Abstract
Universally fatal only four decades ago, the progress in the three-stage palliation of hypoplastic left heart syndrome and related single right ventricular lesions has drastically improved the outlook for these patients. While the stage II operation (hemi-Fontan or bidirectional Glenn) and stage III Fontan procedure have evolved into relatively low risk operations, the stage I Norwood procedure remains one of the highest risk and costliest common operations performed in congenital heart surgery. Yet, despite this fact, experienced centers now report hospital survivals in excess of 90% for the Norwood. This traditional three-stage surgical palliation has seen several innovations in the past decade aimed at improving outcomes, particularly for the Norwood procedure. One significant change is a renewed interest in the right ventricle-to-pulmonary artery shunt as the source of pulmonary blood flow, rather than the modified Blalock-Taussig shunt for the Norwood. The multi-institutional Single Ventricle Reconstruction trial randomized 555 patients to one or the other shunt, and these subjects continue to be followed closely as they now approach 10 years post-randomization. In addition to modifications to the Norwood procedure, the “hybrid procedure,” a combined catheter-based and surgical approach, avoids the Norwood procedure in the newborn period entirely. The initial hybrid procedure is then followed by a “comprehensive” stage II, that combines components of both the Norwood and the traditional stage II, and later completion of the Fontan. Proponents of this approach hope to not only improve short-term survival, but potentially longer-term outcomes, such as neurodevelopment, as well. Regardless of the approach, traditional surgical staged palliation or the hybrid procedure, survivals have vastly improved, and large numbers of these patients are surviving not only through their Fontan in early childhood, but into adolescence and young adulthood. As this population grows, it becomes increasingly important to understand the longer-term outcomes of these Fontan patients, not only in terms of survival, but also burden of disease, neurodevelopmental outcomes, psychosocial development and quality of life.
Keywords: congenital cardiac defect, hypoplastic left heart syndrome, single ventricle, hybrid imaging, surgery
Introduction
Hypoplastic left heart syndrome (HLHS) is characterized by hypoplasia of the left ventricle and systemic outflow tract obstruction [1]. Other common related functional single right ventricle (FSRV) lesions include double outlet right ventricle with mitral atresia and unbalanced atrioventricular septal defect. Without intervention, HLHS and related FSRV lesions are essentially uniformly fatal. Since the first description of the Norwood Procedure in 1981, great strides have been made in the treatment of the HLHS and related lesions [2]. Over the past 35 years, hospital survival for the Norwood procedure has improved from 0% to over 90% in experienced centers [3].
Current Therapy for HLHS and Other Functional Single Right Ventricle Lesions
Traditional surgical staged palliation consists of the Norwood procedure at birth, a stage II superior cavopulmonary connection, generally performed at 4–6 months of age, and a completion Fontan at 18–48 months of age. More recently, the Hybrid procedure has been proposed as an alternative to the initial Norwood procedure. While the physiology remains the same after a Norwood or a Hybrid procedure, the Hybrid is less invasive and does not require as extensive an intervention in the neonatal period. The tradeoff is a more involved second stage operation, and more intensive follow-up and more frequent interventions in the period between stage I and stage II. The following State of the Art review will discuss the current therapy for HLHS and related FSRV lesions, as well as the intermediate-term outcomes for the Fontan procedure.
Traditional Staged Surgical Palliation
The requirements for the Norwood procedure have not changed from Dr. Norwood’s initial description in 1980. These requirements are, (1) unobstructed systemic outflow from the single right ventricle to a reconstructed aorta, (2) unobstructed pulmonary venous return into the right atrium and (3) controlled pulmonary blood flow (PBF) [4]. While the first two requirements have remained largely unchanged, there are now two acceptable options for the source of the measured PBF.
In the classic Norwood procedure, a modified Blalock–Taussig shunt (MBTS) provides PBF from the innominate or subclavian artery to the pulmonary arteries via a polytetrafluoroethylene (PTFE) tube. Due to the placement of the MBTS downstream of the neoaortic valve, there is continuous forward flow from the systemic to the pulmonary circulation in both systole and diastole following the Norwood procedure. “Coronary steal” may result as diastolic retrograde flow occurs in both the coronary arteries and descending aorta [5]. As 70–80% of coronary flow occurs during diastole, coronary insufficiency due to this coronary steal may play an important role in the recognized incidence of mortality between the stage I and II operations. Indeed, decreased coronary arterial flow and oxygen delivery both at rest and after administration of adenosine (simulating exercise) have been shown to be significantly decreased in patients after the Norwood procedure with MBTS compared to patients after anatomic repair of other congenital cardiovascular malformations [6].
Recently, there has been renewed enthusiasm for the right ventricle-to-pulmonary artery shunt (RVPAS) as a source of PBF for the Norwood procedure. Although the initial description was by Norwood and colleagues in 1981 [2] and first current era reports were by Kishimoto and colleagues in 1999 [7], the RVPAS was popularized by Sano in a number of manuscripts in the early 2000’s [8–10], and is often referred to as a “Sano shunt” or a “Sano modification” of the Norwood procedure. The RVPAS eliminates the diastolic runoff and coronary arterial steal associated with the MBTS [5]. The disadvantage of the RVPAS is the need to perform a ventriculotomy, with the potential risk to ventricular function and arrhythmia generation.
Small, non-randomized case series comparing the two sources of PBF demonstrated conflicting results. Some reports showed improved hemodynamics, branch pulmonary artery growth and decreased mortality [11–15]. Others centers, generally those with favorable results with the MBTS, failed to show a benefit with the RVPAS [16–18].
The Single Ventricle Reconstruction Trial
Due to this uncertainty of the impact of the source of PBF on the Norwood procedure, the National Heart, Lung, and Blood Institute (NHLBI)-funded Pediatric Heart Network (www.pediatricheartnetwork.com) Single Ventricle Reconstruction (SVR) trial was undertaken starting in 2005 to compare the MBTS and the RVPAS [19]. The SVR trial enrolled neonates with HLHS or related FSRV lesions from 15 North American centers. The primary outcome was a combined endpoint of death or cardiac transplantation 12 months after randomization. Secondary outcomes included Norwood and stage II post-operative course, right ventricular function at the Stage II operation, branch pulmonary artery dimensions at Stage II, and neurodevelopmental outcomes at 14 months of age.
The SVR trial enrolled 555 eligible neonates of which 549 subjects (275 MBTS and 274 RVPAS) underwent a Norwood procedure and were included in the analysis. At the 12-month primary endpoint, subjects in the RVPAS group had a 74% transplantation-free survival vs 64% for the MBTS group (P=0.01) [20]. However, when all available follow-up data (mean 32±11 months) were analyzed at the conclusion of the trial, a statistically significant transplant-free survival advantage of the RVPAS cohort could no longer be shown (P = 0.06). Anatomic subtype (aortic atresia, aortic stenosis, mitral atresia or mitral stenosis) did not impact outcome. The right ventricular function by echocardiography was similar in both cohorts at 14 months. The RVPAS group required more unplanned surgical and interventional catheter-based cardiovascular interventions to address stenosis in the shunt, branch pulmonary arteries or neoaorta, as compared to the MBTS group (P = 0.003).
A more in depth competing risks analysis revealed that mortality was separated into two phases: early/acute and late/constant [21]. The early/late phase inflection point occurred at 6–7 months, suggesting that risk factors for mortality were a function of time. The early phase, which is more closely associated with the Norwood procedure, showed obstructed pulmonary venous return, single ventricle diagnosis other than HLHS compared to HLHS subtypes aortic stenosis/mitral stenosis and aortic atresia/mitral atresia, lower socioeconomic status, and smaller ascending aorta to be risk factors for death. The highest hazard ratio for mortality in the early/acute phase was obstructed pulmonary venous return. The risk factors for mortality during the late/constant phase were lower gestational age and presence of a genetic syndrome. In reviewing the influence of various subgroups, an interesting interaction between HLHS anatomic subtype, prematurity and shunt type emerged. The benefit of the RVPAS at a mean follow-up of 2.7±0.9 years was largely confined to full-term neonates with aortic atresia, in whom mortality was approximately 1/3 that of similar subjects undergoing an MBTS. Interestingly, pre-term neonates with a patent aortic valve had a better transplant-free survival with an MBTS.
The same publication analyzed risk factors for transplantation [21]. Cardiac transplantations occurred in 19 subjects in the SVR trial. Multivariable analysis revealed decreased pre-Norwood procedure right function as measured by lower fractional area change, HLHS vs. other single ventricle diagnosis, and number of surgeries prior to the Norwood procedure as risk factors for transplantation.
Follow-up Results from the Single Ventricle Reconstruction Trial
Since the initial SVR publications in 2010, the 3-year and 6-year results have been analyzed. At 3 years, the combined death and cardiac transplantation rates for the RVPAS vs. MBTS groups were 33% vs. 39% (P=0.14) [22]. When all available data were examined by Kaplan-Meier analysis (mean follow-up 4.4±1.0 years), there was also no difference between groups (log rank P=0.11). Overall, there were 100 deaths and 10 transplantations in the MBTS cohort and 86 deaths and 11 transplantations in the RVPAS group. For subjects surviving to one year, there were 10 events (9 deaths, 1 transplant) in the MBTS group and 25 events (18 deaths, 7 transplantations) in the RVPAS group. This loss of benefit to transplant-free survival for the RVPAS is also illustrated by the differing non-proportional hazards associated with the two sources of pulmonary blood flow over time. The RVPAS group had a lower hazard before 5 months of age (the mean age for stage II) (Hazard Ratio [HR] 0.63, 95% confidence interval [CI], 0.45–0.88), no difference from 5 months to 1 year, and a higher hazard after 1 year (HR 2.22, 95% CI, 1.07–4.62). As reflected by this increasing hazard over time, the transplantation-free survival conditional on surviving to 1 year was worse in the RVPAS group (log-rank P=0.03). This non-proportional hazard was seen despite no differences between stage II surgery morbidity and growth, neurodevelopmental status, right ventricular ejection fraction, and tricuspid regurgitation grade at 14 months of age or differences in the rates of Fontan completion, medical events and morbidities. Subjects receiving the RVPAS had more interventional catheterization lab visits and catheter-based interventions at 3 years (both P<0.001), a hazard risk which increased over the study time period.
The 6-year results have been presented as an abstract, with the manuscript pending [23]. While the point averages continued to reflect a difference favoring the RVPAS (combined death/transplantation rate, 36%) compared to the MBTS (41%), however the number of subjects are not sufficient to demonstrate a statistically significant difference between the two groups (log-rank P=0.13, Figure 1). Similar to the 3-year results, RVPAS subjects had a higher incidence of any catheter intervention (0.38 vs. 0.23 interventions/patient-year, P<0.001), including balloon angioplasty (P=0.014), stent (P=0.009), and coiling (P<0.001). Morbidities were similar between groups. Common morbidities included, pacemaker 3%, thrombosis 16%, stroke 7%, seizures 13%, protein losing enteropathy 3%, plastic bronchitis 0.5%. Functional performance revealed New York Heart Association Class I in 71% of subjects, Class II in 21%, Class III in 3%, and Class IV in 5%. Risk factors for death or transplantation were low birth weight (<2500 g), higher degree of pre-Norwood tricuspid regurgitation (>2.5 mm jet width), lower surgeon Norwood procedure volume, preterm birth (<37 weeks), and combined aortic atresia and pre-term birth (all P<0.01). By echocardiography, right ventricular ejection fraction was similar in the RVPAS and MBTS groups (46% in both groups, P=0.9).
