Abstract
Sulfur mustard (SM) and nitrogen mustard (NM) are cytotoxic alkylating agents that cause severe and progressive injury to the respiratory tract, resulting in significant morbidity and mortality. Evidence suggests that macrophages and the inflammatory mediators they release play roles in both acute and long-term pulmonary injury caused by mustards. In this article, we review the pathogenic effects of SM and NM on the respiratory tract and potential inflammatory mechanisms contributing to this activity.
Keywords: vesicant, pulmonary toxicity, macrophages, inflammatory mediators
Introduction
Sulfur mustard (SM) and the related analog nitrogen mustard (NM) are bifunctional alkylating agents known to cause severe damage to target organs, including the lung.1–3 Owing to their lipophilic nature, mustards readily penetrate tissues and cells and react with sulfhydryl, carboxyl, and aliphatic amino groups, as well as heterocyclic nitrogen atoms, forming stable adducts and causing alkylation and cross-linking of nucleic acids, proteins, and lipids.4,5 This results in oxidative and nitrosative stress, impairment of cellular functioning, DNA damage, and cytotoxicity.3,6
Acute respiratory complications following inhalation exposure to mustards include rhinorrhea, irritation, coughing, and choking, whereas long-term effects include asthma, bronchitis, bronchiectasis, airway narrowing due to scarring, and pulmonary fibrosis leading to bronchiolitis obliterans and chronic obstructive pulmonary disease (COPD).2,3,7,8 These pathological alterations are correlated with a persistent macrophage-dominant inflammatory response, and increases in proinflammatory/profibrotic mediators, including reactive oxygen species (ROS) and reactive nitrogen species (RNS), tumor necrosis factor α (TNFα), eicosanoids, interleukin (IL)-1, IL-6, IL-8, IL-10, IL-12, IL-13, matrix metalloproteinases (MMPs), connective tissue growth factor (CTGF), and transforming growth factor (TGF)-β, which have been implicated in pulmonary disease pathology and progression.3,9,10 Macrophages are known to play roles in both acute and chronic lung pathologies.11 However, the signaling events regulating macrophage activation and phenotypic differentiation and the role of these cells in initiating, propagating, and resolving mustard-induced pulmonary injury are currently unknown, and this represents the focus of our research.
Mustard-induced histopathological changes in the respiratory tract
To assess the role of macrophages and inflammatory mediators in vesicant-induced lung injury and fibrosis, we developed an experimental rodent model using NM (0.125 mg/kg, i.t.). At this dose, all animals survive and appear clinically normal for at least 4 weeks. Importantly, histopathological alterations induced by NM in the trachea, bronchi, and lung are generally similar to those observed with SM.12–18 In the trachea and bronchi, acute changes, including focal attenuation of the epithelium, detachment of the epithelium from the mucosa, loss of cilia, and an accumulation of fibrin entrapping necrotic epithelial cells and debris in the lumen, are observed 1–3 days postexposure.14 At this time, multifocal hyperplasia, bronchiolized alveoli, perivascular and peribronchial edema, hyperplasia and hypertrophy of goblet cells, blood vessel hemorrhage, fibrin deposits, and inflammatory cell infiltrates, along with patchy, mild thickening of alveolar septa, are also noted in the lower respiratory tract and the lung.14,16–18 These pathological sequelae following NM exposure persist for at least 28 days. With time, erythrophagocytosis, fibroplasia, squamous metaplasia of the bronchial wall, and emphysema-like changes in the alveolar tissue also develop, and, by 7 days after mustard exposure, prominent trichrome staining is evident within inflammatory lesions, particularly around the alveolar septal wall and the peribronchiolar region, with a few areas exhibiting organized fibrin deposits.16 By 28 days postexposure, multiple areas of fibrosis containing organized collagen fibers are observed around airways and bronchioles.16 These alterations are consistent with progressive and persistent histological changes described by others in rodents up to 30 days after NM or SM exposure.12,13,19,20 Similar pathological changes have been described in lungs of Iran–Iraq war veterans exposed to SM or in individuals following accidental pulmonary exposure to mustards.8,21,22 Together, these findings validate our experimental rodent model for investigating mechanisms of mustard-induced pulmonary injury.
