Abstract
Importance
Despite strong biological plausibility, evidence from epidemiologic studies and clinical trials on the relations between intakes of lutein and zeaxanthin and age-related macular degeneration (AMD) has been inconsistent. The roles of other carotenoids are less thoroughly investigated.
Objective
To investigate the associations between intakes of carotenoids and AMD.
Design, Setting, and Participants
Prospective cohort study, with cohorts from the Nurses' Health Study and the Health Professionals Follow-up Study in the United States. A total of 63 443 women and 38 603 men were followed up, from 1984 until May 31, 2010, in the Nurses' Health Study and from 1986 until January 31, 2010, in the Health Professionals Follow-up Study. All participants were aged 50 years or older and were free of diagnosed AMD, diabetes mellitus, cardiovascular disease, and cancer at baseline.
Main outcomes and Measures
Predicted plasma carotenoid scores were computed directly from food intake, assessed by repeated food frequency questionnaires at baseline and follow-up, using validated regression models to account for bioavailability and reporting validity of different foods, and associations between predicted plasma carotenoid scores and AMD were determined.
Results
We confirmed 1361 incident intermediate and 1118 advanced AMD cases (primarily neovascular AMD) with a visual acuity of 20/30 or worse by medical record review. Comparing extreme quintiles of predicted plasma lutein/zeaxanthin score, we found a risk reduction for advanced AMD of about 40% in both women and men (pooled relative risk comparing extreme quintiles = 0.59; 95% CI, 0.48-0.73; P for trend < .001). Predicted plasma carotenoid scores for other carotenoids, including β-cryptoxanthin, α-carotene, and β-carotene, were associated with a 25% to 35% lower risk of advanced AMD when comparing extreme quintiles. The relative risk comparing extreme quintiles for the predicted plasma total carotenoid index was 0.65 (95% CI, 0.53-0.80; P for trend < .001). We did not identify any associations of carotenoids, either as predicted plasma score or calculated intake, with intermediate AMD.
Conclusions and Relevance
Higher intake of bioavailable lutein/zeaxanthin is associated with a long-term reduced risk of advanced AMD. Given that some other carotenoids are also associated with a lower risk, a public health strategy aimed at increasing dietary consumption of a wide variety of fruits and vegetables rich in carotenoids may reduce the incidence of advanced AMD.
Age-related macular degeneration (AMD) is the leading cause of blindness in the developed world,1,2 affecting 6.5% of persons aged 40 years and older in the United States, with 0.8% having advanced AMD.3 The prevalence of AMD is projected to increase by 50% in the next couple of decades4-6 as a consequence of exponential population aging and the lack of a cure or any effective means of primary prevention other than smoking cessation.7
Carotenoids are fat-soluble plant pigments found in red, yellow, orange, and dark green fruits and vegetables. Of more than 600 carotenoids, 6 are commonly found in the human diet and serum: lutein, zeaxanthin, α-carotene, β-carotene, lycopene, and β-cryptoxanthin.8 Lutein and zeaxanthin are selectively concentrated in the macula,9,10 where they are hypothesized to protect against AMD by absorbing blue light, quenching free radicals, and stabilizing cell membranes.11 However, despite compelling biological plausibility, epidemiologic studies have not yielded consistent findings12-15 and long-term, well-powered prospective cohort studies are lacking. The recently concluded Age-Related Eye Disease Study 2 (AREDS2) trial was unable to confidently demonstrate protective effects of lutein/zeaxanthin,16,17 and whether lutein/zeaxanthin may protect against early AMD also remains unknown. Some other carotenoids such as α-carotene, β-carotene, and lycopene found in the retinal pigment epithelium (RPE) and choroid18 have been inconsistently linked to a lower risk of AMD.12-15,19,20
We previously reported a suggestive inverse association of lutein/zeaxanthin with advanced AMD21 and some associations for other carotenoids.22 With an additional decade of follow-up and the occurrence of a large number of additional incident AMD cases, we aimed to provide more detailed insights into the roles of carotenoids in the development of AMD.
Methods
Study Population
The Nurses' Health Study (NHS) is an ongoing prospective cohort initiated in 1976 that includes 121 700 US female registered nurses aged 30 to 55 years at baseline. The Health Professionals Follow-up Study (HPFS) was initiated in 1986 and includes 51 529 US male health professionals aged 40 to 75 years at baseline. Both cohorts are predominantly white (NHS, >98%; HPFS, >91%) and have high rates of long-term follow-up (>95%). Both cohort studies have approval by the human subjects committees at the Brigham and Women's Hospital and the Harvard T. H. Chan School of Public Health. Cohort participants or family members provided written informed consent.
We restricted the study population to participants aged 50 years or older and censored participants at age 90 years to alleviate concerns of low reporting validity (NHS, n = 0; HPFS, n = 526). At baseline, we excluded participants who did not return the initial food frequency questionnaire (FFQ), left the entire fruit and vegetable sections blank or had more than 70 food items blank, reported implausible dietary intake (<500 or >3500 kcal/d for NHS and <800 or >4200 kcal/d for HPFS) (dietary exclusions: NHS, n = 46 142; HPFS, n = 1647), or had prevalent AMD, cancer (except nonmelanoma skin cancer), diabetes mellitus, or cardiovascular disease (disease exclusions: NHS, n = 8536; HPFS, n = 5709). To minimize detection bias, we also excluded participants who never reported an eye examination during follow-up (NHS, n = 3362; HPFS, n = 4763) and excluded from analysis the person-time during any 2-year interval in which a participant did not report an eye examination. In sensitivity analyses including intervals lacking an eye examination, results did not materially change. Participants contributed person-time to the analysis from return of the baseline questionnaire or reaching age 50 years to the confirmed diagnosis of AMD, death, loss to follow-up, or the end of follow-up (May 31, 2010, for the NHS and January 31, 2010, for the HPFS), whichever occurred first. By 2010, a total of 63 443 women and 38 603 men contributed to the analysis.
AMD Ascertainment
Our case definition has been previously validated.23 When a participant reported a diagnosis of AMD on a biennial questionnaire, we requested written informed consent and then contacted the participant's eye care professional to confirm the diagnosis by review of medical records. We excluded cases with only small hard drusen (<63 μm in diameter). We defined intermediate AMD as having at least 1 of the following signs: intermediate drusen (≥63 and <125 μm), pigment abnormalities, large drusen (≥125 μm), or any noncentral geographic atrophy (GA). We defined a subgroup of intermediate AMD as having at least 1 large druse or any noncentral GA, the most likely to progress to advanced AMD.24 We defined neovascular AMD as having any of the following: RPE detachment, sub-retinal neovascular membrane, disciform scar, or history of treatment with laser, photodynamic, or anti–vascular endothelial growth factor therapy for AMD. Central GA was defined as having a central GA lesion involving the center of the macula. Advanced AMD included neovascular AMD and central GA. Additionally, our case definition included a visual acuity of 20/30 or worse due primarily to AMD. The person was used as the unit of analysis, and the worse eye was used for classification.
