Abstract
Background
Cardiac device implantation can be complicated by inability to adequately place leads because of significant lead capture issues. This study sought to determine whether there are genetic bases that underlie poor lead capture.
Methods and Results
Retrospective review of all patients with structurally normal hearts who underwent new device implantation at Texas Children's Hospital between 2009 and 2014 was performed. Patients with inability to capture at 10 V or a final capture threshold ≥3 V at 0.4 ms during implant were analyzed. Among a total of 136 patients (median age, 13 years; range, 3 days to 46 years), 11 patients (8.1%) who underwent dual chamber device implantation had elevated thresholds in the atria (4), ventricle (3), or both chambers (4; atrial-lead threshold, 4.7±4.3 versus 0.7±0.3 V; ventricular-lead, 3.0±3.3 versus 0.7±0.3 V). All 11 patients presented with sinus node dysfunction and 10 had atrial arrhythmias. At implant, inability to find atrial capture was seen in 4 patients. Three demonstrated intermittent complete loss of ventricular capture after implantation: 1 has recurrent syncope, 2 eventually died. Genetic testing performed in 10 demonstrated 7 patients with 6 distinct SCN5A mutations, all predicted to be severe loss-of-function mutations by bioinformatic analyses. In the remaining patients, although putative pathogenic mutations were not found, multiple SCN5A polymorphisms were identified in 2 and a desmin mutation in 1.
Conclusions
This study suggests that significant capture issues at implant may be because of loss-of-function SCN5A mutations, providing new insights into SCN5A function. Recognition of this association may be critical for planning device implantation strategies and patient follow-up.
Keywords: atrial tachyarrhythmia with short PR; Brugada syndrome; cardiac pacemaker, artificial; implantable cardioverter-defibrillators; SCN5A protein, human; sick sinus syndrome; NaV1.5 voltage-gated sodium channel
Significant issues with pacemaker or implantable cardioverter defibrillator (ICD) lead placement can occur during device implantation because of an inability to obtain a reasonable lead capture threshold. Although infrequent, this complication has led to difficultly or even inability to place leads, resulting in compromises to patient care and potentially adverse outcomes. We noted that in some of these patients, capture thresholds undulated over time, leading us to wonder whether these alterations might be secondary to ion channel dysfunction. A previous study associated heterozygous mutations in SCN5A, which codes for the cardiac voltage-gated sodium channel (NaV1.5), with sick sinus syndrome and atrial inexcitability but never elaborated on capture issues.1 Later, our center was the first to report a patient with atrial arrhythmias found to have poor capture thresholds during lead placement.2 Genetic testing revealed that she harbored a homozygous loss-of-function mutation in SCN5A. With this in mind, the present study reviewed all patients with poor pacemaker capture at Texas Children's Hospital from 2009 to 2014 as well as all patients with known SCN5A mutations at our center to test the hypothesis that loss-of-function SCN5A mutations are associated with poor pacemaker capture.
Methods
A retrospective review of all patients at Texas Children's Hospital (Houston, TX) who underwent new pacemaker or ICD implantation between 2009 and 2014 was performed with approval from the internal review board. Dates were chosen in the current era where genetic testing was available and would have been performed at our institution. Patients with congenital heart disease and heart transplant were excluded. Patients with cardiomyopathy, including left ventricular noncompaction (LVNC), were included. Records were reviewed for significant difficulties with lead capture at the time of implant as reported by the implanting physician. Any patient with documented capture thresholds >10 V or a final capture threshold ≥3 V at a pulse width of 0.4–0.5 ms was included. Final capture thresholds reported in this study reflect the final threshold at implant. If multiple sites were attempted with inability to capture at 10 V and the final implant site had a capture threshold of 1.5 V, the final capture threshold was recorded as 1.5 V for the purposes of this study. If a lead was ultimately not placed because of inability to capture at 10 V, the threshold was recorded as 10 V. Lead sensing at implant was also recorded, although this was not the primary focus. Leads with sensing <0.3 mV were considered abnormal. Patients with lead sensing problems without capture issues were excluded. In addition, a retrospective review of all other patients with SCN5A mutations at Texas Children's Hospital was performed. Data collection included demographics, clinical history, review of electrocardiograms, genetic testing results, and device data including implant records.
Genetic Testing
After informed consent was obtained, the patients’ blood sample was sent to either Familion (New Haven, CT) or John Welsh Cardiovascular Diagnostic Laboratory (Houston, TX). For Familion, the genomic DNA isolated from blood was polymerase chain reaction amplified and used for direct sequencing of the complete open reading frame, splice junctions, and flanking regions of the genes of interest. For John Welsh Laboratory, the isolated genomic DNA was used for polymerase chain reaction amplification of the exons and splice junctions of the genes of interest before direct sequencing. Of the 10 patients with genetic testing, 2 were tested by Familion using their long-QT syndrome panel which included KCNQ1, KCNH2, SCN5A, KCNE1, and KCNE2, whereas 8 were tested by the John Welsh Laboratory. Of these 8 patients, 5 were tested using the atrioventricular block panel which included LMNA, DES, EMD, NKX2.5, SCN5A, and SCN1B; 1 was tested using the Brugada syndrome (BrS)/J-Wave Syndrome panel which included CACNA1C, CACNB2, GPD1L, KCND3, KCNE3, KCNJ8, HCN4, SCN1B, SCN2B, SCN3B, and SCN5A; and 2 were tested only for SCN5A.
Statistical Analysis
Data are presented as mean±SD.