Macrophages and inflammatory mediators accumulate in the lung following mustard exposure
In rodents, mustard-induced pulmonary injury is associated with an accumulation of activated macrophages in the lung; these cells appear within 1 day of exposure and persist for at least 28 days.10,14,16,18 Evidence suggests that macrophages play roles in both acute and chronic pulmonary pathologies, including cytotoxicity and fibrosis, and that these responses are mediated by phenotypically distinct macrophage subpopulations, broadly characterized as proinflammatory/cytotoxic M1 macrophages and anti-inflammatory/wound repair M2 macrophages.11,23 Whereas proinflammatory/cytotoxic M1 macrophages release ROS and RNS, eicosanoids, TNFα, IL-12, and MMPs, anti-inflammatory/wound repair M2 macrophages release mediators such as IL-10, which suppresses inflammation, and growth factors that promote wound repair.11 Excessive release of mediators by M1 and M2 macrophages can lead to chronic inflammation, injury, and/or fibrosis. Studies in our laboratory have demonstrated that macrophages accumulating in the lung early (1–3 days) after NM express inducible nitric oxide synthase (iNOS), cyclooxygenase (COX)-2, and TNFα, markers of M1 macrophages;14,16–18 M1 markers, including iNOS, TNFα, COX-2, IL-12α, MMP-9, and MMP-1010 are also upregulated in isolated macrophages (Table 1). The observation that M1 macrophages persist in the lung for at least 28 days after NM exposure suggests a prolonged proinflammatory response. Proinflammatory M1 macrophages have been implicated in lung injury induced by ozone and bleomycin, pulmonary toxicants known to cause acute oxidative injury to the lung,24,25 and we speculate that they play similar roles in mustard toxicity. This is supported by our findings that treatment of rats with gadolinium chloride, an M1 macrophage inhibitor,26 suppressed NM-induced lung injury.
Table 1.
Phenotypic characteristics of macrophage subpopulations accumulating in the lung following nitrogen mustard exposure
| M1 markers | CTL | Days after mustard exposure | |||
|---|---|---|---|---|---|
| 1 | 3 | 7 | 28 | ||
| CD11b | +/− | +++ | ++ | ++ | + |
| iNOS | +/− | +++ | +++ | ++ | + |
| COX-2 | +/− | ++ | +++ | ++ | + |
| TNFα | + | ++ | +++ | + | + |
| MMP-9 | +/− | ++ | +++ | ND | ND |
| iNOS gene | +/− | +++ | ++ | + | + |
| COX-2 gene | +/− | +++ | +++ | ++ | + |
| TNFα gene | + | +++ | ++ | + | + |
| MMP-9 gene | + | +++ | +++ | +++ | ++ |
| MMP-10 gene | + | +++ | + | ++ | + |
| IL-12α gene | +/− | +++ | +++ | ++ | + |
| M2 markers | |||||
| CD68 | + | + | ++ | ++ | +++ |
| CD163 | − | + | + | ++ | +++ |
| CD206 | + | + | ++ | +++ | +++ |
| Arg II | − | ++ | +++ | ++ | + |
| Ym-1 | +/− | + | ++ | ++ | +++ |
| Gal-3 | + | + | ++ | ++ | +++ |
| TGF-β | +/− | ND | +++ | +/− | +/− |
| IL-10 gene | +/− | +++ | +++ | +++ | +/− |
| PTX-2 gene | +/− | +++ | +++ | +++ | +/− |
| CTGF gene | +/− | +++ | +++ | ++ | +/− |
| ApoE gene | +/− | +/− | ++ | +++ | ++ |
Note: Rats were euthanized 1, 3, 7, or 28 days after treatment with nitrogen mustard (NM) or control (CTL). Protein expression was assessed by immunohistochemistry in lung sections. Gene expression was analyzed in frozen lung samples by RT-PCR, and data normalized relative to GAPDH. ND, not determined; +++, high expression; ++, intermediate expression; +, low expression; +/−, low/no expression; −, no expression.