Dietary Assessment of Carotenoids
We began follow-up in 1984 for the NHS and 1986 for the HPFS, when the first comprehensive FFQ with an expanded section on fruit and vegetable intake was administered, and updated dietary intake every 4 years. On the FFQs, commonly used units or portion sizes (eg, 1 orange or half cup of broccoli) are specified for each item. The FFQs contained at least 15 questions for fruit and juice intake and 30 questions for vegetable intake. Participants were asked to report how often, on average during the past year, they had consumed each food item (responses ranging from ≤1 time/mo to ≥6 times/d). Use and dosage of beta carotene and multivitamin supplements were assessed by biennial questionnaires with additional information on brands for multivitamins, whereas lycopene supplements were only inquired about from 2002 onward. We calculated nutrient intakes by multiplying the consumption frequency of each food by the nutrient content of the specified food portion and summing across all foods. Nutrient values were energy adjusted using the residual method.25 The FFQ has been validated in both cohorts and had good reproducibility and validity in measuring a wide range of foods and nutrients.26-30
Because of variation in assessment validity and bioavailability across carotenoid-containing foods, calculated intakes of carotenoids from FFQs may not adequately represent the more biologically relevant internal dosage. We thus used a previously validated empirical prediction model among 4180 nonsmoking women in the NHS that related carotenoid-containing foods directly to the measured plasma carotenoid level using linear regression.31 The regression coefficient of each food in the model reflected a weighted contribution to the bio-available level. We then derived predicted plasma carotenoid scores for all participants by multiplying the consumption frequency of each food by its regression coefficient and summing across all foods. We created a total carotenoid index by first categorizing each predicted plasma score into quintiles and then summing the quintile scores across all carotenoids, yielding a final score ranging from 5 to 25. The empirical prediction model demonstrated improved assessment of carotenoid intakes compared with the conventional food composition–based method.31
Statistical Analysis
We calculated the cumulative average for predicted plasma carotenoid scores by averaging scores from all available FFQs up to the start of each 2-year risk interval. We used time-varying multivariate Cox proportional hazards model to estimate the hazard ratios and 95% confidence intervals controlling for known and suspected risk factors. We assessed the linear trend across categories by modeling the median level of each category as a continuous variable. We examined the possible nonlinear relations between carotenoids and AMD nonparametrically by the likelihood ratio test, comparing a model with only the linear term vs a model with the linear and restricted cubic splines with 4 knots.32
To assess whether the associations between carotenoids and AMD would vary by prespecified risk factors including age, body mass index (calculated as weight in kilograms divided by height in meters squared), smoking status, and postmenopausal hormone use, we created interaction terms between carotenoids and these variables and tested their significance using likelihood ratio tests. In exploratory analysis, we investigated the independent association of each carotenoid adjusting for all other carotenoids as a composite variable (sum of the quintile score of each carotenoid).
We performed the analyses separately in each cohort and pooled the results with an inverse variance-weighted meta-analysis using the fixed-effects model. We used an α level of .05 without adjustment for multiple comparisons. We used SAS version 9.3 statistical software (SAS Institute, Inc) to perform the analyses.
Results
During 26 years of follow-up in the NHS and 24 years in the HPFS, we confirmed 1361 incident intermediate and 1118 advanced AMD cases (>96% neovascular AMD). The median age at AMD onset was 73 years in women and 76 years in men.
In 1996 (the middle of follow-up), participants at the highest cumulative average predicted plasma score of lutein/zeaxanthin were likely to be more physically active, smoke less, consume more fruits and vegetables, and score higher in an alternative healthy eating index. They also had higher calculated intakes of lutein/zeaxanthin and other carotenoids (Table 1).
Table 1. Age-Standardized Characteristics of Participants in the Nurses' Health Study and the Health Professionals Follow-up Study According to the Cumulative Average Predicted Plasma Score of Lutein/Zeaxanthin in 1996.
| Characteristic | Lutein/Zeaxanthin Quintile | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Nurses' Health Study | |||||
| Participants, No. | 10 548 | 10 548 | 10 548 | 10 548 | 10 548 |
| Age, mean, y | 62 | 62 | 62 | 62 | 63 |
| BMI, mean | 27 | 27 | 27 | 27 | 26 |
| White, % | 99 | 98 | 98 | 98 | 97 |
| Current smoker, % | 17 | 13 | 10 | 9 | 8 |
| Pack-years of smoking | 29 | 25 | 23 | 21 | 20 |
| Physical activity, median, MET-h/wk | 8 | 9 | 11 | 13 | 16 |
| Hypertension, % | 39 | 41 | 41 | 41 | 38 |
| Postmenopausal, % | 90 | 90 | 90 | 90 | 90 |
| Current menopausal hormone use, %a | 37 | 41 | 42 | 43 | 44 |
| Current aspirin use, % | 46 | 48 | 50 | 50 | 50 |
| Dietary intake, mean | |||||
| Total energy intake, kcal/d | 1703 | 1670 | 1698 | 1756 | 1877 |
| Alcohol, g/d | 5 | 5 | 6 | 6 | 7 |
| Fruits and vegetables, servings/d | 4 | 5 | 5 | 6 | 8 |
| aHEI excluding fruits and vegetables | 39 | 41 | 42 | 43 | 44 |
| Lutein/zeaxanthin, μg/d | 1657 | 2259 | 2732 | 3338 | 4779 |
| α-Carotene, μg/d | 528 | 662 | 767 | 873 | 1112 |
| β-Carotene, μg/d | 2890 | 4690 | 4324 | 5101 | 6862 |
| β-Cryptoxanthin, μg/d | 110 | 156 | 184 | 208 | 241 |
| Lycopene, μg/d | 5935 | 6314 | 6571 | 6748 | 7093 |
| ALA, g/d | 0.93 | 0.95 | 0.96 | 0.98 | 1.01 |
| DHA, g/d | 0.11 | 0.13 | 0.14 | 0.16 | 0.19 |
| Predicted lutein/zeaxanthin plasma score, mean, μg/L | 152 | 161 | 169 | 179 | 203 |
| Health Professionals Follow-up Study | |||||
| Participants, No. | 4999 | 4999 | 5000 | 4999 | 4999 |
| Age, mean, y | 62 | 63 | 63 | 64 | 64 |
| BMI, mean | 26 | 26 | 26 | 26 | 26 |
| White, % | 97 | 96 | 95 | 96 | 95 |
| Current smoker, % | 8 | 5 | 4 | 4 | 4 |
| Pack-years of smoking | 14 | 13 | 12 | 12 | 11 |
| Physical activity, median, MET-h/wk | 20 | 23 | 25 | 28 | 32 |
| Hypertension, % | 34 | 34 | 34 | 33 | 34 |
| Current aspirin use, % | 68 | 70 | 70 | 68 | 67 |
| Dietary intake, mean | |||||
| Total energy intake, kcal/d | 2068 | 1938 | 1956 | 1998 | 2115 |
| Alcohol, g/d | 12 | 11 | 11 | 11 | 11 |
| Fruits and vegetables, servings/d | 4 | 5 | 6 | 6 | 8 |
| aHEI excluding fruits and vegetables | 41 | 43 | 44 | 45 | 47 |
| Lutein/zeaxanthin, μg/d | 1848 | 2563 | 3091 | 3832 | 5468 |
| α-Carotene, μg/d | 606 | 768 | 879 | 1,024 | 1374 |
| β-Carotene, μg/d | 3413 | 4341 | 5052 | 6013 | 8152 |
| β-Cryptoxanthin, μg/d | 121 | 172 | 211 | 244 | 307 |
| Lycopene, μg/d | 6545 | 7223 | 7532 | 7888 | 8520 |
| ALA, g/d | 1.05 | 1.07 | 1.08 | 1.10 | 1.13 |
| DHA, g/d | 0.16 | 0.19 | 0.20 | 0.23 | 0.27 |
| Predicted lutein/zeaxanthin plasma score, mean, μg/L | 149 | 159 | 167 | 177 | 202 |
Abbreviations: aHEI, alternative healthy eating index; ALA, α-linolenic acid; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); DHA, docosahexaenoic acid; MET-h, hours of metabolic equivalent tasks.
