Abstract
We reviewed published MESA (Multi-Ethnic Study of Atherosclerosis) study articles concerning peripheral arterial disease, subclavian stenosis (SS), aortic artery calcium (AAC), and thoracic artery calcium (TAC). Important findings include, compared to non-Hispanic whites, lower ankle-brachial index (ABI) and more SS in African Americans, and higher ABI and less SS in Hispanic and Chinese Americans. Abnormal ABI and brachial pressure differences were associated with other subclinical cardiovascular disease (CVD) measures. Both very high and low ABI independently predicted increased CVD events. Looking at aortic measures, TAC and AAC were significantly associated with other subclinical CVD measures. Comparisons of AAC with coronary artery calcium (CAC) showed that both were less common in ethnic minority groups. However, although CAC was much more common in men than in women in multivariable analysis, this was not true of AAC. Also, when AAC and CAC were adjusted for each other in multivariable analysis, there was a stronger association for AAC than for CAC with CVD and total mortality.
The MESA (Multi-Ethnic Study of Atherosclerosis) study is a large prospective cohort study designed to include 4 major race or ethnicity groups: non-Hispanic White, Hispanic, African American, and Chinese. Participants at baseline were 6,814 men and women 45 to 84 years of age without clinical cardiovascular disease (CVD). The objective was to study of the epidemiology and prognosis of several different measures of subclinical CVD, as well as the progression of subclinical CVD, and their predictive role in clinical CVD. The ankle-brachial index (ABI) was 1 of the measures studied, and indicates whether one has lower extremity peripheral arterial disease (PAD). Using MESA study data, many aspects of PAD have been explored, including the role of race or ethnicity, the influence of different methods of calculating the ABI on PAD prevalence, standard and novel risk factors, genetics, the association of PAD with other subclinical CVDs, and the ABI as a predictor of incident CVD. The interarm blood pressure difference was used to assess the prevalence of subclavian stenosis.
Thoracic aortic calcium (TAC) was measured in the MESA study using the computed tomography (CT) scans for coronary artery calcium (CAC). Measurement of abdominal aortic calcium (AAC) by CT scan was done on a random subset of approximately 2,000 participants. Within the MESA study many aspects of TAC and AAC have been explored, including aortic lengthening and dilation, standard and novel risk factors, associations with other subclinical CVDs, and the utility of TAC and AAC as predictors of incident CVD.
PERIPHERAL ARTERIAL DISEASE
Race or ethnicity and PAD in the MESA study
Using data from the baseline the MESA study visit, an analysis found significant differences in prevalence rates of PAD by race or ethnicity [1]. Specifically, African Americans had a substantially higher unadjusted prevalence of PAD (7.2%) compared with non-Hispanic Whites (3.6%), Hispanics (2.4%), and Chinese (2.0%; p < 0.01). In a multivariable logistic regression model consisting of age and sex, and compared with non-Hispanic Whites, African Americans were found to have over twice the odds of an ABI <0.90 (odds ratio [OR]: 2.28; p < 0.01) whereas Chinese had significantly lower odds (OR: 0.56; p = 0.03). The odds for Hispanics were also lower (OR: 0.74), but this result was not statistically significant (p = 0.14). The addition of body mass index, diabetes, pack-years of cigarette smoking, hypertension, dyslipidemia, education level, and annual income changed the ORs for all 3 raceethnic groups (African American OR: 1.67; Chinese OR: 0.39; Hispanic OR: 0.49; p < 0.05 for all). Additional adjustment for interleukin-6, fibrinogen, D-dimer, and homocysteine modestly changed the magnitudes of these associations: African Americans 1.47 (95% confidence interval [CI]: 1.07 to 2.02), Hispanics 0.45 (95% CI: 0.29 to 0.70) and Chinese 0.44 (95% CI: 0.24 to 0.78). These results are summarized in Figure 1.
FIGURE 1. Ethnic-specific odds ratios for peripheral arterial disease.
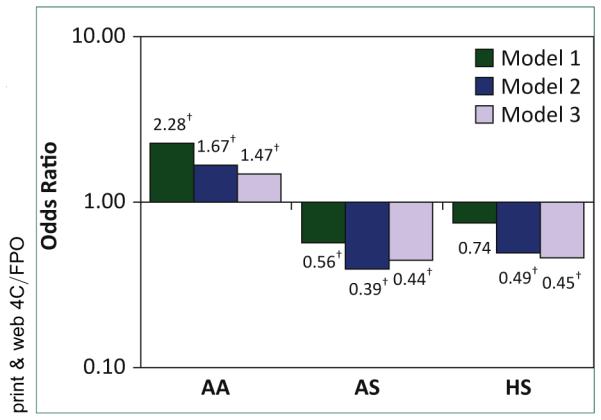
Model 1 was adjusted for age and sex; Model 2 was adjusted for those in Model 1 + diabetes, smoking, hypertension, dyslipidemia, body mass index, education, and income; Model 3 was adjusted for those in Model 2 + interleukin-6, fibrinogen, D-dimer, and homocysteine. *Non-Hispanic (HS) white = reference group. δp < 0.05. AA, African American; AS, Chinese.
Among the MESA study participants without any of the traditional CVD risk factors or the presence of abnormalities for different measures of subclinical atherosclerosis, an analysis of the MESA study data found that African Americans had an ABI 0.02 lower than non-Hispanic Whites, suggesting an intrinsic difference in the ABI [2]. These slight differences, although clinically negligible, could significantly impact the estimation of PAD prevalence in different sex- and race-ethnic-groups.
Using baseline data from the MESA study, an analysis was conducted to determine PAD prevalence within Dominican, Mexican, Puerto Rican, and other Hispanic Americans (n = 1,437), as well as the magnitude and significance of associations between CVD risk factors and PAD within each Hispanic subgroup [3]. In a multivariable logistic model containing the CVD risk factors, only Other Hispanic Americans had significantly lower odds of an ABI >1.0 (OR: 0.51; 95% CI: 0.28 to 0.93), compared with Mexican Americans. In separate Hispanic sub-group–specific multivariable logistic models including the CVD risk factors, increasing age was a significant risk factor for an ABI >1.0 among all Hispanic subgroups except Dominican Americans. In general, female sex was consistently associated with lower odds of PAD, whereas diabetes, current smoking, and hypertension were associated with higher odds of PAD (except for hypertension in Puerto Rican Americans).
Influence of ABI calculation method on PAD prevalence
Both the posterior tibial and dorsalis pedis arteries were used in calculating the ABI. The ABI could be calculated using the highest (ABI-HI), lowest (ABI-LO), or mean (ABI-MN) ankle systolic blood pressure (SBP) in a given leg. In clinical practice, the highest arm and leg SBPs are used to compute the ABI. However, the choice of which pressure to use may have implications for associations between ABI and the underlying burden of atherosclerosis. In an analysis differences were determined in the prevalence of PAD in the MESA study using 3 different methods of calculating the ABI [4].
The ABI was calculated separately in each leg with 3 distinct methods: specifically, for ABI-HI, the higher of the measurements in a given ankle was used; for ABI-LO, the lower of these 2 pressures was used; and for ABI-MN, the average of the 2 pressures was used. This resulted in each participant’s having 6 different ABI values (3 on the right and 3 on the left). From this, 3 index ABI values (ABI-HI, ABI-LO, and ABI-MN) were defined as the lower of the corresponding right and left values for each method. In all cases, the highest brachial artery pressure (right vs. left) was used for the denominator to account for the possible influence of subclavian stenosis.
The prevalence of PAD between men and women was compared by ABI method within each race-ethnic group. In general, the prevalence of PAD was higher among women than among men, regardless of race-ethnic group. When the ABI-LO method was used, a significantly higher prevalence of PAD was identified among women for non-Hispanic Whites, Chinese, and African Americans. When the ABI-MN method was used, a significantly higher prevalence of PAD was identified among non-Hispanic Whites and Chinese. When the ABI-HI method was used, there were no significant sex differences in PAD prevalence within any race-ethnic group.
Among all race-ethnic groups and in both sexes, the prevalence of PAD differed by the calculation method. Overall and compared with ABI-HI, the prevalence of PAD was 3.95 times higher among women when ABI-LO was used, whereas among men the prevalence was 2.74 times higher when this pressure was used. When the ABI-LO method was used, the corresponding (female and male) prevalence among non-Hispanic Whites, Chinese, African Americans, and Hispanics were 4.71 and 3.19, 3.44 and 3.15, 3.39 and 2.56, and 3.94 and 2.04 times higher, respectively. When ABI-MN was used, the prevalence of PAD was intermediate between the prevalence for the highest and lowest ankle pressures.
Standard and novel risk factors for PAD
Using data from the baseline the MESA study visit, the associations of several risk factors for CVD and the presence of PAD defined using the ABI have been examined. Among participants without traditional CVD risk factors or subclinical atherosclerosis, an analysis showed that male sex, weight, and high education level were positively correlated with ABI, whereas triglycerides, pack-years (in past smokers), and pulse pressure were negatively correlated [2].
From a separate analysis of all the MESA study participants using multivariable logistic regression, the associations between risk factors and PAD (ABI ≤0.90) are shown in Table 1. The results indicate that age, diabetes mellitus, any cigarette smoking, hypertension, dyslipidemia, and African American ethnicity are associated with significantly higher odds for the presence of PAD, whereas a higher education level and being Chinese or Hispanic are associated with significantly lower odds. A higher body mass index was marginally associated with lower odds for PAD. When the summary variable for diabetes in the aforementioned multivariable model was replaced by a variable that used 3 categories based on fasting glucose levels, participants with impaired fasting glucose had an 87% higher odds ratio for PAD (p = 0.02). Similarly, each 1-mU/l increment in fasting serum insulin was associated with a 29% higher odds ratio for PAD. When analyzed individually and with adjustment for traditional CVD risk factors, the “novel” risk factors homocysteine, C-reactive protein, interleukin-6, D-dimer, fibrinogen, and plasmin-antiplasmin, were positively associated with PAD. Factor VIII and Chlamydia pneumoniae titer were not significantly associated. Notably, in a model containing interleukin-6, C-reactive protein, and the traditional risk factors, interleukin-6 remained significant, but C-reactive protein did not [1].