Following mustard exposure, increased numbers of M2 macrophages are observed in the lung, characterized by expression of CD163, CD206, and arginase (Arg) II (Table 1). These cells first appear 3 days after exposure, suggesting that the process of fibrosis begins early in the pathogenic response.10,16,17 This is supported by our findings that expression of the profibrotic mitogen TGF-β and fibroplasia are also evident 3 days post-NM. With time following NM exposure, M2 macrophages became enlarged and foamy, a characteristic of profibrotic macrophages, and, by 28 days, they are mainly clustered in the airspaces adjacent to fibrotic regions.10,16,27
To further characterize macrophages responding to NM, cells were analyzed by flow cytometry using markers for inflammatory macrophages (CD11b) and maturity (CD43).10 Following NM exposure, CD11b+ inflammatory cells are observed in the lung within 1 day of exposure and persist for up to 28 days. CD11b+ cells were found to consist of immature macrophages, which express high levels of CD43 (CD43+), and mature macrophages, which express low levels of CD43 (CD43−). While maximum numbers of immature CD11b+CD43+ macrophages are observed in the lung at 3 days after NM exposure, CD11b+CD43− mature macrophages peak at 7 days. At 28 days, only a small number of CD11b+ infiltrating macrophages are present in the lung, and these cells are mainly CD43−. To analyze these subpopulations further, they were sorted and examined for relative expression of M1 and M2 genes.10 At 3 days post-NM, M1 (iNOS, IL-12α) marker expression is prominent in the CD11b+CD43+ subpopulation, while M2 (IL-10, CX3CR1) genes are upregulated in the CD11b+CD43− subpopulation. These findings indicate that M1 macrophages accumulating in the lung after NM are more immature than M2 macrophages and derived largely from blood monocytes.28 In contrast, low expression of CD43 on M2 macrophages indicates a more mature phenotype. We speculate that these cells are mainly derived from immature M1 macrophages that develop an M2 phenotype as they mature.29 This is supported by our findings that ApoE, a protein associated with macrophage activation toward an anti-inflammatory M2 phenotype,30 is increased in immature CD11b+CD43+ macrophages relative to mature CD11b+CD43− cells.10 Similar macrophage maturation and phenotypic switching have been described in renal and peritoneal models of inflammation.31,32
Role of spleen-derived macrophages in mustard-induced lung injury
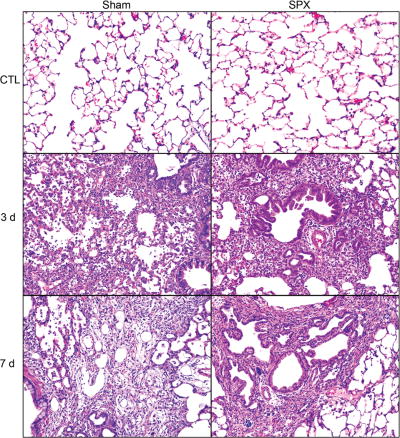
The spleen is the largest lymphatic organ in the body and plays an important role in pathogen recognition, iron recycling, and clearance of effete erythrocytes. The spleen has also been shown to act as a reservoir of inflammatory monocytes, which are readily mobilized to sites of tissue injury by angiotensin-II (AT-2) released from injured tissues and AT-2 receptor-1α (ATR-1α) expressed on inflammatory leukocytes, where they differentiate into macrophages and participate in both pro- and anti-inflammatory responses.33–39 To investigate the contribution of spleen monocytes to NM-induced inflammation and injury, we used splenectomized (SPX) rats. Treatment of SPX rats with NM results in an increase in lung macrophages expressing CCR2 but a decrease in ATR-1α+ cells, receptors important in bone marrow and spleen monocyte trafficking, respectively.40 Although the numbers of CD11b+ inflammatory cells in the lung are not affected by splenectomy, numbers of iNOS+ proinflammatory/cytotoxic M1 macrophages increase. Expression of M1 markers (i.e., iNOS and COX-2) is also upregulated in macrophages isolated from lungs of SPX rats. In contrast, NM-induced accumulation of M2 macrophages (CD68+, CD163+, CD206+, and Ym-1+) is generally unaltered by splenectomy, while expression of M2 proteins, including IL-10, ApoE, pentraxin (PTX) -2 and PTX-3, is reduced. These changes in lung macrophages responding to NM are associated with exacerbated tissue injury and more rapid fibrogenesis (Fig. 1) relative to sham-exposed rats.40 These data indicate that macrophages and mediators derived from the spleen participate in the early-resolution phase of vesicant-induced injury, and that in their absence the balance is shifted towards cytotoxic M1 macrophages, which promote lung injury.
Figure 1.
Histopathologic effects of NM on the lungs of control and splenectomized rats. Lung sections, prepared 3 and 7 days after exposure of sham control and SPX rats to CTL or NM, were stained with hematoxylin and eosin (magnification 200×). Representative sections from three rats/treatment groups are shown. From Ref. 40.