Figure 1.
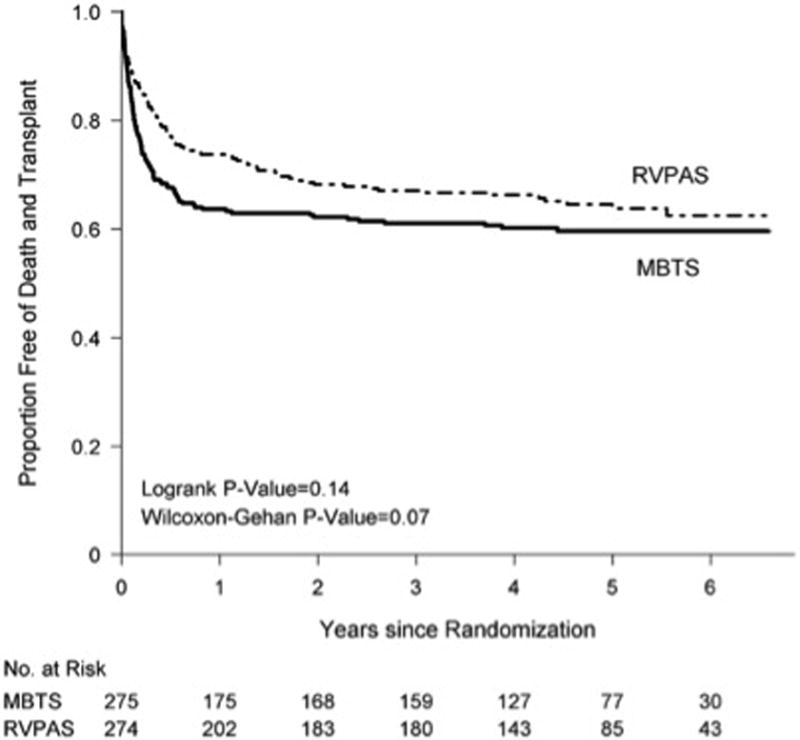
Comparison of the shunt types by intention-to-treat analysis in their freedom from the composite end point of death or cardiac transplantation (ie, transplantation-free survival). MBTS indicates modified Blalock-Taussig shunt; and RVPAS, right ventricle–to–pulmonary artery shunt (22).
Neurodevelopmental Outcomes
As survivals have improved for even the most complex congenital heart malformations, such as HLHS, attention has turned to longer-term outcomes, including neurodevelopment. Neurodevelopmental outcomes were analyzed at 14 months and again at 3 years for the SVR trial [24]. Three hundred twenty-one subjects were available for neurodevelopmental assessment at 14 months following randomization in the SVR trial. The Psychomotor Development Index (PDI) and Mental Development Index (MDI) of the Bayley Scales of Infant Development-II were administered. Overall, the average scores for PDI and MDI were below normal means. Independent risk factors for a lower PDI score included, lower birth weight, center where the Norwood was performed, longer length of stay following the Norwood and more complications between the Norwood procedure and 12 months of age. Independent risk factors for a lower MDI score included lower birth weight, center, genetic syndrome, lower maternal education, longer mechanical ventilation after the Norwood procedure and more complications after the Norwood discharge to 12 months of age. It is notable (and perhaps frustrating) that many of these risk factors are patient-related rather than easily modifiable.
Subjects from the SVR trial were also analyzed for neurodevelopmental outcome at 3 years. The Ages and Stages Questionnaire (ASQ) was utilized to measure neurodevelopment. The ASQ contains five domains, Communication, Gross Motor, Fine Motor, Problem-Solving, and Personal/Social Interaction. Delay in any of these domains is defined as <2 standard deviations below the mean. All ASQ domains for the study subjects demonstrated means lower than the reference population. The percentage of subjects classified as delayed was 20% for the Communication Scale, 30% Gross Motor, 35% Fine Motor, 24% Problem Solving, and 17% Personal/Social. Overall, at least 51% of the cohort demonstrated delay in at least one domain. On multivariable analysis, risk factors which were associated with more than one abnormal ASQ domain were census block with a greater percentage below the federal poverty level, vision or hearing problems, male sex, feeding therapy at 2 years of age, and more complications between the Norwood procedure and 3 years.
Behavior was measured with the Parent-Report Behavior Assessment System for Children, Second Edition (BASC-2), which qualifies adaptive and problem behaviors at home and at school. BASC-2 includes 14 subscales which are used to generate four composite scores: Externalizing Problems, Internalizing Problems, Behavioral Symptoms Index, and Adaptive Skills. At-risk or frankly abnormal behavioral composite scores were seen in 7.8%–21.9%.
Quality of life was assessed using both the Generic and Cardiac Modules of the Pediatric Quality of Life Inventory (PedsQL). The PedsQL measures physical, emotional, social, and school performance. Subjects in the SVR trial had lower mean score in each of the domains of the Generic PedsQL. The scores for the cardiac specific module were not reported.
The Functional Status, 2nd Edition Revised (FSII-R) is a parent-reported questionnaire assessing functional status for children with chronic health problems. FSII-R demonstrated lower total scores (P<0.001) and lower activity scores (P < .001) compared to the reference population.
Practice Pattern Variation
Perhaps one of the more informative manuscripts from the SVR trial, with respect to the ability to alter outcomes, addressed the practice pattern variations seen between centers in the SVR trial [24]. While gestational age, birth weight and proportion of subjects with a diagnosis of HLHS were similar across centers, there was a wide variation in per-operative, intra-operative and post-operative management. The range of these difference were striking and included such basic elements as, use of pre-operative intubation, cardiopulmonary bypass time, route of feeding at discharge, and utilization of a home monitoring program. While variation in care is not unto itself problematic, the mortality following the Norwood procedure varied from 7% to 39% across centers. It seems likely that adoption of best practices would positively influence this wide range in the mortality rate.
The Hybrid Approach
Hypothesized as a less invasive approach to palliate neonates with HLHS, in 1993 Gibbs et al. reported the first stenting of the arterial duct (DA) combined with bilateral pulmonary arterial banding (bPAB) and atrial septostomy as an alternative procedure to Norwood operation [25]. However, based on the first experiences in eight patients, the same group from Leeds, England did not further recommend ductal stenting as a palliation of newborns with duct-dependent systemic blood flow [26]. Later in Giessen, Germany, a more successful collaborative surgical-interventional approach was achieved by surgically performed bPAB via a brief open-chest procedure, followed by a second elective percutaneous transcatheter DA-stenting, which was combined with atrial septum (IAS) manipulation, if necessary [27]. Subsequently, in 1998, Hakan Akintuerk performed the first comprehensive stage II. Postnatal bPAB placement saved the life of the newborn, who had been admitted in cardiogenic shock five months prior. Early promising results of a hybrid stage I, followed by successful comprehensive stage II and Fontan completion were published in 2002 [28]. Independent from the experiences in Giessen, early in the new millennium a second group of collaboratively minded interventionists and surgeons in Columbus, Ohio focused on a one-step Hybrid procedure, which entailed placing a stent within the duct by transpulmonary access immediately after bPAB during the same open chest approach. Percutaneous manipulation of the interatrial septum was delayed until just before the palliated patient was discharged home [29]. In both centers, the Hybrid procedure replaced the Norwood as the preferred first stage of palliation [29, 30]. In Sao Paulo, Brazil, the Columbus Hybrid-variant also supplanted the use of the Norwood procedure [31]. Subsequently, the group in Toronto described an additional variant of the Hybrid approach for HLHS patients with aortic atresia [32]. This approach added a reverse MBTS after bPAB placement, but prior to ductal stent placement. Based on an institutional risk score, a recommendation is made as to whether the patient is best served by a Hybrid or a Norwood procedure. Over time, multiple centers worldwide have established Hybrid programs for high-risk cases [33]. In Japan [34] and London [35], the Hybrid procedure is used in some critical newborns as a palliative step for bridging newborns to a delayed Norwood procedure beyond the neonatal period with or without ductal stenting.
Irrespective of the exact technical approach, a successful Hybrid requires the same three objectives as originally proposed by Norwood for surgical palliation: adequate systemic blood flow, sufficient protection of the pulmonary arterial circulation and unrestrictive flow of pulmonary venous system. After the learning curves and the acquisition of the requisite technical skills, an elective Hybrid procedure treating HLHS can be performed with extremely low mortality [29, 30].
In addition to a complete stage I procedure, the individual components of the Hybrid approach can be utilized in specific situations. Percutaneous duct-stenting is the treatment of choice for prostaglandin refractory ductal obstruction [36]. Balloon atrial septostomy with or without stent placement is also the preferred method to treat severe hypoxemia due to significantly obstructed or intact atrial septum. Obstructed pulmonary venous blood flow associated with a total anomalous pulmonary venous return can be palliated by transcutaneous catheter techniques [37]. Neonates who present in extremis due to low systemic perfusion, can be resuscitated with bPABs [38].
Considering the wide variation of different techniques described for the Hybrid approach, as well as the variable results, it can be challenging to understand the risks and benefits. The primary benefit of a Hybrid approach is as a minimally invasive alternative to a Norwood procedure with a less acute post-procedural hospital course [29, 30]. In addition to the immediate advantages, by postponing the open surgical procedure until after the neonatal period, it has been postulated that there may be potential benefits to long-term outcomes, such as neurodevelopment [39, 40].
The current state of the art of the Hybrid approach in HLHS, might be summarized as following:
Prenatal diagnosis of HLHS is optimal for elective postnatal treatment by a trained and prepared surgical-interventional team.
Ductal patency is maintained open with low dose prostaglandin E1 (2–5ng/kg-min), which decreases the risk of apnea and the rapid decrease of pulmonary vascular resistance (Rp).
If there is no need for immediate atrial septum manipulation, pulmonary blood-flow should be immediately controlled by surgical fashioned bPABs. As described by Galantowicz [30], the PAB can easily constructed by cutting a 3.0 or 3.5 mm PTFE tube graft to a small 1–2mm strip, which are used in patients with body weight above or below 3 kg, respectively. After median sternotomy and partially opened pericardium, PTFE-bands are sewn around the left and right pulmonary artery (Figure 2). We recommend against manipulations such as transpulmonary ductal stenting or placement of a reverse MBTS, which may result in blood loss, circulatory instability, or need for inotropic infusions. Concurrent use of additional interventions at this time have been associated with reported mortality rates between 10 to 25% [32, 35]. Conversely, the surgical approach in Giessen is simply focused on bPAB, the surgical procedure mortality has been less than 1% [29]. In addition, time under anesthesia with slightly permissive hypercapnic ventilation can be held short, followed by immediate spontaneous breathing and early extubation. The heart rate and systemic vascular resistance (Rs) are perioperatively controlled by continuous infusion of the alpha-2 agonist clonidine, followed by early oral application of a ß1-receptor blocker (bisoprolol) together with tissue ACE-inhibitor (lisinopril) without jeopardizing the coronary perfusion pressure [41].
