Abstract
A 77 year-old male is admitted to the hospital after sustaining a hip fracture. He has a past medical history of chronic obstructive pulmonary disease, hypertension, hyperlipidemia, chronic back pain, and hearing loss. Prior to surgery he receives midazolam for agitation and morphine for pain control. He undergoes a general anesthetic for his fracture repair, requiring high doses of fentanyl for pain control. Postoperatively, he has poor pulmonary mechanics and is taken to the ICU intubated and mechanically ventilated. On postoperative day one, his sedation is weaned and he is put on a spontaneous breathing trial. While he appears intermittently awake, he will not follow commands and only intermittently makes eye contact. The patient is left intubated due to his altered mental status.
Delirium is a common problem in critically ill patients but has only recently been recognized as a serious entity associated with important clinical outcomes, including increased days on mechanical ventilation, length of hospital stay, cost of care, long-term cognitive impairment, requirement for post-discharge institutionalization, and mortality.1-3 Validated delirium screening tools for ICU patients, which can used by a wide range of personnel, have improved diagnosis, and routine delirium assessment is now recommended as standard of care in the ICU. Furthermore, potential pharmacologic (e.g., antipsychotics, dexmedetomidine) and non-pharmacologic (e.g., early physical therapy, sleep hygiene) prevention and treatment strategies have been studied to reduce delirium and improve its associated outcomes with varying results. This review will explore the risk factors for ICU delirium, tools for its diagnosis, preventative strategies, and its potential treatments. This information can be utilized throughout the patient's hospitalization, including in the perioperative environment, and can be practiced by anesthesiologists as well as intensivists to improve patient care.
Characterizing Delirium
The term ‘delirium’ is frequently used across clinical settings to describe patients with altered mental status, but its proper diagnosis requires specific manifestations to be present. Delirium is defined in The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5)4 as a reduced ability to direct, focus, sustain, and shift attention. This is coupled with a change in cognition, either in the form of memory deficit, disorientation, or perceptual disturbances. Importantly, the inattention and change in cognition cannot be accounted for by a baseline neurocognitive disorder (e.g., dementia) or a severely reduced level of arousal (e.g., sedative administration, coma). The disturbance in mental status must be an acute change from baseline and fluctuate throughout the day but may occur in addition to baseline disease (e.g., delirium superimposed on dementia, delirium after stroke). Delirium diagnosis identifies the constellation of altered brain function signs but does not identify the etiology. It should, therefore, prompt further investigation into potential patient vulnerability factors and precipitating factors associated with the current illness or hospital course.
Incidence
The incidence of delirium varies widely depending on the patient population examined and the method of diagnosis (e.g., psychiatric evaluation versus nurse screening tool). It has been reported to occur in 16-89% of hospitalized patients, including in up to 45% of post-anesthesia care unit patients and 50% of postoperative patients on the ward.5,6 The incidence, however, seems to be the highest in the ICU, with up to 80% of mechanically ventilated patients having delirium.7,8 Delirium can present with three motor subtypes – hyperactive, hypoactive, and mixed – that may carry different prognoses.9,10 The two most common types of delirium, as studied in a medical ICU, were mixed at 54.9% of delirious patients and hypoactive at 43.5%.11 Hypoactive delirium is characterized by slowed mentation, lethargy, and decreased movement. It is found more commonly in the elderly, with age >65 years being an independent risk factor.11 It typically requires active screening with delirium assessment tools to diagnose since it is often less clinically apparent than the restlessness and agitated behavior of hyperactive delirium. In a study of patients admitted to the ICU postoperatively after elective procedures, patients that suffered from hypoactive delirium had increased 6-month mortality compared to the patients with other subtypes of delirium (32.0% vs. 8.7%, P=0.04).12
Risk factors
Known risk factors for developing delirium are numerous and commonly separated into factors that predispose a patient to delirium and others that precipitate the development of delirium (Table 1). Advanced age and baseline cognitive impairment have been consistently found to increase delirium risk across a variety of hospital settings.13 Similarly, patients with increased comorbid disease burden (especially respiratory disease)14 and frailty15 appear to be at higher risk. Thus, patients with lower cognitive and physical reserve likely possess decreased capacity to maintain normal brain functioning in response to stress (e.g., surgery, critical illness) and are, therefore, at higher risk for delirium.16,17 Similarly, a more significant systemic insult such as sepsis, prolonged mechanical ventilation, or major surgery (in particular complex abdominal, hip fracture, and cardiac surgery), will increase risk of delirium compared to a lesser physiologic insult.13 Increased pain levels have repeatedly been shown to increase delirium, especially in the postoperative setting, potentially due to heightened stress response and altered neurotransmission.18,19
Table 1. Delirium Risk Factors.
| Predisposing Factors | Precipitating Factors | |
|---|---|---|
| Advanced age | Metabolic disturbances | Benzodiazepines |
| Baseline cognitive impairment | Hypotension | Opioids (meperidine, morphine) |
| Increased comorbid disease | Sepsis | Deep versus light sedation |
| Frailty | Poor pain control | Anticholinergics |
| Alcohol and drug abuse | Mechanical ventilation | Steroids |
| High severity of illness | Sleep disturbances | Surgery (abdominal, cardiac, hip) |
Risk factors for delirium are numerous and can be separated into predisposing patient factors and precipitating clinical factors.
Several medications have been associated with delirium. With regard to sedative and analgesic medications, use of lorazepam, midazolam, meperidine, and morphine are most strongly associated with a higher risk of delirium, likely due to their longer duration of actions and increased risk of drug accumulation with altered organ function (e.g., renal and hepatic insufficiency) compared to agents such as propofol, dexmedetomindine, and fentanyl.13,14 Sedation with benzodiazepine infusions for mechanical ventilation, in particular, carries a higher risk of delirium compared to other sedative regimens,20-22 as does deep levels of sedation when compared to light sedation.20 Additionally, medications with anticholinergic properties (e.g., diphenhydramine, promethazine, cyclobenzaprine) can precipitate delirium, potentially through altered neurotransmission or reduced neuronal control of inflammation.23,24 Steroid administration during critical illness, either as a marker of shock severity or due to their known psychological side effects, has been associated with transition to delirium.25 Dopamine is a neurotransmitter, and medications that potentiate its effects can cause psychosis whereas those that block its effects are used as antipsychotics. Dopamine administration for shock greatly increases the odds of requiring treatment for delirium after adjustment for severity of illness factors, although direct comparison to other vasoactive medications was not performed.26
Identifying delirium in the ICU setting
The gold standard for delirium diagnosis is evaluation by a psychiatrist using DSM-5 criteria, which is not feasible on a routine basis. A number of screening tools, therefore, have been developed and validated for clinical use by a wide range of personnel. Importantly, it has been demonstrated that most delirium in the ICU goes undiagnosed without using a regular screening tool,27 and current guidelines recommend the routine screening for delirium in all ICU patients.28 Importantly, a patient must be arousable to voice to assess for delirium. Thus, an arousal/sedation tool such as the Richmond Agitation-Sedation Scale (RASS)29 must be utilized along with a delirium assessment tool. There are seven validated instruments to assess delirium in critically ill patients (Table 2).7,30 Of these, the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU)31 and the Intensive Care Delirium Screening Checklist (ICDSC)32 are the most widely studied. Additionally, based on assessment of psychometric properties, the CAM-ICU and ICDSC are the recommended instruments by the Society of Critical Care Medicine's Pain, Agitation, and Delirium guidelines for monitoring delirium in ICU patients.28
Table 2. Validated Instruments to Assess Delirium in Critically Ill Patients.
| Instrument | Features Assessed | Time Period | Scoring | Initial ICU Validation | |||
|---|---|---|---|---|---|---|---|
| Assessor | Comparator | N | Results | ||||
| Confusion Assessment Method for the ICU (CAM-ICU) | Acute changes or fluctuation in mental status, inattention, disorganized thinking, altered level of consciousness | Short moment in time assessment | Positive or negative | Research nurses | Psychiatric expert assessment with DSM-IV criteria | 111 | 100% and 93% sensitivities, 98% and 100% specificities |
| Intensive Care Delirium Screening Checklist (ICDSC) | Altered level of consciousness, inattention, disorientation, psychosis, altered psychomotor activity, inappropriate speech/mood, sleep disturbance, symptom fluctuation | Assessment over nursing shift or day | 0 to 8 | Research team members completed checklist for 24-hour periods | Psychiatric expert assessment with DSM-IV criteria | 93 | 99% sensitivity and 64% specificity if ≥4 features |
| Cognitive Test for Delirium (CTD) | Orientation, attention span, memory, comprehension and conceptual reasoning, vigilance | Longer moment in time assessment | 0 to 30 | Psychologist technician | Psychiatric expert assessment with DSM-IIIR criteria | 103 | 100% sensitivity and 95% specificity if score ≤18* |
| Abbreviated Cognitive Test for Delirium | Visual attention span, recognition memory | Short moment in time assessment | 0 to 24 | Psychologist technician | Psychiatric expert assessment with DSM-IIIR criteria | 100 | 95% sensitivity and 99% specificity if score ≤10* |
| Delirium Detection Score | Agitation, anxiety, hallucination, orientation, seizures, tremor, paroxysmal sweating, altered sleep-wake rhythm | Longer moment in time assessment | 0 to 56 | Clinical physicians and nurses | Sedation-Agitation Scale and defined clinical assessment | 1073 | 69% sensitivity and 75% specificity if score ≥8 |
| Neelon and Champagne Confusion Scale (NEECHAM) | Attention, command, orientation, appearance, motor, verbal, vital function, oxygen saturation, urinary continence | Short moment in time assessment | 0 to 30 | Clinical nurses | Psychiatric intern assessment with DSM-IV criteria | 105 | 97% sensitivity and 83% specificity if score ≤24 |
| Nursing Delirium Screening Scale (Nu-DESC) | Disorientation, inappropriate behavior, inappropriate communication, hallucination, psychomotor retardation | Assessment over nursing shift or moment in time assessment | 0 to 10 | Research physicians and nurses | Psychiatric expert assessment with DSM-IV criteria | 156 | 82% sensitivity and 83% specificity if score ≥2 |
Assessed delirium vs. dementia, depression, and schizophrenia and not delirium vs. normal mental status or coma
Seven instruments have been validated to assess for delirium in critically ill patients. These instruments vary in the features assessed, time of assessment, scoring scale, and validation. The Society of Critical Care Medicine's Pain, Agitation, and Delirium guidelines recommend the CAM-ICU or ICDSC for routine monitoring of delirium in the intensive care unit.
