Summary
With nearly 30% of the world population infected, hepatitis B virus (HBV) causes significant morbidity and mortality worldwide. Although several potent antiviral agents are currently in use against HBV infection, the majority of chronically infected individuals do not achieve a functional and complete cure, as measured by the clearance of HB surface antigen (HBsAg) from blood and eradication of the covalently closed-circular DNA (cccDNA) from the nuclei of hepatocytes. In addition, even treated persons who achieve a long-term (> 10-15 years) sustained virological response (undetectable HBV DNA), are still at high risk of developing morbidity and mortality from liver complications. This review focuses upon novel, mechanistically diverse anti-HBV therapeutic strategies that are currently in development or in clinical evaluation, and highlights new combination strategies which may contribute to full elimination of HBV DNA and cccDNA from the infected liver, leading to a complete cure of chronic hepatitis B.
Keywords: HBV Cure, siRNA, CRISPR/Cas9, Anti-HBV agents, cccDNA, HBsAg, Antiviral therapy
Introduction
Over the past decades research efforts have led to the development of several potent nucleosides analog inhibitors (NA) such as lamivudine (Epivir), adefovir dipivoxil (Hepsera), entecavir (Baraclude), telbivudine (Tyzeka), and tenofovir disoproxil fumarate (Viread) allowing a large decrease of HBV viremia in chronically infected persons.1 NAs in their 5’-triphosphate form are potent inhibitors of DNA polymerase/reverse transcriptase activities of the viral polymerase enzyme. They compete with natural nucleotides and act on several steps of viral DNA synthesis including initial polymerization, protein priming or the subsequent DNA strand elongation. It has been suggested that combination therapy using one of these nucleoside analogs and interferon could have better virus elimination efficacy than NA monotherapy, but such studies are difficult to perform since the current monotherapy is already very effective at controlling HBV viral load. However, current NA treatment does not lead to HBV cure as indicated by low levels of HBsAg seroconversion (Box 1).2,3
Box 1. Definitions of cures.
Apparent virological cure
Sustained off-drug suppression of serum HBsAg, HBeAg and viral DNA.
cccDNA = undetectable or repressed
Normalization of liver function (Normal levels of serum ALT and AST).
*Risk of death from liver disease: to be determined once long-term survival data have been obtained.
Functional cure
Sustained off-drug suppression of serum HBsAg, HBeAg, viral DNA and cccDNA.
Normalization of liver function (Normal levels of serum ALT and AST).
*Comparable to individuals with naturally resolved infection.
Absolute cure – virological cure
Sustained off-drug suppression of serum HBsAg, HBeAg and viral DNA.
Normalization of liver function (Normal levels of serum ALT and AST).
Elimination of cccDNA.
Presence of HBsAb.
* Comparable to uninfected individuals.
Despite the success of current available therapy, subjects who cleared the virus (HBeAg negative, HBsAg negative) can experience reactivation of HBV on treatment interruption or following the use of anti-inflammatory or immunosuppressant medications.4 This strongly suggests that current anti-HBV therapeutics are unable to eradicate the virus from infected liver cells. These limitations have led researchers to continue their drug development efforts toward finding new viral targets that could potentially lead to the discovery of a functional or absolute cure (Box 1).5
Considering that HBV covalently closed circular DNA (cccDNA) serves as the template for pregenomic RNA (pgRNA) transcription, it is thought to be responsible for virus persistence. At the same time, integration of HBV DNA is thought to be associated with increase risk of hepatocellular carcinoma (HCC) development.6 Accordingly, new therapeutic approaches that target cccDNA directly or indirectly are in development (Fig. 1). Following the recent successes with HCV drug development, the field of viral hepatitis is turning its focus to another major threat to liver health, namely HBV.7 These new therapeutic strategies will have to address the problems of cccDNA elimination, intrahepatic innate immune response stimulation, HBV specific immune response restoration and will probably have to include combination of drugs to target multiple steps of the HBV replication cycle.
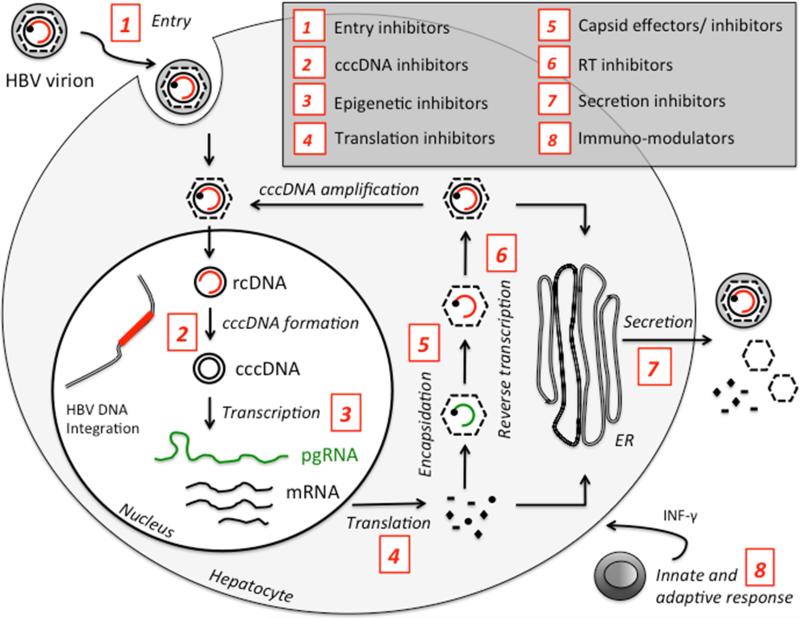
Fig. 1.
