Abstract
In this paper, I explain why I adopted an RDoC approach to study the neurobiology of auditory verbal hallucinations (AVH), or voices. I explain that the RDoC construct of “Agency” fits well with AVH phenomenology. To the extent that voices sound non-self, voice hearers lack a sense of agency over the voices. Using a vocalization paradigm like those used with non-human primates to study mechanisms subserving the sense of agency, we find that the auditory N1 ERP is suppressed during vocalization, that EEG synchrony preceding speech onset is related to N1 suppression, and that both are reduced in patients with schizophrenia. Reduced cortical suppression is also seen across multiple psychotic disorders and in clinically high-risk youth. The motor activity preceding talking and connectivity between frontal and temporal lobes during talking have both proved sensitive to AVH, suggesting neural activity and connectivity associated with intentions to act may be a better way to study agency and predictions based on agency.
Keywords: RDoC, Agency, Auditory Verbal Hallucinations, EEG, ERP, N1
This is one in a series of papers focused on using the RDoC framework to study psychopathology. Here, I discuss why our group has used the RDoC framework to understand auditory verbal hallucinations (AVH) experienced by patients with schizophrenia, the methods we have used, and the successes and challenges we have faced.
Why we did this
Long before RDoC was launched as a framework for research into the neurobiology of mental illnesses, people questioned the validity of schizophrenia as an entity, claiming that schizophrenia is a heterogeneous illness, with a wide variety of symptoms, including disorganization, negative symptoms, and positive symptoms. Recognizing the intractability of studying the neurobiology of ‘schizophrenia’ as an illness, we decided to study a symptom, that crossed diagnostic boundaries but that was tightly associated with schizophrenia. We chose to study AVH, a cardinal symptom of the illness associated with high morbidity and mortality.
Agency
We quickly learned that not only is schizophrenia heterogeneous, so are AVHs. For example, AVHs are often, but not always, reported to be spoken words, memories of previously heard speech, or negative in tone, suggesting the RDoC constructs of language, memory, and acute threat would be relevant in our studies of the neurobiology of AVH. However, regardless of whether AVH are pulled from old memories, have a linguistic quality, or are negative, they are typically experienced as separate from one’s own mental processes and lack ‘self’ attributes. This suggests there may be deficits in recognizing ‘self’ as the agent of the voices, regardless of their content. The RDoC construct of Agency might, thus, be considered a ‘super-construct’. Furthermore, a failure to monitor inner (self) speech is a leading explanatory construct of AVH (Allen, Laroi, McGuire, & Aleman, 2008). Accordingly, we approached our studies believing that understanding the neural mechanism underlying the sense of agency (in this case, the monitoring of experiences that are self-generated) might help us understand the pathophysiology of this aspect of the phenomenon, regardless of its content or tone.
Mechanisms responsible for agency
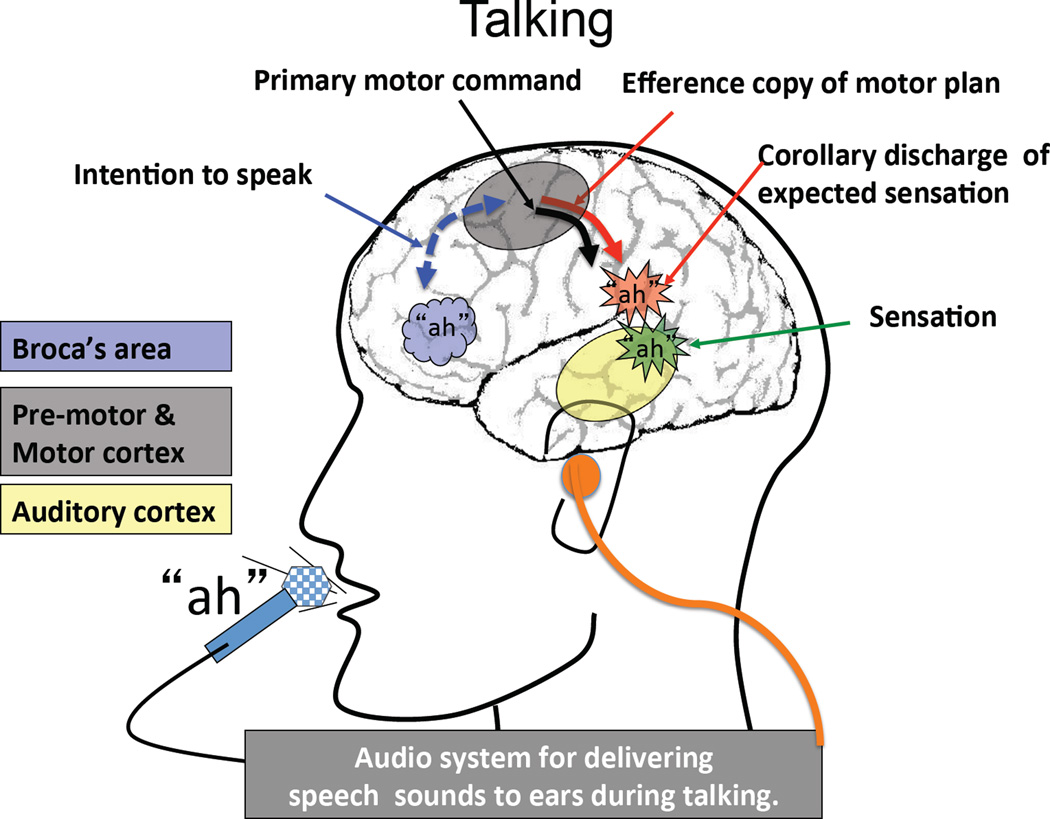
A dominant view about agency is that it “involves a generalisable relation between actions and their consequences, and is triggered by efferent motor commands” (Engbert, Wohlschlager, & Haggard, 2008). This type of mechanism may be responsible for the sense of agency we experience when we move and when we speak, both overtly and covertly. In Figures 1 and 2, this concept is illustrated from the point of view of vocalization during our talking/listening paradigm. Simply, every utterance is accompanied by the transmission of an efference copy of the motor plan to sensory cortex, where a corollary discharge of the expected sensory consequences of the motor act is compared to the actual sensation. Although ancient philosophers recognized the need for such a mechanism (Grösser, 1994), corollary discharge (Sperry, 1950) and efference copy (Von Holst & Mittelstaedt, 1950) were not introduced to modern physiology until 65 years ago.