Abbreviations: DSM, Diagnostic and Statistical Manual of Mental Disorders; ICU, intensive care unit
The CAM-ICU is an abbreviated version of the Confusion Assessment Method (CAM)33 designed to fit the needs of non-verbal and verbal ICU patients. Originally described by Ely et al in 2001, the CAM-ICU tool assesses the same four cardinal features as the CAM – acute changes/fluctuations in mental status, inattention, disorganized thinking, and an altered level of consciousness – but in a condensed manner ideal for the ICU setting. To validate the CAM-ICU, assessments were performed in 111 patients (471 total evaluations) by 2 independent nurses and compared to expert psychiatric assessment using the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) criteria. The study found sensitivities of 100% and 93% and specificities of 98% and 100%.8 The moment in time delirium assessment with the CAM-ICU requires less than 2 minutes to complete, which has prompted its use in hospital settings outside the ICU. While the majority of subsequent studies have shown high sensitivity and specificity for the CAM-ICU across a variety of patients (e.g., medical and surgical) and severities of illness,30,34,35 some studies have found lower sensitivity of the CAM-ICU when used in less severely ill patients outside of the ICU, such as the post-anesthesia care unit (though these studies have shown specificity near or above 90%).36 A recent systematic review of studies in ICU patients demonstrated pooled sensitivity of 80% and specificity of 96% for the CAM-ICU.37 The ICDSC assesses eight diagnostic features of delirium over an entire nursing shift (altered level of consciousness, inattention, disorientation, psychosis, altered psychomotor activity, inappropriate speech/mood, sleep disturbance, and symptom fluctuation).32 In its validation, the ICDSC was performed in 93 patients and compared to psychiatric evaluation. Presence of four or more of the listed features had 99% sensitivity and 64% specificity for delirium.32 A recent systematic review of studies in ICU patients demonstrated pooled sensitivity of 74% and specificity of 82% for the ICDSC.37
Delirium assessment tools such as the CAM-ICU and ICDSC should not be viewed solely as tools for research but rather as pivotal components in the care of patients. Current clinical guidelines recommend using either the CAM-ICU or ICDSC for routine delirium assessment in the critically ill.28 These assessments can be effectively performed outside of the research setting by clinical nursing staff if appropriate education and training is provided (resources available online).38 Successful implementation of routine delirium screening in the ICU requires institutional acknowledgement of the necessity for delirium screening, physician and nurse leaders to serve as delirium experts and resources, didactic instruction, case-based scenarios, bedside demonstrations, adjustment of techniques to fit patient population (e.g., language, questions, visuals used during assessment), follow-up teaching, and routine presentation of results on interdisciplinary rounds (e.g., the Brain Roadmap).39 Large scale implementation trials have shown that nurses can use the CAM-ICU routinely with high levels of compliance and reliability,40,41 and that compliance and reliability of measurements at the bedside can be sustained multiple years after implementation.42 Given the fluctuating course of delirium, it is important that these assessments are performed in a serial nature (for an assessment at any given point in time may not capture complete symptomatology) and combined with chart review and discussion with family and caregivers.
Delirium Prevention
A large portion of ICU patients develop delirium, especially those who are mechanically ventilated or who have other risk factors on admission. While many of these risk factors are often non-modifiable by clinicians, several preventative strategies have been demonstrated to reduce the incidence of ICU delirium.
Pharmacologic prophylaxis
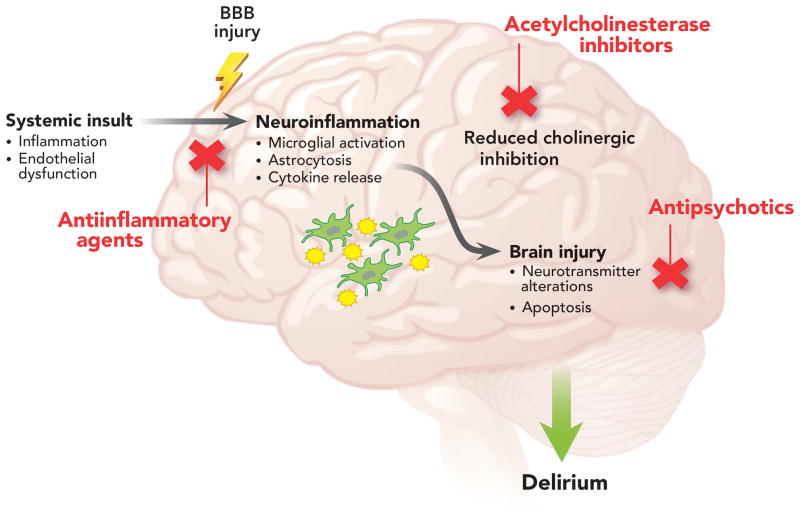
Multiple pathophysiologic processes likely contribute to delirium, and a number of pharmacologic prophylaxis agents have subsequently been studied to decrease delirium incidence (Figure 1).43,44 This includes agents to reduce dopamine activity and improve neurotransmitter imbalances (e.g., antipsychotics) and agents that increase cholinergic activity as low cholinergic activity and anticholinergic medications have been linked to delirium (e.g., acetylcholinesterase inhibitors). The majority of recent animal and human research has focused on systemic insults (e.g., surgery, sepsis) leading to inflammatory signaling through the blood brain barrier, resulting in neuroinflammation and neuronal injury.45-50 Thus agents to reduce systemic inflammation and decrease the neuroinflammatory cascade (e.g., steroids and statins) are also being examined.
Figure 1. Potential Mechanisms and Therapies for ICU Delirium.
Hypothesized mechanisms for intensive care unit (ICU) delirium include systemic inflammation, endothelial dysfunction, increased blood brain barrier permeability, and reduced cholinergic control of the inflammatory response that, along with baseline patient vulnerability factors, predispose patients to neuroinflammation and subsequent neuronal injury. Primed and over activated microglia from these processes may also exacerbate the pathophysiologic changes. Therapeutic agents studied for the prevention or treatment of ICU delirium have targeted these pathways. Abbreviations: BBB, blood brain barrier
Studies investigating whether prophylactic antipsychotic administration reduces the incidence or duration of delirium have had mixed results. Perioperative haloperidol (1.5 mg per day) prophylaxis in elderly hip surgery patients did not affect the incidence of delirium but did decrease the duration (5.4 vs. 11.8 days, P<0.001) compared to placebo.51 A low dose haloperidol bolus (0.5 mg intravenous) followed by an infusion (0.1 mg/hr for 12 hrs) in elderly patients admitted to the ICU after noncardiac surgery decreased the incidence of delirium only after intra-abdominal surgeries (14.5% vs. 24.7%, P=0.018).52 A before-after study of intravenous haloperidol (1 mg every 8 hrs) as prophylaxis in ICU patients deemed high risk for delirium showed significantly less incidence (P=0.01) and duration of delirium (P=0.003).53 A more recent randomized controlled trial – The Haloperidol Effectiveness in ICU Delirium (HOPE-ICU) study – however, showed no difference in days alive and free of delirium or coma between patients prophylactically treated with intravenous haloperidol (2.5 mg every 8 hrs) or placebo.54 Oversedation was the most common adverse event in the trial (15% in the haloperidol group); those treated with haloperidol, however, were less likely to develop agitation defined as a RASS score ≥ +2 (13% vs. 20%, p=0.0075).