Mustard-induced oxidative stress
Oxidative stress has been shown to contribute to vesicant-induced lung injury, in part by triggering the inflammatory cascade.3,41,42 Thus, following exposure to vesicants, antioxidant levels decrease, and markers of oxidative stress, such as malondialdehyde, 8-hydroxyguanosine, and 4-hydroxynonenal, increase.43–45 Additionally, treatment of animals with antioxidants reduces vesicant-induced lung injury and inflammation.19,43,46,47 Heme oxygenase (HO)-1 is a phase II stress response enzyme with antioxidant and anti-inflammatory activity.48,49 After exposure of rodents to mustards, increases in both the intensity of HO-1 expression and the number of macrophages expressing HO-1 are observed in the lung.14,16,17 Increased levels of HO-1 persist for at least 28 days, suggesting that oxidative stress is an ongoing process after vesicant exposure.16 Findings that HO-1 expression is predominantly localized in lung macrophages indicate that these cells may be a significant source of cytotoxic oxidants. Mustard exposure is also associated with upregulation of the antioxidant Mn–superoxide dismutase (SOD) in lung macrophages and epithelial cells; lipocalin-2, a member the lipocalin superfamily with antioxidant activity,50 is also increased in bronchoalveolar lavage (BAL).16,17 These data provide additional support for the idea that vesicant-induced injury results from an imbalance between oxidants and antioxidants.
Approaches to mitigating mustard-induced pulmonary injury and fibrosis
At present, there are no approved drugs to counter pulmonary toxicity induced by mustards. Our approach to identifying potentially efficacious treatments has been to focus on macrophage-derived inflammatory proteins implicated in acute lung injury, fibrosis, and pulmonary disease pathogenesis. Initially, we targeted iNOS, an enzyme mediating the production of RNS by inflammatory macrophages.51 We found that mice deficient in iNOS are less sensitive to the cytotoxic effects of the half-mustard 2-chloroethyl ethyl sulfide (CEES) than wild-type controls.52 CEES-mediated alterations in pulmonary function are also reduced in iNOS−/− mice, suggesting that RNS generated via iNOS play a role in the pathogenic responses to mustards. To explore this further, we studied the effects of blocking iNOS using aminoguanidine (AG), a specific inhibitor of the enzyme,53 in our rat model of NM-induced lung toxicity. Treatment of animals with AG reduces NM-induced histopathological changes in the lung 1 and 3 days postexposure and blunts NM-induced increases in BAL cell and protein content.16 NM-induced increases in proliferating cell nuclear antigen (PCNA) expression and fibroplasia are also reduced by AG, along with NM-induced oxidative stress and the accumulation of COX-2+ and iNOS+ proinflammatory M1 macrophages in the lung. This is correlated with reduced numbers of Ym-1+ and galactin (Gal)-3+ M2 macrophages in the lung.16 Similar effects are observed using 1400W, another selective iNOS inhibitor.54 Antioxidants have been reported to inhibit proliferation of cells within hyperplastic lesions, collagen accumulation, and the development of fibrotic lesions in the lung following exposure to bleomycin or to CEES.55–57 Our findings provide support for a role for ROS and RNS in NM-induced lung toxicity and fibroplasia, and suggest that targeting these mediators may be useful for the treatment of acute lung injury induced by mustard vesicants.
In further studies, we focused on TNFα, a macrophage-derived proinflammatory cytokine known to promote oxidative stress, inflammatory cell influx, cytotoxicity and apoptosis, and pulmonary fibrosis.58–61 Initially, we used transgenic mice lacking TNF receptor (TNFR) 1, the major receptor mediating the proinflammatory actions of TNFα.58,59 Loss of TNFR1 in mice is associated with significant protection from CEES-induced lung injury, oxidative stress, and inflammation.62 CEES-induced expression of iNOS, COX-2, and monocyte chemotactic protein (MCP)-1 mRNA is also attenuated in Tnfr1−/− mice relative to wild-type mice, while CEES-mediated upregulation of CuZn-SOD and Mn-SOD is delayed or absent in Tnfr1−/− mice, and functional alterations are blunted. These findings prompted us to assess the effects of pharmacologic inhibition of TNFα on mustard-induced toxicity in the lung using pentoxifylline, a methyl xanthine phosphodiesterase inhibitor reported to downregulate TNFα production,63 or a specific antibody to TNFα. In these experiments, rats were treated with pentoxifylline (46.7 mg/kg, intraperitoneally) daily for 3 days beginning 15 min after NM exposure, or with anti-TNFα antibody (15 mg/kg, intravenously) once every 7–9 days, beginning 30 min after NM exposure. Inhibition of TNFα with pentoxifylline or anti-TNFα antibody reduces progressive histopathologic alterations in the lung, including acute inflammation, edema, and injury, as well as NM-induced peribronchial and parenchymal fibrotic alterations.17,64 Inhibition of TNFα also reduces NM-induced damage to the alveolar–epithelial barrier, measured by BAL protein and cell content, as well as expression of the oxidative stress markers HO-1 and lipocalin-2. TNFα inhibitors also effectively suppress the accumulation of proinflammatory/cytotoxic M1 macrophages in the lung in response to NM. Treatment of rats with anti-TNFα antibody also reduces NM-induced increases in expression of the profibrotic mediator TGF-β.64 This is associated with marked inhibition of NM-induced fibrosis and collagen deposition in the lung. Together, these findings suggest that TNFα is an important mediator of vesicant-induced pulmonary injury, and blocking TNFα is effective in attenuating the acute and long-term effects of mustards in the lung.