Schematic representation of the inhibitors of Hepatitis B virus replication cycle. cccDNA, covalently closed circular DNA; ER, endoplasmic reticulum; HBV, hepatitis B virus; INF, interferon; mRNA, messenger RNA; pgRNA, pregenomic RNA; rcDNA, relaxed circular DNA; RT, reverse transcriptase.
New antivirals currently in the pipelines include entry inhibitors, relaxed circular (rc)DNA-cccDNA conversion inhibitors and capsid assembly effectors (Fig. 1). Besides these direct acting agents, there has also been a significant development in the area of host-targeting agents (HTA). Examples include small interfering RNA-based strategies (SiRNA), RNAi silencers, CRISPR/Cas9 approaches, HBsAg inhibitors, immunomodulators, therapeutic vaccines and toll like receptor (TLR) agonists.8,9 Using these new investigational approaches, it is hoped that a functional cure for chronic HBV is achieved within the next decade. This review highlights recent progress in developing novel anti-HBV drugs and their mechanisms of action.
Current treatments and limitations for a cure
Nucleoside analogs are relatively potent inhibitors used for the treatment of chronic hepatitis B. These HBV reverse transcriptase inhibitors are usually well tolerated and have excellent bioavailability. They are also cost-effective in comparison to interferon treatments such as peginterferon alfa-2a (Pegasys) & interferon alfa-2b (Intron A). Nevertheless, these drugs have some limitations in terms of HBsAg clearance and cccDNA suppression. Although drug resistance can occur clinically with some of the earlier oral treatment such as Epivir, drug resistant viruses are rarely selected with the more recent drugs such as Baraclude and Viread. It has been thought that combination of NAs could have an additive and synergistic antiviral effect and could reduce the rate of drug resistance. However, combination studies involving two nucleoside analogs did not increase virologic response, since the drugs are already very potent on their own.10 As a result, because pegylated- interferon (PEG-IFN) has a different mechanism of action than NA, their combination (TDF + PEG-IFN for 48 wk) showed a greater viral suppression and higher rates of HBsAg loss.11,12
As new therapeutic strategies are being developed for the treatment of chronic hepatitis B (CHB), uncovering new inhibitory mechanisms and potential targets, it is very likely that NA will have their place in future combinations with future drug candidates.
Viral entry inhibitors
The HBV viral replication cycle consists of a complex multistep mechanism (Fig. 1), starting with the virus entering the hepatocyte, followed by DNA replication, nucleocapsid formation and release of virions. HBV entry represents an essential step for spreading and maintaining virus replication. The process involves two major interactions between the viral envelope protein Pre-S1 and hepatocyte cellular receptors including first, HBV binding to the glycoproteins