Numerous studies have examined agents to prevent delirium after cardiac surgery. In a blinded, placebo-controlled trial of 126 patients undergoing elective cardiac surgery with cardiopulmonary bypass, a single dose of sublingual risperidone (1 mg) upon regaining consciousness reduced incidence of delirium compared to placebo (11% vs. 32%, P=0.009).55 Another study of risperidone in elderly patients requiring cardiac surgery with cardiopulmonary bypass examined whether repeated doses (0.5 mg every 12 hrs) could prevent the development of delirium in patients exhibiting signs of acute brain dysfunction but who did not yet meet delirium criteria (referred to as subsyndromal delirium).56 They found a lower incidence of delirium development in the risperidone group compared to placebo (13.7% vs. 34%, P= 0.031). These studies utilized validated delirium assessments, and the incidence of delirium in the placebo groups was similar to other published cohorts.57 The positive results seen with risperidone need to be confirmed in additional larger cohorts before routine administration can be recommended. Prophylactic administration of dexamethasone upon induction of anesthesia to reduce the subsequent systemic and neurologic inflammatory cascade of surgery and cardiopulmonary bypass did not reduce the incidence or duration of delirium in the first 4 days after surgery compared to placebo in a study of patients undergoing cardiac surgery with cardiopulmonary bypass.58 Gamberini et al performed a randomized controlled trial of a 7-day course of the acetylcholinesterase inhibitor rivastigmine vs. placebo in patients undergoing elective cardiac surgery with cardiopulmonary bypass but found no difference in the incidence of postoperative delirium.59
Donepezil, an acetylcholinesterase inhibitor used commonly in dementia patients, has been studied with regard to delirium prophylaxis but with negative results. In a study of 80 elderly patients undergoing elective total joint replacement surgery, patients were randomized to donepezil or placebo for 14 days before surgery and 14 days afterward.60 No significant difference in delirium incidence was found between the groups. Similar results were seen in a pilot trial of 16 elderly patients undergoing hip fracture repair randomized to donepezil or placebo within 24 hrs of surgery.61 The donepezil treatment group experienced more adverse effects and had no significant improvements in delirium presence or severity. A randomized, double-blind, placebo-controlled trial of 33 patients undergoing elective total hip replacement also found no significant difference in the incidence of delirium.62
There is interest in the pleiotropic anti-inflammatory effects of statin medications with regard to delirium.63 Ongoing statin therapy while in the ICU has been shown in two studies to be associated with lower overall risk of delirium,64,65 and increasing duration of statin withholding in chronic statin users increases the odds of developing delirium.63 Further randomized controlled trials are needed to provide evidence of the ability of statins to prevent delirium.
Despite the multiple agents evaluated covering a variety of pathophysiologic pathways, there remains a lack of proven prophylactic agents to reduce delirium. In addition, many of these agents have significant side effects, in particular the antipsychotics which may prolong the QT interval, lead to oversedation, or cause neuroleptic malignant syndrome. This emphasizes the necessity of nonpharmacological preventative measures to improve delirium outcomes.
Choice of sedation for mechanical ventilation
The type of sedation used in mechanically ventilated patients in the ICU can affect rates of delirium. Currently, it is recommended by the Pain, Agitation, and Delirium guidelines to perform analgesia-first sedation followed by non-benzodiazepine medications if needed for sedation in mechanically ventilated patients in the ICU.28 This is partly based upon evidence demonstrating increased risk of delirium with traditional sedation regimens involving continuous benzodiazepine infusions and deeper levels of sedation. Pandharipande et al compared sedation with dexmedetomidine vs. lorazepam infusion in intubated patients, assessing rates of delirium (as defined by CAM-ICU), coma, ICU length of stay, and mortality.20 This study of 106 critically ill patients found that the patients receiving dexmedetomidine had more delirium/coma-free days than the lorazepam group (7 vs. 3, P=0.01) and less coma (63% vs. 92%, P<0.001). There was no difference in antipsychotic use between the groups. These findings were subsequently confirmed with a multicenter trial by Riker et al, in which dexmedetomidine was compared to midazolam for targeted sedation.21 They found similar results, with 54% of the dexmedetomidine-treated patients developing delirium, while 76.6% of the midazolam-treated patients developed delirium (P≤0.001).21 In comparing sedation with propofol vs. dexmedetomidine, a study found fewer neurocognitive disorders and improved arousal, cooperation, and communication with dexmedetomidine.66 However, they only assessed the CAM-ICU once, 48 hrs after the infusions had been discontinued, and found no difference in delirium. A recent Cochrane meta-analysis pooled seven randomized controlled trials comparing sedation with dexmedetomidine to benzodiazepines, propofol, or “standard care” which included propofol or midazolam.67 The risk of delirium was numerically lower with dexmedetomidine but not statistically significant (risk ratio [RR] 0.85; 95% confidence interval [CI] 0.63 to 1.14). Subgroup analyses showed the risk of delirium was lower in patients receiving dexmedetomidine than in those receiving benzodiazepines (RR 0.81; 95% CI 0.59 to 1.09; 1007 participants) or propofol (RR 0.37; 95% CI 0.16 to 0.87; 495 participants).67 Overall, the authors found high rates of heterogeneity between the studies, including the baseline risk for delirium, time in ICU prior to enrollment and assessment, frequency and duration of delirium assessments, and levels of sedation targeted, all of which would bias delirium outcomes. They recommended that further studies stratify randomized patients based on delirium risk. Dexmedetomidine has additionally been found to reduce delirium rates when used after cardiac surgery.68 Most recently, a randomized controlled trial of dexmedetomidine vs. propofol for ICU sedation in 183 patients after cardiac surgery found a decreased incidence (17.5% vs. 31.5%, P=0.028) and reduced duration of delirium (2 vs. 3 days, P=0.04) in the dexmedetomidine group, leading to a reduction in ICU time and cost related to delirium.69
In the sedation studies outlined in the previous paragraph, analgesia (and likely supplemental sedation) was provided with fentanyl in addition to the sedative medications administered. Similar fentanyl requirements were found between dexmedetomidine, midazolam, and propofol regimens21,66 with the exception of after cardiac surgery, in which dexmedetomidine patients required less analgesic medications.69 There are now data examining analgesia-based sedation regimens and their effect on delirium. One trial showed that patients treated only with intermittent morphine had higher rates of agitated delirium (20% vs. 7%, P=0.04) compared to patients sedated with propofol or midazolam (hypoactive delirium was not assessed in this study), though patients receiving analgosedation had shorter ICU lengths of stay.70 When comparing morphine to dexmedetomidine for sedation after cardiac surgery, patients receiving a morphine-based regimen had similar overall incidence of delirium but had an increased duration of delirium by 3 days (P=0.03).71
Exposure to sedative medications and deeper levels of sedation are associated with increased risk of delirium, but questions have arisen regarding whether delirium that abates quickly after sedative discontinuation – rapidly reversible, sedation-related delirium – portends similar outcomes to delirium that persists after sedative discontinuation – persistent delirium. A prospective cohort study performed delirium assessments before and after sedative discontinuation.72 It found delirium to be extremely prevalent, with 89% of patients developing delirium, but only a small group of patients (12%) had delirium that abated after sedation interruption (rapidly reversible, sedation-related delirium). 72 This group with rapidly reversible, sedation-related delirium had fewer ventilator days (P<0.001), ICU days (P=0.001), and hospital days (P<0.001), was more likely to be discharged home vs. an institution (P<0.001), and had higher survival rates (P<0.001) than those whose delirium persisted. Persistent delirium (77% of the cohort) remained associated with worse outcomes. This study has important clinical implications: 1) recent sedative administration should be carefully considered when evaluating for delirium, 2) the effect of persistent delirium on negative outcomes is likely greater than measured in prior studies as those with rapidly reversible, sedation-related delirium were included, biasing those studies towards the null, and 3) only a small subset of patients on sedative medications resolve their delirium rapidly after discontinuation of those medications, underlining the importance of monitoring for delirium even in patients on sedative medications.
Early mobility
Early physical and occupational therapy in intubated and mechanically ventilated patients coordinated amongst nursing staff, physical therapists, and respiratory therapists is feasible, safe, and has been demonstrated to reduce ICU delirium.73 Therapy can progress from passive range of motion to active range of motion, exercise in bed, sitting, standing, walking, and activity of daily living training depending on a patient's sedation level and physical abilities. Schweickert et al conducted a multi-center randomized controlled trial of 104 hemodynamically stable medical ICU patients to look at the effect of daily sedation interruptions paired with physical and occupational therapy on long-term functional independence, with a secondary outcome that included delirium.73 After patients were randomized, those in the intervention group had regular sessions with the physical and occupational therapists while their sedation was paused, progressing from range of motion exercises to walking. They found a median of 2 days of ICU delirium in the early physical therapy group, whereas the control group had a median of 4 days of delirium (P=0.03). Both groups had similar sedation and analgesia, although the physical therapy group had, on average, more time without sedation than the control group.
Sleep hygiene
Fragmented sleep has been associated with delirium, and studies have evaluated ways to improve sleep hygiene (i.e., habits and practices conducive to sleep) by providing more favorable environments for sleep in the ICU. Providing ear plugs to patients in the ICU has been shown to reduce the incidence of delirium and improve sleep perception.74 A quality improvement project aimed at improving sleep by minimizing sleep disruptions, promoting normal circadian rhythms, using nonpharmacological sleep aids, and implementing alternative sleep medications when necessary (e.g., zolpidem, haloperidol, atypical antipsychotics) has also been shown to decrease the incidence of ICU delirium/coma and improve daily delirium/coma-free status though without improved perceived sleep quality.75 The authors subsequently found no association between daily perceived sleep quality rating and transition to delirium.22 These studies suggest that maintaining practices conducive to sleep is important to prevent delirium in the ICU but highlight the difficulty in monitoring sleep and differentiating between sleep perception and measures of actual sleep.
