Abstract
Cyclooxygenase-2 (COX-2) plays an important role in mediating actions of the renin-angiotensin system (RAS). This review sheds light on the recent developments regarding the complex interactions between components of RAS and COX-2; and their implications on renal function and disease. COX-2 is believed to counter regulate the effects of RAS activation and therefore counter balance the vasoconstriction effect of Ang II. In kidney, under normal conditions, these systems are essential for maintaining a balance between vasodilation and vasoconstriction. However, recent studies suggested a pivotal role for this interplay in pathology. COX-2 increases the renin release and Ang II formation leading to increase in blood pressure. COX-2 is also associated with diabetic nephropathy, where its upregulation in the kidney contributes to glomerular injury and albuminuria. Selective inhibition of COX-2 retards the progression of renal injury. COX-2 also mediates the pathologic effects of the (Pro)renin receptor (PRR) in the kidney. In summary, this review discusses the interaction between the RAS and COX-2 in health and disease.
Keywords: Cyclooxygenase-2, angiotensin II, angitensin type I receptor, angiotensin type II receptor, pro(renin) receptor
2. INTRODUCTION
The Renin-angiotensinsystem (RAS) plays a critical role in regulating blood pressure and fluid balance. An overactive RAS (1) can augment the blood pressure and increase sodium retention, hypertension, and renal injury. Absence of RAS-counter regulatory mechanisms magnifies the RAS effects. In the kidney, prostaglandins serves as a regulator of renin release in the macula densa cells and mediate water and salt homeostasis (1). Selective inhibition of COX-2 prevented increases in renal renin mRNA levels in response to sodium restricted diet (2). In this review we will focus on the recent developments and the physiological and pathological roles of the interactions between PRR, AT1R, and AT2R, and COX system in disease states such as hypertension and diabetic nephropathy.
3. EXPRESSION AND REGULATION OF CYCLOOXYGENASE (COX) IN KIDNEY
COX enzyme synthesizes prostaglandins from arachidonic acid (3). COX-1 was first purified from bovine vesicular glands in 1976 (4). It is constitutively expressed in many tissues including kidney. In kidneys, COX-1 is present along the distal tubule, and mediates the basal synthesis of prostaglandin E2 (PGE2) (5). COX-2 is inducible and shares significant homology and activity with COX-1. Upregulation of COX-2 is associated with inflammation and cytokine production. It is expressed in the kidneys; In the cortex, COX-2 is expressed in macula densa cells which play an important role in mediating sodium balance and fluid volume, and regulation of renin release (6). COX-2 is also expressed in the thick limb of the ascending loop of Henle and medulla (7). Ang II via distinct actions of AT1 and AT2 receptors regulates COX-2 in the kidney (8). Its expression has been reported to be upregulated in hypertensive Ren-2 transgenic rats, which have high levels of Ang II (9). Chronic sodium restriction has been shown to upregulates cortical expression of COX-2 but decreases medullary expression. On the other hand, increased salt intake increases the inner medullalary COX-2 level, but decreases cortical COX-2 expression. This difference in medullary expression shows distinct function of COX-2 to contribute to salt and water reabsorption, while cortical COX-2 mediates renin release and tubuloglomerular feedback (10). In the inner medulla inhibition of COX-2 leads to development of saltsensitive hypertension (11), indicating that COX-2 plays a key protective anti-hypertensive role in inner medulla. Ang II infusion in rats increases COX-2 in the inner medulla and has opposite effect in cortex; however no changes were reported in COX-1 (12).
3.1. COX-2 regulates renin secretion
In the renal cortex COX-2 is expressed in macula densa cells where it regulates the release of renin, particularly during sodium restriction state (10). COX-2 links sodium sensing macula densa cells to synthesis of renin by juxtaglomerular cells. Mice deficient in COX-2 have reduced plasma renin levels, renin expression and response to acute stimuli that normally increase renin secretion (13). Reduction in sodium exposure increases renin secretion through stimulation of PGE2, leading to increased Ang II formation (14). The increase in the Ang II increases vascular tone, which is counterbalanced by COX-2 (15). Under the influence of intrarenal Ang II, COX-1 elicits vasoconstriction, while COX-2 has a vasodilatory effect (16). Previous studies indicated a protective role of COX-2. For instance, renal cortical expression of COX-2 leads to vasodilatation when Ang II is increased during low salt conditions (9). Two recently studied models of upregulated COX-2 indicated the effects of COX-2 inhibition on the intrarenal Ang II activity. The sodium restriction model has augmented Ang II level along with elevated COX-2 expression while the chronic ACE inhibition model has similar high levels of COX-2 activity but decreased Ang II levels (17). In rats fed with a low sodium diet, inhibition of COX-2 and ACE using captopril decreased glomerular filtration rate (GFR) and renal plasma flow, and points to the counter regulation between RAS and COX system (17). Similar results were obtained with COX-2 inhibition alone and demonstrated that cortical COX-2 expression promotes vasodilation in the renal vasculature (9).
4. EXPRESSION OF (PRO)RENIN RECEPTOR (PRR) IN KIDNEY
(Pro)renin receptor (PRR), a new component of renin-angiotensin system (RAS) was first reported and cloned in mesangial cells in 2002 (18). It is a 350 amino-acid protein with a single transmembrane domain which binds with renin and prorenin and is composed of four different domains namely, N-terminal signal peptide (SP), an extracellular domain (EC), a signal transmembrane domain (TM), and a short cytosolic domain (CD) (19). EC domain is responsible for mediating renin and prorenin effects through binding with renin and prorenin.
PRR is expressed in heart, kidney, brain, placenta, liver, lung, smooth muscle, pancreas, brown adipose tissue, and the gastrointestinal tract. In kidney, it is mainly expressed in podocytes, mesangial cell, vascular smooth muscle cells, and proximal and distal renal tubules (20). Activation of PRR mediates Ang II dependent and independent effects in kidney diseases (18). In cultured mesangial cells PRR activates the mitogen-activated protein kinase (MAPK)-extracellular signal-regulated kinase (ERK) signaling pathway leading to proliferation and activation of inflammatory and fibrotic molecules including transforming growth factor β (TGF-β), plasminogen activator inhibitor-1 (PAI-1), IL-1β, nuclear factor-κB (NF-κB) and COX-2, contributing to kidney dysfunction (21).
Recent studies reported that PRR act as an adaptor protein between the Wnt receptor and V-ATPase complex, resulting in activation of Wnt-β-catenin signaling pathway, which regulates embryonic development and disease (22–23).
4.1. Signaling of PRR
High glucose activates PRR-Wnt3a-β-catenin-snail signal pathway in mouse podocytes, leading to reorganization of F-actin, and increases cell albumin permeability, causing structural and functional abnormalities (20). Other reports also suggested that PRR effects autopaghy (24). Under high glucose condition, PRR activates ERK1/2 and PI3K-AKT signaling pathway contributing to decrease in autophagosome formation, induction of cell apoptosis, and organ damage (25). PRR also plays an important role in generation of Ang II through RAS activation, leading to kidney damage (26). This increase in Ang II has been attenuated using PRO 20, a new PRR inhibitory peptide. Knockdown of PRR in brain in salt-sensitive hypertension model decreases Ang II formation (27). In 2-kidney, 1-clip (2K1C) hypertensive rats, PRR expression is augmented in the clip kidney, leading to Ang II formation in distal nephron segments, distal tubular sodium reabsorption, and development of hypertension (28). Recently, we demonstrated that global null deletion of carcinoembryonic antigen-related cell adhesion molecule 1 (Ceacam1) stimulates PI3K-Akt, activates cAMP response element-binding protein 1, ATF-1, ATF-2, NF-κB p65 and RAS along with upregulation of PRR, resulting an increase in blood pressure (29).