heparin sulfate proteoglycans followed by its interaction with the sodium taurocholate co-transporting polypeptide (NTCP). Recently, Hepatera developed a synthetic lipopeptide, called Myrcludex-B, which is derived from the HBV L-protein.13 Studies have shown that the peptide competes with the viral Pre-S1 motif for NTCP binding, blocking de novo HBV infection. Because of its early effect on the HBV replication cycle, according to the authors, the drug may also efficiently block the amplification of the HBV cccDNA. With this new concept, this inhibitor, which is currently in phase II clinical trials, could have a role in the development of an HBV cure regimen (Table 1).14 Although, there are several other HBV entry inhibitors that can block the in vitro interaction of HBV with NTCP such as Cyclosporin A, Ritonavir, Ezetimibe, Vanitaracin A, Irbesartan, among others; these inhibitors alone can not lead to a complete inhibition of cccDNA synthesis as observed with Myrcludex-B. However, they might still play an important role by preventing viral entry into cccDNA free hepatocytes when combined with other antiviral therapies.15
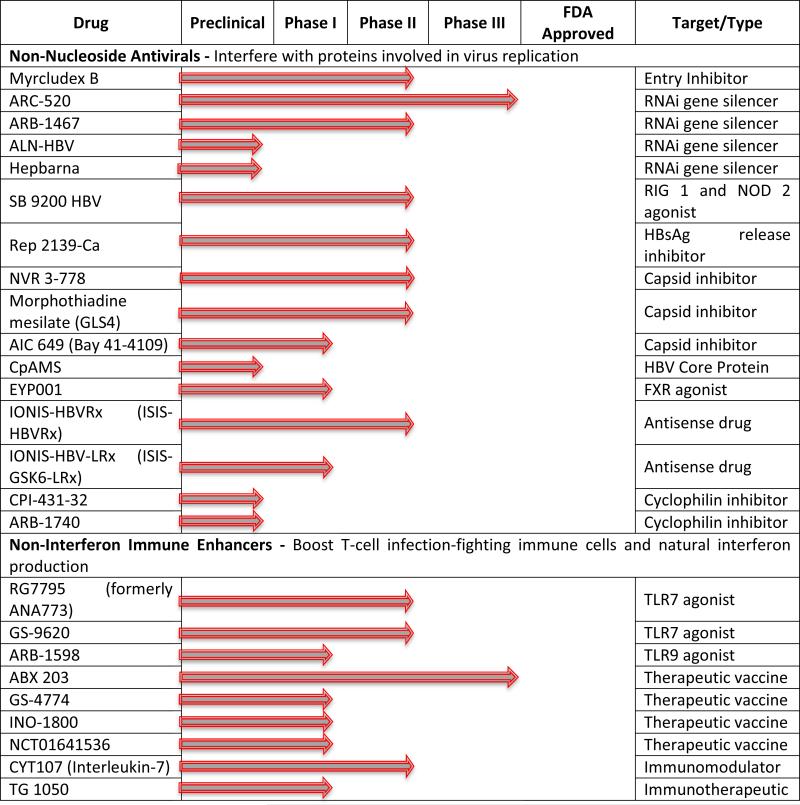
Table 1.
Therapeutics in development for the treatment of hepatitis B chronic Infection. Updated information can be found on the Hepatitis B Foundation website (http://www.hepb.org/professionals/hbf_drug_watch.htm).
Therapies targeting cccDNA
cccDNA formation inhibitor
HBV has evolved a unique replication cycle that results in the production of large viral loads during active replication without actually killing the infected cell directly. Two of the key events in the viral replication cycle of HBV involve first, the generation of cccDNA transcriptional template, either from input genomic DNA or newly replicated capsid-associated DNA and second, reverse transcription of the viral pgRNA to form progeny HBV DNA genomes.16,17 The HBV cccDNA is associated with viral persistence in HBV-infected hepatocytes.18,19 Hepatocytes have a long half-life (> 6 months or even years); therefore, elimination of cccDNA by hepatocyte turnover is not a major means of clearance. The major limitation of current treatment is the failure to eliminate the preexisting cccDNA pool and/or prevent cccDNA formation from trace-levels of wild-type or drug-resistant virus.20 As a consequence, HBV commonly rebounds after cessation of treatment with NA, leading different groups to develop assays to screen libraries of compounds in order to discover new antiviral candidates that can inhibit cccDNA formation.20 Doing so, disubstituted sulfonamides (DSS), such as CCC-0975 and CCC-0346 have been identified as inhibitors of cccDNA production.21 These molecules are believed to interfere with rcDNA conversion to cccDNA in HepDES19 cells, also inhibiting de novo cccDNA formation. Further development of these DSS in combination with other antivirals such as NA might lead to the elimination of HBV cccDNA.