While outwardly appearing to improve sleep, sedative administration in the ICU has been shown to differentially alter sleep patterns when measured by polysomnography. In a study of 12 ICU patients not requiring vasoactive or sedative medications, patients were monitored with polysomnography for 2 nights, one of which they received propofol and the other no sedation to serve as a control.76 They found that propofol administration significantly decreased the number of patients exhibiting rapid eye movement (REM) sleep (P=0.02) and the percentage of REM sleep (P=0.04). In a similar study of 13 hemodynamically stable ICU patients not requiring vasoactive or sedative medications, patients were monitored with polysomnography for 3 nights, receiving dexmedetomidine on the second night only and no sedatives the other 2 nights to serve as a control.77 They found dexmedetomidine improved sleep efficiency (P<0.002) and stage 2 sleep (P=0.006) while decreasing night-time sleep fragmentation (P=0.023). This limited data in critically ill patients is consistent with additional data indicating that sedation with dexmedetomidine more closely resembles natural non-REM sleep than sedation with γ-aminobutyric acid (GABA)ergic agents.78 However, clinical studies investigating the interactions between sedative agents, sleep patterns, and delirium have not yet been performed.
Interest in sleep disturbances in delirium has led to studies investigating the role of melatonin in delirium. Abnormal release of circadian melatonin has been found in septic ICU patients,79 and melatonin levels have been found to be significantly lower in postoperative ICU patients with delirium compared to those without delirium.80 Importantly, data regarding whether melatonin or melatonin agonists improve sleep quality and circadian rhythms in ICU patients are limited and unclear. In a randomized controlled trial of 24 patients on mechanical ventilation after tracheostomy, melatonin supplementation increased nocturnal sleep efficiency as measured by the bispectral index but not by other sleep measurements.81 Another study of 32 patients with tracheostomy found no significant difference in sleep duration as measured by nursing assessment.82
One of the first randomized controlled trials examining melatonin as an agent for delirium prevention found melatonin (0.5 mg nightly) was associated with a lower risk of delirium compared to placebo (12.0% vs. 31.0%, P=0.014) in 145 elderly patients admitted to a medical acute care unit.83 A double-blind randomized controlled trial of melatonin (3 mg nightly) vs. placebo in 378 patients with hip fracture, however, did not demonstrate a difference in incidence of delirium.84 Ramelteon, a melatonin receptor agonist, has been shown to lower risk of delirium (3% vs. 32%; P=0.003) in a randomized controlled trial of 67 elderly patients admitted to a medical ICU or acute care ward who received ramelteon (8 mg nightly) or placebo.85 They did not find any benefit of ramelteon on sleep metrics, making it unclear whether the effects of ramelteon on delirium are related to sleep. Limitations of studies examining melatonin and ramelteon include the lack of sleep measurement via polysomnography or electroencephalogram and inability to adjust for actual sleep differences between groups. While generally well-tolerated, these agents are not benign and may cause headache, daytime sleepiness, dizziness, and depressive symptoms. Thus, large randomized controlled trials with direct sleep measurement are required to clarify the role of pharmacologic agents in sleep and delirium prevention in the ICU before prophylactic administration can be recommended. A recent systemic review concluded that nonpharmacologic and pharmacologic sleep interventions may be a promising approach to improve delirium but that current research is limited by varied methodologies and significant bias, requiring a systematic approach in future research to evaluate the complex interactions between sleep interventions and delirium.86
Sedation bundles
While several studies have identified specific interventions that reduce delirium rates, others have combined the evidence-based prevention techniques into bundles to evaluate if, when applied together in a consistent manner, they could reduce delirium rates even further. The Awakening and Breathing Coordination, Delirium Monitoring/Management, and Early Exercise/Mobility “ABCDE” bundle was originally published in 201187 and studied in a before-after trial between 2010 and 2012 at a tertiary medical center.88 Critically ill patients were enrolled from 5 separate ICUs, one step down unit, and a hematology/oncology specialty unit. The patients in the “before” group were treated as per the standard practice, which included spontaneous awakening trials (SAT) and spontaneous breathing trials (SBT) but no consistent delirium screening by clinical providers. In the “after” group, the ABCDE bundle was implemented and included a daily SAT, which was then coordinated with an SBT, scheduled RASS and CAM-ICU assessments, delirium action plan discussions amongst providers, and early mobility evaluations and performance. Patients in the post-implementation bundle group had less delirium (48.7% vs. 62.3%, P=0.02) and a lower percent of ICU days spent delirious (33% vs. 50%, P=0.002). There was a significant independent effect of the ABCDE bundle on decreasing delirium (P=0.03).
Similar to the ABCDE bundle, another study examined a quality improvement project aimed at reducing benzodiazepine exposure, lightening sedation, and increasing mobility. This study found a significant increase in the days alive without delirium (53% vs. 21%, P=0.003).89 In a before-after study of protocolized de-escalation of sedation and required RASS and CAM-ICU assessments, the authors found a significant reduction in the odds of developing delirium (odds ratio 0.67; P=0.01) along with a reduction in mechanical ventilation duration (P=0.04) and hospital length of stay (P=0.02).90 Thus, current evidence supports the use of sedation bundles to decrease the development of delirium.
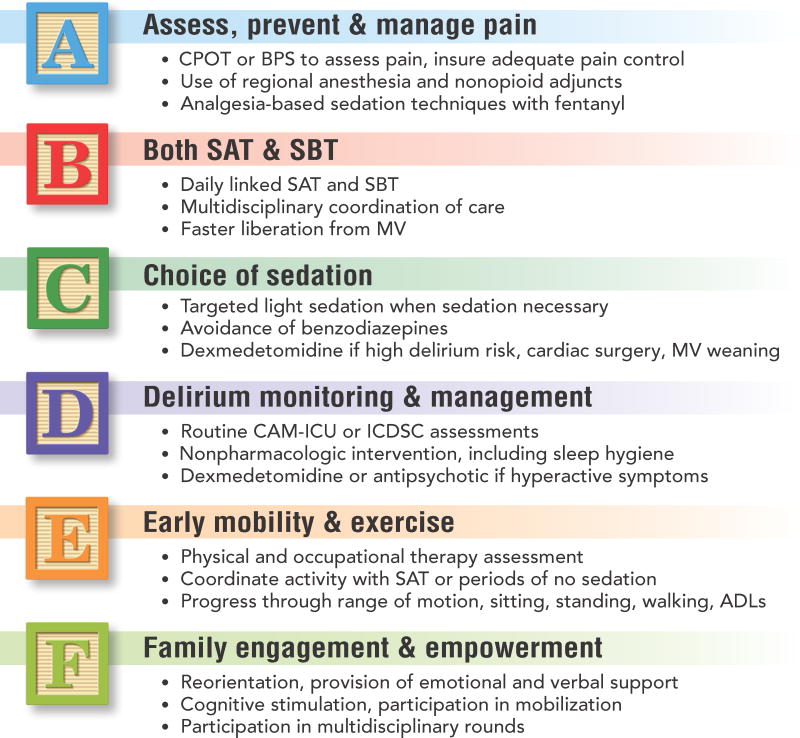
The American Association of Critical Care Nurses has developed a toolkit for implementing the ABCDE bundle at the bedside.91 This toolkit includes resources for the specific components of the bundle as well as tools for overall implementation. The Society of Critical Care Medicine has recently launched the ICU Liberation collaborative with a reworking of the ABCDE bundle.92 The now coined “ABCDEF” bundle involves Assessment and management of Pain, Both SATs and SBTs, Choice of sedation if required, Delirium monitoring and management, Early mobility and exercise, and Family engagement and empowerment (fig. 2).
Figure 2. The ABCDEF Building Blocks of ICU Delirium Management.
Multidisciplinary intensive care unit (ICU) care bundles focusing on pain management, liberation from mechanical ventilation, light sedation or no sedation, avoidance of benzodiazepines, routine delirium monitoring, and early mobility have been shown to reduce delirium and improve patient outcomes. More information on the ABCDEF bundle can be found online.38,92
Abbreviations: ADLs, activities of daily living; BPS, Behavioral Pain Scale; CAM-ICU, Confusion Assessment Method for the Intensive Care Unit; CPOT, Critical-Care Pain Observation Tool; ICDSC, Intensive Care Delirium Screening Checklist; MV, mechanical ventilation; SAT, spontaneous awakening trial; SBT, spontaneous breathing trial
Delirium Treatment
Prevention of delirium is of utmost importance because the number of evidence-based pharmacological treatment options is minimal, and those that exist have significant limitations. Therefore, the cornerstone of delirium treatment is correction of the patient's medical conditions that may be contributing to delirium.