In all elective patients, ductal stenting is performed as an independent percutaneous approach by trans-femoral vascular access. The enormous development of stent technology in Europe resulted in a CE-mark for stents indicated for ductal stenting in newborns. The stent-design, based on a self-expanding nitinol alloy (Sinus-SuperFlexDS, OptiMed Inc., Karlsruhe, Germany) allows duct-stenting by advancing loaded stents with widths from 7 to 9 mm and lengths between 12 and 24 mm deliverable through a 4F vascular sheath. Therefore, stent placement within the duct can performed by femoral venous or preferentially by femoral arterial access. Before stent placement, the extremely variable anatomy of the junction of the duct to descending aorta needs to be precisely delineated by brief episodes of angiography utilizing minimal volume hand injections of contrast medium. The entire length of the duct needs to be fully covered (Figure 3). A further advantage of percutaneous stent placement is the optimal visualization of the isthmus region to determine if an additional intervention to maintain retrograde flow to the proximal aorta is necessary. Ballooning of an obvious “reverse coarctation” should be performed prior to duct stenting and if stent placement becomes necessary, it should performed after stent placement within the duct.
In order to guarantee unrestricted flow of pulmonary venous return into the right atrium, interventions on the atrial septum in advance may be of benefit.
Figure 2.
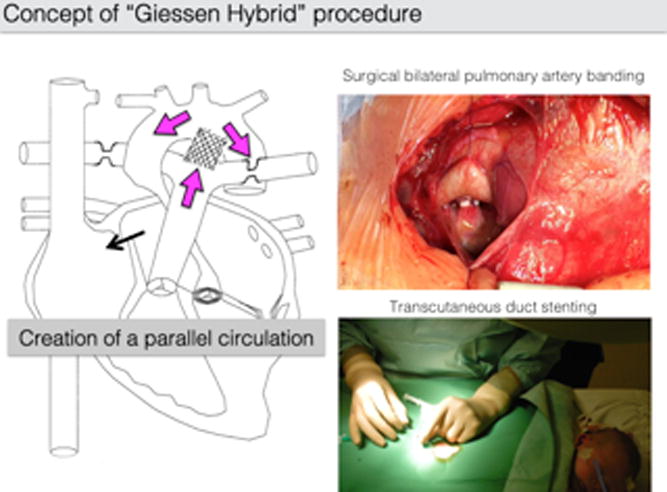
Line drawing (left) of bilateral pulmonary artery banding and ductal stent, with flow characteristics shown by purple arrows, as well as the left-to-right shunting at the atrial communication (black arrow) (Akintuerk et al [28]). The open chest approach with right pulmonary banding (right upper) and percutaneous heart catheterization by femoral access for ductal stenting after surgical bPAB (right lower).
Figure 3.
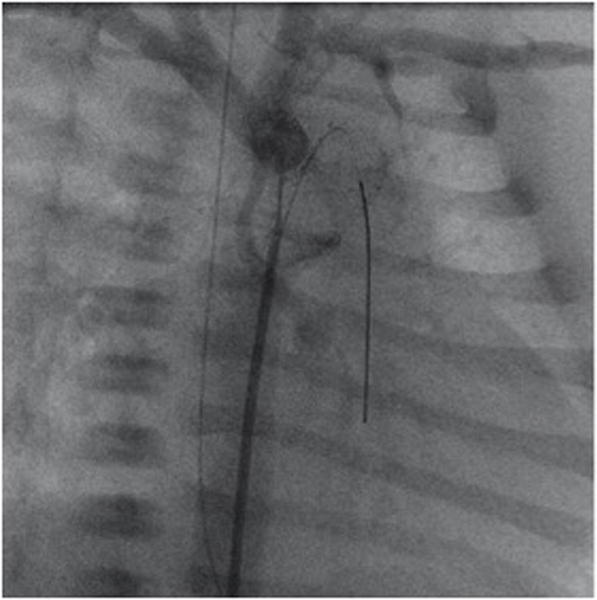
Right anterior oblique angiography performed through a 4F multipurpose catheter positioned at the junction of the arterial duct (stented by an open cell self-expanding SinusSuperflex-DS stent). The extreme hypoplastic ascending aorta is connected to a rather well developed aortic arch. A coronary soft-tip wire is seen passing retrograde through the ductal stent into the right ventricular outflow tract.
The unrestricted atrial-level left-to-right flow and ductal right-to-left flow, combined with a protected pulmonary circulation, need to be maintained until the comprehensive stage II, which is performed in most patients in an age of 4–5months. The fragile interstage I period, between the Hybrid and the comprehensive stage II, requires not only a successful initial Hybrid palliation with a well-balanced circulation, but also appropriate and assiduous follow-up monitoring, which includes detailed instructions for the parents. Medications are titrated to balance the systemic-to-pulmonary vascular resistance ratio, as well as the ratio of oxygen consumption-to-demand. In regard to the important role of the parents, the cardiovascular medications need to be safe, well understood and easily administered. The respiratory rate while sleeping, oral intake, body weight are monitored closely by the parents [30, 41]. In addition, interstage management requires close outpatient evaluation by an experienced pediatric cardiologist, in order to detect any hemodynamic imbalance prior to the development of any crisis [30]. Based on this close outpatient monitoring, interventional procedures should be aggressively pursued for not only detected hemodynamic abnormalities, but any suspected issue during the interstage period. Interventions to address obstructions in the stented duct, atrial septum or even of the descending aortic arch can be effectively performed utilizing various catheter techniques [30]. In summary, this combined parents-physician monitoring program has been able to significantly reduce the interstage morbidity and in particular mortality to less than 5%, for HLHS, which is comparable to that described for interstage surveillance programs following the Norwood procedure [42].
Pre-comprehensive stage II catheterizations have generally been replaced by magnetic resonance imaging in sedated, spontaneously breathing infants, similar to our practice for all elective heart catheterizations. Summarizing the two-center experience for the comprehensive stage II in Giessen [43] and Columbus [44], accounting for more than 200 patients, the comprehensive stage II can be performed with a mortality of less than 5%, despite the early learning curve being included in these reports. The surgical approach consists of bilateral pulmonary de-banding, ductal stent removal, atrial septectomy, reconstruction of the aortic arch and bidirectional cavo-pulmonary connection, which combines components of the Norwood and stage II procedures into one operation, thereby avoiding an extensive open-heart operation in the neonatal period [39]. In addition, anesthetic and intensive care strategies focusing on early extubation and strategies to reduce oxygen consumption facilitate care in the immediate post-operative period. The Achilles heel of the comprehensive stage II is the variable fate of the left pulmonary artery with respect to stenosis. Refinements, such as intra-operative stenting, are warranted to improve the current surgical techniques [30, 43]. Following the comprehensive stage II, the completion Fontan circulation can be performed with the typically expected low mortality. At dedicated centers, an estimated ten-year survival of 78% can currently be achieved by the Hybrid approach in an unselected population of neonates [44].
Late Fontan Outcomes
The outcomes of patients who undergo the Fontan procedure are by far better than initially expected [45, 46]. The current variation in outcomes in various reports is related to variation in the indications for Fontan completion: centers with restrictive indications will present higher survival than those with more permissive indications [47–51]. Technical variations have also been shown to impact outcomes, such that procedures performed in more recent decades resulted in better early and late outcomes [48–52]. Therefore, the interpretation of any outcomes after Fontan surgery should take into consideration the precise technique used and the era when it was performed. Specific long-term outcomes after Fontan for patients born with HLHS have rarely been reported. Only a limited number of studies reported outcomes after Norwood surgery beyond 15 years [53–56]. In the recent years, large series of follow-up of outcomes after Fontan into the third decade have been published and these will be the best guide to give long-term predictions to the families requiring counseling [47,49,57, 58]. The outcomes of patients with HLHS who have reached Fontan completion seem to be somewhat equivalent to the remaining population in the first two decades. We should therefore feel entitled to use current available data from the whole population of patients with a Fontan circulation to draw current predictions of late outcomes for patients born with HLHS.
Survival
Current expectations points towards a 30-year survival of 85% for those operated today with, at this early stage, only minor differences in survival between those with and without previous Norwood surgery [59–62]. Hospital mortality of Fontan completion has also decreased consistently and is identified from large multi-centric data to be comprised between 1 and 2% [63,64]. From longitudinal data from birth, it therefore seems that two thirds of the patients born with HLHS and operated in best centers may hope to reach adulthood [53–56].
The overarching discriminant in late survival is the type of Fontan. Patients with total cavo-pulmonary connections (TCPC), the lateral tunnel and the extra-cardiac conduit seem to have similar outcomes, which are better than those with an atriopulmonary connection [47, 59, 61, 62, 65–67]. Because the introduction of the Norwood procedure was contemporary to the introduction of the total cavo-pulmonary connection, the majority of those surviving initial staged operations had this latter form of Fontan and predictions of their late survival should be based on these latter procedures. The Australia and New Zealand Fontan Registry, collating data from 1423 subjects, reported survival at 26 years of 89% for those operated with the lateral tunnel technique and 92% at 18 years for those with an extra-cardiac conduit, in concordance with recent major series [57,59,67]. Interestingly, the survival of this population has not been subjected to a sudden decline in early adulthood as was previously expected. The attrition rate of this population has been remarkably stable, and, for this reason, we feel emboldened to make prediction of survival to 30 years [59].
Heart Transplantation
Since the conception of the Fontan operation, predictions were made that heart transplantation would be the final end-of-life option and counseling of the families unvariably included this option. In reality, heart transplantation has taken only a very small part in the treatment armentarium of this population with only 4% to 7% reaching this status within 20 years of the Fontan [49, 68]. The reasons for the infrequent use of this ultimate resource are likely the multiplicity of previous operations increasing the risk of reentry and immunoreactivity, the poor general status of the patients and the complexity of the reconstruction necessary [69, 70]. It has been recognized that those with a Fontan circulation did not have equal access to transplantation because this access is dependent on structuring of heart transplantation programs with expertise in congenital heart diseases [68]. A large multi-centric study has suggested that post-transplantation survival in patients with a previous Fontan surgery was inferior to the survival of patients with idiopathic dilated cardiomyopathy and bi-ventricular congenital heart diseases [70]. Numerous single center studies have since demonstrated equal late outcomes after transplantation of patients with a Fontan circulation including those with HLHS. It demonstrates that heart transplantation should be offered to patients with a Fontan circulation and that restriction of this practice to expert centers may be advisable [68,71–73].
Burden of Disease
Even though survival after Fontan is now proven to be superior to previous expectations, the burden of their disease is still considerable. In a study of 529 patients, who had undergone an extra-cardiac conduit in Australia and New Zealand, 47% of the hospital survivors suffered an adverse event within 15 years, when including events such as transplantation, reoperation, stroke, tachy- and bradyarrrhythmia, thromboembolic events, bleeding, pacemaker implantation, protein-losing enteropathy, plastic bronchitis and poor functional status [60].