Current menopausal hormone use among postmenopausal women.
Predicted plasma carotenoid scores were strongly correlated with their respective calculated intakes (Spearman correlation, r = 0.67-0.90) and with each other (eg, r = 0.64 between lutein/zeaxanthin and food-sourced β-carotene; r = 0.67 between α-carotene and food-sourced β-carotene). Lycopene had the weakest correlations with all other carotenoids (r ≤ 0.18).
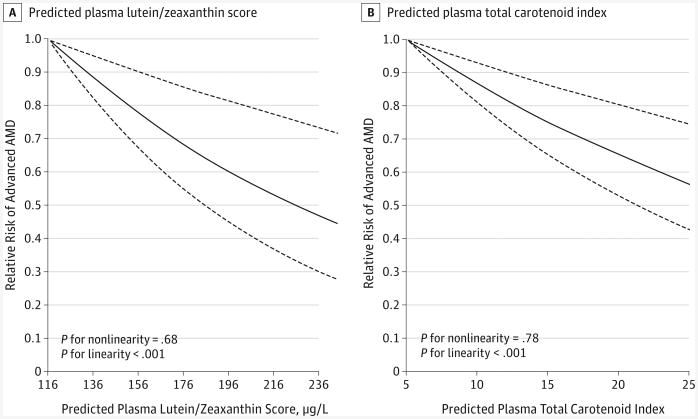
Comparing extreme quintiles, we identified an inverse association with advanced AMD for predicted plasma carotenoid scores of lutein/zeaxanthin (relative risk [RR] = 0.59; 95% CI, 0.48-0.73; P for trend < .001), β-cryptoxanthin (RR = 0.73; 95% CI, 0.60-0.89; P for trend = .002), α-carotene (RR = 0.69; 95% CI, 0.56-0.84; P for trend < .001), food-sourced β-carotene (RR = 0.64; 95% CI, 0.52-0.79; P for trend < .001), total carotene from food (RR = 0.64; 95% CI, 0.51-0.79; P for trend < .001), and total carotenoid index (RR = 0.65; 95% CI, 0.53-0.80; P for trend < .001) (Table 2). Predicted plasma lutein/zeaxanthin score and total carotenoid index had a linear relationship with advanced AMD within the range of dietary intake (Figure 1). Carotenoids other than lycopene had a similar linear relation (all P for linearity < .05; all P for nonlinearity > .10; graphs not shown). For the outcome of intermediate AMD, we did not observe any association for any predicted plasma carotenoid scores (Table 2). The results did not materially change when restricted to a subgroup of intermediate AMD with large drusen or noncentral GA (n = 283 in the NHS and n = 80 in the HPFS; data not shown).
Table 2. Pooled Relative Risks of AMD According to Quintiles of Predicted Plasma Carotenoid Scores and Calculated Intakes in the Nurses' Health Study and the Health Professionals Follow-up Study.
| Carotenoids | Multivariate RR (95% CI)as | |||
|---|---|---|---|---|
| Advanced AMD | Intermediate AMD | |||
| Predicted Plasma Score | Calculated Intakeb | Predicted Plasma Score | Calculated Intakeb | |
| Lutein/zeaxanthin | ||||
| Quintile | ||||
| 1 | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| 2 | 0.82 (0.68-0.99) | 0.90 (0.75-1.08) | 0.97 (0.82-1.15) | 0.92 (0.78-1.10) |
| 3 | 0.94 (0.78-1.12) | 0.86 (0.71-1.03) | 0.95 (0.80-1.13) | 0.96 (0.81-1.14) |
| 4 | 0.83 (0.69-1.00) | 0.81 (0.67-0.99) | 0.93 (0.78-1.11) | 0.96 (0.80-1.14) |
| 5 | 0.59 (0.48-0.73) | 0.79 (0.64-0.97) | 0.93 (0.78-1.12) | 0.97 (0.81-1.16) |
| P value for trend | <.001 | .04 | .42 | .99 |
| P value for heterogeneity | .99 | .04 | .92 | .17 |
| β-Cryptoxanthin | ||||
| Quintile | ||||
| 1 | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| 2 | 0.94 (0.78-1.14) | 0.83 (0.69-1.01) | 0.87 (0.73-1.04) | 0.97 (0.81-1.15) |
| 3 | 0.87 (0.72-1.05) | 0.86 (0.72-1.04) | 1.00 (0.84-1.18) | 0.98 (0.82-1.16) |
| 4 | 0.90 (0.74-1.08) | 0.84 (0.70-1.02) | 0.91 (0.77-1.09) | 0.90 (0.75-1.08) |
| 5 | 0.73 (0.60-0.89) | 0.71 (0.58-0.86) | 0.85 (0.72-1.02) | 0.90 (0.75-1.07) |
| P value for trend | .002 | .002 | .12 | .12 |
| P value for heterogeneity | .97 | .73 | .20 | .13 |
| Lycopene | ||||
| Quintile | ||||
| 1 | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| 2 | 0.98 (0.83-1.16) | 1.02 (0.86-1.21) | 0.94 (0.81-1.10) | 0.98 (0.84-1.15) |
| 3 | 0.93 (0.78-1.11) | 0.97 (0.81-1.16) | 1.01 (0.86-1.19) | 0.98 (0.83-1.15) |
| 4 | 0.85 (0.71-1.03) | 0.77 (0.63-0.94) | 0.95 (0.80-1.12) | 1.04 (0.88-1.23) |
| 5 | 0.93 (0.76-1.13) | 1.00 (0.83-1.21) | 1.04 (0.87-1.23) | 1.05 (0.88-1.24) |
| P value for trend | .17 | .38 | .64 | .44 |
| P value for heterogeneity | .08 | .59 | .86 | .96 |
| α-Carotene | ||||
| Quintile | ||||
| 1 | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| 2 | 0.99 (0.82-1.19) | 0.81 (0.67-0.98) | 0.88 (0.73-1.04) | 0.94 (0.78-1.13) |
| 3 | 0.92 (0.76-1.11) | 0.82 (0.68-0.99) | 0.91 (0.77-1.08) | 0.95 (0.79-1.13) |
| 4 | 0.88 (0.73-1.07) | 0.82 (0.68-0.99) | 0.87 (0.73-1.04) | 1.13 (0.95-1.34) |
| 5 | 0.69 (0.56-0.84) | 0.68 (0.56-0.83) | 0.94 (0.79-1.12) | 0.98 (0.82-1.17) |
| P value for trend | <.001 | .002 | .86 | .69 |
| P value for heterogeneity | .36 | .08 | .68 | .24 |
| β-Carotene | ||||
| Quintile | ||||
| 1 | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| 2 | 0.97 (0.80-1.17) | 1.04 (0.86-1.26) | 1.02 (0.85-1.22) | 0.99 (0.82-1.18) |
| 3 | 0.90 (0.74-1.09) | 0.96 (0.79-1.16) | 1.08 (0.90-1.29) | 1.09 (0.91-1.30) |
| 4 | 0.80 (0.65-0.97) | 0.86 (0.70-1.05) | 0.99 (0.82-1.19) | 1.02 (0.85-1.23) |