TABLE 1.
Traditional risk factors for peripheral arterial disease
| Model 1 | Model 2 | |||
|---|---|---|---|---|
|
|
|
|||
| Risk Factor | Odds Ratio | 95% CI | Odds Ratio | 95% CI |
| Age (1 year) | 1.10 | 1.08–1.11 | 1.09 | 1.07–1.11 |
|
| ||||
| Male | 0.99 | 0.77–1.27 | 0.95 | 0.73–1.23 |
|
| ||||
| Race/ethnicity | ||||
| Non-Hispanic White | 1.00 | – | 1.00 | – |
| Chinese | 0.56 | 0.32–0.96 | 0.39 | 0.22–0.69 |
| African American | 2.28 | 1.72–3.01 | 1.67 | 1.23–2.26 |
| Hispanic | 0.74 | 0.50–1.10 | 0.49 | 0.32–0.76 |
|
| ||||
| Diabetes mellitus | 2.12 | 1.57–2.87 | ||
|
| ||||
| Smoking | 3.42 | 2.48–4.73 | ||
|
| ||||
| Hypertension | 1.63 | 1.22–2.18 | ||
|
| ||||
| Dyslipidemia | 1.58 | 1.22–2.05 | ||
|
| ||||
| Body mass index | 0.97 | 0.94–0.99 | ||
|
| ||||
| Education* | 0.74 | 0.55–0.98 | ||
|
| ||||
| Income† | 0.75 | 0.56–1.01 | ||
Model 1 adjusted for age, sex, and race or ethnicity; Model 2 adjusted for age, sex, race or ethnicity, diabetes, smoking, hypertension, dyslipidemia, body mass index, education, and income. CI, confidence interval.
Greater than high school compared with high school or less.
Greater than $25,000 compared with less than this amount.
The associations between measures of kidney function and PAD has been explored [4]. Using baseline the MESA study data, there were 813 participants (12.0%) with microalbuminuria and 100 (1.5%) with macroalbuminuria. After adjustment for demographic factors, among diabetics, and compared to those with no albuminuria, those with albuminuria had a 1.90-fold greater odds of PAD (95% CI: 1.19 to 3.04). After further adjustment for major CVD risk factors, the odds ratio modestly attenuated to 1.65 (95% CI: 1.00 to 2.74). For nondiabetic subjects, Model 1 was suggestive of an increased risk, with an odds ratio of 1.44 (95% CI: 0.99 to 2.05), but Model 2 indicated the excess risk was largely due to confounding by major CVD risk factors. The degree of albuminuria (micro vs. macro) was not associated with PAD in either diabetic or nondiabetic subjects [5].
Contributions of the MESA study toward understanding genetic and biomarker determinants of lower extremity PAD
Genetics
In the pre–genome-wide association study (GWAS) era, genetics studies of PAD were largely candidate gene, and were conducted primarily in Europeandescent participants. These studies identified genes primarily in inflammation, coagulation, and reninangiotensin pathways associated with either the ABI or PAD [6-13]. A few additional studies found genes important in nicotine metabolism and dependence pathways important for PAD [14,15].
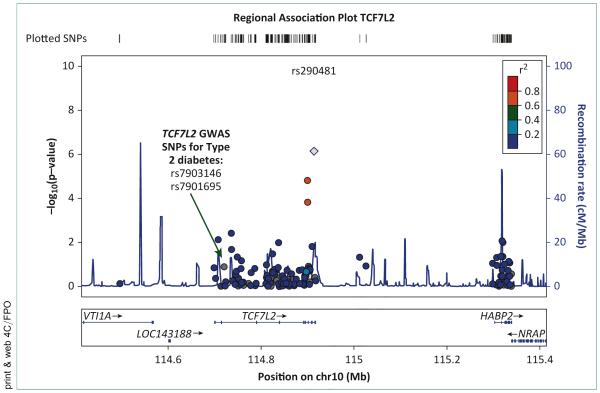
During the GWAS era, the MESA study has participated in 2 consortia, the CARe (Candidate Gene Association Resource) and the CHARGE (Cohorts for Heart and Aging Research in Genomic Epidemiology) consortia, which have informed the current literature on genetic variants underlying PAD. In the discovery phase meta-analysis of 8 studies (n = 21,547), using the CARe CVD-centric gene chip [16], an analysis found 2 genetic variants, rs2171209 in SYTL3 and rs290481 in TCF7L2, significantly associated with lower ABI (Fig. 2) [17]. TCF7L2 is involved in the Wnt signaling pathway and is associated with impaired beta cell function, impaired insulin secretion and increased hepatic glucose production [18] and is a well-known and replicated gene in several race-ethnic groups for type 2 diabetes susceptibility [19-25]. The TCF7L2 variant, rs290481, was not located near, or in linkage disequilibrium with, previously confirmed variants for type 2 diabetes (Fig. 2). SYTL3 has been linked to lipoprotein(a) (Lp(a)) levels [26].
FIGURE 2. Regional association of TCF7L2 with the ankle-brachial index (ABI) in the CARe (Candidate Gene Association Resource) consortia.
GWAS, genome-wide association study; SNP, ■■■.
Both type 2 diabetes and Lp(a) are known risk factors for PAD. However, neither the variant in TCF7L2, nor the variant in SYTL3, replicated with the addition of 9 further studies (n = 15,440). One genetic variant in CYP2B6, a gene linked to smoking behavior [15], was among the strongest associations for PAD in participants of European descent; however, it did not meet array-wide significance in the discovery phase, and thus was not pursued for replication. In African Americans from CARe, no genetic variants were associated with ABI or PAD at an array-wide level of significance.
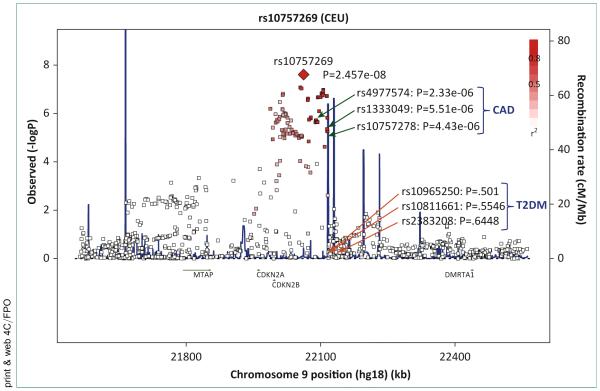
Another large effort to identify genetic variants for PAD was the CHARGE consortium, to which the MESA study contributed European-descent participants as a replication study. A GWAS analysis of 41,692 European and European American participants from 10 discovery and 8 replication studies including the MESA study found that rs10757269 in CDKN2B in 9p21 region was significantly associated with the ABI (Fig. 3) [27], a region of known association with coronary artery disease (CAD) and atherosclerosis [28-31]. The association of rs10757269 with the ABI was modestly attenuated when CAD was accounted for.
FIGURE 3. Regional association of 9p21 with the ankle-brachial index (ABI) in the CHARGE consortia.
CAD, coronary artery disease; CEU, ■■■; T2DM, type 2 diabetes mellitus.
In the United States, many race-ethnic groups are a mixture of genetic and ancestral backgrounds. For example, African Americans in the United States are a mixture of European and African ancestral groups, whereas Hispanics in the United States are an admixed group with European, African, and Native American backgrounds [32,33]. Allele frequency differences, particularly in disease variants, resulting from this admixture among groups can affect expression of disease phenotypes. This in turn could be partly responsible for differences in disease prevalence and incidence among race-ethnic groups. In the MESA study, an analysis [34] found that higher percent global Native American ancestry was associated with significantly lower odds of PAD among Hispanics. However, European ancestry was not associated with PAD among either African Americans or Hispanics.
Biomarkers
In examining associations with markers of inflammation, coagulation, and adhesion, the MESA study has contributed above and beyond the well-known associations of more traditional biomarkers for PAD such as C-reactive protein, D-dimer, and fibrinogen. In a subgroup with normal CVD risk factor and subclinical disease measures in the MESA study higher interleukin-6 per natural log unit was associated with a 1.4-fold greater odds of PAD [35]. Also in the MESA study, higher p-selectin per standard deviation was significantly associated with a 30% increased risk of incident PAD and a lower ABI over time [36].
The MESA study has particularly made a contribution in the area of race-ethnic differences in associations of biomarkers with PAD. Higher L-selectin was marginally associated with a higher ABI in a subsample of 2,403 MESA study participants [37], and this association did not differ by race-ethnic group. Higher Lp(a) (natural log units) was significantly associated with a 1.7-fold higher odds of PAD in Hispanic men, and a 1.5-fold higher odds of PAD in Hispanic women [38]. There were no significant associations for European descent, African Americans, or Chinese men and women. Additionally, Lp(a) exhibited a J-shape association with ABI categories with Lp(a) elevated in the lowest and highest ABI groups.
PAD associations with other subclinical CVD
Lower extremity PAD affects approximately 10% to 15% of community-dwelling men and women 65 years of age and older [39]. In primary-care medical practices, PAD affects 29% of men and women who are 70 years of age and older or 50 to 69 years of age with a history of diabetes or smoking [40]. Identifying the association of PAD with the prevalence of other subclinical CVDs is important for understanding the clinical significance of PAD and for identifying potential targets for interventions to prevent adverse outcomes in people with PAD. Because of its thorough characterization of subclinical diseases, the MESA study cohort provided an ideal opportunity to relate a low ABI and PAD with other subclinical diseases.
The MESA study cohort established that a low ABI is associated with an increased prevalence of subclinical atherosclerosis in other vascular beds, and with reduced upper extremity vessel compliance and increased left ventricular mass. Among men participating in the MESA study, a low ABI was also associated with reduced bone density [41-44].