cccDNA targeted endonuclease
New promising systems that specifically use sequence-specific endonucleases to cleave cccDNA and eradicate it from infected hepatocytes have been developed, including the programmable RNA-guided DNA endonucleases (CRISPR/Cas9), transcription activator-like effector nuclease (TALEN) or zinc-finger nuclease. Promising studies in cell and mouse models with CRIPSR/Cas9 have shown that these systems have the potential to serve as effective tools for the depletion of the cccDNA pool in chronically HBV infected subjects.22,23,24
CRIPSR/Cas9 specifically reduced total viral DNA levels by up to ~1,000-fold and HBV cccDNA levels by up to ~10-fold, in addition, it also mutationally inactivated the majority of the residual viral DNA in the stably transfected HepAD38 system. Moreover, these Spy Cas9/sgRNA systems showed additive inhibition of HBV DNA accumulation when used in combination with known pharmacological inhibitors of the HBV RT enzyme in the Hep2.2.15 cells, and in the infected HepaRG cells, reduced both viral production and up to 67% cccDNA formation.23 In a HBV hydrodynamics-mouse model, CRISPR/Cas9 system was capable of disrupting the intrahepatic HBV genome (~28%), with significant reduction but not complete elimination of HBsAg.24
siRNA approach
Persistence of chronic HBV infection is markedly demonstrated by an absence of antiviral immune response against the virus. As a result, a continuous production of surface antigen (HBsAg) in the plasma of chronically infected individuals is observed.25 Three forms of HBsAg are secreted from infected hepatocytes, comprising of filaments and spherical particles, with or without virion. The empty, non-infectious particles are the most abundant in the plasma, and may play a role in preventing the immune system from building a specific immune response against HBV. One way to stop secretion of HBsAg from infected hepatocytes is to cease transcription of messenger RNA (mRNA) by using small interfering RNA (siRNA). These short sequences of nucleotides (siRNA) knock- down expression of genes of interest by promoting gene silencing at the posttranscriptional level. Several siRNA-based regimens are currently being developed and evaluated. The promising ARC-520 from Arrowhead Pharmaceuticals is in a phase II/III clinical studies (Table 1). This new molecule is comprised of two distinct siRNA sequences, which was designed to reduce all transcripts of HBV cccDNA and with wide genotype coverage of the HBV genome. To enhance delivery to hepatocytes, ARC-520 was conjugated with cholesterol and then coinjected with a hepatocyte-targeted membrane-active peptide. In chimpanzees, ARC-520 treatment resulted in a remarkable 95% decline in HBV DNA levels and, as high as 90% inhibition of secreted HBeAg and HBsAg.26,27 Similar results were demonstrated in HBeAg-positive patients, however, insignificant suppression of HBsAg was observed in HBeAg-negative chimpanzees or patients, supporting the hypothesis that HBsAg in this case was produced from integrated DNA which is not targeted by ARC-520.
TKM-HBV/ARB-001467 developed by Arbutus Biopharma is another siRNA regimen in a phase II clinical trial (Table 1), which is currently being evaluated for its safety and tolerability in HBeAg-negative or –positive subjects receiving nucleoside analog therapy. This molecule targets three conserved regions within the HBV genome and appears to clear HBsAg expression from both cccDNA and integrated HBV. Lipid nanoparticles are utilized to transport it to the hepatocytes giving it more stability against nucleases.
siRNA-based approaches for HBV are especially beneficial for the fact that the HBV viral RNA transcripts have their sequences overlapped. This facilitates the synthesis of one single siRNA trigger that could degrade all viral transcripts simultaneously and prevent viral proteins secretion. However, there are three main drawbacks with regard to siRNA approach for HBV therapeutics: (i) Specific delivery to hepatocytes in vivo: because of their small size and highly negatively charged hydrophilic phosphate backbone, siRNA are rapidly filtrated by the kidney and are cleared from the blood stream before achieving their target. (ii) siRNA that reach the cell membrane of hepatocytes can be easily trapped in the endosome and undergo degradation by nucleolytic enzymes. (iii) Undesirable off-target effects of siRNA and innate system stimulation are also a concern. Despite the aforementioned obstacles, novel chemical modifications seem to minimize the chance of cross-reactivity with human mRNAs to occur. These approaches can also enhance efficient delivery of siRNA to the cytoplasm where they can react with RNA-induced silencing complex (RISC) and prompt specific degradation of the HBV mRNAs.27
More recently, Benitec Biopharma developed BB-HB-331 based on a similar approach pertaining to DNA-directed RNA interfering strategy (ddRNAi).28 BB-HB-331 is a recombinant DNA construct, capable of continuously expressing short hairpin RNA (shRNA), which in turn can permanently silence the targeted viral messenger RNA expression with a single treatment. They revealed the results of an in vivo study conducted in humanized mouse PhoenixBio (PXB), showing a 98.5% elimination of circulating HBV (reduced serum HBV DNA by 1.83 logs), a 94.5% reduction of intracellular liver HBV DNA, and almost complete suppression of serum antigens HBeAg and HBsAg (92.6% and 97.6%), and reduction of HBV viral RNA and cccDNA levels.