Results
Patient Population
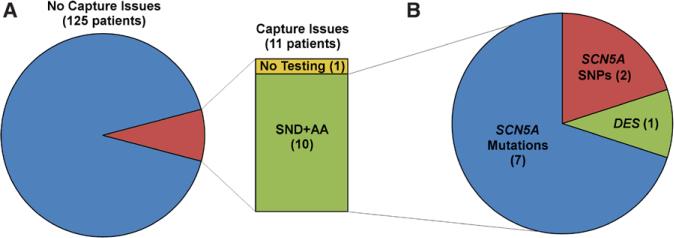
During the time period between 2009 and 2014, a total of 425 patients underwent a device-related procedure and 136 of these underwent new device implant (60 pacemaker, 76 ICD) at a median age of 13 years (range, 3 days to 46 years). New devices were implanted for the following primary reasons: congenital or surgical atrioventricular block (43), hypertrophic cardiomyopathy (29), long-QT syndrome (27), sinus node dysfunction (15), dilated cardiomyopathy (DCM; 8), BrS (4), LVNC (2), catecholaminergic polymorphic ventricular tachycardia (VT; 2), Becker cardiomyopathy (2), arrhythmogenic right ventricular dysplasia (1), and other (3). A total of 200 leads were placed (131 endocardial, 69 epicardial). Of the 136 patients, 11 had significant capture issues at implant (median age, 11 years; range, 3 days to 17 years) and met study criteria (Figure 1A). Five of the 11 patients were males and 8 were of Hispanic origin. Dual chamber pacemakers were attempted in 9 (6 epicardial, 3 transvenous) and dual chamber ICDs were placed in 2 (1 epicardial, 1 transvenous). At implantation, inadequate capture was seen in the atrium in 4 patients, ventricle in 3 patients, and in both atrium and ventricle in 4 patients (Table). Both mean atrial (4.7±4.3 versus 0.7±0.3 V) and ventricular thresholds (3.0±3.3 versus 0.7±0.3 V) at implant were higher when compared with those without reported capture issues. In addition, 8 of 11 (73%) patients had extremely low or essentially no sensing at implant (Table). Median follow-up from implant for the 11 patients was 16 months (range, 15 days to 5 years). There were 2 deaths at 15 days and 11 months after device implant.
Figure 1.
Retrospective review of all patients with new pacemaker or implantable cardioverter defibrillator implant from 2009 to 2014 at Texas Children's Hospital. A, Of these 136 patients, 11 had capture issues. Of these, 1 did not undergo genetic testing, whereas the remaining 10 exhibited sinus node dysfunction (SND) and atrial arrhythmia (AA). B, Genetic testing of these 10 patients revealed that 7 of them (70%) harbored SCN5A mutations, 2 had multiple SCN5A single-nucleotide polymorphisms (SNPs), and 1 had a variant of unknown significant in the DES gene, which codes for desmin.
Table.
Eleven Patients With Poor Pacemaker Capture of 136 Reviewed at Texas Children's Hospital
| Case | Sex | Age at Implant in Years and Device |
Phenotype | Sensing Issue |
Capture Issue |
SCN5A SNP or Mutation | NaV1.5* | PloyPhen-2 |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 4.7 PM | SND, atrial flutter; alive | A | A+V | G to A substitution at the donor splice site of intron 5 (IVS5+1G>A) | Truncation to 204 aa | N/A |
| 2 | M | Newborn PM | SND, AT, AVB, LVNC, VT, loss of V capture; alive | ... | V | Nucleotide substitution (5227G>A) | G1743R (trafficking defect) | Probably damaging |
| 3 | M | 1.6 PM | SND, atrial flutter/fibrillation, VT, loss of V capture; died | A+V | A+V | Nucleotide substitution (4534C>T) | R1512W (impaired fast inactivation) | Probably damaging |
| 4 | M | 15.6 PM | SND, atrial flutter; alive | A | A | Nucleotide substitution (3823G>A) | D1275N (reduced INa) | Probably damaging |
| 5 | F | 11.8 ICD | SND, atrial flutter/fibrillation, VT; alive | A | A+V | Nucleotide substitution (5770G>A)† | A1924T (impaired activation) | Possibly damaging or benign |
| 6 | F | 12.3 ICD | SND, AT; alive | V | A+V | Nucleotide substitution (5770G>A)† | A1924T (impaired activation) | Possibly damaging or benign |
| 7 | F | 5.9 PM | SND, atrial flutter; alive | ... | V | Deletion of C at position 100 in exon 2 (100delC) | Truncation to 95 aa | N/A |
| ... | F | 1.6 PM | LVNC, LQT, AT, VT, loss of V capture; died | ... | A | SNPs: 1673A>G, 100C>T | N/A | N/A |
| ... | M | 17.2 PM | Sinus pauses, intermittent AVB, A flutter; alive | A | A | SNPs: 87A>G, IVS5-86G>A, IVS27_28ins28, 3183A>G, IVS21+73G>A, 5457T>C, 5844C>T | N/A | N/A |
| ... | M | 12.5 PM | SND, AT; alive | A | A | None | N/A | N/A |
| ... | F | 6.8 PM | SND, AVB; alive | V | V | (No genetic testing) | N/A | N/A |
A indicates atrial; AT, atrial tachycardia; AVB, atrioventricular block; ICD, implantable cardioverter defibrillator; LQT, long QT; LVNC, left ventricular noncompaction; N/A, not applicable; PM, pacemaker; SND, sinus node dysfunction; SNP, single-nucleotide polymorphism; V, ventricular; and VT, ventricular tachycardia.