5. ANGIOTENSIN II TYPE 1 RECEPTOR
In the kidney, renin converts angiotensinogen to angiotensin I (Ang I), that is further converted by the angiotensin converting enzyme (ACE) yielding angiotensin II (Ang II). The major biological actions of Ang II are mediated through the stimulation of AT1R. It is 359 amino acid residues with a calculated molecular weight of 41 kDa. The AT1R mediates the effects of Ang II leading to vasoconstriction, angiogenesis, sodium and water reabsorption, cell growth, proliferation, inflammatory responses, and oxidative stress (30). It is expressed in almost all of the body tissues including vascular smooth muscle cells (VSMCs), endothelial cells, kidney, liver, adrenal gland, ovary, brain, testis, lung, heart and adipose tissue. In kidney AT1R is predominetly expressed in mesangial cells, podocytes, and all nephron segments.
5.1. Signaling of AT1 receptor
Ang II activates various signaling pathways through AT1R, including G-protein-derived second messengers, protein kinases and small G-proteins. It also signals through mitogen-activated protein kinase (MAPK), JAK-STAT, NADH/NADPH, Akt/PKB, PKC and nitric oxide signaling pathways. Ang II activates of tyrosine kinases, which in turn phosphorylate down stream targets including the Ras/Raf/MAPK cascade and translocation of MAPK into the nucleus, thereby modulating various cells growth, proliferation, apoptosis, differentiation, transformation and vascular contraction. AT1 receptor can activate the JAK-STAT signaling pathway transducing cell surface signals into the cell cytoplasm and nucleus (31). Stimulation of AT1R leads to vasoconstriction via inhibition of adenylate cyclase causing a decrease in the vasodilator cAMP (31).
5.2. AT1R and COX-2
AT1R regulates COX-2 expression and prostaglandins production (Figure 1). Ang II stimulates phospholipase A2 (PLA2) activity, which releases arachidonic acid from cell membrane phospholipids and these effects are mediated via AT1R (32–33). Arachidonic acid is then processed by cyclooxygenases, lipoxygenases, or cytochrome P450 oxygenases to many different eicosanoids, which influence vascular and renal mechanisms important in the regulation of blood pressure and cell growth, possibly by activating redox sensitive pathways (34).
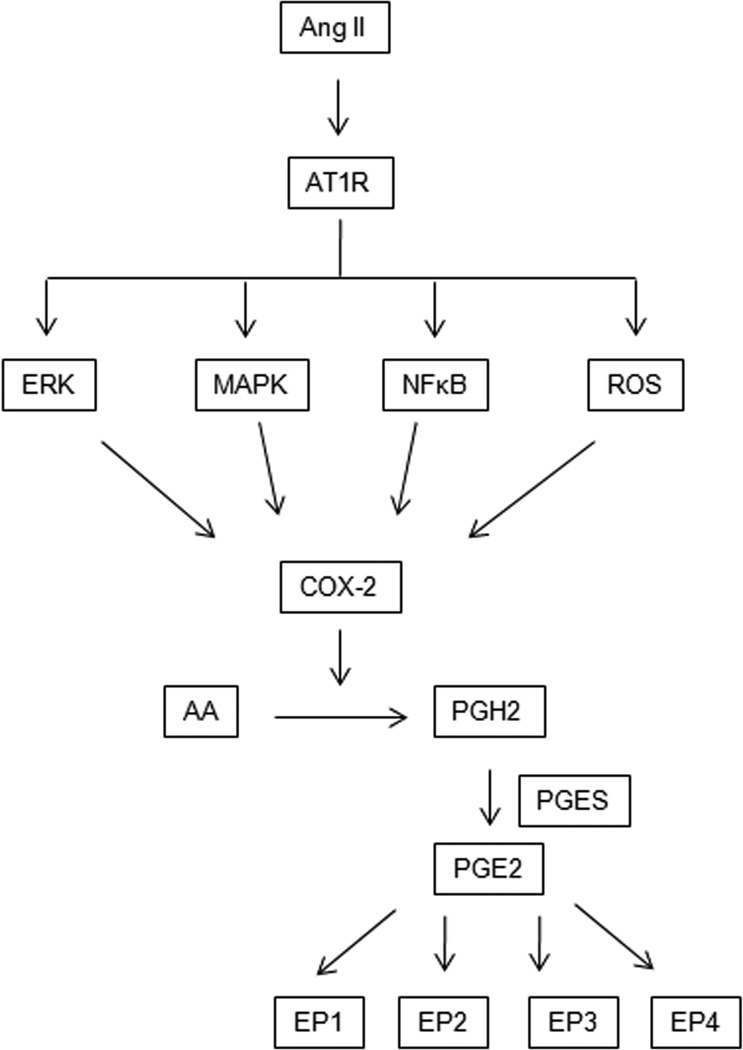
Figure 1.
Angiotensin type I receptor (AT1R) Regulates Prostaglandin E2 (PGE2) signaling. Ang II activates AT1R, which then initiates signaling cascade involving the activation of MAPK/ROS/NF-κB, which in turn activates inducible cyclooxygenase-2 (COX-2). COX-2 catalyzes the conversion of arachidonic acid (AA) to prostaglandin H2 (PGH2). PGH2 is converted into PGE2 by terminal PGE synthase (PGES). PGE2 signals occur via four different G-protein coupled receptors, EP1R-EP4R, each of which has multiple splice variants with different signaling properties.
In primary culture of cTALH Ang II administration significantly inhibited COX-2 expression induced by phorbol ester suggesting a direct role of Ang II regulating cortical COX-2 expression (1). In kidney, prostaglandins (PGE2 and PGI2) act locally on the glomerulus to maintain GFR by dilating the afferent arteriole, therefore counter regulating vasoconstrictive actions of AT1R (35). Co-expression of COX-2 and Prostaglandin E synthase (PGES) in the macula densa further supports a critical vasodilatory role of PGE2 (36). In addition to the direct vasodilator effect of prostaglandins on arterioles, the prostaglandins synthesize renin to generate Ang II. Ang II constricts the glomerular efferent arteriole (37) and increases intraglomerular pressure, maintaining GFR. This efferent arteriolar effect of Ang II is reinforced by afferent vasodilation induced by PGE2 (35).
AT1R plays a potent vasoconstrictive role in renovascular hypertension, and previous studies demonstrated that PGE2 and PGF2α was significantly increased in both clipped and contra lateral non-clipped kidney and plays a protective role to renal ischemia, hypertension and increases the sodium excretion (38–39).
Conversion of PGE2 to PGF2α is an important step in mediating vasodilatory effect of prostaglandins, and that in AT1R under low salt conditions enhances the formation of PGE2, but has no effect on conversion of PGE2 to PGF2α (40). However, in diabetes conversion of PGE2 to PGF2α is impaired due to increased AT1R activity and a reduction in PGE 9-ketoreductase leading to inflammatory state, and blockade of AT1R reverses its effect and increases the conversion of PGE2 to PGF2α which could be due to increase activity of functional AT2R, indicating a close relationship between prostaglandins and components of RAS.
6. ANGIOTENSIN II TYPE 2 RECEPTOR
The AT2 receptor is a 363 amino acid protein receptor with 7 transmembrane domains but which has only about 34% homology with the AT1 receptor. Its genetic sequence is uniformly found on the long arm of the X-chromosome in multiple species. The expression of AT2R changes dramatically over the course of development. Fetal organisms have high expression of AT2R, which then decreases in most organ systems after birth. Exceptions to this include the kidney, vascular tissue, adrenal gland, heart, ovary, sections of brain and non-pregnant uterine myometrium where higher levels of expression are maintained (41). Expression of AT2 receptor can vary considerably between species. In the brain, for example, the AT2R is found in multiple sensory and processing centers in the rat while in humans it is found only in the cerebellum (42). In the heart, AT2 receptor has been detected in the ventricles as well as the adventitia of arteries in rats (42). In the adult kidney, AT2R expression is generally low although it is highly expressed in adventitia of preglomerular blood vessels as well as proximal tubules and interstitial cells (43). As discussed below, renal expression of the AT2 receptor also changes significantly in various disease states and under certain physiologic conditions. For example, low sodium increases renal AT2 receptor expression and a similar increase are observed in both diabetes and obesity (43–44).
