R, an individual who acquired bilateral damage to the mPFC, in addition to other cortical and subcortical regions including the insula, anterior cingulate cortex, and MTL, from herpes simplex encephalitis. Interestingly, patient R performed within normal limits on the vast majority of self-concept tests given to him. In regard to personal trait knowledge, he was administered the Big Five Inventory twice, one year apart, and he also was given an experimental personality trait rating task, which involved judging traits ranging in positivity. Critically, patient R’s ratings of his own personality on the Big Five Inventory were as stable as controls across the two testing sessions. Moreover, patient R showed a normal positivity bias in his trait self-ratings. At first glance, these results would seem to refute the notion that personal trait knowledge depends on the mPFC for storage and retrieval. However, patient R’s ratings of his own personality were not completely in line with ratings made of him by his mother and sister, namely they diverged in describing his overall agreeableness, conscientiousness, and introversion. Therefore, patient R’s personal trait knowledge, although stable across time, was not entirely accurate, as determined by how he was viewed by family members. Nevertheless, given the extent of patient R’s brain damage, it is difficult to isolate the contribution of the mPFC to his impaired personal trait knowledge accuracy. Moreover, although patient R demonstrated stable personal trait knowledge, his mPFC damage was largely right lateralized, sparing portions of the left ventral and dorsal mPFC. As such, intact regions of the mPFC may have supported his stable personal trait knowledge.
Therefore, despite evidence from functional neuroimaging and neuropsychology indicating that the mPFC supports retrieval of certain forms of personal memory, much remains uncertain about the contribution of this brain region to personal trait knowledge. To help close this gap in knowledge, we investigated this type of personal semantic memory in J.S., an individual with bilateral lesions to the mPFC and adjacent cortical and subcortical regions (Rapcsak et al., 1998; Rapcsak, Reminger, Glisky, Kaszniak, & Comer, 1999). Consistent with Philippi and colleagues’ (2012) investigation of patient R, we measured both consistency and accuracy of personal trait knowledge in J.S. We similarly focused specifically on personal trait knowledge, as opposed to personal role knowledge. Moreover, to better understand the contribution of the mPFC to trait knowledge retrieval we also investigated other person trait knowledge in J.S..
II. Methods
II.A Participants
Patient J.S
J.S., a right-handed white male, was 74 years old when he participated in this study. He was originally referred to our group for memory impairment following surgical repair of a ruptured anterior communicating artery aneurysm when he was 63 years old (Rapcsak et al., 1998; 1999). J.S. had completed 16 years of formal education and worked as a salesman prior to his aneurysm.
Figure 1 shows a CT scan of J.S.’s brain. CT revealed bilateral infarction of the mPFC, including orbitofrontal cortex, anterior cingulate gyrus, and the genu of the corpus callosum. Although mPFC damage was extensive bilaterally, frontal lesion was more extensive on the right side, including anterior (frontopolar) and inferior dorsolateral prefrontal areas. The lesion also affected the basal forebrain-septal area bilaterally. A single photon emission computed tomographic (SPECT) study was consistent with the CT findings and demonstrated severe reduction of blood flow in bilateral anterior cingulate gyrus and prefrontal cortex.
Figure 1.
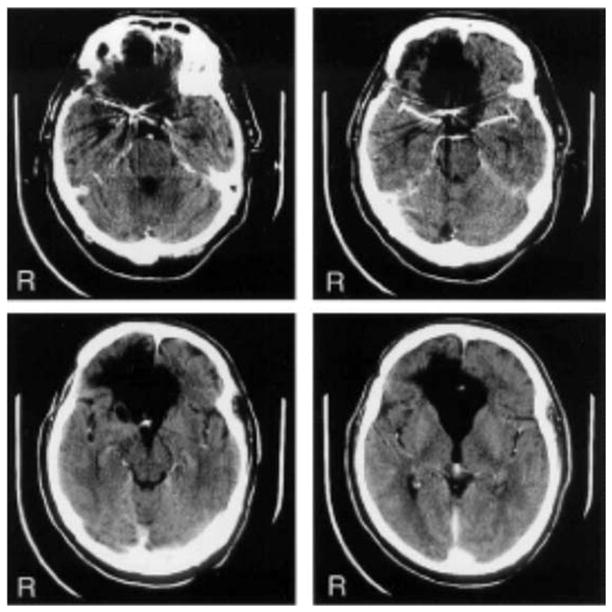
CT scan of J.S.’s brain
Table 1 shows J.S.’s neuropsychological test performance, which was completed when he was 74 years old. His performance on a test of premorbid intellectual function (North American Adult Reading Test, NAART, Spreen & Strauss, 1998) was average (NAART Full Scale IQ [FSIQ] = 105). His current intellectual function, as measured by the Wechsler Abbreviated Scale of Intelligence (WASI, Wechsler, 1999) was lower than expected (WASI FSIQ = 85) given his estimated premorbid abilities. J.S. showed no difficulties on tests of confrontational naming (Boston Naming Test, BNT, Kaplan, Goodglass, & Weintrab, 1983) and category fluency (Animal Naming). His performance on tests of memory (i.e. Wechsler Memory Scale-Third Edition [WMS-III; Wechsler, 1997], the California Verbal Learning Test [CVLT; Delis, Kramer, Kaplan, & Ober, 1987], and the Visual Paired Associates subtest from the Wechsler Memory Scale-Revised [WMS-R; Wechsler, 1987) ranged from low average to severely impaired. His memory performance is best characterized by difficulty in acquiring both visual and verbal information. He also showed a striking tendency to endorse lure items as previously presented. For example, in the recognition portion of the CVLT he recognized 15 out of 16 target items, but he also made 16 out of 16 possible false alarms.
Table 1.