Among 3,458 women and 3,112 men (average 62.8 years of age) in the MESA study, lower ABI values were associated with higher carotid artery intima-media thickness and an elevated CAC score among men and women [41]. In these analyses, ABI values were categorized as follows: ABI <0.90 (definite PAD), 0.90 to 0.99 (border-line ABI), 1.00 to 1.09 (low-normal ABI), 1.10 to 1.29 (normal ABI), and ABI ≥1.30 (high ABI). Because a truly normal ABI is between 1.10 and 1.30, an ABI value of 1.00 to 1.09 can be considered low normal and an ABI of 0.90 to 0.99 is borderline. Adjusting for age, race or ethnicity, cholesterol, SBP, body mass index, diabetes, smoking, and cholesterol-lowering medication, the presence of ABI <0.90 and borderline ABI were each associated with significantly higher internal carotid artery intima-media thickness compared to a normal ABI value in both men and women [41]. Among men, and compared to a normal ABI, a low normal ABI value was also associated with a higher internal carotid artery intima-media thickness. In fully adjusted analyses, among men and women, respectively, an ABI <0.90 was associated with significantly higher odds of having a CAC score >20 compared with a normal ABI value [41]. In addition, men with a borderline ABI had higher odds of a CAC score >20 compared with men with a normal ABI.
In the MESA study a high ABI value was associated with greater left ventricular mass [42]. High ABI values occur in the setting of stiff lower extremity arteries, requiring substantively higher pressures to occlude the lower extremity arteries. Among the MESA study participants, ABI values <0.90 and values >1.40 were associated with significantly increased MRI-measured left ventricular mass, adjusting for age, sex, race or ethnicity, diabetes, hypertension, smoking, and other potential confounders. After additional adjustment for measures of subclinical disease including CAC score and carotid artery intimamedia thickness, the association of high ABI with higher left ventricular mass remained highly statistically significant, but there was no remaining association of low ABI with left ventricular mass. These findings demonstrate that lower extremity arterial stiffness is associated with higher left ventricular mass. Further study is needed to determine whether this association occurs due to a nonatherosclerotic pathway and whether greater left ventricular mass may be a causal intermediary between high ABI values and heart failure [42].
Using tonometry-derived measures of small- and largevessel compliance, a lower ABI value in the MESA study was associated with poorer small-vessel compliance [43] and greater impairment in small-vessel compliance was associated with a higher incidence of significant ABI decline over time. In contrast, tonometry-measured largevessel compliance was not associated with either lower ABI values or with faster declines in the ABI over time. A previous cross-sectional study also demonstrated an association of lower ABI values with poorer small-vessel compliance [44]. However, the MESA study cohort established this association in a much larger cohort, and demonstrated that the association remained significant even after multivariable adjustment. Only the MESA study has demonstrated an association of poorer vessel compliance with faster rates of ABI decline.
Finally, in a randomly selected sample of 904 female and 929 male MESA study participants, the association of CT-measured bone mineral density in the spine with the ABI and carotid artery intima-media thickness was assessed. Lower ABI values were associated significantly with lower bone mineral density in men. However, this association was not demonstrated in women [45]. The association of a lower ABI with reduced bone mineral density has been demonstrated in several other studies [46-48]. However, the MESA study was the first study to demonstrate the association of lower ABI values with lower lumbar spine bone mineral density and the first to suggest that this association occurred in men but not women. The link between lower ABI and reduced bone density may be related to the fact that people with lower ABI values have lower physical activity and lower activity is associated with lower bone density.
High ABI
A high ABI is a marker of abnormal stiffening of the posterior tibial or dorsalis pedis arteries. When compared to those with an ABI between 0.90 and 1.40, individuals with a high ABI (>1.40) have been shown to be at significantly higher risk of incident CVD [49].
In the MESA study, an analysis examined the risk factors associated with conversion from a normal (0.90 to 1.40) ABI to a high (>1.4) ABI value [50]. In the analyses of prospective changes, the ABIs from the same leg were compared from baseline to visit 3. They defined progression to a high ABI as going from a normal ABI at baseline to an ABI >1.40 at visit 3. Individuals with an incompressible ankle artery at either study visit were excluded from the study.
The mean time between visit 1 and visit 3 ABI measurements was 3.2 ± 0.32 years. Of the 5,514 MESA study participants with a baseline ABI between 0.90 and 1.40, 71 (1.3%) had an ABI >1.40 at visit 3. Compared with those in the normal ABI group, the significant baseline risk factors for progressing into the high-ABI group included a higher baseline ABI, body mass index, and insulin level but a lower high-density lipoprotein cholesterol (HDL-C) value. Baseline smoking was less prevalent among those who progressed into the high-ABI group. In a multivariable logistic regression model, the odds of progression into the high-ABI group were only significant for male sex (OR: 1.78; 95% CI: 1.06 to 3.00) and baseline body mass index (1-unit increment; OR: 1.08; 95% CI: 1.03 to 1.13). Statin use was associated with a significant reduction in the odds for progression to a high ABI (OR: 0.29; 95% CI: 0.09 to 0.94). None of the 3-year changes in other CVD risk factors were significantly association with progression into the high-ABI group.
With adjustment for age and sex and compared with non-Hispanic Whites, African Americans had a lower odds for progressing to the high ABI group after adjustment for the traditional CVD risk factors (OR: 0.50; 95% CI: 0.24 to 1.00). Chinese and Hispanic participants were not at a different risk than non-Hispanic Whites for progression to the high ABI group.
ABI as a predictor of incident CVD
It is now well recognized that an abnormally low ABI is a strong and independent predictor of incident coronary heart disease (CHD), total CVD, and CVD- and all-cause mortality [51,52]. In addition, this is true of both symptomatic and asymptomatic persons [53]. Evidence now supports a normal range of ABI from about 1.00 to 1.40, with higher and lower values both associated with increased CVD risk [52,54].
The MESA study provided the opportunity to study the predictive value of the ABI for CVD compared to multiple other subclinical measures. 6,647 MESA study men and women were followed for 5.3 years [54]. The cutpoints of <1.00 (arterial obstruction) and >1.40 (arterial stiffness) were used to define a low and a high ABI, and both were associated with CVD events, with a hazard ratio (HR) of 1.77 for a low and 1.85 for a high ABI after adjustment for both traditional and newer biomarker risk factors when compared with the reference group (1.00 ≤ AB1 ≤ 1.4). The ABI also significantly improved risk discrimination beyond the standard risk factors. Uniquely, the MESA study allowed Criqui et al. to further adjust the ABI-CVD association for CAC, common and internal carotid intima-media thickness, and major ECG abnormalities, which only modestly attenuated the ABI associations with CVD. Figure 4 shows the association of high, low, and normal ABIs with incident CVD by follow-up year, showing similarly elevated risks for a low and a high ABI over time compared to the normal ABI group. Subgroup analyses showed that HRs for CHD were similar for a low and high ABI, 1.87 and 2.15 respectively, but a high ABI had a stronger association with stroke (HR: 2.69) than a low ABI (HR: 1.56). Consistent with these findings, a prior study using pulse wave velocity as a measure of vascular stiffness showed a stronger association with stroke than with other CVD events [55].
FIGURE 4.
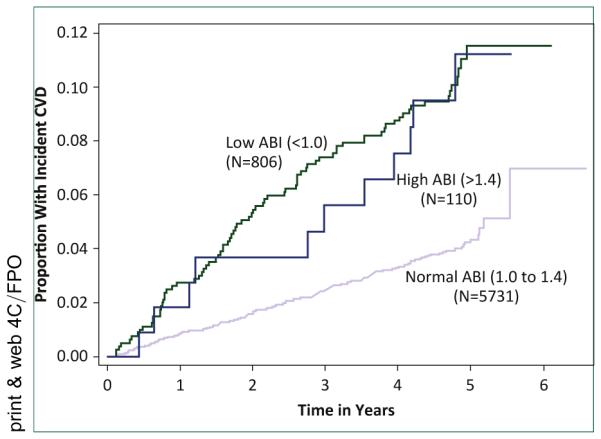
ABI, ankle-brachial index; CVD, cardiovascular disease.
Analyses using the ABIs <1.40 as a continuous variable also showed a significant association with CVD events. Additionally, analyses stratified by the 4 race-ethnic groups in the MESA study, non-Hispanic Whites, African Americans, Hispanics, and Chinese showed a similar association between ABI levels and CVD events in each group. In a model with all race-ethnic groups, the p-value for an interaction term for ABI by race-ethnic group was not significant.
The ABI has been evaluated in the MESA study for its ability to further discriminate risk in persons at intermediate risk by the Framingham CHD risk score (i.e., >5% to <20% 10-year risk) [56]. This group is thought to be the most likely to benefit clinically from additional risk discrimination beyond risk scores based on CVD risk factors. In this group of 1,330 MESA study participants, 6 risk markers including subclinical CVD measures were compared. These were the ABI, brachial flow-mediated dilation, CAC, carotid intima-media thickness, family history of CHD, and highly sensitive C-reactive protein. For CVD prediction, CAC showed the highest net reclassification index beyond the risk model, and the ABI showed the second best net reclassification index. The ABI is now recommended by the 2013 American College of Cardiology/American Heart Association Guideline on the Assessment of Cardiovascular Risk as 1 of 4 measures that could be used to inform dyslipidemia treatment when a risk-based treatment decision is uncertain [57].
Subclavian stenosis, another presentation of peripheral atherosclerosis
Atherosclerosis affects also the upper extremities, although symptoms are infrequent. The disease is typically localized proximally, primarily in the subclavian arteries, including the innominate artery. The comparison of systolic pressures between arms can be the first step to diagnose hemodynamically significant disease, because severe bilateral disease is uncommon. Two angiographic series reported intermediate sensitivity but high specificity of interarm difference of SBPs (IADSBP) to identify >50% subclavian stenosis (SCS) [58,59]. Hence, the identification of IADSBP is an easily accessible tool to detect SCS in epidemiological studies and primary care.
The epidemiology and clinical significance of IADSBP have only been determined recently. In a study combining data from 2 population-based and 2 clinical studies, an IADSBP ≥15 mm Hg was associated with most traditional risk factors (smoking, SBP, low HDL) as well as concomitant PAD and clinical CAD [60]. Another analysis in this population showed a significant association between SCS and total and CVD mortality [61]. Other studies have since concurred with these findings and a meta-analysis confirmed the utility of SCS detection to identify a subset of individuals at high risk of CVD mortality [62].