Capsid assembly and core protein effectors
The HBV nucleocapsid is well recognized to have an important role in the viral replication cycle. It is believed to play an essential role in HBV genome packaging, reverse transcription, intracellular trafficking and maintenance of chronic infection.29 Several small molecules including heteroarylpyrimidines (HAP) have been shown to target the capsid protein (Cp) homo-dimers that rearrange to form the nucleocapsid. They have been identified to disrupt the capsid assembly, thus leading to inhibition of HBV replication both in vitro and in vivo.30,31 BAY 41-4109 (AiCuris) was the first HAP to be developed and reached phase I, but because of toxicity, solubility and other issues, it seems to have been abandoned.8 Despite hepatotoxicity in rats at high dosage,32 it was shown to inhibit the virus replication in HBV transgenic mouse,33 and more importantly effectiveness against lamivudine (Epivir) and adefovir dipivoxil (Hepsera) resistant viruses.34,33 Based on these results, HEC Pharm developed more recently another heteroarylpyrimidine named morphothiadine mesilate GLS4 which entered a phase II clinical trial in China.35 Early studies have demonstrated that this new HAP was more potent and significantly less toxic than analog BAY41-4109.36 GLS4 was found to misdirect capsid assembly leading to the formation of aberrant capsids without primarily affecting core protein levels.37 As these molecules may also have an impact on cccDNA stability, it is suggested that they may contribute to discovery of an HBV cure.38
Another class of small molecules known as sulfamoylbenzamides has been identified to interfere with the capsid, and potently inhibit the formation of pgRNA-containing capsids.39 NVR 3-778 is a sulfamoylbenzamide compound having a pangenotypic antiviral activity, developed by Novira (later acquired by Johnson & Johnson) that recently reached human phase IIa producing significant virus loads reduction (1.7 log reduction of serum HBV DNA and 0.86 log for HBV RNA, at 600 mg BID for 41 days). NVR 3-778 has shown encouraging pharmacokinetic properties, and was well tolerated in human volunteers.40 It has also been shown to inhibit the production of HBV DNA and RNA particles, especially in combination with PEG-IFN. As their mechanism of action is still not completely clear, this new class of small molecules represent a promising cohort of molecules with curative potential when combined with other small molecule inhibitors.
Toll-like receptor (TLR-7)
Toll-like receptors (TLR) agonists have antiviral effects. TLR-7 agonist activates the innate immunity by stimulating plasmacytoid dendritic cells to produce IFN-alpha and other cytokines/chemokines and induce the activation of killer cells as well as cytotoxic lymphocytes. Therefore, this new approach with agonist-induced activation of TLR7 can trigger both innate and adaptive immune responses and may represent a new strategy to treat chronic viral infections. GS-9620 (Gilead) is a small molecule, which has agonist activity. It binds to TLR7 leading to subsequent activation of several transcription factors, including nuclear factor κB (NF-κB) and interferon regulatory factors. GS-9620 has recently entered phase II clinical trials in combination with Tenofovir versus Tenofovir monotherapy.41,42
Other therapeutics with potential
Caspase activators, RIG 1 activators, cyclophilin inhibitors, RNase H inhibitors and therapeutic vaccines are also being evaluated (Table 1.). Some of these strategies such as therapeutic vaccines seem very promising, but are still in development and will have to overcome any possible toxicity and problems related to immune-enhancing approaches variable in treated subjects.9 An impressive reduction of HBsAg has been demonstrated with the novel nucleic acid polymer Rep 2139-Ca (Replicor) alone or in combination with pegylated interferon alpha 2a in subjects chronically infected with HBV or co-infected with hepatitis delta virus (HDV). 43 This compound is in a phase II clinical trial and has the ability to block the formation of surface antigen protein by inhibiting the interaction of apolipoproteins with these subviral particles.44 Recently, a new in vitro approach was developed to facilitate the direct interaction of small molecules with the human HBV polymerase. With a large-scale production of this enzyme coupled with its structural and biophysical characterizations,45 Voros et al., validated their new system using a small molecule – metal-dependent and -binding modulator of HBV polymerase, calcomine orange 2R – which inhibits not only the duck HBV polymerase, but also human HBV polymerase. It remains to be determined whether this drug would interact synergistically with NAs that also target the viral polymerase.
Another approach targeting microRNA could also have a role toward an HBV cure. MicroRNA-122 (miR-122) is a non-coding RNA involved in liver development and hepatic function, which has also been found to play a role in the regulation of HBV replication. It has been shown that miR-122 plays a role in viral persistence, as a decrease of miR-122 is correlated with enhancement of HBV replication through a cyclin G1-P53 dependent pathway. Based on these observations, Li et al. found that all four HBV mRNAs were harboring an miR-122 complementary site, revealing a novel mechanism by which viral mRNAs mediate host miRNA activity, contributing to the regulation of liver cancer cell proliferation, invasion and tumor growth.46 Moreover, recent studies have shown that transfection of miR-122 expression vector into HepG2.2.15 cells repressed the transcription and expression of the protein N-myc downstream-regulated gene 3 (NDRG3), contributing to HBV-related hepatocarcinogenesis.47 Thus, given the broad interactions of miR-122 in HBV chronic infection and HBV-related hepatocarcinomas, this microRNA represents a target for the development of new anti-HBV therapies.
Conclusion
Compared to the current available therapies that decrease and suppress the HBV viral DNA levels to undetectable levels, the new investigational drugs and approaches described herein have the potential to decrease or eliminate cccDNA and/or HBsAg. It is believed that combinations of antiviral agents targeting HBV replication and drugs restoring or increasing the host immune response could lead to a functional and perhaps an absolute cure within a decade.9 Following the recent success of HCV therapy, the viral hepatitis community has turned its focus on the discovery of novel HBV-associated biomarkers and therapeutic targets. It is hoped that the recent surge in anti-HBV drug discovery efforts will lead to the development of novel therapeutic strategies that could represent a path to cure for the more than 300 million individuals who are suffering from chronic hepatitis B infection worldwide.