Summary
Mustard exposure involves a complex cascade of events, including oxidative stress, acute injury and disruption of tissue architecture, remodeling, and fibrosis. Studies from our laboratory have shown that M1and M2 macrophages sequentially accumulate in the lung following mustard exposure, and that they release mediators that contribute to both acute and long-term pathogenic responses. Our studies also demonstrate that inflammatory mediators, including RNS, ROS and TNFα, are important in vesicant-induced pulmonary injury. Thus, targeting these molecules may represent an efficacious approach to mitigating the toxic effects of mustards in humans.
Acknowledgments
This work was supported by National Institute of Health Grants U54AR055073, R01ES004738, and P30ES005022.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Ekstrand-Hammarstrom B, Wigenstam E, Bucht A. Inhalation of alkylating mustard causes long-term T cell-dependent inflammation in airways and growth of connective tissue. Toxicology. 2011;280:88–97. doi: 10.1016/j.tox.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 2.Razavi SM, Ghanei M, Salamati P, et al. Long-term effects of mustard gas on respiratory system of Iranian veterans after Iraq-Iran war: a review. Chin J Traumatol. 2013;16:163–168. [PubMed] [Google Scholar]
- 3.Weinberger B, Laskin JD, Sunil VR, et al. Sulfur mustard-induced pulmonary injury: therapeutic approaches to mitigating toxicity. Pulm Pharmacol Ther. 2011;24:92–99. doi: 10.1016/j.pupt.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giuliani I, Boivieux-Ulrich E, Houcine O, et al. Toxic effects of mechlorethamine on mammalian respiratory mucociliary epithelium in primary culture. Cell Biol Toxicol. 1994;10:231–246. doi: 10.1007/BF00756763. [DOI] [PubMed] [Google Scholar]
- 5.Smith KJ, Smith WJ, Hamilton T, et al. Histopathologic and immunohistochemical features in human skin after exposure to nitrogen and sulfur mustard. Am J Dermatopathol. 1998;20:22–28. doi: 10.1097/00000372-199802000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Shakarjian MP, Heck DE, Gray JP, et al. Mechanisms mediating the vesicant actions of sulfur mustard after cutaneous exposure. Toxicol Sci. 2010;114:5–19. doi: 10.1093/toxsci/kfp253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balali-Mood M, Hefazi M. The pharmacology, toxicology, and medical treatment of sulphur mustard poisoning. Fundam Clin Pharmacol. 2005;19:297–315. doi: 10.1111/j.1472-8206.2005.00325.x. [DOI] [PubMed] [Google Scholar]
- 8.Wang GQ, Xia ZF. Tissue injury by hot fluid containing nitrogen mustard. Burns. 2007;33:923–926. doi: 10.1016/j.burns.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Aghanouri R, Ghanei M, Aslani J, et al. Fibrogenic cytokine levels in bronchoalveolar lavage aspirates 15 years after exposure to sulfur mustard. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1160–1164. doi: 10.1152/ajplung.00169.2003. [DOI] [PubMed] [Google Scholar]
- 10.Venosa A, Malaviya R, Choi H, et al. Characterization of distinct macrophage subpopulations during nitrogen mustard-induced injury and fibrosis. Am J Respir Cell Mol Biol. 2015;54:436–446. doi: 10.1165/rcmb.2015-0120OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laskin DL, Sunil VR, Gardner CR, et al. Macrophages and tissue injury: agents of defense or destruction? Annu Rev Pharmacol Toxicol. 2011;51:267–288. doi: 10.1146/annurev.pharmtox.010909.105812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allon N, Amir A, Manisterski E, et al. Inhalation exposure to sulfur mustard in the guinea pig model: clinical, biochemical and histopathological characterization of respiratory injuries. Toxicol Appl Pharmacol. 2009;241:154–162. doi: 10.1016/j.taap.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Calvet JH, Jarreau PH, Levame M, et al. Acute and chronic respiratory effects of sulfur mustard intoxication in guinea pig. J Appl Physiol. 1994;76:681–688. doi: 10.1152/jappl.1994.76.2.681. [DOI] [PubMed] [Google Scholar]
