Abstract
Activated protein C (APC), derived from the plasma protease zymogen, protein C, is antithrombotic and anti-inflammatory. In preclinical injury models, recombinant APC provides neuroprotection for multiple injuries, including ischemic stroke. APC acts directly on brain endothelial cells and neurons by initiating cell signaling that requires multiple receptors. Two or more major APC receptors mediate APC’s neuroprotective cell signaling. When bound to endothelial cell protein C receptor, APC can cleave protease activated receptor 1 causing biased cytoprotective signaling that reduces ischemia-induced injury. Pharmacologic APC alleviates bleeding induced by tissue plasminogen activator (tPA) in murine ischemic stroke studies. Remarkably, APC’s signaling promotes neurogenesis. The signaling-selective recombinant variant of APC, “3K3A-APC”, was engineered to lack most of APC’s anticoagulant activity but retain APC’s cell signaling actions. Recombinant 3K3A-APC is in ongoing NIH-funded clinical trials for ischemic stroke.
Preface
It is a very special honor to present this year’s Sol Sherry Distinguished Lecture in Thrombosis. Not only did Dr. Sherry significantly contribute to important research on fibrinolysis which was eventually translated to clinical therapy but also he was an international leader for developing organizations which enabled communications and cooperation among basic and clinical investigators. He was a cofounder of the International Society on Thrombosis and Hemostasis and contributed to the origins of the ATVB Council within the AHA. For the very first international meeting on thrombosis research that I attended, Dr. Sherry was the President of that International Congress on Thrombosis and Hemostasis in Philadelphia. At that meeting, I met many colleagues whose research spanned from basic laboratory studies (Desire Collen and Victor Marder who studied plasminogen activator) to clinical trials (Vijay Kakkar who pioneered heparin antithrombotic therapies). In large part, Dr. Sherry made possible such broadly-purposed meetings which we now take for granted. His leadership for societies like the ISTH and ATVB Council and their meetings helped to define his powerful legacy, and our societies and the world have greatly benefited from Dr. Sherry’s contributions to thrombosis research.
Introduction
Stroke is a leading cause of morbidity and mortality, and > 80 % of strokes are ischemic in nature and caused by thrombosis. In spite of much effort, only one drug has been FDA-approved for treating acute ischemic stroke in the past two decades, namely tissue plasminogen activator (tPA).1–6 Extensive trial data indicate that the standard of care for all eligible patients is intravenous tPA within 4.5 h of stroke symptom onset. Neuroimaging and various other criteria are critical for selection of tPA-eligible patients. Currently the vast majority of acute stroke patients do not receive tPA therapy. Recent trial data indicate that endovascular reperfusion therapy can improve outcomes for some patients, with or without tPA therapy.1–5 Success of mechanical neurothrombectomy employing newer devices requires excellent neuroimaging and considerations of multiple patient parameters. Ongoing research will hopefully enable more extensive utilization of endovascular procedures for acute ischemic stroke, though this is not the topic for this review.
The pathophysiology of acute ischemic stroke in treated patients is very complex. When a thrombotic occlusion is cleared either by tPA or thrombectomy or by both treatments, the normal functioning of the brain can be damaged not only by ischemia, per se, but paradoxically it can also be damaged by reperfusion, by the toxicities of tPA, and/or by other sequelae, including oxidative and nitrosative stress, mitochondrial dysfunction, apoptosis of multiple cell types, and inflammatory processes.2, 7–11 While it is clear that, when used appropriately, tPA thrombolytic therapy is beneficial, it remains problematic that thrombolytic therapy increases the risk of serious intracranial hemorrhage and that early death after thrombolysis seems mostly attributable to intracranial hemorrhage.6 Clearly, mitigation of tPA’s hemorrhagic effects by neuroprotective adjunctive therapies could be very useful.6 Moreover, there is a general consensus that future efforts for improving acute ischemic stroke therapies may come from combining reperfusion techniques with one or more neuroprotective agents that target the diverse ischemia and post-ischemia pathologic processes.2, 4, 7–9, 11
Researchers have previously considered >1,000 experimental treatments for stroke,12 and they have evaluated more than 600 in animals and more than 100 in humans.12–14 Yet only tPA has achieved FDA approval.1–6 Against this background of failed compounds, activated protein C (APC) presents a truly novel type of neuroprotective agent as summarized in this review. For a more detailed review of the extensive in vitro and preclinical data for APC’s neuroprotective actions, the reader is referred to the review in reference.11
This review and the Sol Sherry lecture is focused on the promise of APC for neuroprotection in ischemic stroke based on its multiple-action-multiple-target properties which are extensively reviewed elsewhere.11 For this more narrowly focused review, first, the protein C pathways and the diverse biologic activities of APC will be presented. Second, the neuroprotective actions of wild type (wt)-APC and of an engineered signaling-selective APC mutant will be discussed. And, third, the unusual mechanism of action for APC’s neuroprotection which involves initiation of biased signaling via the G-protein coupled receptor, protease activated receptor 1 (PAR1) will be highlighted. Finally, clinical trials of recombinant 3K3A-APC for ischemic stroke will be discussed.