J.S.’s neuropsychological test performance
| Function | Test | Performance | Interpretation |
|---|---|---|---|
| Intellectual Function | |||
| “Premorbid” | NAARTa Full Scale IQ | 105 | average |
| Current | WASIa | ||
| Verbal IQ | 95 | average | |
| Performance IQ | 79 | borderline | |
| Full scale IQ | 85 | low average | |
| Language | Animal Namingb | 16 | average |
| Boston Naming Testb | 57/60 | average | |
| Memory | WMS-IIIa | ||
| Auditory Immediate | 86 | low average | |
| Visual Immediate | 68 | impaired | |
| Immediate Memory | 73 | borderline | |
| Auditory Delay | 83 | low average | |
| Visual Delay | 88 | low average | |
| Auditory Recognition Delay | 65 | impaired | |
| General Memory | 77 | borderline | |
| CVLTb | |||
| List A Trial 1 | 5/16 | average | |
| List A Trial 5 | 6/16 | borderline | |
| List A Short-Delay Free Recall | 2/16 | impaired | |
| List A Short-Delay Cued Recall | 5/16 | borderline | |
| List A Long-Delay Free Recall | 1/16 | impaired | |
| List A Long-Delay Cued Recall | 3/16 | impaired | |
| Recognition Measures | |||
| Recognition Hits | 15/16 | high average | |
| False Positives | 16/16 | impaired | |
| WMS-Rb | |||
| Visual Paired Associates I | 6/18 | impaired | |
| Visual Paired Associates II | 2/6 | impaired | |
| Executive | WCSTb | ||
| Categories completed | 0/6 | impaired | |
| Correct Responses | 0 | -- | |
| Errors | 72/72 | impaired | |
| Perseverative Errors | 71 | impaired | |
| Verbal Fluencyb | 20 | borderline | |
| Mental Controlb | 11 | borderline | |
| BDSb (longest span) | 3 | low average | |
| WAIS-R Arithmeticb | 9 | average | |
Note. BDS = Backwards Digit Span; CVLT = California Verbal Learning Test (Delis, Kramer, Kaplan, & Ober, 1987); NAART = North American Adult Reading Test (Spreen & Strauss, 1998); WAIS-R = Wechsler Adult Intelligence Scale-Revised (Wechsler, 1981); WASI = Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999); WCST = Wisconsin Card Sorting Test (Hart, Kwentus, Wade, & Taylor, 1988); WMS-R = Wechsler Memory Scale-Revised (Wechsler, 1987); WMS-III = Wechsler Memory Scale-Third Edition (Wechsler, 1997).
Index scores;
Raw scores;
The following tests were administered to assess his executive function: the modified Wisconsin Card Sorting Test (WCST; Hart, Kwentus, Wade, & Taylor, 1988), a word fluency test, using initial letters F, A, and S (Spreen & Benton, 1977), Mental Arithmetic subtest from the Wechsler Adult Intelligence Scale-Revised (WAIS-R; Wechsler, 1981), and Mental Control and Backward Digit Span from the WMS-III (Wechsler, 1997). J.S.’s performance on the WCST was dramatically impaired, mainly as a result of a vast number of perseverative incorrect responses in this task. He also exhibited difficulties on tasks of verbal fluency and manipulating things in mind. Overall, J.S.’s cognitive function is best characterized by impairments in executive function, which are highlighted by difficulties in set shifting, problem solving, working memory, acquiring and retrieving information, and verbal fluency.
J.S. developed a confabulatory syndrome as a result of his aneurysm, which had persisted at the time when he participated in the present study. J.S. exhibited a vast amount of confabulations, both spontaneous and provoked, which included both plausible information and bizarre or implausible accounts. Some of his confabulations focused on particular themes and repeated themselves during different testing sessions. For example, by the end of the sessions he would ask experimenters to check with his secretary (a female nurse) whether he was going to be available for testing the following week since he had a trip planned in the near future, usually either to Africa or South America. He would also frequently add that he had visited a few continents during the past few days. He also exhibited spontaneous confabulations that did not seem to repeat themselves during different sessions. For instance, he once said his grandmother had come to visit him the day before the evaluation. Another time he said he had been to the university to give a talk about medical devices the week before. J.S. had lost all contact with his family and hence it was impossible to determine the origins of many of his confabulations.
Controls and Informants
A group of nine healthy older adults (five males) matched in age (mean = 74.8, standard deviation = 6.04) and education (mean = 15.2, standard deviation = 3.03) to J.S. and without a history of neurological or psychiatric illness served as control participants. Controls were recruited from a pool of healthy, community-dwelling adults, who had performed within normal limits on a comprehensive battery neuropsychological tests within two years of experimental testing. Included in the neuropsychological battery were standardized tests that have been found through confirmatory factor analysis to load on one of two factors: one measuring executive functioning (EF factor) and the other episodic memory (EM factor; for a detailed description of these tests and factor analyses, see Glisky et al., 1995; 2001; 2008). Each participant received a composite score on each factor, which was the average z score for the five tests loading on each factor after variance for age was removed, relative to a normative sample of 227 older adults. The mean z scores for controls on these factors were well within normal limits (mean [standard deviation]: EF factor = 0.11 [0.70]; EM factor = −0.08 [0.43]).
A nurse who worked at the nursing home where J.S. had been living for 9 years, nurse P, served as his informant. Nurse P was a 47-year-old man, who had 14 years of education and had known J.S. for 5 years. We asked him to participate in the study after we learned that he was the caregiver with whom J.S. had the most contact with. Nurse P reported knowing J.S. very well.
A group of nine individuals who reported knowing controls very well (i.e. family members and friends) without a history of dementia, stroke, alcohol abuse, or current psychiatric illness, served as informants of controls. The number of years that informants had known their controls ranged from 10 to 55.
II.B Materials and Procedure
Consistent with prior research (Klein et al., 1996; 1999; 2002; 2003; Tulving, 1993), we used a personality trait questionnaire to test participants’ personal trait knowledge. The questionnaire consisted of 84 words selected from a pool of normalized personality trait adjectives (Anderson, 1968). Half of the traits in the questionnaire were positive (e.g. considerate, trusting, intelligent) and half negative (e.g. moody, prejudiced, obstinate). In Anderson (1968), words were ordered according to likeability ratings made by 100 participants. Based on the distribution of these ratings, a word was considered positive if it was one of the first 252 words in the list, and negative if it had a ranking between 253 and 555.