It has been already demonstrated that the burden of late complications is higher in patients with HLHS [60,74]. It does not seem at this stage that the higher severity of this condition is related to the intrinsic inability of a single right ventricle to support a Fontan circulation for several decades, but rather to the fact that patients with HLHS tend to have a higher incidence of decreased ventricular function and atrioventricular valve regurgitation, and have a higher propensity of developing arrhythmias [60,74–76]. Because patients with HLHS have clearly a higher burden of disease, it is likely that some difference will appear in the future in the survival rate of these patients compared to the remaining Fontan population.
Tachy- and Bradyarrhythmias
Dysrhythmias has been one of the most prevalent complications after Fontan surgery with quoted incidence varying between 13% and 54% at 20 years [47,77,78]. Unfortunately, the relative incidence of brady- and tachyarrhythmias and their relative type have rarely been specified. The incidence of supraventricular tachycardia is higher in patients with atriopulmonary connection type of Fontan because of the progressive dilatation of their atrial cavity. It seems that patients with an extra-cardiac conduit might have a lower incidence of dysrhythmias than those with a lateral tunnel [47,79,80].
Thrombo-embolic events
Both the extra-cardiac conduit and the lateral tunnel technique result in the exposure of PTFE in the venous flow, predisposing to the formation of clots and requiring the administration of anti-thrombotic agents [81]. There is still no formal consensus on the ideal anti-thrombotic agent and patients are either placed under warfarin or aspirin. A prospective randomized trial, a propensity score matched analysis and a meta-analysis all points towards the equivalence of both strategies or to the superiority of aspirin in the prevention of thromboembolic events [81–84]. The incidence of thromboembolic events seems to be limited to 18% to 21% at 10 years [85,86].
Protein-losing enteropathy and plastic bronchitis
These two feared complications consist in the loss of proteins in the lumen of either the intestine or the bronchi, and seem to occur between 5% and 10% of cases with an incidence increasing with time [47,49]. A few years ago, the development of this complications was associated with a risk of death of 49% at five years, but it seems that the introduction of an enteric-coated form of steroid has now improved this prognosis at least temporarily [86–88]. While various interventions have been attempted for these complications, heart transplantation has been shown to remain the most effective treatment, likely because it normalizes the systemic venous pressures of these patients [89,90].
Liver and Renal Failure
The key driver of the Fontan circulation is the increased systemic venous pressure, which over time has been noted to impact liver and renal function. Within years of Fontan completion, almost all patients are noted to develop hepatic fibrosis with some evolving to cirrhosis [91]. Some rare cases of hepatocarcinomas have also been described [49,92]. Similarly, decreased glomerular filtration has been noted in patients with a Fontan circulation [93]. At this stage, it is difficult to appreciate to what extent liver fibrosis and cirrhosis will affect the survival and quality of life of those with a Fontan circulation. While the existence of liver fibrosis is widespread, it has not yet been associated with profound hepatic functional impairment. The impact of the Fontan on the liver remains nonetheless one of the primary concern for the long-term survivors with a Fontan circulation.
Exercise Capacity
It has been consistently reported that patients with Fontan circulation have decreased maximal exercise capacity with peak oxygen consumption averaging around 65% of predicted values [94,95]. There is a wide variation in the capacity of these individuals to participate to individual and even group sporting activities, with some being able to function at levels that are surprising for patients bearing only one functional ventricle. The American Heart Association has recently lifted a ban on exercise for these patients [96]. It has now been demonstrated that resistance exercise training is actually beneficial for these patients [97]. An increased leg muscle mass is responsible for increased cardiac output on exercise, acting as an additional pump at maximal exercise. There is growing belief that a lifestyle including regular exercise training will be beneficial for this population.
Pregnancy
The majority of practitioners recommend the avoidance of pregnancy [98]. It is remarkable that some women with a Fontan circulation have nonetheless achieved successful pregnancies, even though the number of those with HLHS remain anecdotal [98,99]. Women with a Fontan procedure, who have attempted to become pregnant or carry to delivery, have faced issues of infertility, miscarriages and pregnancies resulting in small and premature babies. The maternal mortality has remained low, but the impact of pregnancy on long-term outcomes remains to be elucidated.
Neurodevelopmental Outcomes and Quality of Life
The late functional outcomes of patients living with a Fontan circulation remains the topic of heated debate. The majority of studies report them to have decreased scores of quality of life, but several optimistic studies have also been published depicting normal quality of life and normal level of emotional functioning [95,100–102]. The performances of patients with HLHS do not seem to differ from those with other single ventricle conditions or from other patients with congenital heart diseases [103]. There is no doubt that we should focus on improving the quality of life and provide psychological support to this population at risk. One should not, however, see this population as being profoundly debilitated. The majority of this population is able to function normally without perceived restrictions. It was remarkable to note that, in a yet unpublished work, 28 years after an atriopulmonary Fontan performed in Australia and New Zealand, not only two thirds of the patients were still alive, but two thirds of those were working.
Medical therapy
Large variations in medical therapy have been described [104,105]. As an example, it has long been debated whether ACE inhibitors, a cornerstone medical therapy of heart failure, would be of interest in single ventricle circulation. In a randomized study of enalapril performed in 230 patients of the Paediatric Heart Network, no benefits could be demonstrated [106]. The recent TEMPO study suggested the benefits of the administration of bosentan [107]. The ideal medical therapy for these patients remain elusive.
Comment and Future Considerations
Tremendous progress has been made over the past three decades in the care of children with functional single ventricle malformations. Progress has been dramatic at times, such as the development of the Norwood procedure before which HLHS was universally fatal, and incremental, but no less important in improving outcomes. Our understanding of the long-term implications of a Fontan circulation continues to evolve as the number of adolescent and young adult survivors grows.
The state of the art for the care of the single ventricle patient is a moving target, constantly changing and improving. Currently, there are a variety of valid approaches to the management of the patient with HLHS and related FSRV malformations particularly in the first stage. For the first stage of traditional surgical staged palliation (the Norwood procedure), there are two alternatives for the source of PBF, the MBTS and the RVPAS. While the 12-month end point comparing transplantation-free survival showed a benefit associated with the RVPAS and the point averages continue to favor the RVPAS at 6 years, the difference is no longer statistically significant. Concerns over the ventriculotomy required for the RVPAS and the development of subsequent late right ventricular function do not seem to be warranted, as right ventricular function by echocardiogram is equivalent at 6 years. Thus, either approach seems reasonable at this time. Continued follow-up of this large, well characterized cohort of patients will be important, not only to detect any late effects of shunt choice, but also to understand long-term outcomes and how they may be useful to inform early management decisions beyond shunt type.
The Hybrid approach is also an equally viable alternative to traditional staged palliation. As stated in the section on the Hybrid approach, one of the keys to success with this management strategy is the requirement for a dedicated, experienced and collaborative team of cardiologist and surgeons. The results for the Hybrid outlined in this manuscript are the outcomes from arguably the two most experienced centers in the world. The results from the SVR trial represents a 15-center sampling of large and moderate-sized institutions with varying traditions of success with surgical single ventricle palliation. The highest performing centers in the SVR trial have results superior to those reported for the Hybrid approach, while standard performing centers have lower survivals. The potential long-term benefits of avoiding an extensive neonatal open-heart surgery, particularly in neurodevelopment, remain to be determined. Thus, the selection of one or the other approach depends largely on local experience and resources. However, an important caveat is that optimal results for the Hybrid approach requires equal dedication and expertise as is required for optimal surgical results and simply switching from one to the other or vice versa does not guarantee success.
A common theme is the need for institutional commitment, expertise and experience for optimal results for the care of these fragile and complex patients, whether the approach is the traditional staged surgical palliation and an initial Norwood procedure or the Hybrid approach. There are multiple publications demonstrating a positive correlation between volumes and outcomes for high complexity lesions [108,109]. However, these results are on aggregate and do not indicate that any individual center, large, moderate or small, by virtue of volume alone insures superior results, nor that a moderate or small program cannot achieve excellent outcomes. In addition, the concept that regionalization to the small number of centers with superior results can take all cases of FSRV malformations each year, which number over 1000 cases/year in the United States alone, is unrealistic in many countries.
If neither the type of shunt nor the development of the Hybrid approach has dramatically decreased mortality and regionalization seems unlikely in some countries, what potential avenues for improvement exist? As noted above, there was wide variation in practices across centers in the SVR trial. While this may seem unimportant, the similarly wide variation in Norwood procedure mortality across centers, from 7% to 39%, is sobering [20]. It is possible that determining and sharing of best practices would be effective in improving the results at all centers. The Northern New England Cardiovascular Disease Study Group and the Michigan Society of Thoracic and Cardiovascular Surgeons Quality Collaborative have demonstrated that this approach has been effective in decreasing mortality and morbidity in adult cardiac surgery [110, 111]. The Pediatric Heart Network funded a study on collaborative learning aimed at decreasing length of intubation following congenital cardiovascular surgery, which showed the method to be effective in promoting early extubation [112].
Future directions include the use of stem cell therapy to improve cardiac performance in the single ventricle patient. Several groups have either demonstrated efficacy in small numbers of patients or are starting trials on the use of stem cells in this population [113, 114]. Techniques may include intracoronary injection, intramyocardial injection or onlay patches containing stem cells aimed at improving both systolic and diastolic function of the myocardium.
Universally fatal only 35 years ago, improvements in the treatment of HLHS and related FSRV malformations has been transformative. The survival of this population of babies born with HLHS has now surpassed initial expectations. The current state of the art reflects these remarkable achievements. Yet, despite this progress, the enthusiasm for improvement in survivals and long-term quality of life remains. As the first generation of these patients enter adulthood, the focus must now be on improving not only their longevity, but also their quality of life. In that respect, it becomes increasingly important to address the still considerable burden of disease associated with the Fontan circulation. It is likely that these improvements will be dependent on not only ongoing progress in care for those living with a single ventricle malformation, but also on the improvement of the initial stages of their palliation.
Acknowledgments
Funding Sources: RGO- The Single Ventricle Reconstruction trial was supported by grants (HL068269, HL068270, HL068279, HL068281, HL068285, HL068288, HL068290, HL068292, and HL085057) from the National Heart, Lung, and Blood Institute (NHLBI). This work is solely the responsibility of the authors and do not necessarily represent the official views of NHLBI or NIH.
Footnotes
Journal Subject Terms: Congenital Heart Disease, Cardiovascular Surgery
Conflict of Interest Disclosures: None.