6.1. Signaling of AT2 receptor
Ang II has traditionally been considered the primary ligand for both the AT1 and AT2R. Further investigation has however revealed this signaling pathway in the kidney to be more complex. As demonstrated by Padia et al, Ang III is actually the preferred AT2R ligand in the kidney and is in fact necessary for AT2R mediated processes such as pressure natriuresis (44). Additionally, Ang (1–7) is a peptide formed by ACE2 from Ang I and Ang II that appears to mediate at least some of its effects via an AT2R dependent mechanism while also acting through its own Mas receptor (45).
The AT2R signals through several established mechanisms, most often in a manner that counteracts the AT1R. Perhaps the most important such pathway involves signaling through bradykinin-nitric oxide (NO) and cGMP which plays a role in several processes including AT2R mediated vasodilation (44). In a mechanism that is dependent on this same NO-cGMP pathway, the AT2R has been found to directly inhibit AT1R activity by decreasing AT1R transcription (46), while further inhibition occurs through dimerization between the AT1R and AT2R themselves. The AT2R also opposes AT1R mediated activation of MAP kinases ERK 1 and ERK2, which have a large effect in cellular growth and proliferation. AT2R does this by activating phosphotyrosine phosphatases via Giα2 and Giα3 G-protein coupling as well as through a G protein independent mechanism (44). These phosphatases then inactivate MAP kinases to counteract AT1R mediated activation.
6.2. AT2R and COX-2
AT2R receptor stimulates the release of arachidonic acid through activation of phospholipase A2 (Figure 2) and induces downstream production of prostacyclins such as PGI2 (47). AT2R also acts to counteract AT1R regulation of COX-2 expression. Inhibition of Ang II activity at the AT1R leads to an increase in COX-2 expression at the macula densa. In contrast, AT2R stimulated COX-2 in the macula densa (8). This represents another possible counter regulatory balance to the vasoactive effects of Ang II and may also be a negative feedback mechanism to control renin release.
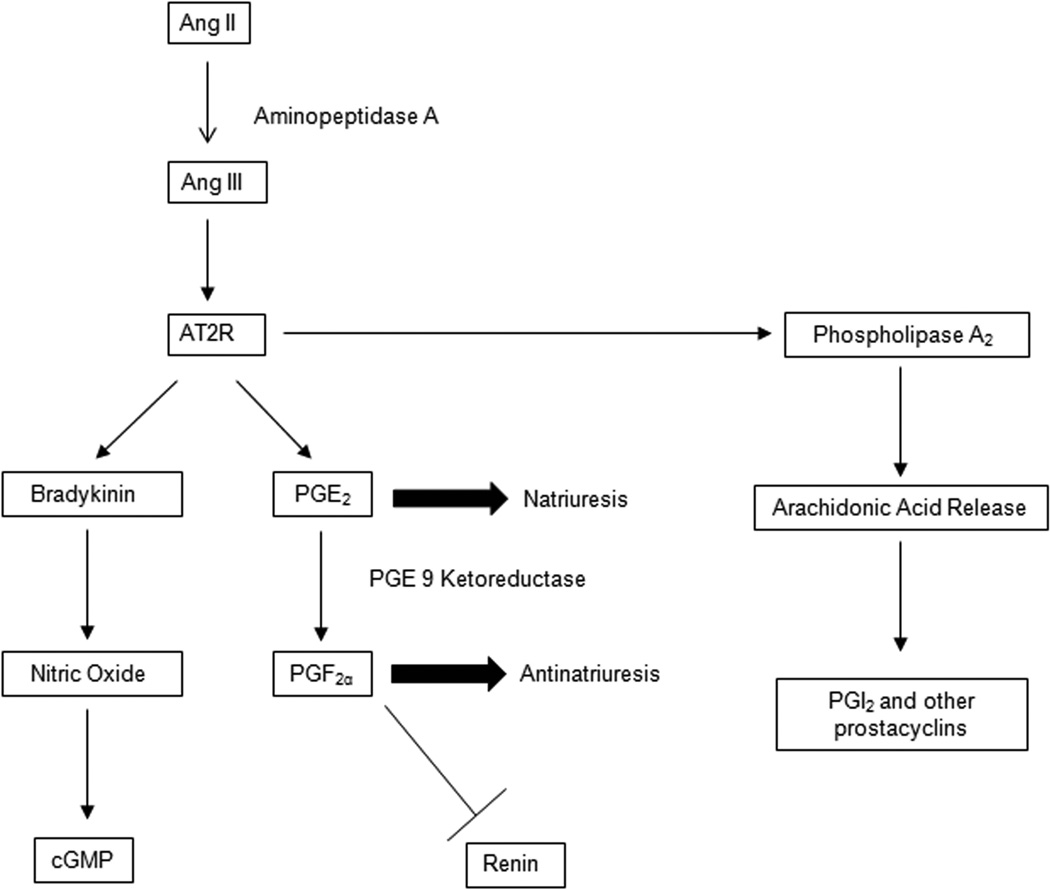
Figure 2.
AT2 Receptor signaling pathways: Ang II is converted to Ang III, the main ligand for the AT2 receptor. AT2 receptor signals primarily through the bradykinin-NO-cGMP and increases PGE2, a pronatriuresis prostaglandin. AT2 receptor also increases conversion of PGE2 to PGF2α, an antinatriuritic prostaglandin that also acts as an inhibitor of renin secretion. AT2 is known to activate phospholipase A2, increasing arachidonic acid release and conversion to downstream prostaglandins including PGI2.
In the kidney, the AT2 receptor mediates several effects through manipulation of prostaglandins. AT2 receptor expression is increased in the setting of low sodium intake. Bradykinin, cGMP and PGE2 are also increased by low sodium (48). AT2 receptor stimulation leads to production of bradykinin, NO and cGMP which opposes AT1 receptor mediated effects on vascular tone and antinaturiuresis (49). Bradykinin in turn signals through the B2 receptor to increase production of PGE2 (Figure 3) that counter the antinatriuretic/antidiuretic effects of AT1R. AT2R stimulation leads to the conversion of PGE2 to PGF2α via the PGE 9-ketoreductase under low sodium conditions (40). This provides another counter regulation to the activation of RAS as PGF2α inhibits the release of renin and downstream vasoconstriction (40). Interestingly, AT2R-NO-cGMP pathway has also been shown to play a role outside the kidney. For example, stimulation of the AT2 receptor in the jejunum leads to increased jejunal absorption via a cGMP dependent mechanism (50).
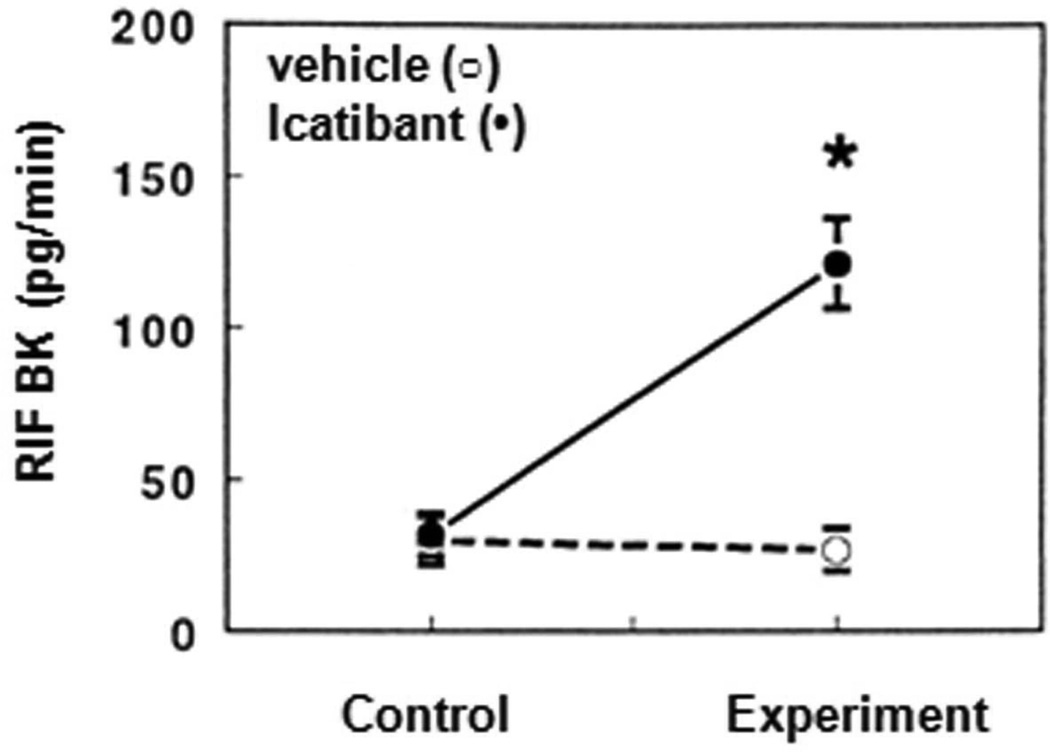
Figure 3.
