Abstract
Hypertension and associated cardiovascular diseases represent the most common health complication of obesity and the leading cause of morbidity and mortality in overweight and obese patients. Emerging evidence suggests a critical role for the central nervous system particularly the brain action of the adipocyte-derived hormone leptin in linking obesity and hypertension. The preserved ability of leptin to cause cardiovascular sympathetic nerve activation despite the resistance to the metabolic actions of the hormone appears essential in this pathological process. This review describes the evidence supporting the neurogenic bases for obesity-associated hypertension with a particular focus on the neuronal and molecular signaling pathways underlying leptin’s effects on sympathetic nerve activity and blood pressure.
Keywords: Blood pressure, adiposity, sympathetic nervous system, leptin
Introduction
The modern obesity epidemic is threatening to reduce life expectancy due to the many health complications associated with this condition. Indeed, an increase in fat mass is associated with a number of comorbidities including diabetes and cardiovascular disease. Hypertension, which is a major risk factor for cardiovascular diseases, is commonly associated with obesity. In fact, estimates suggest that excess adiposity accounts for 65–75% of essential hypertension (i.e. high blood pressure with unknown secondary cause) [1]. Thus, understanding the underlying mechanisms of obesity-induced hypertension has the potential to reduce cardiovascular disease.
Obesity is not only a significant risk factor for hypertension but an indicator of poor response to treatment, as obese patients are more likely to have resistant hypertension, that is, to have uncontrolled blood pressure despite the use of three or more antihypertensive medications [2]. This highlights the need to understand the mechanisms linking obesity and hypertension to develop more effective treatment strategies.
While peripheral mechanisms including chronic inflammation, physical compression of the kidneys, and vascular dysfunction have been proposed as potential mediators of obesity-associated hypertension, there has been substantial evidence supporting a critical role for the central nervous system in driving this linkage. Data from human studies as well as animal models have suggested that obesity leads to alterations in autonomic regulation, particularly an increase in sympathetic nerve activity to various tissues [3]. Indeed, obesity-induced hypertension has been linked to increased sympathetic outflow to the kidney, which leads to increased fluid retention and vasoconstriction, as well as activation of the renin-angiotensin system; together, these promote an increase in arterial pressure [4]. Current research suggests that these changes during obesity are driven by neurohumoral processes. In this review, we will discuss the neurogenic mechanisms of obesity-induced hypertension with a particular focus on the role of leptin and the signaling pathways associated with the leptin receptor.
Obesity as a major risk factor for hypertension
Epidemiological and longitudinal studies have documented a strong relationship between obesity and hypertension [5]. Moreover, clinical and animal studies have shown that weight gain increases blood pressure whereas weight loss through diet and exercise or bariatric surgery lowers blood pressure, establishing a causal relationship between obesity and hypertension [6–8]. In fact, several studies have demonstrated that weight loss results in a decrease in blood pressure even among obese individuals with clinically normal blood pressure [9]. Weight loss decreases not only the autonomic contribution to blood pressure but also improves vascular function, which is frequently impaired in obesity [10].
Strikingly, obesity is associated with cardiometabolic dysfunction even in children, with increasing body mass index correlating with higher systolic blood pressure, total cholesterol levels, and fasting blood glucose in obese 3–19 year olds [11]. Consistent with this, a large study of 2.3 million Israeli adolescents has recently shown that childhood obesity and even elevated body mass index within the normal range correlates with increased risk of cardiovascular-related death in adulthood [12]. Visceral adiposity in particular carries an increased risk of adverse cardiovascular events, insulin resistance, and resistant hypertension [13–15].
Sympathetic nerve activation in obesity
The increased sympathetic tone in human obesity was documented by different means including catecholamine spillover, microneurography and pharmacological approaches [5]. All these methods have shown elevated sympathetic nerve activity (SNA) in obese hypertensive subjects compared to lean controls with the SNA returning to normal levels after weight loss implicating adiposity as the main cause of the sympathetic nerve activation [16, 17]. This is further supported by longitudinal studies as well as feeding experiments demonstrating that weight gain is commonly associated with an increase in SNA. It is interesting to note that elevated SNA is frequently detected in obese normotensive subjects [5, 18, 19]. This sympathetic overactivity observed in obese normotensives may contribute to several complications that are found in these subjects including an increase in arterial stiffness and atherosclerosis [20, 21]. A decrease in the SNA may explain the weight loss-induced decrease in blood pressure commonly observed in obese normotensive subjects [22].
Additional evidence implicating the sympathetic nervous system in obesity-induced hypertension derives from animal studies. Several animal models of obesity and particularly those with diet-induced obesity exhibit increased baseline sympathetic tone, which can be revealed by catecholamine spillover or by direct recording of sympathetic neural discharge from the nerves subserving different tissues. In a longitudinal study using radiotelemetry recording of SNA in rats, Muntzel et al. showed that lumbar SNA begins to increase shortly after starting an obesogenic diet and become significant by the 12th day of the diet [23]. Blood pressure, plasma norepinephrine, and renal SNA were also found to be elevated after only 1–3 weeks of high fat feeding in rabbits [24]. Renal denervation leads to a rapid and sustained reversal of hypertension in dogs with diet-induced obesity, indicating that elevated renal SNA is driving this pathology [25]. It should be noted, however, that renal denervation studies in humans with resistant hypertension, many of whom are obese, have had mixed results, with the large, double-blinded Simplicity-3 trial showing a similar decrease in blood pressure after renal denervation and sham operations [26]. This was surprising as smaller trials had shown positive results after renal denervation. Furthermore, autonomic inhibition via ganglionic blockade leads to a more significant reduction in blood pressure in obese patients compared to lean controls [27].