Protein C Pathways and Diverse Multiple Biologic Activities of Activated Protein C (APC)
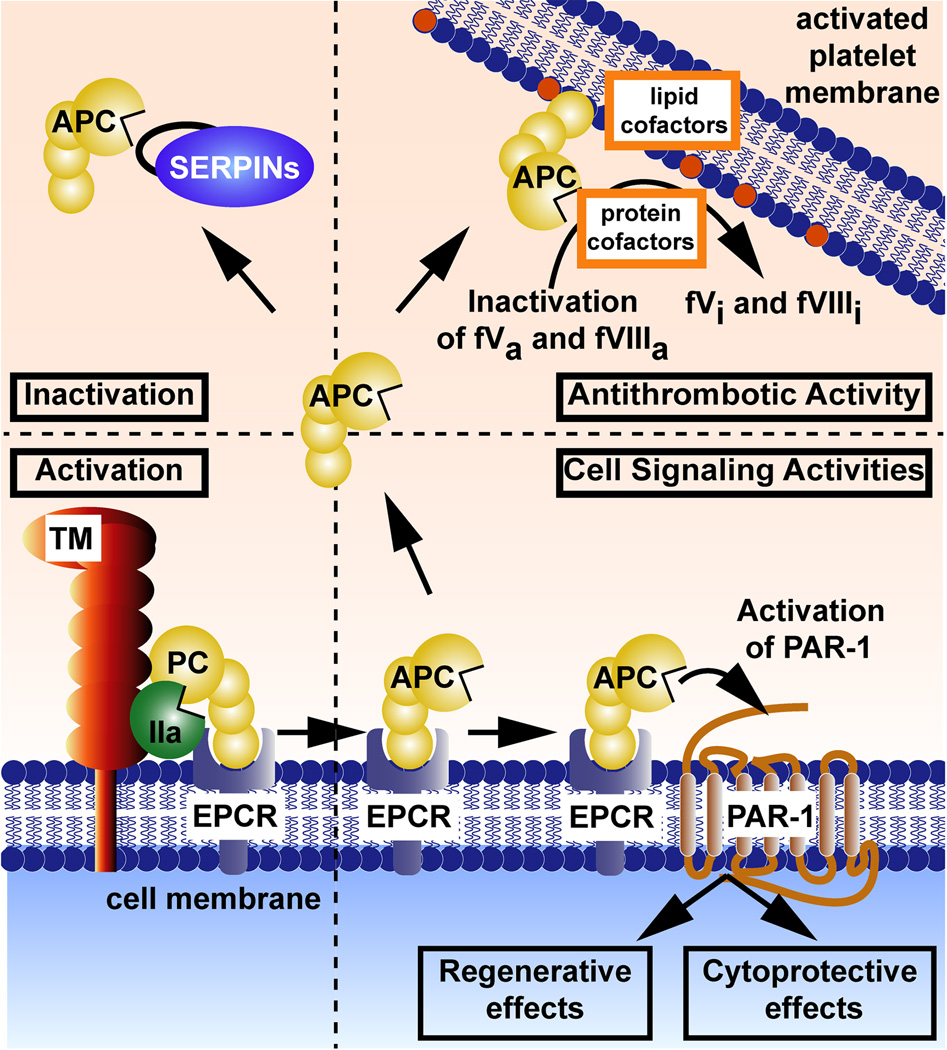
Protein C is a plasma protease zymogen with a normal plasma level of 70 nM in man, and in its active form, APC has a normal plasma level of ~ 40 pM.15 The pathways responsible for APC’s anticoagulant activities have been known for decades while the basis for its cell signaling activities continue to be elucidated. The schemes in Fig. 1 summarize reactions and molecules which mediate activation of protein C, inhibition of APC, expression of APC’s anticoagulant activity and APC’s initiation of cell signaling (see reviews15–17). For activation of protein C zymogen, thrombomodulin-bound thrombin cleavage at Arg169 in protein C which is bound to endothelial cell protein C receptor (EPCR) converts the zymogen to its active protease form. Following dissociation from EPCR, APC can circulate and provide a variety of different activities. For anticoagulant effects, APC binds to phospholipid membranes with its cofactors (including inter alia, protein S, certain lipids, HDL, etc) where it cleaves factors Va and VIIIa at one or only a few Arg peptide bonds thereby irreversibly inactivating these procoagulant cofactors. For provision of its cytoprotective actions, including anti-apoptotic and anti-inflammatory activities, stabilization of endothelial barriers to prevent vascular leakage, or for its regenerative actions such as neurogenesis, APC may bind to EPCR and then activate PAR 1 (Fig. 1). These cytoprotective and neurogenerative activities may also involve other receptors such as PAR2, PAR3, S1P1, Mac-1, apolipoprotein E receptor 2, epidermal growth factor receptor, and Tie-2 (tunica intima endothelial receptor tyrosine kinase 2).11–15–16,18–25 As a consequence of APC-induced signaling, the expression of hundreds of mRNAs are altered such that the beneficial actions of APC may be related to altered gene expression profiles.15,26, 27 The half-life of circulating APC is significantly determined, at least in large part, by its irreversible reaction with plasma serine protease inhibitors (SERPINS, Fig. 1).
Figure 1. Pathways and molecules for protein C activation, expression of APC activities, and inhibition of APC.
Activation (bottom left): EPCR-bound protein C (PC) zymogen is activated by thrombomodulin (TM)-bound thrombin. Anticoagulant Activity (upper right): APC’s anticoagulant action is based on limited proteolysis of the activated (a) clotting factors Va and VIIIa which causes irreversible inactivation (i). Various lipid cofactors and protein cofactors can enhance APC’s cleavages of factors Va and VIIIa, as depicted for reactions on platelet membranes. Cell Signaling Activities (bottom right): APC-induced cell signaling is responsible for its cytoprotective effects and regenerative effects. These include, inter alia, anti-apoptotic and anti-inflammatory activities, an ability to stabilize endothelial barriers to prevent vascular leakage, and an ability to alter gene expression profiles for many genes which help to mediate neuronal regenerative activities. APC’s cell signaling actions often also require other receptors (not shown here), including PAR3, Mac-1, S1P receptor 1, etc. Inactivation (upper left): The proteolytic activity of circulating APC is irreversibly inhibited by plasma serine protease inhibitors (SERPINS) which provide a major mechanism, in part, for clearance of this enzyme. In these schemes, coloring of molecules is as follows: protein C (PC) zymogen and active protease, APC (yellow); thrombin (IIa) (green); thrombomodulin (TM) (red); the endothelial protein C receptor (EPCR) (blue-shaded); and serine protease inhibitors (SERPINS) (purple). This figure is modified from Griffin et al, Blood, 2015.17
Since the substrates for APC’s protease actions for its anticoagulant actions (e.g., factors Va and VIIIa) differ completely from the substrates for its cell signaling actions (e.g., PAR1 PAR3, etc.), we initiated studies of APC mutants to identify variants with highly selective loss of one or the other type of action for their use in studies intended to clarify which kinds of APC activities were essential for its beneficial biologic effects.15,17 These efforts produced both signaling-selective and anticoagulant-selective APC variants that gave the intended types of clarifications. One of the most valuable mutants was recombinant human 3K3A-APC in which a single loop containing three consecutive Lys residues (191–193) was replaced with three Ala residues and which retained normal cell signaling actions in every assay done to date but which lost > 90 % anticoagulant activity.28 This loss was rationalized as due to loss of interactions of this positive loop of APC with negatively charged residues Asp577 and Asp578 on the surface of the Factor Va substrate.29
APC’s Neuroprotective Activities and Reduction of tPA-induced Bleeding