“Reproduced with permission from Siragy et.al.(80)”. Renal interstitial fluid PGE2 before and after intrarenal administration of the bradykinin antagonist icatibant (•) or vehicle (○) in uninephrectomized conscious dogs. PGE2 decreased during icatibant administration from 11.4.±0.4. pg/min during the control periods to 5.1.±0.3. during drug infusion (P<0.0.1).
7. HYPERTENSION
7.1. Role of Ang II/AT1R/PRR and COX-2 in hypertension
PGE2 has either vasodilatory or vasoconstrictive effects in the kidney depending upon binding to specific PGE2 receptors (EP). EP-4 is the most abundant EP receptor and promotes vasodilation, while EP-3 mediates vasoconstriction. In addition to PGE2, other prostaglandins, PGI2 has a predominantly vasodilatory effect and TxA2 and PGF2α are vasoconstrictors. In kidney, PGE2 plays an important role in regulating fluid reabsorption in the collecting duct and thick ascending limb of the loop of Henle. Inhibition of PGE2, increases tubular sodium and water reabsorption thus contributing to development of hypertension. This effect is mostly COX-2 mediated as clinical studies demonstrated similar levels of sodium retention in patients on NSAIDs (51).
The vasodilator effects of COX-2 derived prostanoids in the vasculature to counteract the Ang II-AT1R induced vasoconstriction are controversial. Okumura et al, reported that COX-2 inhibition decreases arterial pressure (52). A Similar decrease in the arterial pressure was reported by Wang J-L et.al, with COX-2 inhibition, this could be due to decrease in renin release by COX-2 inhibition (53). These results suggest that COX-2 has pro-hypertensive effects. In Cx40−/− mice, nonselective COX inhibition was shown to lower blood pressure without affecting renin release (54). In chronic Ang II infused mice where renin levels are suppressed, inhibition of COX-2 decreases blood pressure (16). In addition to previous reports, in early stage of Ang II-dependent hypertension, PRR and COX-2 were reported to be augmented in the renal medullary tissues and to mediate vasoconstriction effects of Ang II (55). In renal inner medullary cells, activation of PRR upregulates COX-2 expression through MAPK-ERK1/2 signaling pathway, and (PRR and COX-2) was shown to colocalize in interstial and intercalated cells. Downregulation of PRR attenuated the increase in COX-2 expression, suggesting a role of PRR in regulating COX-2, independent of Ang II pathway (56). Taken together, these studies suggest that COX-2 contributes to the hypertension independently of the renin or Ang II levels.
In transgenic rats (TGRCyp1a1-Ren2)) with Ang II dependent hypertension, selective COX-2 inhibition led to pronounced decreases in GFR, suggesting that the enhanced intrarenal COX-2 counteracts the vasoconstrictor action of Ang II in the kidney and play an important role in maintaining GFR. However, inhibition of COX-2 decreased arterial blood pressure, indicating that the systemic COX-2 derived prostanoids have vasoconstrictive action and contributes to the development of hypertension (57). In Cyp1a1-Ren2 rats, inhibition of COX-2 with blockade of neuronal nitric oxide synthase (nNOS) increased renal vascular resistance, demonstrating the renal vasodilator effects of COX-2 (57).
Activation of RAS activity via increased ROS generation during chronic Ang II infusion and in salt sensitive hypertension leads to upregulation of COX-2 (58). Elevated ROS activity could alter the relationship between COX-2 and Ang II from antagonistic to cooperative with regard to the blood pressure effects (59).
7.2. Role of AT2R and COX-2 in hypertension
AT2 receptor mediated response to hypertension involves regulation of eicosanoids. Arachidonic acid in the kidney is processed by cytochrome p450 CYP2C to create 20-HETE which acts as a vasoconstrictor and inhibitor of sodium transport while CYP 2J produces a group of molecules called EETs (epoxyeicosatrienoic acids) which are vasodilators and also inhibitors of sodium transport (60). 20-HETE promotes the development of hypertension but also appears to be protective against glomerular injury while EETs promote vasodilation in an NO and COX-2 independent mechanism (60). As demonstrated by Bautista et al, in the setting of kidney injury the AT2 receptor triggers a vasodilator effect that is mediated by increased production of epoxyeicosatrienoic acids (EETs) and decreased production of 20-HETE (61). 20-HETE production in the rat kidney is meanwhile mediated by the AT2 receptor and 20-HETE has been shown to contribute to the vasoconstrictor response to Ang II (62). Both sets of compounds also have effects on natriuresis. EETs prevent sodium retention and their blockade leads to increased blood pressure in a process dependent on serine/threonine protein phosphatase (60). In one study, inhibition of arachidonic acid metabolism prevents renal vasodilation while COX inhibition with indomethacin does not, suggesting that the vasodilator effect is dependent on CYP 450 arachidonic metabolism but not on COX (61). However, Brouwers et al showed that in hypertensive rats treated with an ACE inhibitor, the AT2 receptor agonist C21 induced a vasodilator response that was partially inhibited by indomethacin, suggesting at least a partial role for prostaglandins in this AT2 mediated response (63). In either case, AT2 receptor stimulation appears to be central to renal homeostasis.
8. DIABETIC NEPHROPATHY
8.1. Role of Ang II/AT1R/PRR and COX-2 in diabetic nephropathy
Upregulation of COX-2 in kidney has been associated with glomerular injury, and its inhibition reduces proteinuria, and decreases progression of diabetic nephropathy (64). In vivo animal and human studies indicate the effects of COX-2 in diabetic nephropathy and selective inhibition of COX-2 reduce proteinuria in patients without effecting systolic blood pressure (65). In diabetic rats acute and chronic inhibition of COX-2 does not have any influence on plasma glucose levels, but they have successfully prevented hyperfiltration and considerably reduced albuminuria (66).
Hyperglycemia has been shown to augment COX-2 mediated-hyperfiltration but decreases the ability of COX-2 to increase GFR in hyperfiltering patients (67). These data signify increased compromise of the glomerular barrier mediated by COX-2. Upregulation of COX-2 expression in podocytes has been demonstrated in streptozotocin-induced diabetic model (68) where it contributes to podocyte injury, possibly via activation of thromboxane receptors (69). Furthermore, the glomerular hyperfiltration-associated increase in sheer stress and increases podocyte COX-2 expression and PGE2 production (70). In addition, activation of the EP4 receptor increases PGE2 production, thus potentially mediating a positive feedback in the course of kidney injury similar to that observed in diabetes.
Further evidence indicates that COX-2 is a primary mediator of renal injury, which is attributed to RAS activity during high glucose conditions associated with diabetes. In female diabetic patients, inhibition of COX-2 prohibits AngII-mediated reductions in GFR (71). Studies have also shown an association between PRR and COX-2 expression. PRR upregulation augments cortical expression of COX-2 via activation the ERK1/2 pathway (72). In the rat mesangial cells (RMCs), the increase in PRR under high glucose treatment resulted in an increase in IL-1β and COX-2 expression via Ang II and ERK1/2-NF-kappaB signaling cascade (35). Downregulation of PRR attenuated this increase in COX-2 expression (21). Diabetic COX-2-transgenic mice showed progressive albuminuria, significant foot-process effacement, mesangial expansion, thickening of the glomerular basement membrane and increased PRR expression (95). COX-2 inhibitor abrogated the upregulation of PRR and reduced renal injury, suggesting positive feedback mechanism of COX-2 and PRR that contributes to renal injury in diabetes (Figure 4) (73). The relevance of diabetes to RAS promotion of COX-2 activity is demonstrated in mesangial cell COX-2 via the AT1 receptor (74) as well as renin and PRR (21).Though reactive oxygen species have been implicated in mediating glucose and Ang II augmentation of COX-2 expression in glomerular endothelial cells, the mechanism remains to be fully elucidated.