Full length NaV1.5 is 2016 amino acids (aa) long.
Homozygous.
Genetic Analyses
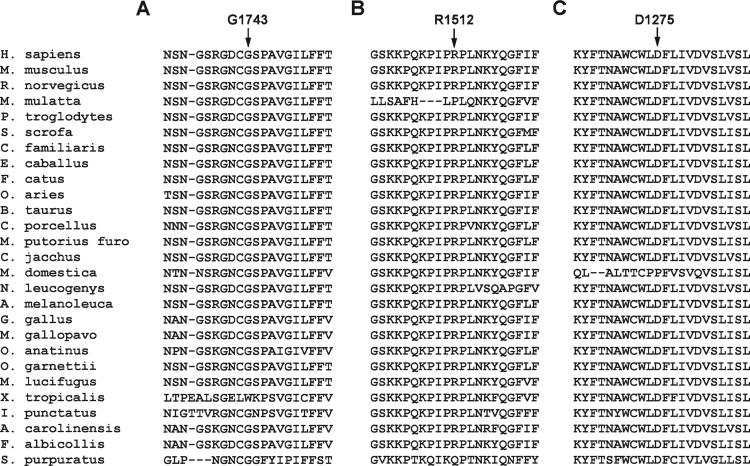
Genetic testing was performed in 10 of the 11 patients with capture issues; results were not available before device implantation in any of the patients. Among the 10 patients with genetic testing, 6 distinct class I SCN5A mutations (including 1 novel mutation) were found in 7 patients (70%; Table). In 2 patients, in whom no putative mutations were found, multiple single nucleotide polymorphisms in SCN5A were reported (Table). In the last patient, no SCN5A mutations or single nucleotide polymorphisms were identified; however, a variant of unknown significance in DES, which encodes desmin, was seen (Figure 1B). The cohort of 7 patients with putative class I SCN5A mutations consisted of 4 females, 2 of whom are identical twins, and 3 males, with age of presentation ranging from newborn to 15 years. Six of the 7 patients were Hispanic. Mis-sense mutations were analyzed in terms of conservation across species (Figure 2) as well as using PolyPhen-2, which predicts the severity of the resultant amino acid substitution (Table).3
Figure 2.
Conservation of the amino acids that are mutated in patients from case 3 (A), case 4 (B), and case 5 (C). Left, Lists the species; arrows point to the amino acid and position that are mutated, respectively, in cases 3, 4, and 5.
Clinical Features
Among these 11 patients, 1 patient did not undergo genetic testing. This patient had sinus node dysfunction and complete heart block with poor sensing and capture in the ventricle necessitating placement of a left ventricular epicardial lead (Figure 1A). In the remaining 10 patients, all of whom were genetically tested, 9 had isolated cardiac issues, whereas one had multiple medical issues including developmental delay, seizure disorder, prolonged QT (with negative genetic testing), and DCM. Echocardiography demonstrated structurally normal hearts with normal chamber size and normal biventricular function in 9 patients. Two patients (including one with multiple medical problems) demonstrated LVNC (Table).
Despite these individual differences, all 11 patients demonstrated sinus node dysfunction, 10 of whom had documented atrial arrhythmias including 4 with atrial flutter, 2 with atrial flutter/atrial fibrillation, and 4 with ectopic atrial tachycardia (Figure 1A). The single patient without atrial arrhythmia was also the one without genetic testing. However, that patient did have multiple atrial high rate episodes at 225 bpm (higher than expected sinus tachycardia rates) at times consistent with sleep although definitive atrial tachycardia was not documented by electrograms. At implant, there was complete quiescence of atrial activity and inability to find atrial capture in 4 patients. In 2 patients, an epicardial atrial lead could not be placed because of inadequate capture threshold and therefore an atriotomy was performed and the lead inserted into the endocardium via an epicardial approach through the right atrial appendage. Three patients demonstrated intermittent complete loss of ventricular capture after implantation, 2 of whom eventually died. The remaining patient has a history of undulating capture thresholds with recurrent syncope because of intermittent loss of ventricular capture during times of illness and is currently undergoing evaluation for possible heart transplantation.
Inclusion Analysis of All Patients With SCN5A Mutations
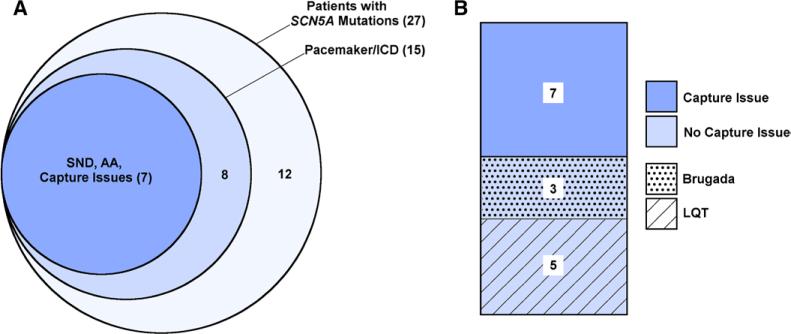
Because of the high incidence of SCN5A mutations among patients with poor pacemaker capture (7 of 10 tested), all patients with known SCN5A mutations at Texas Children's Hospital were reviewed to determine how many patients with known SCN5A mutations also had pacemaker capture issues. There were a total of 27 patients at our institution with 20 distinct SCN5A mutations (Figure 3A, large circle). Of these 27 patients, 15 underwent placement of a pacemaker or ICD. Seven of these 15 patients demonstrated pacemaker capture issues and are included in our cohort of 11 patients. The remaining 8 patients did not exhibit sinus node dysfunction, atrial arrhythmias, or pacemaker capture issues (Figure 3A, medium circle). In other words, 26% of all patients with confirmed SCN5A mutations at our institution had capture issues (7 of 27; Figure 3A, small circle).