| 5 | 0.82 (0.67-1.01) | 0.86 (0.69-1.06) | 1.03 (0.85-1.24) | 0.99 (0.82-1.20) |
| P value for trend | .03 | .05 | .92 | .88 |
| P value for heterogeneity | .28 | .99 | .87 | .65 |
| β-Carotene from food | ||||
| Quintile | ||||
| 1 | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| 2 | 0.84 (0.69-1.02) | 0.97 (0.80-1.18) | 0.93 (0.77-1.11) | 0.86 (0.71-1.03) |
| 3 | 0.80 (0.66-0.97) | 0.89 (0.73-1.09) | 0.97 (0.80-1.16) | 0.96 (0.80-1.15) |
| 4 | 0.79 (0.64-0.96) | 0.86 (0.70-1.05) | 1.00 (0.83-1.21) | 1.01 (0.84-1.21) |
| 5 | 0.64 (0.52-0.79) | 0.68 (0.55-0.85) | 1.02 (0.84-1.24) | 0.92 (0.76-1.12) |
| P value for trend | <.001 | <.001 | .47 | .88 |
| P value for heterogeneity | .67 | .60 | .94 | .90 |
| Total carotene from food | ||||
| Quintile | ||||
| 1 | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| 2 | 0.89 (0.74-1.08) | 0.97 (0.80-1.17) | 0.88 (0.73-1.06) | 0.87 (0.72-1.05) |
| 3 | 0.76 (0.62-0.93) | 0.89 (0.74-1.09) | 0.95 (0.79-1.14) | 0.98 (0.82-1.17) |
| 4 | 0.77 (0.63-0.94) | 0.78 (0.64-0.95) | 0.94 (0.78-1.14) | 0.96 (0.80-1.16) |
| 5 | 0.64 (0.51-0.79) | 0.72 (0.58-0.89) | 0.99 (0.82-1.19) | 0.94 (0.78-1.13) |
| P value for trend | <.001 | <.001 | .64 | .89 |
| P value for heterogeneity | .59 | .65 | .96 | .40 |
| Total carotenoid index c | ||||
| Quintile | ||||
| 1 | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| 2 | 0.95 (0.80-1.14) | 0.83 (0.69-1.00) | 0.77 (0.64-0.91) | 0.95 (0.80-1.13) |
| 3 | 0.84 (0.70-1.01) | 0.82 (0.68-0.99) | 0.93 (0.79-1.10) | 0.97 (0.82-1.15) |
| 4 | 0.77 (0.64-0.94) | 0.79 (0.65-0.95) | 0.87 (0.73-1.03) | 1.06 (0.90-1.26) |
| 5 | 0.65 (0.53-0.80) | 0.72 (0.59-0.87) | 0.92 (0.77-1.10) | 0.99 (0.83-1.18) |
| P value for trend | <.001 | .001 | .80 | .73 |
| P value for heterogeneity | .67 | .51 | .98 | .56 |
Abbreviations: AMD, age-related macular degeneration; RR, relative risk.
Multivariate models were adjusted for age (continuous), body mass index (<18.5, 18.5-23, 23-25, 25-30, 30-35, ≥35; calculated as weight in kilograms divided by height in meters squared), pack-years of smoking (never, 1-9, 10-24, 25-44, 45-64, ≥65 pack-years), physical activity (<3, 3-8.9, 9-17.9, 18-26.9, ≥27 hours of metabolic equivalent tasks per week), current aspirin use (≥1 tablet/wk or none), history of hypertension, diabetes mellitus, and cardiovascular disease, dietary variables including alternative healthy eating index (excluding fruits and vegetables), alcohol intake, docosahexaenoic acid, and α-linolenic acid (all in quintiles). In the Nurses' Health Study, models were additionally adjusted for postmenopausal status and menopausal hormone use (never, current, and past); in the Health Professionals Follow-up Study, additional adjustment was made for race (white vs nonwhite).
Multivariate models were additionally adjusted for total calorie intake (in quintiles).
Total carotenoid index was created by summing the quintile score of each carotenoid.
Figure 1. Dose-Response Relationship Between Predicted Plasma Carotenoid Scores and the Relative Risk of Advanced Age-Related Macular Degeneration (AMD).
Dose-response relationships were calculated for predicted plasma lutein/zeaxanthin score (A) and predicted plasma total carotenoid index (B) with the risk of advanced AMD. Multivariate models were adjusted for age (continuous), body mass index (≥30; calculated as weight in kilograms divided by height in meters squared), current aspirin use (≥1 tablet/wk), history of hypertension, pack-years of smoking, physical activity, and modified alternative healthy eating index (all in categories). Solid lines represent relative risks; dashed lines, 95% CIs.
Because AREDS2 raised the concern for the competitive absorption between lutein/zeaxanthin and β-carotene, in a sensitivity analysis we excluded β-carotene supplement users (4.5% of the person-years in the NHS and 10% in the HPFS) from the analysis of predicted plasma lutein/zeaxanthin score. However, neither the RR for advanced AMD (RR = 0.58; 95% CI, 0.47-0.72; P for trend < .001) nor the RR for intermediate AMD (RR = 0.90; 95% CI, 0.75-1.09; P for trend = .26) was essentially altered.
In secondary analyses using calculated intakes of carotenoids, lutein/zeaxanthin (P for trend = .003), β-cryptoxanthin (P for trend = .009), α-carotene (P for trend < .001), and food-sourced β-carotene (P for trend < .001) were also inversely related to advanced AMD in the NHS (eTable 1 in the Supplement). However, none were associated with advanced AMD in the HPFS (eTable 2 in the Supplement).