Leptin effects on blood pressure
Substantial evidence points to leptin as a critical link between obesity, sympathetic overdrive and hypertension. Leptin is a 16kDa adipocyte-derived hormone that circulates in proportion to fat mass and acts as a negative regulator of energy homeostasis. Leptin action in the brain decreases food intake and increases energy expenditure. Additionally, leptin increases SNA to a number of tissues including those involved in cardiovascular regulation such as the kidneys and blood vessels. These sympathoexcitatory effects of leptin have been shown to increase arterial pressure [28]. In addition, transgenic mice that overexpress leptin independently from adipose tissue mass are lean, but develop hypertension that is sympathetically-mediated, confirming that this hormone is a critical link between adiposity, sympathetic activation and blood pressure elevation [29].
In the context of obesity, exogenous administration of leptin fails to effectively regulate energy homeostasis while maintaining its effects on cardiovascular sympathetic outflow and blood pressure. This “selectivity” in leptin resistance may explain the ability of leptin to drive hypertension and sympathetic overactivity in obesity [30]. This is further supported by the sympathoinhibition and hypotension induced by central blockade of leptin signaling in obese rabbits [31]. It should be noted that leptin deficient ob/ob mice as well as db/db mice that lack a functional leptin receptor develop severe obesity, but are normotensive [28, 32]. While mutations in the genes encoding the leptin protein or its receptor are rare in humans, these individuals develop severe obesity, but have low or normal sympathetic tone and arterial pressure [28, 33]. Together, this evidence demonstrates the requirement of leptin for hypertension to develop in obesity.
Interestingly, several studies have shown that especially among obese patients, serum leptin levels correlate best with subcutaneous abdominal fat mass, as opposed to visceral fat mass [34–36]. On the other hand, it is well established that visceral adiposity is strongly associated with cardiovascular risks. Thus, although it has been proposed that this is due to concurrent accumulation of fat at both depots, it is also likely that additional factors derived from visceral adipose tissue such as inflammatory mediators may contribute to the effect of body fat distribution on obesity-associated hypertension and other cardiovascular diseases. However, while it may not be the only contributing factor, as discussed above leptin is necessary for the development of obesity-associated hypertension in humans and animal models.
Neuronal substrates of leptin action
Leptin receptors are expressed in multiple regions of the brain as well as several other peripheral tissues. Interestingly, selective deletion of leptin receptors in neurons largely recapitulate the metabolic phenotype of whole-body leptin receptor deficient mice, pointing to the central nervous system as the predominant site underlying the metabolic actions of leptin [37]. Within the brain, the leptin receptor is present in several nuclei with the highest expression observed in the hypothalamus particularly the arcuate nucleus (Arc). Within the Arc, two molecularly distinct neuronal populations have emerged as major targets of leptin: the stimulatory neurons expressing proopiomelanocortin (POMC) and inhibitory neurons expressing agouti-related protein (AgRP). Recent studies have identified other neuronal populations within the Arc that are sensitive to leptin, but these are less well characterized.
POMC neurons produce melanocortin peptides including α-melanocyte stimulating hormone (α-MSH), an agonist of the melanocortin-3 (MC3R) and melanocortin-4 (MC4R) receptors located in the second-order neurons whereas AgRP neurons release AgRP the strong MC3R and MC4R antagonist. Thus, POMC and AgRP neurons are considered as critical antagonistic components of the melanocortin system. Leptin activates anorexigenic POMC neurons while simultaneously inhibit orexigenic AgRP neurons [38, 39].
Substantial efforts have been directed at determining the importance of leptin receptors in various brain nuclei. Deletion or knockdown of the leptin receptor in many regions of the hypothalamus including the Arc, paraventricular nucleus (PVN), dorsomedial hypothalamus (DMH), ventromedial hypothalamus (VMH), and lateral hypothalamus (LH) was found to alter one or more aspects of energy homeostasis and impair the metabolic and/or anorectic effects of leptin. However, the obesity phenotypes developed by mice bearing disruption of the leptin receptor in various nuclei were modest when compared to the mice that lack the leptin receptor in the whole body or in the central nervous system. For example, although the Arc was widely considered a critical site of leptin action, leptin receptor deletion from POMC or AgRP neurons or both results in only mild, additive, weight gain [38]. Given the marked obesity of both leptin receptor and MC4R knockout mice, it is unclear why the phenotype of mice lacking leptin receptors in POMC and AgRP neurons is so mild, as these are thought to be the primary cells that act on MC4Rs. This has been interpreted as an indication that leptin act through a distributed brain network to regulate peripheral metabolism and that loss of leptin receptors in one brain region or specific population of neurons is not enough to cause metabolic derangements. Another potential explanation may relate to potential adaptations triggered by the loss of leptin receptors in specific neuronal populations such as POMC and AgRP neurons. Alternatively, it is possible that leptin action upstream of POMC and AgRP neurons and/or other factors that contribute to the activity of these cells contribute to obesity in the MC4R knockout mouse.
In contrast to the involvement of a distributed brain network in mediating leptin’s effects on metabolism and energy homeostasis, many studies have shown that leptin action on a single brain nucleus is sufficient to drive its cardiovascular sympathetic effects. Several hypothalamic (e.g. Arc, DMH and VMH) and extra-hypothalamic (e.g. the subfornical organ and the nucleus tractus solitarii) have been implicated in mediating the effect of leptin on renal SNA and/or blood pressure. These studies have for the most part utilized direct injection of leptin or pharmacological antagonists into specific brain nuclei or conditional knockout mouse models to selectively delete either the leptin receptor or its downstream components in specific nuclei or neuronal populations (Table 1).