Figure 3.
Retrospective review of all patients with confirmed SCN5A mutations at Texas Children's Hospital. A, The large circle represents the 27 patients with SCN5A mutations. Of these, 15 patients had either a pacemaker or implantable cardioverter defibrillator (ICD; medium circle). Of the 15 patients, 7 had sinus node dysfunction (SND), atrial arrhythmia (AA), and pacemaker capture issues (small circle). B, Of the 8 patients with SCN5A mutation without capture issues, 3 had Brugada and 5 had LQT syndrome.
Of the 7 patients with SCN5A mutations and pacemaker capture issues, only 1 had a clinical diagnosis of BrS; however, during review of all ECGs for this study, in one other patient, a single ECG (among many performed) demonstrated a type I Brugada pattern. The other 5 patients demonstrated nonspecific conduction delays and ST changes without definitive Brugada changes. None of these patients underwent provocative drug testing to evaluate for BrS. In contrast, of the 8 patients with SCN5A mutations without capture issues, 3 had a definitive clinical diagnosis of BrS and 5 had a diagnosis of long QT syndrome (Figure 3B). Bioinformatic analysis using PolyPhen-2 of the SCN5A mutations in these patients could not distinguish between patients with and without capture issues.3 The 7 cases with definitive SCN5A mutations and poor capture are described below as short vignettes.
Case 1
A 4-year-old Hispanic female initially presented with sinus node dysfunction. During placement of a dual-chamber epicardial pacemaker, an atrial lead could not be placed because of inability to capture despite multiple sites including both right and left atrium at maximal outputs. A single epicardial ventricular lead with an implant pacing threshold of 8.6 V was placed. Her ventricular threshold improved with time, remaining elevated but stable (2.75 V at 0.4 ms). During the ensuing 4 years, she has been admitted multiple times for atrial flutter requiring direct current cardioversion. She has remained clinically stable for >1 year on metoprolol and warfarin.
Case 2
A newborn Hispanic male was initially diagnosed with ventricular ectopy, atrioventricular block, and LVNC with depressed function. Three days after birth, he underwent placement of an uncomplicated epicardial, dual-chamber pacemaker with normalization of systolic function. At the age of 2 years, he had several episodes of syncope and seizures associated with a viral illness, as well as runs of atrial tachycardia. Pacemaker interrogation showed complete noncapture by the ventricular lead. During pacemaker revision, an adequate epicardial ventricular pacing site could not be found, until an area was finally located after 2 hours in the left ventricle with a final threshold of 1.4 V at 1 ms. The existing atrial lead was left untouched with a threshold of 1.25 V at 0.52 ms. A few days later, the ventricular threshold increased to 2.25 V at 1 ms and has since fluctuated with thresholds >5 V at times. The thresholds wax and wane with highest thresholds occurring during times of acute illness. Recently, he had another episode of syncope associated with mild viral symptoms and his ventricular lead demonstrated complete lack of capture at maximal output. During admission, he demonstrated episodes of nonsustained atrial and VT for which he was treated with amiodarone and metoprolol. With resolution of his viral illness, all arrhythmias quiesced and his myocardial capture returned.
Case 3
A 20-month-old Hispanic boy initially presented with an unresponsive episode after developing a fever. Work-up revealed atrial flutter/fibrillation with variable conduction, sinus bradycardia with a junctional escape, and sinus pauses up to 4 seconds. Because of a single ECG noted to demonstrate type I Brugada changes he was diagnosed with BrS. During epicardial lead placement, poor sensing and capture of both the right atrium and right ventricle were encountered resulting in the need to convert a subxiphoid incision into a full sternotomy. After much trial, the ventricular lead was placed on the left ventricular apex. The atrial lead was secured into the right atrial endocardium through a right atrial appendage incision. Both leads demonstrated only intermittent capture at full 10 V output.
Postoperatively, he experienced an acute and extensive thrombotic stroke. Four days later, the patient had multiple episodes of pacemaker capture loss and hypotension. Despite maximal pacemaker output, in a nonacidotic, nonhypokalemic state, intermittent loss of capture persisted. Because of brady-cardia and low cardiac output with multiorgan system failure and evidence of irreversible neurological injury, support was withdrawn.
Case 4
A 15-year-old Hispanic male presented with atrial flutter requiring direct current cardioversion. He was subsequently found to have sinus node dysfunction with multiple sinus pauses up to 4.5 seconds. Intracardiac mapping during an electrophysiology study found significantly decreased atrial voltages, inability to capture portions of atrial tissue even at maximum output, and intra-atrial and interatrial conduction delays. During subsequent placement of a transvenous dual-chamber pacemaker, the atrial lead had to be placed along the low atrial septum to achieve capture. The ventricular lead was placed without issues.