- 14.Malaviya R, Sunil VR, Cervelli J, et al. Inflammatory effects of inhaled sulfur mustard in rat lung. Toxicol Appl Pharmacol. 2010;248:89–99. doi: 10.1016/j.taap.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malaviya R, Sunil VR, Venosa A, et al. Inflammatory mechanisms of pulmonary injury induced by mustards. Toxicol Lett. 2015;244:2–7. doi: 10.1016/j.toxlet.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malaviya R, Venosa A, Hall L, et al. Attenuation of acute nitrogen mustard-induced lung injury, inflammation and fibrogenesis by a nitric oxide synthase inhibitor. Toxicol Appl Pharmacol. 2012;265:279–291. doi: 10.1016/j.taap.2012.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sunil VR, Vayas KN, Cervelli JA, et al. Pentoxifylline attenuates nitrogen mustard-induced acute lung injury, oxidative stress and inflammation. Exp Mol Pathol. 2014;97:89–98. doi: 10.1016/j.yexmp.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sunil VR, Patel KJ, Shen J, et al. Functional and inflammatory alterations in the lung following exposure of rats to nitrogen mustard. Toxicol Appl Pharmacol. 2011;250:10–18. doi: 10.1016/j.taap.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ucar M, Korkmaz A, Reiter RJ, et al. Melatonin alleviates lung damage induced by the chemical warfare agent nitrogen mustard. Toxicol Lett. 2007;173:124–131. doi: 10.1016/j.toxlet.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Yaren H, Mollaoglu H, Kurt B, et al. Lung toxicity of nitrogen mustard may be mediated by nitric oxide and peroxynitrite in rats. Res Vet Sci. 2007;83:116–122. doi: 10.1016/j.rvsc.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Taghaddosinejad F, Fayyaz AF, Behnoush B. Pulmonary complications of mustard gas exposure: a study on cadavers. Acta Med Iran. 2011;49:233–236. [PubMed] [Google Scholar]
- 22.Rowell M, Kehe K, Balszuweit F, et al. The chronic effects of sulfur mustard exposure. Toxicology. 2009;263:9–11. doi: 10.1016/j.tox.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 23.Martinez FO, Sica A, Mantovani A, et al. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 24.Fakhrzadeh L, Laskin JD, Laskin DL. Deficiency in inducible nitric oxide synthase protects mice from ozone-induced lung inflammation and tissue injury. Am J Respir Cell Mol Biol. 2002;26:413–419. doi: 10.1165/ajrcmb.26.4.4516. [DOI] [PubMed] [Google Scholar]
- 25.Genovese T, Cuzzocrea S, Di Paola R, et al. Inhibition or knock out of inducible nitric oxide synthase result in resistance to bleomycin-induced lung injury. Respir Res. 2005;6:58. doi: 10.1186/1465-9921-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pendino KJ, Meidhof TM, Heck DE, et al. Inhibition of macrophages with gadolinium chloride abrogates ozone-induced pulmonary injury and inflammatory mediator production. Am J Respir Cell Mol Biol. 1995;13:125–132. doi: 10.1165/ajrcmb.13.2.7542894. [DOI] [PubMed] [Google Scholar]
- 27.Wang L, Lyerla T. Histochemical and cellular changes accompanying the appearance of lung fibrosis in an experimental mouse model for Hermansky Pudlak syndrome. Histochem Cell Biol. 2010;134:205–213. doi: 10.1007/s00418-010-0724-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Howell DN, Ahuja V, Jones L, et al. Differential expression of CD43 (leukosialin, sialophorin) by mononuclear phagocyte populations. J Leukoc Biol. 1994;55:536–544. doi: 10.1002/jlb.55.4.536. [DOI] [PubMed] [Google Scholar]
- 29.Arnold L, Henry A, Poron F, et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204:1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baitsch D, Bock HH, Engel T, et al. Apolipoprotein E induces antiinflammatory phenotype in macrophages. Arterioscler Thromb Vasc Biol. 2011;31:1160–1168. doi: 10.1161/ATVBAHA.111.222745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin SL, Castano AP, Nowlin BT, et al. Bone marrow Ly6Chigh monocytes are selectively recruited to injured kidney and differentiate into functionally distinct populations. J Immunol. 2009;183:6733–6743. doi: 10.4049/jimmunol.0901473. [DOI] [PubMed] [Google Scholar]