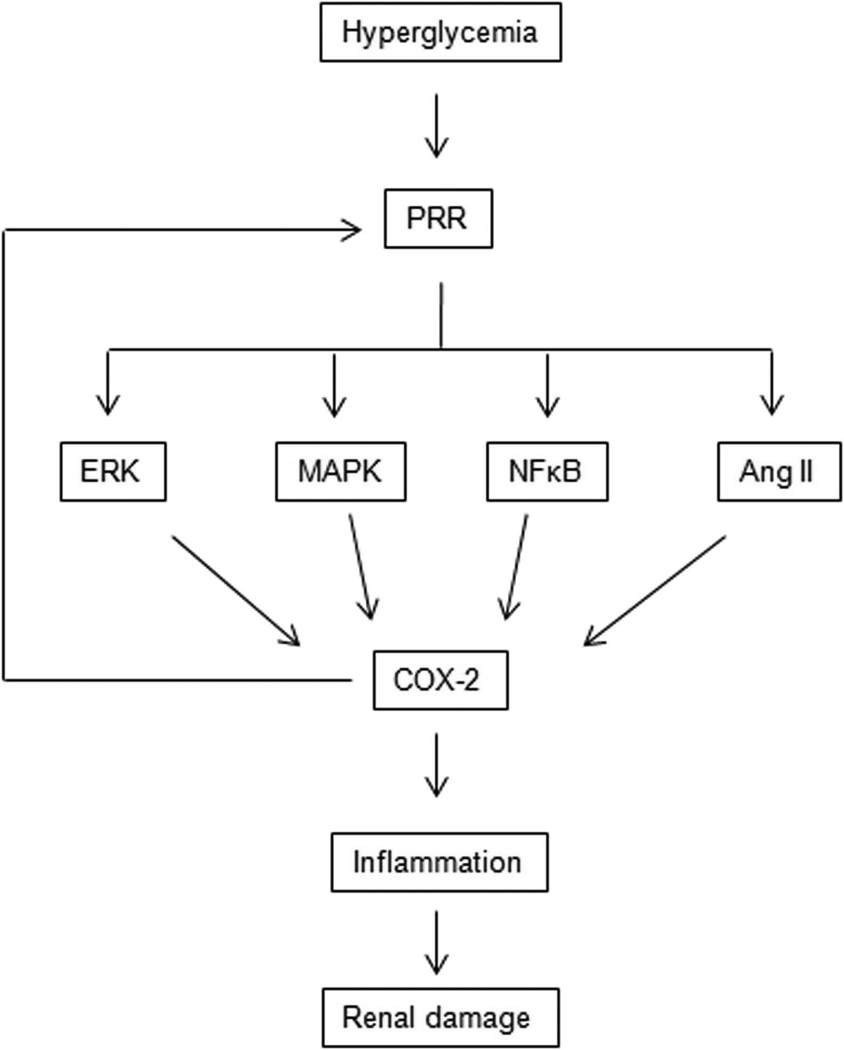
Figure 4.
(Pro)renin (PRR) Induces Renal Damage Via cyclooxygenase-2 (COX-2) signaling. High glucose upregulates PRR, which in turn augments renal expression of COX-2 via activation the Ang II, ERK1/2, MAPK and NF-κB pathway leading to renal damage. COX-2 feedbacks on PRR further amplifying the renal injury.
8.2. Role of AT2R and COX-2 in diabetic nephropathy
Diabetic nephropathy is characterized by increased renal inflammation and fibrosis, and is accompanied by activation of the local renal RAS (75). Likewise blockade of RAS with angiotensin converting enzyme inhibitors or angiotensin receptor blockers is now standard of care for patients with diabetic nephropathy. Several studies have suggested that the AT2 receptor is upregulated in the kidneys of diabetic animals although not all reports have agreed with this. Stimulation of the AT2 receptor decreased markers of renal inflammation and fibrosis in hypertensive nondiabetic rats (75) and use of the AT2 receptor agonist C21 in diabetic mice reduces renal injury and albuminuria as well as markers of oxidative stress, inflammation and fibrosis (76). Similarly, treatment with the AT2 receptor agonist reduces cytokine markers of renal inflammation as well as proteinuria in streptozotocin induced diabetic rats while increasing renal production of NO and cGMP (75). Conversely, AT2 receptor knockout in a mouse model of type 1 diabetes increases the pace at which diabetic nephropathy develops (77). Our group has therefore previously hypothesized that the explanation for the failure of AT2 receptors protective role in diabetic nephropathy despite its increased expression may be a loss of function that is not yet fully explained (75).
The renal protective effects of the AT2 receptor are also mediated in part through its effect on eicosanoids. 12(S)-HETE is a renal metabolite of arachidonic acid formed by the 12-lipoxygenase and is increased in the setting of diabetic nephropathy (78). 12(S)-HETE enhances Ang II mediated vasoconstriction and renal inflammation and renal 12(S)-HETE levels rise in the setting of elevated blood glucose which correlate with increased Ang II levels and albuminuria (79). The AT2 receptor activation decreases 12(S)-HETE production which may be protective under normal conditions (79). Either decreased expression or decreased function of the AT2R receptor in diabetic nephropathy, as previously discussed, may allow for increased 12-lipoxygenase production of 12(S)-HETE and thus progression of renal disease.
9. SUMMARY
The interaction between the RAS and COX-2 in the kidney is complex. Studies have demonstrated the role for COX-2 in the augmentation and restriction of the RAS. On one hand, COX-2 mediates renin release. On the other hand, COX-2 restricts the Ang II mediated increases in renovascular resistance by eliciting direct vasodilation. These studies demonstrate the potential conversion of beneficial effects of COX-2 to a dysfunctional regulator of the RAS that potentiate Ang II mediated hypertension and kidney injury. Data suggested oxidative stress as a potential mechanism in the pro- hypertensive effects of COX-2. Diabetes also appears to transform beneficial effects of COX-2 to pathophysiologic degradation of the glomerular integrity via a PRR-COX-2 receptor positive feedback loop. Future studies should evaluate the role of prostaglandin E synthase and specific EP receptors that could help develop novel strategies for management of hypertension and diabetic kidney disease.