References
- 1.Noonan JA, Nadas AS. The hypoplastic left heart syndrome; an analysis of 101 cases. Pediatr Clin North Am. 1958;5:1029–1056. doi: 10.1016/s0031-3955(16)30727-1. [DOI] [PubMed] [Google Scholar]
- 2.Norwood WI, Lang P, Casteneda AR, Campbell DN. Experience with operations for hypoplastic left heart syndrome. J Thorac Cardiovasc Surg. 1981;82:511–519. [PubMed] [Google Scholar]
- 3.Tweddell JS, Hoffman GM, Fedderly RT, Berger S, Thomas JP, Jr, Ghanayem NS, Kessel MW, Litwin SB. Phenoxybenzamine improves systemic oxygen delivery after the Norwood procedure. Ann Thorac Surg. 1999;67:161–167. doi: 10.1016/s0003-4975(98)01266-1. [DOI] [PubMed] [Google Scholar]
- 4.Norwood WI, Kirklin JK, Sanders SP. Hypoplastic left heart syndrome: experience with palliative surgery. Am J Cardiol. 1980;45:87–91. doi: 10.1016/0002-9149(80)90224-6. [DOI] [PubMed] [Google Scholar]
- 5.Ohye RG, Ludomirsky A, Devaney EJ, Bove EL. Comparison of right ventricle to pulmonary artery conduit and modified Blalock–Taussig shunt hemodynamics after the Norwood operation. Ann Thorac Surg. 2004;78:1090–1093. doi: 10.1016/S0003-4975(03)01386-9. [DOI] [PubMed] [Google Scholar]
- 6.Donnelly JP, Raffel DM, Shulkin BL, Corbett JR, Bove EL, Mosca RS, Kulik TJ. Resting coronary flow and coronary flow reserve in human infants after repair or palliation of congenital heart defects as measured by positron emission tomography. J Thorac Cardiovasc Surg. 1998;115:103–101. doi: 10.1016/s0022-5223(98)70448-9. [DOI] [PubMed] [Google Scholar]
- 7.Kishimoto H, Kawahira Y, Kawata H, Miura T, Iwai S, Mori T. The modified Norwood palliation on a beating heart. J Thorac Cardiovasc Surg. 1999;118:1130–1132. doi: 10.1016/S0022-5223(99)70118-2. [DOI] [PubMed] [Google Scholar]
- 8.Sano S, Ishino K, Kado H, Shiokawa Y, Sakamoto K, Yokota M, Kawada M. Outcome of right ventricle-to-pulmonary artery shunt in first-stage palliation of hypoplastic left heart syndrome: a multi-institutional study. Ann Thorac Surg. 2004;78:1951–1957. doi: 10.1016/j.athoracsur.2004.05.055. [DOI] [PubMed] [Google Scholar]
- 9.Sano S, Ishino K, Kawada M, Honjo O. Right ventricle-pulmonary artery shunt in first-stage palliation of hypoplastic left heart syndrome. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annual. 2004;7:22–31. doi: 10.1053/j.pcsu.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 10.Sano S, Ishino K, Kawada M, Arai S, Kasahara S, Asai T, Masuda Z, Takeuchi M, Ohtsuki S. Right ventricle-pulmonary artery shunt in first-stage palliation of hypoplastic left heart syndrome. J Thorac Cardiovasc Surg. 2003;126:504–509. doi: 10.1016/s0022-5223(02)73575-7. [DOI] [PubMed] [Google Scholar]
- 11.Pizarro C, Malec E, Maher KO, Januszewska K, Gidding SS, Murdison KA, Baffa JM, Norwood WI. Right ventricle to pulmonary artery conduit improves outcome after stage I Norwood for hypoplastic left heart syndrome. Circulation. 2003;108(Suppl 1):II155–160. doi: 10.1161/01.cir.0000087390.94142.1d. [DOI] [PubMed] [Google Scholar]
- 12.Pizarro C, Norwood WI. Right ventricle to pulmonary artery conduit has a favorable impact on postoperative physiology after Stage I Norwood: preliminary results. Eur J Cardiothorac Surg. 2003;23:991–995. doi: 10.1016/s1010-7940(03)00158-1. [DOI] [PubMed] [Google Scholar]
- 13.Januszewska K, Kolcz J, Mroczek T, Procelewska M, Malec E. Right ventricle-to-pulmonary artery shunt and modified Blalock-Taussig shunt in preparation to hemi-Fontan procedure in children with hypoplastic left heart syndrome. Eur J Cardiothorac Surg. 2005;27:956–961. doi: 10.1016/j.ejcts.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Rumball EM, McGuirk SP, Stümper O, Laker SJ, de Giovanni JV, Wright JG, Barron DJ, Brawn WJ. The RV-PA conduit stimulates better growth of the pulmonary arteries in hypoplastic left heart syndrome. Eur J Cardiothorac Surg. 2005;27:801–806. doi: 10.1016/j.ejcts.2005.01.061. [DOI] [PubMed] [Google Scholar]
- 15.Caspi J, Pettitt TW, Mulder T, Stopa A. Development of the pulmonary arteries after the Norwood procedure: comparison between Blalock-Taussig shunt and right ventricular-pulmonary artery conduit. Ann Thorac Surg. 2008;86:1299–1304. doi: 10.1016/j.athoracsur.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 16.Azakie A, Martinez D, Sapru A, Fineman J, Teitel D, Karl TR. Impact of right ventricle to pulmonary artery conduit on outcome of the modified Norwood procedure. Ann Thorac Surg. 2004;77:1727–1733. doi: 10.1016/j.athoracsur.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Mahle WT, Cuadrado AR, Tam VK. Early experience with a modified Norwood procedure using right ventricle to pulmonary artery conduit. Ann Thorac Surg. 2003;76:1084–1089. doi: 10.1016/s0003-4975(03)00343-6. [DOI] [PubMed] [Google Scholar]
- 18.Tabbutt S, Dominguez TE, Ravishankar C, Marino BS, Gruber PJ, Wernovsky G, Gaynor JW, Nicolson SC, Spray TL. Outcomes after the stage I reconstruction comparing the right ventricular to pulmonary artery conduit with the modified Blalock–Taussig shunt. Ann Thorac Surg. 2005;80:1582–1591. doi: 10.1016/j.athoracsur.2005.04.046. [DOI] [PubMed] [Google Scholar]
- 19.Ohye RG, Gaynor JW, Ghanayem NS, Goldberg CS, Laussen PC, Frommelt PC, Newburger JW, Pearson GD, Tabbutt S, Wernovsky G, Wruck LM, Atz AM, Colan SD, Jaggers J, McCrindle BW, Prakash A, Puchalski MD, Sleeper LA, Stylianou MP, Mahony L, for the Pediatric Heart Network Investigators Design and rationale of a randomized trial comparing the Blalock-Taussig and right ventricle-pulmonary artery shunts in the Norwood procedure. J Thorac Cardiovasc Surg. 2008;136:968–975. doi: 10.1016/j.jtcvs.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohye RG, Sleeper LA, Mahoney L, Newburger JW, Pearson GD, Lu M, Goldberg CS, Tabbutt S, Frommelt PC, Ghanayem NS, Laussen PC, Rhodes JF, Lewis AB, Mital S, Ravishankar C, Williams IA, Dunbar-Masterson C, Atz AM, Colan S, Minich LL, Pizarro C, Kanter KR, Jaggers J, Jacobs JP, Krawczeski CD, Pike N, McCrindle BW, Virzi L, Gaynor JW, for the Pediatric Heart Network Investigators Comparison of shunt types in the Norwood Procedure for single-ventricle lesions. N Engl J Med. 2010;362:1980–1992. doi: 10.1056/NEJMoa0912461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tweddell JS, Sleeper LA, Ohye RG, Williams IA, Mahony L, Pizarro C, Pemberton VL, Frommelt PC, Bradley SM, Cnota JF, Hirsch J, Kirshbom PM, Li JS, Pike N, Puchalski M, Ravishankar C, Jacobs JP, Laussen PC, McCrindle BW, for the Pediatric Heart Network Investigators Intermediate-term mortality and cardiac transplantation in infants with single-ventricle lesions: risk factors and their interaction with shunt type. J Thorac Cardiovasc Surg. 2012;144:152–159. doi: 10.1016/j.jtcvs.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newburger JW, Sleeper LA, Frommelt PC, Pearson GD, Mahle WT, Chen S, Dunbar-Masterson C, Mital S, Williams IA, Ghanayem NS, Goldberg CS, Jacobs JP, Krawczeski CD, Lewis AB, Pasquali SK, Pizarro C, Gruber PJ, Atz AM, Khaikin S, Gaynor JW, Ohye RG, for the Pediatric Heart Network Investigators Transplant-Free Survival and Interventions at 3 Years in the Single Ventricle Reconstruction Trial. Circulation. 2014;129:2013–2020. doi: 10.1161/CIRCULATIONAHA.113.006191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newburger JW, Sleeper LA, Gaynor JW, Hollenbeck-Pringle D, Frommelt P, Li J, Mahle WT, WIllliams IA, Atz AM, Burns KM, Chen S, Cnota JF, Dunbar-Masterson C, Ghanayem NS, Goldberg CS, Jacobs JP, Lewis AB, Mital S, Pizarro C, Eckhauser AW, Stark P, Ohye RG, for the Pediatric Heart Network Investigators . American Heart Association Scientific Sessions 2015. Orlando, Florida: Nov 10, 2015. The Single Ventricle Reconstruction (SVR) Trial at 6 Years: Transplant-Free Survival, Catheter Interventions, and Morbidity. [Google Scholar]
- 24.Pasquali SK, Ohye RG, Lu M, Kaltman J, Caldarone CA, Pizarro C, Dunbar-Masterson C, Gaynor JW, Jacobs JP, Kaza AK, Newburger J, Rhodes JF, Scheurer M, Silver E, Sleeper LA, Tabbutt S, Tweddell J, Uzark K, Wells W, Mahle WT, Pearson GD, for the Pediatric Heart Network Investigators Variation in perioperative care across centers for infants undergoing the Norwood procedure. J Thorac Cardiovasc Surg. 2012;144:915–921. doi: 10.1016/j.jtcvs.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gibbs JL, Wren C, Watterson KG, Hunter S, Hamilton JR. Stenting of the arterial duct combined with banding of the pulmonary arteries and atrial septectomy or septostomy: a new approach to palliation for the hypoplastic left heart syndrome. Br Heart J. 1993;69:551–555. doi: 10.1136/hrt.69.6.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gibbs JL, Uzon D, Blackburn MEC. Fate of the stented arterial duct. 8HLHS and 10PAt. Ductal stenting cannot be recommended. Circulation. 1999;99:2621–2625. doi: 10.1161/01.cir.99.20.2621. [DOI] [PubMed] [Google Scholar]
- 27.Michel-Behnke, Akintürk H, Schranz D. Fate of the stented arterial duct. Circulation. 2000;102:E178. doi: 10.1161/01.cir.102.22.e178. [DOI] [PubMed] [Google Scholar]