Case 5
As previously reported,2 a 6-year-old Hispanic female initially presented with sinus bradycardia and frequent sinus pauses but was lost to follow-up for 5 years. At the age of 11 years, she presented with recurrent sustained VT after an aborted sudden cardiac death, requiring extracorporeal membrane oxygenation support for severely depressed biventricular function. Because her arrhythmia was refractory to medications, she underwent placement of a pacemaker/defibrillator. Temporary atrial leads could not be placed because of inability to capture at full 10 V output. Temporary ventricular leads paced intermittently at full 10 V output. The following day, a dual-chamber epicardial ICD was attempted. Once again, despite multiple attempts to place an atrial lead, the atria were entirely quiescent and atrial capture could not be achieved. Nevertheless, an epicardial lead was placed in case capture could be later achieved. Similarly for the ventricular lead, only intermittent ventricular capture was obtained despite attempts at multiple sites. Over time, the patient's ventricular function improved and her epicardial system demonstrated signifi-cant improvement with her most recent atrial and ventricular capture thresholds 0.75 V at 0.6 ms and 1.5 V at 0.6 ms, respectively, at the latest interrogation. She has demonstrated nonsustained atrial tachycardia on pacemaker interrogation and a single ECG was found to demonstrate a type I Brugada pattern. This patient has an identical twin sister described in the next case.
Case 6
Like her identical twin from case 6, this patient at the age of 12 years presented with sinus pauses of ≤5 seconds associated with lightheadedness and dizziness.2 Given her sinus node dysfunction and symptoms as well as her family history, a dual chamber transvenous ICD was implanted for primary prevention. Atrial and ventricular capture thresholds at implant were poor and variable. Pacemaker interrogation has revealed episodes of atrial tachycardia. Subsequent genetic testing confirmed the same A1924T homozygous mutation in SCN5A.2
Case 7
A 5-year-old Caucasian female presented with near syncope in sustained atrial tachycardia. She underwent successful direct current cardioversion and subsequent Holter monitoring demonstrated episodes of atrial flutter, sinus node dys-function, and sinus pauses. Atrial and ventricular thresholds shortly after implant of a dual chamber pacemaker were 2 V at 0.09 ms and 3 V at 0.15 ms, respectively, and have remained persistently high in the succeeding months although occasionally the thresholds have improved to 1 V at 0.15 ms and 2 V at 0.27 ms, respectively.
Mutation Analyses
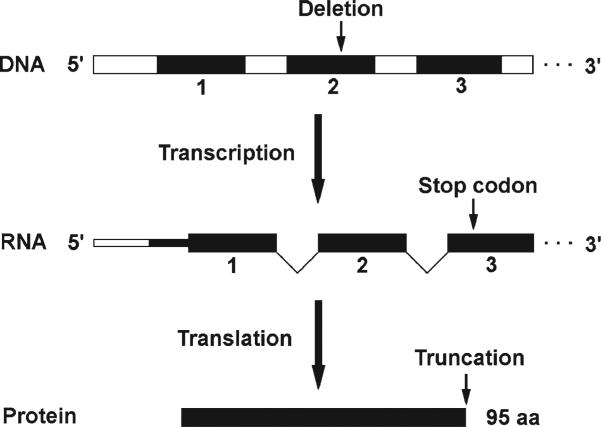
A novel SCN5A mutation was discovered in patient 7 of our cohort and is predicted to result in a truncated NaV1.5 (Figure 4). This mutation is a deletion of nucleotide number 100 (100delC) in exon 2 of SCN5A (counting from the start codon). This leads to a premature translation stop codon corresponding to the 63rd amino acid after the deletion (R34Afs*63), which would lead to a protein product of only 95 amino acids long (Figure 4), with the following sequence: MANFLLPRGTSSFRRFTRESLAAIEKRMAEKQAAAQPPCRRAERGCPRRRLPGPSWTCRPPKSCQISMAIHPKSSSESPWRTWTPSIAPKRLSSY.
Figure 4.
Novel SCN5A mutation and its predicted effect. This mutation is a single nucleotide deletion at position 100 of SCN5A exon 2 (100delC), which is predicted to lead to a frame-shift of the downstream message. This introduces a premature stop codon resulting in a truncated protein product of 95 amino acids (aa) long. The wild-type product (NaV1.5) is 2016 aa long. Black boxes, exons; white boxes, introns.
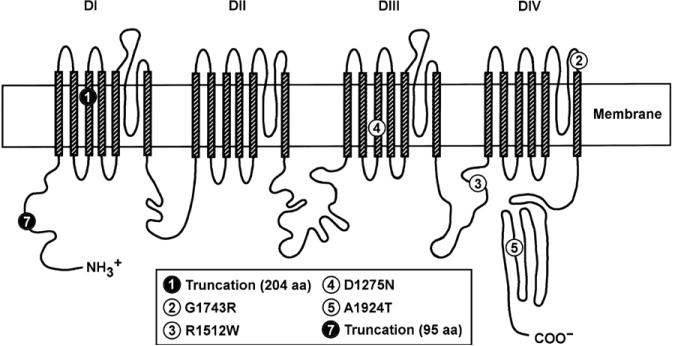
Thus, in our cohort, both cases 1 and 7 harbor mutations that predict a severely truncated NaV1.5 which would most likely undergo nonsense-mediated decay, leaving the patients with at most 50% functional NaV 1.5 (Figures 3 and 5; Table).4
Figure 5.