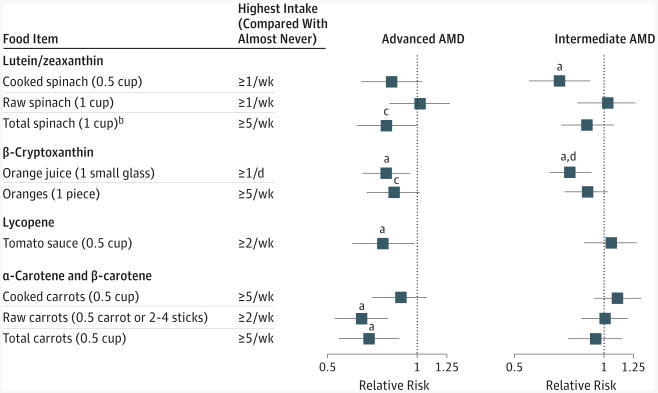
The primary food sources for measured plasma carotenoid levels in the NHS31 were cooked and raw spinach for lutein/zeaxanthin, oranges and orange juice for β-cryptoxanthin, oked and raw carrots for both α- and β-carotene, and tomato sauce for lycopene (Figure 2), consistent with the National Health and Nutrition Examination Survey.33 These foods were generally inversely related to advanced AMD, although with variation for specific forms of these foods (Figure 2). The inverse association between to matosauce and advanced AMD was primarily attributed to that in the HPFS (comparing ≥2 servings/wk vs almost never, RR = 0.60; 95% CI, 0.39-0.93; P for trend = .01). Cooked spinach had an inverse association with intermediate AMD (comparing ≥1 serving/wk vs almost never, RR = 0.71; 95% CI, 0.56-0.90; P for trend = .02), as did orange juice (comparing ≥1 small glass/d vs almost never, RR = 0.77; 95% CI, 0.66-0.91; P for trend = .003) (Figure 2). Although not the primary source of α-carotene, consumption of bananas, which predicted plasma α-carotene level in our sample, was inversely relate to intermediate AMD (comparing ≥5 pieces/wk vs almost never, RR = 0.83; 95% CI, 0.70-1.00; P for trend = .003).
Figure 2. Relative Risks of Age-Related Macular Degeneration (AMD) According to Primary Carotenoid-Containing Foods.
Bars surrounding point estimates indicate 95% CIs. Multivariate models were adjusted for the same variables as in Table 2.
aP for linear trend < .05.
bCooked spinach (0.5 cup) is approximately equivalent to 2.5 × raw spinach (1 cup); total spinach (1 cup) = raw spinach (1 cup) + 2.5 × cooked spinach (0.5 cup).
cP for linear trend < .10.
dP for heterogeneity between the Nurses' Health Study and the Health Professionals Follow-up Study < .05.
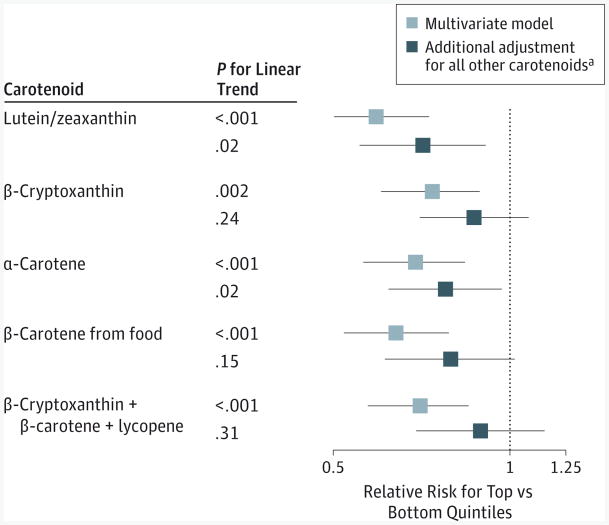
In an exploratory analysis adjusted for all other carotenoids as a composite score, only lutein/zeaxanthin and α-carotene persisted with an inverse association with advanced AMD (Figure 3). Collectively, β-cryptoxanthin, β-carotene, and lycopene did not have an inverse association after accounting for lutein/zeaxanthin and α-carotene, nor did β-cryptoxanthin and β-carotene combined after accounting for all other carotenoids. In the sensitivity analysis in which we entered each individual carotenoid in the same model, the inverse association for lutein/zeaxanthin and α-carotene persisted (lutein/zeaxanthin: RR = 0.66; 95% CI, 0.50-0.87; α-carotene: RR = 0.75; 95% CI, 0.59-0.96).
Figure 3. Independent Associations of Predicted Plasma Carotenoid Scores With Advanced Age-Related Degeneration.
Bars surrounding point estimates indicate 95% CIs. Multivariate models were adjusted for the same variables as in Table 2. P for heterogeneity between the Nurses' Health Study and the Health Professionals Follow-up Study > .10 for all the relative risks.
aAll other carotenoids were a composite score derived by totaling the quintile score of each carotenoid.
Plasma carotenoid scores except lycopene seemed to be associated with a lower risk of total AMD among postmenopausal women currently using exogenous hormones compared with those not currently using them (eFigure 1 in the Supplement). We also found a suggestive stronger inverse association for all predicted plasma carotenoid scores except lycopene with advanced AMD in those aged 75 years and older, and this was most pronounced for lutein/zeaxanthin (P for interaction = .04) (eFigure 2A in the Supplement). We found similar RRs in never smokers compared with ever smokers (all P for interaction > .25) (eFigure 2B in the Supplement).
Discussion
Our findings from 2 large, long-running prospective cohorts with repeated dietary assessments suggest that a higher intake of bioavailable lutein/zeaxanthin is associated with a 40% lower risk of advanced AMD. Higher intakes of other bioavailable carotenoids also contribute to a reduced risk of advanced AMD. In contrast, intakes of carotenoids were not associated with intermediate AMD, suggesting an effect on AMD progression rather than initiation.
Although the inverse association between lutein/zeaxanthin and advanced AMD was consistent with a number of previous studies,12,13,19-21,34 the observational nature of our study precludes the level of causal inference that could be derived from a randomized trial. Unfortunately, the primary analyses of the AREDS2 trial failed to prove a protective effect of lutein/zeaxanthin.16 However, when restricted to participants at the bottom 20% of dietary intake of lutein/zeaxanthin, there was a 26% risk reduction.17 The subgroup result is consistent with the hypothesis that supplements may be more effective when the background dietary intake is below a biologically sufficient threshold. Given the unlikely occurrence of another well-designed large-scale randomized trial, long-running large prospective cohort studies like ours provide the best available evidence to further strengthen the evidence base for a protective role of lutein/zeaxanthin.
Lutein and zeaxanthin form macular pigments that may protect against AMD by reducing oxidative stress, absorbing blue light, and stabilizing cell membranes.11 Cross-sectional studies (reviewed by Beatty et al35) and experimental studies36-38 have shown a significant correlation between serum lutein and zeaxanthin and macular pigment optical density. Increasing evidence also suggests that genetic variants related to lutein and zeaxanthin metabolism are associated with macular pigment optical density or AMD.39-42 Therefore, multiple independent lines of evidence point to a protective role of lutein and zeaxanthin in the development of advanced AMD.
Several mechanisms could explain the protective roles of other carotenoids including α-carotene, β-carotene, and β-cryptoxanthin, which are nonmacular pigment carotenoids. All carotenoids are potent antioxidants, which could reduce systemic oxidative stress that indirectly influences the macula. The original AREDS formula containing β-carotene, antioxidant vitamins, and minerals but not lutein and zeaxanthin reduced the risk of AMD progression by a quarter.43 Carotenoids including α-carotene, β-carotene, and lycopene have been found in human RPE and choroid18 and could protect this tissue against light-induced oxidative damage and locally produced free radicals. The integrity of the RPE and choroid could further affect the retina's uptake of lutein and zeaxanthin from the circulating blood. We also speculate that other carotenoids may directly protect lutein and zeaxanthin from oxidative damage in both blood and the RPE/choroid. Among a subsample of women in the NHS, we found that measured plasma lutein/zeaxanthin could be significantly predicted by every other plasma carotenoid apart from its own food sources (all P < .001), in accordance with a separate study.44
We did not find an association between carotenoids and intermediate AMD. While one previous case-control study13 and one cross-sectional study45 reported an inverse association, only one34 of 3 prospective cohort studies15,34,46 reported a significant inverse association between intake of lutein/zeaxanthin and intermediate AMD. One nested case-control study based on 41 cases found an inverse association for serum lutein/zeaxanthin but not for other carotenoids,47 whereas 2 other case-control studies14,20 found an inverse association only for serum lycopene.