Initially, multiple clinical observations in the 1990’s were consistent with a hypothetical neuroprotective function for APC. For example, circulating APC levels were measured in stroke patients and found to be decreased in patients with antecedent infection/inflammation compared to controls.30 Then it was determined that during the short ischemic phase of routine carotid endarterectomy in the human brain, APC blood levels were increased,31 implying APC could be generated in order to provide some degree of neuroprotective activity. Prospective epidemiologic studies of ischemic stroke in the ARIC study showed that plasma protein C levels were inversely associated with the incidence of stroke.32 These clinical findings set the stage for our subsequent discovery of human APC’s neuroprotection in a murine ischemic stroke model which employed middle cerebral artery occlusion (MCAO).33
The discovery of human APC’s neuroprotective activity in the mouse MCAO model led us to clone murine protein C34 for subsequent studies to avoid cross species artefacts in mechanistic studies of APC’s neuroprotective actions.11,17,35,36 Studies of recombinant human and murine APCs indicated that wild type (wt)-APC had remarkable anti-apoptotic activity for ischemic brain microvascular endothelial cells by reducing p53, normalizing the proapoptotic Bax/Bcl-2 ratio, and lowering caspase-3 signaling, and that APC’s in vivo neuroprotection required PAR1 and EPCR, strongly implying that APC’s neuroprotection requires APC-initiated cell signaling.36
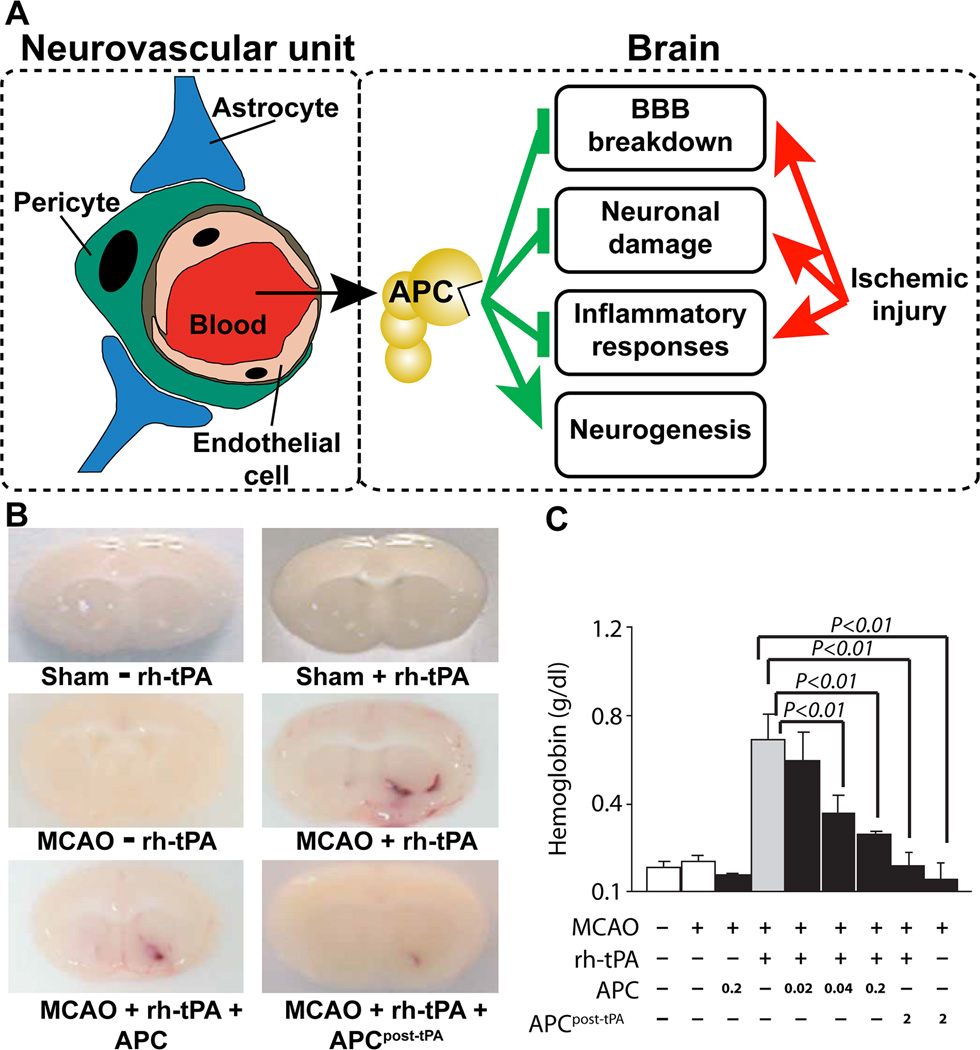
After finding APC’s cytoprotective benefits for brain endothelial cells, we discovered that APC was also neuronal protective in both in vitro and in vivo studies;37 mechanistic studies showed that neuronal protection requires PAR-1, PAR-3 and EPCR.11,37–42 Extensive studies showed that APC protects multiple cell types in the neurovascular unit as well as neurons (Fig. 2A). To determine if APC could protect neurons from tPA’s toxicity and also reduce tPA-induced bleeding, we used the murine MCAO model for combined therapies using tPA and APC. 11,43,44 Paradoxically, although wt-APC is known as an anticoagulant, it reduced tPA-induced hemorrhage, as imaged in Fig. 2B and quantified in Fig. 2C. wt-APC also reduced neuronal damage (e.g., neuronal apoptosis) caused by tPA or by NMDA (N-methyl-D-aspartate).11,37,43,44 These findings provided support for exploration of APC for adjunctive therapy with tPA for stroke.
Figure 2. APC neuroprotective effects following brain ischemia target multiple cell types in the neurovascular unit and involve APC’s inhibition of blood-brain-barrier breakdown, neuronal damage, and inflammation and APC’s promotion of neurogenesis.
Panel A schematically identifies multiple cells in the neurovascular unit which are targeted by APC when it reduces ischemia-induced brain injury because APC can inhibit endothelial vascular barrier disruption, provide endothelial cells and neurons with anti-apoptotic activity, and inhibit inflammatory responses of multiple cell types. APC also can promote neurogenesis over days following ischemic injury. Panel B shows images for brains at 24 h post-MCAO injury for animals which received recombinant human tPA without APC or with APC (0.02, 0.04 or 0.2 mg/kg) or which received 2 mg/kg APC at 3 h post-MCAO injury (as indicated). Images were obtained at 24 h after MCAO and visualize hemoglobin leakage, i.e., tPA-induced bleeding. Panel C presents data for quantification of bleeding and shows that APC dose-dependently reduced tPA-induced bleeding in the ischemic hemisphere. Similar studies using PAR1 null mice showed that PAR1 was required for the ability of APC to prevent tPA-induced bleeding (data not shown). This figure is modified from Griffin et al, Blood, 2015.17
The potential for APC to enhance murine neurogenesis in vivo was described first in MCAO studies45 and then followed by the description of APC’s ability to promote neurogenesis in vitro in cultures of human embryo-derived neural stem/progenitor cells.19 For promotion of neurogenesis of human neural progenitor cells in vitro, 3K3A-APC needs PAR-1, PAR-3 and sphingosine phosphate receptor-1, indicating that this activity is derived from characteristic APC-induced cell signaling actions. Recent studies show that late post-ischemic treatment of mice with 3K3A-APC stimulates neuronal production by transplanted human neural stem/progenitor cells, thereby promoting neural circuit restoration and improving functional recovery.46 Thus, the 3K3A-APC variant not only reduces damage to neurons but also promotes growth and regeneration of neurons.19,45,46
APC’s Biased Activation of Protease Activated Receptor 1 (PAR1)
The central importance of APC-induced cell signaling for neuroprotection is consistent with discoveries that signaling-selective recombinant APC mutants,47–49 including 3K3A-APC, are neuroprotective.50–55 So what is known in some detail about the mechanisms for APC-induced neuroprotective cell signaling? Discovery of the requirement for two receptors, PAR1 and EPCR, for APC’s ability to alter gene expression profiles56 and to inhibit apoptosis of endothelial cells57 in vitro was followed by the first in vivo proof of concept for critical roles for these receptors for APC’s neuroprotective actions in mice for endothelial cells and neurons.36, 37 Other receptors, as noted above, sometimes have also been identified as required for APC’s beneficial effects on cells.15–18,58–62 However, the two receptors, PAR1 and EPCR, provide the initial, simplest paradigm for understanding APC-initiated cell signaling (Fig. 1).