Schematic of NaV1.5 encoded by SCN5A and the mutations in this study. The filled circles refer to mutations leading to NaV1.5 truncations, whereas the open circles refer to missense mutations. The numbers inside the circles correspond to the case numbers, except for 5, which refers to both cases 5 and 6 because they are identical. Adapted from Splawski et al4 with permission of the publisher. Copyright © 2000, Wolters Kluwer Health, Inc. Authorization for this adaptation has been obtained both from the owner of the copyright in the original work and from the owner of copyright in the translation or adaptation. DI–IV indicates domains 1 to 4.
Case 2 harbors a NaV1.5 mutation, G1743R, which is predicted to completely abolish its trafficking to the cell membrane based on in vitro studies in HEK293 cells.5 The same study also reports that this trafficking defect may be partially rescued with mexiletine or quinidine treatment, although the underlying mechanism was not explored.5 Therefore, case 2 is predicted to have only 50% of functional NaV1.5, similar to cases 1 and 7 (Table).
Case 3 harbors the R1512W mutation which based on an earlier in vitro study demonstrated a left-shift in both activation and inactivation curves at room temperature.6 Although that study argues that the R1512W mutation would lead to an overall increase in INa against conventional wisdom, a more likely explanation (as conceded by the authors in their discussion) is that because of the left shift, some NaV1.5 channels are already activated and then inactivated during resting membrane potential. Therefore, when the cell is depolarized, these channels will not be available to fire, leading to a decreased INa and blunting of the action potential upstroke. Furthermore, this left shift may be even more pronounced at body temperature (37°C) because this study was conducted at room temperature (22°C).6 In other words, the R1512W mutation may also severely compromise NaV1.5 function although further studies, especially in vivo, are needed to rectify the current controversial findings.
The D1275N mutation in case 4 was initially reported in a Dutch family who presented with atrial standstill but was subsequently associated with a range of cardiac diseases including DCM, sinus node dysfunction, atrial and ventricular tachyarrhythmias, conduction disease, and ventricular enlargement without impaired contractility.7–10 However, several in vitro studies that compared wild-type with D1275N channels in heterologous expression systems failed to demonstrate major differences in the biophysical characteristics of the D1275N mutant channel.7,11 To resolve these apparently contradictory findings, a recent study generated a mouse model expressing the human D1275N mutant channel and showed that not only did the animals develop slowed conduction, heart block, atrial fibrillation, VT, and a DCM phenotype, cardiomyocytes isolated from these animals showed a significantly depressed INa, when compared with mice expressing the wild-type human channel.12 This study underscores the importance of not over-interpreting in vitro studies and the need to use multiple model systems especially higher organisms, when trying to understand how a particular mutation may affect a patient in vivo. With this study in mind, the D1275N mutation in case 4 most likely causes a severe decrease in NaV1.5, perhaps not dissimilar in magnitude to the mutations in cases 1 to 3 (Table).
The final mutation, A1924T, found in the twins of cases 5 and 6, was previously characterized in vitro. When expressed in Xenopus oocytes, the A1924T NaV1.5 mutant showed a 9-mV negative voltage shift of the steady-state activation curve at room temperature.6 This could potential lead to a decrease in INa because at resting membrane potential a greater fraction of NaV1.5 would already be opened and therefore unavailable when the myocyte is actually depolarized. Given that our patients are homozygous for this mutation, this could lead to an even greater decrease in total INa during phase 0 of the cardiac action potential, and thereby a more severe phenotype.
Discussion
In this study, we identified a cohort of patients with poor pacemaker capture at implant. We found that all demonstrated sinus node dysfunction and nearly all of them had clinically significant atrial arrhythmias (Figure 1; Table). A majority of these patients demonstrated loss-of-function SCN5A mutations or multiple SCN5A polymorphisms (Figure 5; Table).
Among known mutations of key ion channel genes, mutations in SCN5A are unique in that they have been implicated in a multitude of cardiac diseases including ones with dissimilar electrophysiological profiles.13 For examples, gain-of-function SCN5A mutations may cause long QT syndrome type 3, whereas loss-of-function mutations may cause conduction disease, BrS, and sick sinus syndrome.1,13,14 To complicate matters further, the same SCN5A mutation may also present differently even within the same family, for example, with features of long QT syndrome, BrS, and conduction disease, in a phenomenon now referred to as overlap syndromes.15 Finally, common benign variants or single nucleotide polymorphisms can also potentially modulate phenotype severity by affecting both the biophysical properties of NaV1.5 or its expression level.13
A secondary review of all SCN5A patients in our institution did not demonstrate poor pacemaker capture among patients with definitive clinical diagnosis of BrS or long QT syndrome (Figure 3). These findings raise the question whether patients with sinus node dysfunction, atrial arrhythmias, and poor pacemaker capture represent a unique clinical phenotype, early BrS, or whether they are part of this continuum in the spectrum of SCN5A overlap syndromes.13,15
In our cohort, pacemaker capture difficulties were seen in both the atrium and the ventricle. Although we acknowledge the possibility that atrial capture issues may not be recognized in patients with SCN5A mutations who undergo a single chamber ICD, interestingly there have been no reported cases of inability to capture the ventricle among patients with SCN5A mutations. Similarly, Conte et al16 reported that 7.5% of their children with drug-induced Brugada changes had sinus node dysfunction; however, they did not comment on whether their patients had any complications during ICD implant.