Figure 1.
The intention to say “ah” during the Talk condition is represented as a thought bubble in the speech production areas of the frontal lobe. A command is sent to pre-motor and motor cortical areas, shown with a dotted bi-directional line. In motor cortical areas, two commands are issued: One is the primary command to initiate the motor act of speaking, and one is an efference copy of that command which is sent to auditory cortex. In auditory cortex, a corollary discharge of the expected “ah” sound is generated. This is represented as a red burst, overlaid with “ah” in the cartoon. An auditory re-afference is produced by the vocalized speech and represented as an “ah” entering the ear. The neural representation of the sensation in auditory cortex is shown as a green burst. When auditory re-afference (what you hear) matches the corollary discharge (what you intended to say), auditory cortical responsiveness is suppressed.
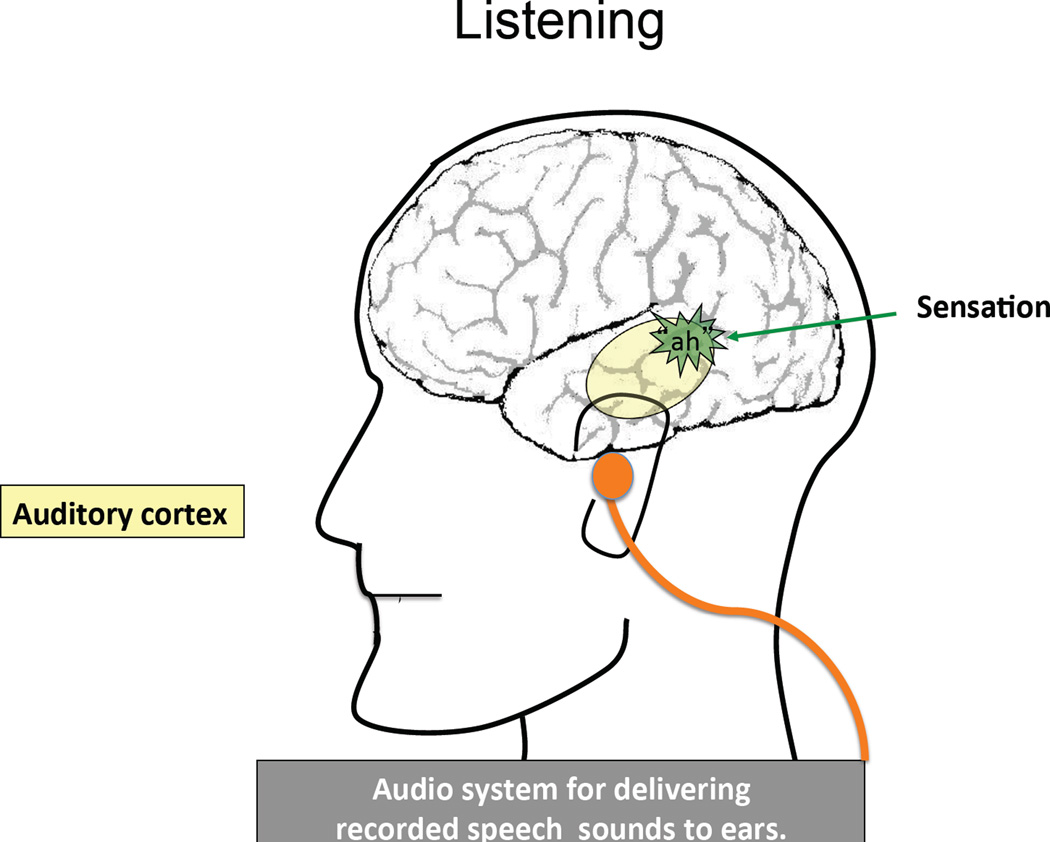
Figure 2.
During the Listen condition, the speech sounds the subject vocalized during the Talk condition are played to the subject who passively listens to them.
Many scientists use the terms “efference copy” and “corollary discharge” interchangeably perhaps because their studies do not focus on neural activity preceding motor actions, and those that do, typically do not record neural activity from motor areas of the brain (Eliades & Wang, 2005). Because of the millisecond timing information available in EEG coupled with our ability to record from many sites on the scalp at once, we have been able to observe neural activity over frontal cortex preceding the onset of a speech sound. Thus, we have been able to take advantage of this resolution to distinguish between the neural activity preceding speech sound onset and the neural activity associated with processing the predicted speech sound itself.
Importantly, both the proprioceptive and acoustic sensations preceding and during speech are predicted, although we are not necessarily aware of these predictions, and are only aware of predictions when they fail, or when there is a prediction error. Theoretically, prediction errors can be detected at any point during the action from the intention to speak to the first quantum of energy reaching the ears. Accordingly, a failed sense of agency can result from mismatches between predictions and sensations at any point in the action-sensation path. The intention to act may not be conscious until after the act is initiated (Libet, Gleason, Wright, & Pearl, 1983). For more discussion about when the intention to act is conscious, see Engbert et al. (2008).