Participants rated the extent to which each trait applied to them and their informant using a four-point scale with the following options: not at all, somewhat, quite a bit, and definitely. Instructions were typed at the top of each page and read as follows for self-ratings of J.S. and controls: “Please indicate to what extent each of the following traits applies to you, by circling the appropriate response for each trait.” Instructions were appropriately modified for other-person ratings of J.S. and controls, and for informants. J.S. and controls were asked to indicate how well each trait described them and their informant on two separate occasions, spaced a week to 10 days apart. The same adjectives, but in a different order, were presented on each occasion. Informants of J.S. and controls rated how well each trait described J.S. (or control) at the present time, and rated how well each trait described oneself.
III. Results
III.A Accuracy and consistency of personal trait knowledge
Table 2 shows accuracy and consistency scores for J.S. and control participants. Accuracy of the participants’ personal trait knowledge was calculated with two-way mixed intraclass correlation coefficients (ICCs), absolute agreement, between the ratings of participants on the personality trait questionnaire on the first and second occasions and their informants’ ratings of the participants. Consistency of participants’ personal trait knowledge was calculated with one-way ICCs, single measure, between the first and second ratings of participants’ self-ratings in the personality trait questionnaire.
Table 2.
Personal and other-person trait knowledge measures for J.S. and controls
| Personal Trait Knowledge
|
Other-Person Trait Knowledge
|
|||
|---|---|---|---|---|
| Consistency | Accuracy | Consistency | Accuracy | |
| J.S. | −.02 | S1=.10; S2=.25 | .58 | S1=.65; S2=.77 |
| Controls | .84 (.08) | S1=.73 (.07) S2=.71 (.09) |
.81 (.20) | S1=.67 (.16) S2=.71 (.11) |
Notes: S1 = session 1; S2 = session 2; numbers in parenthesis are standard deviations.
J.S.’s accuracy and consistency scores were compared to control participants with a Bayesian single-case method developed by Crawford and Garthwaite (2007) and expanded on by Crawford, Garthwaite, and Porter (2010). The 95 percent confidence interval (CI) represents z-scores for J.S.’s performance relative to the control group (i.e. the normative sample).
In regard to the integrity of J.S.’s personal trait knowledge, his accuracy for his own personality traits (.10 and .25 in session 1 and 2, respectively) was significantly worse than accuracy in controls in session 1 (.73), p < .00001, CI = −13.368 to −4.643; and session 2 (.71), p = . 0012, CI = −7.642 to −2.571. Moreover, J.S.’s personal trait knowledge consistency (−.02) was remarkably worse than controls (.84), p < .000001, CI = −15.953 to −5.565.
To gain additional insight into the nature of J.S.’s impaired personal trait knowledge, we investigated whether he, like patient R, showed a normal positivity bias in his trait ratings. Proportionally, J.S. endorsed more positive than negative traits in both sessions (.76 vs. .48 in session 1 and .59 vs. .14 in session 2). The controls on average also endorsed more positive than negative traits in both sessions (.87 vs .08 in session 1 and .84 vs. .09 in session 2). The proportion of positive traits endorsed by J.S. was not significantly different from controls in session 1, p =.45, CI = −1.596 to −0.058, or session 2, p = .22, CI = −2.301 to −0.434. J.S. endorsed significantly more negative traits than controls in session 1, p < .0001, CI = 4.112 to 11.886; but not in session 2, p = .37, CI = 0.167 to 1.790.
III.B Accuracy and consistency of trait knowledge of another person
Accuracy and consistency of trait knowledge of another was calculated in the same fashion as personal trait knowledge and analyzed with the Bayesian single-case method. In contrast with J.S.’s impaired personal trait knowledge, J.S.’s other-person trait knowledge accuracy in session 1 (.65) was not significantly different from controls (.67), p = .91 CI = −0.776 to 0.535; nor was his other-person trait knowledge accuracy in session 2 (.77) significantly different from controls (.71), p = .62 CI = −0.174 to 1.234. Moreover, J.S.’ other person knowledge consistency (.58) was not significantly different from controls (.81), p = .30 CI = −1.983 to −0.274.
J.S.’s accurate and consistent description of his caregiver’s traits could indicate that J.S. has acquired and stored trait knowledge of his caregiver. However, J.S. also could have rated his caregiver on the basis of general semantic knowledge about male nurses. Nurse P may have fit the stereotype and thus rated himself in a similar fashion. To test this hypothesis we showed J.S. a picture of a male nurse he had never met. We hypothesized that if J.S. had rated his caregiver on the basis of general semantic knowledge, but did not really know who his caregiver was, he would rate the unknown nurse in a similar fashion on the personality trait questionnaire.
Two months after J.S. had rated his caregiver, we presented to J.S. the picture of the lure nurse, told him this was a male nurse and that we would like for him to rate the nurse on a list of personality traits. JS responded, “I’m sorry, but I don’t know him”. Even after repeated encouragement of the experimenter, JS emphatically denied ever meeting the lure nurse and added “…but I do know a male nurse, [nurse P]. I can do the questionnaire about him”. The conversation went on as follows:
JS: “…I met him up here, I come here often”
Experimenter: “Why do you come here?”
JS: “I come to identify how to stop being an alcoholic. I come here because I don’t want to go to AA meetings. My wife is also an alcoholic, she’s part Korean and part Japanese, she comes here too…”
Experimenter: “How did you meet nurse P?”
JS: “I don’t remember how I met him, it was 4 or 5 years ago, but I like him very much. He and I identify quite a bit. We grew up in the same environment and I have to respect him for what he does.”
Experimenter: “How are you and he alike?”
JS: “[P‘s] grandfather was rich and so was mine. We both have equal education, although he thinks I am smarter, and we’ve both traveled. I trust him, we converse a lot.”
While J.S.’s report was filled with confabulations, the fact that J.S. denied knowing the lure nurse and that he mentioned nurse P specifically, suggests that he did not complete the questionnaire about his caregiver’s personality based solely on general semantic knowledge.