A clinically important observation from the present study is that the pacemaker capture threshold seems to be highest during acute episodes of illness, which is often at the time of implantation. However, once the patients recover, capture thresholds tend to improve but may fluctuate over time. Although the mechanism by which fever elicits Brugada ECG changes is not clear, fever and elevated temperatures are thought to inactivate NaV1.5 channels.15 Acute increases in metabolic demand secondary to illness may result in alterations within the cardiac milieu such that cardiomyocytes require full functioning of all available NaV1.5 channels to remain excitable and conductive. Because our patients have a decreased reserve of functional NaV1.5 channels secondary to their genetic mutations, cardiomyocyte (especially atrial myocyte) NaV1.5 channel dysfunction may become prominent in the setting of fever, acute stress, or metabolic demand. At implant, capture thresholds are tested by pacing the heart rates above baseline sinus rhythm. We speculate whether pacing at heart rates above baseline may result in the inability to depolarize a critical threshold of atrial myocytes and thus result in loss of pacemaker capture. Whether pacing at slower heart rates during implant would result in pacemaker capture has not been tested.
This study also underscores the importance of planning before pacemaker implantation in this patient population. If capture thresholds are unacceptably high, we recommend making all efforts to try several different pacing sites. As in many of our cases, we were eventually able to achieve a successful site for pacing, although this may have required multiple attempts. Ultimately if no adequate location is found, we suggest lead implantation be considered even if pacing thresholds are unacceptable, because thresholds may subsequently normalize once the patient improves clinically (as illustrated by case 5). A dilemma arises when considering the possibility of complete loss of ventricular capture as was seen in 3 of the patients, which were not secondary to significant hyperkalemia and acidosis based on clinical history. In case 3, inability to capture and low cardiac output contributed to multi-system organ failure. In the surviving patient (case 2), syncope and seizures as a result of complete loss of ventricular capture have resulted in consideration for possible heart transplant. At this time he is being managed by weekly threshold interrogation and inpatient admission for any illness. The remaining patient with intermittent loss of ventricular capture had multiple medical problems including DCM. A hospital admission for respiratory distress and seizures was notable for VT and bradycardia with heart rates dropping to the 30s suggesting loss of ventricular capture (lower rate limit of pacemaker was 70 bpm). Unfortunately, documentation of bradycardia was not available to confirm loss of pacemaker capture. Interrogation of the pacemaker the following day demonstrated appropriate capture thresholds. She was ultimately discharged home. Four days later she died in her sleep. Although we cannot confirm the cause of death, this case highlights the fact that at a minimum, close follow-up and monitoring may be necessary in these cases, particularly during times of acute illness. If the clinical phenotype becomes more malignant and unpredictable, more aggressive approaches, including evaluation for transplant, may need to be considered.
Limitations
This is a single-center retrospective study of patients who underwent device implantation and with known SCN5A mutations. Genetic testing was not sent on all patients who underwent device implantation and the testing was not uniform across patients. Therefore, we cannot exclude the possibility of additional mutations that have not been discovered.
Conclusions and Significance
In this study, we identified an association between sinus node dysfunction, atrial arrhythmias, elevated or fluctuating poor pacemaker capture, and SCN5A mutations (5 heterozygous and 1 homozygous) that are predicted to cause severely compromised NaV1.5 function. Our findings underscore the importance of maintaining a high index of suspicion for genetic mutations when working up patients with complex arrhythmias or poor pacemaker capture, especially when they exhibit sinus node dysfunction and atrial arrhythmias. Whether these findings represent a unique clinical manifestation of SCN5A mutations or are part of the continuum of the Brugada phenotype and overlap syndrome warrants further investigation. Recognition of this association can have important implications for device implantation strategies to improve clinical outcomes.
WHAT IS KNOWN
Significant issues with pacemaker or implantable cardioverter defibrillator lead placement may complicate device implantation because of an inability to obtain a reasonable lead capture threshold.
Mutations in SCN5A underlie a multitude of cardiac diseases including long-QT syndrome type 3, Brugada syndrome, and sick sinus syndrome.
WHAT THE STUDY ADDS
Further insight into the function of SCN5A: SCN5A loss-of-function may result in signifi-cant capture issues at implant in addition to sinus node dysfunction and atrial arrhythmias.
Recognition of this clinical entity may help cardiac electrophysiologists understand the spectrum of SCN5A-related diseases, guide potential genetic screening, and better plan device implantation and patient follow-up strategies.
This new understanding of SCN5A function may also fuel future research into novel therapeutics to improve lead capture and safety in patients with loss-of-function SCN5A mutations.
Acknowledgments
Sources of Funding
Dr Chiang is supported by the Baylor College of Medicine Medical Scientist Training Program Caskey Scholarship. Dr Wehrens is supported by the National Institutes of Health grant R01-HL089598 and R01-HL117641 and the American Heart Association grant 13EIA14560061.
Footnotes
Disclosures
None.