Agency and hallucinations
Feinberg (1978) suggested that dysfunction of the efference copy/corollary discharge mechanisms may contribute to the positive symptoms of schizophrenia. These mechanisms evolved for motor control, and the link between thinking (the presumed raw material of AVH) and motor mechanisms has been described by psychologists for a century or more (Jackson, 1958; Washburn, 1916). Feinberg linked thinking and the corollary discharge mechanism, when he said, “These discharges may assist in the distinction between self-generated and externally produced movements; they also allow (or represent) monitoring of the motor commands before the effector response has occurred. Here, I hypothesize that this mechanism of control and integration is also present in thinking, which as Hughlings Jackson pointed out, may be considered the highest and most complex form of motor activity. I speculate that if corollary discharges are normally part of the motor mechanisms of thought, their derangement could produce many of the symptoms of schizophrenia.” Thus, thinking and overt speech may utilize the same action-based motor system mechanisms to tag self-generated sensations and distinguish them from sensations with an external source. If this mechanism is dysfunctional, auditory sensations resulting from these inner experiences may not be tagged as “self” and may be experienced as AVH. Frith (1987) expanded this concept and prompted a series of experiments confirming failures of agency or self-monitoring in schizophrenia patients who tend to experience AVH (e.g. Brebion et al., 2000; Shergill, Samson, Bays, Frith, & Wolpert, 2005; Stirling, Hellewell, & Quraishi, 1998) and delusions (e.g. Lindner, Thier, Kircher, Haarmeier, & Leube, 2005; Stirling et al., 1998).
What we did
Assay of agency—simple vocalization
My colleagues and I adopted a human neuroscience approach to study agency (Ford, Roach, & Mathalon, 2010) by adapting a non-human primate vocalization paradigm (Eliades & Wang, 2003, 2005, 2008) to humans to study the function of the corollary discharge/efference copy mechanism in schizophrenia. The neurobiology of the mechanism associated with vocalization has been elegantly described in crickets (Poulet & Hedwig, 2002, 2006, 2007), bats (Suga & Shimozawa, 1974), and non-human primates (Eliades & Wang, 2003, 2005, 2008). Fewer laboratories have attempted to study it in humans, perhaps because vocalizing produces artifacts in the scalp recorded EEG. However, many have been successful by using simple vocalizations (Behroozmand, Karvelis, Liu, & Larson, 2009; Curio, Neuloh, Numminen, Jousmaki, & Hari, 2000; Ford, Gray, Faustman, Roach, & Mathalon, 2007; Ford, Roach, Faustman, & Mathalon, 2007; Greenlee et al., 2011; Heinks-Maldonado, Mathalon, Gray, & Ford, 2005; Heinks-Maldonado et al., 2007) and rigorous data cleaning algorithms (Ford et al., 2010).
In all our studies, we report suppression of auditory cortex during vocalization in healthy controls, as seen in a reduction of the N1 amplitude of the event related brain potential (ERP) to the onset of the spoken sound as it is being spoken. This provides a direct test of the corollary discharge hypothesis and is similar to methods used by others with human (Behroozmand et al., 2009; Curio et al., 2000; Greenlee et al., 2011; Houde, Nagarajan, Sekihara, & Merzenich, 2002) and non-human primates (Eliades & Wang, 2003, 2005, 2008). This suppression is considered to result from a match between the corollary discharge and the sensory reafference, or side tones, entering the ear, as shown in Figure 1.
Next, we looked at what happens before speech by estimating the phase locking of the neural activity, about 100ms before speech onset, from trial to trial. This cross trial synchrony in the beta band (12–30Hz) was greater during vocalizing than listening, and the degree of synchrony predicted N1 suppression to the speech sound (Ford, Roach, et al., 2007). Based on that relationship, we suggested the activity preceding speech sound onset was the neural instantiation of the efference copy.
Because of the importance of this action-based signal to the sense of agency, we attempted to better localize it anatomically. Using EEG with anatomical MRI to facilitate source localization in 36 healthy subjects, we demonstrated that inferior frontal gyrus (IFG) activity during the 300ms before speaking was associated with suppression of N1 to the speech sound (Wang et al., 2014), confirming the relationship we had observed previously (Ford, Roach, et al., 2007), but with more precise anatomical localization.
In addition, Wang et al. (2014) also reported that there was greater spatial coherence of neural activity in the theta band between IFG and auditory cortex during speaking than during listening, as we had shown for theta band activity, using less precise anatomical information and a different speaking paradigm, as described below (Ford, Mathalon, Whitfield, Faustman, & Roth, 2002). Recording directly from the human brain, we reported that neural phase synchrony in the gamma band between Broca’s area and auditory cortex in the 50ms time window preceding speech onset was greater during speaking than listening and was correlated with suppression of N1 recorded from auditory cortex (Chen et al., 2011). While details of these studies vary with regard to the paradigm, time window preceding speech onset, frequency band, and method of calculating phase synchrony, all showed greater synchrony during speaking than listening. Thus, phase synchrony of neural activity during the motor act of speaking may contribute to transmission of the efference copy of that motor act, heralding the expected sensations.
Using the N1 component of the ERP in the talking/listening paradigm illustrated in Figures 1 and 2, we find that the normal dampening of the auditory cortical response during talking is less evident in patients with schizophrenia (Ford, Gray, et al., 2007; Ford et al., 2001; Ford et al., 2013; Ford, Roach, et al., 2007; Heinks-Maldonado et al., 2007). This is consistent with corollary discharge dysfunction in schizophrenia as predicted by Feinberg (1978). However, the degree of N1 suppression was not related to AVH severity. That is, corollary discharge dysfunction was sensitive to diagnosis but not to AVH. Efference copy preceding vocalization, however, was related to diagnosis and to AHV severity. That is, there was less pre-speech neural synchrony in schizophrenia patients, especially those with greater hallucination severity (Ford, Roach, et al., 2007).
In another earlier study (Ford et al., 2002), we asked subjects to read aloud hallucinatory statements like “why are you trying to annoy me?” while we recorded their speech and then played it back, similar to our approach with our vocalization paradigm described above. We found greater spatial coherence between the neural activity in frontal and temporal regions during talking than listening in the theta band. This effect was not seen in patients who tended to hallucinate. Because this analysis captured activity during the entire speech sample, it necessarily involved both the neural activity associated with the action of speech and the acoustic consequences of it. I mention it here because it is another successful attempt to find relationships with AVH.