IV. Discussion
Although functional neuroimaging research has established that processing of personal trait knowledge is reliably and robustly associated with increased activation in the mPFC (Martinelli et al., 2013; van der Meer et al., 2010), the extent to which this brain region is necessary for storage and retrieval of personal trait knowledge has remained unclear. The present case study investigated retrieval of personal and other-person trait knowledge in J.S., an individual with extensive damage to the mPFC. There were two main findings: 1) accuracy and consistency of personal trait knowledge was severely impaired in J.S. relative to control participants, and 2) J.S.’s accuracy and consistency of trait knowledge of another person was not significantly different from other-person knowledge in controls. Interestingly, J.S. exhibited a normal positivity bias in rating his own personality traits. As discussed below, the results indicate that the mPFC is likely critical for retrieval of personal trait knowledge.
This study measured J.S’s personal trait knowledge accuracy by comparing his self-ratings to an informant’s ratings of his personality. Findings revealed that J.S. had significantly impaired personal trait knowledge accuracy, as there was less agreement between J.S. and his informant in comparison to the degree of agreement found between control participants and their informants. This finding is similar to the results reported in Philippi and colleagues’ (2012) study of patient R, as that individual’s judgments of his own personality characteristics also were discrepant from his informants’ ratings of him. Therefore, these results, along with the findings from patient R, indicate that accurate retrieval of personal trait knowledge depends on the mPFC.
Before this conclusion is accepted, we should note that J.S.’s brain lesion extended into right dorsolateral PFC and basal forebrain. There is considerable evidence that right dorsolateral PFC supports post-retrieval monitoring in laboratory-based memory tasks (Cruse & Wilding, 2009; Hayama & Rugg, 2009; Henson, Rugg, Shallice, & Dolan, 2000). However, meta-analyses of functional neuroimaging studies have revealed that dorsolateral PFC plays a secondary role in retrieval of memories of real-world personal events (Gilboa, 2004; Svoboda et al., 2006). Moreover, functional neuroimaging studies have not implicated this brain region in retrieval of personal trait knowledge (Martinelli et al., 2013; van der Meer et al., 2010). Damage to basal forebrain can result in disorders of memory retrieval as well, including impaired temporal organization of memory (O’Connor & Verfaellie, 2002). However, personal trait knowledge is thought to be devoid of spatiotemporal context (Conway, 2005; Grilli & Verfaellie, 2014; Klein, 2012; Renoult et al., 2012). Therefore, it is unlikely that damage to dorsolateral PFC or basal forebrain are the root causes of J.S.’s impaired personal trait knowledge.
Given that personal trait knowledge accuracy is calculated on the basis of informant responses, it is also important to consider whether an informant has adequate knowledge of an individual to provide valid ratings. This is noteworthy since Nurse P had known J.S. for five years, whereas controls and their informants had known each other from 10 to 55 years. Based on this difference in length of relationships, one might argue that Nurse P may not have had enough time to learn J.S.’s personality and thus provided inaccurate responses himself. We think this is unlikely, since five years of close contact between a nurse and patient would seem to be adequate time to learn the personality of another; indeed, it was enough time for J.S. to learn Nurse P’s personality. Nonetheless, to strengthen the conclusion that J.S. had impaired personal trait knowledge accuracy, it would have been useful to determine whether Nurse P’s accuracy of another, non-brain injured patient in the nursing home, whom he also had known for approximately five years, was comparable to the informants’ ratings of the controls1. It would be important for future studies of personal trait knowledge to incorporate this methodology.
This study also investigated stability of J.S.’s personal trait knowledge by measuring his ability to consistently rate his personality traits across two sessions. Interestingly, there was virtually no relation between J.S.’s self-ratings across two sessions, whereas healthy adults were highly consistent in their self-ratings. Importantly, J.S.’s other person ratings on a parallel version of the task were consistent over time, indicating that the lack of consistency in self-ratings cannot be attributed to J.S. being unable to understand the task at hand. J.S.’s lack of consistency is discrepant from patient R, who was as consistent in his self-ratings as a group of controls on a different personality trait rating measure. There are methodological differences between these case studies, namely the studies differed on the instruments used to measure personal trait knowledge and the length of interval across the repeated assessments (1 year in patient R and 1 week in J.S.). However, self-rating consistency among control participants in the present study was similar to self-rating consistency among controls reported in Philippi and colleagues (2012) (.84 vs. .71), indicating that these methodological differences might play a small role in the different findings across studies. Damage to the mPFC was more right lateralized in both patient R and J.S.. However, CT and SPECT results revealed that lesion to the mPFC was extensive bilaterally in J.S., which may explain why personal trait knowledge was stable in patient R and severely impaired in J.S. It seems unlikely that brain damage beyond the mPFC is the cause of this discrepancy in findings, as patient R had far more diffuse brain damage than J.S. outside of the mPFC region, and patient R also had damage to the dorsolateral PFC and basal forebrain. Taken together, the findings of patient R and J.S. may indicate that personal trait knowledge stability is more resilient than accuracy to mPFC damage, and thus a more extensive lesion to the mPFC is necessary to impair the former relative to the latter.
The present results expand on prior research linking damage to the mPFC with impaired episodic memory (Kurczek et al., 2015; Bertossi et al., in press) and autobiographical fact knowledge (Philippi et al., 2015) and show that lesions to this brain region can disrupt another type of self-relevant memory, namely personal trait knowledge. The mPFC has been implicated in the formation and retrieval of schemas, which are “adaptable associative networks of knowledge extracted over multiple similar experiences” (Gosh, Moscovitch, Melo Colella, & Gilboa, 2014). Although non-personal schemas have been the focus of prior work, the mPFC also may support the storage and retrieval of a self-schema, which could be defined as an abstract knowledge structure of one’s defining personal identity features (related to the notion of the “working self” described in Conway, 2005). Based on this idea, damage to the mPFC may impair the ability to instantiate the self-schema while retrieving personal memories. This could result in an impoverished use of a self-perspective in episodic memory (Kurczek et al., 2015), or in the case of J.S., an impaired ability to judge one’s personal trait knowledge.