Our study has some limitations. Although our results did not appreciably change after adjusting for many known and suspected risk factors including an alternative healthy eating index, an indicator of a healthy dietary pattern,48 residual confounding from unaccounted or imprecise measurement cannot be excluded. However, similar associations among ever and never smokers assured us that results were unlikely to be confounded by smoking, the strongest modifiable risk factor for advanced AMD.49 Because our nutrient and blood database assessed lutein and zeaxanthin together, we were unable to estimate the individual effect of each nutrient to inform the optimal ratio for supplementation. Although the relationship between lutein/zeaxanthin and advanced AMD was linear within the range consumed in our cohorts (0.8-10.7 mg/d), we could not evaluate the effect of the higher dosage (10 mg of lutein plus 2 mg of zeaxanthin) used in the AREDS2 formula. Some patients with intermediate AMD in the later follow-up may have been using the AREDS formula, which was not ascertained by our FFQs; this may have resulted in underestimation of the true associations between carotenoids and advanced AMD because dietary effects of carotenoids could be masked under intake of pharmacological doses of antioxidant vitamins and minerals.
Strengths of this study included a prospective cohort design with high follow-up that minimized recall and selection biases. Another strength lies in our creation of predicted plasma carotenoid scores to better estimate the true variation of carotenoid exposures accounting for variations in bioavailability across different foods,50,51 preparation methods,52 accuracy of responses to various FFQ items, and food composition databases. Analyses using the predicted plasma scores strengthened the association between lutein/zeaxanthin and advanced AMD and modestly improved associations with other carotenoids. Differences in the impact of substituting estimated bioavailable nutrient levels among specific carotenoids might be attributable to variation in the accuracy of FFQ data for specific food items. The predicted plasma score for lutein/zeaxanthin (median, 16.9 μg/dL; eTable 1 and eTable 2 in the Supplement) in our cohorts was comparable with the baseline serum level in AREDS2 participants (mean, 17.9 μg/dL) and the general population participants older than 60 years sampled from the 2005-2006 National Health and Nutrition Examination Survey (mean, 15.0 μg/dL).16
Conclusions
Higher intakes of bioavailable carotenoids, particularly lutein/zeaxanthin and α-carotene, are associated with reduced risk of advanced AMD. This study lends further support to the causal role of lutein/zeaxanthin in protecting against the development of advanced AMD. Because other carotenoids may also have a protective role, a public health strategy of increasing the consumption of a wide variety of fruits and vegetables rich in carotenoids could be most beneficial and is compatible with current dietary guidelines.
Supplementary Material
eFigure 1. Predicted plasma carotenoid scores and RRs of total AMD according to current menopausal hormone use status among postmenopausal women
There were 1,221 cases in non-current users group and 413 cases in current users group
All the RRs were comparing the fifth to the bottom quintile
Multivariate models were adjusted for: age (continuous), BMI (≥30 kg/m2), pack-years of smoking (never, 1-9, 10-24, 25-44, 45-64, ≥65y), physical activity (<3, 3-8.9, 9-17.9, 18-26.9, ≥27 MET-h/wk), current aspirin use (≥1 tablets/wk or none), aHEI (excluding fruits and vegetables)
eFigure 2. Predicted plasma carotenoid scores and pooled RRs of advanced AMD according to A) age and B) smoking status
A) Multivariate models were adjusted for the same variables as in supplement Figure 1 and additionally adjusted for race in HPFS; 651 cases in age<75 group (477 from NHS and 174 cases from HPFS); 467 cases in age≥75 group (296 from NHS and 171 cases from HPFS).
B) Multivariate models were adjusted for the same variables as in A) except pack-year of smoking; 425 cases in never smokers group (289 from NHS and 136 cases from HPFS); 693 cases in ever smokers group (484 from NHS and 209 cases from HPFS)
eTable 1. RRs of AMD according to quintiles of predicted plasma carotenoid scores and calculated intakes in NHS
eTable 2. RRs of AMD according to quintiles of predicted plasma carotenoid scores and calculated intakes in HPFS
At a Glance.
Antioxidant carotenoids are hypothesized to lower the risk of age-related macular degeneration (AMD); however, results from prior epidemiologic studies have been inconsistent.
Comparing extreme quintiles, intake of bioavailable lutein and zeaxanthin was associated with a pooled relative risk of advanced AMD of 0.59 (95% CI, 0.48-0.73).
An association of any of these carotenoids with development of intermediate AMD was not identified.
Although not yet supported by randomized clinical trials, this study suggests that carotenoids may slow worsening of AMD once it occurs.
Acknowledgments
Funding/Support: This study was supported by grants EY017362, EY021900, UM1 CA186107, and UM1 CA167552 from the National Institutes of Health.
Role of the Funder/Sponsor: The National Institutes of Health had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Ms Wu and Dr Schaumberg had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Wu, Cho, Willett, Schaumberg.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Wu.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Wu, Willett, Schaumberg.
Obtained funding: Schaumberg.
Administrative, technical, or material support: Schaumberg.
Study supervision: Cho, Sastry, Schaumberg.
Author Audio Interview at jamaophthalmology.com
Supplemental content at jamaophthalmology.com
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported.