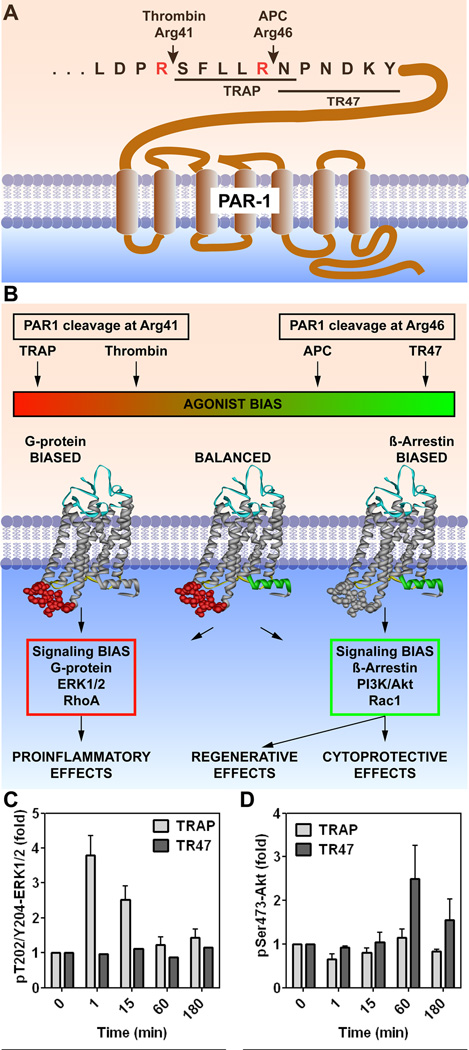
The idea that APC’s cell signaling activities involve direct activation of PAR1 was quite enigmatic for a decade because thrombin activation of PAR1 involving canonical cleavage of PAR1 at Arg4163 (Fig. 3A) was known to be proinflammatory and disruptive of endothelial barrier integrity whereas APC’s actions were anti-inflammatory and promoted vascular integrity. The enigma was how to explain that PAR1 activation by two different proteases could cause completely opposing effects? In 2011 at the American Society of Hematology meeting, we first described the ability of APC to cleave PAR1 at Arg46 and that this specific cleavage by APC carried functional consequences which differentiated it from thrombin’s cleavage (Fig. 3A).64,65 Thrombin’s cleavage at Arg41 generates a new N-terminus which begins with Ser42, and this identified PAR1 sequence(s) known as “thrombin receptor activating peptides” (TRAP) (peptides beginning with SFLLRN-etc.) that extensively mimic thrombin’s biologic effects (Fig. 3B). In contrast, cleavage at Arg46 generates a new N-terminus which begins with Asn47 and represents a PAR1 sequence known as TR47 (a 20-mer peptide beginning with NPNDKY-etc.) which agonizes PAR1 similar to APC’s effects (Fig. 3B). When added to cultured endothelial cells, TRAP, but not TR47, causes rapid ERK1/2 phosphorylation as does thrombin (Fig. 3C); but, in contradistinction, TR47, but not TRAP, causes delayed Akt phosphorylation as does APC (Fig. 3D). Thus, cleavages of PAR1 by either thrombin or APC at residues separated by only five amino acids generates tethered agonist ligands which cause completely opposing biologic effects for PAR1, a situation that may be termed biased agonism of this G protein coupled receptor.66–68 As depicted in Fig. 3B, signaling bias for activated PAR1 can either initiate proinflammatory effects, ERK1/2 activation and Ras homolog gene family member A activation linked to disruption of endothelial barriers or initiate β-arrestin 2-dependent Rac1 (Ras-related C3 botulinum toxin substrate 1) activation linked to endothelial barrier stabilization69 and survival signaling via Akt activation. Thus, in summary, substantial data support the hypothesis that APC’s neuroprotection involves biased activation of PAR1 due to cleavage at Arg46 which induces biased PAR1-mediated signaling (Fig. 3). Further studies using mice carrying single point mutations in PAR1 at either Arg41 or Arg46 are in progress to test this hypothesis.
Figure 3. PAR1-dependent biased signaling initiated by thrombin or APC.
(A) PAR1 cleavage at Arg41 by thrombin results in the N-terminal tethered peptide agonist which begins with residue Ser42 (SFLLRN-etc.) whereas cleavage at Arg46 by APC results in a different N-terminal tethered agonist which begins with residue Asn47 (NPNDKY-etc.). (B) Thrombin’s cleavage results in G protein-dependent signaling (red box) whereas APC’s cleavage results in β-arrestin 2-dependent signaling (green box). (C) and (D) Synthetic peptides designated TRAP (Thrombin Receptor Activating Peptide) that begin with amino acid Ser42 cause thrombin-like effects on cells whereas a 20-mer synthetic peptide that begins with amino acid Asn47 (TR47) causes APC-like effects on cells.58,63–65 These consequences are illustrated by the differences in phosphorylations of ERK1/2 (C) compared to Akt (D); notably, TRAP induces phosphorylation of ERK1/2 but not Akt whereas TR47 induces phosphorylation of Akt but not ERK1/2.64 This figure is modified from Griffin et al, Blood, 2015.17
Recombinant 3K3A-APC and Clinical Trials for Ischemic Stroke
To distinguish the contributions of APC’s antithrombotic activity from its cell signaling activities in various in vitro studies or animal injury studies, recombinant APC mutants have been engineered with selective loss or gain of either of these activities.17, 47–49, 58,70,71 In every case where we studied recombinant APC mutants, signaling-selective APC mutants were neuroprotective while a highly anticoagulant APC mutant was neurotoxic.50–53, 72,73 Hence, clinical trial efforts were undertaken on the assumption that APC-initiated cell signaling provides all of its neuroprotective activities.
Translation from bench to beside for neuroprotective APC for ischemic stroke was based on knowledge of 3K3A-APC’s mechanism of action and the property that this APC mutant has < 10 % of APC’s anticoagulant activity.74,75 Preclinical studies of recombinant 3K3A-APC for toxicity in two animals74 and for efficacy in two animal stroke models51,52,72,73,76 justified the phase 1 safety study in healthy normal adults.75 That study indicated that high dose bolus regimens which resulted in very high transient levels of 3K3A-APC (e.g., plasma levels of 4.5 µg/ml) were safe, including when the same high dose boluses were administered every 12 h over 2.5 days. Based on mild effects of moderately severe headache, nausea and vomiting in one or two subjects treated with the maximum dose studied of 0.72 mg/kg, a maximum dose of 0.54 mg/kg was considered the maximum tolerated dose. No concerns were raised in the phase 1 study, and so clinical studies were advanced to phase 2.
The ‘‘Safety Evaluation of 3K3A-APC in Ischemic Stroke (RHAPSODY)’’ trial (NCT02222714, NN104) is a multicenter, prospective, double-blinded, dose-escalation Phase 2 RCT, as noted.2 The trial is being conducted within the NeuroNEXT clinical trials network at 15 sites funded by NINDS. It intends to assess the safety, pharmacokinetics and efficacy of 3K3A-APC following treatment with tPA, mechanical neurothrombectomy, or both (for subjects undergoing neurothrombectomy, onset time to arterial puncture must be < 6 h). Four different doses of 3K3A-APC are being tested to establish the maximum tolerated dose. Eligibility criteria include age 18–90 and NIHSS ≥5. The trial started in October 2014 and will enroll at least 100 subjects over a two or more year target period.
In summary, basic and clinical discoveries over 3 decades ago clarified the physiologic antithrombotic activity of protein C15,16 while the neuroprotective activity of APC was discovered only this century.33 Extensive in vitro and preclinical studies showed that APC-initiated cell signaling underlies APC’s beneficial effects on different types of brain cells,11,35 including stimulation of neural progenitor cells.19,45,46 Engineering of 3K3A-APC resulted in loss of > 90 % anticoagulant activity28 with retention of its full neuroprotective actions,35 setting the stage for further preclinical work that led to ongoing clinical trial efforts employing 3K3A-APC for ischemic stroke therapy.75
HIGHLIGHTS.
The plasma serine protease, activated protein C (APC), can exert a broad range of biologic actions including antithrombotic activity and initiation of cell signaling which provides anti-inflammatory, anti-apoptotic, and endothelial barrier stabilization.