REFERENCES
- 1.Cheng HF, Wang JL, Zhang MZ, Miyazaki Y, Ichikawa I, McKanna JA, Harris RC. Angiotensin II attenuates renal cortical cyclooxygenase-2 expression. J Clin Invest. 1999;103(7):953–961. doi: 10.1172/JCI5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harding P, Sigmon DH, Alfie ME, Huang PL, Fishman MC, Beierwaltes WH, Carretero OA. Cyclooxygenase-2 mediates increased renal renin content induced by low-sodium diet. Hypertension. 1997;29(1 Pt 2):297–302. doi: 10.1161/01.hyp.29.1.297. [DOI] [PubMed] [Google Scholar]
- 3.Francois H, Facemire C, Kumar A, Audoly L, Koller B, Coffman T. Role of microsomal prostaglandin E synthase 1 in the kidney. J Am Soc Nephrol. 2007;18(5):1466–1475. doi: 10.1681/ASN.2006040343. [DOI] [PubMed] [Google Scholar]
- 4.Miyamoto T, Ogino N, Yamamoto S, Hayaishi O. Purification of prostaglandin endoperoxide synthetase from bovine vesicular gland microsomes. J Biol Chem. 1976;251(9):2629–2636. [PubMed] [Google Scholar]
- 5.Khan KN, Paulson SK, Verburg KM, Lefkowith JB, Maziasz TJ. Pharmacology of cyclooxygenase-2 inhibition in the kidney. Kidney Int. 2002;61(4):1210–1219. doi: 10.1046/j.1523-1755.2002.00263.x. [DOI] [PubMed] [Google Scholar]
- 6.Harris RC. The macula densa: recent developments. J Hypertens. 1996;14(7):815–822. doi: 10.1097/00004872-199607000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Hao CM, Breyer MD. Physiological regulation of prostaglandins in the kidney. Annu Rev Physiol. 2008;70:357–377. doi: 10.1146/annurev.physiol.70.113006.100614. [DOI] [PubMed] [Google Scholar]
- 8.Zhang MZ, Yao B, Cheng HF, Wang SW, Inagami T, Harris RC. Renal cortical cyclooxygenase 2 expression is differentially regulated by angiotensin II AT(1) and AT(2) receptors. Proc Natl Acad Sci U S A. 2006;103(43):16045–16050. doi: 10.1073/pnas.0602176103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roig F, Llinas MT, Lopez R, Salazar FJ. Role of cyclooxygenase-2 in the prolonged regulation of renal function. Hypertension. 2002;40(5):721–728. doi: 10.1161/01.hyp.0000036451.76323.29. [DOI] [PubMed] [Google Scholar]
- 10.Yang T, Singh I, Pham H, Sun D, Smart A, Schnermann JB, Briggs JP. Regulation of cyclooxygenase expression in the kidney by dietary salt intake. Am J Physiol. 1998;274(3 Pt 2):F481–F489. doi: 10.1152/ajprenal.1998.274.3.F481. [DOI] [PubMed] [Google Scholar]
- 11.Zewde T, Mattson DL. Inhibition of cyclooxygenase-2 in the rat renal medulla leads to sodium-sensitive hypertension. Hypertension. 2004;44(4):424–428. doi: 10.1161/01.HYP.0000140924.91479.03. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez AA, Cespedes C, Villanueva S, Michea L, Vio CP. E Prostanoid-1 receptor regulates renal medullary alphaENaC in rats infused with angiotensin II. Biochem Biophys Res Commun. 2009;389(2):372–377. doi: 10.1016/j.bbrc.2009.08.157. [DOI] [PubMed] [Google Scholar]
- 13.Kim SM, Chen L, Mizel D, Huang YG, Briggs JP, Schnermann J. Low plasma renin and reduced renin secretory responses to acute stimuli in conscious COX-2-deficient mice. Am J Physiol Renal Physiol. 2007;292(1):F415–F422. doi: 10.1152/ajprenal.00317.2006. [DOI] [PubMed] [Google Scholar]
- 14.Yang T, Park JM, Arend L, Huang Y, Topaloglu R, Pasumarthy A, Praetorius H, Spring K, Briggs JP, Schnermann J. Low chloride stimulation of prostaglandin E2 release and cyclooxygenase-2 expression in a mouse macula densa cell line. J Biol Chem. 2000;275(48):37922–37929. doi: 10.1074/jbc.M006218200. [DOI] [PubMed] [Google Scholar]
- 15.Hanner F, Chambrey R, Bourgeois S, Meer E, Mucsi I, Rosivall L, Shull GE, Lorenz JN, Eladari D, Peti-Peterdi J. Increased renal renin content in mice lacking the Na+/H+ exchanger NHE2. Am J Physiol Renal Physiol. 2008;294(4):F937–F944. doi: 10.1152/ajprenal.00591.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Araujo M, Welch WJ. Tubuloglomerular feedback is decreased in COX-1 knockout mice after chronic angiotensin II infusion. Am J Physiol Renal Physiol. 2010;298(4):F1059–F1063. doi: 10.1152/ajprenal.00547.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green T, Rodriguez J, Navar LG. Augmented cyclooxygenase-2 effects on renal function during varying states of angiotensin II. Am J Physiol Renal Physiol. 2010;299(5):F954–F962. doi: 10.1152/ajprenal.00609.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen G, Delarue F, Burckle C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest. 2002;109(11):1417–1427. doi: 10.1172/JCI14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quadri S, Siragy HM. Regulation of (pro)renin receptor expression in mIMCD via the GSK-3beta-NFAT5-SIRT-1 signaling pathway. Am J Physiol Renal Physiol. 2014;307(5):F593–F600. doi: 10.1152/ajprenal.00245.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li C, Siragy HM. High glucose induces podocyte injury via enhanced (pro)renin receptor-Wnt-beta-catenin-snail signaling pathway. PLoS One. 2014;9(2):e89233. doi: 10.1371/journal.pone.0089233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang J, Siragy HM. Glucose promotes the production of interleukine-1beta and cyclooxygenase-2 in mesangial cells via enhanced (Pro)renin receptor expression. Endocrinology. 2009;150(12):5557–5565. doi: 10.1210/en.2009-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cruciat CM, Ohkawara B, Acebron SP, Karaulanov E, Reinhard C, Ingelfinger D, Boutros M, Niehrs C. Requirement of prorenin receptor and vacuolar H+-ATPase-mediated acidification for Wnt signaling. Science. 2010;327(5964):459–463. doi: 10.1126/science.1179802. [DOI] [PubMed] [Google Scholar]
- 23.Kinouchi K, Ichihara A, Itoh H. Functional characterization of (pro)renin receptor in association with V-ATPase. Front Biosci (Landmark Ed) 2011;16:3216–3223. doi: 10.2741/3907. [DOI] [PubMed] [Google Scholar]
- 24.Binger KJ, Muller DN. Autophagy and the (Pro)renin Receptor. Front Endocrinol (Lausanne) 2013;4:155. doi: 10.3389/fendo.2013.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang J, Siragy HM. Regulation of (pro)renin receptor expression by glucose-induced mitogen-activated protein kinase, nuclear factor-kappaB, and activator protein-1 signaling pathways. Endocrinology. 2010;151(7):3317–3325. doi: 10.1210/en.2009-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson S, Rennke HG, Brenner BM. Therapeutic advantage of converting enzyme inhibitors in arresting progressive renal disease associated with systemic hypertension in the rat. J Clin Invest. 1986;77(6):1993–2000. doi: 10.1172/JCI112528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li W, Sullivan MN, Zhang S, Worker CJ, Xiong Z, Speth RC, Feng Y. Intracerebroventricular infusion of the (Pro)renin receptor antagonist PRO20 attenuates deoxycorticosterone acetate-salt-induced hypertension. Hypertension. 2015;65(2):352–361. doi: 10.1161/HYPERTENSIONAHA.114.04458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prieto MC, Botros FT, Kavanagh K, Navar LG. Prorenin receptor in distal nephron segments of 2-kidney, 1-clip goldblatt hypertensive rats. Ochsner J. 2013;13(1):26–32. [PMC free article] [PubMed] [Google Scholar]