Potential treatment options
Despite the abundance of literature and research on delirium, there remains a paucity of large, randomized controlled trials of pharmacologic treatments for delirium. The clinical approach to pharmacological treatment often includes the use of typical (e.g., haloperidol) and atypical (e.g., olanzapine, quetiapine, ziprasidone) antipsychotics, but evidence on their efficacy is limited and conflicting. In a pilot study of 101 ICU patients at risk for delirium, placebo vs. haloperidol (5 mg) vs. ziprasidone (40 mg) in repeated doses showed no difference in delirium-free days among all three groups.93 Another evaluation of haloperidol (2.5-5 mg every 8 hrs) vs. olanzapine (5 mg daily) showed no difference in length of delirium in 73 ICU patients, although the patients receiving haloperidol did have more extrapyramidal side effects.94 In a study of 36 ICU patients with delirium requiring intravenous haloperidol, patients were randomized to scheduled quetiapine (50 mg every 12 hrs) or placebo in addition to the “as-needed” haloperidol. The group that received quetiapine had a faster resolution of the first episode of delirium (36 vs. 120 hrs, P=0.006), though mortality and ICU length of stay were similar.95 Based on evidence that impaired cholinergic neurotransmission may lead to the development of delirium, rivastigmine (1.5-6 mg every 12 hrs) was studied as an adjunct to haloperidol vs. a combination of placebo and haloperidol.96 This study found no decrease in the duration of delirium, and there was a trend toward increased mortality in the rivastigmine group.96
In a small pilot study of patients with agitated delirium preventing tracheal extubation, 20 patients were randomized to a dexmedetomidine (0.2-0.7 μg/kg/hr) or haloperidol (0.5-2 mg/hr) infusion. The patients receiving dexmedetomidine had a shorter time to extubation (19.9 vs. 42.5 hrs, P=0.016), ICU length of stay (1.5 vs. 6.5 days, P=0.004), and less requirement for tracheostomy.97 The recently published Dexmedetomidine to Lessen ICU Agitation (DahLIA) trial randomized patients whose critical illness had otherwise resolved, but for whom weaning from mechanical ventilation was hampered by hyperactive or agitated delirium, to receive up to 7 days of intravenous dexmedetomidine up to 1.5 μg/kg/hr (n = 41) or placebo (n = 33).98 Patients treated with dexmedetomidine had increased ventilator-free time at 7 days (144.8 vs. 127.5 hrs, P=0.01) and had faster resolution of their delirium symptoms (23.3 vs. 40.0 hrs, P=0.01). No difference was found in bradycardia requiring interruption of study drug or hypotension requiring vasopressor support between groups. A recently published, non-randomized study, examined the effectiveness of dexmedetomidine as a rescue therapy for non-intubated ICU patients with hyperactive delirium.99 Patients whose agitated delirium failed to be controlled with up to 30 mg of intravenous haloperidol (n=46) received dexmedetomidine (0.2-0.7 μg/kg/hr). Patients whose agitated delirium improved after haloperidol (n=86) received a haloperidol infusion (0.5-1 mg/hr). Patients receiving dexmedetomidine had a higher percentage of time at target sedation (92% vs. 59%, P=0.001), less over-sedation (0 vs. 11.6%, P=0.01), and a shorter ICU length of stay (3.1 vs. 6.4, P<0.001) without increased incidence of hemodynamic side effects.99 Additionally, the overall failure rate for haloperidol in this study was 43% when including patients with delirium refractory to haloperidol and those in whom haloperidol administration resulted in adverse events, demonstrating the limited efficacy of antipsychotic agents in the treatment of ICU delirium.
Treatment algorithms
Overall, the evidence does not support a single effective pharmacologic approach to treatment of delirium in the ICU. The treatment options available also have significant side effects. Antipsychotic agents can cause sedation, respiratory depression, and prolonged QT intervals and may lead to rare but life-threatening neuroleptic malignant syndrome. One of the most common clinical concerns with dexmedetomidine is bradycardia. While bradycardia was commonly seen in several trials, there were no significant differences between the dexmedetomidine and the comparator groups (benzodiazepines, propofol, placebo, haloperidol) with regards to bradycardia necessitating treatment (e.g., atropine, glycopyrrolate, or pacing).20,21,66,98,99 Current evidence supports the use of dexmedetomidine for prevention of delirium and for treatment of refractory delirium across a wide variety of ICU patients, including those not on mechanical ventilation, but further studies are needed on dexmedetomidine as the first-line therapy for the treatment of delirium once it develops. Additionally, studies examining the effectiveness of alpha2-agonists administered orally or by intermittent intravenous bolus (e.g., guanfacine, clonidine) are needed with regard to delirium, as one of the limitations of dexmedetomidine therapy is its administration by continuous infusion. In general, pharmacologic measures should only be considered once non-pharmacologic prevention strategies have failed and the patient is a risk to self or others. Synthesizing the evidence, we recommend the algorithm in Figure 2, built upon the Society of Critical Care Medicine's ABCDEF bundle, for delirium prevention and treatment in the ICU.
Conclusions
Delirium is now recognized to be a common and serious clinical manifestation of acute brain organ dysfunction with long-term consequences. This recognition has led to routine screening and increased attention on prevention. Intensivists now routinely assess for it, and many ICUs around the country are implementing preventative strategies, such as the ABCDEF bundle. While this is a large problem in the ICU, it is increasingly recognized throughout the hospital as patients age and polypharmacy worsens. Preventing delirium will become the responsibility of many clinicians who will have the ability to avoid precipitating factors. Further studies are needed on effective treatments, differences between the motor subtypes, and long-term consequences of ICU delirium.
Acknowledgments
Funding sources: Dr. Pandharipande is supported by the National Institutes of Health AG035117, HL111111 (Bethesda, Maryland, USA). Dr. Hughes is supported by American Geriatrics Society Jahnigen Career Development Award and National Institutes of Health HL111111, AG045085 (Bethesda, Maryland, USA).
Footnotes
Summary Statement: This review examines the most recent evidence for the diagnosis, prevention, and treatment of delirium in the ICU.
Conflicts of Interest in past 36 months: Christina J Hayhurst: None
Pratik P Pandharipande: Research grant from Hospira Inc. in collaboration with National Institutes of Health
Christopher G Hughes: None
References
- 1.Lat I, McMillian W, Taylor S, Janzen JM, Papadopoulos S, Korth L, Ehtisham A, Nold J, Agarwal S, Azocar R, Burke P. The impact of delirium on clinical outcomes in mechanically ventilated surgical and trauma patients. Crit Care Med. 2009;37:1898–905. doi: 10.1097/CCM.0b013e31819ffe38. [DOI] [PubMed] [Google Scholar]
- 2.Ely EW, Shintani A, Truman B, Speroff T, Gordon SM, Harrell FE, Jr, Inouye SK, Bernard GR, Dittus RS. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291:1753–62. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 3.Pandharipande PP, Girard TD, Jackson JC, Morandi A, Thompson JL, Pun BT, Brummel NE, Hughes CG, Vasilevskis EE, Shintani AK, Moons KG, Geevarghese SK, Canonico A, Hopkins RO, Bernard GR, Dittus RS, Ely EW, BRAIN-ICU Study Investigators Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369:1306–16. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Association AP. Diagnostic and statistical manual of mental disorders. fifth edition. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 5.Vasilevskis EE, Han JH, Hughes CG, Ely EW. Epidemiology and risk factors for delirium across hospital settings. Best Pract Res Clin Anaesthesiol. 2012;26:277–87. doi: 10.1016/j.bpa.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Card E, Pandharipande P, Tomes C, Lee C, Wood J, Nelson D, Graves A, Shintani A, Ely EW, Hughes C. Emergence from general anaesthesia and evolution of delirium signs in the post-anaesthesia care unit. Br J Anaesth. 2015;115:411–7. doi: 10.1093/bja/aeu442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Devlin JW, Fong JJ, Fraser GL, Riker RR. Delirium assessment in the critically ill. Intensive Care Med. 2007;33:929–40. doi: 10.1007/s00134-007-0603-5. [DOI] [PubMed] [Google Scholar]