Key Points.
Despite current treatments available for HBV infection, the inability of the host immune system to completely clear the virus can lead to the establishment of incurable chronic infection.
Identification of new targets and development of novel, curative drugs are necessary.
Development of new therapeutic strategies that address the problems of cccDNA elimination, intrahepatic innate immune response stimulation, and HBV-specific immune response restoration are critical components of a path toward a possible cure.
Combination of antiviral agents targeting HBV replication, and drugs restoring or increasing the host immune response could lead to a functional cure.
Novel modalities such as CRISPR/Cas9 could disrupt HBV cccDNA and also target integrated viral DNA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have nothing to disclose.
References
- 1.Kang L, Pan J, Wu J, Hu J, Sun Q, Tang J. Anti-HBV drugs: progress, unmet needs, and new hope. Viruses. 2015;7(9):4960–77. doi: 10.3390/v7092854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wei W, Wu Q, Zhou J, Kong Y, You H. A better antiviral efficacy found in nucleos(t)ide analog (NA) combinations with interferon therapy than NA monotherapy for HBeAg positive chronic hepatitis B: a meta-analysis. International journal of environmental research and public health. 2015;12(8):10039–55. doi: 10.3390/ijerph120810039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang J, Guo F, Zhao X, Guo JT. Therapeutic strategies for a functional cure of chronic hepatitis B virus infection. Acta pharmaceutica Sinica B. 2014;4(4):248–57. doi: 10.1016/j.apsb.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang YJ, Yang L, Zuo JP. Recent developments in antivirals against hepatitis B virus. Virus Res. 2016;213:205–13. doi: 10.1016/j.virusres.2015.12.014. [DOI] [PubMed] [Google Scholar]
- 5.Block TM, Gish R, Guo H, Mehta A, Cuconati A, Thomas London W, et al. Chronic hepatitis B: What should be the goal for new therapies? Antiviral Research. 2013;98(1):27–34. doi: 10.1016/j.antiviral.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hai H, Tamori A, Kawada N. Role of hepatitis B virus DNA integration in human hepatocarcinogenesis. World journal of gastroenterology. 2014;20(20):6236–43. doi: 10.3748/wjg.v20.i20.6236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lucifora J, Trepo C. Hepatitis: After HCV cure, HBV cure? Nature Reviews Gastroenterology & hepatology. 2015;12(7):376–8. doi: 10.1038/nrgastro.2015.103. [DOI] [PubMed] [Google Scholar]
- 8.Block TM, Rawat S, Brosgart CL. Chronic hepatitis B: A wave of new therapies on the horizon. Antiviral Research. 2015;121:69–81. doi: 10.1016/j.antiviral.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeisel MB, Lucifora J, Mason WS, Sureau C, Beck J, Levrero M, et al. Towards an HBV cure: state-of-the-art and unresolved questions--report of the ANRS workshop on HBV cure. Gut. 2015;64(8):1314–26. doi: 10.1136/gutjnl-2014-308943. [DOI] [PubMed] [Google Scholar]
- 10.Sung JJY, Lai JY, Zeuzem S, Chow WC, Heathcote E, Perrillo R, et al. A randomised double-blind phase II study of lamivudine (LAM) compared to lamivudine plus adefovir dipivoxil (ADV) for treatment naïve patients with chronic hepatitis B (CHB): Week 52 analysis. J Hepatol. 2003;38(Suppl 2):25–6. [Google Scholar]
- 11.Perrillo RP. Current treatment of chronic hepatitis B: benefits and limitations. Seminars in liver disease. 2005;25(S1):20–8. doi: 10.1055/s-2005-915647. [DOI] [PubMed] [Google Scholar]
- 12.Marcellin P, Ahn SH, Ma X, Caruntu FA, Tak WY, Elkashab M, et al. Combination of tenofovir disoproxil fumarate and peginterferon alpha-2a increases loss of hepatitis B surface antigen in patients with chronic hepatitis B. Gastroenterology. 2016;150(1):134–44. doi: 10.1053/j.gastro.2015.09.043. [DOI] [PubMed] [Google Scholar]
- 13.Urban S, Schulze A, Schieck A, Gähler C, Ni Y, Meier A, et al. 10 preclinical studies on Myrcludex B, a novel entry inhibitor for hepatitis B and hepatitis delta virus (HDV) infections. J Hepatol. 2010;52(Supplement 1):S5. [Google Scholar]