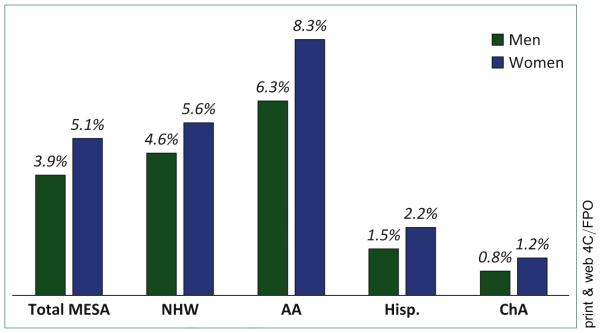
From this perspective, the MESA study provided important insights, identifying sex- and race-ethnic–specific data in the prevalence of this condition and its association with other major atherosclerotic localizations [63]. The MESA study revealed a prevalence of 4.6% of SCS defined as IADSBP ≥15 mm Hg, which was more frequent in women (5.1%) than in men (3.9%), with increasing rates in the elderly. The prevalence, at 2.5% and 3.7% in men and women 45 to 54 years of age, rose to 4.4% and 6.9% in those over 75 years of age, respectively. The MESA study also highlighted important disparities in different race-ethnic groups showing higher rates in African Americans and lower rates in Hispanic and Chinese participants (Fig. 5). Risk factors found associated with this condition were pulse pressure, C-reactive protein levels and body mass index. Other traditional risk factors had statistically inconsistent or borderline association with SCS. However, adjusted for these risk factors, SCS was significantly associated with other presentations of atherosclerosis in other localizations. Among subjects with SCS, 11.4% also had an ABI ≤0.90 (vs. 3.6% in those without SCS; p < 0.001). In turn, 13.1% of those with PAD had SCS, compared with 4.2% in those without PAD. Adjusted for risk factors, SCS was associated with PAD (OR: 2.35), the highest common carotid artery intima-media thickness quartile (OR: 1.32) and high CAC (score >100 vs. score = 0; OR: 1.43) [63]. The MESA study was the first study to qualify SCS as marker of subclinical atherosclerosis in other arterial beds.
FIGURE 5. Prevalence of subclavian stenosis in the MESA (Multi-Ethnic Study of Atherosclerosis) study cohort, 2000 to 2002.
AA, African American; ChA, Chinese; Hisp, Hispanic; NHW, non-Hispanic White.
THE AORTA
Aorta lengthening and dilation
Epidemiologic studies have reported a significant downward shift of the bifurcation position with increasing age, and 1 study reported this downward shift was independent of age-related changes in lumbar length [64,65]. Although most studies have found a more pronounced downward shift after the fifth and sixth decades of life, reports on whether this shift is more prominent in men or women have been mixed, and no race-ethnic differences have been observed.
In the MESA study, a more caudal bifurcation position was independently associated with CVD risk factors of older age, male sex, smoking, and hypertension [65]. All are recognized risk factors for abdominal aortic aneurysm (AAA). AAA is thought to be caused by weakening of the aortic wall, from pathologic degradation of elastin fibers. Shear forces from blood flow coupled with loss of elastin cause the aortic diameter to increase and potentially rupture. Some have postulated that a similar mechanism of elastin fiber loss due to the natural aging process may also cause aortic lengthening, and the observed caudal positioning of the bifurcation over time. This hypothesis is supported by the observation in the MESA study that a more cephalad position of the bifurcation was independently associated with diabetes and elevated triglycerides. Diabetes and elevated triglycerides are the only CVD risk factors commonly found to be inversely associated with aortic aneurysmal disease. Hypertriglyceridemia is common problem in diabetics. Diabetes is associated with glycation products thought to cause rigidity and stiffening of the arterial walls. Aortic stiffening, thought to impede dilation in AAA, may also impede age-related bifurcation descent.
Epidemiologic studies have also reported significant age-associated increases in abdominal aortic diameter at the bifurcation [66,67]. In a study of 504 patients, increasing aortic diameter was independently associated with CVD risk factors of older age, male sex, and higher body mass index [66]. Results were similar in the MESA study, where increasing aortic diameter was associated with hypertension as well. In both studies diabetes and abnormal lipids were inversely associated with increasing aortic diameter, though results were only significant for diabetes in 1 study [66], and abnormal lipids in the other study [67]. Taken together, epidemiologic studies of age-related changes in the bifurcation position and the aortic diameter at the bifurcation suggest lengthening and dilation in the abdominal aorta over time. CVD risk factors commonly associated with plaque formation are positively associated with both lengthening and dilation, whereas diabetes, which a known risk factor for arterial stiffness, is inversely associated with both.
Measurement of TAC and AAC
A key component of the initial protocol for the MESA study was CT. Field centers assessed CAC using an electron beam CT scanner at 3 field centers and a multidetector CT scanner at 3 centers, both with electrocardiography triggering [68]. These scans were completed in nearly all MESA study participants. Scans were brightness adjusted using a phantom and read at the MESA study CT reading center (UCLA Harbor, Los Angeles, California, USA). Because TAC was read from the cardiac CTs, the aorta arch was largely not visualized. The parts of the aorta imaged were the ascending thoracic aorta measured from the aortic annulus to the lower edge of the pulmonary artery, and the descending thoracic aorta measured from the lower edge of the pulmonary artery to the cardiac apex. Because calcium was scored throughout the visible thoracic aorta, and body size varies, there was minor variability from participant to participant related to the length of aorta imaged [69].
Prior to the second and third MESA study exams, additional funding was obtained to image AAC [70]. To measure AAC, electron-beam CT scanners were used at 3 field centers. These were set as follows: scan collimation of 3 mm, slice thickness of 6 mm, and reconstruction using twenty-five 6-mm slices with 35-cm field of view and normal kernel. Multidetector CT mode scanners were used at the remaining 3 field centers. Images were reconstructed in a 35-cm field of view with 5 mm slice thickness. All scan scores were brightness adjusted with a standard phantom. Images were taken from 15 cm cephalad to the L5-S1 lumbar plate as determined from the scout film, with the aortic bifurcation presumed to be at the L5-S1 plate. This presumption was correct on average, but there was much variability in the cohort. Empirical analysis revealed that images obtained showed at least 8 cm of abdominal aorta cephalad to the aortic bifurcation in most subjects. Thus, AAC was only measured in that 8-cm segment for comparability across participants. Noncontrast CT images were analyzed centrally using a standard protocol by the MESA study CT Reading Center. Calcification was identified as a plaque of ≥1 mm2 with a density of >130 Hounsfield units, and was quantified using the Agatston scoring method.
Risk factors for AAC and TAC, compared with CAC, standard and novel
Risk factors for AAC
Considerable data have been published showing the associations of traditional and novel CVD risk factors with CAC, but much less so for AAC [71,72]. In the MESA study, risk factor associations for AAC were compared with risk factor associations for CAC in 1,974 men and women [70]. For AAC and CAC logistic regression models were used for categorical comparisons of AAC and CAC (present or absent) and multiple linear regression for continuous comparisons. Slightly more than one fifth of persons (21.1%) had neither AAC nor CAC, 21.1% had AAC only, 7.3% had CAC only, and 50.5% had both. Table 2 shows the results. In general, both AAC and CAC were more common in non-Hispanic Whites than in the other 3 race-ethnic groups. AAC was significantly associated with cigarette smoking, high LDL-C, and low HDL-C, and showed no sex difference. In contrast, CAC showed much weaker associations with smoking and dyslipidemia and a strong male predominance. Age and hypertension were associated similarly and significantly with AAC and CAC. Novel risk factors generally showed no independent association with either AAC or CAC. The area under the receiver operating characteristic curves was much higher for AAC, 0.859, than for CAC, 0.794. The results for analysis of AAC and CAC as continuous variables were similar. Thus, AAC showed stronger correlations with most CVD risk factors than did CAC. There was no lipid-independent association of testosterone, estradiol, dehydroepiandrosterone, or sex hormone binding globulin with AAC in the MESA study [73]. In a separate study, a subset of 1,147 persons in the MESA study with AAC measured also had determination of fine particulate matter (PM2.5) levels [74]. A slightly elevated risk of aortic calcification (RR = 1.06; p = NS) was observed with a 10 μg/m3 contrast in PM2.5. The PM2.5-associated risk of aortic calcification was stronger among participants with long-term residence near a PM2.5 monitor (RR: 1.11; p = 0.05), and among participants not recently employed outside the home (RR: 1.10; p = 0.05). PM2.5 associations were observed most consistently among Hispanics.
TABLE 2.