- 8.Ely EW, Inouye SK, Bernard GR, Gordon S, Francis J, May L, Truman B, Speroff T, Gautam S, Margolin R, Hart RP, Dittus R. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286:2703–10. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 9.Grover S, Sharma A, Aggarwal M, Mattoo SK, Chakrabarti S, Malhotra S, Avasthi A, Kulhara P, Basu D. Comparison of symptoms of delirium across various motoric subtypes. Psychiatry Clin Neurosci. 2014;68:283–91. doi: 10.1111/pcn.12131. [DOI] [PubMed] [Google Scholar]
- 10.Meagher D. Motor subtypes of delirium: past, present and future. Int Rev Psychiatry. 2009;21:59–73. doi: 10.1080/09540260802675460. [DOI] [PubMed] [Google Scholar]
- 11.Peterson JF, Pun BT, Dittus RS, Thomason JW, Jackson JC, Shintani AK, Ely EW. Delirium and its motoric subtypes: a study of 614 critically ill patients. J Am Geriatr Soc. 2006;54:479–84. doi: 10.1111/j.1532-5415.2005.00621.x. [DOI] [PubMed] [Google Scholar]
- 12.Robinson TN, Raeburn CD, Tran ZV, Brenner LA, Moss M. Motor subtypes of postoperative delirium in older adults. Arch Surg. 2011;146:295–300. doi: 10.1001/archsurg.2011.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vasilevskis EE, Han JH, Hughes CG, Ely EW. Epidemiology and risk factors for delirium across hospital settings. Best Pract Res Clin Anaesthesiol. 2012;26:277–287. doi: 10.1016/j.bpa.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Rompaey B, Schuurmans MJ, Shortridge-Baggett LM, Truijen S, Bossaert L. Risk factors for intensive care delirium: a systematic review. Intensive Crit Care Nurs. 2008;24:98–107. doi: 10.1016/j.iccn.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Jung P, Pereira MA, Hiebert B, Song X, Rockwood K, Tangri N, Arora RC. The impact of frailty on postoperative delirium in cardiac surgery patients. J Thorac Cardiovasc Surg. 2015;149:869–75. e1–2. doi: 10.1016/j.jtcvs.2014.10.118. [DOI] [PubMed] [Google Scholar]
- 16.Jones RN, Fong TG, Metzger E, Tulebaev S, Yang FM, Alsop DC, Marcantonio ER, Cupples LA, Gottlieb G, Inouye SK. Aging, brain disease, and reserve: implications for delirium. Am J Geriatr Psychiatry. 2010;18:117–27. doi: 10.1097/JGP.0b013e3181b972e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quinlan N, Marcantonio ER, Inouye SK, Gill TM, Kamholz B, Rudolph JL. Vulnerability: the crossroads of frailty and delirium. J Am Geriatr Soc. 2011;59(Suppl 2):S262–8. doi: 10.1111/j.1532-5415.2011.03674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lynch EP, Lazor MA, Gellis JE, Orav J, Goldman L, Marcantonio ER. The impact of postoperative pain on the development of postoperative delirium. Anesth Analg. 1998;86:781–5. doi: 10.1097/00000539-199804000-00019. [DOI] [PubMed] [Google Scholar]
- 19.Vaurio LE, Sands LP, Wang Y, Mullen EA, Leung JM. Postoperative delirium: the importance of pain and pain management. Anesth Analg. 2006;102:1267–73. doi: 10.1213/01.ane.0000199156.59226.af. [DOI] [PubMed] [Google Scholar]
- 20.Pandharipande PP, Pun BT, Herr DL, Maze M, Girard TD, Miller RR, Shintani AK, Thompson JL, Jackson JC, Deppen SA, Stiles RA, Dittus RS, Bernard GR, Ely EW. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298:2644–53. doi: 10.1001/jama.298.22.2644. [DOI] [PubMed] [Google Scholar]
- 21.Riker RR, Shehabi Y, Bokesch PM, Ceraso D, Wisemandle W, Koura F, Whitten P, Margolis BD, Byrne DW, Ely EW, Rocha MG, Group SS. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301:489–99. doi: 10.1001/jama.2009.56. [DOI] [PubMed] [Google Scholar]
- 22.Kamdar BB, Niessen T, Colantuoni E, King LM, Neufeld KJ, Bienvenu OJ, Rowden AM, Collop NA, Needham DM. Delirium transitions in the medical ICU: exploring the role of sleep quality and other factors. Crit Care Med. 2015;43:135–41. doi: 10.1097/CCM.0000000000000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zaal IJ, Devlin JW, Peelen LM, Slooter AJ. A systematic review of risk factors for delirium in the ICU. Crit Care Med. 2015;43:40–7. doi: 10.1097/CCM.0000000000000625. [DOI] [PubMed] [Google Scholar]
- 24.Steinberg BE, Sundman E, Terrando N, Eriksson LI, Olofsson PS. Neural control of inflammation: implications for perioperative and critical care. Anesthesiology. 2016;124:1174–89. doi: 10.1097/ALN.0000000000001083. [DOI] [PubMed] [Google Scholar]
- 25.Schreiber MP, Colantuoni E, Bienvenu OJ, Neufeld KJ, Chen KF, Shanholtz C, Mendez-Tellez PA, Needham DM. Corticosteroids and transition to delirium in patients with acute lung injury. Crit Care Med. 2014;42:1480–6. doi: 10.1097/CCM.0000000000000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sommer BR, Wise LC, Kraemer HC. Is dopamine administration possibly a risk factor for delirium? Crit Care Med. 2002;30:1508–11. doi: 10.1097/00003246-200207000-00019. [DOI] [PubMed] [Google Scholar]
- 27.van Eijk MM, van Marum RJ, Klijn IA, de Wit N, Kesecioglu J, Slooter AJ. Comparison of delirium assessment tools in a mixed intensive care unit. Crit Care Med. 2009;37:1881–5. doi: 10.1097/CCM.0b013e3181a00118. [DOI] [PubMed] [Google Scholar]
- 28.Barr J, Fraser GL, Puntillo K, Ely EW, Gelinas C, Dasta JF, Davidson JE, Devlin JW, Kress JP, Joffe AM, Coursin DB, Herr DL, Tung A, Robinson BR, Fontaine DK, Ramsay MA, Riker RR, Sessler CN, Pun B, Skrobik Y, Jaeschke R, American College of Critical Care M Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41:263–306. doi: 10.1097/CCM.0b013e3182783b72. [DOI] [PubMed] [Google Scholar]
- 29.Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O'Neal PV, Keane KA, Tesoro EP, Elswick RK. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338–44. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 30.Luetz A, Heymann A, Radtke FM, Chenitir C, Neuhaus U, Nachtigall I, von Dossow V, Marz S, Eggers V, Heinz A, Wernecke KD, Spies CD. Different assessment tools for intensive care unit delirium: which score to use? Crit Care Med. 2010;38:409–18. doi: 10.1097/CCM.0b013e3181cabb42. [DOI] [PubMed] [Google Scholar]
- 31.Ely EW, Inouye SK, Bernard GR, Gordon S, Francis J, May L, Truman B, Speroff T, Gautam S, Margolin R, Hart RP, Dittus R. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286:2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 32.Bergeron N, Dubois MJ, Dumont M, Dial S, Skrobik Y. Intensive Care Delirium Screening Checklist: evaluation of a new screening tool. Intensive Care Med. 2001;27:859–864. doi: 10.1007/s001340100909. [DOI] [PubMed] [Google Scholar]
- 33.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941–948. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 34.Lin SM, Liu CY, Wang CH, Lin HC, Huang CD, Huang PY, Fang YF, Shieh MH, Kuo HP. The impact of delirium on the survival of mechanically ventilated patients. Crit Care Med. 2004;32:2254–2259. doi: 10.1097/01.ccm.0000145587.16421.bb. [DOI] [PubMed] [Google Scholar]
- 35.Guenther U, Popp J, Koecher L, Muders T, Wrigge H, Ely EW, Putensen C. Validity and reliability of the CAM-ICU Flowsheet to diagnose delirium in surgical ICU patients. J Crit Care. 2010;25:144–51. doi: 10.1016/j.jcrc.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 36.Neufeld KJ, Leoutsakos JS, Sieber FE, Joshi D, Wanamaker BL, Rios-Robles J, Needham DM. Evaluation of two delirium screening tools for detecting post-operative delirium in the elderly. Br J Anaesth. 2013;111:7. doi: 10.1093/bja/aet167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gusmao-Flores D, Figueira Salluh JI, Chalhub RA, Quarantini LC. The confusion assessment method for the intensive care unit (CAM-ICU) and intensive care delirium screening checklist (ICDSC) for the diagnosis of delirium: a systematic review and meta-analysis of clinical studies. Crit Care. 2012;16:R115. doi: 10.1186/cc11407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.www.icudelirium.org (last date accessed: August 24, 2016)
- 39.Brummel NE, Vasilevskis EE, Han JH, Boehm L, Pun BT, Ely EW. Implementing delirium screening in the ICU: secrets to success. Crit Care Med. 2013;41:2196–208. doi: 10.1097/CCM.0b013e31829a6f1e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pun BT, Gordon SM, Peterson JF, Shintani AK, Jackson JC, Foss J, Harding SD, Bernard GR, Dittus RS, Ely EW. Large-scale implementation of sedation and delirium monitoring in the intensive care unit: a report from two medical centers. Crit Care Med. 2005;33:1199–1205. doi: 10.1097/01.ccm.0000166867.78320.ac. [DOI] [PubMed] [Google Scholar]