Activated protein C initiates neuroprotective cell signaling on multiple types of brain cells that requires Protease Activated Receptors 1 and 3, endothelial cell protein C receptor and other receptors.
Activated protein C initiates biased signaling when activating Protease activated Receptor 1.
Recombinant activated protein C variants, such as 3K3A-activated protein C, were engineered to retain neuroprotective cell signaling but lose anticoagulant activity to reduce bleeding risks when used for therapy.
3K3A-activated protein C, in combination with tissue-plasminogen activator and/or neurothrombectomy, is in clinical trial studies for ischemic stroke.
Why did you choose the profession of scientific investigation?
During high school at Seattle Prep, my favorite subjects were physics, chemistry and math; there it was emphasized that scholarship required a deep and fundamental knowledge of subjects. In the Jesuit tradition, academic excellence, curiosity and service are inextricably entwined. During those years, I decided that my mission in life should be to discover new knowledge that would be intrinsically satisfying and that could also contribute to improving society. It seemed likely that being an eternal student in the academic life with the goal of making truly pioneering discoveries of potential utility could be fulfilling.
What have been important influences on your professional life?
My very supportive family including my inspiring sisters plus my mentors, close professional friends, and trainees have been critically important influences on my career development and to all of them I am very grateful. For decades, my wife has provided limitless support to enable my own intense work efforts even while also providing her own model of leading a devoted academic life at UCSD.
My movement from protein biophysics to the subfield of thrombosis and hemostasis came from encouragement by fellow postdocs and friends, Deane Mosher and Bruce and Barbara Furie. Long term collaborations with dear friends like Bonno Bouma, Berislav Zlokovic and Hardy Weiler have helped to influence the directions for my research trajectories. My trainees taught me much of what I have learned and contributed substantially to my life.
Broadly influencing my approach to science were several readings – Thomas Kuhn's book, The Structure of Scientific Revolutions, and Jacques Monod's comment that "Without fundamental anxiety, there is no fundamental science". I have always been fundamentally anxiety to understand things more clearly. When traveling across subfields of science, I have tried first to learn the prevailing paradigms and, second, to assess what are the most important questions and their implications. Then it became challenging to incorporate new technologies and their new data into efforts to advance knowledge, including sometimes deconstructing and reconstructing paradigms.
How have mentors contributed to your professional development?
Elkan Blout at Harvard told me he didn’t care what I did but that it had to be important and significant, and then gave me 30 days to think about what to do. He emphasized that one must think deeply about problems, and he used the Socratic method to bring out the best from his students and postdocs. He modeled working harder than anyone I had ever met. Chris Anfinsen at the NIH generously provided support, advice and knowledge. His intense drive to understand protein structure-activity relationships was contagious. Understanding protein or enzyme SAR has driven my research on blood clotting factors and led to efforts to translate 2nd generation recombinant biologics, like 3K3A-APC, to the clinic. Chris also wisely advised changing research topics, directions, or technologies every 5–7 years, as I have done.
At Scripps, my initial group chief, Charles Cochrane, and my best friend, Ted Zimmerman, taught me to view problems as they did as physician scientists. Ted's encouragement led me to undertake clinical research that led to discovery of mild and severe protein C deficiency which then converted me to a translational research mentality.
Who are your scientific inspirations?
Each of my mentors was very inspiring as models for their particular skills. Individuals who have struck me with awe for their accomplishments in clinically relevant basic science accomplishments include Desire Collen for his tPA work, Ted Zimmerman for his factor VIII/vWF work, Barry Coller for his anti-platelet Mab work, and Hans Peter Schwarz for bringing plasma protein C concentrate from a dream to a reality for protein C deficient patients. I admire greatly many colleagues whose research has changed ideas and fundamental knowledge, like previous Sol Sherry and AHA distinguished lecturers. Over the years, I have also been inspired by the highly idealistic, devoted, hard-working administrative NIH staff who support and promote the American research mission. Similarly, the devoted staff of the AHA and of other non-profit societies and organizations who enable the pursuit of knowledge for the benefit of patients and of the world are inspiring by their lives.
What are your non-scientific activities?
Over the years, I have taken great pleasure in tennis, windsurfing and skiing with my three children, colleagues, and family friends. Visits with adult children and their families and weekly gatherings with my wife and close friends to play bridge or just enjoy time together is most enjoyable . Travel enriches my life, and regular trips to interesting cities like Paris, Kyoto and London with their fascinating cultural differences, museums, theater, gardens, and cuisines have become essential activities.
What wisdom do you impart on new investigators?
Choose your research areas and problems which are important for science and medicine and are very engaging to your mind. Work very hard and be tenacious about pursuit of your research and of general knowledge. Read broadly and attend seminars outside your own research topic for your continuing education. Cross fields and seek interdisciplinary collaborations with the best people. Reevaluate your personal goals every five years for their intrinsic value and for their value to the meaning of your life. Do not let rejection by journals or triage by study sections deter you from your dreams. Use each rejection as a learning experience and bounce back, work harder, and remember, if you are doing research, you are very lucky.
Acknowledgments
John H. Griffin drafted this review which was edited and approved by all coauthors. Laurent O. Mosnier and John H. Griffin prepared the Figures.
Sources of Funding
This work was supported in part by the NIH grants HL031950 and HL052246 (J.H.G.), HL104165 and HL130678 (L.O.M.) and NS090904 (B.V.Z.).
Abbreviations
- APC
activated protein C
- EPCR
endothelial protein C receptor
- PAR
protease activated receptor
- tPA
tissue plasminogen activator
- wt
wild type
- MCAO
middle cerebral artery occlusion
- TRAP
thrombin receptor activating peptide
- ERK1/2
extracellular signal-regulated kinase 1/2
- Mac-1
Macrophage-1 antigen (aka integrin αMβ2 or CD11b/CD18)
- Akt
Protein kinase B
- TR47
20-mer peptide that begins with Asn-47
- TM
thrombomodulin
Biography

Footnotes
Disclosure of Conflicts of Interest
Dr. Griffin is a consultant for ZZ Biotech LLC and inventor for some uses of 3K3A-APC. Dr. Mosnier is an inventor for some uses of 3K3A-APC. Dr. Zlokovic is a founder and the Chief Scientific Officer of ZZ Biotech LLC, a biotechnology company with a mission to develop APC and its functional mutants for the treatment of stroke and other neurological disorders.