To develop a more complete picture of the contribution of the mPFC to personal trait knowledge, it will be important for future research to elucidate the degree to which confabulation is associated with deficits in this type of personal memory. Damage to the mPFC is strongly associated with confabulatory syndromes (Gilboa & Moscovitch, 2002). Moreover, prior research has shown that both episodic and personal semantic memory can be distorted by confabulation (Kopelman, Ng, & Van Den Brouke, 1997). There also is evidence that damage to the mPFC, when accompanied by a confabulatory syndrome, can result in impaired judgments of the relevance or relatedness of semantic knowledge to active general schemas (Ghosh et al., 2014). Therefore, confabulation also may influence the ability to separate the self-schema from other schematic knowledge structures. Patient R, like J.S., confabulated (Feinstein et al., 2010), which casts doubt on the possibility that confabulation is a prerequisite for impaired personal trait knowledge. Nevertheless, the notion that confabulation and the self are related has long been of theoretical interest (Conway & Tacchi, 1996; Kopelman, 1999). Thus, a larger study could investigate the importance of confabulation by investigating the integrity of personal trait knowledge in individuals with mPFC damage who either do or do not confabulate.
Another challenge for future studies is to determine the extent to which damage to the mPFC impairs pre-existing relative to post-morbid personal trait knowledge. The fact that J.S. was inconsistent in rating his personality traits suggests that J.S. might have experienced severe impairment to his repository of pre-existing personal trait knowledge. However, it is also possible that J.S. has an impaired ability to update what knowledge remains accessible to him. Although we have limited information regarding J.S. before his illness, given his premorbid occupation and functional status we can infer that his general demeanor has likely changed since his ruptured aneurysm. Moreover, findings from functional neuroimaging (Leshikar & Duarte, 2014; Macrae, Moran, Heatherton, Banfield, & Kelley, 2004; Zhu et al., 2012) and neuropsychological research (Philippi et al., 2012) have indicated that the mPFC supports the advantage of self-referential processing on new episodic learning. Therefore, the mPFC also may be critical for new personal semantic learning. Based on this notion, J.S.’s inaccurate self-ratings could, to a certain extent, reflect reliance on preexisting personal trait knowledge that is not only limited in scope but also does not accurately reflect his current characteristics.
Although J.S.’s deficits in personal trait knowledge retrieval are remarkable, in one respect he approached the trait questionnaire task similarly to healthy adults. Specifically, J.S. endorsed a normal proportion of positive traits in both sessions. Moreover, although he endorsed more negative traits than control participants in session 1, he endorsed a similar proportion of negative traits to controls in session 2; and in both sessions, he endorsed more positive than negative traits. One interpretation of this finding is that J.S. used general knowledge of what constitutes desirable traits to rate himself, with the assumption that most people would possess such traits. Another possibility is that J.S. maintains enough of a self-schema to be biased to judge positive traits as self-descriptive. As such, while the exact meaning of his positivity bias remains uncertain, it might reflect an area of relative preservation of the self-schema.
In addition to personal trait knowledge, this study also investigated the integrity of other-person trait knowledge to determine whether both were impaired by damage to the mPFC. Interestingly, J.S. exhibited intact trait knowledge consistency of another person: Nurse P. Although J.S.’s other-person trait knowledge consistency was numerically lower relative to healthy adults, this difference was not statistically significant. Moreover, consistency for other-person knowledge was notably greater than it was for personal trait knowledge. His other-person trait knowledge accuracy also was very similar to controls. Therefore, these findings speak against the idea that other-person trait knowledge relies on the mPFC for retrieval. They also suggest that new learning of trait knowledge of another person likely can be acquired in the face of severe damage to the mPFC, as J.S. met Nurse P after his aneurysm. The fact that J.S. would not attempt to describe an unknown male nurse supports the conclusion that he based his trait judgments on knowledge that was specific to Nurse P, as opposed to only general knowledge of male nurses.
How could J.S. acquire and reliably retrieve trait knowledge of another person while exhibiting impairment in personal trait knowledge? Prior investigation of J.S. has shown that, like other individuals with prefrontal cortex lesions, he is capable of acquiring gist-based semantic information, despite impaired new episodic learning (Rapzsak et al., 1999). Thus, perhaps a gist-based learning mechanism supports acquisition of highly abstract trait knowledge of another person. Such a learning mechanism may be more effective at acquiring new knowledge in comparison to updating pre-existing knowledge, which may be particularly relevant to personal trait knowledge in individuals who experience life changes after brain injury.
Functional neuroimaging research provides another explanation based on the neural correlates of self and other-person reflection. A recent meta-analysis comparing self- and other-referential processing revealed a ventral to dorsal gradient within mPFC that corresponded to a shift from self to other-oriented cognitive processes and representations (Denny et al., 2012). Given that J.S.’s lesion does not extend into dorsal mPFC, his learning of another’s personality traits may have been supported by his ability to engage in cognitive processes involved in representing others. However, it is worth noting that meta-analyses comparing personal and other-person trait knowledge have revealed that dorsal mPFC also is recruited during personal trait knowledge retrieval (Martinelli et al., 2013; van der Meer et al., 2010). Moreover, Denny and colleagues (2012) also showed that processing knowledge of others was strongly correlated with activity in posterior neural regions, including the temporoparietal junction. Thus, given that J.S.’s brain lesion was isolated to anterior neural regions, his ability to consistently retrieve knowledge of another person may have been supported by posterior neural regions. Clearly, additional research is needed to determine the neural bases of other-person trait knowledge.
With a primary focus on elucidating the role of the mPFC in personal and other-person trait knowledge retrieval, this case study leaves open questions about the extent to which other brain regions work in concert with the mPFC to support personal trait knowledge retrieval. For instance, the lateral temporal lobe has been implicated in storage and retrieval of other forms of personal and general semantic memory (Renoult et al., 2012). However, the degree to which personal trait knowledge relies on this brain region remains underspecified. One possibility is that personal trait knowledge, like other forms of abstract semantic memory, is stored in the lateral temporal lobe and retrieved by the mPFC during schema formation. Future research could address this idea by investigating personal trait knowledge in individuals with relatively isolated lesions to the lateral temporal lobe, such as adults in the early stages of semantic dementia.