- 41.Soja SL, Pandharipande PP, Fleming SB, Cotton BA, Miller LR, Weaver SG, Lee BT, Ely EW. Implementation, reliability testing, and compliance monitoring of the Confusion Assessment Method for the Intensive Care Unit in trauma patients. Intensive Care Med. 2008;34:1263–1268. doi: 10.1007/s00134-008-1031-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vasilevskis EE, Morandi A, Boehm L, Pandharipande PP, Girard TD, Jackson JC, Thompson JL, Shintani A, Gordon SM, Pun BT, Wesley EE. Delirium and sedation recognition using validated instruments: reliability of bedside intensive care unit nursing assessments from 2007 to 2010. J Am Geriatr Soc. 2011;59(Suppl 2):S249–S255. doi: 10.1111/j.1532-5415.2011.03673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Gool WA, van de Beek D, Eikelenboom P. Systemic infection and delirium: when cytokines and acetylcholine collide. Lancet. 2010;375:773–775. doi: 10.1016/S0140-6736(09)61158-2. [DOI] [PubMed] [Google Scholar]
- 44.Hughes CG, Patel MB, Pandharipande PP. Pathophysiology of acute brain dysfunction: what's the cause of all this confusion? Curr Opin Crit Care. 2012;18:518–26. doi: 10.1097/MCC.0b013e328357effa. [DOI] [PubMed] [Google Scholar]
- 45.Cerejeira J, Firmino H, Vaz-Serra A, Mukaetova-Ladinska EB. The neuroinflammatory hypothesis of delirium. Acta Neuropathol. 2010;119:737–754. doi: 10.1007/s00401-010-0674-1. [DOI] [PubMed] [Google Scholar]
- 46.Terrando N, Eriksson LI, Ryu JK, Yang T, Monaco C, Feldmann M, Jonsson Fagerlund M, Charo IF, Akassoglou K, Maze M. Resolving postoperative neuroinflammation and cognitive decline. Ann Neurol. 2011;70:986–95. doi: 10.1002/ana.22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hughes CG, Pandharipande PP, Thompson JL, Chandrasekhar R, Ware LB, Ely EW, Girard TD. Endothelial activation and blood-brain barrier injury as risk factors for delirium in critically ill patients. Crit Care Med. 2016;44:e809–17. doi: 10.1097/CCM.0000000000001739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cunningham C, Wilcockson DC, Campion S, Lunnon K, Perry VH. Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. J Neurosci. 2005;25:9275–84. doi: 10.1523/JNEUROSCI.2614-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Terrando N, Yang T, Ryu JK, Newton PT, Monaco C, Feldmann M, Ma D, Akassoglou K, Maze M. Stimulation of the alpha7 nicotinic acetylcholine receptor protects against neuroinflammation after tibia fracture and endotoxemia in mice. Mol Med. 2014;20:667–75. doi: 10.2119/molmed.2014.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vacas S, Degos V, Tracey KJ, Maze M. High-mobility group box 1 protein initiates postoperative cognitive decline by engaging bone marrow-derived macrophages. Anesthesiology. 2014;120:1160–7. doi: 10.1097/ALN.0000000000000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kalisvaart KJ, de Jonghe JF, Bogaards MJ, Vreeswijk R, Egberts TC, Burger BJ, Eikelenboom P, van Gool WA. Haloperidol prophylaxis for elderly hip-surgery patients at risk for delirium: a randomized placebo-controlled study. J Am Geriatr Soc. 2005;53:1658–66. doi: 10.1111/j.1532-5415.2005.53503.x. [DOI] [PubMed] [Google Scholar]
- 52.Wang W, Li HL, Wang DX, Zhu X, Li SL, Yao GQ, Chen KS, Gu XE, Zhu SN. Haloperidol prophylaxis decreases delirium incidence in elderly patients after noncardiac surgery: a randomized controlled trial*. Crit Care Med. 2012;40:731–9. doi: 10.1097/CCM.0b013e3182376e4f. [DOI] [PubMed] [Google Scholar]
- 53.van den Boogaard M, Schoonhoven L, van Achterberg T, van der Hoeven JG, Pickkers P. Haloperidol prophylaxis in critically ill patients with a high risk for delirium. Crit Care. 2013;17:R9. doi: 10.1186/cc11933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Page VJ, Ely EW, Gates S, Zhao XB, Alce T, Shintani A, Jackson J, Perkins GD, McAuley DF. Effect of intravenous haloperidol on the duration of delirium and coma in critically ill patients (Hope-ICU): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2013;1:515–23. doi: 10.1016/S2213-2600(13)70166-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Prakanrattana U, Prapaitrakool S. Efficacy of risperidone for prevention of postoperative delirium in cardiac surgery. Anaesth Intensive Care. 2007;35:714–9. doi: 10.1177/0310057X0703500509. [DOI] [PubMed] [Google Scholar]
- 56.Hakim SM, Othman AI, Naoum DO. Early treatment with risperidone for subsyndromal delirium after on-pump cardiac surgery in the elderly: a randomized trial. Anesthesiology. 2012;116:987–97. doi: 10.1097/ALN.0b013e31825153cc. [DOI] [PubMed] [Google Scholar]
- 57.McPherson JA, Wagner CE, Boehm LM, Hall JD, Johnson DC, Miller LR, Burns KM, Thompson JL, Shintani AK, Ely EW, Pandharipande PP. Delirium in the cardiovascular ICU: exploring modifiable risk factors. Crit Care Med. 2013;41:405–13. doi: 10.1097/CCM.0b013e31826ab49b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sauer AM, Slooter AJ, Veldhuijzen DS, van Eijk MM, Devlin JW, van Dijk D. Intraoperative dexamethasone and delirium after cardiac surgery: a randomized clinical trial. Anesth Analg. 2014;119:1046–52. doi: 10.1213/ANE.0000000000000248. [DOI] [PubMed] [Google Scholar]
- 59.Gamberini M, Bolliger D, Lurati Buse GA, Burkhart CS, Grapow M, Gagneux A, Filipovic M, Seeberger MD, Pargger H, Siegemund M, Carrel T, Seiler WO, Berres M, Strebel SP, Monsch AU, Steiner LA. Rivastigmine for the prevention of postoperative delirium in elderly patients undergoing elective cardiac surgery--a randomized controlled trial. Crit Care Med. 2009;37:1762–8. doi: 10.1097/CCM.0b013e31819da780. [DOI] [PubMed] [Google Scholar]
- 60.Liptzin B, Laki A, Garb JL, Fingeroth R, Krushell R. Donepezil in the prevention and treatment of post-surgical delirium. Am J Geriatr Psychiatry. 2005;13:1100–6. doi: 10.1176/appi.ajgp.13.12.1100. [DOI] [PubMed] [Google Scholar]
- 61.Marcantonio ER, Palihnich K, Appleton P, Davis RB. Pilot randomized trial of donepezil hydrochloride for delirium after hip fracture. J Am Geriatr Soc. 2011;59(Suppl 2):S282–S288. doi: 10.1111/j.1532-5415.2011.03691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sampson EL, Raven PR, Ndhlovu PN, Vallance A, Garlick N, Watts J, Blanchard MR, Bruce A, Blizard R, Ritchie CW. A randomized, double-blind, placebo-controlled trial of donepezil hydrochloride (Aricept) for reducing the incidence of postoperative delirium after elective total hip replacement. Int J Geriatr Psychiatry. 2007;22:343–349. doi: 10.1002/gps.1679. [DOI] [PubMed] [Google Scholar]
- 63.Morandi A, Hughes CG, Girard TD, McAuley DF, Ely EW, Pandharipande PP. Statins and brain dysfunction: a hypothesis to reduce the burden of cognitive impairment in patients who are critically ill. Chest. 2011;140:580–585. doi: 10.1378/chest.10-3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Page VJ, Davis D, Zhao XB, Norton S, Casarin A, Brown T, Ely EW, McAuley DF. Statin use and risk of delirium in the critically ill. Am J Respir Crit Care Med. 2014;189:666–73. doi: 10.1164/rccm.201306-1150OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Morandi A, Hughes CG, Thompson JL, Pandharipande PP, Shintani AK, Vasilevskis EE, Han JH, Jackson JC, Laskowitz DT, Bernard GR, Ely EW, Girard TD. Statins and delirium during critical illness: a multicenter, prospective cohort study. Crit Care Med. 2014;42:1899–909. doi: 10.1097/CCM.0000000000000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jakob SM, Ruokonen E, Grounds RM, Sarapohja T, Garratt C, Pocock SJ, Bratty JR, Takala J, Dexmedetomidine for Long-Term Sedation I Dexmedetomidine vs midazolam or propofol for sedation during prolonged mechanical ventilation: two randomized controlled trials. JAMA. 2012;307:1151–60. doi: 10.1001/jama.2012.304. [DOI] [PubMed] [Google Scholar]
- 67.Chen K, Lu Z, Xin YC, Cai Y, Chen Y, Pan SM. Alpha-2 agonists for long-term sedation during mechanical ventilation in critically ill patients. Cochrane Database Syst Rev. 2015;1:CD010269. doi: 10.1002/14651858.CD010269.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ji F, Li Z, Nguyen H, Young N, Shi P, Fleming N, Liu H. Perioperative dexmedetomidine improves outcomes of cardiac surgery. Circulation. 2013;127:1576–84. doi: 10.1161/CIRCULATIONAHA.112.000936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Djaiani G, Silverton N, Fedorko L, Carroll J, Styra R, Rao V, Katznelson R. Dexmedetomidine versus propofol sedation reduces delirium after cardiac surgery: a randomized controlled trial. Anesthesiology. 2016;124:362–8. doi: 10.1097/ALN.0000000000000951. [DOI] [PubMed] [Google Scholar]
- 70.Strom T, Martinussen T, Toft P. A protocol of no sedation for critically ill patients receiving mechanical ventilation: a randomised trial. Lancet. 2010;375:475–480. doi: 10.1016/S0140-6736(09)62072-9. [DOI] [PubMed] [Google Scholar]