Table 1.
Cardiovascular Sympathetic Effects of Manipulating Leptin Signaling in Various Brain Regions
| Brain Region | Manipulation | Result | Reference |
|---|---|---|---|
| Arcuate Nucleus | Microinjection of adCre in LepRflox mice | Impaired RSNA, BAT SNA and BP responses to leptin | [41] |
| Arcuate Nucleus | Direct injection of leptin in rats | Increased RSNA and MAP | [40] |
| Arcuate Nucleus - POMC neurons | POMCCre/LepRFlox mice | Resistant to BP response to leptin | [42] |
| Dorsomedial Hypothalamus | Direct injection of leptin receptor antagonist in rabbits | No significant effect on HR, MAP or RSNA | [49] |
| Dorsomedial Hypothalamus | Direct injection of leptin in rats | Increased BP and HR but not RSNA | [47] |
| Dorsomedial Hypothalamus | Direct injection of leptin receptor antagonist in DIO mice | Decreased HR and SBP | [28] |
| Dorsomedial Hypothalamus | Reactivation of LepR in LepR TB mice by adCre microinjection | Increased HR and SBP | [28] |
| Paraventricular nucleus | Direct injection of leptin in rats | No effect on HR, BP or RSNA | [47] |
| Paraventricular nucleus | Injection of antisense oligonucleotide against LepR mRNA in rats | Impaired BP response to direct leptin injection | [48] |
| Ventromedial Hypothalamus | Direct injection of leptin receptor antagonist in rabbits | Decreased HR, MAP and RSNA only in | [49] |
| Ventromedial Hypothalamus | Direct injection of leptin in rats | HFD treated animals Increased BP and RSNA but not HR | [47] |
| Nucleus Tractus Solitarii | Direct injection of leptin in rats | Increase MAP and RSNA | [50] |
| Subfornical organ | Direct injection of leptin in rats | Decrease in BP | [52] |
| Subfornical organ | Microinjection of adCre in LepRflox mice | Impaired RSNA and BP responses to leptin | [51] |
Abbreviations: BAT SNA, brown adipose tissue sympathetic nerve activity; BP, blood pressure; HFD, high fat diet; HR, heart rate; MAP, mean arterial pressure; RSNA, renal sympathetic nerve activity
Direct injection of leptin into the Arc increased renal SNA as well as arterial pressure in rats demonstrating that action in this nucleus is sufficient to evoke the sympathetic and cardiovascular effects of leptin [40]. Conversely, Arc-specific deletion of the leptin receptor abolishes the ability of leptin to increase renal SNA and protects mice from obesity-associated increase in blood pressure [41]. Conditional knockout mice that lack leptin receptors in POMC neurons are also resistant to leptin-induced increases in blood pressure and remain normotensive despite the presence of obesity and hyperleptinemia [42]. Thus, the leptin receptors in the Arc and more specifically POMC neurons appear critical for the sympathoexcitatory and pressor effects of leptin and for obesity-induced hypertension.
Additional evidence implicating the Arc in obesity-induced increases in sympathetic tone and blood pressure derives from studying patients and animal models bearing mutations in the MC4R which is thought to be the most common cause of human monogenic obesity accounting for as many as 6% of early onset severely obese cases [43]. Strikingly, patients with MC4R mutations have a much lower incidence of hypertension, and on average have lower systolic and diastolic blood pressure and normal or decreased sympathetic tone as compared to obese-matched controls [44]. Mouse models of MC4R deficiency display similar sympathetic and hemodynamic phenotypes, exhibiting extreme obesity without an increase in sympathetic tone or blood pressure [45, 46]. Conversely, MC4R stimulation increased blood pressure even in obese patients, indicating that this axis is preserved in obesity [44]. Together, these findings strongly suggest that over-activation of the melanocortin system which is downstream to leptin signaling may drive obesity-associated hypertension.
The various studies investigating the role of the hypothalamic nuclei other than the Arc in mediating the sympathetic and cardiovascular actions of leptin action have yielded somewhat conflicting findings (Table 1). For instance, one study demonstrated that leptin injection directly into the DMH or VMH but not the PVN is sufficient to increase blood pressure in rats [47]. Other investigators have shown no effect on baseline blood pressure of leptin receptor knockdown in the PVN, but that this prevented an increase in blood pressure after direct injection of leptin into the PVN [48]. On the other hand, injection of a leptin receptor antagonist into the VMH but not the DMH reversed the increases in blood pressure, heart rate, and renal SNA in high fat fed rabbits [49]. Conversely, reactivation of leptin receptors only in the DMH increased blood pressure in high fat fed mice while DMH-restricted administration of a leptin receptor antagonist decreased blood pressure in mice fed a high fat diet [28]. Leptin injection into the NTS also increases SNA to the kidney but not the brown adipose tissue [50] while deletion of leptin receptors from the subfornical organ prevents leptin-induced renal but not brown adipose sympathetic nerve activation [51]. By contrast, leptin injection directly into the subfornical organ caused a decrease in blood pressure in young lean rats, an effect that was blunted in diet-induced rats [52]. The reasons behind the conflicting cardiovascular effects evoked from these various brain regions are unclear, but may be due to variations in methodologies, off target effects or unique physiology of the different species studied. Moreover, the mechanism whereby multiple brain sites are both sufficient and required for leptin to increase SNA and arterial pressure remain unclear and may be related to endogenous leptin action or compensatory mechanisms.