According to Conway’s (2005) model of self and memory, personal memory is organized hierarchically, with personal trait and role knowledge supported by autobiographical facts and episodic memories. Given that J.S. exhibited impaired personal trait knowledge, it would be interesting to know to what extent this individual could access other forms of personal memory in the hierarchy. Other forms of episodic and personal semantic memory could be assessed with structured measures, such as the Autobiographical Memory Interview (Kopelman, Baddeley, & Wilson, 1989) or the Autobiographical Interview (Levine, Svoboda, Hay, Winocur, & Moscovitch, 2002), as well as newer self-memory assessment methods that specifically focus on personal semantics (Rathbone, Moulin, & Conway, 2009; Grilli & Verfaellie, 2015). Further investigation of all forms of personal memory in memory-impaired populations could inform conceptual models of the organization and neural bases of personal memory. Structured interview measures also could be adapted to investigate episodic and semantic knowledge of other people, as opposed to investigating only other-person trait knowledge. Unfortunately, we were not able to take these next steps with J.S., as he moved to another assisted-living facility after he took part in the trait-rating questionnaire task, and our contact with the individual was lost. Thus, it will be important for future research to replicate the findings of the present case study, and to expand on the results by investigating all forms of personal memory in the same individuals. Such information could shed light on the degree of cognitive and neural interdependence between various forms of personal episodic and semantic memory.
In conclusion, this case study reveals that bilateral lesions to the mPFC can result in severe deficits to retrieval of personal trait knowledge. The fact that J.S. demonstrated accurate and consistent retrieval of another, frequently-seen person’s traits suggests that trait knowledge of others may not depend on the mPFC to the same degree as personal trait knowledge. These findings may have implications for family members and other individuals working with adults with mPFC damage, as it suggests that certain aspects of social cognition may be preserved in the face of severe impairment to personal memory and other cognitive deficits. Future research can expand on these results by investigating whether personal role knowledge (e.g. I am a father; I am a psychologist) also depends on the mPFC or if it relies on dissociable neural regions. Regardless, these results corroborate evidence from functional neuroimaging to implicate the mPFC in self-reflection. This case study also adds to a growing body of neuropsychological research to show that damage to the mPFC disrupts the self-referential nature of memory.
Footnotes
We thank an anonymous reviewer for raising this point.
References
- Addis DR, Moscovitch M, Crawley AP, McAndrews MP. Recollective qualities modulate hippocampal activation during autobiographical memory retrieval. Hippocampus. 2004;14(6):752–762. doi: 10.1002/hipo.10215. [DOI] [PubMed] [Google Scholar]
- Anderson NH. Likableness ratings of 555 personality trait words. Journal of Personality and Social Psychology. 1968;9(3):272–279. doi: 10.1037/h0025907. [DOI] [PubMed] [Google Scholar]
- Berryhill ME, Phuong L, Picasso L, Cabeza R, Olson IR. Parietal lobe and episodic memory: Bilateral damage causes impaired free recall of autobiographical memory. The Journal of Neuroscience. 2007;27(25):14415–14423. doi: 10.1523/JNEUROSCI.4163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertossi E, Tesini C, Cappelli A, Ciaramelli E. Ventromedial prefrontal damage causes a pervasive impairment of episodic memory and future thinking. Neuropsychologia. doi: 10.1016/j.neuropsychologia.2016.01.034. in press. [DOI] [PubMed] [Google Scholar]
- Conway MA. Memory and the self. Journal of Memory and Language. 2005;53(4):594–628. [Google Scholar]
- Conway MA, Tacchi PC. Motivated confabulation. Neurocase. 1996;2:325–339. [Google Scholar]
- Coronel JC, Federmeier KD. The N400 reveals how personal semantics is processed: Insights into the nature and organization of self-knowledge. Neuropsychologia. 2016;84:36–43. doi: 10.1016/j.neuropsychologia.2016.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik FIM, Moroz TM, Moscovitch M, Stuss DT, Winocur G, Tulving E, Kapur S. In search of the self: A positron emission tomography study. Psychological Science. 1999;10(1):26–34. [Google Scholar]
- Crawford JR, Garthwaite PH, Porter S. Point and interval estimates of effect sizes for the case-controls design in neuropsychology: Rationale, methods, implementations, and proposed reporting standards. Cognitive Neuropsychology. 2010;27:245–260. doi: 10.1080/02643294.2010.513967. [DOI] [PubMed] [Google Scholar]
- Crawford JR, Garthwaite PH. Comparison of a single case to a control or normative sample in neuropsychology: Development of a Bayesian approach. Cognitive Neuropsychology. 2007;24:343–372. doi: 10.1080/02643290701290146. [DOI] [PubMed] [Google Scholar]
- D’Argembeau A, Ruby P, Collette F, Degueldre C, Balteau E, Luxen A, Maquet P, Salmon E. Distinct regions of the medial prefrontal cortex are associated with self-referential processing and perspective taking. Journal of Cognitive Neuroscience. 2007;19(6):935–944. doi: 10.1162/jocn.2007.19.6.935. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. The California Verbal Learning Test: Adult version manual. San Antonio, TX: Psychological Corporation; 1987. [Google Scholar]