- 71.Shehabi Y, Grant P, Wolfenden H, Hammond N, Bass F, Campbell M, Chen J. Prevalence of delirium with dexmedetomidine compared with morphine based therapy after cardiac surgery: a randomized controlled trial (DEXmedetomidine COmpared to Morphine-DEXCOM Study) Anesthesiology. 2009;111:1075–84. doi: 10.1097/ALN.0b013e3181b6a783. [DOI] [PubMed] [Google Scholar]
- 72.Patel SB, Poston JT, Pohlman A, Hall JB, Kress JP. Rapidly reversible, sedation-related delirium versus persistent delirium in the intensive care unit. Am J Respir Crit Care Med. 2014;189:658–65. doi: 10.1164/rccm.201310-1815OC. [DOI] [PubMed] [Google Scholar]
- 73.Schweickert WD, Pohlman MC, Pohlman AS, Nigos C, Pawlik AJ, Esbrook CL, Spears L, Miller M, Franczyk M, Deprizio D, Schmidt GA, Bowman A, Barr R, McCallister KE, Hall JB, Kress JP. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373:1874–82. doi: 10.1016/S0140-6736(09)60658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Van Rompaey B, Elseviers MM, Van Drom W, Fromont V, Jorens PG. The effect of earplugs during the night on the onset of delirium and sleep perception: a randomized controlled trial in intensive care patients. Crit Care. 2012;16:R73. doi: 10.1186/cc11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kamdar BB, King LM, Collop NA, Sakamuri S, Colantuoni E, Neufeld KJ, Bienvenu OJ, Rowden AM, Touradji P, Brower RG, Needham DM. The effect of a quality improvement intervention on perceived sleep quality and cognition in a medical ICU. Crit Care Med. 2013;41:800–9. doi: 10.1097/CCM.0b013e3182746442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kondili E, Alexopoulou C, Xirouchaki N, Georgopoulos D. Effects of propofol on sleep quality in mechanically ventilated critically ill patients: a physiological study. Intensive Care Med. 2012;38:1640–6. doi: 10.1007/s00134-012-2623-z. [DOI] [PubMed] [Google Scholar]
- 77.Alexopoulou C, Kondili E, Diamantaki E, Psarologakis C, Kokkini S, Bolaki M, Georgopoulos D. Effects of dexmedetomidine on sleep quality in critically ill patients: a pilot study. Anesthesiology. 2014;121:801–7. doi: 10.1097/ALN.0000000000000361. [DOI] [PubMed] [Google Scholar]
- 78.Sanders RD, Maze M. Contribution of sedative-hypnotic agents to delirium via modulation of the sleep pathway. Can J Anaesth. 2011;58:149–56. doi: 10.1007/s12630-010-9421-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mundigler G, Delle-Karth G, Koreny M, Zehetgruber M, Steindl-Munda P, Marktl W, Ferti L, Siostrzonek P. Impaired circadian rhythm of melatonin secretion in sedated critically ill patients with severe sepsis. Crit Care Med. 2002;30:536–40. doi: 10.1097/00003246-200203000-00007. [DOI] [PubMed] [Google Scholar]
- 80.Yoshitaka S, Egi M, Morimatsu H, Kanazawa T, Toda Y, Morita K. Perioperative plasma melatonin concentration in postoperative critically ill patients: its association with delirium. J Crit Care. 2013;28:236–42. doi: 10.1016/j.jcrc.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 81.Bourne RS, Mills GH, Minelli C. Melatonin therapy to improve nocturnal sleep in critically ill patients: encouraging results from a small randomised controlled trial. Crit Care. 2008;12:R52. doi: 10.1186/cc6871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ibrahim MG, Bellomo R, Hart GK, Norman TR, Goldsmith D, Bates S, Egi M. A double-blind placebo-controlled randomised pilot study of nocturnal melatonin in tracheostomised patients. Crit Care Resusc. 2006;8:187–91. [PubMed] [Google Scholar]
- 83.Al-Aama T, Brymer C, Gutmanis I, Woolmore-Goodwin SM, Esbaugh J, Dasgupta M. Melatonin decreases delirium in elderly patients: a randomized, placebo-controlled trial. Int J Geriatr Psychiatry. 2011;26:687–94. doi: 10.1002/gps.2582. [DOI] [PubMed] [Google Scholar]
- 84.de Jonghe A, van Munster BC, Goslings JC, Kloen P, van Rees C, Wolvius R, van Velde R, Levi M, de Haan RJ, de Rooij SE, Amsterdam Delirium Study G Effect of melatonin on incidence of delirium among patients with hip fracture: a multicentre, double-blind randomized controlled trial. CMAJ. 2014;186:E547–56. doi: 10.1503/cmaj.140495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hatta K, Kishi Y, Wada K, Takeuchi T, Odawara T, Usui C, Nakamura H, Group D-J. Preventive effects of ramelteon on delirium: a randomized placebo-controlled trial. JAMA Psychiatry. 2014;71:397–403. doi: 10.1001/jamapsychiatry.2013.3320. [DOI] [PubMed] [Google Scholar]
- 86.Flannery AH, Oyler DR, Weinhouse GL. The impact of interventions to improve sleep on delirium in the ICU: a systematic review and research framework. Crit Care Med. 2016 doi: 10.1097/CCM.0000000000001952. Epub Aug 9. [DOI] [PubMed] [Google Scholar]
- 87.Morandi A, Brummel NE, Ely EW. Sedation, delirium and mechanical ventilation: the ‘ABCDE’ approach. Curr Opin Crit Care. 2011;17:43–9. doi: 10.1097/MCC.0b013e3283427243. [DOI] [PubMed] [Google Scholar]
- 88.Balas MC, Vasilevskis EE, Olsen KM, Schmid KK, Shostrom V, Cohen MZ, Peitz G, Gannon DE, Sisson J, Sullivan J, Stothert JC, Lazure J, Nuss SL, Jawa RS, Freihaut F, Ely EW, Burke WJ. Effectiveness and safety of the awakening and breathing coordination, delirium monitoring/management, and early exercise/mobility bundle. Crit Care Med. 2014;42:1024–36. doi: 10.1097/CCM.0000000000000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Needham DM, Korupolu R, Zanni JM, Pradhan P, Colantuoni E, Palmer JB, Brower RG, Fan E. Early physical medicine and rehabilitation for patients with acute respiratory failure: a quality improvement project. Arch Phys Med Rehabil. 2010;91:536–42. doi: 10.1016/j.apmr.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 90.Dale CR, Kannas DA, Fan VS, Daniel SL, Deem S, Yanez ND, 3rd, Hough CL, Dellit TH, Treggiari MM. Improved analgesia, sedation, and delirium protocol associated with decreased duration of delirium and mechanical ventilation. Ann Am Thorac Soc. 2014;11:367–74. doi: 10.1513/AnnalsATS.201306-210OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.www.aacn.org/wd/practice/content/actionpak/withlinks-ABCDE-ToolKit.content (last date accessed: August 24, 2016)
- 92.www.iculiberation.org (last date accessed: August 24, 2016)
- 93.Girard TD, Pandharipande PP, Carson SS, Schmidt GA, Wright PE, Canonico AE, Pun BT, Thompson JL, Shintani AK, Meltzer HY, Bernard GR, Dittus RS, Ely EW, Investigators MT Feasibility, efficacy, and safety of antipsychotics for intensive care unit delirium: the MIND randomized, placebo-controlled trial. Crit Care Med. 2010;38:428–37. doi: 10.1097/ccm.0b013e3181c58715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Skrobik YK, Bergeron N, Dumont M, Gottfried SB. Olanzapine vs haloperidol: treating delirium in a critical care setting. Intensive Care Med. 2004;30:444–9. doi: 10.1007/s00134-003-2117-0. [DOI] [PubMed] [Google Scholar]
- 95.Devlin JW, Roberts RJ, Fong JJ, Skrobik Y, Riker RR, Hill NS, Robbins T, Garpestad E. Efficacy and safety of quetiapine in critically ill patients with delirium: a prospective, multicenter, randomized, double-blind, placebo-controlled pilot study. Crit Care Med. 2010;38:419–27. doi: 10.1097/CCM.0b013e3181b9e302. [DOI] [PubMed] [Google Scholar]
- 96.van Eijk MM, Roes KC, Honing ML, Kuiper MA, Karakus A, van der Jagt M, Spronk PE, van Gool WA, van der Mast RC, Kesecioglu J, Slooter AJ. Effect of rivastigmine as an adjunct to usual care with haloperidol on duration of delirium and mortality in critically ill patients: a multicentre, double-blind, placebo-controlled randomised trial. Lancet. 2010;376:1829–37. doi: 10.1016/S0140-6736(10)61855-7. [DOI] [PubMed] [Google Scholar]
- 97.Reade MC, O'Sullivan K, Bates S, Goldsmith D, Ainslie WR, Bellomo R. Dexmedetomidine vs. haloperidol in delirious, agitated, intubated patients: a randomised open-label trial. Crit Care. 2009;13:R75. doi: 10.1186/cc7890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Reade MC, Eastwood GM, Bellomo R, Bailey M, Bersten A, Cheung B, Davies A, Delaney A, Ghosh A, van Haren F, Harley N, Knight D, McGuiness S, Mulder J, O'Donoghue S, Simpson N, Young P, Dah LIAI, the A, New Zealand Intensive Care Society Clinical Trials G Effect of dexmedetomidine added to standard care on ventilator-free time in patients with agitated delirium: a randomized clinical trial. JAMA. 2016;315:1460–8. doi: 10.1001/jama.2016.2707. [DOI] [PubMed] [Google Scholar]
- 99.Carrasco G, Baeza N, Cabre L, Portillo E, Gimeno G, Manzanedo D, Calizaya M. Dexmedetomidine for the treatment of hyperactive delirium refractory to haloperidol in nonintubated ICU patients: a nonrandomized controlled trial. Crit Care Med. 2016;44:1295–306. doi: 10.1097/CCM.0000000000001622. [DOI] [PubMed] [Google Scholar]