Multivariable logistic model analyses for the presence of AAC and CAC
| Multivariable Adjusted Models* | ||
|---|---|---|
| AAC (0 vs. Nonzero) |
CAC (0 vs. Nonzero) |
|
| n | 552 vs. 1,371 | 813 vs. 1,110 |
| Area under the ROC curve/C-statistic | ||
|
| ||
| Age-, sex-, race/ethnicity-adjusted model | 0.794 | 0.770 |
|
| ||
| Fully adjusted model | 0.859 | 0.794 |
|
| ||
| Age at baseline (per 10 years) | 3.41 (2.89–4.02) | 2.43 (2.14–2.77) |
|
| ||
| Male (vs. female) | 0.91 (0.69–1.20) | 2.83 (2.23–3.60) |
|
| ||
| Race/ethnic group (vs. non-Hispanic White) | ||
| Chinese | 1.19 (0.79–1.80) | 0.79 (0.55–1.11) |
| African American | 0.29 (0.21–0.41) | 0.43 (0.32–0.58) |
| Hispanic | 0.49 (0.36–0.68) | 0.71 (0.54–0.94) |
|
| ||
| Smoking status (vs. never) | ||
| Former | 1.72 (1.24–2.40) | 1.79 (1.38–2.31) |
| Current | 3.32 (2.04–5.41) | 1.51 (1.05–2.17) |
|
| ||
| Pack-years (per 20 pack-years) | 1.88 (1.44–2.45) | 1.02 (0.93–1.12) |
|
| ||
| Systolic BP (per SD, 20.8 mm Hg) | 1.21 (1.05–1.39) | 1.23 (1.09–1.38) |
|
| ||
| Any antihypertensive medications (vs. no) | 2.09 (1.54–2.83) | 1.69 (1.33–2.16) |
|
| ||
| Glycemic status (vs. normoglycemia) | ||
| Impaired fasting glucose | 1.28 (0.96–1.72) | 1.01 (0.79–1.30) |
| Diabetes | 1.37 (0.89–2.10) | 1.42 (0.99–2.03) |
|
| ||
| HDL-C (per SD, 15.2 mg/dl) | 0.77 (0.67–0.88) | 0.91 (0.81–1.03) |
|
| ||
| LDL-C (per SD, 31.3 mg/dl) | 1.52 (1.34–1.74) | 1.22 (1.09–1.36) |
|
| ||
| Any lipid-lowering medications (vs. no) | 2.43 (1.59–3.72) | 1.62 (1.18–2.23) |
|
| ||
| Calcium (per SD, 0.51 mg/dl)† | 1.09 (0.90–1.31) | 1.10 (0.94–1.29) |
|
| ||
| Phosphorus (per SD, 0.52 mg/dl)† | 0.96 (0.79–1.17) | 1.11 (0.95–1.31) |
AAC, abdominal aortic calcium; CAC, coronary artery calcium; BP, blood pressure; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; ROC, receiver-operating characteristic; SD, standard deviation.
Estimated from multivariable logistic regression models. All variables shown in the table were entered in the models simultaneously.
Subset (n = 1,098), with calcium and phosphorous measurements.
Risk factors for TAC
Several MESA study papers have examined the epidemiology and prognostic significance of TAC. Age, sex, race or ethnicity, and risk factor relationships with TAC have been examined in the MESA study [75]. It was noted that at all age groups, women had a higher prevalence of TAC than did men (overall 29.1% vs. 26.8%), with smoking the strongest predictor of TAC, along with diabetes, hypercholesterolemia, elevated low-density lipoprotein cholesterol, low HDL-C, family history of myocardial infarction, and elevated C-reactive protein. Of interest, TAC prevalence was similar in Chinese as in non-Hispanic Whites (both 32.4%), higher than that found in Hispanics (24.9%) and African Americans (22.4%). TAC incidence in the MESA study over 2.4 years (from those without TAC at baseline) was significantly lower in African Americans than in non-Hispanic Whites and relates as well to age, SBP, antihypertensive medication and smoking [76]. Further, age, SBP, lipid-lowering medication, diabetes, and smoking were all associated with the progression of TAC. Although 1 analysis has previously shown a close correspondence of TAC with CAC [77], another [78] subsequently showed in the MESA study the presence of TAC to be associated with both the incidence of CAC (11 per 100 patient-years vs. 6 per 100 patient-years in those without TAC) and progression of CAC.
In the MESA study, traditional CVD risk factor associations have been compared with TAC versus AAC using ordinal logistic regression with the categories for both TAC and AAC defined as none, and the first through fourth quartiles [79]. The results are shown in Table 3, and can be compared with results for AAC vs. CAC in Table 2. TAC was actually significantly less common in men (OR: 0.61). Race-ethnic differences were similar for TAC and AAC. Similar to the previously reported comparisons with CAC, AAC showed a stronger association with cigarette smoking than TAC, and slightly stronger associations for dyslipidemia. However, hypertension showed a somewhat stronger association with TAC than AAC. The association with diabetes was similar and significant for both TAC and AAC. Additional analyses showed little effect modification for these associations by race or ethnicity.
TABLE 3.
Odds for increasing increments of AAC and TAC: the MESA (Multi-Ethnic Study of Atherosclerosis) study (1990 to 2000)
| AAC | TAC | |||
|---|---|---|---|---|
|
|
|
|||
| Variable | OR | 95% CI | OR | 95% CI |
| Age (per 10 years) | 3.36 | 3.00–3.75 | 4.14 | 3.59–4.76 |
|
| ||||
| Men vs. women | 1.11 | 0.91–1.34 | 0.61 | 0.48–0.77 |
|
| ||||
| Race/ethnic group | ||||
| Non-Hispanic White | 1.00 (Reference) | – | 1.00 (Reference) | – |
| Chinese | 0.95 | 0.71–1.28 | 1.12 | 0.78–1.60 |
| African American | 0.41 | 0.32–0.53 | 0.39 | 0.29–0.54 |
| Hispanic | 0.66 | 0.53–0.83 | 0.62 | 0.47–0.83 |
|
| ||||
| Smoking | ||||
| Never smoker | 1.00 (Reference) | – | 1.00 (Reference) | – |
| Former smoker | 2.31 | 1.90–2.81 | 1.60 | 1.26–2.03 |
| Current smoker | 4.42 | 3.25–6.01 | 2.93 | 1.98–4.34 |
|
| ||||
| Hypertension | 1.82 | 1.50–2.21 | 2.69 | 2.11–3.41 |
|
| ||||
| Diabetes mellitus | 1.54 | 1.18–2.00 | 1.52 | 1.12–2.06 |
|
| ||||
| Family history of coronary heart disease |
1.39 | 1.16–1.67 | 1.44 | 1.15–1.80 |
|
| ||||
| LDL-C (per SD 31) | 1.26 | 1.15–1.38 | 1.09 | 0.97–1.23 |
|
| ||||
| HDL-C (per SD 15) | 0.83 | 0.75–0.92 | 0.82 | 0.73–0.93 |
| Body mass index (per SD 5) |
1.01 | 0.91–1.1 | 0.94 | 0.83–1.06 |
|
| ||||
| Cholesterol medication | 2.08 | 1.67–2.59 | 1.35 | 1.04–1.73 |
For AAC and thoracic aortic calcium (TAC), all listed independent variables are in the same model. CI, confidence interval; OR, odds ratio; other abbreviations as in Table 2.
AAC and TAC associations with other subclinical CVD
It is not surprising that both AAC and TAC have been shown to be associated with other measures of subclinical disease. In the MESA study, those with versus without AAC have been shown to be much more likely to have CAC (60% vs. 16% for women and 80% vs. 35% for men), carotid intima-media thickness ≥1 mm (38% vs. 7% for women and 43% vs. 16% for men), ABI <0.9 (5% vs. 1% in women and 4% vs. 2% in men; all p < 0.01 except p < 0.05 for ABI in men) [80]. Moreover, greater AAC among both women and men has been shown to be associated with lower volumetric trabecular bone mineral density in the MESA study after full adjustment for age, race or ethnicity, risk, and inflammatory factors [81]. Distal aortic calcification, but not proximal aortic calcification has been shown to be associated with forced expiratory volume from pulmonary function testing; this important association may help to explain the strong relation between lung function and CVD [82].
Decreased arterial compliance, estimated by aortic distensibility measured by magnetic resonance imaging in the MESA study has also been shown, even after adjustment for risk factors, CAC, and C-reactive protein to be associated with increased left ventricular area (each standard deviation decrease in aortic distensibility associated with an 18 cm2 increase in left ventricular area) [83]. Moreover, reduced aortic distensibility was shown in another report from the MESA study to be associated with increased odds of retinal arteriolar narrowing (odds ratio 1.7) even after adjustment for risk and inflammatory factors and other measures of subclinical disease [84]. Finally, the prevalence of TAC is greater with decreasing quartiles of aortic distensibility (7%, 17%, 31%, and 42%; p < and the relation of TAC with aortic distensibility remains after adjustment for age, sex, race or ethnicity, and other risk factors [85].
AAC and TAC as predictors of incident CVD
AAC measured in plain lumbar radiographs has predicted CVD in studies prior to the MESA study [86-89]. However, prior to the MESA study, little such data existed on AAC quantified by CT. In addition, AAC is significantly correlated with CAC [70], and no prior study had attempted to see whether the predictive value of AAC was independent of coexisting CAC. In the MESA study, the association of AAC with CAC was examined in a subset of 1,966 participants who had scans for both measures. As AAC is much more prevalent than CAC, and in order for a fair comparison of AAC to CAC in predicting CHD and CVD, percentile categories were used for both measures: 0 to 50th percentile (reference category), 51st to 75th percentile (third quartile), and >75th percentile (fourth quartile). Reference categories could not be smaller than half the participants because CAC was absent in half the participants. Rates of hard CHD, hard CVD, CVD mortality, and total mortality each increased monotonically across increasing categories of both AAC and CAC. The highest crude rates for each of the 4 endpoints were in the highest category (fourth quartile) of AAC. Table 4 [90] shows the results of the multivariate analysis, with all results adjusted for the general Framingham risk score and race or ethnicity. The first 3 rows show the results for AAC, and the next 3 rows the results for CAC. Comparing the fourth quartile to the reference category, the HRs were significantly elevated for each calcium measure for each of the 4 endpoints. The remaining rows show the results for AAC and CAC additionally adjusted for each other. For CHD, the results are somewhat stronger for CAC, though AAC remains significant. For CVD, the results for AAC and CAC are both significant and of similar magnitude. For CVD mortality, the HR for AAC is 5.9 (p = 0.009) whereas for CAC the HR of 2.1 is not significant. Finally, for total mortality the HR for AAC is 2.7 (p ≤ .001), whereas for CAC the HR is 1.9 (p = 0.036). There was no significant interaction of AAC with either sex or race or ethnicity. Additional models using continuous definitions of AAC and CAC gave similar results. Thus, with simultaneous consideration of AAC and CAC, CAC was a stronger predictor of CHD, but AAC was a stronger predictor of CVD mortality and total mortality. These results suggest the possible utility of using AAC in CVD risk prediction.
TABLE 4.