- 32.Liu G, Ma H, Qiu L, et al. Phenotypic and functional switch of macrophages induced by regulatory CD4+CD25+ T cells in mice. Immunol Cell Biol. 2011;89:130–142. doi: 10.1038/icb.2010.70. [DOI] [PubMed] [Google Scholar]
- 33.Auffray C, Fogg D, Garfa M, et al. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- 34.Filippatos G, Tilak M, Pinillos H, et al. Regulation of apoptosis by angiotensin II in the heart and lungs (Review) Int J Mol Med. 2001;7:273–280. doi: 10.3892/ijmm.7.3.273. [DOI] [PubMed] [Google Scholar]
- 35.Wang R, Zagariya A, Ibarra-Sunga O, et al. Angiotensin II induces apoptosis in human and rat alveolar epithelial cells. Am J Physiol. 1999;276:L885–889. doi: 10.1152/ajplung.1999.276.5.L885. [DOI] [PubMed] [Google Scholar]
- 36.Hiroyoshi T, Tsuchida M, Uchiyama K, et al. Splenectomy protects the kidneys against ischemic reperfusion injury in the rat. Transpl Immunol. 2012;27:8–11. doi: 10.1016/j.trim.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 37.Li X, Molina-Molina M, Abdul-Hafez A, et al. Extravascular sources of lung angiotensin peptide synthesis in idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2006;291:L887–895. doi: 10.1152/ajplung.00432.2005. [DOI] [PubMed] [Google Scholar]
- 38.Robbins CS, Chudnovskiy A, Rauch PJ, et al. Extramedullary hematopoiesis generates Ly-6Chigh monocytes that infiltrate atherosclerotic lesions. Circulation. 2012;125:364–374. doi: 10.1161/CIRCULATIONAHA.111.061986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swirski FK, Nahrendorf M, Etzrodt M, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Venosa A, Malaviya R, Gow AJ, et al. Protective role of spleen-derived macrophages in lung inflammation, injury and fibrosis induced by nitrogen mustard. Am J Physiol Lung Cell Mol Physiol. 2015;309:L1487–1498. doi: 10.1152/ajplung.00276.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mukhopadhyay S, Rajaratnam V, Mukherjee S, et al. Modulation of the expression of superoxide dismutase gene in lung injury by 2-chloroethyl ethyl sulfide, a mustard analog. J Biochem Mol Toxicol. 2006;20:142–149. doi: 10.1002/jbt.20128. [DOI] [PubMed] [Google Scholar]
- 42.Gould NS, White CW, Day BJ. A role for mitochondrial oxidative stress in sulfur mustard analog 2-chloroethyl ethyl sulfide-induced lung cell injury and antioxidant protection. J Pharmacol Exp Ther. 2009;328:732–739. doi: 10.1124/jpet.108.145037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kumar O, Sugendran K, Vijayaraghavan R. Protective effect of various antioxidants on the toxicity of sulphur mustard administered to mice by inhalation or percutaneous routes. Chem Biol Interact. 2001;134:1–12. doi: 10.1016/s0009-2797(00)00209-x. [DOI] [PubMed] [Google Scholar]
- 44.Mukherjee S, Stone WL, Yang H, et al. Protection of half sulfur mustard gas-induced lung injury in guinea pigs by antioxidant liposomes. J Biochem Mol Toxicol. 2009;23:143–153. doi: 10.1002/jbt.20279. [DOI] [PubMed] [Google Scholar]
- 45.O’Neill HC, White CW, Veress LA, et al. Treatment with the catalytic metalloporphyrin AEOL 10150 reduces inflammation and oxidative stress due to inhalation of the sulfur mustard analog 2-chloroethyl ethyl sulfide. Free Radic Biol Med. 2010;48:1188–1196. doi: 10.1016/j.freeradbiomed.2010.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McClintock SD, Till GO, Smith MG, et al. Protection from half-mustard-gas-induced acute lung injury in the rat. J Appl Toxicol. 2002;22:257–262. doi: 10.1002/jat.856. [DOI] [PubMed] [Google Scholar]
- 47.Wigenstam E, Rocksen D, Ekstrand-Hammarstrom B, et al. Treatment with dexamethasone or liposome-encapsuled vitamin E provides beneficial effects after chemical-induced lung injury. Inhal Toxicol. 2009;21:958–964. doi: 10.1080/08958370802596298. [DOI] [PubMed] [Google Scholar]
- 48.Otterbein LE, Lee PJ, Chin BY, et al. Protective effects of heme oxygenase-1 in acute lung injury. Chest. 1999;116:61S–63S. doi: 10.1378/chest.116.suppl_1.61s-a. [DOI] [PubMed] [Google Scholar]