References
- 1.Zerna C, Hegedus J, Hill MD. Evolving treatments for acute ischemic stroke. Circ Res. 2016;118:1425–1442. doi: 10.1161/CIRCRESAHA.116.307005. [DOI] [PubMed] [Google Scholar]
- 2.Amar AP, Griffin JH, Zlokovic BV. Combined neurothrombectomy or thrombolysis with adjunctive delivery of 3K3A-activated protein C in acute ischemic stroke. Front Cell Neurosci. 2015;9:344. doi: 10.3389/fncel.2015.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell BC, Donnan GA, Lees KR, Hacke W, Khatri P, Hill MD, Goyal M, Mitchell PJ, Saver JL, Diener HC, Davis SM. Endovascular stent thrombectomy: The new standard of care for large vessel ischaemic stroke. Lancet Neurol. 2015;14:846–854. doi: 10.1016/S1474-4422(15)00140-4. [DOI] [PubMed] [Google Scholar]
- 4.Marshall RS. Progress in intravenous thrombolytic therapy for acute stroke. JAMA Neurol. 2015;72:928–934. doi: 10.1001/jamaneurol.2015.0835. [DOI] [PubMed] [Google Scholar]
- 5.Prabhakaran S, Ruff I, Bernstein RA. Acute stroke intervention: A systematic review. JAMA. 2015;313:1451–1462. doi: 10.1001/jama.2015.3058. [DOI] [PubMed] [Google Scholar]
- 6.Wardlaw JM, Murray V, Berge E, del Zoppo GJ. Thrombolysis for acute ischaemic stroke. Cochrane Database Syst Rev. 2014:CD000213. doi: 10.1002/14651858.CD000213.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chamorro A, Dirnagl U, Urra X, Planas AM. Neuroprotection in acute stroke: Targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol. 2016;15:869–881. doi: 10.1016/S1474-4422(16)00114-9. [DOI] [PubMed] [Google Scholar]
- 8.Bai J, Lyden PD. Revisiting cerebral postischemic reperfusion injury: New insights in understanding reperfusion failure, hemorrhage, and edema. Int J Stroke. 2015;10:143–152. doi: 10.1111/ijs.12434. [DOI] [PubMed] [Google Scholar]
- 9.Tobin MK, Bonds JA, Minshall RD, Pelligrino DA, Testai FD, Lazarov O. Neurogenesis and inflammation after ischemic stroke: What is known and where we go from here. J Cereb Blood Flow Metab. 2014;34:1573–1584. doi: 10.1038/jcbfm.2014.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niego B, Medcalf RL. Plasmin-dependent modulation of the blood-brain barrier: A major consideration during tPA-induced thrombolysis? J Cereb Blood Flow Metab. 2014;34:1283–1296. doi: 10.1038/jcbfm.2014.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zlokovic BV, Griffin JH. Cytoprotective protein C pathways and implications for stroke and neurological disorders. Trends Neurosci. 2011;34:198–209. doi: 10.1016/j.tins.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Collins VE, Macleod MR, Donnan GA, Horky LL, van der Worp BH, Howells DW. 1,026 experimental treatments in acute stroke. Ann Neurol. 2006;59:467–477. doi: 10.1002/ana.20741. [DOI] [PubMed] [Google Scholar]
- 13.Howells DW, Sena ES, O'Collins V, Macleod MR. Improving the efficiency of the development of drugs for stroke. Int J Stroke. 2012;7:371–377. doi: 10.1111/j.1747-4949.2012.00805.x. [DOI] [PubMed] [Google Scholar]
- 14.Kikuchi K, Tanaka E, Murai Y, Tancharoen S. Clinical trials in acute ischemic stroke. CNS Drugs. 2014;28:929–938. doi: 10.1007/s40263-014-0199-6. [DOI] [PubMed] [Google Scholar]
- 15.Mosnier LO, Zlokovic BV, Griffin JH. The cytoprotective protein C pathway. Blood. 2007;109:3161–3172. doi: 10.1182/blood-2006-09-003004. [DOI] [PubMed] [Google Scholar]
- 16.Esmon CT. Protein C anticoagulant system-anti-inflammatory effects. Semin Immunopathol. 2012;34:127–132. doi: 10.1007/s00281-011-0284-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffin JH, Zlokovic BV, Mosnier LO. Activated protein C: Biased for translation. Blood. 2015;125:2898–2907. doi: 10.1182/blood-2015-02-355974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKelvey K, Jackson CJ, Xue M. Activated protein C: A regulator of human skin epidermal keratinocyte function. World J Biol. Chem. 2014;5:169–179. doi: 10.4331/wjbc.v5.i2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo H, Zhao Z, Yang Q, Wang M, Bell RD, Wang S, Chow N, Davis TP, Griffin JH, Goldman SA, Zlokovic BV. An activated protein C analog stimulates neuronal production by human neural progenitor cells via a PAR1-PAR3-S1PR1-akt pathway. J Neurosci. 2013;33:6181–6190. doi: 10.1523/JNEUROSCI.4491-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xue M, Chow SO, Dervish S, Chan YK, Julovi S, Jackson CJ. Activated protein C enhances human keratinocyte barrier integrity via sequential activation of epidermal growth factor receptor and Tie2. J Biol. Chem. 2011;286:6742–6750. doi: 10.1074/jbc.M110.181388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta A, Williams MD, Macias WL, Molitoris BA, Grinnell BW. Activated protein C and acute kidney injury: Selective targeting of PAR-1. Curr. Drug Targets. 2009;10:1212–1226. doi: 10.2174/138945009789753291. [DOI] [PubMed] [Google Scholar]
- 22.Danese S, Vetrano S, Zhang L, Poplis VA, Castellino FJ. The protein C pathway in tissue inflammation and injury: Pathogenic role and therapeutic implications. Blood. 2010;115:1121–1130. doi: 10.1182/blood-2009-09-201616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rezaie AR. The occupancy of endothelial protein C receptor by its ligand modulates the PAR-1 dependent signaling specificity of coagulation proteases. IUBMB. Life. 2011;63:390–396. doi: 10.1002/iub.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feistritzer C, Riewald M. Endothelial barrier protection by activated protein C through PAR1-dependent Sphingosine 1-Phosphate Receptor-1 crossactivation. Blood. 2005;105:3178–3184. doi: 10.1182/blood-2004-10-3985. [DOI] [PubMed] [Google Scholar]
- 25.Finigan JH, Dudek SM, Singleton PA, Chiang ET, Jacobson JR, Camp SM, Ye SQ, Garcia JG. Activated protein C mediates novel lung endothelial barrier enhancement: Role of Sphingosine 1-Phosphate receptor transactivation. J Biol. Chem. 2005;280:17286–17293. doi: 10.1074/jbc.M412427200. [DOI] [PubMed] [Google Scholar]
- 26.Riewald M, Ruf W. Protease-Activated Receptor-1 signaling by activated protein C in cytokine perturbed endothelial cells is distinct from thrombin signaling. J Biol. Chem. 2005;280:19808–19814. doi: 10.1074/jbc.M500747200. [DOI] [PubMed] [Google Scholar]
- 27.Kerschen EJ, Hernandez I, Zogg M, Jia S, Hessner MJ, Fernandez J, Griffin JH, Huettner CS, Castellino FJ, Weiler H. Activated protein C targets CD8+ dendritic cells to reduce the mortality of endotoxemia in mice. J Clin. Invest. 2010;120:3167–3178. doi: 10.1172/JCI42629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gale AJ, Tsavaler A, Griffin JH. Molecular characterization of an extended binding site for coagulation factor Va in the positive exosite of activated protein C. J Biol Chem. 2002;277:28836–28840. doi: 10.1074/jbc.M204363200. [DOI] [PubMed] [Google Scholar]