Molecular pathways underlying leptin action
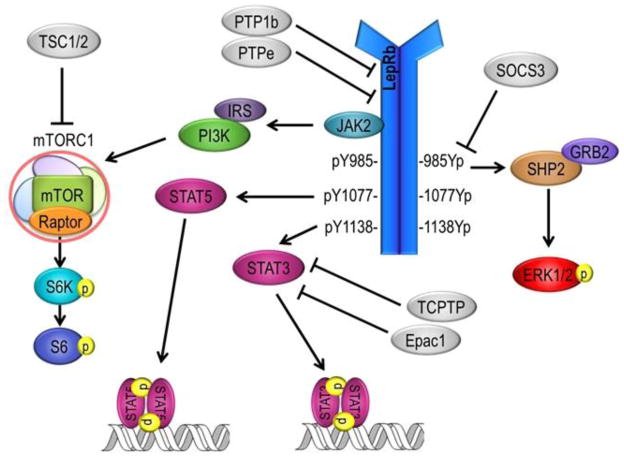
Leptin action on a single neuron can activate multiple distinct intracellular signaling cascades (Figure 1) which may explain the differential effects of leptin on metabolism and cardiovascular function. Leptin action on the long signaling b isoform of the receptor leads to the recruitment of janus kinase 2 (Jak2) and subsequent phosphorylation of several tyrosine sites on the receptor itself as well as phosphorylation of signal transducer and activator of transcription 3 (STAT3) and STAT5. STAT3 activation leads to its dimerization and translocation to the nucleus, where it regulates transcription of neuronal excitation-related gene products including the neuropeptides POMC and AgRP. Leptin also activates the extracellular signal–regulated kinase 1/2 (ERK1/2) pathway as well as phosphoinositide 3-kinase (PI3K) axis, which induces a number of downstream signaling cascades including mammalian target of rapamycin complex 1 (mTORC1) and subsequently the ribosomal proteins S6 kinase and S6. These signaling pathways have been shown to have differential contributions to the metabolic and cardiovascular sympathetic effects of leptin, which will be described further below. Negative regulators of leptin signaling such as suppressor of cytokine signaling 3 (SOCS3) and protein tyrosine phosphatase 1b (PTP1b) may also play an important role in mediating obesity-associated hypertension.
Figure 1.
Activation of the long signaling form of the leptin receptor (LepRb) initiates multiple distinct intracellular signaling cascades, which are thought to differentially contribute to the metabolic and cardiovascular sympathetic effects of leptin. Abbreviations: Epac1, exchange factor directly activated by cAMP 1; ERK1/2, extracellular signal-regulated kinase 1 and 2; GRB2, growth factor receptor-bound protein 2; IRS, insulin receptor substrate; JAK2, janus kinase 2; LepRb, leptin receptor b; mTOR, mechanistic target of rapamycin; mTORC1, mTOR complex 1; PI3K, phosphatidylinositol-3-kinase; PTP1b, protein tyrosine phosphatase 1b; PTPe, protein tyrosine phosphatase e; Raptor, regulator-associated protein of mTOR; S6, ribosomal protein S6; S6K, S6 kinase; SHP2, Src-homology 2 domain-containing phosphatase 2; SOCS3, suppressor of cytokine signaling 3; Stat3 and 5, Signal transducer and activator of transcription 3 and 5; TCPTP, T-cell protein tyrosine phosphatase; TSC1/2, Tuberous sclerosis protein 1 and 2.
STATs
This signaling cascade is the most well studied downstream mediator of leptin receptor activation. Mice that have disruption in Tyr1138 of the of leptin receptor making it unable to activation selectively STAT3 develop severe obesity and hyperphagia presumably due to loss of leptin ability to control metabolic function [53]. Similarly, disruption of STAT3 signaling specifically in neurons or in leptin receptor expressing cells cause weight gain in mice [54]. Surprisingly, mice in which Tyr1138 of the leptin receptor is mutated displayed normal renal sympathetic response to leptin indicating that STAT3 pathway is not required for renal sympathoexcitatory effect of leptin [55].
POMC neuron-specific STAT3 deletion leads to impairment of both the metabolic and blood pressure responses to leptin [56]. Interestingly, despite the evidence implicating POMC neurons in leptin-mediated sympathoexcitation and blood pressure elevation, it has been proposed that POMC neurons are unlikely to be the source of leptin-mediated sympathoexcitation in obesity, as these neurons become leptin resistant during dietary obesity as indicated by the inability of leptin to induce STAT3 phosphorylation in the Arc neurons of diet-induced obese mice [57].
Leptin activation of STAT5 has been implicated in the control of reproduction function, but not energy homeostasis [58]. The contribution of STAT5 to leptin control of SNA and blood pressure remain unknown.
PI3K
Manipulation of leptin dependent signaling pathways pharmacologically or through neuronal population specific ablation points to differential roles for leptin-induced molecular signaling in metabolic and cardiovascular regulation, with the PI3K/mTORC1 axis in particular being important for leptin to increase renal SNA and blood pressure. Given the impaired STAT3 phosphorylation in some neurons during obesity, it is possible that other molecular pathways downstream of the leptin receptor such as the PI3K/mTORC1 axis mediate a preserved effect of leptin on cardiovascular SNA in obesity.
Pharmacological studies have pointed to mTORC1 signaling as an important pathway underlying the anorectic effect of leptin in rats [59]. Paradoxically, mice with increased mTORC1 activity in POMC neurons due to deletion of the endogenous mTORC1 inhibitor TSC1 are hyperphagic and obese, indicating that a precise balance of mTORC1 activity is necessary for appropriate energy balance [60]. Interestingly, blockade of hypothalamic mTORC1 prevents the blood pressure and renal sympathetic responses to leptin, which is consistent with the requirement of upstream PI3K signaling for leptin’s cardiovascular sympathetic action [61, 62].