- 29.Huang J, Ledford KJ, Pitkin WB, Russo L, Najjar SM, Siragy HM. Targeted deletion of murine CEACAM 1 activates PI3K-Akt signaling and contributes to the expression of (Pro)renin receptor via CREB family and NF-kappaB transcription factors. Hypertension. 2013;62(2):317–323. doi: 10.1161/HYPERTENSIONAHA.113.01324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Atlas SA. The renin-angiotensin aldosterone system: pathophysiological role and pharmacologic inhibition. J Manag Care Pharm. 2007;13(8 Suppl B):9–20. doi: 10.18553/jmcp.2007.13.s8-b.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higuchi S, Ohtsu H, Suzuki H, Shirai H, Frank GD, Eguchi S. Angiotensin II signal transduction through the AT1 receptor: novel insights into mechanisms and pathophysiology. Clin Sci (Lond) 2007;112(8):417–428. doi: 10.1042/CS20060342. [DOI] [PubMed] [Google Scholar]
- 32.Freeman EJ, Ruehr ML, Dorman RV. ANG II-induced translocation of cytosolic PLA2 to the nucleus in vascular smooth muscle cells. Am J Physiol. 1998;274(1 Pt 1):C282–C288. doi: 10.1152/ajpcell.1998.274.1.C282. [DOI] [PubMed] [Google Scholar]
- 33.Bonventre JV. Phospholipase A2 and signal transduction. J Am Soc Nephrol. 1992;3(2):128–150. doi: 10.1681/ASN.V32128. [DOI] [PubMed] [Google Scholar]
- 34.Dulin NO, Alexander LD, Harwalkar S, Falck JR, Douglas JG. Phospholipase A2-mediated activation of mitogen-activated protein kinase by angiotensin II. Proc Natl Acad Sci U S A. 1998;95(14):8098–8102. doi: 10.1073/pnas.95.14.8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edwards RM. Effects of prostaglandins on vasoconstrictor action in isolated renal arterioles. Am J Physiol. 1985;248(6 Pt 2):F779–F784. doi: 10.1152/ajprenal.1985.248.6.F779. [DOI] [PubMed] [Google Scholar]
- 36.Tomida T, Numaguchi Y, Matsui H, Toki Y, Ito T, Okumura K, Hayakawa T. Altered expression of prostacyclin synthase in a subset of the thick ascending limb cells and mesangial cells in 5/6-nephrectomized rats. Hypertens Res. 2001;24(4):411–419. doi: 10.1291/hypres.24.411. [DOI] [PubMed] [Google Scholar]
- 37.Edwards RM. Segmental effects of norepinephrine and angiotensin II on isolated renal microvessels. Am J Physiol. 1983;244(5):F526–F534. doi: 10.1152/ajprenal.1983.244.5.F526. [DOI] [PubMed] [Google Scholar]
- 38.Soejima H, Nomura Y, Tsuruta K, Ikegami K. Intrarenal prostaglandins E2 and F2 alpha in experimental renovascular hypertension. Urol Int. 1981;36(3):158–165. doi: 10.1159/000280407. [DOI] [PubMed] [Google Scholar]
- 39.Yoshida M, Soejima H, Ueda S, Ikegami K. Role of renal prostaglandin E2 in two-kidney, one-clip renovascular hypertension in rabbits. Nephron. 1986;44(2):142–149. doi: 10.1159/000184219. [DOI] [PubMed] [Google Scholar]
- 40.Siragy HM, Carey RM. The subtype 2 angiotensin receptor regulates renal prostaglandin F2 alpha formation in conscious rats. Am J Physiol. 1997;273(3 Pt 2):R1103–R1107. doi: 10.1152/ajpregu.1997.273.3.R1103. [DOI] [PubMed] [Google Scholar]
- 41.de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev. 2000;52(3):415–472. [PubMed] [Google Scholar]
- 42.Zhuo J, Moeller I, Jenkins T, Chai SY, Allen AM, Ohishi M, Mendelsohn FA. Mapping tissue angiotensin-converting enzyme and angiotensin AT1, AT2 and AT4 receptors. J Hypertens. 1998;16(12 Pt 2):2027–2037. doi: 10.1097/00004872-199816121-00026. [DOI] [PubMed] [Google Scholar]
- 43.Carey RM, Siragy HM. Newly recognized components of the renin-angiotensin system: potential roles in cardiovascular and renal regulation. Endocr Rev. 2003;24(3):261–271. doi: 10.1210/er.2003-0001. [DOI] [PubMed] [Google Scholar]
- 44.Carey RM, Padia SH. Role of angiotensin AT(2) receptors in natriuresis: Intrarenal mechanisms and therapeutic potential. Clin Exp Pharmacol Physiol. 2013;40(8):527–534. doi: 10.1111/1440-1681.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Santos RA, Ferreira AJ, Verano-Braga T, Bader M. Angiotensin-converting enzyme 2, angiotensin-(1-7) and Mas: new players of the renin-angiotensin system. J Endocrinol. 2013;216(2):R1–R17. doi: 10.1530/JOE-12-0341. [DOI] [PubMed] [Google Scholar]
- 46.Yang J, Chen C, Ren H, Han Y, He D, Zhou L, Hopfer U, Jose PA, Zeng C. Angiotensin II AT(2) receptor decreases AT(1) receptor expression and function via nitric oxide/cGMP/Sp1 in renal proximal tubule cells from Wistar-Kyoto rats. J Hypertens. 2012;30(6):1176–1184. doi: 10.1097/HJH.0b013e3283532099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jacobs LS, Douglas JG. Angiotensin II type 2 receptor subtype mediates phospholipase A2-dependent signaling in rabbit proximal tubular epithelial cells. Hypertension. 1996;28(4):663–668. doi: 10.1161/01.hyp.28.4.663. [DOI] [PubMed] [Google Scholar]
- 48.Siragy HM, Ibrahim MM, Jaffa AA, Mayfield R, Margolius HS. Rat renal interstitial bradykinin, prostaglandin E2, and cyclic guanosine 3',5'-monophosphate. Effects of altered sodium intake. Hypertension. 1994;23(6 Pt 2):1068–1070. doi: 10.1161/01.hyp.23.6.1068. [DOI] [PubMed] [Google Scholar]
- 49.Siragy HM, Carey RM. The subtype-2 (AT2) angiotensin receptor regulates renal cyclic guanosine 3', 5'-monophosphate and AT1 receptor-mediated prostaglandin E2 production in conscious rats. J Clin Invest. 1996;97(8):1978–1982. doi: 10.1172/JCI118630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jin XH, Wang ZQ, Siragy HM, Guerrant RL, Carey RM. Regulation of jejunal sodium and water absorption by angiotensin subtype receptors. Am J Physiol. 1998;275(2 Pt 2):R515–R523. doi: 10.1152/ajpregu.1998.275.2.R515. [DOI] [PubMed] [Google Scholar]
- 51.Frishman WH. Effects of nonsteroidal anti-inflammatory drug therapy on blood pressure and peripheral edema. Am J Cardiol. 2002;89(6A):18D–25D. doi: 10.1016/s0002-9149(02)02233-6. [DOI] [PubMed] [Google Scholar]
- 52.Okumura T, Hayashi I, Ikezawa T, Yamanaka M, Takata T, Fujita Y, Saigenji K, Yamashina S, Majima M. Cyclooxygenase-2 inhibitors attenuate increased blood pressure in renovascular hypertensive models, but not in deoxycorticosterone-salt hypertension. Hypertens Res. 2002;25(6):927–938. doi: 10.1291/hypres.25.927. [DOI] [PubMed] [Google Scholar]
- 53.Wang JL, Cheng HF, Harris RC. Cyclooxygenase-2 inhibition decreases renin content and lowers blood pressure in a model of renovascular hypertension. Hypertension. 1999;34(1):96–101. doi: 10.1161/01.hyp.34.1.96. [DOI] [PubMed] [Google Scholar]
- 54.Krattinger N, Alonso F, Capponi A, Mazzolai L, Nicod P, Meda P, Haefliger JA. Increased expression of renal cyclooxygenase-2 and neuronal nitric oxide synthase in hypertensive Cx40-deficient mice. J Vasc Res. 2009;46(3):188–198. doi: 10.1159/000156704. [DOI] [PubMed] [Google Scholar]
- 55.Gonzalez AA, Green T, Luffman C, Bourgeois CR, Gabriel Navar L, Prieto MC. Renal medullary cyclooxygenase-2 and(pro)reninreceptorexpressionduringangiotensinII-dependenthypertension. Am J Physiol Renal Physiol. 2014;307(8):F962–F970. doi: 10.1152/ajprenal.00267.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gonzalez AA, Luffman C, Bourgeois CR, Vio CP, Prieto MC. Angiotensin II-independent upregulation of cyclooxygenase-2 by activation of the (Pro)renin receptor in rat renal inner medullary cells. Hypertension. 2013;61(2):443–449. doi: 10.1161/HYPERTENSIONAHA.112.196303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patterson ME, Mullins JJ, Mitchell KD. Renoprotective effects of neuronal NOS-derived nitric oxide and cyclooxygenase-2 metabolites in transgenic rats with inducible malignant hypertension. Am J Physiol Renal Physiol. 2008;294(1):F205–F211. doi: 10.1152/ajprenal.00150.2007. [DOI] [PubMed] [Google Scholar]