- 29.Pellequer JL, Gale AJ, Getzoff ED, Griffin JH. Three-dimensional model of coagulation factor Va bound to activated protein C. Thromb Haemost. 2000;84:849–857. [PubMed] [Google Scholar]
- 30.Macko RF, Ameriso SF, Gruber A, Griffin JH, Fernandez JA, Barndt R, Quismorio FP, Jr, Weiner JM, Fisher M. Impairments of the protein C system and fibrinolysis in infection-associated stroke. Stroke. 1996;27:2005–2011. doi: 10.1161/01.str.27.11.2005. [DOI] [PubMed] [Google Scholar]
- 31.Macko RF, Killewich LA, Fernandez JA, Cox DK, Gruber A, Griffin JH. Brain-specific protein C activation during carotid artery occlusion in humans. Stroke. 1999;30:542–545. doi: 10.1161/01.str.30.3.542. [DOI] [PubMed] [Google Scholar]
- 32.Folsom AR, Rosamond WD, Shahar E, Cooper LS, Aleksic N, Nieto FJ, Rasmussen ML, Wu KK. Prospective study of markers of hemostatic function with risk of ischemic stroke. The Atherosclerosis Risk In Communities (ARIC) study investigators. Circulation. 1999;100:736–742. doi: 10.1161/01.cir.100.7.736. [DOI] [PubMed] [Google Scholar]
- 33.Shibata M, Kumar SR, Amar A, Fernandez JA, Hofman F, Griffin JH, Zlokovic BV. Anti-inflammatory, antithrombotic, and neuroprotective effects of activated protein C in a murine model of focal ischemic stroke. Circulation. 2001;103:1799–1805. doi: 10.1161/01.cir.103.13.1799. [DOI] [PubMed] [Google Scholar]
- 34.Fernandez JA, Xu X, Liu D, Zlokovic BV, Griffin JH. Recombinant murine-activated protein C is neuroprotective in a murine ischemic stroke model. Blood Cells Mol Dis. 2003;30:271–276. doi: 10.1016/s1079-9796(03)00034-2. [DOI] [PubMed] [Google Scholar]
- 35.Griffin JH, Fernandez JA, Lyden PD, Zlokovic BV. Activated protein C promotes neuroprotection: Mechanisms and translation to the clinic. Thromb Res. 2016;141(Suppl 2):S62–S64. doi: 10.1016/S0049-3848(16)30368-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng T, Liu D, Griffin JH, Fernandez JA, Castellino F, Rosen ED, Fukudome K, Zlokovic BV. Activated protein C blocks p53-mediated apoptosis in ischemic human brain endothelium and is neuroprotective. Nat Med. 2003;9:338–342. doi: 10.1038/nm826. [DOI] [PubMed] [Google Scholar]
- 37.Guo H, Liu D, Gelbard H, Cheng T, Insalaco R, Fernandez JA, Griffin JH, Zlokovic BV. Activated protein C prevents neuronal apoptosis via Protease Activated Receptors 1 and 3. Neuron. 2004;41:563–572. doi: 10.1016/s0896-6273(04)00019-4. [DOI] [PubMed] [Google Scholar]
- 38.Gorbacheva L, Davidova O, Sokolova E, Ishiwata S, Pinelis V, Strukova S, Reiser G. Endothelial protein C receptor is expressed in rat cortical and hippocampal neurons and is necessary for protective effect of activated protein C at glutamate excitotoxicity. J Neurochem. 2009;111:967–975. doi: 10.1111/j.1471-4159.2009.06380.x. [DOI] [PubMed] [Google Scholar]
- 39.Gorbacheva L, Pinelis V, Ishiwata S, Strukova S, Reiser G. Activated protein C prevents glutamate- and thrombin-induced activation of Nuclear Factor-Kappab in cultured hippocampal neurons. Neuroscience. 2010;165:1138–1146. doi: 10.1016/j.neuroscience.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 40.Gorbacheva L, Strukova S, Pinelis V, Ishiwata S, Stricker R, Reiser G. NF-Kappab-dependent and -independent pathways in the protective effects of activated protein C in hippocampal and cortical neurons at excitotoxicity. Neurochem Int. 2013;63:101–111. doi: 10.1016/j.neuint.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 41.Gorbacheva LR, Storozhevykh TP, Pinelis VG, Davydova ON, Ishiwata S, Strukova SM. Activated protein C via PAR1 receptor regulates survival of neurons under conditions of glutamate excitotoxicity. Biochemistry (Mosc) 2008;73:717–724. doi: 10.1134/s0006297908060138. [DOI] [PubMed] [Google Scholar]
- 42.Gorbacheva LR, Storozhevykh TP, Pinelis VG, Ishiwata S, Strukova SM. Protease-Activated Receptor (PAR)1-mediated anti-apoptotic effect of activated protein C on glutamate excitotoxicity in hippocampal neurons. Thromb Haemost. 2007;98:1150–1152. doi: 10.1160/th07-03-0228. [DOI] [PubMed] [Google Scholar]
- 43.Liu D, Cheng T, Guo H, Fernandez JA, Griffin JH, Song X, Zlokovic BV. Tissue plasminogen activator neurovascular toxicity is controlled by activated protein C. Nat Med. 2004;10:1379–1383. doi: 10.1038/nm1122. [DOI] [PubMed] [Google Scholar]
- 44.Cheng T, Petraglia AL, Li Z, Thiyagarajan M, Zhong Z, Wu Z, Liu D, Maggirwar SB, Deane R, Fernandez JA, LaRue B, Griffin JH, Chopp M, Zlokovic BV. Activated protein C inhibits tissue plasminogen activator-induced brain hemorrhage. Nat. Med. 2006;12:1278–1285. doi: 10.1038/nm1498. [DOI] [PubMed] [Google Scholar]
- 45.Thiyagarajan M, Fernandez JA, Lane SM, Griffin JH, Zlokovic BV. Activated protein C promotes neovascularization and neurogenesis in postischemic brain via Protease-Activated Receptor 1. J Neurosci. 2008;28:12788–12797. doi: 10.1523/JNEUROSCI.3485-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang YZZ, Rege SV, Wang M, Si G, Zhou Y, Wang S, Griffin JH, Goldman SA, Zlokovic BV. 3K3A-APC stimulates post ischemic neuronal repair by human neuronal stem cells in mice. Nature Medicine. doi: 10.1038/nm.4154. Published Online August 22, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mosnier LO, Gale AJ, Yegneswaran S, Griffin JH. Activated protein C variants with normal cytoprotective but reduced anticoagulant activity. Blood. 2004;104:1740–1745. doi: 10.1182/blood-2004-01-0110. [DOI] [PubMed] [Google Scholar]
- 48.Mosnier LO, Yang XV, Griffin JH. Activated protein C mutant with minimal anticoagulant activity, normal cytoprotective activity, and preservation of thrombin activable fibrinolysis inhibitor-dependent cytoprotective functions. J Biol. Chem. 2007;282:33022–33033. doi: 10.1074/jbc.M705824200. [DOI] [PubMed] [Google Scholar]
- 49.Mosnier LO, Zlokovic BV, Griffin JH. Cytoprotective-selective activated protein C therapy for ischaemic stroke. Thromb. Haemost. 2014;112:883–892. doi: 10.1160/TH14-05-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walker CT, Marky AH, Petraglia AL, Ali T, Chow N, Zlokovic BV. Activated protein C analog with reduced anticoagulant activity improves functional recovery and reduces bleeding risk following controlled cortical impact. Brain Res. 2010;1347:125–131. doi: 10.1016/j.brainres.2010.05.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Y, Thiyagarajan M, Chow N, Singh I, Guo H, Davis TP, Zlokovic BV. Differential neuroprotection and risk for bleeding from activated protein C with varying degrees of anticoagulant activity. Stroke. 2009;40:1864–1869. doi: 10.1161/STROKEAHA.108.536680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Y, Zhang Z, Chow N, Davis TP, Griffin JH, Chopp M, Zlokovic BV. An activated protein C analog with reduced anticoagulant activity extends the therapeutic window of tissue plasminogen activator for ischemic stroke in rodents. Stroke. 2012;43:2444–2449. doi: 10.1161/STROKEAHA.112.658997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang Y, Zhao Z, Chow N, Ali T, Griffin JH, Zlokovic BV. Activated protein C analog promotes neurogenesis and improves neurological outcome after focal ischemic stroke in mice via Protease Activated Receptor 1. Brain Res. 2013;1507:97–104. doi: 10.1016/j.brainres.2013.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gleeson EM, Dichiara MG, Salicio A, Quinn LM, Drakeford C, Russell SE, Walsh PT, Orbe J, Hermida J, Smith OP, O'Donnell JS, Montes R, Preston RJ. Activated protein C beta-glycoform promotes enhanced noncanonical PAR1 proteolysis and superior resistance to ischemic injury. Blood. 2015;126:915–919. doi: 10.1182/blood-2015-03-632877. [DOI] [PubMed] [Google Scholar]