Assay of agency—vocalization with pitch perturbations
In an attempt to understand the precision of the mechanism, we have perturbed the sensory consequences by shifting the pitch of the sound, as subjects are speaking and as has been done with songbirds and non-human primates (Eliades & Wang, 2008; Keller & Hahnloser, 2009) and humans (e.g., Greenlee et al., 2013). Although technically challenging, we have managed this with only an imperceptible delay of 6ms (Heinks-Maldonado et al., 2005). We find greater N1 suppression when healthy controls hear exactly what they said than when the sound is pitch shifted downward. N1 is still suppressed during talking compared to listening even when the sound is pitch shifted. Importantly, N1 suppression was not affected by pitch perturbations in SZ patients with AVH, but was affected in SZ patients without AVH and in healthy controls (Heinks-Maldonado et al., 2007).
Also, we recently showed that N1 is remarkably sensitive to self-generated, small, incidental and unwitting perturbations in speech formant production, during talking but not during playback (Sitek et al., 2013). That is, there was no external manipulation of the speech sound; we simply capitalized on naturally occurring variations in speech. While speakers may not be aware of these variations, auditory cortex seems to be. Data from patients with schizophrenia are still being analyzed.
How it cuts across multiple diagnostic categories
In collaboration with John Sweeney at the University of Illinois B-SNIP (Bipolar & Schizophrenia Network on Intermediate Phenotypes) site in Chicago, we collected data using the talking/listening paradigm illustrated in Figures 1 and 2 from patients with a diagnosis of schizophrenia, psychotic bipolar disease, and schizoaffective disease (Ford et al., 2013). Although the groups differed significantly in AVH severity, they did not differ in the degree of N1 suppression. Accordingly, AVH severity did not correlate with N1 suppression. Thus, it seems to reflect a more general alteration across these three psychotic disorders and may reflect the trait rather than the state of psychosis. The pre-speech onset data are still being analyzed using the anatomical localization methods described by Wang et al (2014).
How it cuts across the wellness spectrum
First-degree relatives of psychotic patients and heritability
In the same study with the BSNIP group, data were also collected from unaffected first-degree family members of the three groups of patients (Ford et al., 2013). We found that amount of N1 suppression was intermediate between that of healthy controls and the patients, being not different from either. Further, although there was a trend for N1 suppression to be heritable, it was not significant. While the sample may not have been large enough, it was large enough to show heritability of N1 amplitude during passive listening. Thus, it takes more than a genetic risk for psychosis to produce a deficit in N1 amplitude suppression during talking.
Clinically high-risk young adults
In a study of young people at clinical high-risk (CHR) for developing schizophrenia, we found evidence of intermediate deficits in N1 suppression, with suppression values falling between those of healthy controls and schizophrenia patients early in their illness (Perez et al., 2012).
Different units of analysis we have used
We have used a number of different units of analysis from the RDoC matrix, which I briefly mention here. “Genes” is the first unit of analysis listed in the RDoC matrix. As mentioned above, we did a heritability analysis and found no convincing evidence that N1 suppression was heritable (Ford et al., 2013). “Molecules” appears next; although we have unpublished data with our talking paradigm from people undergoing an infusion of ketamine, a powerful NMDA receptor antagonist, these data are still being analyzed. “Cells” follows next; although we have not been able to study this neural mechanism at the level of cells, a recent paper in rodents shows data that the inhibition resulting from motor activity is due to the action of parvalbumin positive interneurons (Schneider, Nelson, & Mooney, 2014). “Circuits” follows next in the matrix. As mentioned above, we have data suggesting that there is greater communication between frontal and temporal lobes during talking than listening (Ford et al., 2002), that neural synchrony between frontal and temporal lobes before speech onset is associated with N1 suppression during talking (Wang et al., 2014), and that the degree of trial to trial synchrony preceding speech onset is related to N1 suppression (Ford, Roach, et al., 2007). “Physiology” is next and is a constituent of the definition of ERPs, which are a measure of neurophysiology. Thus, N1 itself is an exemplar of this unit of analysis.
“Self-report” is another unit of analysis and an important one for the study of AVH. Until we have a blood test for AVH, the presence or absence of voices is determined exclusively via self-report. As Kozak and Cuthbert point out, this unit of analysis may serve as a proxy for symptoms, in spite of its lack of dimensionality (Kozak & Cuthbert, In press). However, individual items that reflect the phenomenology of AVH can be rated for their severity with instruments such as the Scale for the Assessment of Positive Symptoms (SAPS) (Andreasen, 1984), where we ask if the voices are experienced as comments on current activity (‘voices commenting’) or as conversations between different voices (‘voices conversing’). Although more sophisticated than commonly used instruments such as the Positive and Negative Symptom scale (PANSS) (Kay, Fiszbein, & Opler, 1987), the SAPS is less detailed than the more comprehensive Psychotic Symptom Rating Scales (PSYRATS) (Drake, Haddock, Tarrier, Bentall, & Lewis, 2007), which we have begun to use.
Issues encountered in trying to incorporate and inter-relate units of analyses
That we consistently find less suppression of N1 during motor-sensory tasks in psychotic patients, yet rarely find relationships between N1 suppression and AVH, embarrasses our theory that AVH are due to failures of corollary discharge, associated with intentions to act. This raises a series of questions.
First, is the theory that agency plays a role in AVH wrong? Agency implies intention to act, whether it is unconscious or goal directed, and the intention to act is associated with an efference copy of that intention. The concept can apply to conscious inner speech and to unconscious, unbidden thoughts. So, while our thoughts are not preceded by a conscious ‘intention’ to think, some mechanism must be alerting our subconsciousness that our inner experiences are self-generated and predictable, because we are not typically surprised by the content of our thoughts. If there is something wrong with this alerting mechanism, there might be a prediction error. These ‘unpredicted’ thoughts would be more highly processed and elaborated than necessary, and they would get more attention than they deserve. They may even seem to be coming from external sources. Thus, agency continues to be a reasonable explanatory construct of AVH.