- 28.Akintuerk H, Michel-Behnke I, Valeske K, Mueller M, Thul J, Bauer J, Hagel KJ, Kreuder J, Vogt P, Schranz D. Stenting of the arterial duct and banding of the pulmonary arteries: basis for combined Norwood stage I and II repair in hypoplastic left heart. Circulation. 2002;105:1099–1103. doi: 10.1161/hc0902.104709. [DOI] [PubMed] [Google Scholar]
- 29.Galantowicz M, Cheatham JP, Phillips A, Cua CL, Hoffman TM, Hill SL, Rodeman R. Hybrid approach for hypoplastic left heart syndrome: intermediate results after the learning curve. Ann Thorac Surg. 2008;85:2063–2070. doi: 10.1016/j.athoracsur.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 30.Schranz D, Bauer A, Reich B, Steinbrenner B, Recla S, Schmidt D, Apitz C, Thul J, Valeske K, Bauer J, Müller M, Jux C, Michel-Behnke I, Akintürk H. Fifteen-year single center experience with the “Giessen Hybrid” approach for hypoplastic left heart and variants: current strategies and outcomes. Pediatr Cardiol. 2015;36:365–373. doi: 10.1007/s00246-014-1015-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pedra CA, Pedra S, Jatene MB. In: The Brazilian hybrid approach for hypoplastic left heart syndrome. Butera, Cheatham, Schranz, Tulzer, editors. Springer; Chapter 30. in press. [Google Scholar]
- 32.Baba K, Honjo O, Chaturvedi R, Lee KJ, Van Arsdell G, Caldarone CA, Benson LN. “Reverse Blalock- Taussig shunt”: application in single ventricle hybrid palliation. J Thorac Cardiovasc Surg. 2013;146:352–357. doi: 10.1016/j.jtcvs.2012.11.029. [DOI] [PubMed] [Google Scholar]
- 33.Karamlou T, Diggs BS, Ungerleider RM, Welke KF. Evolution of treatment options and outcomes for hypoplastic left heart syndrome over an 18-year period. J Thorac Cardiovasc Surg. 2010;139:119–127. doi: 10.1016/j.jtcvs.2009.04.061. [DOI] [PubMed] [Google Scholar]
- 34.Ota N, Muramata M, Tosaka Y, Ide Y, Tachi M, Ito H, Sugimoto A, Sakamoto K. Is routine rapid-staged bilateral pulmonary artery banding before stage 1 Norwood a viable strategy? J Thorac Cardiovasc Surg. 2014;148:1519–1525. doi: 10.1016/j.jtcvs.2013.11.053. [DOI] [PubMed] [Google Scholar]
- 35.Murphy MO, Bellsham-Revell H, Morgan GJ, Krasemann T, Rosenthal E, Qureshi SA, Salih C, Austin CB, Anderson DR. Hybrid Procedure for Neonates With Hypoplastic Left Heart Syndrome at High-Risk for Norwood: Midterm Outcomes. Ann Thorac Surg. 2015;100:2286–2290. doi: 10.1016/j.athoracsur.2015.06.098. [DOI] [PubMed] [Google Scholar]
- 36.Schranz D, Michel-Behnke I. Advances in interventional and hybrid therapy in neonatal congenital heart disease. Semin Fetal Neonatal Med. 2013;8:311–321. doi: 10.1016/j.siny.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 37.Schranz D, Jux C, Akintuerk H. Novel catheter-interventional strategy for intracardiac connecting of total anomalous pulmonary venous return (TAPVR) in newborns with hypoplastic left heart-syndrome (HLHS) prior to hybrid approach. Catheter Cardiovasc Interv. 2013;82:564–568. doi: 10.1002/ccd.24783. [DOI] [PubMed] [Google Scholar]
- 38.Ishizaka T, Ohye RG, Suzuki T, Devaney EJ, Bove EL. Bilateral pulmonary artery banding for resuscitation in hypoplastic left heart syndrome. Ann Thorac Surg. 2003;75:277–279. doi: 10.1016/s0003-4975(02)04302-3. [DOI] [PubMed] [Google Scholar]
- 39.Licht DJ, Shera DM, Clancy RR, Wernovsky G, Montenegro LM, Nicolson SC, Zimmerman RA, Spray TL, Gaynor JW, Vossough A. Brain maturation is delayed in infants with complex congenital heart defects. J Thorac Cardiovasc Surg. 2009;137:536–537. doi: 10.1016/j.jtcvs.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knirsch WL, Liamlahi R, Hug MI, Hoop R, von Rhein M, Prêtre R, Kretschmar O, Latal B. Mortality and neurodevelopmental outcome at 1 year of age comparing hybrid and Norwood procedures. Eur J Cardiothorac Surg. 2012;42:33–39. doi: 10.1093/ejcts/ezr286. [DOI] [PubMed] [Google Scholar]
- 41.Schranz D, Voelkel NF. “Nihilism” of chronic heart failure therapy in children and why effective therapy is withheld. Eur J Pediatr. 2016;175:445–455. doi: 10.1007/s00431-016-2700-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rudd NA, Frommelt MA, Tweddell JS, Hehir DA, Mussatto KA, Frontier KD, Slicker JA, Bartz PJ, Ghanayem NS. Improving interstage survival after Norwood operation: outcomes from 10 years of home monitoring. J Thorac Cardiovasc Surg. 2014;148:1540–1547. doi: 10.1016/j.jtcvs.2014.02.038. [DOI] [PubMed] [Google Scholar]
- 43.Yerebakan C, Valeske K, Elmontaser H, Yörüker U, Mueller M, Thul J, Mann V, Latus H, Villanueva A, Hofmann K, Schranz D, Akintuerk H. Hybrid therapy for hypoplastic left heart syndrome: Myth, alternative, or standard? J Thorac Cardiovasc Surg. 2016;151:1112–1123. doi: 10.1016/j.jtcvs.2015.10.066. [DOI] [PubMed] [Google Scholar]
- 44.Galantowicz M, Yates AR. Improved outcomes with the comprehensive stage 2 procedure after an initial hybrid stage 1. J Thorac and Cardiovasc Surg. 2016;151:424–429. doi: 10.1016/j.jtcvs.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 45.Fontan F, Baudet E. Surgical repair of tricuspid atresia. Thorax. 1971;26:240–248. doi: 10.1136/thx.26.3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Leval MR, Deanfield JE. Four decades of Fontan palliation. Nat Rev Cardiol. 2010;7:520–527. doi: 10.1038/nrcardio.2010.99. [DOI] [PubMed] [Google Scholar]
- 47.d’Udekem Y, Iyengar AJ, Galati JC, Forsdick V, Weintraub RG, Wheaton GR, Bullock A, Justo RN, Grigg LE, Sholler GF, Hope S, Radford DJ, Gentles TL, Celermajer DS, Winlaw DS. Redefining Expectations of Long-Term Survival After the Fontan Procedure: Twenty-Five Years of Follow-Up From the Entire Population of Australia and New Zealand. Circulation. 2014;130:S32–S38. doi: 10.1161/CIRCULATIONAHA.113.007764. [DOI] [PubMed] [Google Scholar]
- 48.Gentles TL, Mayer JE, Gauvreau K, Newburger JW, Lock JE, Kupferschmid JP, Burnett J, Jonas RA, Castaneda AR, Wernovsky G. Fontan operation in five hundred consecutive patients: factors influencing early and late outcome. J Thorac Cardiovasc Surg. 1997;114:376–391. doi: 10.1016/s0022-5223(97)70183-1. [DOI] [PubMed] [Google Scholar]
- 49.Pundi KN, Johnson JN, Dearani JA, Pundi KN, Li Z, Hinck CA, Dahl SH, Cannon BC, O’Leary PW, Driscoll DJ, Cetta F. 40-Year Follow-Up After the Fontan Operation. J Am Coll Cardiol. 2015;66:1700–1710. doi: 10.1016/j.jacc.2015.07.065. [DOI] [PubMed] [Google Scholar]
- 50.Ohuchi H, Kagisaki K, Miyazaki A, Kitano M, Yazaki S, Sakaguchi H, Ichikawa H, Yamada O, Yagihara T. Impact of the evolution of the Fontan operation on early and late mortality: a single-center experience of 405 patients over 3 decades. Ann Thorac Surg. 2011;92:1457–1466. doi: 10.1016/j.athoracsur.2011.05.055. [DOI] [PubMed] [Google Scholar]
- 51.Fontan F, Kirklin JW, Fernandez G, Costa F, Naftel DC, Tritto F, Blackstone EH. Outcome after a “perfect” Fontan operation. Circulation. 1990;81:1520–1536. doi: 10.1161/01.cir.81.5.1520. [DOI] [PubMed] [Google Scholar]
- 52.d’Udekem Y, Iyengar AJ, Cochrane AD, Grigg LE, Ramsay JM, Wheaton GR, Penny DJ, Brizard CP. The Fontan procedure: contemporary techniques have improved long-term outcomes. Circulation. 2007;116:I157–164. doi: 10.1161/CIRCULATIONAHA.106.676445. [DOI] [PubMed] [Google Scholar]
- 53.d’Udekem Y, Xu MY, Galati JC, Lu S, Iyengar AJ, Konstantinov IE, Wheaton GR, Ramsay JM, Grigg LE, Millar J, Cheung MM, Brizard CP. Predictors of Survival After Single-Ventricle Palliation. J Am Coll Cardiol. 2012;59:1178–1185. doi: 10.1016/j.jacc.2011.11.049. [DOI] [PubMed] [Google Scholar]
- 54.Hansen JH, Petko C, Bauer G, Voges I, Kramer H-H, Scheewe J. Fifteen-year single-center experience with the Norwood operation for complex lesions with single-ventricle physiology compared with hypoplastic left heart syndrome. J Thorac Cardiovasc Surg. 2012;144:166–172. doi: 10.1016/j.jtcvs.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 55.Furck AK, Uebing A, Hansen JH, Scheewe J, Jung O, Fischer G, Rickers C, Holland-Letz T, Kramer H-H. Outcome of the Norwood operation in patients with hypoplastic left heart syndrome: a 12-year single-center survey. J Thorac Cardiovasc Surg. 2010;139:359–365. doi: 10.1016/j.jtcvs.2009.07.063. [DOI] [PubMed] [Google Scholar]
- 56.McGuirk SP, Griselli M, Stumper OF, Rumball EM, Miller P, Dhillon R, de Giovanni JV, Wright JG, Barron DJ, Brawn WJ. Staged surgical management of hypoplastic left heart syndrome: a single institution 12 year experience. Heart. 2006;92:364–370. doi: 10.1136/hrt.2005.068684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ono M, Boethig D, Goerler H, Lange M, Westhoff-Bleck M, Breymann T. Clinical outcome of patients 20 years after Fontan operation–effect of fenestration on late morbidity. Eur J Cardiothorac Surg. 2006;30:923–929. doi: 10.1016/j.ejcts.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 58.Elder RW, McCabe NM, Veledar E, Kogon BE, Jokhadar M, Rodriguez FH, McConnell ME, Book WM. Risk factors for major adverse events late after Fontan palliation. Congenit Heart Dis. 2015;10:159–168. doi: 10.1111/chd.12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schilling C, Dalziel K, Nunn R, Plessis Du K, Shi WY, Celermajer D, Winlaw D, Weintraub RG, Grigg LE, Radford DJ, Bullock A, Gentles TL, Wheaton GR, Hornung T, Justo RN, d’Udekem Y. Int J Cardiol. 2016;219:14–19. doi: 10.1016/j.ijcard.2016.05.035. [DOI] [PubMed] [Google Scholar]