Cox models for hard CHD, hard CVD, CVD mortality, and total mortality for categorical definition of AAC and CAC, adjusted for the general Framingham risk score and race or ethnicity
| Hard CHD* | Hard CVD† | CVD Mortality‡ | Total Mortality§ | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| Percentile Category | HR | p Value | HR | p Value | HR | p Value | HR | p Value |
| AAC only | ||||||||
| 0–50th | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| 51st-75th | 1.49 | 0.383 | 1.87 | 0.069 | 3.77 | 0.054 | 1.43 | 0.232 |
| >75th | 4.06 | <0.001 | 4.00 | <0.001 | 7.83 | 0.002 | 3.51 | <0.001 |
|
| ||||||||
| CAC only | ||||||||
| 0–50th | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| 51st-75th | 4.74 | 0.001 | 3.67 | <0.001 | 4.03 | 0.019 | 2.28 | 0.003 |
| >75th | 6.14 | <0.001 | 4.21 | <0.001 | 3.92 | 0.021 | 2.79 | <0.001 |
|
| ||||||||
| AAC and CAC, percentile | ||||||||
| AAC 0–50th | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| AAC 51st-75th | 1.08 | 0.875 | 1.46 | 0.279 | 3.11 | 0.104 | 1.23 | 0.506 |
| AAC >75th | 2.38 | 0.038 | 2.66 | 0.003 | 5.89 | 0.009 | 2.71 | <0.001 |
| CAC 0–50th | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| CAC 51st-75th | 3.91 | 0.006 | 2.92 | 0.003 | 2.75 | 0.096 | 1.82 | 0.037 |
| CAC >75th | 4.35 | 0.004 | 2.90 | 0.003 | 2.10 | 0.231 | 1.85 | 0.036 |
CHD, coronary heart disease; CVD, cardiovascular disease; HR, hazard ratio; other abbreviations as in Table 2.
Events = 50; total = 1,930.
Events = 83; total = 1,930.
Events = 30; total = 1,966.
Events = 105; total = 1,966.
Limited data have suggested a possible relationship between TAC and CVD events [91]. In the MESA study, the TAC measurement was limited to the cardiac CT slices. Twenty-eight percent of the 6,807 participants had TAC present. After adjustment for CAC categories, TAC presence was associated an increased risk of all CHD in women (HR: 2.15), but not in men (HR: 0.92) [69]. When restricting the analysis to hard CHD (nonfatal MI or CHD related death), the HR for TAC was 1.74 (p = NS). Other endpoints were not evaluated.
In a comprehensive analysis in the MESA study assessing improvement in discrimination from the addition of TAC, aortic valve calcification, mitral annular calcification, pericardial adipose tissue volume, and liver attenuation to the Framingham risk score and CAC, it was noted that the addition of any of these measures resulted in an actual and significant decrease in the area under the curve (all p < 0.01 for change in area under the curve), and concluding that these measures are unlikely to be useful for improving cardiovascular risk prediction [92]. Although follow-up from the MESA study now extends beyond 10 years, with longer follow-up it will be important to examine whether individual components of CVD (e.g., stroke, PAD, heart failure) might be more closely related to TAC and other noncoronary measures of calcification than the composite CHD and CVD endpoints that have been examined thus far, especially for risk prediction in women, where TAC appears to be most closely related to vascular event risk.
In summary, AAC appears to be a somewhat stronger predictor than TAC for CHD, but the associations of TAC with CVD and mortality have yet to be evaluated in the MESA study.
SUMMARY
Study of the upper and lower extremity peripheral arteries in the MESA study has led to unique insights into the significance of subclinical CVD. Risk factors for subclinical disease in these arteries has been determined, including genetic factors and new biomarkers, as well as their association with subclinical CVD in other arterial beds. Importantly, both high and low levels of the ABI have been shown to predict increased CVD events.
Study of the aorta has similarly provided new insights. Predictors of both elongation of the aorta and dilation of the distal abdominal aorta have been published. Risk factors for TAC and AAC have been determined, and differences in risk factors for these 2 subclinical CVD measures detailed, as well as the association of TAC and AAC with other subclinical CVD measures. A major new observation is a stronger association for AAC than for CAC with CVD and total mortality.
Highlights.
Low and high ABI were predictive of incident CVD events after cIMT and CAC adjustment.
TAC was related to new or progressive CAC and inversely with aortic distensibility.
AAC was a better predictor of CVD and all-cause mortality than was CAC.
AAC was more related to CVD risk factors than CAC.
TAC and TAC progression were related to CVD risk factors.
Lower aortic bifurcation & larger aortic diameter were related to CVD risk factors.
ACKNOWLEDGMENTS
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA study investigators and institutions can be found at http://www.mesa-nhlbi.org.
This research was supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040 and UL1-TR-001079 from the National Center for Research Resources. Additional support included AHA 0430032N from the American Heart Association; R01 DK080015 from the National Institutes of Diabetes and Digestive and Kidney Diseases; and R01 HL066075, R01 HL074338, R01 HL074406, R01 HL077449, R01 HL077612, R01 HL088451, R01 HL086719, R01 HL093081, R01 HL096875, R01 HL098077, R01 HL10161-01A1, R21 HL109924, R01 HL076831, R21 HL091217, R21 DA024273, and RC1 HL100543 from the National Heart, Lung, and Blood Institute.
Footnotes
The authors report no relationships that could be construed as a conflict of interest.
REFERENCES
- 1.Allison MA, Criqui MH, McClelland RL, et al. The effect of novel cardiovascular risk factors on the ethnic-specific odds for peripheral arterial disease in the Multi-Ethnic Study of Atherosclerosis (MESA) J Am Coll Cardiol. 2006;48:1190–7. doi: 10.1016/j.jacc.2006.05.049. [DOI] [PubMed] [Google Scholar]
- 2.Aboyans V, Criqui MH, McClelland RL, et al. Intrinsic contribution of gender and ethnicity to normal ankle-brachial index values: the Multi-Ethnic Study of Atherosclerosis (MESA) J Vasc Surg. 2007;45:319–27. doi: 10.1016/j.jvs.2006.10.032. [DOI] [PubMed] [Google Scholar]
- 3.Allison MA, Budoff MJ, Wong ND, Blumenthal RS, Schreiner PJ, Criqui MH. Prevalence of and risk factors for subclinical cardiovascular disease in selected US Hispanic ethnic groups: the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2008;167:962–9. doi: 10.1093/aje/kwm402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allison MA, Aboyans V, Granston T, et al. The relevance of different methods of calculating the ankle-brachial index: the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2010;171:368–76. doi: 10.1093/aje/kwp382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wattanakit K, Folsom AR, Criqui MH, et al. Albuminuria and peripheral arterial disease: results from the multi-ethnic study of atherosclerosis (MESA) Atherosclerosis. 2008;201:212–6. doi: 10.1016/j.atherosclerosis.2007.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fowkes FG, Connor JM, Smith FB, Wood J, Donnan PT, Lowe GD. Fibrinogen genotype and risk of peripheral atherosclerosis. Lancet. 1992;339:693–6. doi: 10.1016/0140-6736(92)90596-u. [DOI] [PubMed] [Google Scholar]
- 7.Fowkes FG, Lee AJ, Hau CM, Cooke A, Connor JM, Lowe GD. Methylene tetrahydrofolate reductase (MTHFR) and nitric oxide synthase (ecNOS) genes and risks of peripheral arterial disease and coronary heart disease: Edinburgh Artery Study. Atherosclerosis. 2000;150:179–85. doi: 10.1016/s0021-9150(99)00366-4. [DOI] [PubMed] [Google Scholar]
- 8.Monsalve MV, Young R, Jobsis J, et al. DNA polymorphisms of the gene for apolipoprotein B in patients with peripheral arterial disease. Atherosclerosis. 1988;70:123–9. doi: 10.1016/0021-9150(88)90106-2. [DOI] [PubMed] [Google Scholar]
- 9.Morrison AC, Doris PA, Folsom AR, Nieto FJ, Boerwinkle E. Atherosclerosis Risk in Communities Study. G-protein beta3 subunit and alpha-adducin polymorphisms and risk of subclinical and clinical stroke. Stroke. 2001;32:822–9. doi: 10.1161/01.str.32.4.822. [DOI] [PubMed] [Google Scholar]
- 10.Kardia SL, Greene MT, Boerwinkle E, Turner ST, Kullo IJ. Investigating the complex genetic architecture of ankle-brachial index, a measure of peripheral arterial disease, in non-Hispanic whites. BMC Med Genomics. 2008;1:16. doi: 10.1186/1755-8794-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flex A, Gaetani E, Pola R, et al. The −174 G/C polymorphism of the interleukin-6 gene promoter is associated with peripheral artery occlusive disease. Eur J Vasc Endovasc Surg. 2002;24:264–8. doi: 10.1053/ejvs.2002.1711. [DOI] [PubMed] [Google Scholar]
- 12.Kullo IJ, Greene MT, Boerwinkle E, Chu J, Turner ST, Kardia SL. Association of polymorphisms in NOS3 with the ankle-brachial index in hypertensive adults. Atherosclerosis. 2008;196:905–12. doi: 10.1016/j.atherosclerosis.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zintzaras E, Zdoukopoulos N. A field synopsis and meta-analysis of genetic association studies in peripheral arterial disease: the CUMAGAS-PAD database. Am J Epidemiol. 2009;170:1–11. doi: 10.1093/aje/kwp094. [DOI] [PubMed] [Google Scholar]
- 14.Li R, Folsom AR, Sharrett AR, Couper D, Bray M, Tyroler HA. Interaction of the glutathione S-transferase genes and cigarette smoking on risk of lower extremity arterial disease: the Atherosclerosis Risk in Communities (ARIC) study. Atherosclerosis. 2001;154:729–38. doi: 10.1016/s0021-9150(00)00582-7. [DOI] [PubMed] [Google Scholar]