Second, is our assessment of agency wrong? Perhaps we need to consider other paradigms for capturing the agency signal. We chose talking instead of thinking for practical reasons: we cannot capture thoughts and play them back, although there are clever ways to assess the auditory quality of inner speech (Tian & Poeppel, 2013, 2015). In spite of the possibility of common motor mechanisms (Jackson, 1958; Washburn, 1916), talking may be a bad proxy for unbidden thoughts. It will be important to ask whether our measure of agency (i.e., N1 suppression) is correlated with other measures of agency. That is, agency may not be a unitary construct and may have many facets that contribute to its definition.
Third, do we need break down our assay of agency into its constituent temporal parts and relate these to AVH? For example, the motor activity preceding talking has proved sensitive to AVH severity (Ford, Roach, et al., 2007), and dysconnectivity between motor and sensory areas during talking has also proven modestly sensitive to AVH (Ford et al., 2002). Studying the neural activity associated with actions seems a reasonable way to study agency and predictions based on agency. Indeed, action-based studies put the perceiver in the action seat, as the initiator of thoughts/ruminations—the raw material of AVH. Sub-fields of psychology, such as enactivism, have long embraced the importance of the motor system to cognition and experience (Varela, Thompson, & Rosch, 1991), and some have argued that cognitive processes and the neural activity associated with them should primarily be studied with respect to action (Engel, Maye, Kurthen, & Konig, 2013).
Fourth, is our assessment of AVH inadequate? If patients are guarded, they may not want to discuss the voices they hear. Or, there may be a tendency to over-endorse due to the demand characteristics inherent the symptom interview. The experienced patient will agree that he hears voices instead of describing the real experience he feels, which possibly is more akin to a feeling that someone is trying to communicate with him or to a feeling of presence of another person than to hearing audible voices. When we ask patients to describe their voices, we are asking them to do a meta-cognitive task. It is like asking friends to describe the nature of their thoughts. “Are they verbal? Are they just ideas? Are they words? Are they wisps of meaning? Do they have sentence structure?” Like us, patients may not have the right words in their lexicon to describe the inner experiences they are having. Finally, even if we get valid assessments of the experience, relating symptoms to neurobiology is fraught with difficulty for other reasons ranging from failure to distinguish between state and trait effects and medication confounds (Mathalon & Ford, 2012).
Fifth, if N1 suppression failure in psychosis is not due to AVH, what is it related to? AVH are a cardinal symptom of psychosis, and a deficit in corollary discharge, as reflected in N1 suppression failure, is a persistent feature in patients with a history of psychosis. What feature of psychosis is reflected in deficits in the efference copy and corollary discharge mechanisms? Our efforts to relate N1 suppression to other positive symptoms assessed with the SAPS (Andreasen, 1984), and negative symptoms assessed with the Schedule for the Assessment of Negative Symptoms (SANS)(Andreasen, 1983) have been disappointing. It would appear that the relative failure of talking to suppress auditory cortical responses is more strongly related to the diagnosis of psychosis than to the positive and negative symptoms that contribute to the diagnosis. Perhaps, there is something about the diagnosis of psychosis, other than symptoms, that we are not considering (Howes, Fusar-Poli, Bloomfield, Selvaraj, & McGuire, 2012). However, the dominance of diagnosis over symptoms is not new and is reminiscent of the finding that thought disorder was less heritable in twins than the global diagnosis of schizophrenia (Berenbaum, Oltmanns, & Gottesman, 1985).
Sixth, do we need to add in other constructs in our search for the neurobiological underpinnings of AVH? As mentioned above and pointed out by others (Ford, Morris, et al., 2014; Kozak & Cuthbert, In press), there are other RDoC constructs that are relevant to the experience of AVH. Each construct may contribute a different and possibly unique amount of variance to the experience of AVH. More elaborate models involving several constructs may give us traction on the neural underpinnings of AVH (Allen et al., 2008). Armed with manipulations and measures of these constructs paired with good assessments of the phenomenology and adequate statistical models, we may be able to determine which construct is responsible for which feature of the AVH experience.
Future directions of this research program
We have been attempting to extend our studies beyond the motor act of talking by asking if we can find the same pattern of findings when subjects press a button to deliver a pure tone. Like others before us using a self-delivery task (Baess, Horvath, Jacobsen, & Schroger, 2011; Martikainen, Kaneko, & Hari, 2005; McCarthy & Donchin, 1976; Schafer & Marcus, 1973; Sowman, Kuusik, & Johnson, 2012), we find that N1 is suppressed during self-delivery compared to passive playback (Ford, Palzes, Roach, & Mathalon, 2014). We have extended the self-delivery literature by showing that the lateralized readiness potential (LRP) preceding the button press is related to the degree of N1 suppression, reminiscent of the relationship we showed between pre-talking synchrony and N1 suppression in our talking paradigm (Ford, Roach, et al., 2007). We found smaller LRPs and less N1 suppression in patients, but in this case, neither metric was related to AVH. Although N1 suppression is not as strong during button pressing as talking (Ford, Gray, et al., 2007), we argue that this paradigm can be used with lab animals who do not vocalize, but who can be trained to press a lever, trip a light switch, or nose-poke to deliver a tone, as already shown by others (Schneider et al., 2014). Using this task, we have started using fMRI and assessing functional connectivity between motor and sensory cortex, as that link might be most relevant to the sense of agency and perhaps AVH.