- 60.Iyengar AJ, Winlaw DS, Galati JC, Wheaton GR, Gentles TL, Grigg LE, Justo RN, Radford DJ, Weintraub RG, Bullock A, Celermajer DS, d’Udekem Y, The Australia and New Zealand Fontan Registry The extracardiac conduit Fontan procedure in Australia and New Zealand: hypoplastic left heart syndrome predicts worse early and late outcomes. Eur J Cardiothorac Surg. 2014;46:465–473. doi: 10.1093/ejcts/ezu015. [DOI] [PubMed] [Google Scholar]
- 61.Rogers LS, Glatz AC, Ravishankar C, Spray TL, Nicolson SC, Rychik J, Rush CH, Gaynor JW, Goldberg DJ. 18 Years of the Fontan Operation at a Single Institution. J Am Coll Cardiol. 2012;60:1018–1025. doi: 10.1016/j.jacc.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 62.Hirsch JC, Goldberg C, Bove EL, Salehian S, Lee T, Ohye RG, Devaney EJ. Fontan operation in the current era: a 15-year single institution experience. Ann Surg. 2008;248:402–410. doi: 10.1097/SLA.0b013e3181858286. [DOI] [PubMed] [Google Scholar]
- 63.Stewart RD, Pasquali SK, Jacobs JP, Benjamin DK, Jaggers J, Cheng J, Mavroudis C, Jacobs ML. Contemporary Fontan Operation: Association Between Early Outcome and Type of Cavopulmonary Connection. Ann Thorac Surg. 2012;93:1254–1261. doi: 10.1016/j.athoracsur.2012.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Iyengar AJ, Winlaw DS, Galati JC, Celermajer DS, Wheaton GR, Gentles TL, Grigg LE, Weintraub RG, Bullock A, Justo RN, d’Udekem Y. Trends in Fontan surgery and risk factors for early adverse outcomes after Fontan surgery: The Australia and New Zealand Fontan Registry experience. J Thorac Cardiovasc Surg. 2014;148:566–575. doi: 10.1016/j.jtcvs.2013.09.074. [DOI] [PubMed] [Google Scholar]
- 65.de Leval MR, Kilner P, Gewillig M, Bull C. Total cavopulmonary connection: a logical alternative to atriopulmonary connection for complex Fontan operations. Experimental studies and early clinical experience. J Thorac Cardiovasc Surg. 1988;96:682–695. [PubMed] [Google Scholar]
- 66.Marcelletti C, Corno A, Giannico S, Marino B. Inferior vena cava-pulmonary artery extracardiac conduit. A new form of right heart bypass. J Thorac Cardiovasc Surg. 1990;100:228–232. [PubMed] [Google Scholar]
- 67.Khairy P, Fernandes SM, Mayer JE, Triedman JK, Walsh EP, Lock JE, Landzberg MJ. Long-Term Survival, Modes of Death, and Predictors of Mortality in Patients With Fontan Surgery. Circulation. 2008;117:85–92. doi: 10.1161/CIRCULATIONAHA.107.738559. [DOI] [PubMed] [Google Scholar]
- 68.Shi WY, Yong MS, McGiffin DC, Jain P, Ruygrok PN, Marasco SF, Finucane K, Keogh A, d’Udekem Y, Weintraub RG, Konstantinov IE. Heart transplantation in Fontan patients across Australia and New Zealand. Heart. 2016;102:1120–1126. doi: 10.1136/heartjnl-2015-308848. [DOI] [PubMed] [Google Scholar]
- 69.Iyengar AJ, Sharma VJ, Weintraub RG, Shipp A, Brizard CP, d’Udekem Y, Konstantinov IE. Surgical strategies to facilitate heart transplantation in children after failed univentricular palliations: the role of advanced intraoperative surgical preparation. Eur J Cardiothorac Surg. 2014;46:480–485. doi: 10.1093/ejcts/ezu004. [DOI] [PubMed] [Google Scholar]
- 70.Lamour JM, Kanter KR, Naftel DC, Chrisant MR, Morrow WR, Clemson BS, Kirklin JK. The Effect of Age, Diagnosis, and Previous Surgery in Children and Adults Undergoing Heart Transplantation for Congenital Heart Disease. J Am Coll Cardiol. 2009;54:160–165. doi: 10.1016/j.jacc.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 71.Alsoufi B, Deshpande S, McCracken C, Kogon B, Vincent R, Mahle W, KANTER K. Outcomes and risk factors for heart transplantation in children with congenital heart disease. J Thorac Cardiovasc Surg. 2015;150:1455–1462.e3. doi: 10.1016/j.jtcvs.2015.06.029. [DOI] [PubMed] [Google Scholar]
- 72.Alsoufi B, Mahle WT, Manlhiot C, Deshpande S, Kogon B, McCrindle BW, KANTER K. Outcomes of heart transplantation in children with hypoplastic left heart syndrome previously palliated with the Norwood procedure. J Thorac Cardiovasc Surg. 2016;151:167–175.e1–2. doi: 10.1016/j.jtcvs.2015.09.081. [DOI] [PubMed] [Google Scholar]
- 73.Davies RR, Sorabella RA, Yang J, Mosca RS, Chen JM, Quaegebeur JM. Outcomes after transplantation for “failed” Fontan: a single-institution experience. J Thorac Cardiovasc Surg. 2012;143:1183–1192.e4. doi: 10.1016/j.jtcvs.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 74.Gaynor JW, Bridges ND, Cohen MI, Mahle WT, DeCampli WM, Steven JM, Nicolson SC, Spray TL. Predictors of outcome after the Fontan operation: is hypoplastic left heart syndrome still a risk factor? J Thorac Cardiovasc Surg. 2002;123:237–245. doi: 10.1067/mtc.2002.119337. [DOI] [PubMed] [Google Scholar]
- 75.Anderson PAW, Sleeper LA, Mahony L, Colan SD, Atz AM, Breitbart RE, Gersony WM, Gallagher D, Geva T, Margossian R, McCrindle BW, Paridon S, Schwartz M, Stylianou M, Williams RV, Clark BJ, Pediatric Heart Network Investigators Contemporary outcomes after the Fontan procedure: a Pediatric Heart Network multicenter study. J Am Coll Cardiol. 2008;52:85–98. doi: 10.1016/j.jacc.2008.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Trivedi B, Smith PB, Barker PCA, Jaggers J, Lodge AJ, Kanter RJ. Arrhythmias in patients with hypoplastic left heart syndrome. Am Heart J. 2011;161:138–144. doi: 10.1016/j.ahj.2010.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Weipert J, Noebauer C, Schreiber C, Kostolny M, Zrenner B, Wacker A, Hess J, Lange R. Occurrence and management of atrial arrhythmia after long-term Fontan circulation. J Thorac Cardiovasc Surg. 2004;127:457–464. doi: 10.1016/j.jtcvs.2003.08.054. [DOI] [PubMed] [Google Scholar]
- 78.Idorn L, Juul K, Jensen AS, Hanel B, Nielsen KG, Andersen H, Reimers JI, Sørensen KE, Søndergaard L. Arrhythmia and exercise intolerance in Fontan patients: current status and future burden. Int J Cardiol. 2013;168:1458–1465. doi: 10.1016/j.ijcard.2012.12.055. [DOI] [PubMed] [Google Scholar]
- 79.Azakie A, McCrindle BW, Van Arsdell G, Benson LN, Coles J, Hamilton R, Freedom RM, Williams WG. Extracardiac conduit versus lateral tunnel cavopulmonary connections at a single institution: impact on outcomes. J Thorac Cardiovasc Surg. 2001;122:1219–1228. doi: 10.1067/mtc.2001.116947. [DOI] [PubMed] [Google Scholar]
- 80.Balaji S, Daga A, Bradley DJ, Etheridge SP, Law IH, Batra AS, Sanatani S, Singh AK, Gajewski KK, Tsao S, Singh HR, Tisma-Dupanovic S, Tateno S, Takamuro M, Nakajima H, Roos-Hesselink JW, Shah M. An international multicenter study comparing arrhythmia prevalence between the intracardiac lateral tunnel and the extracardiac conduit type of Fontan operations. J Thorac Cardiovasc Surg. 2014;148:576–581. doi: 10.1016/j.jtcvs.2013.08.070. [DOI] [PubMed] [Google Scholar]
- 81.Alsaied T, Alsidawi S, Allen CC, Faircloth J, Palumbo JS, Veldtman GR. Strategies for thromboprophylaxis in Fontan circulation: a meta-analysis. Heart. 2015;101:1731–1737. doi: 10.1136/heartjnl-2015-307930. [DOI] [PubMed] [Google Scholar]
- 82.Iyengar AJ, Winlaw DS, Galati JC, Wheaton GR, Gentles TL, Grigg LE, Justo RN, Radford DJ, Attard C, Weintraub RG, Bullock A, Sholler GS, Celermajer DS, d’Udekem Y. No difference between aspirin and warfarin after extracardiac Fontan in a propensity score analysis of 475 patients. Eur J Cardiothorac Surg. 2016 doi: 10.1093/ejcts/ezw159. pii:ezw159 (epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 83.Monagle P, Cochrane A, Roberts R, Manlhiot C, Weintraub R, Szechtman B, Hughes M, Andrew M, McCrindle BW, Fontan Anticoagulation Study Group A multicenter, randomized trial comparing heparin/warfarin and acetylsalicylic acid as primary thromboprophylaxis for 2 years after the Fontan procedure in children. J Am Coll Cardiol. 2011;58:645–651. doi: 10.1016/j.jacc.2011.01.061. [DOI] [PubMed] [Google Scholar]
- 84.McCrindle BW, Manlhiot C, Cochrane A, Roberts R, Hughes M, Szechtman B, Weintraub R, Andrew M, Monagle P, Fontan Anticoagulation Study Group Factors associated with thrombotic complications after the Fontan procedure: a secondary analysis of a multicenter, randomized trial of primary thromboprophylaxis for 2 years after the Fontan procedure. J Am Coll Cardiol. 2013;61:346–353. doi: 10.1016/j.jacc.2012.08.1023. [DOI] [PubMed] [Google Scholar]
- 85.Coon PD, Rychik J, Novello RT, Ro PS, Gaynor JW, Spray TL. Thrombus formation after the Fontan operation. Ann Thorac Surg. 2001;71:1990–1994. doi: 10.1016/s0003-4975(01)02472-9. [DOI] [PubMed] [Google Scholar]
- 86.Kaulitz R, Ziemer G, Rauch R, Girisch M, Bertram H, Wessel A, Hofbeck M. Prophylaxis of thromboembolic complications after the Fontan operation (total cavopulmonary anastomosis) J Thorac Cardiovasc Surg. 2005;129:569–575. doi: 10.1016/j.jtcvs.2004.08.045. [DOI] [PubMed] [Google Scholar]
- 87.Thacker D, Patel A, Dodds K, Goldberg DJ, Semeao E, Rychik J. Use of oral budesonide in the management of protein-losing enteropathy after the Fontan operation. Ann Thorac Surg. 2010;89:837–842. doi: 10.1016/j.athoracsur.2009.09.063. [DOI] [PubMed] [Google Scholar]