- 14.Volz T, Allweiss L, Ben MM, Warlich M, Lohse AW, Pollok JM, et al. The entry inhibitor Myrcludex-B efficiently blocks intrahepatic virus spreading in humanized mice previously infected with hepatitis B virus. J Hepatol. 2013;58(5):861–7. doi: 10.1016/j.jhep.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 15.Verrier ER, Colpitts CC, Sureau C, Baumert TF. Hepatitis B virus receptors and molecular drug targets. Hepatology International. 2016:1–7. doi: 10.1007/s12072-016-9718-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Locarnini S, Hatzakis A, Chen D-S, Lok A. Strategies to control hepatitis B: Public policy, epidemiology, vaccine and drugs. J Hepatol. 2015;62:S76–S86. doi: 10.1016/j.jhep.2015.01.018. [DOI] [PubMed] [Google Scholar]
- 17.Schadler S, Hildt E. HBV life cycle: entry and morphogenesis. Viruses. 2009;1(2):185–209. doi: 10.3390/v1020185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Block TM, Guo H, Guo JT. Molecular virology of hepatitis B virus for clinicians. Clin Liver Dis. 2007;11(4):685–706. doi: 10.1016/j.cld.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo H, Jiang D, Zhou T, et al. Characterization of the intracellular deproteinized relaxed circular DNA of hepatitis B virus: an intermediate of covalently closed circular DNA formation. J Virol. 2007;81(22):12472–84. doi: 10.1128/JVI.01123-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou T, Guo H, Guo JT, Cuconati A, Mehta A, Block TM. Hepatitis B virus e antigen production is dependent upon covalently closed circular (ccc) DNA in HepAD38 cell cultures and may serve as a cccDNA surrogate in antiviral screening assays. Antiviral Res. 2006;72(2):116–24. doi: 10.1016/j.antiviral.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Cai D, Mills C, Yu W, Yan R, Aldrich CE, Saputelli JR, et al. Identification of disubstituted sulfonamide compounds as specific inhibitors of hepatitis B virus covalently closed circular DNA formation. Antimicrob Agents chemother. 2012;56(8):4277–88. doi: 10.1128/AAC.00473-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kennedy EM, Bassit LC, Mueller H, et al. Suppression of hepatitis B virus DNA accumulation in chronically infected cells using a bacterial CRISPR/Cas RNA-guided DNA endonuclease. Virology. 2015;476:196–205. doi: 10.1016/j.virol.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weber ND, Stone D, Sedlak RH, et al. AAV-mediated delivery of zinc finger nucleases targeting hepatitis B virus inhibits active replication. PLoS One. 2014;9(5):e97579. doi: 10.1371/journal.pone.0097579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin SR, Yang HC, Kuo YT, et al. The CRISPR/Cas9 system facilitates clearance of the intrahepatic HBV templates in vivo. Mol Ther Nucleic Acids. 2014;3:e186. doi: 10.1038/mtna.2014.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marcellin P, Castelnau C, Martinot-Peignoux M, Boyer N. Natural history of hepatitis B. Minerva Gastroenterol. Dietol. 2005;51(1):63–75. [PubMed] [Google Scholar]
- 26.Gish RG, Yuen MF, Chan HL, et al. Synthetic RNAi triggers and their use in chronic hepatitis B therapies with curative intent. Antiviral Res. 2015;121:97–108. doi: 10.1016/j.antiviral.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 27.Sebestyén MG, Wong SC, Trubetskoy V, et al. Targeted in vivo delivery of siRNA and an endosome-releasing agent to hepatocytes. Methods Mol Biol. 2015;1218:163–86. doi: 10.1007/978-1-4939-1538-5_10. [DOI] [PubMed] [Google Scholar]
- 28.Mao T, Graham M, Kao SC, Strings V, Cock TA, Lindell P, Roelvink P, Suhy D. BB-HB-331, a DNA-directed RNA interference agent (ddRNAi) for the treatment of subjects infected with hepatitis B virus (HBV), can effectively suppress HBV in a primary hepatocyte model. Global Antiviral Journal. 2015;11(Suppl 3) Abstract 111. [Google Scholar]
- 29.Alaluf MB, Shlomai A. New therapies for chronic hepatitis B. Liver international: official journal of the International Association for the Study of the Liver. 2016 doi: 10.1111/liv.13086. [DOI] [PubMed] [Google Scholar]
- 30.Bourne C, Lee S, Venkataiah B, Lee A, Korba B, Finn MG, et al. Small-molecule effectors of hepatitis B virus capsid assembly give insight into virus life cycle. J Virol. 2008;82(20):10262–70. doi: 10.1128/JVI.01360-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stray SJ, Zlotnick A. BAY 41-4109 has multiple effects on hepatitis B virus capsid assembly. J Mol Rec. 2006;19(6):542–8. doi: 10.1002/jmr.801. [DOI] [PubMed] [Google Scholar]