We continue to welcome the opportunity to study AVH with our assays of agency in people without a diagnosis of schizophrenia, as we have been doing in schizoaffective and bipolar patients (Ford et al., 2013). AVH have been reported by patients with borderline personality disorder (Slotema et al., 2012), trauma (Bentall, Wickham, Shevlin, & Varese, 2012), psychotic depression (Choong, Hunter, & Woodruff, 2007), social isolation (Hoffman, 2007), and the normal population (Diederen, van Lutterveld, & Sommer, 2012). Superficially it seems reasonable to extend the studies to such groups, but we have to ask whether these groups all have the same type of phenomenology. Ultimately, wedding phenomenology with a relevant RDoC construct may provide the most successful path forward.
Acknowledgments
Funding & Acknowledgements
This work was supported by grants to JMF from Department of Veterans Affairs (I01CX000497) and National Institute of Mental Health (MH-58262).
I would like to thank all of my co-authors of the work discussed in this paper. Special thanks go to: Daniel H. Mathalon for on-going inspiration and intellectual guidance; Brian J. Roach for making it all happen and inspired data analysis; John Sweeney and his team for collecting data with our paradigm at his BSNIP site at the University of Illinois in Chicago; James Reilly for the heritability analysis of the BSNIP data; Jun Wang for his MRI-guided source analysis of the BSNIP data; Chi-Ming Chen for his analysis of the intra-cranial data; Veronica Perez for her analysis of the data from the clinical-high risk sample; Theda Heinks for her work on the pitch perturbation paradigm; Kevin Sitek for his work on the naturalistic formant variation analysis; Vanessa Palzes for her work on the button press paradigms; and to all the patients and their families for participating in this research.
References
- Allen P, Laroi F, McGuire PK, Aleman A. The hallucinating brain: a review of structural and functional neuroimaging studies of hallucinations. Neurosci Biobehav Rev. 2008;32(1):175–191. doi: 10.1016/j.neubiorev.2007.07.012. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS) Iowa City, IA: University of Iowa; 1983. [Google Scholar]
- Andreasen NC. Scale for the Assessment of Positive Symptoms. Iowa City, IA: University of Iowa; 1984. [Google Scholar]
- Baess P, Horvath J, Jacobsen T, Schroger E. Selective suppression of self-initiated sounds in an auditory stream: An ERP study. Psychophysiology. 2011;48(9):1276–1283. doi: 10.1111/j.1469-8986.2011.01196.x. [DOI] [PubMed] [Google Scholar]
- Behroozmand R, Karvelis L, Liu H, Larson CR. Vocalization-induced enhancement of the auditory cortex responsiveness during voice F0 feedback perturbation. Clin Neurophysiol. 2009;120(7):1303–1312. doi: 10.1016/j.clinph.2009.04.022. doi: S1388-2457(09)00340-X [pii] 10.1016/j.clinph.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentall RP, Wickham S, Shevlin M, Varese F. Do specific early-life adversities lead to specific symptoms of psychosis? A study from the 2007 the Adult Psychiatric Morbidity Survey. Schizophr Bull. 2012;38(4):734–740. doi: 10.1093/schbul/sbs049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenbaum H, Oltmanns TF, Gottesman II. Formal thought disorder in schizophrenics and their twins. J Abnorm Psychol. 1985;94(1):3–16. doi: 10.1037//0021-843x.94.1.3. [DOI] [PubMed] [Google Scholar]
- Brebion G, Amador X, David A, Malaspina D, Sharif Z, Gorman JM. Positive symptomatology and source-monitoring failure in schizophrenia--an analysis of symptom-specific effects. Psychiatry Res. 2000;95(2):119–131. doi: 10.1016/s0165-1781(00)00174-8. [DOI] [PubMed] [Google Scholar]
- Chen CM, Mathalon DH, Roach BJ, Cavus I, Spencer DD, Ford JM. The corollary discharge in humans is related to synchronous neural oscillations. J Cogn Neurosci. 2011;23(10):2892–2904. doi: 10.1162/jocn.2010.21589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choong C, Hunter MD, Woodruff PW. Auditory hallucinations in those populations that do not suffer from schizophrenia. Curr Psychiatry Rep. 2007;9(3):206–212. doi: 10.1007/s11920-007-0020-z. [DOI] [PubMed] [Google Scholar]
- Curio G, Neuloh G, Numminen J, Jousmaki V, Hari R. Speaking modifies voice-evoked activity in the human auditory cortex. Hum Brain Mapp. 2000;9(4):183–191. doi: 10.1002/(SICI)1097-0193(200004)9:4<183::AID-HBM1>3.0.CO;2-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diederen KM, van Lutterveld R, Sommer IE. Neuroimaging of voice hearing in non-psychotic individuals: a mini review. Front Hum Neurosci. 2012;6:111. doi: 10.3389/fnhum.2012.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake R, Haddock G, Tarrier N, Bentall R, Lewis S. The Psychotic Symptom Rating Scales (PSYRATS): their usefulness and properties in first episode psychosis. Schizophr Res. 2007;89(1–3):119–122. doi: 10.1016/j.schres.2006.04.024. doi: S0920-9964(06)00213-1 [pii] 10.1016/j.schres.2006.04.024. [DOI] [PubMed] [Google Scholar]
- Eliades SJ, Wang X. Sensory-motor interaction in the primate auditory cortex during self-initiated vocalizations. J Neurophysiol. 2003;89(4):2194–2207. doi: 10.1152/jn.00627.2002. [DOI] [PubMed] [Google Scholar]
- Eliades SJ, Wang X. Dynamics of auditory-vocal interaction in monkey auditory cortex. Cereb Cortex. 2005;15(10):1510–1523. doi: 10.1093/cercor/bhi030. [DOI] [PubMed] [Google Scholar]