References
- 1.Pascolini D, Mariotti SP, Pokharel GP, et al. 2002 Global update of available data on visual impairment: a compilation of population-based prevalence studies. Ophthalmic Epidemiol. 2004;11(2):67–115. doi: 10.1076/opep.11.2.67.28158. [DOI] [PubMed] [Google Scholar]
- 2.Congdon N, O'Colmain B, Klaver CC, et al. Eye Diseases Prevalence Research Group. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122(4):477–485. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- 3.Klein R, Chou CF, Klein BE, Zhang X, Meuer SM, Saaddine JB. Prevalence of age-related macular degeneration in the US population. Arch Ophthalmol. 2011;129(1):75–80. doi: 10.1001/archophthalmol.2010.318. [DOI] [PubMed] [Google Scholar]
- 4.Friedman DS, O'Colmain BJ, Muñoz B, et al. Eye Diseases Prevalence Research Group. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122(4):564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 5.Rein DB, Wittenborn JS, Zhang X, Honeycutt AA, Lesesne SB, Saaddine J Vision Health Cost-Effectiveness Study Group. Forecasting age-related macular degeneration through the year 2050: the potential impact of new treatments. Arch Ophthalmol. 2009;127(4):533–540. doi: 10.1001/archophthalmol.2009.58. [DOI] [PubMed] [Google Scholar]
- 6.Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(2):e106–e116. doi: 10.1016/S2214-109X(13)70145-1. [DOI] [PubMed] [Google Scholar]
- 7.Lim LS, Mitchell P, Seddon JM, Holz FG, Wong TY. Age-related macular degeneration. Lancet. 2012;379(9827):1728–1738. doi: 10.1016/S0140-6736(12)60282-7. [DOI] [PubMed] [Google Scholar]
- 8.Mangels AR, Holden JM, Beecher GR, Forman MR, Lanza E. Carotenoid content of fruits and vegetables: an evaluation of analytic data. J Am Diet Assoc. 1993;93(3):284–296. doi: 10.1016/0002-8223(93)91553-3. [DOI] [PubMed] [Google Scholar]
- 9.Bone RA, Landrum JT, Tarsis SL. Preliminary identification of the human macular pigment. Vision Res. 1985;25(11):1531–1535. doi: 10.1016/0042-6989(85)90123-3. [DOI] [PubMed] [Google Scholar]
- 10.Handelman GJ, Dratz EA, Reay CC, van Kuijk JG. Carotenoids in the human macula and whole retina. Invest Ophthalmol Vis Sci. 1988;29(6):850–855. [PubMed] [Google Scholar]
- 11.Krinsky NI, Landrum JT, Bone RA. Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annu Rev Nutr. 2003;23:171–201. doi: 10.1146/annurev.nutr.23.011702.073307. [DOI] [PubMed] [Google Scholar]
- 12.Seddon JM, Ajani UA, Sperduto RD, et al. Eye Disease Case-Control Study Group. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. JAMA. 1994;272(18):1413–1420. [PubMed] [Google Scholar]
- 13.SanGiovanni JP, Chew EY, Clemons TE, et al. Age-Related Eye Disease Study Research Group. The relationship of dietary carotenoid and vitamin A, E, and C intake with age-related macular degeneration in a case-control study: AREDS report No. 22. Arch Ophthalmol. 2007;125(9):1225–1232. doi: 10.1001/archopht.125.9.1225. [DOI] [PubMed] [Google Scholar]
- 14.Mares-Perlman JA, Brady WE, Klein R, et al. Serum antioxidants and age-related macular degeneration in a population-based case-control study. Arch Ophthalmol. 1995;113(12):1518–1523. doi: 10.1001/archopht.1995.01100120048007. [DOI] [PubMed] [Google Scholar]
- 15.van Leeuwen R, Boekhoorn S, Vingerling JR, et al. Dietary intake of antioxidants and risk of age-related macular degeneration. JAMA. 2005;294(24):3101–3107. doi: 10.1001/jama.294.24.3101. [DOI] [PubMed] [Google Scholar]
- 16.Age-Related Eye Disease Study 2 Research Group. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013;309(19):2005–2015. doi: 10.1001/jama.2013.4997. [DOI] [PubMed] [Google Scholar]
- 17.Chew EY, Clemons TE, Sangiovanni JP, et al. Age-Related Eye Disease Study 2 (AREDS2) Research Group. Secondary analyses of the effects of lutein/zeaxanthin on age-related macular degeneration progression: AREDS2 report No. 3. JAMA Ophthalmol. 2014;132(2):142–149. doi: 10.1001/jamaophthalmol.2013.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bernstein PS, Khachik F, Carvalho LS, Muir GJ, Zhao DY, Katz NB. Identification and quantitation of carotenoids and their metabolites in the tissues of the human eye. Exp Eye Res. 2001;72(3):215–223. doi: 10.1006/exer.2000.0954. [DOI] [PubMed] [Google Scholar]
- 19.Eye Disease Case-Control Study Group. Antioxidant status and neovascular age-related macular degeneration. Arch Ophthalmol. 1993;111(1):104–109. doi: 10.1001/archopht.1993.01090010108035. [DOI] [PubMed] [Google Scholar]
- 20.Zhou H, Zhao X, Johnson EJ, et al. Serum carotenoids and risk of age-related macular degeneration in a Chinese population sample. Invest Ophthalmol Vis Sci. 2011;52(7):4338–4344. doi: 10.1167/iovs.10-6519. [DOI] [PubMed] [Google Scholar]
- 21.Cho E, Hankinson SE, Rosner B, Willett WC, Colditz GA. Prospective study of lutein/zeaxanthin intake and risk of age-related macular degeneration. Am J Clin Nutr. 2008;87(6):1837–1843. doi: 10.1093/ajcn/87.6.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cho E, Seddon JM, Rosner B, Willett WC, Hankinson SE. Prospective study of intake of fruits, vegetables, vitamins, and carotenoids and risk of age-related maculopathy. Arch Ophthalmol. 2004;122(6):883–892. doi: 10.1001/archopht.122.6.883. [DOI] [PubMed] [Google Scholar]
- 23.Seddon JM, Willett WC, Speizer FE, Hankinson SE. A prospective study of cigarette smoking and age-related macular degeneration in women. JAMA. 1996;276(14):1141–1146. [PubMed] [Google Scholar]
- 24.Chew EY, Clemons TE, Agrón E, et al. Age-Related Eye Disease Study Research Group. Ten-year follow-up of age-related macular degeneration in the Age-Related Eye Disease Study: AREDS report No. 36. JAMA Ophthalmol. 2014;132(3):272–277. doi: 10.1001/jamaophthalmol.2013.6636. [DOI] [PubMed] [Google Scholar]
- 25.Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. 1986;124(1):17–27. doi: 10.1093/oxfordjournals.aje.a114366. [DOI] [PubMed] [Google Scholar]
- 26.Salvini S, Hunter DJ, Sampson L, et al. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol. 1989;18(4):858–867. doi: 10.1093/ije/18.4.858. [DOI] [PubMed] [Google Scholar]
- 27.Feskanich D, Rimm EB, Giovannucci EL, et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc. 1993;93(7):790–796. doi: 10.1016/0002-8223(93)91754-e. [DOI] [PubMed] [Google Scholar]
- 28.Michaud DS, Giovannucci EL, Ascherio A, et al. Associations of plasma carotenoid concentrations and dietary intake of specific carotenoids in samples of two prospective cohort studies using a new carotenoid database. Cancer Epidemiol Biomarkers Prev. 1998;7(4):283–290. [PubMed] [Google Scholar]
- 29.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122(1):51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 30.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135(10):1114–1126. doi: 10.1093/oxfordjournals.aje.a116211. [DOI] [PubMed] [Google Scholar]