- Denny BT, Kober H, Wager TD, Ochsner KN. A meta-analysis of functional neuroimaging studies of self and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. Journal of Cognitive Neuroscience. 2012;24(8):1742–1752. doi: 10.1162/jocn_a_00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein JS, Rudrauf D, Khalsa SS, Cassell MD, Bruss J, Grabowski TJ, Tranel D. Bilateral limbic system destruction in man. Journal of Clinical & Experimental Neuropsychology. 2010;32(1):88–106. doi: 10.1080/13803390903066873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JH, Addis DR, Giovanello KS. Differential neural activity during search of specific and general autobiographical memories elicited by musical cues. Neuropsychologia. 2011;49:2514–2526. doi: 10.1016/j.neuropsychologia.2011.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa A. Autobiographical and episodic memory – One and the same? Evidence from prefrontal activation in neuroimaging studies. Neuropsychologia. 2004;42:1336–1349. doi: 10.1016/j.neuropsychologia.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Gilboa A, Moscovitch M. The cognitive neuroscience of confabulation: A review and a model. In: Baddeley AD, MDKopelman MD, Wilson BA, editors. The Handbook of Memory Disorders. John Wiley & Sons, Ltd; 2002. pp. 315–342. [Google Scholar]
- Glisky EL, Kong LL. Do young and older adults rely on different processes in source memory tasks? A neuropsychological study. Journal of Experimental Psychology: Learning, Memory, & Cognition. 2008;34(4):809–822. doi: 10.1037/0278-7393.34.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glisky EL, Polster MR, Routhieaux BC. Double dissociation between item and source memory. Neuropsychology. 1995;9:229–235. [Google Scholar]
- Glisky EL, Rubin SR, Davidson PSR. Source memory in older adults: An encoding or retrieval problem? Journal of Experimental Psychology: Learning, Memory, and Cognition. 2001;27:1131–1146. doi: 10.1037//0278-7393.27.5.1131. [DOI] [PubMed] [Google Scholar]
- Gosh VE, Moscovitch M, Melo Colella B, Gilboa A. Schema representation in patients with ventromedial PFC lesions. The Journal of Neuroscience. 2014;34(36):12057–12070. doi: 10.1523/JNEUROSCI.0740-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilli MD, Verfaellie M. Experience-near but not experience-far autobiographical facts depend on the medial temporal lobe for retrieval: Evidence from amnesia. Neuropsychologia. 2016;81(29):180–185. doi: 10.1016/j.neuropsychologia.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilli MD, Verfaellie M. Supporting the self-concept with memory: Insight from amnesia. Social Cognitive and Affective Neuroscience. 2015;10(12):1684–1692. doi: 10.1093/scan/nsv056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilli MD, Verfaellie M. Personal semantic memory: Insights from neuropsychological research on amnesia. Neuropsychologia. 2014;61:56–64. doi: 10.1016/j.neuropsychologia.2014.06.012. [DOI] [PubMed] [Google Scholar]
- Hart RP, Kwentus JA, Wade JB, Taylor JR. Modified Wisconsin card sorting test in elderly normal, depressed and demented patients. Clinical Neuropsychologist. 1988;2(1):49–56. [Google Scholar]
- Holland AC, Addis DR, Kensinger EA. The neural correlates of specific versus general autobiographical memory construction and elaboration. Neuropsychologia. 2011;49:3164–3177. doi: 10.1016/j.neuropsychologia.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintrab S. The Boston Naming Test. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. Journal of Cognitive Neuroscience. 2002;14(5):785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Klein SB. Self, memory, and the self-reference effect: An examination of conceptual and methodological issues. Personality and Social Psychology Review. 2012;16(3):283–300. doi: 10.1177/1088868311434214. [DOI] [PubMed] [Google Scholar]
- Klein SB, Chan RL, Loftus J. Independence of episodic and semantic self-knowledge: The case from autism. Social Cognition. 1999;17(4):413–436. [Google Scholar]
- Klein SB, Cosmides L, Costabile KA. Preserved knowledge of self in a case of Alzheimer’s dementia. Social Cognition. 2003;21(2):157–165. [Google Scholar]
- Klein SB, Loftus J, Kihlstrom JF. Self-knowledge of an amnesic patient: Toward a neuropsychology of personality and social psychology. Journal of Experimental Psychology: General. 1996;125(3):250–260. doi: 10.1037//0096-3445.125.3.250. [DOI] [PubMed] [Google Scholar]
- Klein SB, Loftus J, Kihlstrom JF. Memory and temporal experience: The effects of episodic memory loss on an amnesic patient’s ability to remember the past and imagine the future. Social Cognition. 2002;20(5):353–379. [Google Scholar]
- Kopelman MD. Varieties of false memory. Cognitive Neuropsychology (Special Issue: The Cognitive Neuropsychology of False Memories) 1999;16:197–214. [Google Scholar]
- Kopelman MD, Ng N, Van Den Brouke O. Confabulation extending across episodic, personal, and general semantic memory. Cognitive Neuropsychology. 1997;14:683–712. [Google Scholar]
- Kopelman MD, Stanhope N, Kinglsey D. Retrograde amnesia in patients with diencephalic, temporal lobe, or frontal lesions. Neuropsychologia. 1999;37:939–958. doi: 10.1016/s0028-3932(98)00143-2. [DOI] [PubMed] [Google Scholar]
- Kopelman MD, Wilson BA, Baddeley AD. The autobiographical memory interview: A new assessment of autobiographical and personal semantic memory in amnesic patients. Journal of Clinical & Experimental Neuropsychology. 1989;11(5):724–744. doi: 10.1080/01688638908400928. [DOI] [PubMed] [Google Scholar]
- Kurczek J, Wechsler E, Ahuja S, Jensen U, Cohen NJ, Tranel D, Duff M. Differential contributions of hippocampus and medial prefrontal cortex to self-projection and self-referential processing. Neuropsychologia. 2015;73:116–126. doi: 10.1016/j.neuropsychologia.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshikar ED, Duarte A. Medial prefrontal cortex supports source memory for self-referenced materials in young and older adults. Cognitive, Affective, & Behavioral Neuroscience. 2014;14(1):236–252. doi: 10.3758/s13415-013-0198-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B. Autobiographical memory and the self in time: Brain lesion effects, functional neuroanatomy, and the lifespan development. Brain and Cognition. 2004;55:54–68. doi: 10.1016/S0278-2626(03)00280-X. [DOI] [PubMed] [Google Scholar]
- Levine B, Svoboda E, Hay JF, Winocur G, Moscovitch M. Aging and autobiographical memory: Dissociating episodic from semantic retrieval. Psychology & Aging. 2002;17(4):677–689. [PubMed] [Google Scholar]