- Eliades SJ, Wang X. Neural substrates of vocalization feedback monitoring in primate auditory cortex. Nature. 2008;453(7198):1102–1106. doi: 10.1038/nature06910. [DOI] [PubMed] [Google Scholar]
- Engbert K, Wohlschlager A, Haggard P. Who is causing what? The sense of agency is relational and efferent-triggered. Cognition. 2008;107(2):693–704. doi: 10.1016/j.cognition.2007.07.021. [DOI] [PubMed] [Google Scholar]
- Engel AK, Maye A, Kurthen M, Konig P. Where's the action? The pragmatic turn in cognitive science. Trends Cogn Sci. 2013;17(5):202–209. doi: 10.1016/j.tics.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Feinberg I. Efference copy and corollary discharge: implications for thinking and its disorders. Schizophr Bull. 1978;4(4):636–640. doi: 10.1093/schbul/4.4.636. [DOI] [PubMed] [Google Scholar]
- Ford JM, Gray M, Faustman WO, Roach BJ, Mathalon DH. Dissecting corollary discharge dysfunction in schizophrenia. Psychophysiology. 2007;44(4):522–529. doi: 10.1111/j.1469-8986.2007.00533.x. [DOI] [PubMed] [Google Scholar]
- Ford JM, Mathalon DH, Kalba S, Whitfield S, Faustman WO, Roth WT. Cortical responsiveness during talking and listening in schizophrenia: an event-related brain potential study. Biol Psychiatry. 2001;50:540–549. doi: 10.1016/s0006-3223(01)01166-0. [DOI] [PubMed] [Google Scholar]
- Ford JM, Mathalon DH, Roach BJ, Keedy SK, Reilly JL, Gershon ES, Sweeney JA. Neurophysiological evidence of corollary discharge function during vocalization in psychotic patients and their nonpsychotic first-degree relatives. Schizophr Bull. 2013;39(6):1272–1280. doi: 10.1093/schbul/sbs129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JM, Mathalon DH, Whitfield S, Faustman WO, Roth WT. Reduced communication between frontal and temporal lobes during talking in schizophrenia. Biol Psychiatry. 2002;51(6):485–492. doi: 10.1016/s0006-3223(01)01335-x. doi: [DOI] [PubMed] [Google Scholar]
- Ford JM, Morris SE, Hoffman RE, Sommer I, Waters F, McCarthy-Jones S, Cuthbert BN. Studying hallucinations within the NIMH RDoC framework. Schizophr Bull. 2014;40(Suppl 4):S295–S304. doi: 10.1093/schbul/sbu011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JM, Palzes VA, Roach BJ, Mathalon DH. Did I do that? Abnormal predictive processes in schizophrenia when button pressing to deliver a tone. Schizophr Bull. 2014;40(4):804–812. doi: 10.1093/schbul/sbt072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JM, Roach BJ, Faustman WO, Mathalon DH. Synch before you speak: auditory hallucinations in schizophrenia. Am J Psychiatry. 2007;164(3):458–466. doi: 10.1176/ajp.2007.164.3.458. [DOI] [PubMed] [Google Scholar]
- Ford JM, Roach BJ, Mathalon DH. Assessing corollary discharge in humans using noninvasive neurophysiological methods. Nat Protoc. 2010;5(6):1160–1168. doi: 10.1038/nprot.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CD. The positive and negative symptoms of schizophrenia reflect impairments in the perception and initiation of action. Psychol Med. 1987;17(3):631–648. doi: 10.1017/s0033291700025873. [DOI] [PubMed] [Google Scholar]
- Greenlee JD, Behroozmand R, Larson CR, Jackson AW, Chen F, Hansen DR, Howard MA., 3rd Sensory-motor interactions for vocal pitch monitoring in non-primary human auditory cortex. PLoS One. 2013;8(4):e60783. doi: 10.1371/journal.pone.0060783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenlee JD, Jackson AW, Chen F, Larson CR, Oya H, Kawasaki H, Howard MA., 3rd Human auditory cortical activation during self-vocalization. PLoS One. 2011;6(3):e14744. doi: 10.1371/journal.pone.0014744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grösser OJ. On the History of the Ideas of Efference Copy and Reafference. In: Debru Claude., editor. Essays in the History of Physiological Sciences. 1994. pp. 35–56. [Google Scholar]
- Heinks-Maldonado TH, Mathalon DH, Gray M, Ford JM. Fine-tuning of auditory cortex during speech production. Psychophysiology. 2005;42(2):180–190. doi: 10.1111/j.1469-8986.2005.00272.x. [DOI] [PubMed] [Google Scholar]
- Heinks-Maldonado TH, Mathalon DH, Houde JF, Gray M, Faustman WO, Ford JM. Relationship of imprecise corollary discharge in schizophrenia to auditory hallucinations. Arch Gen Psychiatry. 2007;64(3):286–296. doi: 10.1001/archpsyc.64.3.286. [DOI] [PubMed] [Google Scholar]
- Hoffman RE. A social deafferentation hypothesis for induction of active schizophrenia. Schizophr Bull. 2007;33(5):1066–1070. doi: 10.1093/schbul/sbm079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houde JF, Nagarajan SS, Sekihara K, Merzenich MM. Modulation of the auditory cortex during speech: an MEG study. J Cogn Neurosci. 2002;14(8):1125–1138. doi: 10.1162/089892902760807140. [DOI] [PubMed] [Google Scholar]
- Howes OD, Fusar-Poli P, Bloomfield M, Selvaraj S, McGuire P. From the prodrome to chronic schizophrenia: the neurobiology underlying psychotic symptoms and cognitive impairments. Curr Pharm Des. 2012;18(4):459–465. doi: 10.2174/138161212799316217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson JH. Selected writings of John Hughlings Jackson. New York: Basic Books; 1958. [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Keller GB, Hahnloser RH. Neural processing of auditory feedback during vocal practice in a songbird. Nature. 2009;457(7226):187–190. doi: 10.1038/nature07467. doi: nature07467 [pii] 10.1038/nature07467. [DOI] [PubMed] [Google Scholar]