- 55.Andreou AP, Efthymiou M, Yu Y, Watts HR, Noormohamed FH, Ma D, Lane DA, Crawley JT. Protective effects of non-anticoagulant activated protein C variant (D36A/L38D/A39V) in a murine model of ischaemic stroke. PLoS One. 2015;10:e0122410. doi: 10.1371/journal.pone.0122410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Riewald M, Petrovan RJ, Donner A, Mueller BM, Ruf W. Activation of endothelial cell Protease Activated Receptor 1 by the protein C pathway. Science. 2002;296:1880–1882. doi: 10.1126/science.1071699. [DOI] [PubMed] [Google Scholar]
- 57.Mosnier LO, Griffin JH. Inhibition of staurosporine-induced apoptosis of endothelial cells by activated protein C requires Protease-Activated Receptor-1 and endothelial cell protein C receptor. Biochem. J. 2003;373:65–70. doi: 10.1042/BJ20030341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rezaie AR. Protease-Activated Receptor signalling by coagulation proteases in endothelial cells. Thromb. Haemost. 2014;112:876–882. doi: 10.1160/TH14-02-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bock F, Shahzad K, Vergnolle N, Isermann B. Activated protein C based therapeutic strategies in chronic diseases. Thromb. Haemost. 2014;111:610–617. doi: 10.1160/TH13-11-0967. [DOI] [PubMed] [Google Scholar]
- 60.Griffin JH, Zlokovic BV, Mosnier LO. Protein C anticoagulant and cytoprotective pathways. Int J Hematol. 2012;95:333–345. doi: 10.1007/s12185-012-1059-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang XV, Banerjee Y, Fernandez JA, Deguchi H, Xu X, Mosnier LO, Urbanus RT, de Groot PG, White-Adams TC, McCarty OJ, Griffin JH. Activated protein C ligation of ApoER2 (lrp8) causes Dab1-dependent signaling in U937 cells. Proc. Natl. Acad. Sci. U. S. A. 2009;106:274–279. doi: 10.1073/pnas.0807594106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sinha RK, Yang XV, Fernandez JA, Xu X, Mosnier LO, Griffin JH. Apolipoprotein E receptor 2 mediates activated protein C-induced endothelial Akt activation and endothelial barrier stabilization. Arterioscler Thromb Vasc Biol. 2016;36:518–524. doi: 10.1161/ATVBAHA.115.306795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Coughlin SR. Protease-Activated Receptors in hemostasis, thrombosis and vascular biology. J Thromb Haemost. 2005;3:1800–1814. doi: 10.1111/j.1538-7836.2005.01377.x. [DOI] [PubMed] [Google Scholar]
- 64.Mosnier LO, Sinha RK, Burnier L, Bouwens EA, Griffin JH. Biased agonism of Protease-Activated Receptor 1 by activated protein C caused by noncanonical cleavage at Arg46. Blood. 2012;120:5237–5246. doi: 10.1182/blood-2012-08-452169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mosnier LO, Griffin JH. Non-canonical cleavage of protease activated receptor 1 (PAR1) by activated protein C provides novel insights into the repertoire of cytoprotective and proinflammatory PAR1 signaling. Blood. 2011;118(21):534. [Google Scholar]
- 66.Hollenberg MD, Mihara K, Polley D, Suen JY, Han A, Fairlie DP, Ramachandran R. Biased signalling and Proteinase-Activated Receptors (PARs): Targeting inflammatory disease. British journal of pharmacology. 2014;171:1180–1194. doi: 10.1111/bph.12544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rajagopal S, Rajagopal K, Lefkowitz RJ. Teaching old receptors new tricks: Biasing seven-transmembrane receptors. Nat. Rev. Drug Discov. 2010;9:373–386. doi: 10.1038/nrd3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Katritch V, Cherezov V, Stevens RC. Diversity and modularity of G protein-coupled receptor structures. Trends Pharmacol. Sci. 2012;33:17–27. doi: 10.1016/j.tips.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Soh UJ, Trejo J. Activated protein C promotes Protease-Activated Receptor-1 cytoprotective signaling through Beta-Arrestin and Dishevelled-2 scaffolds. Proc. Natl. Acad. Sci. U. S. A. 2011;108:E1372–E1380. doi: 10.1073/pnas.1112482108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wildhagen KC, Lutgens E, Loubele ST, ten Cate H, Nicolaes GA. The structure-function relationship of activated protein C. Lessons from natural and engineered mutations. Thromb Haemost. 2011;106:1034–1045. doi: 10.1160/TH11-08-0522. [DOI] [PubMed] [Google Scholar]
- 71.Quinn LM, Drakeford C, O'Donnell JS, Preston RJ. Engineering activated protein C to maximize therapeutic efficacy. Biochem Soc Trans. 2015;43:691–695. doi: 10.1042/BST20140312. [DOI] [PubMed] [Google Scholar]
- 72.Guo H, Singh I, Wang Y, Deane R, Barrett T, Fernandez JA, Chow N, Griffin JH, Zlokovic BV. Neuroprotective activities of activated protein C mutant with reduced anticoagulant activity. Eur J Neurosci. 2009;29:1119–1130. doi: 10.1111/j.1460-9568.2009.06664.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 73.Wang Y, Sinha RK, Mosnier LO, Griffin JH, Zlokovic BV. Neurotoxicity of the anticoagulant-selective E149A-activated protein C variant after focal ischemic stroke in mice. Blood Cells Mol Dis. 2013;51:104–108. doi: 10.1016/j.bcmd.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Williams PD, Zlokovic BV, Griffin JH, Pryor KE, Davis TP. Preclinical safety and pharmacokinetic profile of 3K3A-APC, a novel, modified activated protein C for ischemic stroke. Curr. Pharm. Des. 2012;18:4215–4222. doi: 10.2174/138161212802430413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lyden P, Levy H, Weymer S, Pryor K, Kramer W, Griffin JH, Davis TP, Zlokovic B. Phase 1 safety, tolerability and pharmacokinetics of 3K3A-APC in healthy adult volunteers. Curr Pharm Des. 2013;19:7479–7485. doi: 10.2174/1381612819666131230131454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang Y, Zhao Z, Chow N, Rajput PS, Griffin JH, Lyden PD, Zlokovic BV. Activated protein C analog protects from ischemic stroke and extends the therapeutic window of tissue-type plasminogen activator in aged female mice and hypertensive rats. Stroke. 2013;44:3529–3536. doi: 10.1161/STROKEAHA.113.003350. [DOI] [PMC free article] [PubMed] [Google Scholar]