- 88.Mertens L, Hagler DJ, Sauer U, Somerville J, Gewillig M. Protein-losing enteropathy after the Fontan operation: an international multicenter study. PLE study group. J Thorac Cardiovasc Surg. 1998;115:1063–1073. doi: 10.1016/s0022-5223(98)70406-4. [DOI] [PubMed] [Google Scholar]
- 89.Backer CL, Russell HM, Pahl E, Mongé MC, Gambetta K, Kindel SJ, Gossett JG, Hardy C, Costello JM, Deal BJ. Heart transplantation for the failing Fontan. Ann Thorac Surg. 2013;96:1413–1419. doi: 10.1016/j.athoracsur.2013.05.087. [DOI] [PubMed] [Google Scholar]
- 90.Gossett JG, Almond CS, Kirk R, Zangwill S, Richmond ME, Kantor PF, Tresler MA, Lenderman SM, Naftel DC, Matthews KL, Pahl E. Outcomes of cardiac transplantation in single-ventricle patients with plastic bronchitis: a multicenter study. JACC. 2013;61:985–986. doi: 10.1016/j.jacc.2012.10.042. [DOI] [PubMed] [Google Scholar]
- 91.Rychik J. The Relentless Effects of the Fontan Paradox. Seminars in Thoracic and Cardiovascular Surgery: Pediatric Cardiac Surgery Annual. 2016;19:37–43. doi: 10.1053/j.pcsu.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 92.Jitta DJ, Wagenaar LJ, Mulder BJM, Guichelaar M, Bouman D, van Melle JP. Three cases of hepatocellular carcinoma in Fontan patients: Review of the literature and suggestions for hepatic screening. Int J Cardiol. 2016;206:21–26. doi: 10.1016/j.ijcard.2015.12.033. [DOI] [PubMed] [Google Scholar]
- 93.Anne P, Du W, Mattoo TK, Zilberman MV. Nephropathy in patients after Fontan palliation. Int J Cardiol. 2009;132:244–247. doi: 10.1016/j.ijcard.2007.11.079. [DOI] [PubMed] [Google Scholar]
- 94.Paridon SM, Mitchell PD, Colan SD, Williams RV, Blaufox A, Li JS, Margossian R, Mital S, Russell J, Rhodes J, for the Pediatric Heart Network Investigators A cross-sectional study of exercise performance during the first 2 decades of life after the Fontan operation. J Am Coll Cardiol. 2008;52:99–107. doi: 10.1016/j.jacc.2008.02.081. [DOI] [PubMed] [Google Scholar]
- 95.d’Udekem Y, Cheung MMH, Setyapranata S, Iyengar AJ, Kelly P, Buckland N, Grigg LE, Weintraub RG, Vance A, Brizard CP, Penny DJ. How Good Is a Good Fontan? Quality of Life and Exercise Capacity of Fontans Without Arrhythmias. Ann Thorac Surg. 2009;88:1961–1969. doi: 10.1016/j.athoracsur.2009.07.079. [DOI] [PubMed] [Google Scholar]
- 96.Van Hare GF, Ackerman MJ, Evangelista J-AK, Kovacs RJ, Myerburg RJ, Shafer KM, Warnes CA, Washington RL, American Heart Association Electrocardiography and Arrhythmias Committee of the Council on Clinical Cardiology, Council on Cardiovascular Disease in the Young, Council on Cardiovascular and Stroke Nursing, Council on Functional Genomics and Translational Biology, and the American College of Cardiology Eligibility and Disqualification Recommendations for Competitive Athletes With Cardiovascular Abnormalities: Task Force 4: Congenital Heart Disease: A Scientific Statement From the American Heart Association and American College of Cardiology. Circulation. 2015;132:e281–91. doi: 10.1161/CIR.0000000000000240. [DOI] [PubMed] [Google Scholar]
- 97.Cordina RL, O’Meagher S, Karmali A, Rae CL, Liess C, Kemp GJ, Puranik R, Singh N, Celermajer DS. Resistance training improves cardiac output, exercise capacity and tolerance to positive airway pressure in Fontan physiology. Int J Cardiol. 2013;168:780–788. doi: 10.1016/j.ijcard.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 98.Zentner D, Kotevski A, King I, Grigg L, d’Udekem Y. Fertility and pregnancy in the Fontan population. Int J Cardiol. 2016;208:97–101. doi: 10.1016/j.ijcard.2016.01.180. [DOI] [PubMed] [Google Scholar]
- 99.Gouton M, Nizard J, Patel M, Sassolas F, Jimenez M, Radojevic J, Mathiron A, Amedro P, Barre E, Labombarda F, Vaksmann G, Chantepie A, Le Gloan L, Ladouceur M. Maternal and fetal outcomes of pregnancy with Fontan circulation: A multicentric observational study. Int J Cardiol. 2015;187:84–89. doi: 10.1016/j.ijcard.2015.03.344. [DOI] [PubMed] [Google Scholar]
- 100.Uzark K, Zak V, Shrader P, McCrindle BW, Radojewski E, Varni JW, Daniels K, Handisides J, Hill KD, Lambert LM, Margossian R, Pemberton VL, Lai WW, Atz AM, for the Pediatric Heart Network Investigators Assessment of Quality of Life in Young Patients with Single Ventricle after the Fontan Operation. J Pediatr. 2016;170:166–172.e1. doi: 10.1016/j.jpeds.2015.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Saliba Z, Butera G, Bonnet D, Bonhoeffer P, Villain E, Kachaner J, Sidi D, Iserin L. Quality of life and perceived health status in surviving adults with univentricular heart. Heart. 2001;86:69–73. doi: 10.1136/heart.86.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.McCrindle BW, Williams RV, Mitchell PD, Hsu DT, Paridon SM, Atz AM, Li JS, Newburger JW, for the Pediatric Heart Network Investigators Relationship of patient and medical characteristics to health status in children and adolescents after the Fontan procedure. Circulation. 2006;113:1123–1129. doi: 10.1161/CIRCULATIONAHA.105.576660. [DOI] [PubMed] [Google Scholar]
- 103.Gaynor JW, Ittenbach RF, Gerdes M, Bernbaum J, Clancy RR, McDonald-McGinn DM, Zackai EH, Wernovsky G, Nicolson SC, Spray TL. Neurodevelopmental outcomes in preschool survivors of the Fontan procedure. J Thorac Cardiovasc Surg. 2014;147:1276–1282. doi: 10.1016/j.jtcvs.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Anderson PAW, Breitbart RE, McCrindle BW, Sleeper LA, Atz AM, Hsu DT, Lu M, Margossian R, Williams RV. The Fontan Patient: Inconsistencies in Medication Therapy Across Seven Pediatric Heart Network Centers. Pediatr Cardiol. 2010;31:1219–1228. doi: 10.1007/s00246-010-9807-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wilson TG, Iyengar AJ, Winlaw DS, Weintraub RG, Wheaton GR, Gentles TL, Ayer J, Grigg LE, Justo RN, Radford DJ, Bullock A, Celermajer DS, Dalziel K, Schilling C, d’Udekem Y, Registry TAANZF Int J Cardiol. 2016;210:95–99. doi: 10.1016/j.ijcard.2016.02.089. [DOI] [PubMed] [Google Scholar]
- 106.Hsu DT, Zak V, Mahony L, Sleeper LA, Atz AM, Levine JC, Barker PC, Ravishankar C, McCrindle BW, Williams RV, Altmann K, Ghanayem NS, Margossian R, Chung WK, Border WL, Pearson GD, Stylianou MP, Mital S, Pediatric Heart Network Investigators Enalapril in infants with single ventricle: results of a multicenter randomized trial. Circulation. 2010;122:333–340. doi: 10.1161/CIRCULATIONAHA.109.927988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hebert A, Mikkelsen UR, Thilen U, Idorn L, Jensen AS, Nagy E, Hanséus K, Sørensen KE, Søndergaard L. Bosentan Improves Exercise Capacity in Adolescents and Adults After Fontan Operation: The TEMPO (Treatment With Endothelin Receptor Antagonist in Fontan Patients, a Randomized, Placebo-Controlled, Double-Blind Study Measuring Peak Oxygen Consumption) Study. Circulation. 2014;130:2021–2030. doi: 10.1161/CIRCULATIONAHA.113.008441. [DOI] [PubMed] [Google Scholar]
- 108.Hornik CP, He X, Jacobs JP, Li JS, Jaquiss RDB, Jacobs ML, O’Brien SM, Welke K, Peterson ED, Pasquali SK. Relative Impact of Surgeon and Center Volume on Early Mortality After the Norwood Operation. Ann Thorac Surg. 2012;93(6):1992–1997. doi: 10.1016/j.athoracsur.2012.01.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hirsch JC, Gurney JG, Donohue JE, Gebremarian A, Bove EL, Ohye RG. Hospital Mortality for Norwood and Arterial Switch Operations as a Function of Institutional Volume. Ped Cardiol. 2008;29:713–717. doi: 10.1007/s00246-007-9171-2. [DOI] [PubMed] [Google Scholar]
- 110.O’Connor GT, Plume SK, Olmstead EM, Coffin LH, Morton JR, Maloney CT, Nowicki ER, Tryzelaar JF, Hernandez F, Adrian L, Casey KJ, Soule DN, Marrin CAS, Nugent WC, Charlesworth DC, Clough R, Katz S, Leavitt BJ, Wennberg JE. A regional prospective study of in-hospital mortality associated with coronary artery bypass grafting. JAMA. 1991;266:803–809. [PubMed] [Google Scholar]
- 111.Share DA, Campbell DA, Birkmeyer N, Prager RL, Gurm HS, Moscucci M, Udow-Phillips M, Birkmeyer JD. How a regional collaborative of hospitals and physicians in Michigan cut costs and improved the quality of care. Health Aff (Millwood) 2011;30:636–645. doi: 10.1377/hlthaff.2010.0526. [DOI] [PubMed] [Google Scholar]
- 112.Mahle WT, Nicolson S, Shekerdemian LS, Gaies MG, Witte MK, Lee EK, Goldsworthy M, Stark P, Burns KM, Scheurer MA, Cooper DS, Thiagarajan R, Sivarajan B, Hollenbeck-Pringle D, Colan S. American Heart Association Scientific Sessions 2015. Orlando, Florida: Nov 10, 2015. Utilizing a Collaborative Learning Model to Decrease Length of Postoperative Ventilation Following Infant Heart Surgery. [Google Scholar]
- 113.Ishigami S, Ohtsuki S, Tarui S, Ousaka D, Eitoku T, Kondo M, Okuyama M, Kobayashi J, Baba K, Arai S, Kawabata T, Yoshizumi K, Tateishi A, Kuroko Y, Iwasaki T, Sato S, Kasahara S, Sano S, Oh H. Intracoronary Autologous Cardiac Progenitor Cell Transfer in Patients With Hypoplastic Left Heart Syndrome. The TICAP Prospective Phase 1 Controlled Trial. Circ Res. 2015;116:653–664. doi: 10.1161/CIRCRESAHA.116.304671. [DOI] [PubMed] [Google Scholar]
- 114.Chery J, Wong J, Huang S, Wang S, Si MS. Regenerative Medicine Strategies for Hypoplastic Left Heart Syndrome. Tissue Eng Part B Rev. 2016 doi: 10.1089/ten.TEB.2016.0136. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]