- Macrae CN, Moran JM, Heatherton TF, Banfield JF, Kelley WM. Medial prefrontal activity predicts memory for self. Cerebral Cortex. 2004;14(6):647–654. doi: 10.1093/cercor/bhh025. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Frith CD. Aging affects the engagement of the hippocampus during autobiographical memory retrieval. Brain. 2003;126:1511–1523. doi: 10.1093/brain/awg157. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Mummery CJ. Differential modulation of a common memory retrieval network revealed by position emission tomography. Hippocampus. 1999;9(1):54–61. doi: 10.1002/(SICI)1098-1063(1999)9:1<54::AID-HIPO6>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Martinelli P, Sperduti M, Piolino P. Neural substrates of the self-memory system: New insights from a meta-analysis. Human Brain Mapping. 2013;34(7):1515–1529. doi: 10.1002/hbm.22008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray RJ, Debbane M, Fox PT, Bzdok D, Eickhoff SB. Functional connectivity mapping of regions associated with self- and other-processing. Human Brain Mapping. 2015;36:1304–1324. doi: 10.1002/hbm.22703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray RJ, Schaer M, Debbane M. Degrees of separation: A quantitative neuroimaging meta-analysis investigating self-specificity and shared neural activation between self- and other-reflection. Neuroscience and Biobehavioral Reviews. 2012;36:1043–1059. doi: 10.1016/j.neubiorev.2011.12.013. [DOI] [PubMed] [Google Scholar]
- O’Connor M, Verfaellie M. The amnesic syndrome: Overview and subtypes. In: Baddeley AD, Kopelman MD, Wilson BA, editors. The Handbook of Memory Disorders. 2. John Wiley & Sons, Ltd; England: 2002. pp. 145–166. [Google Scholar]
- Philippi CL, Duff MC, Denburg NL, Tranel D, Rudrauf D. Medial PFC damage abolishes the self-reference effect. Journal of Cognitive Neuroscience. 2011;24(2):475–481. doi: 10.1162/jocn_a_00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippi CL, Feinstein JS, Khalsa SS, Damasio A, Tranel D, Landini G, Williford K, Rudrauf D. Preserved self-awareness following extensive bilateral brain damage to the insula, anterior cingulate, and medial prefrontal cortices. PLOS one. 2012;7(8):1–17. doi: 10.1371/journal.pone.0038413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippi CL, Tranel D, Duff M, Rudrauf D. Damage to the default mode network disrupts autobiographical memory retrieval. Social Cognitive and Affective Neuroscience. 2015;10:318–326. doi: 10.1093/scan/nsu070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin P, Northoff G. How is our self related to midline regions and the default-mode network? Neuroimage. 2011;57(3):1221–1233. doi: 10.1016/j.neuroimage.2011.05.028. [DOI] [PubMed] [Google Scholar]
- Rapcsak SZ, Kaszniak AW, Reminger SL, Glisky ML, Glisky EL, Comer JF. Dissociation between verbal and autonomic measures of memory following frontal lobe damage. Neurology. 1998;50:1258–1265. doi: 10.1212/wnl.50.5.1259. [DOI] [PubMed] [Google Scholar]
- Rapcsak Reminger, Glisky Kaszniak, Comer Neuropsychological mechanisms of false facial recognition following frontal lobe damage. Cognitive Neuropsychology. 1999;16:267–292. [Google Scholar]
- Rathbone CJ, Moulin CJA, Conway MA. Autobiographical memory and amnesia: Using conceptual knowledge to ground the self. Neurocase. 2009;15(5):405–418. doi: 10.1080/13554790902849164. [DOI] [PubMed] [Google Scholar]
- Renoult T, Davidson PSR, Palombo DJ, Moscovitch M, Levine B. Personal semantics: At the crossroads of semantic and episodic memory. Trends in Cognitive Sciences. 2012;16(11):550–558. doi: 10.1016/j.tics.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Renoult L, Tanguay A, Beaudry M, Tavakoli P, Rabipour S, Campbell K, Moscovitch M, Levine B, Davidson PSR. Personal semantics: Is it distinct from episodic and semantic memory? An electrophysiological study of memory for autobiographical facts and repeated events in honor of Shlomo Bentin. Neuropsychologia. doi: 10.1016/j.neuropsychologia.2015.08.013. in press. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. Journal of Neurology, Neurosurgery, and Psychiatry. 1957;20(1):11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon S, Levine B. Same as it ever was: Vividness modulates the similarities and differences between the neural networks that support retrieving remote and recent autobiographical memories. NeuroImage. 2013;83:880–891. doi: 10.1016/j.neuroimage.2013.06.082. [DOI] [PubMed] [Google Scholar]
- Spreen O, Benton AL. Neurosensory Center Comprehensive Examination for Aphasia (NCCEA) Victoria: University of Victoria Neuropsychology Laboratory; 1977. [Google Scholar]
- Spreen O, Strauss E. A Compendium of Neuropsychological Tests: Second Edition. New York, NY: Oxford University Press; 1998. [Google Scholar]
- Svoboda E, McKinnon MC, Levine B. The functional neuroanatomy of autobiographical memory: A meta-analysis. Neuropsychologia. 2006;44:2189–2208. doi: 10.1016/j.neuropsychologia.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E. Episodic and semantic memory. In: Tulving E, Donaldson W, editors. Organization of memory. NewYork: Academic Press; 1972. pp. 381–403. [Google Scholar]
- Tulving E. Elements of episodic memory. New York: Oxford University Press; 1983. [Google Scholar]
- Tulving E. Memory and consciousness. Canadian Psychology. 1985;26(1):1–12. [Google Scholar]
- Tulving E. Self-knowledge of an amnesic individual is represented abstractly. In: Srull TK, Wyer RS, editors. Advances in Social Cognition. Vol. 5. Hillsdale, NJ: Erlbaum; 1993. pp. 147–156. [Google Scholar]
- van der Meer L, Costafreda S, Aleman A, David AS. Self-reflection and the brain: A theoretical review and meta-analysis of neuroimaging studies with implications for schizophrenia. Neuroscience and Biobehavioral Reviews. 2010;34:935–946. doi: 10.1016/j.neubiorev.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- Wechsler D. Wechsler Memory Scale-Third Edition: Administration and scoring manual. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- Wechsler D. The Wechsler Memory Scale-Revised. San Antonio, TX: The Psychological Corporation; 1987. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-Revised. San Antonio, TX: The Psychological Corporation; 1981. [Google Scholar]
- Zhu L, Guo X, Li J, Zheng L, Wang Q, Yang Z. Hippocampal activity is associated with self-descriptiveness effect in memory, whereas self-reference effect in memory depends on medial prefrontal activity. 2012 doi: 10.1002/hipo.20994. [DOI] [PubMed] [Google Scholar]