ERK1/2
In contrast to PI3K/mTORC1 axis, pharmacological inhibition of the ERK1/2 pathway prevents leptin-evoked sympathoexcitation to brown adipose tissue, but not the kidney [63]. Thus, the ERK1/2 pathway appears to be more important for metabolic processes such as thermogenesis, as opposed to leptin’s cardiovascular sympathetic effects. However, deletion of the protein tyrosine phosphatase Shp2 which is upstream to the ERK1/2 signaling from the forebrain or POMC neurons not only prevented the anorectic effects of leptin but also attenuated its pressor effect [64, 65]. While Shp2 regulates a number of intracellular processes with respect to leptin signaling it is generally recognized as a critical component of the ERK1/2 pathway, indicating that ERK1/2 may not exclusively regulate the metabolic effects of leptin.
Negative regulators of leptin signaling
SOCS3 expression is induced by leptin receptor activation and acts as a negative feedback inhibitor of leptin signaling (Figure 1). SOCS3 is often considered to be an important mechanism underlying leptin resistance, as its expression is elevated in the hypothalamus during obesity [66]. Inactivation of SOCS3 in neurons sensitizes mice to leptin and protect from diet-induced obesity. In contrast, SOCS3 deletion specifically in leptin receptor expressing cells unexpectedly fails to protect against diet-induced obesity [67]. It is likely that SOCS3 inhibition also results in sensitization to the cardiovascular sympathetic effects of leptin making it an ineffective target for treatment of obesity. Indeed, mice carrying a mutation in Tyr985 of the leptin receptor which prevents its interaction with SOCS3 have elevated baseline blood pressure and are more sensitive to the effects of leptin on arterial pressure and renal SNA [55].
The protein tyrosine phosphatase PTP1b also acts as a negative regulator of leptin signaling, and its deletion from the whole body or specifically from neurons sensitizes mice to metabolic and cardiovascular effects of leptin; protecting against diet-induced obesity while increasing baseline blood pressure [68, 69]. PTP1b is unique in that it may interact with multiple leptin receptor dependent signaling cascades. While PTP1b is best known for its action in dephosphorylating Jak2, it can also interact with insulin receptor substrate 1, which contributes to the PI3K/mTORC1 axis. Paradoxically, despite its seemingly ubiquitous role in leptin sensitization, PTP1b deletion specifically from POMC neurons impairs the blood pressure response to leptin, further supporting the idea that a precise balance of leptin signaling cascades is required for appropriate regulation of blood pressure [70].
There are many other proteins that are known to inhibit leptin receptor signaling through various mechanisms (Figure 1). However, the significance of these inhibitory proteins for the cardiovascular and sympathetic effects of leptin has not been investigated.
Conclusions
Obesity-associated hypertension is a major risk factor for cardiovascular disease that affects a growing proportion of the population. Recent evidence supports a critical role for the leptin-melanocortin system in the pathogenesis of this disease. Data from obese humans as well as animal models have unequivocally established the close relationship between weight gain and hypertension and further demonstrate the importance of neurogenic mechanisms for this association. Elevated serum leptin levels combined with selective leptin resistance have emerged as potential mechanisms driving sympathetic activation and hypertension in obesity. Rare mutations in components of the leptin-melanocortin system as well as genetically modified mouse models have demonstrated a critical role for leptin in the development of obesity-associated hypertension, although it is unclear whether leptin alone is sufficient or if other processes are required.
More recent work has focused on specific neuronal and molecular pathways underlying the effects of leptin on metabolism and cardiovascular function, demonstrating several key leptin responsive regions of the hypothalamus that may differentially contribute to these processes. The Arc, DMH, and VMH have all been proposed as critical brain regions for the cardiovascular effects of leptin, whereas leptin’s anorectic and thermogenic effects appear to involve neurons distributed across multiple brain nuclei.
The ability of the leptin receptor to activate various downstream signaling cascades is consistent with the ability of leptin to influence different physiological processes. Whereas the ERK1/2 and PI3K pathways appear to uniquely underlie leptin’s metabolic and cardiovascular sympathetic effects, respectively, STAT3 phosphorylation has been shown to play an important role in both of these processes. Furthermore, the interactions between these distinct neuronal and molecular signaling mechanisms in mediating obesity-associated hypertension is still under investigation, as it is unclear how individual molecular signaling cascades differentially contribute to leptin action in various neuronal populations.