References
- 1.Benson DW, Wang DW, Dyment M, Knilans TK, Fish FA, Strieper MJ, Rhodes TH, George AL., Jr Congenital sick sinus syndrome caused by recessive mutations in the cardiac sodium channel gene (SCN5A). J Clin Invest. 2003;112:1019–1028. doi: 10.1172/JCI18062. doi: 10.1172/JCI18062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lopez KN, Decker JA, Friedman RA, Kim JJ. Homozygous mutation in SCN5A associated with atrial quiescence, recalcitrant arrhythmias, and poor capture thresholds. Heart Rhythm. 2011;8:471–473. doi: 10.1016/j.hrthm.2010.10.014. doi: 10.1016/j.hrthm.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 3.Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7:248–249. doi: 10.1038/nmeth0410-248. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Splawski I, Shen J, Timothy KW, Lehmann MH, Priori S, Robinson JL, Moss AJ, Schwartz PJ, Towbin JA, Vincent GM, Keating MT. Spectrum of mutations in long-QT syndrome genes. KVLQT1, HERG, SCN5A, KCNE1, and KCNE2. Circulation. 2000;102:1178–1185. doi: 10.1161/01.cir.102.10.1178. [DOI] [PubMed] [Google Scholar]
- 5.Valdivia CR, Tester DJ, Rok BA, Porter CB, Munger TM, Jahangir A, Makielski JC, Ackerman MJ. A trafficking defective, Brugada syndrome-causing SCN5A mutation rescued by drugs. Cardiovasc Res. 2004;62:53–62. doi: 10.1016/j.cardiores.2004.01.022. doi: 10.1016/j.cardiores.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 6.Rook MB, Bezzina Alshinawi C, Groenewegen WA, van Gelder IC, van Ginneken AC, Jongsma HJ, Mannens MM, Wilde AA. Human SCN5A gene mutations alter cardiac sodium channel kinetics and are associated with the Brugada syndrome. Cardiovasc Res. 1999;44:507–517. doi: 10.1016/s0008-6363(99)00350-8. [DOI] [PubMed] [Google Scholar]
- 7.Groenewegen WA, Firouzi M, Bezzina CR, Vliex S, van Langen IM, Sandkuijl L, Smits JP, Hulsbeek M, Rook MB, Jongsma HJ, Wilde AA. A cardiac sodium channel mutation cosegregates with a rare connexin40 genotype in familial atrial standstill. Circ Res. 2003;92:14–22. doi: 10.1161/01.res.0000050585.07097.d7. [DOI] [PubMed] [Google Scholar]
- 8.McNair WP, Ku L, Taylor MR, Fain PR, Dao D, Wolfel E, Mestroni L, Familial Cardiomyopathy Registry Research Group SCN5A mutation associated with dilated cardiomyopathy, conduction disorder, and arrhythmia. Circulation. 2004;110:2163–2167. doi: 10.1161/01.CIR.0000144458.58660.BB. doi: 10.1161/01.CIR.0000144458.58660.BB. [DOI] [PubMed] [Google Scholar]
- 9.Olson TM, Michels VV, Ballew JD, Reyna SP, Karst ML, Herron KJ, Horton SC, Rodeheffer RJ, Anderson JL. Sodium channel mutations and susceptibility to heart failure and atrial fibrillation. JAMA. 2005;293:447–454. doi: 10.1001/jama.293.4.447. doi: 10.1001/jama.293.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laitinen-Forsblom PJ, Mäkynen P, Mäkynen H, Yli-Mäyry S, Virtanen V, Kontula K, Aalto-Setälä K. SCN5A mutation associated with cardiac conduction defect and atrial arrhythmias. J Cardiovasc Electrophysiol. 2006;17:480–485. doi: 10.1111/j.1540-8167.2006.00411.x. doi: 10.1111/j.1540-8167.2006.00411.x. [DOI] [PubMed] [Google Scholar]
- 11.Gui J, Wang T, Jones RP, Trump D, Zimmer T, Lei M. Multiple loss-of-function mechanisms contribute to SCN5A-related familial sick sinus syndrome. PLoS One. 2010;5:e10985. doi: 10.1371/journal.pone.0010985. doi: 10.1371/journal.pone.0010985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watanabe H, Yang T, Stroud DM, Lowe JS, Harris L, Atack TC, Wang DW, Hipkens SB, Leake B, Hall L, Kupershmidt S, Chopra N, Magnuson MA, Tanabe N, Knollmann BC, George AL, Jr, Roden DM. Striking In vivo phenotype of a disease-associated human SCN5A mutation producing minimal changes in vitro. Circulation. 2011;124:1001–1011. doi: 10.1161/CIRCULATIONAHA.110.987248. doi: 10.1161/CIRCULATIONAHA.110.987248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barc J, Bezzina CR. Role of rare and common genetic variation in SCN5A in cardiac electrical function and arrhythmia. In: Abriel H, editor. Cardiac Sodium Channel Disorders. Elsevier; Philadelphia, PA: 2014. pp. 665–677. [Google Scholar]
- 14.Meregalli PG, Tan HL, Probst V, Koopmann TT, Tanck MW, Bhuiyan ZA, Sacher F, Kyndt F, Schott JJ, Albuisson J, Mabo P, Bezzina CR, Le Marec H, Wilde AA. Type of SCN5A mutation determines clinical severity and degree of conduction slowing in loss-of-function sodium channelopathies. Heart Rhythm. 2009;6:341–348. doi: 10.1016/j.hrthm.2008.11.009. doi: 10.1016/j.hrthm.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 15.Remme CA. Cardiac sodium channel overlap syndrome. In: Abriel H, editor. Cardiac Sodium Channel Disorders. Elsevier; Philadelphia, PA: 2014. pp. 761–776. [Google Scholar]
- 16.Conte G, Dewals W, Sieira J, de Asmundis C, Ciconte G, Chierchia GB, Di Giovanni G, Baltogiannis G, Saitoh Y, Levinstein M, La Meir M, Wellens F, Pappaert G, Brugada P. Drug-induced Brugada syndrome in children: clinical features, device-based management, and long-term follow-up. J Am Coll Cardiol. 2014;63:2272–2279. doi: 10.1016/j.jacc.2014.02.574. doi: 10.1016/j.jacc.2014.02.574. [DOI] [PubMed] [Google Scholar]