- 15.Thorgeirsson TE, Geller F, Sulem P, et al. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature. 2008;452:638–42. doi: 10.1038/nature06846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keating BJ, Tischfield S, Murray SS, et al. Concept, design and implementation of a cardiovascular gene-centric 50 k SNP array for large-scale genomic association studies. PLoS One. 2008;3:e3583. doi: 10.1371/journal.pone.0003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wassel CL, Lamina C, Nambi V, et al. Genetic determinants of the ankle-brachial index: A meta-analysis of a cardiovascular candidate gene 50K SNP panel in the candidate gene association resource (CARe) consortium. Atherosclerosis. 2012;222:138–47. doi: 10.1016/j.atherosclerosis.2012.01.039. PMCID3596171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin T, Liu L. The Wnt signaling pathway effector TCF7L2 and type 2 diabetes mellitus. Mol Endocrinol. 2008;22:2383–92. doi: 10.1210/me.2008-0135. [DOI] [PubMed] [Google Scholar]
- 19.Lettre G, Palmer CD, Young T, et al. Genome-wide association study of coronary heart disease and its risk factors in 8,090 African Americans: the NHLBI CARe Project. PLoS Genet. 2011;7:e1001300. doi: 10.1371/journal.pgen.1001300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeggini E, Weedon MN, Lindgren CM, et al. Replication of genomewide association signals in UK samples reveals risk loci for type 2 diabetes. Science. 2007;316:1336–41. doi: 10.1126/science.1142364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scott LJ, Mohlke KL, Bonnycastle LL, et al. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science. 2007;316:1341–5. doi: 10.1126/science.1142382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saxena R, Gianniny L, Burtt NP, et al. Common single nucleotide polymorphisms in TCF7L2 are reproducibly associated with type 2 diabetes and reduce the insulin response to glucose in nondiabetic individuals. Diabetes. 2006;55:2890–5. doi: 10.2337/db06-0381. [DOI] [PubMed] [Google Scholar]
- 23.Sladek R, Rocheleau G, Rung J, et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007;445:881–5. doi: 10.1038/nature05616. [DOI] [PubMed] [Google Scholar]
- 24.Steinthorsdottir V, Thorleifsson G, Reynisdottir I, et al. A variant in CDKAL1 influences insulin response and risk of type 2 diabetes. Nat Genet. 2007;39:770–5. doi: 10.1038/ng2043. [DOI] [PubMed] [Google Scholar]
- 25.Takeuchi F, Serizawa M, Yamamoto K, et al. Confirmation of multiple risk Loci and genetic impacts by a genome-wide association study of type 2 diabetes in the Japanese population. Diabetes. 2009;58:1690–9. doi: 10.2337/db08-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ober C, Nord AS, Thompson EE, et al. Genome-wide association study of plasma lipoprotein(a) levels identifies multiple genes on chromosome 6q. J Lipid Res. 2009;50:798–806. doi: 10.1194/jlr.M800515-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murabito JM, White CC, Kavousi M, et al. Association between chromosome 9p21 variants and the ankle brachial index identified by a meta-analysis of 21 genome-wide association studies. Circ Cardiovasc Genet. 2012;5:100–12. doi: 10.1161/CIRCGENETICS.111.961292. PMID:22199011. PMCID: PMC 3303225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Helgadottir A, Thorleifsson G, Manolescu A, et al. A common variant on chromosome 9p21 affects the risk of myocardial infarction. Science. 2007;316:1491–3. doi: 10.1126/science.1142842. [DOI] [PubMed] [Google Scholar]
- 29.Cluett C, McDermott MM, Guralnik J, et al. The 9p21 myocardial infarction risk allele increases risk of peripheral artery disease in older people. Circ Cardiovasc Genet. 2009;2:347–53. doi: 10.1161/CIRCGENETICS.108.825935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Samani NJ, Raitakari OT, Sipila K, et al. Coronary artery diseaseassociated locus on chromosome 9p21 and early markers of atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28:1679–83. doi: 10.1161/ATVBAHA.108.170332. [DOI] [PubMed] [Google Scholar]
- 31.Ye S, Willeit J, Kronenberg F, Xu Q, Kiechl S. Association of genetic variation on chromosome 9p21 with susceptibility and progression of atherosclerosis: a population-based, prospective study. J Am Coll Cardiol. 2008;52:378–84. doi: 10.1016/j.jacc.2007.11.087. [DOI] [PubMed] [Google Scholar]
- 32.Burchard EG, Ziv E, Coyle N, et al. The importance of race and ethnic background in biomedical research and clinical practice. N Engl J Med. 2003;348:1170–5. doi: 10.1056/NEJMsb025007. [DOI] [PubMed] [Google Scholar]
- 33.Risch N, Burchard E, Ziv E, Tang H. Categorization of humans in biomedical research: genes, race and disease. Genome Biol. 2002:3. doi: 10.1186/gb-2002-3-7-comment2007. comment2007.1ecomment2007.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allison MA, Peralta CA, Wassel CL, et al. Genetic ancestry and lower extremity peripheral arterial disease in the Multi-Ethnic Study of Atherosclerosis. Vasc Med. 2010;15:351–9. doi: 10.1177/1358863X10375586. PMCID4077267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aboyans V, McClelland RL, Allison MA, et al. Lower extremity peripheral artery disease in the absence of traditional risk factors. The Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2011;214:169–73. doi: 10.1016/j.atherosclerosis.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wassel CL, Rasmussen-Torvik LJ, Callas PW, et al. A genetic risk score comprising known venous thromboembolism loci is associated with chronic venous disease in a multi-ethnic cohort. Thromb Res. 2015;136:966–73. doi: 10.1016/j.thromres.2015.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berardi C, Decker PA, Kirsch PS, et al. Plasma and serum L-selectin and clinical and subclinical cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis (MESA) Transl Res. 2014;163:585–92. doi: 10.1016/j.trsl.2014.02.001. PMID: 2463106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Forbang NI, Criqui MH, Allison MA, et al. Sex and ethnic differences in the associations between lipoprotein(a) and peripheral arterial disease in the Multi-Ethnic Study of Atherosclerosis. J Vasc Surg. 2016;63:453–8. doi: 10.1016/j.jvs.2015.08.114. [DOI] [PubMed] [Google Scholar]
- 39.Newman AB, Siscovick DS, Manolio TA, et al. Ankle-arm index as a marker of atherosclerosis in the Cardiovascular Health Study. Cardiovascular Heart Study (CHS) Collaborative Research Group. Circulation. 1993;88:837–45. doi: 10.1161/01.cir.88.3.837. [DOI] [PubMed] [Google Scholar]
- 40.Hirsch AT, Criqui MH, Treat-Jacobson D, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317–24. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 41.McDermott MM, Liu K, Criqui MH, et al. Ankle-brachial index and subclinical cardiac and carotid disease: the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2005;162:33–41. doi: 10.1093/aje/kwi167. [DOI] [PubMed] [Google Scholar]
- 42.Ix JH, Katz R, Peralta CA, et al. A high ankle brachial index is associated with greater left ventricular mass. J Am Coll Cardiol. 2010;55:342–9. doi: 10.1016/j.jacc.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilkins JT, McDermott MM, Liu K, Chan C, Criqui MH, Lloyd-Jones DM. Associations of noninvasive measures of arterial compliance and ankle brachial index: the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Hypertens. 2012;25:535–41. doi: 10.1038/ajh.2012.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duprez DA, De Buyzere MM, De Bruyne L, Clement DL, Cohn JN. Small and large artery elasticity indices in peripheral arterial occlusive disease (PAOD) Vasc Med. 2001;6:211–4. doi: 10.1177/1358836x0100600402. [DOI] [PubMed] [Google Scholar]
- 45.Hyder JA, Allison MA, Barrett-Connor E, et al. Bone mineral density and atherosclerosis: the Multi-Ethnic Study of Atherosclerosis, Abdominal Aortic Calcium Study. Atherosclerosis. 2010;209:283–9. doi: 10.1016/j.atherosclerosis.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vogt MT, Cauley JA, Kuller LH, Nevitt MC. Bone mineral density and blood flow to the lower extremities: the study of Osteoporotic fractures. J Bone Miner Res. 1997;12:283–9. doi: 10.1359/jbmr.1997.12.2.283. [DOI] [PubMed] [Google Scholar]
- 47.Wong SY, Kwok T, Woo J, et al. Bone mineral density and the risk of peripheral arterial disease in men and women: results from Mr. and Mrs. Os. Hong Kong. Osteoporos Int. 2005;16:1933–8. doi: 10.1007/s00198-005-1968-3. [DOI] [PubMed] [Google Scholar]
- 48.Riggs BL, Melton LJ, III, Robb RA, et al. Population-based study of age and sex differences in bone volumetric density, size, geometry, and structure at different skeletal sites. J Bone Miner Res. 2004;19:1945–54. doi: 10.1359/JBMR.040916. [DOI] [PubMed] [Google Scholar]
- 49.Resnick HE, Lindsay RS, McDermott MM, et al. Relationship of high and low ankle brachial index to all-cause and cardiovascular disease mortality: the Strong Heart Study. Circulation. 2004;109:733–9. doi: 10.1161/01.CIR.0000112642.63927.54. [DOI] [PubMed] [Google Scholar]
- 50.Allison MA, Cushman M, Solomon C, et al. Ethnicity and risk factors for change in the ankle-brachial index: the Multi-Ethnic Study of Atherosclerosis. J Vasc Surg. 2009;50:1049–56. doi: 10.1016/j.jvs.2009.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Golomb BA, Dang TT, Criqui MH. Peripheral arterial disease: morbidity and mortality implications. Circulation. 2006;114:688–99. doi: 10.1161/CIRCULATIONAHA.105.593442. PMID16908785. [DOI] [PubMed] [Google Scholar]
- 52.Ankle Brachial Index Collaboration. Fowkes FG, Murray GD, et al. Ankle brachial index combined with Framingham risk score to predict cardiovascular events and mortality. A meta-analysis. JAMA. 2008;300:197–208. doi: 10.1001/jama.300.2.197. PMCID2932628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. New Engl J Med. 1992;326:381–6. doi: 10.1056/NEJM199202063260605. PMID1729621. [DOI] [PubMed] [Google Scholar]