Acknowledgments
Sources of Funding
The authors’ research is supported by the US National Institutes of Health (HL084207), the American Heart Association (Award #14EIA18860041), the University of Iowa Fraternal Order of Eagles Diabetes Research Center, and the University of Iowa Center for Hypertension Research.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
Balyssa B. Bell and Kamal Rahmouni declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
*Of importance
**Of outstanding importance
- 1.Garrison RJ, et al. Incidence and precursors of hypertension in young adults: the Framingham Offspring Study. Prev Med. 1987;16(2):235–51. doi: 10.1016/0091-7435(87)90087-9. [DOI] [PubMed] [Google Scholar]
- 2.Holecki M, Dulawa J, Chudek J. Resistant hypertension in visceral obesity. Eur J Intern Med. 2012;23(7):643–8. doi: 10.1016/j.ejim.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 3.Fidan-Yaylali G, et al. The Association between Central Adiposity and Autonomic Dysfunction in Obesity. Med Princ Pract. 2016 doi: 10.1159/000446915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall JE, et al. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res. 2015;116(6):991–1006. doi: 10.1161/CIRCRESAHA.116.305697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Esler M, et al. Mechanisms of sympathetic activation in obesity-related hypertension. Hypertension. 2006;48(5):787–96. doi: 10.1161/01.HYP.0000242642.42177.49. [DOI] [PubMed] [Google Scholar]
- 6.Neter JE, et al. Influence of weight reduction on blood pressure: a meta-analysis of randomized controlled trials. Hypertension. 2003;42(5):878–84. doi: 10.1161/01.HYP.0000094221.86888.AE. [DOI] [PubMed] [Google Scholar]
- 7.Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53(21):1925–32. doi: 10.1016/j.jacc.2008.12.068. [DOI] [PubMed] [Google Scholar]
- 8.Straznicky NE, et al. Effects of dietary weight loss on sympathetic activity and cardiac risk factors associated with the metabolic syndrome. J Clin Endocrinol Metab. 2005;90(11):5998–6005. doi: 10.1210/jc.2005-0961. [DOI] [PubMed] [Google Scholar]
- 9.Fortmann SP, Haskell WL, Wood PD. Effects of weight loss on clinic and ambulatory blood pressure in normotensive men. Am J Cardiol. 1988;62(1):89–93. doi: 10.1016/0002-9149(88)91370-7. [DOI] [PubMed] [Google Scholar]
- 10.Williams IL, et al. Endothelial function and weight loss in obese humans. Obes Surg. 2005;15(7):1055–60. doi: 10.1381/0960892054621134. [DOI] [PubMed] [Google Scholar]
- 11**.Skinner AC, et al. Cardiometabolic Risks and Severity of Obesity in Children and Young Adults. N Engl J Med. 2015;373(14):1307–17. doi: 10.1056/NEJMoa1502821. The work reported in this paper demonstrate the close link between obesity, hypertension and other cardiovascular risks in children. [DOI] [PubMed] [Google Scholar]
- 12.Twig G, et al. Body-Mass Index in 2.3 Million Adolescents and Cardiovascular Death in Adulthood. N Engl J Med. 2016;374(25):2430–40. doi: 10.1056/NEJMoa1503840. [DOI] [PubMed] [Google Scholar]
- 13.Hubert HB, et al. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation. 1983;67(5):968–77. doi: 10.1161/01.cir.67.5.968. [DOI] [PubMed] [Google Scholar]
- 14.Kurajoh M, et al. Plasma leptin level is associated with cardiac autonomic dysfunction in patients with type 2 diabetes: HSCAA study. Cardiovasc Diabetol. 2015;14:117. doi: 10.1186/s12933-015-0280-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Selvaraj S, et al. Association of Central Adiposity With Adverse Cardiac Mechanics: Findings From the Hypertension Genetic Epidemiology Network Study. Circ Cardiovasc Imaging. 2016;9(6) doi: 10.1161/CIRCIMAGING.115.004396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grassi G, Mark A, Esler M. The sympathetic nervous system alterations in human hypertension. Circ Res. 2015;116(6):976–90. doi: 10.1161/CIRCRESAHA.116.303604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alvarez GE, et al. Sympathetic neural activation in visceral obesity. Circulation. 2002;106(20):2533–6. doi: 10.1161/01.cir.0000041244.79165.25. [DOI] [PubMed] [Google Scholar]
- 18.Lambert E, et al. Differing pattern of sympathoexcitation in normal-weight and obesity-related hypertension. Hypertension. 2007;50(5):862–8. doi: 10.1161/HYPERTENSIONAHA.107.094649. [DOI] [PubMed] [Google Scholar]
- 19.Grassi G, et al. Sympathetic activation in obese normotensive subjects. Hypertension. 1995;25(4 Pt 1):560–3. doi: 10.1161/01.hyp.25.4.560. [DOI] [PubMed] [Google Scholar]
- 20.Cooper JN, et al. Associations between arterial stiffness and platelet activation in normotensive overweight and obese young adults. Clin Exp Hypertens. 2014;36(3):115–22. doi: 10.3109/10641963.2013.789045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robinson MR, et al. Uncomplicated obesity is associated with abnormal aortic function assessed by cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2008;10:10. doi: 10.1186/1532-429X-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Masuo K, et al. Differences in mechanisms between weight loss-sensitive and - resistant blood pressure reduction in obese subjects. Hypertens Res. 2001;24(4):371–6. doi: 10.1291/hypres.24.371. [DOI] [PubMed] [Google Scholar]
- 23.Muntzel MS, et al. Cafeteria diet increases fat mass and chronically elevates lumbar sympathetic nerve activity in rats. Hypertension. 2012;60(6):1498–502. doi: 10.1161/HYPERTENSIONAHA.112.194886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Armitage JA, et al. Rapid onset of renal sympathetic nerve activation in rabbits fed a high-fat diet. Hypertension. 2012;60(1):163–71. doi: 10.1161/HYPERTENSIONAHA.111.190413. [DOI] [PubMed] [Google Scholar]