- Kozak MJ, Cuthbert BN. The NIMH Research Domain Criteria Initiative: Background, Issues, and Pragmatics. Psychophysiology. doi: 10.1111/psyp.12518. (In press) [DOI] [PubMed] [Google Scholar]
- Libet B, Gleason CA, Wright EW, Pearl DK. Time of conscious intention to act in relation to onset of cerebral activity (readiness-potential). The unconscious initiation of a freely voluntary act. Brain. 1983;106(Pt 3):623–642. doi: 10.1093/brain/106.3.623. [DOI] [PubMed] [Google Scholar]
- Lindner A, Thier P, Kircher TT, Haarmeier T, Leube DT. Disorders of agency in schizophrenia correlate with an inability to compensate for the sensory consequences of actions. Curr Biol. 2005;15(12):1119–1124. doi: 10.1016/j.cub.2005.05.049. [DOI] [PubMed] [Google Scholar]
- Martikainen MH, Kaneko K, Hari R. Suppressed responses to self-triggered sounds in the human auditory cortex. Cereb Cortex. 2005;15(3):299–302. doi: 10.1093/cercor/bhh131. [DOI] [PubMed] [Google Scholar]
- Mathalon DH, Ford JM. Neurobiology of schizophrenia: search for the elusive correlation with symptoms. Front Hum Neurosci. 2012;6:136. doi: 10.3389/fnhum.2012.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy G, Donchin E. The effects of temporal and event uncertainty in determining the waveforms of the auditory event related potential (ERP) Psychophysiology. 1976;13(6):581–590. doi: 10.1111/j.1469-8986.1976.tb00885.x. [DOI] [PubMed] [Google Scholar]
- Perez VB, Ford JM, Roach BJ, Loewy RL, Stuart BK, Vinogradov S, Mathalon DH. Auditory cortex responsiveness during talking and listening: early illness schizophrenia and patients at clinical high-risk for psychosis. Schizophr Bull. 2012;38(6):1216–1224. doi: 10.1093/schbul/sbr124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulet JF, Hedwig B. A corollary discharge maintains auditory sensitivity during sound production. Nature. 2002;418(6900):872–876. doi: 10.1038/nature00919. [DOI] [PubMed] [Google Scholar]
- Poulet JF, Hedwig B. The cellular basis of a corollary discharge. Science. 2006;311(5760):518–522. doi: 10.1126/science.1120847. [DOI] [PubMed] [Google Scholar]
- Poulet JF, Hedwig B. New insights into corollary discharges mediated by identified neural pathways. Trends Neurosci. 2007;30(1):14–21. doi: 10.1016/j.tins.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Schafer EW, Marcus MM. Self-stimulation alters human sensory brain responses. Science. 1973;181(95):175–177. doi: 10.1126/science.181.4095.175. [DOI] [PubMed] [Google Scholar]
- Schneider DM, Nelson A, Mooney R. A synaptic and circuit basis for corollary discharge in the auditory cortex. Nature. 2014;513(7517):189–194. doi: 10.1038/nature13724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shergill SS, Samson G, Bays PM, Frith CD, Wolpert DM. Evidence for sensory prediction deficits in schizophrenia. Am J Psychiatry. 2005;162(12):2384–2386. doi: 10.1176/appi.ajp.162.12.2384. [DOI] [PubMed] [Google Scholar]
- Sitek KR, Mathalon DH, Roach BJ, Houde JF, Niziolek CA, Ford JM. Auditory cortex processes variation in our own speech. PLoS One. 2013;8(12):e82925. doi: 10.1371/journal.pone.0082925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotema CW, Daalman K, Blom JD, Diederen KM, Hoek HW, Sommer IE. Auditory verbal hallucinations in patients with borderline personality disorder are similar to those in schizophrenia. Psychol Med. 2012;42(9):1873–1878. doi: 10.1017/S0033291712000165. [DOI] [PubMed] [Google Scholar]
- Sowman PF, Kuusik A, Johnson BW. Self-initiation and temporal cueing of monaural tones reduce the auditory N1 and P2. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 2012;222(1–2):149–157. doi: 10.1007/s00221-012-3204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperry RW. Neural basis of the spontaneous optokinetic response produced by visual inversion. Journal of Comparative and Physiological Psychology. 1950;43:482–489. doi: 10.1037/h0055479. [DOI] [PubMed] [Google Scholar]
- Stirling JD, Hellewell JS, Quraishi N. Self-monitoring dysfunction and the schizophrenic symptoms of alien control. Psychological Medicine. 1998;28(3):675–683. doi: 10.1017/s0033291798006679. [DOI] [PubMed] [Google Scholar]
- Suga N, Shimozawa T. Site of neural attenuation of responses to self-vocalized sounds in echolocating bats. Science. 1974;183(130):1211–1213. doi: 10.1126/science.183.4130.1211. [DOI] [PubMed] [Google Scholar]
- Tian X, Poeppel D. The effect of imagination on stimulation: the functional specificity of efference copies in speech processing. J Cogn Neurosci. 2013;25(7):1020–1036. doi: 10.1162/jocn_a_00381. [DOI] [PubMed] [Google Scholar]
- Tian X, Poeppel D. Dynamics of self-monitoring and error detection in speech production: evidence from mental imagery and MEG. J Cogn Neurosci. 2015;27(2):352–364. doi: 10.1162/jocn_a_00692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela FJ, Thompson E, Rosch E. The Embodied Mind: Cognitive Science and Human Experience. Cambridge, MA, USA: MIT Press; 1991. [Google Scholar]
- Von Holst E, Mittelstaedt H. Das Reafferenzprinzip. Naturwissenschaften. 1950;37:464–476. [Google Scholar]
- Wang J, Mathalon DH, Roach BJ, Reilly J, Keedy SK, Sweeney JA, Ford JM. Action planning and predictive coding when speaking. Neuroimage. 2014;91:91–98. doi: 10.1016/j.neuroimage.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn MF. Movement and mental imagery: Outlines of a motor theory of the complexer mental processes. Boston, MA, USA: Houghton Mifflin Company; 1916. [Google Scholar]