- 54.Criqui MH, McClelland RL, McDermott MM, et al. The ankle-brachial index and incident cardiovascular events in the MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2010;56:1506–12. doi: 10.1016/j.jacc.2010.04.060. PMCID2962558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sutton-Tyrrell K, Najjar SS, Boudreau RM, et al. Elevated aortic pulse wave velocity a marker of arterial stiffness, predicts cardiovascular event in well-functioning older adults. Circulation. 2005;111:3384–90. doi: 10.1161/CIRCULATIONAHA.104.483628. [DOI] [PubMed] [Google Scholar]
- 56.Yeboah J, McClelland RL, Polonsky TS, et al. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA. 2012;308:788–95. doi: 10.1001/jama.2012.9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goff DC, Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S49–73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 58.English JA, Carell ES, Guidera SA, Tripp HF. Angiographic prevalence and clinical predictors of left subclavian stenosis in patients undergoing diagnostic cardiac catheterization. Catheter Cardiovasc Interv. 2001;54:8–11. doi: 10.1002/ccd.1230. [DOI] [PubMed] [Google Scholar]
- 59.Osborn LA, Vernon SM, Reynolds B, Timm TC, Allen K. Screening for subclavian artery stenosis in patients who are candidates for coronary bypass surgery. Catheter Cardiovasc Interv. 2002;56:162–5. doi: 10.1002/ccd.10198. [DOI] [PubMed] [Google Scholar]
- 60.Shadman R, Criqui MH, Bundens WP, et al. Subclavian artery stenosis: prevalence, risk factors, and association with cardiovascular diseases. J Am Coll Cardiol. 2004;44:618–23. doi: 10.1016/j.jacc.2004.04.044. [DOI] [PubMed] [Google Scholar]
- 61.Aboyans V, Criqui MH, McDermott MM, et al. The vital prognosis of subclavian stenosis. J Am Coll Cardiol. 2007;49:1540–5. doi: 10.1016/j.jacc.2006.09.055. [DOI] [PubMed] [Google Scholar]
- 62.Clark CE, Taylor RS, Shore AC, Ukoumunne OC, Campbell JL. Association of a difference in systolic blood pressure between arms with vascular disease and mortality: a systematic review and meta-analysis. Lancet. 2012;379:905–14. doi: 10.1016/S0140-6736(11)61710-8. [DOI] [PubMed] [Google Scholar]
- 63.Aboyans V, Kamineni A, Allison MA, et al. The epidemiology of subclavian stenosis and its association with markers of subclinical atherosclerosis: the Multi-Ethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2010;211:266–70. doi: 10.1016/j.atherosclerosis.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Forbang NI, Nguyen T, Ix JH, Criqui MH, Allison MA. The downward shift of the aortic bifurcation, a possible marker for vascular aging. J Surg Radiol. 2011;2:372–7. [PMC free article] [PubMed] [Google Scholar]
- 65.Forbang NI, Ix JH, Allison MA, Criqui MH. Associations of cardiovascular disease risk factors and calcified atherosclerosis with aortoiliac bifurcation position: the MultiEthnic Study of Atherosclerosis. Angiology. 2015;66:90–5. doi: 10.1177/0003319713516669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Allison MA, Kwan K, DiTomasso D, Wright CM, Criqui MH. The epidemiology of abdominal aortic diameter. J Vasc Surg. 2008;48:121–7. doi: 10.1016/j.jvs.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 67.Laughlin GA, Allison MA, Jensky NE, et al. Abdominal aortic diameter and vascular atherosclerosis: the Multi-Ethnic Study of Atherosclerosis. Eur J Vasc Endovasc Surg. 2011;41:481–7. doi: 10.1016/j.ejvs.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carr JJ, Nelson JC, Wong ND, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 69.Budoff MJ, Nasir K, Katz R, et al. Thoracic aortic calcification and coronary heart disease events: the multiethnic study of atherosclerosis (MESA) Atherosclerosis. 2011;215:196–202. doi: 10.1016/j.atherosclerosis.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Criqui MH, Kamineni A, Allison MA, et al. Risk factor differences for aortic versus coronary calcified atherosclerosis: the multiethnic study of atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30:2289–96. doi: 10.1161/ATVBAHA.110.208181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Matthews KA, Kuller LH, Chang Y, Edmundowicz D. Premenopausal risk factors for coronary and aortic calcification: a 20-year follow-up in the healthy women study. Prev Med. 2007;45:302–8. doi: 10.1016/j.ypmed.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Newman AB, Naydeck BL, Sutton-Tyrrell K, et al. Coronary artery calcification in older adults to age 99: prevalence and risk factors. Circulation. 2001;104:2679–84. doi: 10.1161/hc4601.099464. [DOI] [PubMed] [Google Scholar]
- 73.Michos ED, Vaidya D, Gapstur SM, et al. Sex hormones, sex hormone binding globulin, and abdominal aortic calcification in women and men in the multi-ethnic study of atherosclerosis (MESA) Atherosclerosis. 2008;200:432–8. doi: 10.1016/j.atherosclerosis.2007.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Allen RW, Criqui MH, Diez Roux AV, et al. Fine particulate matter air pollution, proximity to traffic, and aortic atherosclerosis. Epidemiology. 2009;20:254–64. doi: 10.1097/EDE.0b013e31819644cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Takasu J, Katz R, Nasir K, et al. Relationships of thoracic aortic wall calcification to cardiovascular risk factors: the Multiethnic Study of Atheroslerosis. Am Heart J. 2008;155:765–71. doi: 10.1016/j.ahj.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Youssef G, Guo M, McClelland RL, et al. Risk factors for the development and progression of thoracic aorta calcification: the Multiethnic Study of Atherosclerosis. Acad Radiol. 2015;22:1536–45. doi: 10.1016/j.acra.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wong ND, Gransar H, Shaw L, et al. Thoracic aortic calcium versus coronary artery calcium for the prediction of coronary heart disease and cardiovascular disease events. J Am Coll Cardiol Img. 2009;2:319–26. doi: 10.1016/j.jcmg.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 78.Rivera JJ, Nasir K, Katz R, et al. Relationship of thoracic aortic calcium to coronary calcium and its progression (from the Multiethnic Study of Atherosclerosis) Am J Cardiol. 2009;103:1562–7. doi: 10.1016/j.amjcard.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Allison MA, Budoff MJ, Nasir K, et al. Ethnic-specific risks for atherosclerotic calcification of the thoracic and abdominal aorta (from the Multi-Ethnic Study of Atherosclerosis) Am J Cardiol. 2009;104:812–7. doi: 10.1016/j.amjcard.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wong ND, Lopez VA, Allison M, et al. Abdominal aortic calcium and multi-site atherosclerosis: the Multiethnic Study of Atherosclerosis. Atherosclerosis. 2011;214:436–41. doi: 10.1016/j.atherosclerosis.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hyder JA, Allison MA, Wong N, et al. Association of coronary artery and aortic alcium with lumbar bone density: the MESA Abdominal Aortic Calcium Study. Am J Epidemiol. 2009;169:186–94. doi: 10.1093/aje/kwn303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McAllister DA, MacNee W, Duprez D, et al. Pulmonary function is associated with distal aortic calcium, not proximal aortic disensibility. MESA lung study. COPD. 2011;8:71–8. doi: 10.3109/15412555.2011.558543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Al-Mallah MH, Nasir K, Katz R, et al. Relation of thoracic aortic distensibility to left ventricular area (from the Multi-Ethnic Study of Atheroslerosis [MESA]) Am J Cardiol. 2014;113:178–82. doi: 10.1016/j.amjcard.2013.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cheung N, Sharrett AR, Klein R, et al. Aortic distensibility sand retinal arteriolar narrowing: the multi-ethnic study of atherosclerosis. Hypertension. 2007;50:617–22. doi: 10.1161/HYPERTENSIONAHA.107.091926. [DOI] [PubMed] [Google Scholar]
- 85.Al-Mallah MH, Nasir K, Katz R, et al. Thoracic aortic distensibility and thoracic aortic calcium (from the Multiethnic Study of Atherosclerosis [MESA]) Am J Cardiol. 2010;106:575–80. doi: 10.1016/j.amjcard.2010.03.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Witteman JC, Kok FJ, van Saase JL, Valkenburg HA. Aortic calcification as a predictor of cardiovascular mortality. Lancet. 1986;2:1120–2. doi: 10.1016/s0140-6736(86)90530-1. [DOI] [PubMed] [Google Scholar]
- 87.Wilson PW, Kauppila LI, O’Donnell CJ, et al. Abdominal aortic calcific deposits are an important predictor of vascular morbidity and mortality. Circulation. 2001;103:1529–34. doi: 10.1161/01.cir.103.11.1529. [DOI] [PubMed] [Google Scholar]
- 88.Levitzky YS, Cupples LA, Murabito JM, et al. Prediction of intermittent claudication, ischemic stroke, and other cardiovascular disease by detection of abdominal aortic calcific deposits by plain lumbar radiographs. Am J Cardiol. 2008;101:326–31. doi: 10.1016/j.amjcard.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 89.Bastos Gonçalves F, Voûte MT, Hoeks SE, et al. Calcification of the abdominal aorta as an independent predictor of cardiovascular events: a meta-analysis. Heart. 2012;98:988–94. doi: 10.1136/heartjnl-2011-301464. [DOI] [PubMed] [Google Scholar]
- 90.Criqui MH, Denenberg JO, McClelland R, et al. Abdominal aortic calcium, coronary artery calcium, and cardiovascular morbidity and mortality in the Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2014;34:1574–9. doi: 10.1161/ATVBAHA.114.303268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Witteman JC, Kannel WB, Wolf PA, et al. Aortic calcified plaques and cardiovascular disease (the Framingham Study) Am J Cardiol. 1990;66:1060–4. doi: 10.1016/0002-9149(90)90505-u. [DOI] [PubMed] [Google Scholar]
- 92.Yeboah J, Carr JJ, Terry JG, et al. Computed tomographyderived cardiovascular risk markers, incident cardiovascular events, and all-cause mortality in nondiabetics: the Multi-Ethnic Study of Atherosclerosis. Eur J Prev Cardiol. 2014;21:1233–41. doi: 10.1177/2047487313492065. [DOI] [PMC free article] [PubMed] [Google Scholar]