- 49.Rahman I, Biswas SK, Kode A. Oxidant and antioxidant balance in the airways and airway diseases. Eur J Pharmacol. 2006;533:222–239. doi: 10.1016/j.ejphar.2005.12.087. [DOI] [PubMed] [Google Scholar]
- 50.Roudkenar MH, Kuwahara Y, Baba T, et al. Oxidative stress induced lipocalin 2 gene expression: addressing its expression under the harmful conditions. J Radiat Res. 2007;48:39–44. doi: 10.1269/jrr.06057. [DOI] [PubMed] [Google Scholar]
- 51.Laskin JD, Heck DE, Laskin DL. Nitric oxide pathways in toxic responses. In: Ballantine B, Marrs T, Syversen T, editors. General and Applied Toxicology. UK: Wiley-Blackwell; 2010. pp. 425–438. [Google Scholar]
- 52.Sunil VR, Shen J, Patel-Vayas K, et al. Role of reactive nitrogen species generated via inducible nitric oxide synthase in vesicant-induced lung injury, inflammation and altered lung functioning. Toxicol Appl Pharmacol. 2012;261:22–30. doi: 10.1016/j.taap.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Southan GJ, Szabo C. Selective pharmacological inhibition of distinct nitric oxide synthase isoforms. Biochem Pharmacol. 1996;51:383–394. doi: 10.1016/0006-2952(95)02099-3. [DOI] [PubMed] [Google Scholar]
- 54.Malaviya R, Sunil V, Venosa A, et al. Attenuation of nitrogen mustard (NM)-induced pulmonary injury and inflammation by antitumor necrosis factor (TNF)α antibody and the inducible nitric oxide synthase (iNOS) inhibitor, N-(3-(aminomethyl)benzyl) acetamidine (1400W) [abstract] The Toxicologist. 2014;138(S1):A561:p146. [Google Scholar]
- 55.Ikezaki S, Nishikawa A, Enami T, et al. Inhibitory effects of the dietary antioxidants butylated hydroxyanisole and butylated hydroxytoluene on bronchioloalveolar cell proliferation during the bleomycin-induced pulmonary fibrosing process in hamsters. Food Chem Toxicol. 1996;34:327–335. doi: 10.1016/0278-6915(96)00127-5. [DOI] [PubMed] [Google Scholar]
- 56.Giri SN, Biring I, Nguyen T, et al. Abrogation of bleomycin-induced lung fibrosis by nitric oxide synthase inhibitor, aminoguanidine in mice. Nitric Oxide. 2002;7:109–118. doi: 10.1016/s1089-8603(02)00102-7. [DOI] [PubMed] [Google Scholar]
- 57.Hoesel LM, Flierl MA, Niederbichler AD, et al. Ability of antioxidant liposomes to prevent acute and progressive pulmonary injury. Antioxid Redox Signal. 2008;10:973–981. doi: 10.1089/ars.2007.1878. [DOI] [PubMed] [Google Scholar]
- 58.Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3:745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- 59.Bradley JR. TNF-mediated inflammatory disease. J Pathol. 2008;214:149–160. doi: 10.1002/path.2287. [DOI] [PubMed] [Google Scholar]
- 60.Mukhopadhyay S, Hoidal JR, Mukherjee TK. Role of TNFα in pulmonary pathophysiology. Respir Res. 2006;7:125. doi: 10.1186/1465-9921-7-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thrall RS, Vogel SN, Evans R, et al. Role of tumor necrosis factor-α in the spontaneous development of pulmonary fibrosis in viable motheaten mutant mice. Am J Pathol. 1997;151:1303–1310. [PMC free article] [PubMed] [Google Scholar]
- 62.Sunil VR, Patel-Vayas K, Shen J, et al. Role of TNFR1 in lung injury and altered lung function induced by the model sulfur mustard vesicant, 2-chloroethyl ethyl sulfide. Toxicol Appl Pharmacol. 2011;250:245–255. doi: 10.1016/j.taap.2010.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fernandes JL, de Oliveira RT, Mamoni RL, et al. Pentoxifylline reduces pro-inflammatory and increases anti-inflammatory activity in patients with coronary artery disease–a randomized placebo-controlled study. Atherosclerosis. 2008;196:434–442. doi: 10.1016/j.atherosclerosis.2006.11.032. [DOI] [PubMed] [Google Scholar]
- 64.Malaviya R, Sunil VR, Venosa A, et al. Attenuation of nitrogen mustard-induced pulmonary injury and fibrosis by anti-tumor necrosis factor-α antibody. Toxicol Sci. 2015;148:71–88. doi: 10.1093/toxsci/kfv161. [DOI] [PMC free article] [PubMed] [Google Scholar]