- 32.Shi C, Wu CQ, Cao AM, Sheng HZ, Yan XZ, Liao MY. NMR spectroscopy-based metabonomic approach to the analysis of Bay41-4109, a novel anti-HBV compound, induced hepatotoxicity in rats. Toxicol Lett. 2007;173:161–167. doi: 10.1016/j.toxlet.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 33.Weber O, Schlemmer KH, Hartmann E, Hagelschuer I, Paessens A, Graef E, Deres K, Goldmann S, Niewoehner U, Stoltefuss J, Haebich D, Ruebsamen-Waigmann H, Wohlfeil S. Inhibition of human hepatitis B virus (HBV) by a novel non-nucleosidic compound in a transgenic mouse model. Antiviral Res. 2002;54:69–78. doi: 10.1016/s0166-3542(01)00216-9. [DOI] [PubMed] [Google Scholar]
- 34.Billioud G, Pichoud C, Puerstinger G, Neyts J, Zoulim F. The main hepatitis B virus (HBV) mutants resistant to nucleoside analogs are susceptible in vitro to nonnucleoside inhibitors of HBV replication. Antiviral Res. 2011;92:271–276. doi: 10.1016/j.antiviral.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 35.Manzoor S, Saalim M, Imran M, Resham S, Ashraf J. Hepatitis B virus therapy: What's the future holding for us? World j gastroenterol. 2015;21(44):12558–75. doi: 10.3748/wjg.v21.i44.12558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu G, Liu B, Zhang Y, Li J, Arzumanyan A, Clayton MM, et al. Preclinical characterization of GLS4, an inhibitor of hepatitis B virus core particle assembly. Antimicrob Agents chemother. 2013;57(11):5344–54. doi: 10.1128/AAC.01091-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang XY, Wei ZM, Wu GY, Wang JH, Zhang YJ, Li J, et al. In vitro inhibition of HBV replication by a novel compound, GLS4, and its efficacy against adefovir-dipivoxil-resistant HBV mutations. Antiviral ther. 2012;17(5):793–803. doi: 10.3851/IMP2152. [DOI] [PubMed] [Google Scholar]
- 38.Belloni L, Li L, Palumbo GA, Chirapu SR, Calvo L, Finn M, et al. HAPs hepatitis B virus (HBV) capsid inhibitors block core protein interaction with the viral minichromosome and host cell genes and affect cccDNA transcription and stability.. AASLD Liver Meeting 2013; Abstract 138. [Google Scholar]
- 39.Lam A, Ren S, Vogel R, et al. Inhibition of hepatitis B virus replication by the HBV core inhibitor NVR 3-778.. AASLD Liver Meeting 2015.; San Francisco. November 13-17, 2015; Abstract 33. [Google Scholar]
- 40.Gane EJ, Schwabe C, Walker K, Flores L, Hartman G, Klumpp K, et al. Phase 1a safety and pharmacokinetics of NVR 3-778, a potential first-in-class HBV core inhibitor. AASLD. 2014:LB–19. [Google Scholar]
- 41.Gane EJ, Lim YS, Gordon SC, Visvanathan K, et al. The oral toll-like receptor-7 agonist GS-9620 in patients with chronic hepatitis B virus infection. J Hepatol. 2015;63(2):320–8. doi: 10.1016/j.jhep.2015.02.037. [DOI] [PubMed] [Google Scholar]
- 42.Rebbapragada I, Birkus G, Perry J, et al. Molecular determinants of GS-9620-dependent TLR7 activation. PLoS One. 2016;11(1):e0146835. doi: 10.1371/journal.pone.0146835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Al-Mahtab M, Bazinet M, Vaillant A. Effects of nucleic acid polymer therapy alone or in combination with immunotherapy on the establishment of SVR in patients with chronic HBV infection. J Clin Virol. 2015;69:228. [Google Scholar]
- 44.REP 2139-Ca / Pegasys™ combination therapy in hepatitis B / hepatitis D co-infection. U.S. National Institutes of Health ClinicalTrials.gov; 2015. Available from: clinicaltrials.gov/ct2/show/NCT02233075. [Google Scholar]
- 45.Vörös J, Urbanek A, Rautureau GJP, et al. Large-scale production and structural and biophysical characterizations of the human hepatitis B virus polymerase. J Virol. 2014;88(5):2584–99. doi: 10.1128/JVI.02575-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li C, Wang Y, Wang S, Wu B, Hao J, Fan H, et al. Hepatitis B virus mRNA-mediated miR-122 inhibition upregulates PTTG1-binding protein, which promotes hepatocellular carcinoma tumor growth and cell invasion. J Virol. 2013;87(4):2193–205. doi: 10.1128/JVI.02831-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fan CG, Wang CM, Tian C, Wang Y, Li L, Sun WS, et al. miR-122 inhibits viral replication and cell proliferation in hepatitis B virus-related hepatocellular carcinoma and targets NDRG3. Oncology Reports. 2011;26(5):1281–6. doi: 10.3892/or.2011.1375. [DOI] [PubMed] [Google Scholar]