- 58.Jaimes EA, Zhou MS, Pearse DD, Puzis L, Raij L. Upregulation of cortical COX-2 in salt-sensitive hypertension: role of angiotensin II and reactive oxygen species. Am J Physiol Renal Physiol. 2008;294(2):F385–F392. doi: 10.1152/ajprenal.00302.2007. [DOI] [PubMed] [Google Scholar]
- 59.Kopkan L, Huskova Z, Vanourkova Z, Thumova M, Skaroupkova P, Maly J, Kramer HJ, Dvorak P, Cervenka L. Reduction of oxidative stress does not attenuate the development of angiotensin II-dependent hypertension in Ren-2 transgenic rats. Vascul Pharmacol. 2009;51(2–3):175–181. doi: 10.1016/j.vph.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 60.Fan F, Muroya Y, Roman RJ. Cytochrome P450 eicosanoids in hypertension and renal disease. Curr Opin Nephrol Hypertens. 2015;24(1):37–46. doi: 10.1097/MNH.0000000000000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bautista R, Sanchez A, Hernandez J, Oyekan A, Escalante B. Angiotensin II type AT(2) receptor mRNA expression and renal vasodilatation are increased in renal failure. Hypertension. 2001;38(3 Pt 2):669–673. doi: 10.1161/hy09t1.096186. [DOI] [PubMed] [Google Scholar]
- 62.Fan F, Sun CW, Maier KG, Williams JM, Pabbidi MR, Didion SP, Falck JR, Zhuo J, Roman RJ. 20-Hydroxyeicosatetraenoic acid contributes to the inhibition of K+ channel activity and vasoconstrictor response to angiotensin II in rat renal microvessels. PLoS One. 2013;8(12):e82482. doi: 10.1371/journal.pone.0082482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brouwers S, Smolders I, Massie A, Dupont AG. Angiotensin II type 2 receptor-mediated and nitric oxide-dependent renal vasodilator response to compound 21 unmasked by angiotensin-converting enzyme inhibition in spontaneously hypertensive rats in vivo. Hypertension. 2013;62(5):920–926. doi: 10.1161/HYPERTENSIONAHA.112.00762. [DOI] [PubMed] [Google Scholar]
- 64.Kumar Bhatt L, Addepalli V. Minocycline with aspirin: an approach to attenuate diabetic nephropathy in rats. Ren Fail. 2011;33(1):72–78. doi: 10.3109/0886022X.2010.528117. [DOI] [PubMed] [Google Scholar]
- 65.Vogt L, de Zeeuw D, Woittiez AJ, Navis G. Selective cyclooxygenase-2 (COX-2) inhibition reduces proteinuria in renal patients. Nephrol Dial Transplant. 2009;24(4):1182–1189. doi: 10.1093/ndt/gfn644. [DOI] [PubMed] [Google Scholar]
- 66.Quilley J, Santos M, Pedraza P. Renal protective effect of chronic inhibition of COX-2 with SC-58236 in streptozotocin-diabetic rats. Am J Physiol Heart Circ Physiol. 2011;300(6):H2316–H2322. doi: 10.1152/ajpheart.01259.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cherney DZ, Miller JA, Scholey JW, Bradley TJ, Slorach C, Curtis JR, Dekker MG, Nasrallah R, Hebert RL, Sochett EB. The effect of cyclooxygenase-2 inhibition on renal hemodynamic function in humans with type 1 diabetes. Diabetes. 2008;57(3):688–695. doi: 10.2337/db07-1230. [DOI] [PubMed] [Google Scholar]
- 68.Komers R, Lindsley JN, Oyama TT, Schutzer WE, Reed JF, Mader SL, Anderson S. Immunohistochemical and functional correlations of renal cyclooxygenase-2 in experimental diabetes. J Clin Invest. 2001;107(7):889–898. doi: 10.1172/JCI10228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cheng H, Fan X, Guan Y, Moeckel GW, Zent R, Harris RC. Distinct roles for basal and induced COX-2 in podocyte injury. J Am Soc Nephrol. 2009;20(9):1953–1962. doi: 10.1681/ASN.2009010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Srivastava T, McCarthy ET, Sharma R, Cudmore PA, Sharma M, Johnson ML, Bonewald LF. Prostaglandin E(2) is crucial in the response of podocytes to fluid flow shear stress. J Cell Commun Signal. 2010;4(2):79–90. doi: 10.1007/s12079-010-0088-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cherney DZ, Scholey JW, Nasrallah R, Dekker MG, Slorach C, Bradley TJ, Hebert RL, Sochett EB, Miller JA. Renal hemodynamic effect of cyclooxygenase 2 inhibition in young men and women with uncomplicated type 1 diabetes mellitus. Am J Physiol Renal Physiol. 2008;294(6):F1336–F1341. doi: 10.1152/ajprenal.00574.2007. [DOI] [PubMed] [Google Scholar]
- 72.Kaneshiro Y, Ichihara A, Takemitsu T, Sakoda M, Suzuki F, Nakagawa T, Hayashi M, Inagami T. Increased expression of cyclooxygenase-2 in the renal cortex of human prorenin receptor gene-transgenic rats. Kidney Int. 2006;70(4):641–646. doi: 10.1038/sj.ki.5001627. [DOI] [PubMed] [Google Scholar]
- 73.Cheng H, Fan X, Moeckel GW, Harris RC. Podocyte COX-2 exacerbates diabetic nephropathy by increasing podocyte (pro)renin receptor expression. J Am Soc Nephrol. 2011;22(7):1240–1251. doi: 10.1681/ASN.2010111149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Naito M, Shenoy A, Aoyama I, Koopmeiners JS, Komers R, Schnaper HW, Bomsztyk K. High ambient glucose augments angiotensin II-induced proinflammatory gene mRNA expression in human mesangial cells: effects of valsartan and simvastatin. Am J Nephrol. 2009;30(2):99–111. doi: 10.1159/000203619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Matavelli LC, Zatz R, Siragy HM. A nonpeptide angiotensin II type 2 receptor agonist prevents renal inflammation in early diabetes. J Cardiovasc Pharmacol. 2015;65(4):371–376. doi: 10.1097/FJC.0000000000000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Koulis C, Chow BS, McKelvey M, Steckelings UM, Unger T, Thallas-Bonke V, Thomas MC, Cooper ME, Jandeleit-Dahm KA, Allen TJ. AT2R agonist, compound 21, is reno-protective against type 1 diabetic nephropathy. Hypertension. 2015;65(5):1073–1081. doi: 10.1161/HYPERTENSIONAHA.115.05204. [DOI] [PubMed] [Google Scholar]
- 77.Chang SY, Chen YW, Chenier I, Tran Sle M, Zhang SL. Angiotensin II type II receptor deficiency accelerates the development of nephropathy in type I diabetes via oxidative stress and ACE2. Exp Diabetes Res. 2011;2011:521076. doi: 10.1155/2011/521076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Antonipillai I, Nadler J, Vu EJ, Bughi S, Natarajan R, Horton R. A 12-lipoxygenase product, 12- hydroxyeicosatetraenoic acid, is increased in diabetics with incipient and early renal disease. J Clin Endocrinol Metab. 1996;81(5):1940–1945. doi: 10.1210/jcem.81.5.8626861. [DOI] [PubMed] [Google Scholar]
- 79.Abdel-Rahman EM, Abadir PM, Siragy HM. Regulation of renal 12(S)-hydroxyeicosatetraenoic acid in diabetes by angiotensin AT1 and AT2 receptors. Am J Physiol Regul Integr Comp Physiol. 2008;295(5):R1473–R1478. doi: 10.1152/ajpregu.90699.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Siragy HM, Jaffa AA, Margolius HS. Bradykinin B2 receptor modulates renal prostaglandin E2 and nitric oxide. Hypertension. 1997;29(3):757–762. doi: 10.1161/01.hyp.29.3.757. [DOI] [PubMed] [Google Scholar]