- 25.Henegar JR, et al. Catheter-based radiorefrequency renal denervation lowers blood pressure in obese hypertensive dogs. Am J Hypertens. 2014;27(10):1285–92. doi: 10.1093/ajh/hpu048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26*.Bhatt DL, et al. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370(15):1393–401. doi: 10.1056/NEJMoa1402670. This study reported the finding from SIMPLICITY-3 trials showing that in patients with resistant hypertension, the effect of renal denervation is similar to sham intervention. [DOI] [PubMed] [Google Scholar]
- 27.Shibao C, et al. Autonomic contribution to blood pressure and metabolism in obesity. Hypertension. 2007;49(1):27–33. doi: 10.1161/01.HYP.0000251679.87348.05. [DOI] [PubMed] [Google Scholar]
- 28*.Simonds SE, et al. Leptin mediates the increase in blood pressure associated with obesity. Cell. 2014;159(6):1404–16. doi: 10.1016/j.cell.2014.10.058. This paper confirmed the critical role for leptin in mediating obesity-associated hypertension both in humans and animal models. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aizawa-Abe M, et al. Pathophysiological role of leptin in obesity-related hypertension. J Clin Invest. 2000;105(9):1243–52. doi: 10.1172/JCI8341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mark AL. Selective leptin resistance revisited. Am J Physiol Regul Integr Comp Physiol. 2013;305(6):R566–81. doi: 10.1152/ajpregu.00180.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lim K, Burke SL, Head GA. Obesity-related hypertension and the role of insulin and leptin in high-fat-fed rabbits. Hypertension. 2013;61(3):628–34. doi: 10.1161/HYPERTENSIONAHA.111.00705. [DOI] [PubMed] [Google Scholar]
- 32.Mark AL, et al. Contrasting blood pressure effects of obesity in leptin-deficient ob/ob mice and agouti yellow obese mice. J Hypertens. 1999;17(12 Pt 2):1949–53. doi: 10.1097/00004872-199917121-00026. [DOI] [PubMed] [Google Scholar]
- 33.Ozata M, Ozdemir IC, Licinio J. Human leptin deficiency caused by a missense mutation: multiple endocrine defects, decreased sympathetic tone, and immune system dysfunction indicate new targets for leptin action, greater central than peripheral resistance to the effects of leptin, and spontaneous correction of leptin-mediated defects. J Clin Endocrinol Metab. 1999;84(10):3686–95. doi: 10.1210/jcem.84.10.5999. [DOI] [PubMed] [Google Scholar]
- 34.Minocci A, et al. Leptin plasma concentrations are dependent on body fat distribution in obese patients. Int J Obes Relat Metab Disord. 2000;24(9):1139–44. doi: 10.1038/sj.ijo.0801385. [DOI] [PubMed] [Google Scholar]
- 35.Van Harmelen V, et al. Leptin secretion from subcutaneous and visceral adipose tissue in women. Diabetes. 1998;47(6):913–7. doi: 10.2337/diabetes.47.6.913. [DOI] [PubMed] [Google Scholar]
- 36.Cnop M, et al. The concurrent accumulation of intra-abdominal and subcutaneous fat explains the association between insulin resistance and plasma leptin concentrations : distinct metabolic effects of two fat compartments. Diabetes. 2002;51(4):1005–15. doi: 10.2337/diabetes.51.4.1005. [DOI] [PubMed] [Google Scholar]
- 37.Cohen P, et al. Selective deletion of leptin receptor in neurons leads to obesity. J Clin Invest. 2001;108(8):1113–21. doi: 10.1172/JCI13914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van de Wall E, et al. Collective and individual functions of leptin receptor modulated neurons controlling metabolism and ingestion. Endocrinology. 2008;149(4):1773–85. doi: 10.1210/en.2007-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rahmouni K. Cardiovascular Regulation by the Arcuate Nucleus of the Hypothalamus: Neurocircuitry and Signaling Systems. Hypertension. 2016;67(6):1064–71. doi: 10.1161/HYPERTENSIONAHA.115.06425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rahmouni K, Morgan DA. Hypothalamic arcuate nucleus mediates the sympathetic and arterial pressure responses to leptin. Hypertension. 2007;49(3):647–52. doi: 10.1161/01.HYP.0000254827.59792.b2. [DOI] [PubMed] [Google Scholar]
- 41.Harlan SM, et al. Ablation of the leptin receptor in the hypothalamic arcuate nucleus abrogates leptin-induced sympathetic activation. Circ Res. 2011;108(7):808–12. doi: 10.1161/CIRCRESAHA.111.240226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.do Carmo JM, et al. Control of blood pressure, appetite, and glucose by leptin in mice lacking leptin receptors in proopiomelanocortin neurons. Hypertension. 2011;57(5):918–26. doi: 10.1161/HYPERTENSIONAHA.110.161349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Farooqi IS, et al. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med. 2003;348(12):1085–95. doi: 10.1056/NEJMoa022050. [DOI] [PubMed] [Google Scholar]
- 44.Greenfield JR, et al. Modulation of blood pressure by central melanocortinergic pathways. N Engl J Med. 2009;360(1):44–52. doi: 10.1056/NEJMoa0803085. [DOI] [PubMed] [Google Scholar]
- 45.Tallam LS, et al. Melanocortin-4 receptor-deficient mice are not hypertensive or salt-sensitive despite obesity, hyperinsulinemia, and hyperleptinemia. Hypertension. 2005;46(2):326–32. doi: 10.1161/01.HYP.0000175474.99326.bf. [DOI] [PubMed] [Google Scholar]
- 46.Rahmouni K, et al. Role of melanocortin-4 receptors in mediating renal sympathoactivation to leptin and insulin. J Neurosci. 2003;23(14):5998–6004. doi: 10.1523/JNEUROSCI.23-14-05998.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marsh AJ, et al. Cardiovascular responses evoked by leptin acting on neurons in the ventromedial and dorsomedial hypothalamus. Hypertension. 2003;42(4):488–93. doi: 10.1161/01.HYP.0000090097.22678.0A. [DOI] [PubMed] [Google Scholar]