- 31.Hendrickson SJ, Willett WC, Rosner BA, Eliassen AH. Food predictors of plasma carotenoids. Nutrients. 2013;5(10):4051–4066. doi: 10.3390/nu5104051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8(5):551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 33.Murphy MM, Barraj LM, Herman D, Bi X, Cheatham R, Randolph RK. Phytonutrient intake by adults in the United States in relation to fruit and vegetable consumption. J Acad Nutr Diet. 2012;112(2):222–229. doi: 10.1016/j.jada.2011.08.044. [DOI] [PubMed] [Google Scholar]
- 34.Tan JS, Wang JJ, Flood V, Rochtchina E, Smith W, Mitchell P. Dietary antioxidants and the long-term incidence of age-related macular degeneration: the Blue Mountains Eye Study. Ophthalmology. 2008;115(2):334–341. doi: 10.1016/j.ophtha.2007.03.083. [DOI] [PubMed] [Google Scholar]
- 35.Beatty S, Nolan J, Kavanagh H, O'Donovan O. Macular pigment optical density and its relationship with serum and dietary levels of lutein and zeaxanthin. Arch Biochem Biophys. 2004;430(1):70–76. doi: 10.1016/j.abb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 36.Bone RA, Landrum JT, Guerra LH, Ruiz CA. Lutein and zeaxanthin dietary supplements raise macular pigment density and serum concentrations of these carotenoids in humans. J Nutr. 2003;133(4):992–998. doi: 10.1093/jn/133.4.992. [DOI] [PubMed] [Google Scholar]
- 37.Connolly EE, Beatty S, Thurnham DI, et al. Augmentation of macular pigment following supplementation with all three macular carotenoids: an exploratory study. Curr Eye Res. 2010;35(4):335–351. doi: 10.3109/02713680903521951. [DOI] [PubMed] [Google Scholar]
- 38.Johnson EJ, Hammond BR, Yeum KJ, et al. Relation among serum and tissue concentrations of lutein and zeaxanthin and macular pigment density. Am J Clin Nutr. 2000;71(6):1555–1562. doi: 10.1093/ajcn/71.6.1555. [DOI] [PubMed] [Google Scholar]
- 39.Meyers KJ, Mares JA, Igo RP, Jr, et al. Genetic evidence for role of carotenoids in age-related macular degeneration in the Carotenoids in Age-Related Eye Disease Study (CAREDS) Invest Ophthalmol Vis Sci. 2014;55(1):587–599. doi: 10.1167/iovs.13-13216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meyers KJ, Johnson EJ, Bernstein PS, et al. Genetic determinants of macular pigments in women of the Carotenoids in Age-Related Eye Disease Study. Invest Ophthalmol Vis Sci. 2013;54(3):2333–2345. doi: 10.1167/iovs.12-10867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loane E, McKay GJ, Nolan JM, Beatty S. Apolipoprotein E genotype is associated with macular pigment optical density. Invest Ophthalmol Vis Sci. 2010;51(5):2636–2643. doi: 10.1167/iovs.09-4397. [DOI] [PubMed] [Google Scholar]
- 42.Borel P, de Edelenyi FS, Vincent-Baudry S, et al. Genetic variants in BCMO1 and CD36 are associated with plasma lutein concentrations and macular pigment optical density in humans. Ann Med. 2011;43(1):47–59. doi: 10.3109/07853890.2010.531757. [DOI] [PubMed] [Google Scholar]
- 43.Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report No. 8. Arch Ophthalmol. 2001;119(10):1417–1436. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mares JA, LaRowe TL, Snodderly DM, et al. CAREDS Macular Pigment Study Group and Investigators. Predictors of optical density of lutein and zeaxanthin in retinas of older women in the Carotenoids in Age-Related Eye Disease Study, an ancillary study of the Women's Health Initiative. Am J Clin Nutr. 2006;84(5):1107–1122. doi: 10.1093/ajcn/84.5.1107. [DOI] [PubMed] [Google Scholar]
- 45.Moeller SM, Parekh N, Tinker L, et al. CAREDS Research Study Group. Associations between intermediate age-related macular degeneration and lutein and zeaxanthin in the Carotenoids in Age-Related Eye Disease Study (CAREDS): ancillary study of the Women's Health Initiative. Arch Ophthalmol. 2006;124(8):1151–1162. doi: 10.1001/archopht.124.8.1151. [DOI] [PubMed] [Google Scholar]
- 46.VandenLangenberg GM, Mares-Perlman JA, Klein R, Klein BE, Brady WE, Palta M. Associations between antioxidant and zinc intake and the 5-year incidence of early age-related maculopathy in the Beaver Dam Eye Study. Am J Epidemiol. 1998;148(2):204–214. doi: 10.1093/oxfordjournals.aje.a009625. [DOI] [PubMed] [Google Scholar]
- 47.Delcourt C, Carrière I, Delage M, Barberger-Gateau P, Schalch W POLA Study Group. Plasma lutein and zeaxanthin and other carotenoids as modifiable risk factors for age-related maculopathy and cataract: the POLA Study. Invest Ophthalmol Vis Sci. 2006;47(6):2329–2335. doi: 10.1167/iovs.05-1235. [DOI] [PubMed] [Google Scholar]
- 48.McCullough ML, Feskanich D, Stampfer MJ, et al. Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance. Am J Clin Nutr. 2002;76(6):1261–1271. doi: 10.1093/ajcn/76.6.1261. [DOI] [PubMed] [Google Scholar]
- 49.Tomany SC, Wang JJ, Van Leeuwen R, et al. Risk factors for incident age-related macular degeneration: pooled findings from 3 continents. Ophthalmology. 2004;111(7):1280–1287. doi: 10.1016/j.ophtha.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 50.van Het Hof KH, West CE, Weststrate JA, Hautvast JG. Dietary factors that affect the bioavailability of carotenoids. J Nutr. 2000;130(3):503–506. doi: 10.1093/jn/130.3.503. [DOI] [PubMed] [Google Scholar]
- 51.Zaripheh S, Erdman JW., Jr Factors that influence the bioavailability of xanthophylls. J Nutr. 2002;132(3):531S–534S. doi: 10.1093/jn/132.3.531S. [DOI] [PubMed] [Google Scholar]
- 52.Yeum KJ, Russell RM. Carotenoid bioavailability and bioconversion. Annu Rev Nutr. 2002;22:483–504. doi: 10.1146/annurev.nutr.22.010402.102834. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Predicted plasma carotenoid scores and RRs of total AMD according to current menopausal hormone use status among postmenopausal women
There were 1,221 cases in non-current users group and 413 cases in current users group
All the RRs were comparing the fifth to the bottom quintile
Multivariate models were adjusted for: age (continuous), BMI (≥30 kg/m2), pack-years of smoking (never, 1-9, 10-24, 25-44, 45-64, ≥65y), physical activity (<3, 3-8.9, 9-17.9, 18-26.9, ≥27 MET-h/wk), current aspirin use (≥1 tablets/wk or none), aHEI (excluding fruits and vegetables)
eFigure 2. Predicted plasma carotenoid scores and pooled RRs of advanced AMD according to A) age and B) smoking status
A) Multivariate models were adjusted for the same variables as in supplement Figure 1 and additionally adjusted for race in HPFS; 651 cases in age<75 group (477 from NHS and 174 cases from HPFS); 467 cases in age≥75 group (296 from NHS and 171 cases from HPFS).
B) Multivariate models were adjusted for the same variables as in A) except pack-year of smoking; 425 cases in never smokers group (289 from NHS and 136 cases from HPFS); 693 cases in ever smokers group (484 from NHS and 209 cases from HPFS)
eTable 1. RRs of AMD according to quintiles of predicted plasma carotenoid scores and calculated intakes in NHS
eTable 2. RRs of AMD according to quintiles of predicted plasma carotenoid scores and calculated intakes in HPFS