- 48.Shih CD, Au LC, Chan JY. Differential role of leptin receptors at the hypothalamic paraventricular nucleus in tonic regulation of food intake and cardiovascular functions. J Biomed Sci. 2003;10(4):367–78. doi: 10.1159/000071156. [DOI] [PubMed] [Google Scholar]
- 49*.Lim K, et al. Origin of Aberrant Blood Pressure and Sympathetic Regulation in Diet-Induced Obesity. Hypertension. 2016;68(2):491–500. doi: 10.1161/HYPERTENSIONAHA.116.07461. This paper investigates the role of various hypothalamic regions in mediating leptin's contribution to obesity-associated hypertension. [DOI] [PubMed] [Google Scholar]
- 50.Mark AL, et al. Leptin signaling in the nucleus tractus solitarii increases sympathetic nerve activity to the kidney. Hypertension. 2009;53(2):375–80. doi: 10.1161/HYPERTENSIONAHA.108.124255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Young CN, et al. The brain subfornical organ mediates leptin-induced increases in renal sympathetic activity but not its metabolic effects. Hypertension. 2013;61(3):737–44. doi: 10.1161/HYPERTENSIONAHA.111.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith PM, Ferguson AV. Cardiovascular actions of leptin in the subfornical organ are abolished by diet-induced obesity. J Neuroendocrinol. 2012;24(3):504–10. doi: 10.1111/j.1365-2826.2011.02257.x. [DOI] [PubMed] [Google Scholar]
- 53.Bates SH, et al. STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature. 2003;421(6925):856–9. doi: 10.1038/nature01388. [DOI] [PubMed] [Google Scholar]
- 54.Gao Q, et al. Disruption of neural signal transducer and activator of transcription 3 causes obesity, diabetes, infertility, and thermal dysregulation. Proc Natl Acad Sci U S A. 2004;101(13):4661–6. doi: 10.1073/pnas.0303992101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harlan SM, et al. Cardiovascular and sympathetic effects of disrupting tyrosine 985 of the leptin receptor. Hypertension. 2011;57(3):627–32. doi: 10.1161/HYPERTENSIONAHA.110.166538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56*.Dubinion JH, et al. Role of proopiomelanocortin neuron Stat3 in regulating arterial pressure and mediating the chronic effects of leptin. Hypertension. 2013;61(5):1066–74. doi: 10.1161/HYPERTENSIONAHA.111.00020. This paper demonstrates a critical role for STAT3 signaling in POMC neurons in mediating both the metabolic and cardiovascular sympathetic effects of leptin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Munzberg H, Flier JS, Bjorbaek C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology. 2004;145(11):4880–9. doi: 10.1210/en.2004-0726. [DOI] [PubMed] [Google Scholar]
- 58.Patterson CM, et al. Leptin action via LepR-b Tyr1077 contributes to the control of energy balance and female reproduction. Mol Metab. 2012;1(1–2):61–9. doi: 10.1016/j.molmet.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cota D, et al. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312(5775):927–30. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- 60.Mori H, et al. Critical role for hypothalamic mTOR activity in energy balance. Cell Metab. 2009;9(4):362–74. doi: 10.1016/j.cmet.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61**.Harlan SM, et al. Hypothalamic mTORC1 signaling controls sympathetic nerve activity and arterial pressure and mediates leptin effects. Cell Metab. 2013;17(4):599–606. doi: 10.1016/j.cmet.2013.02.017. This is the first report to implicate hypothalamic mTORC1 in sympathetic and cardiovascula regulation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harlan SM, Rahmouni K. PI3K signaling: A key pathway in the control of sympathetic traffic and arterial pressure by leptin. Mol Metab. 2013;2(2):69–73. doi: 10.1016/j.molmet.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rahmouni K, et al. Hypothalamic ERK mediates the anorectic and thermogenic sympathetic effects of leptin. Diabetes. 2009;58(3):536–42. doi: 10.2337/db08-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.do Carmo JM, et al. Shp2 signaling in POMC neurons is important for leptin's actions on blood pressure, energy balance, and glucose regulation. Am J Physiol Regul Integr Comp Physiol. 2014;307(12):R1438–47. doi: 10.1152/ajpregu.00131.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.do Carmo JM, et al. Role of Shp2 in forebrain neurons in regulating metabolic and cardiovascular functions and responses to leptin. Int J Obes (Lond) 2014;38(6):775–83. doi: 10.1038/ijo.2013.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bjorbaek C, et al. Identification of SOCS-3 as a potential mediator of central leptin resistance. Mol Cell. 1998;1(4):619–25. doi: 10.1016/s1097-2765(00)80062-3. [DOI] [PubMed] [Google Scholar]
- 67.Pedroso JA, et al. Inactivation of SOCS3 in leptin receptor-expressing cells protects mice from diet-induced insulin resistance but does not prevent obesity. Mol Metab. 2014;3(6):608–18. doi: 10.1016/j.molmet.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bence KK, et al. Neuronal PTP1B regulates body weight, adiposity and leptin action. Nat Med. 2006;12(8):917–24. doi: 10.1038/nm1435. [DOI] [PubMed] [Google Scholar]
- 69.Belin de Chantemele EJ, et al. Protein tyrosine phosphatase 1B, a major regulator of leptin-mediated control of cardiovascular function. Circulation. 2009;120(9):753–63. doi: 10.1161/CIRCULATIONAHA.109.853077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bruder-Nascimento T, et al. Deletion of protein tyrosine phosphatase 1b in proopiomelanocortin neurons reduces neurogenic control of blood pressure and protects mice from leptin- and sympatho-mediated hypertension. Pharmacol Res. 2015;102:235–44. doi: 10.1016/j.phrs.2015.10.012. [DOI] [PubMed] [Google Scholar]